
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 14 March 2025
Sec. Parasitology
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1519188
 Ru-Jin Liu1
Ru-Jin Liu1 Xian-Guo Guo1*
Xian-Guo Guo1* Pei-Ying Peng2
Pei-Ying Peng2 Yan Lv1
Yan Lv1 Peng-Wu Yin1
Peng-Wu Yin1 Wen-Yu Song1
Wen-Yu Song1 Rong Xiang1
Rong Xiang1 Yan-Ling Chen1
Yan-Ling Chen1 Bei Li1
Bei Li1 Dao-Chao Jin3
Dao-Chao Jin3Objective: The Asian house rat (Rattus tanezumi) is an important infectious source and reservoir host for many zoonotic diseases, and its associated mites (chigger mites and gamasid mites) can act as vectors for these diseases. The present study aimed to elucidate the infestation patterns and related ecology of the mites on the body surface of R. tanezumi in southwest China and explore relevant risk models.
Methods: Field surveys and taxonomic identification of the mites were conducted across five provincial regions in southwest China between 2001 and 2022. The constituent ratio (Cr), prevalence (Pm), mean abundance (MA), and mean intensity (MI) were calculated to reflect the mite infestation status. The species richness index (Mf), Shannon–Wiener diversity index (H´), Pielou’s evenness (E), and Simpson’s dominance index (D) were used to analyze the mite communities. A multiple logistic regression model was employed to identify potential risk factors associated with the mite infestation. The “corrplot” R software (version 4.3.1) package was used to analyze interspecific relationships among some mite species.
Results: A total of 75,023 mites were collected from 3,114 R. tanezumi rats, representing 12 families, 46 genera, and 252 species. Among these, 173 were the chigger mite species and 79 were the gamasid mite species. The species richness and community diversity of the chigger mites were higher than those of the gamasid mites, but the infestation indexes of the gamasid mites on the rats were higher than those of the chigger mites. Several vector mite species co-existed on R. tanezumi, with Laelaps nuttalli, L. echidninus, and Leptotrombidium deliense identified as the three dominant mite species, exhibiting high infestation indexes. The multiple logistic regression analysis showed that the mite infestation was influenced by a series of environmental factors and host-related factors (potential risk factors), with temperature and relative humidity identified as the most important risk factors. The impact of these potential risk factors on the infestation of a single mite group (chigger mites or gamasid mites) was different from the impact on the co-infestation of both mite groups together. Based on the logistic regression analysis, three predictive models were developed to predict the risk probability of each R. tanezumi rat being infested with chigger mites alone, gamasid mites alone, and both mite groups together. A positive correlation existed between any two of the following species: L. deliense, L. rubellum, and L. imphalum.
Conclusion: Rattus tanezumi rats are highly susceptible to mite infestation, hosting a variety of mite species and multiple vector mite species. The presence of multiple vector mite species on these rats increases the potential risk of transmission and persistence of related zoonotic diseases. A series of environmental factors and host factors, especially temperature and relative humidity, can influence mite infestation. The predictive models developed can estimate the likelihood of each rat being infested with mites. Some mite species show a preference for co-existing on R. tanezumi.
Rodents (rats, mice, voles, etc.) often harbor two groups of rodent-associated mites—chigger mites and gamasid mites—on their body surface (1, 2). Chigger mites belong to the suborder Trombidiformes, order Acariformes, subclass Acari, and class Arachnidia within the phylum Arthropoda, with over 3,000 species recorded globally (3, 4). Gamasid mites belong to the suborder Mesostigmata (or Gamasida) and order Parasitiformes within Acari, with more than 8,000 species known worldwide (5, 6). Rodents are not only pests in agriculture and forestry but also infectious sources of many zoonotic diseases (zoonoses) (7, 8). Rodent-associated mites can act as vectors or reservoir hosts for a number of zoonotic diseases (1, 9). Chigger mites are the exclusive vector of scrub typhus (tsutsugamushi disease), and some of them can act as potential vectors of hemorrhagic fever with renal syndrome (HFRS) (10, 11). Gamasid mites can act as vectors, potential vectors, or reservoir hosts for rickettsialpox, HFRS, and other zoonotic diseases (2, 12). Southwest China, covering five provincial regions—Sichuan, Chongqing, Guizhou, Yunnan, and Tibet (Xizang Autonomous Region)—is characterized by its vast territory, complex topography, diverse ecological environments, and different climate types. It has become a hot spot for ecological research on plants and animals (13, 14). In addition, southwest China is an important focus for scrub typhus, HFRS, and other zoonotic diseases, with serious epidemics occurring in some local areas (15, 16). The Asian house rat, Rattus tanezumi (Temminck, 1844), is a very common rodent species with a large population and wide distribution in southwest China. In addition to causing significant damage to agricultural and forestry plants, R. tanezumi also serves as an animal infectious source and reservoir host for many zoonotic diseases, including scrub typhus and HFRS (8, 17). Similar to most species of rodents, R. tanezumi is an important host for both chigger mites and gamasid mites. Through their biting activity, these mites can transmit and preserve pathogens of zoonotic diseases among rats (e.g., R. tanezumi), other rodents, and even from rodents to humans (8, 18).
Due to the medical significance of rodents and their associated mites, it is very important to study the infestation and related ecology of the mites on the body surface of rodents including R. tanezumi. However, the taxonomic identification of chigger mites and gamasid mites (especially chigger mites) is challenging due to their tiny size and the large number of species. To accurately identify these two groups of mites at the species level, numerous careful observations and comparisons must be conducted under a microscope. Many fine structures of chigger mites need to be measured individually under high power (×400) and oil immersion (×1,000), making the identification process particularly challenging. This challenging taxonomic identification process often makes it difficult to study both mite groups simultaneously (2, 19). In some previous studies, chigger mites and gamasid mites on rodents were separately analyzed and reported and they were not regarded as a whole mite community (8, 20). Although chigger mites and gamasid mites belong to different taxonomic groups in zoological taxonomy, they often coexist on the body surface of rodents to form a mite community (21, 22). Therefore, it is necessary to study both mite groups as a whole, including their co-infestation and the related ecological aspects of the entire mite community.
Based on field surveys and taxonomic identification conducted in southwest China between 2001 and 2022, this study is the first to examine chigger mites and gamasid mites on R. tanezumi rats as a whole. The present study aimed to elucidate the species composition, infestation status, related ecology, and risk models of these two mite groups on R. tanezum in southwest China, providing scientific information and guidance for the surveillance and control of vector mites and their rat hosts.
Between 2001 and 2022, field surveys and collections of rodent-associated mites (chigger mites and gamasid mites) were conducted at 112 survey sites across five provincial regions in southwest China (21°08′-33°41’N, 97°21′-110°11′E). The five provincial regions were Yunnan, Guizhou, Sichuan, Chongqing, and Tibet (Xizang Autonomous Region). However, the 112 survey sites did not cover the vast western part of Tibet due to its expansive and sparsely populated territory, relatively inconvenient transport, plateau hypoxia, potential risks in alpine areas, and limitations in manpower and financial support (see Figure 1 in “Results”). At each survey site, rodent hosts were routinely captured using metal wire mousetraps (18 × 12 × 9 cm, Guixi Mousetrap Apparatus Factory, Guixi, Jiangxi, China) in the afternoon or evening (20, 23). The captured rodent hosts were collected in white cloth bags the following morning and transported to a temporary field laboratory for mite collection. Each rodent host was routinely anesthetized with ether and placed on a large white square plate. Chigger mites and gamasid mites were then collected from the host’s body surface using a magnifier. The collected mites were preserved in 70% (or 75%) ethanol for subsequent use (11, 24). After each host was examined, the white square plate, along with the mousetrap, was cleaned with water and then disinfected with 75% ethanol. The white cloth bags, however, were soaked in a 5% Lysol solution for at least 24 h for disinfection and then rinsed with clean water (25–27). After the collection of the mites, each rodent host was identified to the species level according to its morphology (28–31). In the laboratory, the preserved mites were prepared as glass slide specimens using Hoyer’s medium after undergoing dehydration, transparency processing, and drying. Using taxonomic books and literature with taxonomic keys and detailed morphological descriptions, each mite specimen was eventually identified to the species level under a microscope (Olympus Corporation, Tokyo, Japan) (4, 32–37). After the identification of all the mites and their rodent hosts, R. tanezumi and its associated mites were selected as the subject of the present study. The use of animals (including rodent anesthesia and euthanasia) for our research was officially approved by the Animals’ Ethics Committee of Dali University, and the representative specimens were deposited in the specimen repository of the Institute of Pathogens and Vectors, Dali University.
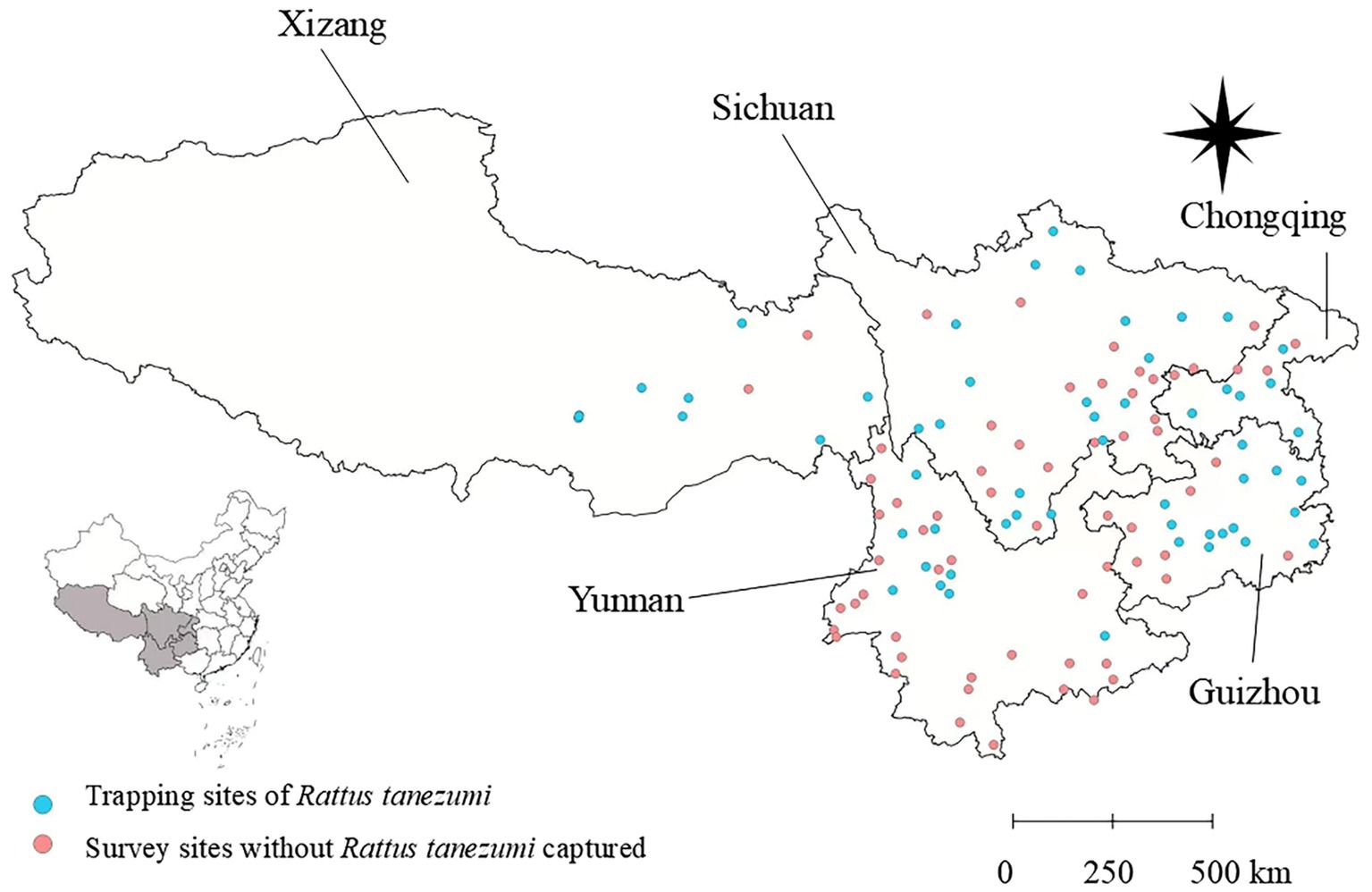
Figure 1. A total of 112 survey sites and 63 positive sites where the Asian house rats (Rattus tanezumi) were captured across the five provincial regions of southwest China between 2001 and 2022. This map was created using the standard map no. GS (2019) 1822, downloaded from the national standard map service system. The base map remained unchanged, and the geographical coordinate system was WGS84.
Based on the taxonomic identification, the number of the species and individual mites was counted. Following conventional methods, the constituent ratio (Cr), prevalence (Pm), mean abundance (MA), and mean intensity (MI) were calculated to assess the mite infestation status on R. tanezumi (19, 38). A chi-squared test was used to assess the statistical significance of Pm, while the non-parametric Kruskal–Wallis test was used to assess the statistical significance of MA and MI. A p-value of <0.05 was considered statistically significant; otherwise, the result was deemed non-significant. The species richness index (Mf), Shannon–Wiener diversity index (H′), Pielou’s evenness (E), and Simpson’s dominance index (D) were calculated to analyze the structure of the mite community (2, 27).
In the above formulas, S is the number of the species in the community, Ni is the number of a certain species (species i), N is the total number of all the species, Hi is the number of the hosts infested with mites, and H is the total number of the hosts examined.
The relative fatness (K) was used to evaluate the nutritional status of the hosts. A higher relative fatness value indicates better nutritional status, while a lower value suggests poorer nutritional status. The formula for calculating the relative fatness was as follows:
In the above formula, K = relative fatness (g/cm3), W = body weight (g), and L = body length (cm).
Multiple logistic regression analysis was conducted using SPSS 23.0 to analyze potential risk factors that may influence the mite infestation on R. tanezumi. In the analysis, the infestation status of the mites on R. tanezumi was regarded as the response variable (dependent variable), with infestation defined as 1 and non-infestation as 0 (1 = infested, 0 = uninfested). The explanatory variables (independent variables) included environmental factors and host factors. The backward stepwise logistic regression method was used to remove factors that were not statistically significant, and the remaining effective factors were included in the multiple logistic regression model (39, 40). Each factor was analyzed with the first level assumed as the reference (OR = 1), against which the other levels were compared and OR values were calculated. Statistically significant factors were screened (p < 0.05 was considered statistically significant, otherwise not) (41, 42).
In the above formula, P is the probability of the mite infestation and β0 is a constant. The β1, β2, … and βn represent partial regression coefficients, and x1, x2, … and xn stand for the independent variables.
Referring to the logic of the points system, a series of new partial regression coefficients (Sn) were calculated. The integer parts of the newly produced partial regression coefficients (S1, S2, …, Sn) were then used in the following calculation for the risk score (Sc). Based on the calculated risk score (Sc), risk assessment models for the probability of the mite infestation on R. tanezumi were finally established using the following formulas (40, 41, 43):
In the above formulas, βmin represents the smallest partial regression coefficient from the multiple logistic regression analysis, Sc is the sum of the risk scores (the sum of the newly produced partial regression coefficients, S1, S2, …, Sn), and P′ is the risk probability of the mite infestation on R. tanezumi.
Based on Spearman’s correlation coefficient (r), the “corrplot” statistical package in R software (version 4.3.1) was used to visualize the interspecific relationships among some vector species of the mites on R. tanezumi (44, 45).
A total of 3,114 Asian house rats (R. tanezumi) were captured from 63 of the 112 survey sites across the five provincial regions of southwest China, including Yunnan, Guizhou, Sichuan, Tibet (Xizang), and Chongqing (Figure 1). Among the five provincial regions, Yunnan had the largest number of the rats, accounting for 62.30% of the total (constituent ratio Cr = 62.30%, 1940/3114), followed by Sichuan (Cr = 22.13%, 689/3314), Guizhou (Cr = 10.85%, 338/3114), and Chongqing (Cr = 3.28%, 102/3114). Tibet had the lowest number of the R. tanezumi rats (Cr = 1.44%, 45/3114).
A total of 75,023 mites were collected from 3,114 rat hosts (R. tanezumi) and identified, comprising 252 species, 46 genera, and 12 families. This included 173 species of chigger mites from 19 genera in two families and 79 species of gamasid mites from 27 genera in 10 families. Although there were more species of the chigger mites (173 species) than the gamasid mites (79 species), the individual constituent ratio (Cr) of the gamasid mites (Cr = 67.11%, 50,348/75023) was significantly higher than that of the chigger mites (Cr = 32.89%, 24,675/75023). Among the 19 genera and two families of the chigger mites, the majority of the species (71) and individuals (14316) belonged to the genus Leptotrombidium in the family Trombiculidae. Among the 27 genera and 10 families of the gamasid mites, the majority of the species (12) and individuals (45454) belonged to the genus Laelaps in the family Laelapidae (Table 1; Figure 2). Among the 252 identified mite species, there were 22 vector species capable of serving as vectors or potential vectors for scrub typhus, HFRS, and other zoonotic diseases (zoonoses). These 22 vector mite species included 17 species of the chigger mites and five species of the gamasid mites. The 17 vector species of the chigger mites were as follows: Leptotrombidium deliense (Walch, 1922), L. rubellum (Wang and Liao, 1984), L. akamushi (Barumpt, 1910), L. pallidum (Nagayo et al., 1919), L. scutellare (Nagayo et al., 1921), L. wenense (Yang et al., 1959) (synonyms: L. kaohuense, L. kaohuensis or L. gaohuensis), L. sialkotense (Vercammen-Grandjean and Langston) (synonym: L. jishoum), L. yui (Chen and Hsu, 1955), Ascoschoengastia indica (Hirst, 1915), Walchia chinensis (Chen and Hsu, 1955), Odontaearus majestivus (Chen and Hsu, 1955), L. guzhangense (Wang et al., 1985), L. apodeme (Wen and Sun, 1984), L. intermedium (Nagayo et al., 1920), L. fuji (Kuwata et al., 1950), L. imphalum (Vercammen-Grandjean and Langston, 1975), and W. pacifica (Chen and Hsu, 1955). These species are known to act as vectors or potential vectors for scrub typhus. The five vector species of the gamasid mites were as follows: Ornithonyssus bacoti (Hirst, 1913), Haemolaelaps glasgowi (Ewing 1925), Tricholaelaps myonysognathus (Grochovskaya and Nguen-Xuan-Hoe, 1961), Eulaelaps stabularis (Koch, 1836), and H. casalis (Berlese, 1887). These can serve as vectors or potential vectors for rickettsialpox, HFRS, and other zoonoses (10, 11, 33, 35, 46, 47).
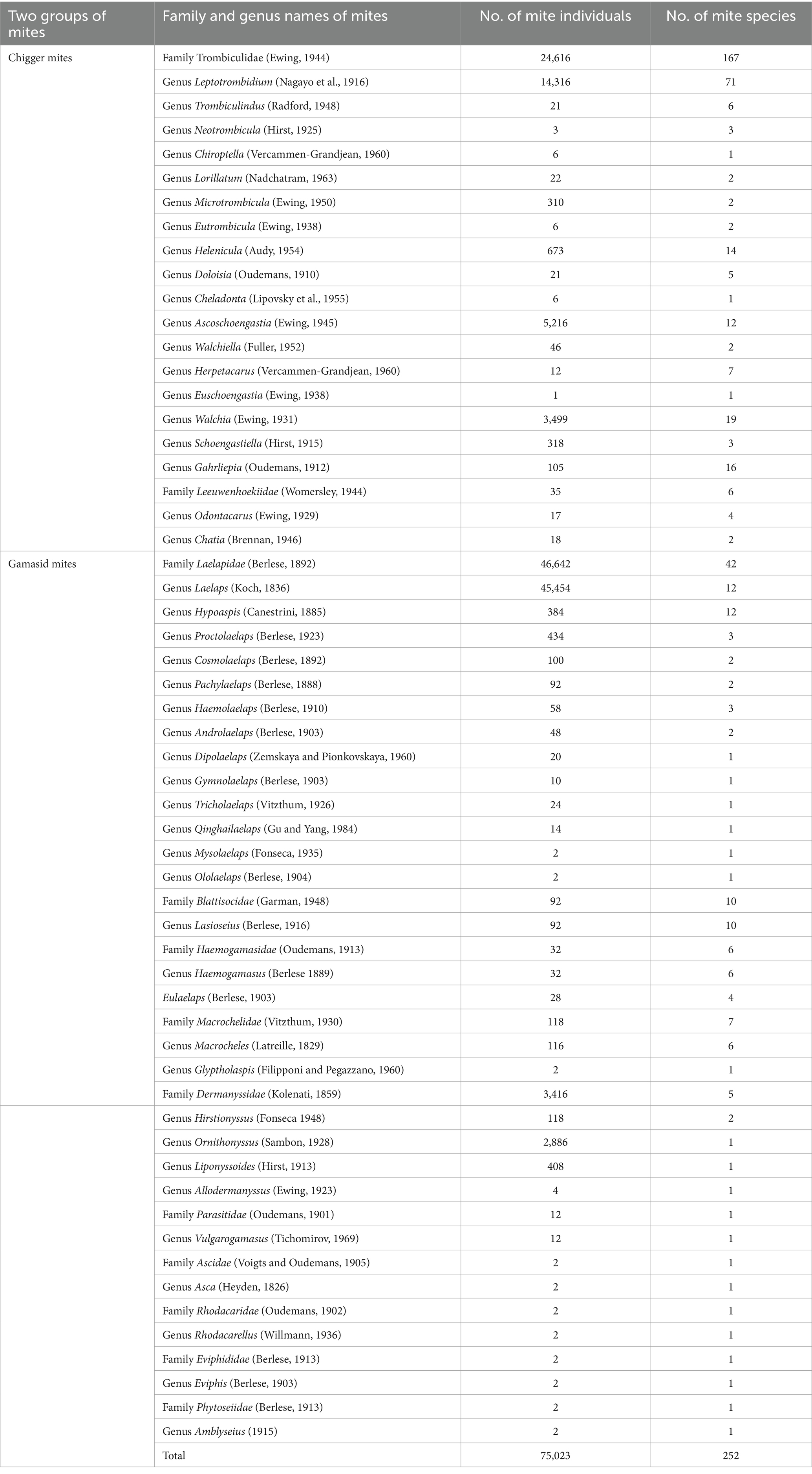
Table 1. Results of the taxonomic identification of the mites on R. tanezumi in southwest China (2001–2022).
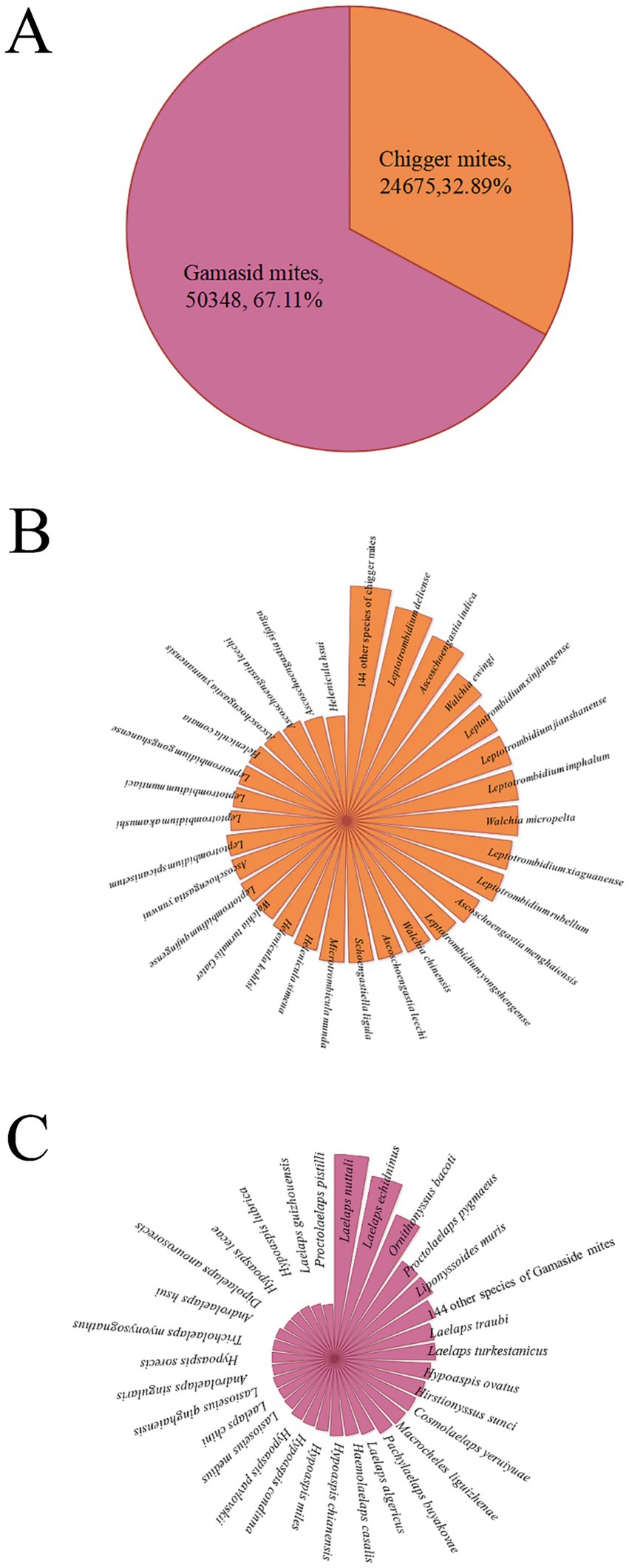
Figure 2. Composition of the mites on R. tanezumi in southwest China, 2001–2022 [annotations: (A). Two groups of mites; (B). species of the chigger mites; (C). species of the gamasid mites. The radius length of each small sector in B and C represents the number of corresponding mite species].
More than half of the R. tanezumi rats were infested with the mites, showing a high overall prevalence (Pm = 71.77%), overall mean abundance (MA = 24.09 mites/per examined rat), and overall mean intensity (MI = 33.57 mites/per infested rat). The infestation indexes (Pm, MA, and MI) for the gamasid mites were higher than those for the chigger mites (p < 0.05). However, the Shannon–Wiener diversity index (H′), Pielou’s evenness (E), and species richness index (Mf) for the chigger mite community were higher than those for the gamasid mite community (Table 2).
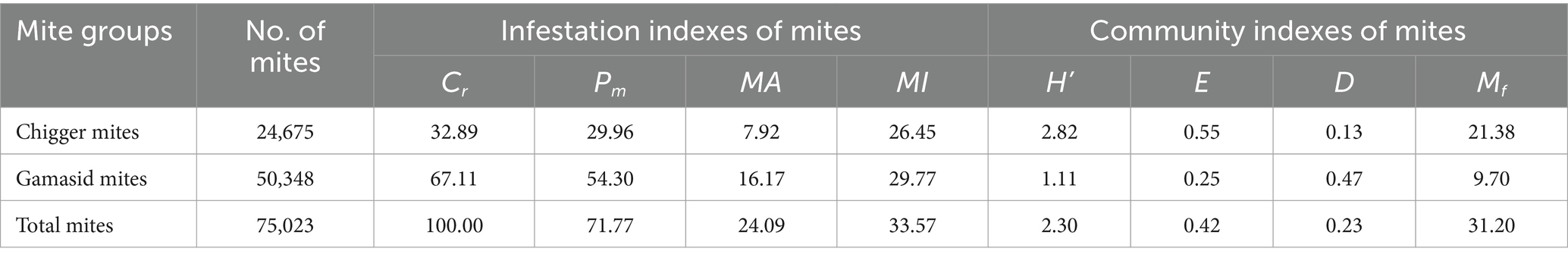
Table 2. Infestation and community indexes of the mites on R. tanezumi in southwest China (2001–2022).
Of the 252 mite species identified, 12 were dominant species, including nine species of the chigger mites and three species of the gamasid mites (Table 3). Among the nine dominant species of the chigger mites, Leptotrombidium deliense had the highest prevalence (Pm = 13.49%) and mean abundance (MA = 2.34) (p < 0.05). Among the three dominant species of the gamasid mites, Laelaps echidninus had the highest prevalence (Pm = 36.90%, p < 0.05) and L. nuttalli had the highest mean intensity (MI = 30.54, p < 0.05) (Table 3). The 12 dominant mite species included six vector species (four chigger species and two gamasid mite species), which are the primary, secondary, or potential vectors for scrub typhus, rickettsialpox, HFRS, and other zoonoses (Table 3) (10, 11, 33, 35, 46, 47).
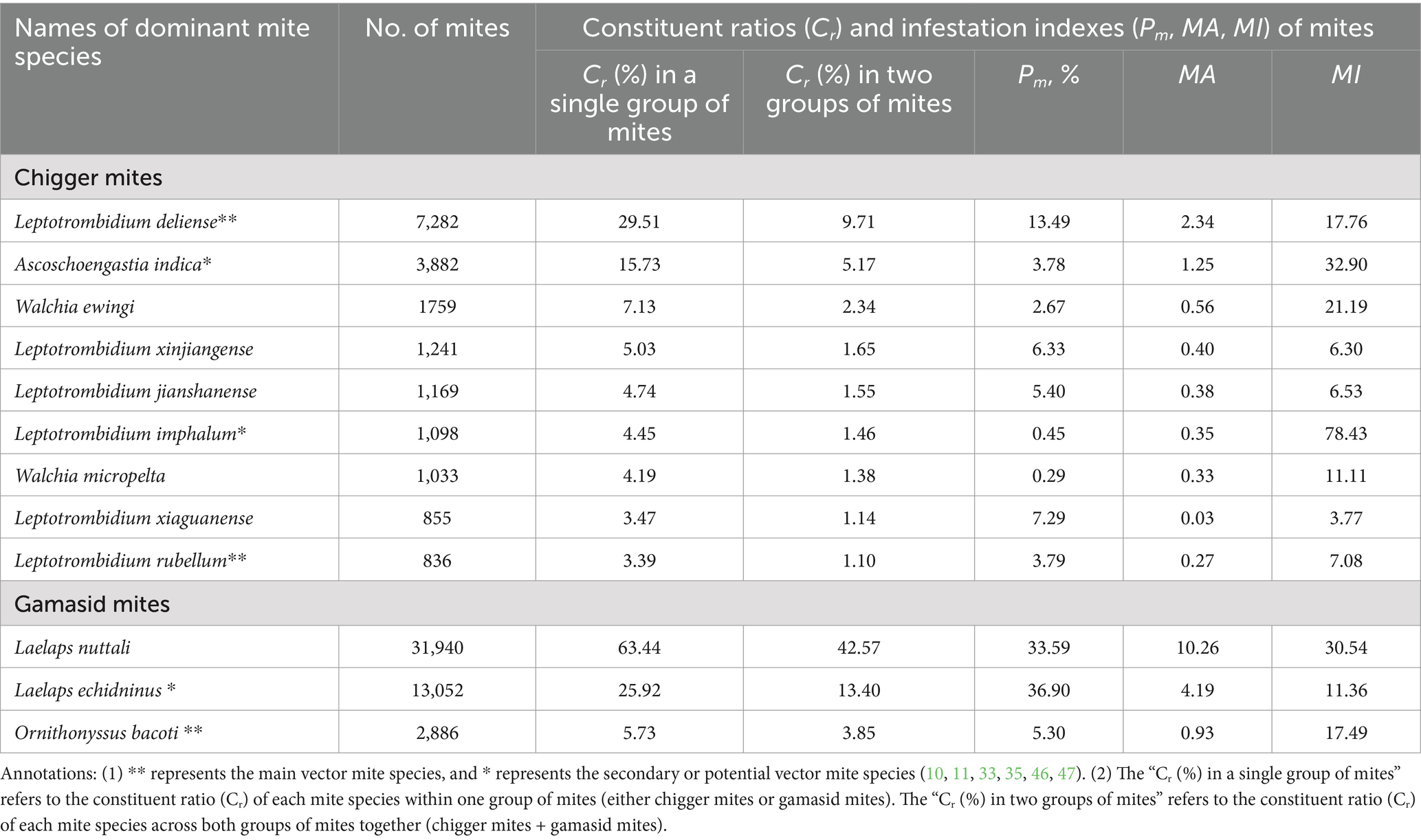
Table 3. Infestation indexes of the 12 dominant species of the mites on R. tanezumi in southwest China (2001–2022).
In the multiple logistic regression analysis, the explanatory variables (independent variables) included eight environmental factors and three host factors, and the variable assignment is listed in Table 4. The relative fatness that lacked statistical significance was removed based on the backward stepwise multiple logistic regression analysis. The remaining ten risk factors were included in the multiple logistic regression model. The Hosmer–Lemeshow goodness-of-fit test showed a good model fit (p = 0.12). The results showed that temperature, relative humidity, landscape, longitude, latitude, habitat, and host age were the risk factors for the chigger mite infestation (Table 5). Based on the above analyses, risk scores (Sc) for the seven risk factors were calculated using a scoring assessment system (Table 6). A predictive model for the chigger mite infestation on R. tanezumi was established as follows: . In the predictive model, P’1 represents the risk probability of each rat host (R. tanezumi) being infested with chigger mites.
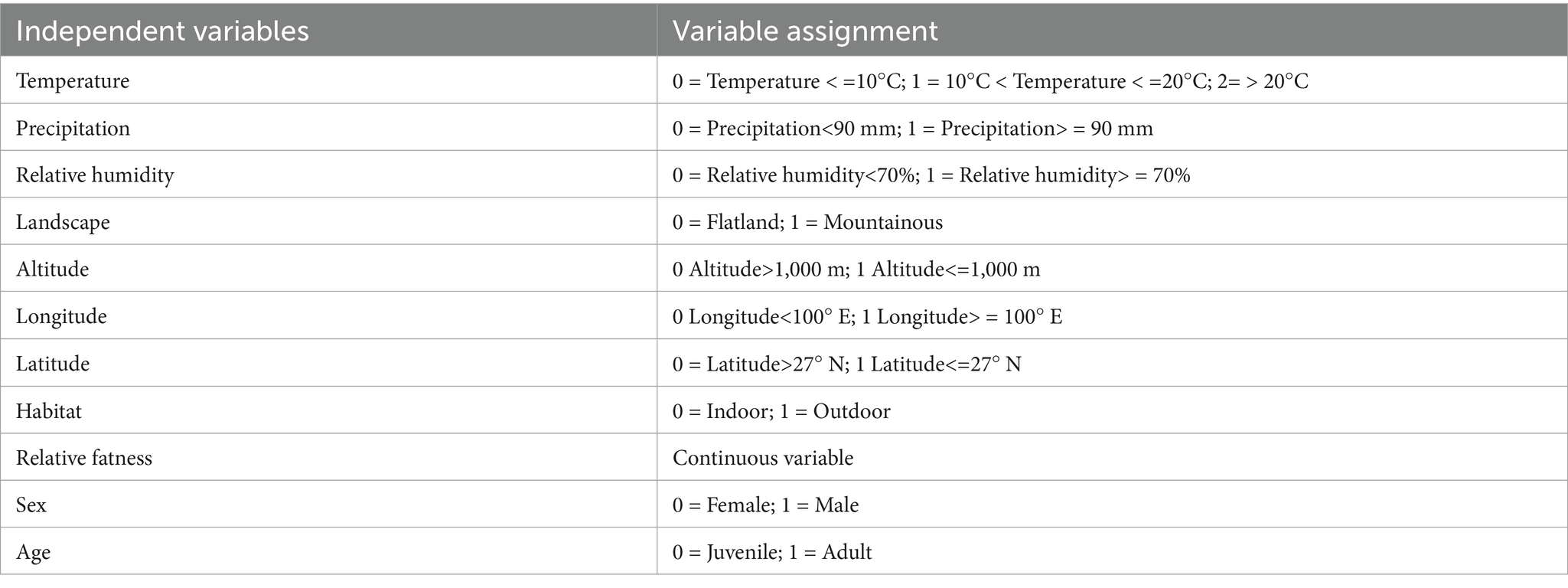
Table 4. Variable assignment in the multiple logistic regression analysis of the chigger mite infestation on R. tanezumi in southwest China (2001–2022).
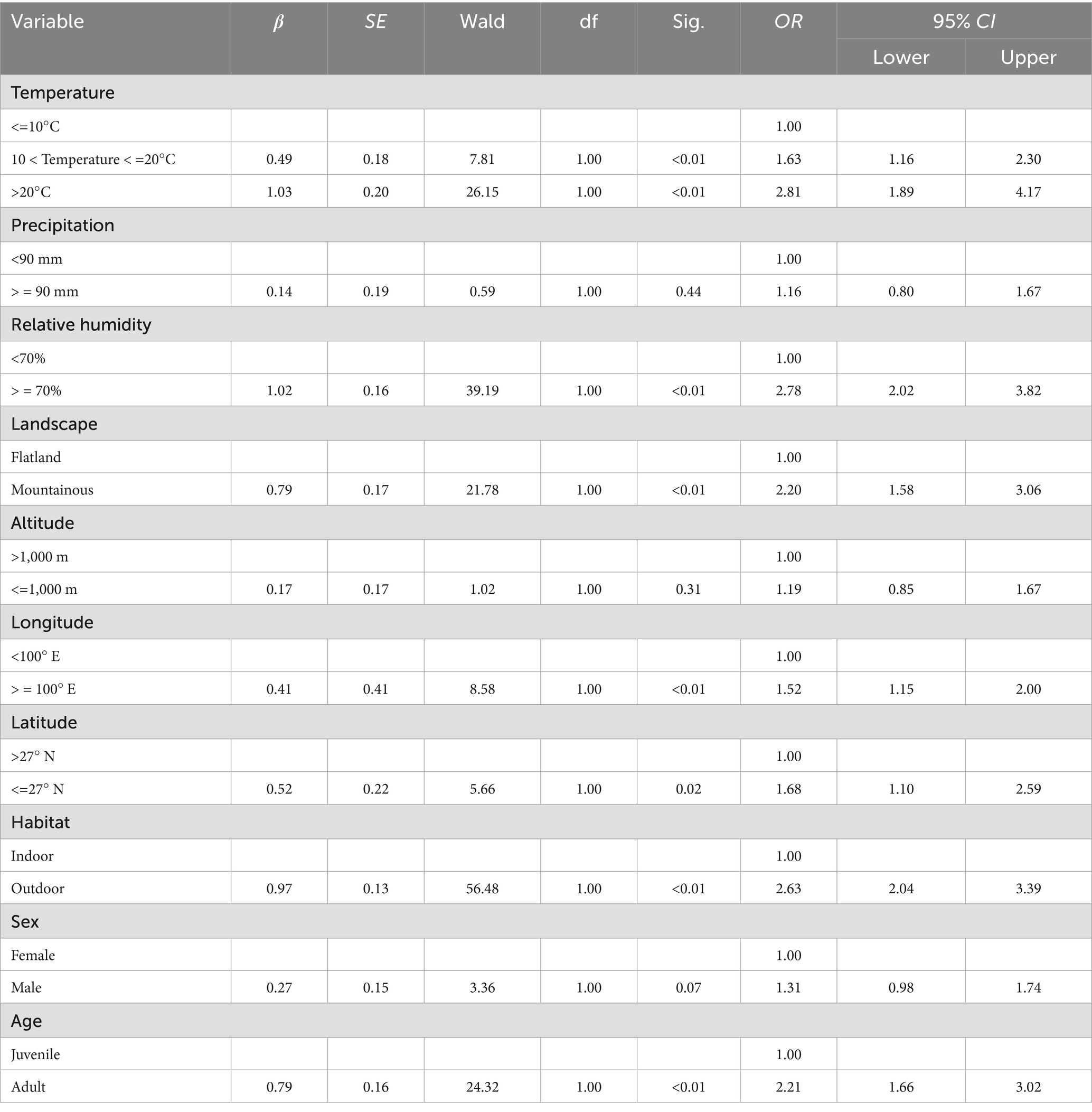
Table 5. Results of the multiple logistic regression analysis of the chigger mite infestation on R. tanezumi in southwest China (2001–2022).
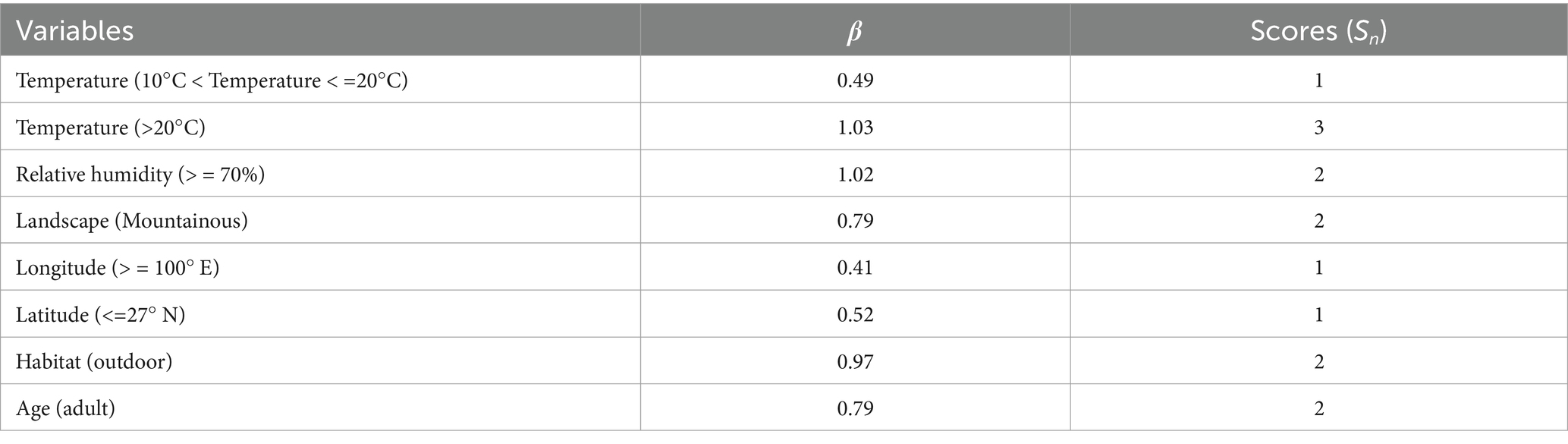
Table 6. Risk scores (Sc) for the chigger mite infestation on R. tanezumi in southwest China (2001–2022).
The variable assignment is listed in Table 7. Six factors that lacked statistical significance were removed, and five risk factors were included in the multiple logistic regression model. The five risk factors were temperature, relative humidity, altitude, habitat, and host sex. The Hosmer–Lemeshow test indicated a good fit (p = 0.60) for the established multiple logistic regression model. All OR values for the different factors are shown in Table 8. The results showed that temperature, relative humidity, altitude, habitat, and host sex were the risk factors for the gamasid mite infestation. Based on the above analyses, risk scores (Sc) for the five risk factors were calculated using a scoring assessment system (Table 9), and a predictive model for the gamasid mite infestation on R. tanezumi was developed as follows: . In the predictive model, P’2 represents the risk probability of each rat host (R. tanezumi) being infested with gamasid mites.
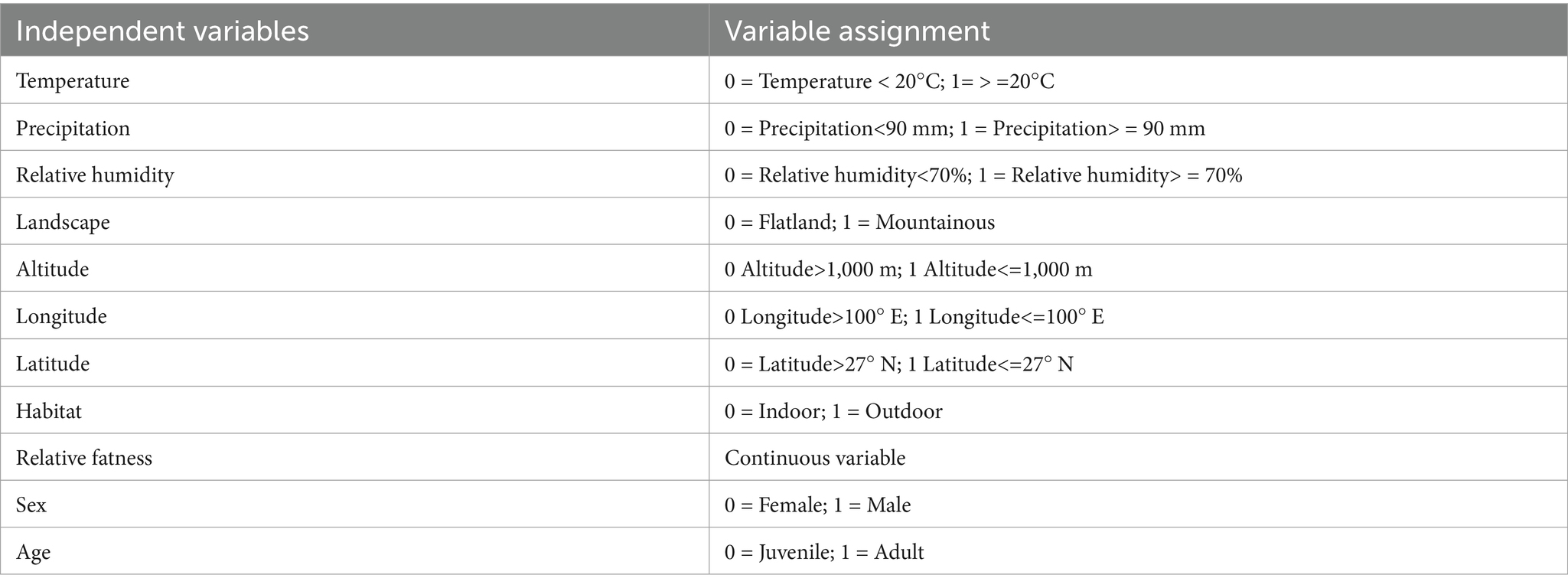
Table 7. Variable assignment in the multiple logistic regression analysis of the gamasid mite infestation on R. tanezumi in southwest China (2001–2022).
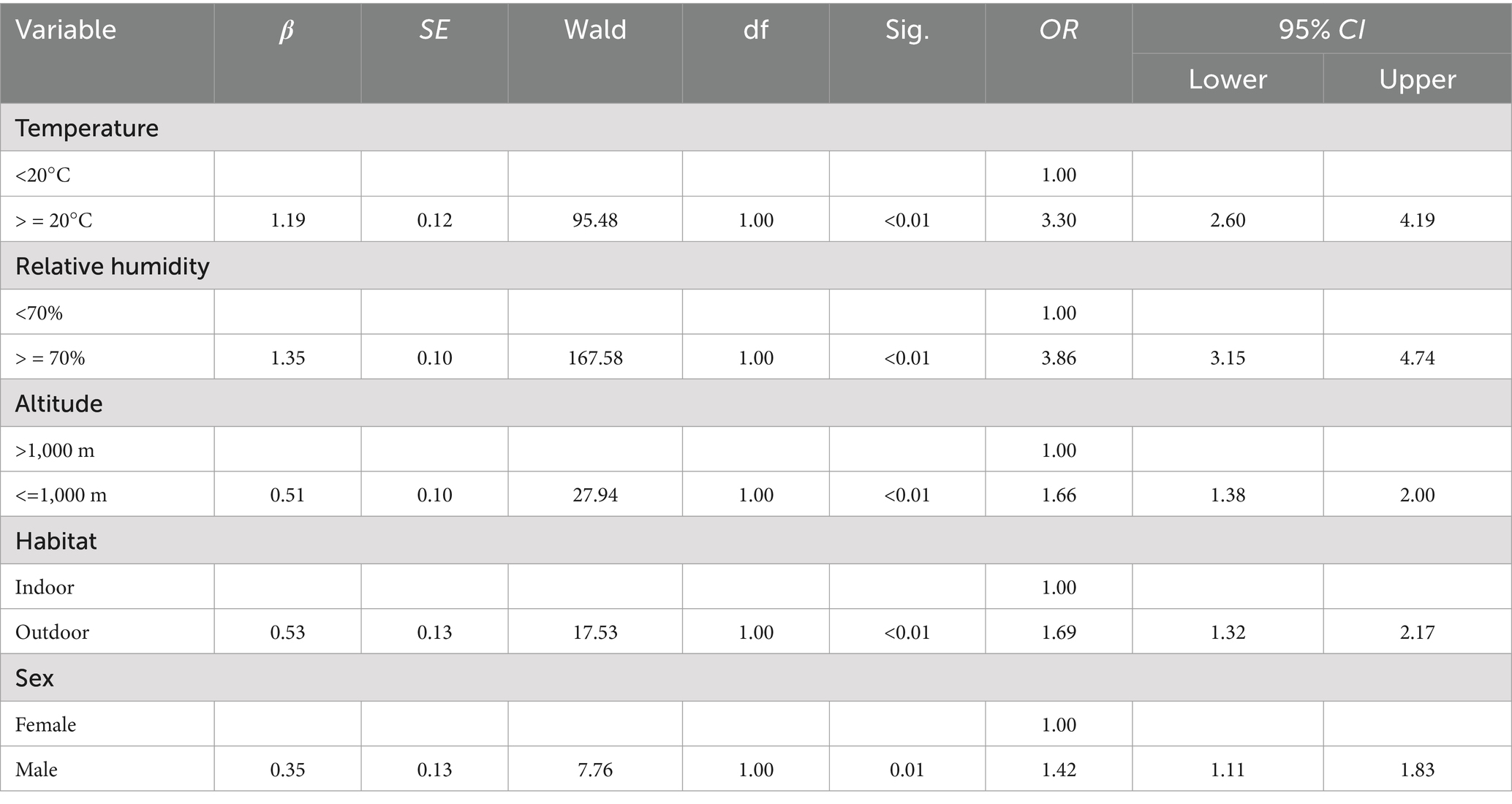
Table 8. Results of the multiple logistic regression analysis of the gamasid mite infestation on R. tanezumi in southwest China (2001–2022).
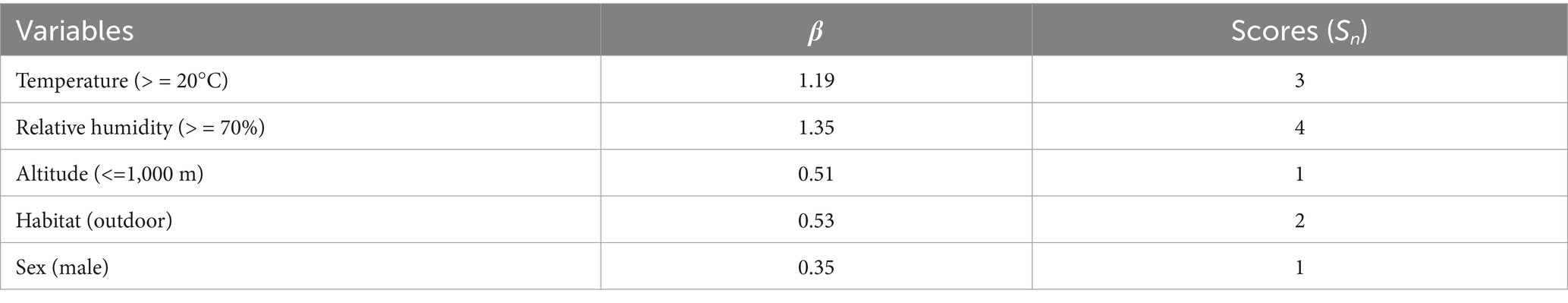
Table 9. Risk scores (Sc) for the gamasid mite infestation on R. tanezumi in southwest China (2001–2022).
The variable assignment is listed in Table 10. Three factors without statistical significance were removed, and eight risk factors were included in the multiple logistic regression model. The eight risk factors were temperature, precipitation, relative humidity, landscape, altitude, longitude, habitat, and host sex (Table 11). The Hosmer–Lemeshow test showed a good fit (p = 0.34) for the established multiple logistic regression model, and all OR values for the different factors are shown in Table 11. The results showed that temperature, precipitation, relative humidity, landscape, altitude, habitat, host sex, and host age were the risk factors for the co-infestation of the two mite groups. Based on the above analyses, risk scores (Sc) for the eight risk factors were calculated using a scoring assessment system (Table 12), and a predictive model for the co-infestation of the two mite groups on R. tanezumi was established as follows: . In the predictive model, P’3 represents the risk probability of each rat host (R. tanezumi) being infested with both mite groups.
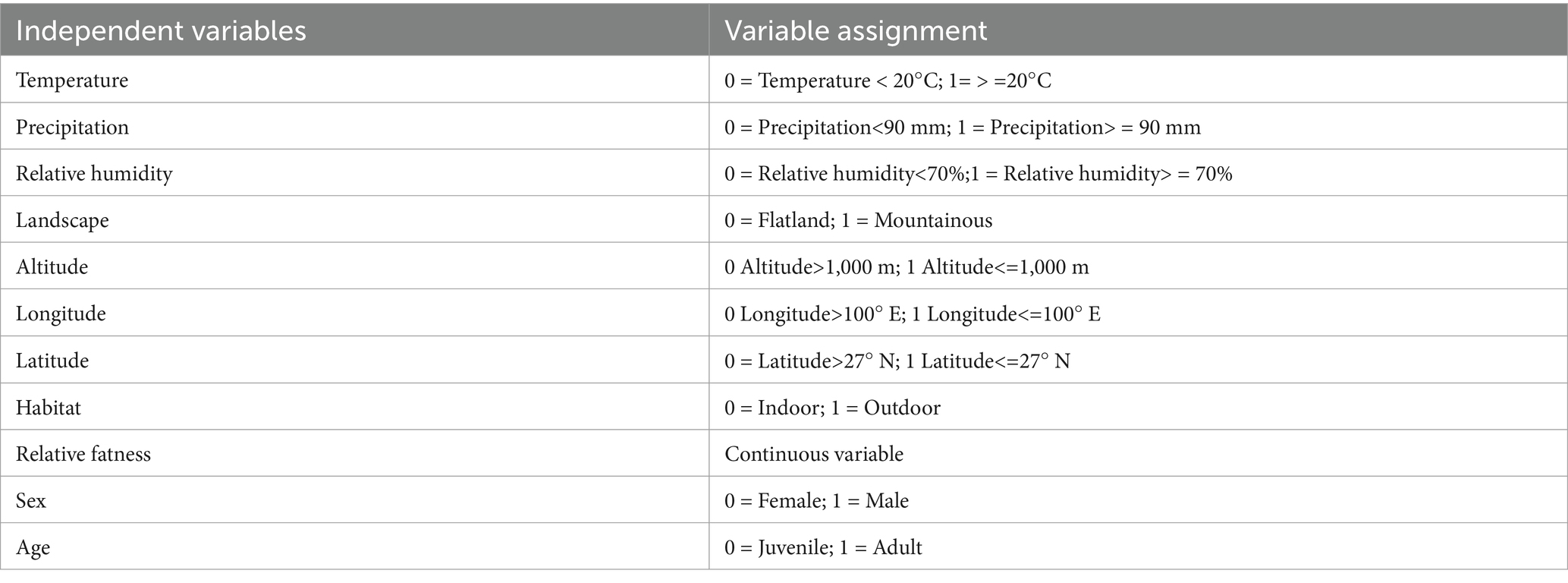
Table 10. Variable assignment in the multiple logistic regression analysis of the combined infestation of the two mite groups on R. tanezumi in southwest China (2001–2022).
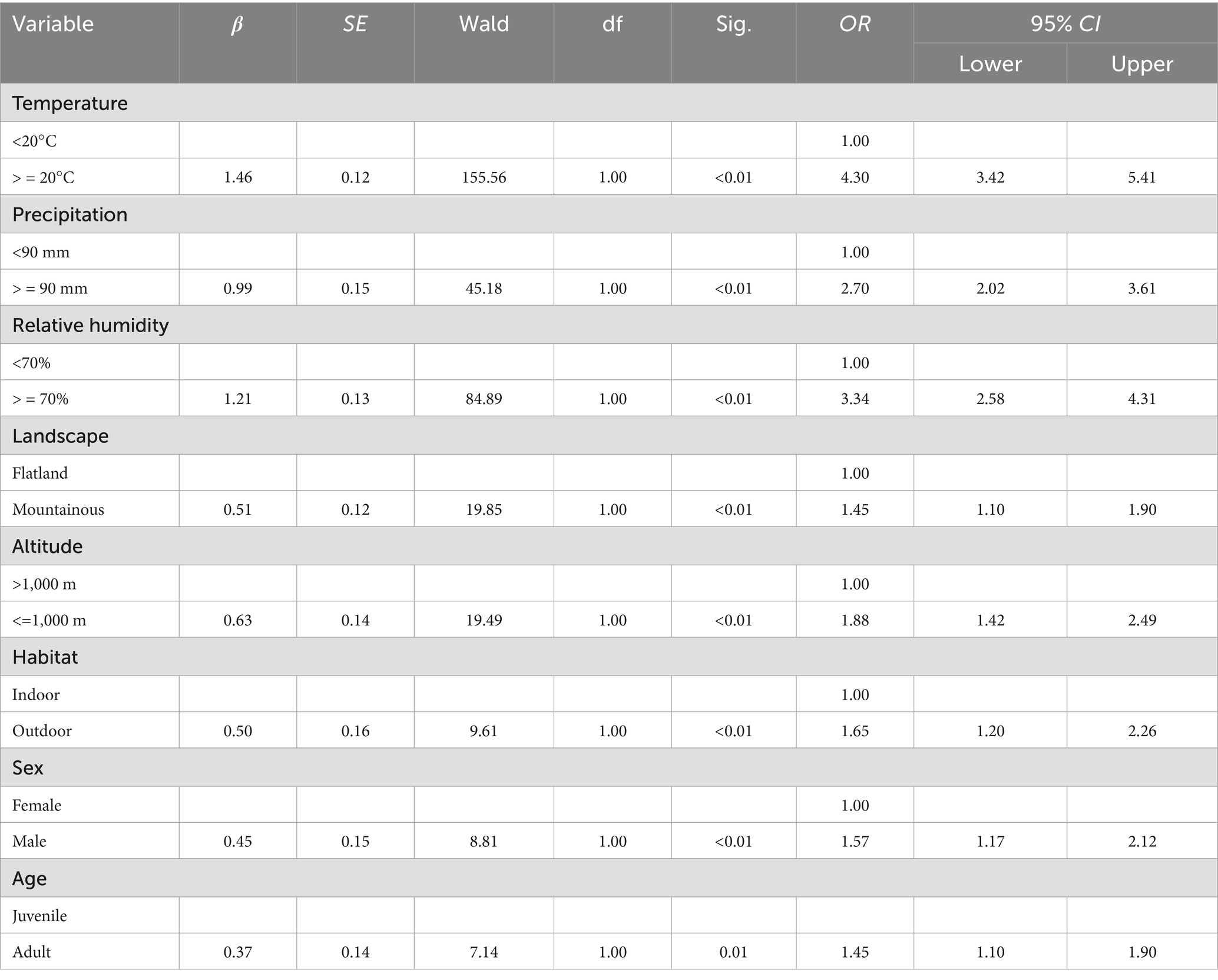
Table 11. Results of the multiple logistic regression analysis of the combined infestation of the two mite groups on R. tanezumi in southwest China (2001–2022).
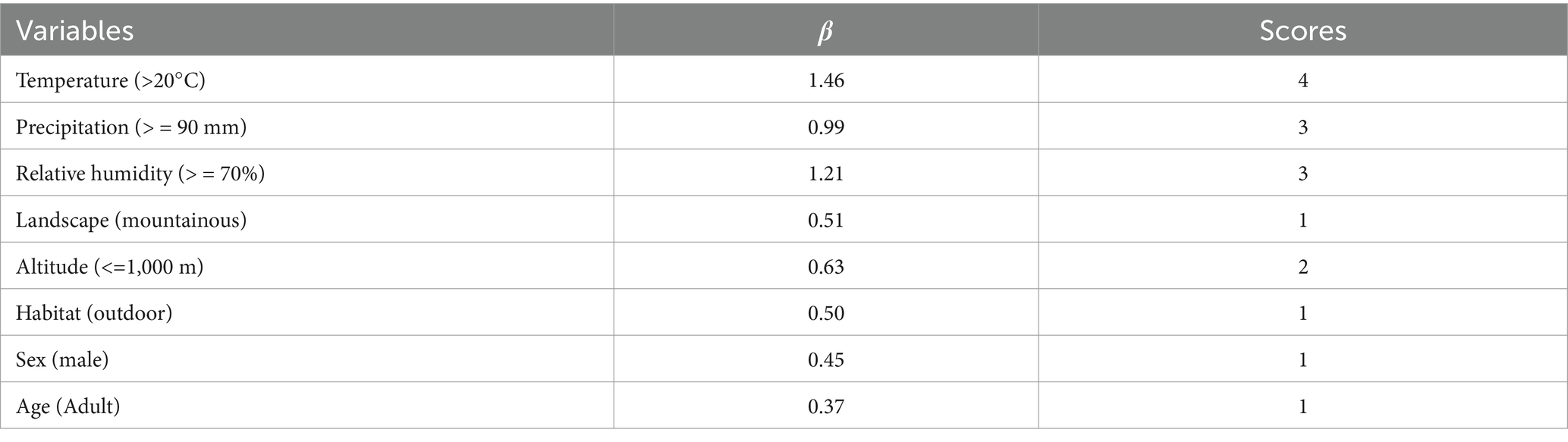
Table 12. Risk scores (Sc) for the combined infestation of the two mite groups on R. tanezumi in southwest China (2001–2022).
The “corrplot” R package was used to analyze the interspecific relationship among 19 important vector mite species on R. tanezumi, with a confidence interval of 0.95. The result is shown in Figure 3. The 19 important mite species included 12 species of the chigger mites and seven species of the gamasid mites. The 12 species of the chigger mites were as follows: L. scutellare, L. deliense, L. rubellum, L. jishoum, L. wenense, L. akamush, L. pallidum, L. guzhangense, L. yui, L. imphalum, A. indica, and W. chinensis. The seven species of gamasid mites are O. bacoti, H. glasgowi, T. myonyssognathus, E. stabularis, L. turkestanicus, L. echidninus, and H. caslis. In Figure 3, the blue squares represent a positive correlation between any two mite species (values ranging from 0 to 1), while the pink squares represent a negative correlation (values ranging from 0 to −1). The color depth indicates the strength of the positive or negative correlation. The result showed that there was a positive correlation between any two of the following species: L. deliense, L. rubellum, and L. imphalum (Figure 3).
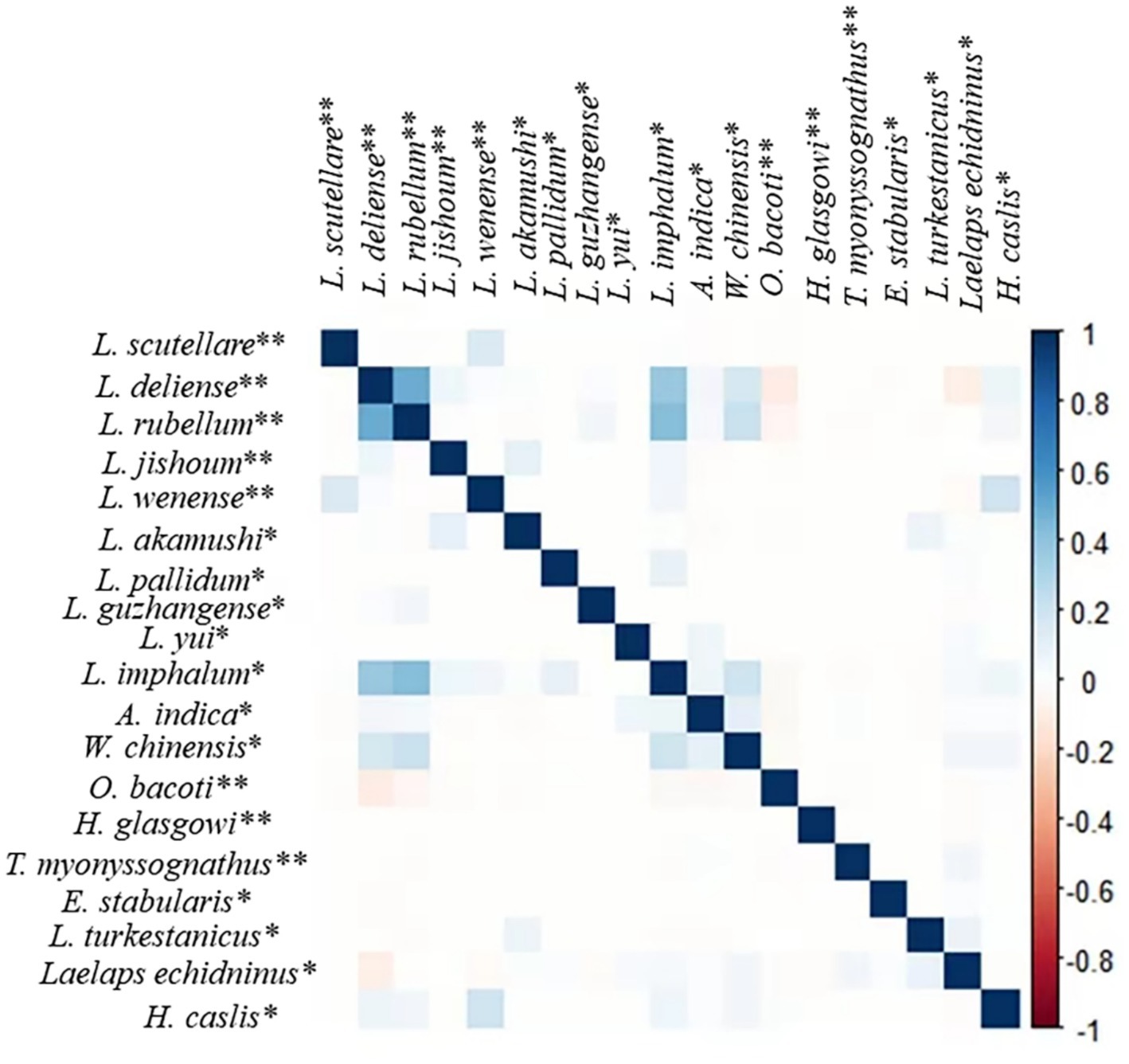
Figure 3. Interspecific relationships among some vector mite species on R. tanezumi in southwest China (2001–2022). Annotation: ** represents the main vector mite species, and * represents the secondary or potential vector mite species (10, 11, 33, 35, 46, 47).
As a very common rodent species, R. tanezumi is an important infectious source and reservoir host for many zoonotic diseases, such as scrub typhus and HFRS (17, 20). Southwest China is a significant focus for scrub typhus, HFRS, and other zoonotic diseases (15, 16). Rodent-associated mites (chigger mites and gamasid mites) found on R. tanezumi and other rodents can act as vectors or potential vectors for these zoonoses (48, 49). Therefore, studying the mites on R. tanezumi in southwest China holds considerable medical significance (50, 51). Although it is very common for chigger mites and gamasid mites to coexist on the body surface of the same rodent, the significant challenges in identifying species within these two mite groups (especially chigger mites) have often hindered their simultaneous study. As a result, previous reports have typically studied these two mite groups separately (20, 52). Based on the field surveys and taxonomic identification conducted in southwest China from 2001 to 2022, the present study investigated these two mite groups on the R. tanezumi rats as a whole for the first time. The results of the present study suggested that mite infestation is highly prevalent on R. tanezumi in southwest China, with a substantial mite burden (252 mite species and 75,023 mite individuals). The number of the chigger mite species (173) and gamasid mite species (79) found on R. tanezumi—a single rodent species—even exceeded the number of mite species found on several different host species across broader geographical regions of China. For example, 41, 53, and 81 species of chigger mites were recorded in Hubei Province, Fujian Province, and northwest China, which covers five provincial regions: Shanxi, Ningxia, Gansu, Qinghai, and Xinjiang (53–55). Similarly, 53, 21, and 36 species of gamasid mites were documented in Zhejiang, Chongqing, and northwest China (55–57). Southwest China encompasses a vast territory, complex and diverse landforms, rich vegetation and animal species, and a variety of climate types. These factors likely contribute to the high species diversity of mites found on R. tanezumi and other rodents in the region (51, 58). Different host species usually exhibit varying levels of susceptibility to mite and other ectoparasite infestations (9, 10). Rattus tanezumi is highly susceptible to mite infestation, which allows it to harbor a wide variety of mite species with a heavy infestation burden.
Of the 252 mite species identified, 22 species (17 chigger species and five gamasid mite species) can act as vectors or potential vectors for scrub typhus, HFRS, and other zoonotic diseases (10, 11, 33, 35, 46, 47). To date, six chigger species have been confirmed as the major vectors for scrub typhus in China, while several other chigger species are considered potential vectors. The six major vectors are L. deliense, L. scutellare, L. rubellum, L. sialkotense, L. wenense, and L. insulare (Wei et al., 1989). Evidence supporting their role as vectors comes from a series of epidemiological and experimental studies (10, 11, 33, 46). For example, large populations of these vector chigger mites have been identified in the foci of scrub typhus, and the seasonal fluctuation of the mites is highly consistent with the seasonal incidence of the disease (11, 27). Orientia tsutsugamushi (Ot), the causative agent of scrub typhus, has been repeatedly detected in vector chigger mites with natural infection. Ot can be successfully transmitted among laboratory rats and mice through the biting activity of the mites, which exhibit transovarial transmission characteristics (7, 9, 27, 33, 59). In addition, epidemiological and experimental evidence has also demonstrated that L. scutellare can effectively transmit Hantaan virus (hantavirus), the pathogen responsible for HFRS (27, 49). Similarly, through epidemiological and experimental studies, some gamasid mites have been proven to be vectors or potential vectors for rickettsialpox, HFRS, and other zoonotic diseases. For example, the pathogen of HFRS (hantavirus) has been detected or isolated from gamasid mites (12, 35, 47). Except for L. insulare, five of the six major vector chigger species in China were found on R. tanezumi in the present study. As a highly common rat species in human residential areas and nearby farmlands, R. tanezumi is closely associated with human life and agricultural activities (8, 20, 52). The presence of multiple vector mite species on R. tanezumi in southwest China increases the potential risk of transmitting scrub typhus, HFRS, and other zoonotic diseases from rats to humans through the biting activity of the mites. In addition, it increases the risk of focus persistence of the zoonoses in the region (11, 27, 60, 61).
Although multiple logistic regression analysis is widely used in epidemiology, economics, ecology, and other fields (39, 42, 62, 63), it has rarely been used in studies of rodent-associated mites (chigger mites and gamasid mites). The present study used the multiple logistic regression model to analyze the potential risk factors for mite infestation on R. tanezumi in southwest China for the first time. Some previous reports have shown that the infestation of ectoparasites can be simultaneously influenced by both environmental factors and host factors. The influencing factors and their effects on ectoparasites varied across different groups and species of ectoparasites (25, 64, 65). The results of the present study also suggested that a series of environmental factors and host factors can influence the infestation of mites on R. tanezumi. Although the effects of the risk factors on the infestation varied between the single mite group (chigger mites or gamasid mites) and the co-infestation of both mite groups together, temperature and relative humidity were the most important risk factors, with the highest risk scores (Sc) (Tables 6, 9, 12). This is consistent with some previous reports on other ectoparasites (25, 66, 67). This result implies that in areas with higher temperatures and higher relative humidity, R. tanezumi is more likely to be infested with mites, which may further increase the potential risk of zoonosis transmission and focus persistence (25, 66). Based on multiple logistic regression, the present study established three predictive models for the infestation risk of chigger mites, gamasid mites, and both mite groups together on R. tanezumi in southwest China for the first time. In clinical practice, similar predictive models can be developed to estimate the risk probability of individual patients being infested with a specific disease (40, 42). The three predictive models established in the present study aimed to predict the risk probability of each R. tanezumi rat being infested with chigger mites, gamasid mites, or both mite groups together. The method and procedure used to establish these predictive models can also be applied to develop corresponding predictive models for other parasitic infections or infestations, including those caused by other ectoparasites (e.g., fleas and sucking lice) in vector surveillance programs.
The analysis using the “corrplot” R package revealed positive or negative correlations between any two of the 19 important vector mite species on R. tanezumi (Figure 3). A positive correlation existed between any two of the following species: L. deliense, L. rubellum, and L. imphalum. A positive correlation indicated that two mite species tend to co-exist on the same host, R. tanezumi, and that their host selection is the same or similar. A negative correlation indicated that two mite species have different preferences in the selection of hosts and that they may experience interspecifc competition or mutual repulsion in selecting hosts. Leptotrombidium deliense, L. rubellum, and L. imphalum are three important vectors for scrub typhus (10, 11, 59). The positive correlation between any two of these three vector species (Figure 3) revealed their tendency to co-exist on the same rat, R. tanezumi. The co-existence of more than two vector species on the same rat may also increase the potential risk of transmitting scrub typhus among rats and even from rats to humans.
Rattus tanezumi is highly susceptible to mite infestation, hosting a wide variety of mite species and multiple vector mite species. The species diversity of the chigger mites was higher than that of the gamasid mites; however, the infestation indexes of the gamasid mites were higher than those of the chigger mites. The presence of multiple vector mite species on R. tanezumi increases the potential risk of transmission and focus persistence of related zoonotic diseases. A series of environmental factors and host factors can influence the infestation of mites on R. tanezumi, and temperature and relative humidity are the most important risk factors. The established predictive models aimed to estimate the risk probability of each R. tanezumi individual being infested with mites. The positive and negative correlation among some vector mite species revealed their similar and differing preferences for the rat host, respectively.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by the use of animals (including animal euthanasia) for our research was officially approved by the Animals’ Ethics Committees of Dali University, approval codes: DLXY2001-1116 and DLDXLL2020-1104, approval dates: 16 November 2001 and 4 November 2020. The study was conducted in accordance with the local legislation and institutional requirements.
R-JL: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. X-GG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. P-YP: Investigation, Writing – review & editing. YL: Investigation, Writing – review & editing. P-WY: Investigation, Writing – review & editing. W-YS: Investigation, Writing – review & editing. RX: Investigation, Writing – review & editing. Y-LC: Investigation, Writing – review & editing. BL: Investigation, Writing – review & editing. D-CJ: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (no. 82160400) and the Major Science and Technique Programs in Yunnan Province (no. 202102AA310055-X).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Conrad, H, Pollock, NB, and John-Alder, H. Chigger mite (Eutrombicula alfreddugesi) ectoparasitism does not contribute to sex differences in growth rate in eastern fence lizards (Sceloporus undulatus). Ecol Evol. (2023) 13:e10590. doi: 10.1002/ece3.10590
2. Zhou, JX, Guo, XG, Song, WY, Zhao, CF, Zhang, ZW, Fan, R, et al. Preliminary study on species diversity and community characteristics of gamasid mites on small mammals in three parallel rivers area of China. Animals. (2022) 12:3217. doi: 10.3390/ani12223217
3. Sungvornyothin, S, Kumlert, R, Paris, DH, Prasartvit, A, Sonthayanon, P, Apiwathnasorn, C, et al. Geometric morphometrics of the scutum for differentiation of trombiculid mites within the genus Walchia (Acariformes: Prostigmata: Trombiculidae), a probable vector of scrub typhus. Ticks Tick Borne Dis. (2019) 10:495–503. doi: 10.1016/j.ttbdis.2018.11.013
4. Stekolnikov, AA. Leptotrombidium (Acari: Trombiculidae) of the world. Zootaxa. (2013) 3728:1–173. doi: 10.11646/zootaxa.3728.1.1
5. Meehan, ML, Song, ZY, and Proctor, H. Roles of environmental and spatial factors in structuring assemblages of forest-floor Mesostigmata in the boreal region of northern Alberta. Canada Int J Acarol. (2018) 44:300–9. doi: 10.1080/01647954.2018.1520297
6. Moraza, ML. Glandular and non-glandular cuticular organs on the idiosoma of Gamasina mites (Acari: Mesostigmata). Persian J Acarol. (2025) 14:151–71. doi: 10.22073/pja.v14i1.86027
7. Walker, DH, and Mendell, NL. A scrub typhus vaccine presents a challenging unmet need. NPJ Vaccines. (2023) 8:11. doi: 10.1038/s41541-023-00605-1
8. Chen, YL, Guo, XG, Ding, F, Lv, Y, Yin, PW, Song, WY, et al. Infestation of oriental house rat (Rattus tanezumi) with chigger mites varies along environmental gradients across five provincial regions of Southwest China. Int J Environ Res Public Health. (2023) 20:2203. doi: 10.3390/ijerph20032203
9. Li, B, Guo, XG, Ren, TG, Peng, PY, Song, WY, Lv, Y, et al. Analysis on infestation and related ecology of chigger mites on large Chinese voles (Eothenomys miletus) in five provincial regions of Southwest China. Int J Parasitol. (2022) 19:169–79. doi: 10.1016/j.ijppaw.2022.08.013
10. Sadanandane, C, Elango, A, Panneer, D, Mary, KA, Kumar, NP, P Paily, K, et al. Seasonal abundance of Leptotrombidium deliense, the vector of scrub typhus, in areas reporting acute encephalitis syndrome in Gorakhpur district, Uttar Pradesh, India. Exp Appl Acarol. (2021) 84:795–808. doi: 10.1007/s10493-021-00650-2
11. Liu, QY, Fan, R, Song, WY, Peng, PY, Zhao, YF, Jin, DC, et al. The distribution and host-Association of the Vector Chigger Species Leptotrombidium imphalum in Southwest China. Insects. (2024) 15:504. doi: 10.3390/insects15070504
12. Beaulieu, F, Quintero-Gutiérrez, EJ, Sandmann, D, Klarner, B, Widyastuti, R, Cómbita-Heredia, O, et al. Review of the mite genus Ololaelaps (Acari, Laelapidae) and redescription of O. formidabilis Berlese. ZooKeys. (2019) 853:1–36. doi: 10.3897/zookeys.853.29407
13. Guo, Y, Guo, XG, Song, WY, Lv, Y, Yin, PW, and Jin, DC. Comparison of chiggers (Acari: Trombiculidae, Leeuwenhoekiidae) on two sibling mouse species, Apodemus draco and A. ilex (Rodentia: Muridae), in Southwest China. Animals. (2023) 13:1480. doi: 10.3390/ani13091480
14. Zhao, YJ, and Gong, X. Diversity and conservation of plant species in dry valleys, Southwest China. Biodivers Conserv. (2015) 24:2611–23. doi: 10.1007/s10531-015-0952-2
15. Yue, YJ, Ren, DS, Liu, XB, Wang, Y, Liu, Q, and Li, G. Spatio-temporal patterns of scrub typhus in mainland China, 2006-2017. PLoS Negl Trop Dis. (2019) 13:e0007916. doi: 10.1371/journal.pntd.0007916
16. Wang, YC, Zhang, CT, Gao, J, Chen, Z, Liu, Z, Huang, J, et al. Spatiotemporal trends of hemorrhagic fever with renal syndrome (HFRS) in China under climate variation. Proc Natl Acad Sci. (2024) 121:e2312556121. doi: 10.1073/pnas.2312556121
17. Kumlert, R, Chaisiri, K, Anantatat, T, Stekolnikov, AA, Morand, S, Prasartvit, A, et al. Autofluorescence microscopy for paired-matched morphological and molecular identification of individual chigger mites (Acari: Trombiculidae), the vectors of scrub typhus. PLoS One. (2018) 13:e0193163. doi: 10.1371/journal.pone.0193163
18. Xiang, R, Guo, XG, Zhao, CF, Fan, R, Mao, KY, Zhang, ZW, et al. Infestation and distribution of gamasid mites on Himalayan field rat (Rattus nitidus) in Yunnan Province of Southwest China. Biologia. (2021) 76:1763–73. doi: 10.2478/s11756-021-00679-z
19. Li, B, Guo, XG, Zhao, CF, Zhang, ZW, Fan, R, Peng, PY, et al. Infestation of chigger mites on Chinese mole shrew, Anourosorex squamipes, in Southwest China and ecological analysis. Parasite. (2022) 29:39. doi: 10.1051/parasite/2022038
20. Ding, F, Jiang, WL, Guo, XG, Fan, R, Zhao, CF, Zhang, ZW, et al. Infestation and related ecology of chigger mites on the Asian house rat (Rattus tanezumi) in Yunnan Province, Southwest China. Korean J Parasitol. (2021) 59:377–92. doi: 10.3347/kjp.2021.59.4.377
21. Guo, XG, Speakman, JR, Dong, WG, Men, XY, Qian, TJ, Wu, D, et al. Ectoparasitic insects and mites on Yunnan red-backed voles (Eothenomys miletus) from a localized area in Southwest China. Parasitol Res. (2013) 112:3543–9. doi: 10.1007/s00436-013-3537-6
22. Peng, PY, Guo, XG, Song, WY, Huo, P, Zou, YJ, Fan, R, et al. Analysis of ectoparasites (chigger mites, gamasid mites, fleas and sucking lice) of the Yunnan red-backed vole (Eothenomys miletus) sampled throughout its range in Southwest China. Med Vet Entomol. (2015) 29:403–15. doi: 10.1111/mve.12134
23. Ding, F, Guo, XG, Song, WY, Fan, R, Zhao, CF, Mao, KY, et al. Infestation and distribution of chigger mites on Brown rat (Rattus norvegicus) in Yunnan Province, Southwest China. Trop Biomed. (2021) 38:111–21. doi: 10.47665/tb.38.1.020
24. Chen, YL, Guo, XG, Song, WY, Ren, TG, Zhang, L, Fan, R, et al. Infestation and distribution of chigger mites on Confucian white-bellied rat (Niviventer confucianus) in Southwest China. Biologia. (2023) 78:727–36. doi: 10.1007/s11756-022-01261-x
25. Liu, QY, Guo, XG, Fan, R, Song, WY, Peng, PY, Zhao, YF, et al. A retrospective report on the infestation and distribution of chiggers on an endemic rodent species (Apodemus latronum) in Southwest China. Vet Sci. (2024) 11:547. doi: 10.3390/vetsci11110547
26. Ong, KH, Lewis, RD, Dixit, A, MacDonald, M, Yang, M, and Qian, Z. Inactivation of dust mites, dust mite allergen, and mold from carpet. J Occup Environ Hyg. (2014) 11:519–27. doi: 10.1080/15459624.2014.880787
27. Liu, RJ, Guo, XG, Zhao, CF, Zhao, YF, Peng, PY, and Jin, DC. An ecological survey of chiggers (Acariformes: Trombiculidae) associated with small mammals in an epidemic focus of scrub typhus on the China–Myanmar border in Southwest China. Insects. (2024) 15:812. doi: 10.3390/insects15100812
28. Huang, WJ, Chen, YX, and Wen, YX. Rodents of China. Shanghai: Fudan University Press, (1995): 1–286.
29. Wei, FW, Yang, QS, Wu, Y, Jiang, XL, Liu, SY, Li, BG, et al. Catalogue of mammals in China. Acta Theriol Sinica. (2021) 41:487–501. doi: 10.16829/j.slxb.150595
30. Wilson, DE, Lacher, TE, and Mittermeier, RA. Handbook of the mammals of the world: 6: lagomorphs and rodents I. Barcelona: Lynx Edicions, (2016),1–407.
31. Wilson, DE, Lacher, TE, and Mittermeier, RA. Handbook of the mammals of the world: 6: lagomorphs and rodents I. Rodents II. Barcelona: Lynx Edicions in association with Conservation International and IUCN, (2017),1–352.
32. Vercammen-Grandjean, PH, and Langston, RL. The chigger mites of the world: vol. 3, Leptotrombidium complex. San Francisco: George Williams Hooper Foundation, University of California, (1976): 1–108.
33. Li, JC, Wang, DQ, and Chen, XB. Trombiculid mites of China: Studies on vector pathogen of tsutsugamushi disease. Guangzhou: Guangdong Science & Technology Press, (1997): 97–551.
34. Hyatt, KH, and Embersom, RM. A review of the Macrochelidae (Acari: Mesostigmata) of the British Isles. Bull Br Mus Nat History Zool. (1988) 54:63–125. doi: 10.5962/bhl.part.17598
35. Pan, ZW, and Deng, GF. Economic insect Fauna of China. Fasc 17, Acari: Gamasina. Beijing, China: Science Press (1980).
36. Luo, LP, Guo, XG, Qian, TJ, Wu, D, Men, XY, Dong, WG, et al. Distribution of gamasid mites on small mammals in Yunnan Province, China. Insect Sci. (2007) 14:71–8. doi: 10.1111/j.1744-7917.2007.00128.x
37. Evans, GO, and Till, WM. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes): an introduction to their external morphology and classification. Transact Zool Soc London. (1979) 35:139–262. doi: 10.1111/j.1096-3642.1979.tb00059.x
38. Yin, PW, Guo, XG, Jin, DC, Song, WY, Zhang, L, Zhao, CF, et al. Infestation and seasonal fluctuation of gamasid mites (Parasitiformes: Gamasida) on indochinese forest rat, Rattus andamanensis (Rodentia: Muridae) in southern Yunnan of China. Biology. (2021) 10:1297. doi: 10.3390/biology10121297
39. Park, JW, Yu, DS, Lee, GS, Seo, JJ, Chung, JK, and Lee, JI. Epidemiological characteristics of rodents and chiggers with Orientia Tsutsugamushi in the Republic of Korea. Korean J Parasitol. (2020) 58:559–64. doi: 10.3347/kjp.2020.58.5.559
40. Shimamoto, T, Komiya, T, and Tsuneyoshi, H. Fate of uncomplicated acute type B aortic dissection and impact of concurrent aortic dilatation on remote aortic events. J Thorac Cardiovasc Surg. (2019) 157:854–63. doi: 10.1016/j.jtcvs.2018.05.126
41. Wang, QG, Koval, JJ, Mills, CA, and Lee, KID. Determination of the selection statistics and best significance level in backward stepwise logistic regression. Commun Stat Simul Comput. (2007) 37:62–72. doi: 10.1080/03610910701723625
42. Vieira-Andrade, JD, Rocha-Neves, JP, Macedo, JP, and Dias-Neto, MF. Onset of neurological deficit during carotid clamping with carotid endarterectomy under regional anesthesia is not a predictor of carotid restenosis. Ann Vasc Surg. (2019) 61:193–202. doi: 10.1016/j.avsg.2019.05.025
43. Ding, WY, Chen, YJ, and Wang, P. Construction and clinical value analysis of a risk warning model for poor prognosis of uterine corpus endometrial cancer. J Modern Oncol. (2023) 31:4377–82.
44. Chen, YL, Guo, XG, Ren, TG, Zhang, L, Fan, R, Zhao, CF, et al. Infestation and distribution of chigger mites on Chevrieri's field mouse (Apodemus chevrieri) in Southwest China. Int J Parasitol. (2022) 17:74–82. doi: 10.1016/j.ijppaw.2021.12.003
45. Wu, YC, Qian, Q, Magalhaes, RJS, Han, ZH, Haque, U, Weppelmann, TA, et al. Rapid increase in scrub typhus incidence in mainland China, 2006–2014. Am J Trop Med Hyg. (2016) 94:532–6. doi: 10.4269/ajtmh.15-0663
46. Xiang, R, Ren, TG, and Guo, XG. Research history and progress of six vector chigger species of scrub typhus in China. Syst Appl Acarol. (2022) 27:1841–56. doi: 10.11158/saa.27.9.11
47. Manucharyan, A, Achenbach, J, Paronyan, L, Avetisyan, L, Danielyan, R, and Melik-Andreasyan, G. Gamasid ticks as vectors of tularemia in the southeast of Armenia. Vector Borne Zoon Dis. (2023) 23:284–90. doi: 10.1089/vbz.2022.0082
48. Lamichhane, S, Achhami, E, Mahaju, S, Gautam, R, and Adhikari, A. A case of acute encephalitis syndrome and cranial nerve palsy secondary to scrub typhus: a rare presentation from Western Nepal. Clin Case Reports. (2023) 11:e7376. doi: 10.1002/ccr3.7376
49. Song, WY, Lv, Y, Yin, PW, Yang, YY, and Guo, XG. Potential distribution of Leptotrombidium scutellare in Yunnan and Sichuan provinces, China, and its association with mite-borne disease transmission. Parasit Vectors. (2023) 16:164. doi: 10.1186/s13071-023-05789-y
50. Lv, Y, Guo, XG, Jin, DC, Song, W, Peng, P, Lin, H, et al. Infestation and seasonal fluctuation of chigger mites on the southeast Asian house rat (Rattus brunneusculus) in southern Yunnan Province, China. Int J Parasitol. (2021) 14:141–9. doi: 10.1016/j.ijppaw.2021.02.005
51. Li, Q, Li, XY, Hu, WQ, Song, WY, He, SW, Wang, HJ, et al. Mammals of Gaoligong Mountain in China: diversity, distribution, and conservation. Zool Res. (2024) 1:3–19. doi: 10.24272/j.issn.2097-3772.2023.005
52. Huang, LQ, Guo, XG, Speakman, JR, and Dong, WG. Analysis of gamasid mites (Acari: Mesostigmata) associated with the Asian house rat, Rattus tanezumi (Rodentia: Muridae) in Yunnan Province, Southwest China. Parasitol Res. (2013) 112:1967–72. doi: 10.1007/s00436-013-3354-y
53. Yang, ZQ, and Liu, YR. A preliminary list of chigger mites in Hubei Province. Acta Arachnologica Sinica. (2003) 12:112–6. doi: 10.3969/j.issn.1005-9628.2003.02.012
54. Wang, DQ, and Liao, HR. A list of the trombiculid mites of Fujian Province. Wuyi Sci J. (1981) 3:104–10.
55. Liu, ZJ, Tian, Y, and Zhou, L. List of vector species in Northwest China. Beijing: Military Medical Science Press, (2011): 205–215.
56. Lu, MG, Jiang, QL, Gong, ZY, Ni, QX, and Ma, LM. A list of gamasid mites (Acari: Gamasina) in Zhejiang province. Chin J Vector Biol Control. (2017) 28:269–73. doi: 10.11853/j.issn.1003.8280.2017.03.019
57. Ji, HQ, Feng, SQ, Liu, N, He, YM, Li, H, Zhu, B, et al. Species and geographical distribution of fleas and gamasid mites on the rat-shape animals in Chongqing city. Chin J Hyg Insect Equip. (2012) 18:413–5. doi: 10.19821/j.1671-2781.2012.05.015
58. Li, F, Huang, XY, Zhang, XC, Zhao, XX, Yang, JH, and Chan, BPL. Mammals of Tengchong section of Gaoligongshan national nature reserve in Yunnan Province. China J Threat Taxa. (2019) 11:14402–14. doi: 10.11609/jott.4439.11.11.14402-14414
59. Lv, Y, Guo, XF, Jin, DC, Song, W, Fan, R, Zhao, C, et al. Host selection and seasonal fluctuation of Leptotrombidium deliense (Walch, 1922) (Trombidiformes: Trombiculidae) at a localized area of southern Yunnan, China. Syst Appl Acarol. (2019) 24:2253–71. doi: 10.11158/saa.24.11.15
60. Wulandhari, SA, Charoennitiwat, V, Samung, Y, Sonthayanon, P, Kumlert, R, Morand, S, et al. Intraspecific sensilla dimorphism in Ascoschoengastia indica (Prostigmata, Trombiculidae). Heliyon. (2024) 10:e33908. doi: 10.1016/j.heliyon.2024.e33908
61. Jiang, J, and Richards, AL. Scrub typhus: no longer restricted to the tsutsugamushi triangle. Tropical Med Infect Dis. (2018) 3:11. doi: 10.3390/tropicalmed3010011
62. Kingsland, S. The refractory model: the logistic curve and the history of population ecology. Q Rev Biol. (1982) 57:29–52. doi: 10.1086/412574
63. Vilken, V, Kalinina, O, Barykin, S, and Zotova, E. Logistic methodology of development of the regional digital economy In: IOP conference series: Materials science and engineering, vol. 497. Britain: IOP Publishing (2019). 012037.
64. Krasnov, BR, Shenbrot, GI, Korallo-Vinarskaya, NP, Vinarski, MV, Warburton, EM, and Khokhlova, IS. The effects of environment, hosts and space on compositional, phylogenetic and functional beta-diversity in two taxa of arthropod ectoparasites. Parasitol Res. (2019) 118:2107–20. doi: 10.1007/s00436-019-06371-1
65. Ming, M, Yuan, S, Fu, H, Li, X, Zhang, H, Liu, T, et al. Influence of biotic and abiotic factors on flea species population dynamics on Lasiopodomys brandtii. Int J Parasitol. (2023) 21:185–91. doi: 10.1016/j.ijppaw.2023.05.006
66. Chang, T, Min, KD, Cho, S, and Kim, Y. Associations of meteorological factors and dynamics of scrub typhus incidence in South Korea: a nationwide time-series study. Environ Res. (2024) 245:117994. doi: 10.1016/j.envres.2023.117994
67. García-del Río, M, Cantarero, A, Castaño-Vázquez, F, Merino, Y, García-Velasco, J, and Merino, S. Experimental manipulation of nest temperature and relative humidity reduces ectoparasites and affects body condition of blue tits (Cyanistes caeruleus). Ibis. (2025) 167:212–24. doi: 10.1111/ibi.13346
Keywords: chigger mite, ecology, gamasid mite, infestation, logistic regression, rodent, statistic model
Citation: Liu R-J, Guo X-G, Peng P-Y, Lv Y, Yin P-W, Song W-Y, Xiang R, Chen Y-L, Li B and Jin D-C (2025) Mite Infestation on Rattus tanezum rats in southwest China concerning risk models. Front. Vet. Sci. 12:1519188. doi: 10.3389/fvets.2025.1519188
Received: 29 October 2024; Accepted: 10 February 2025;
Published: 14 March 2025.
Edited by:
María Teresa Gómez-Muñoz, Complutense University of Madrid, SpainReviewed by:
Pablo Colunga-Salas, Universidad Veracruzana, MexicoCopyright © 2025 Liu, Guo, Peng, Lv, Yin, Song, Xiang, Chen, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Guo Guo, eGdndW8yMDAyQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.