
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 03 March 2025
Sec. Veterinary Epidemiology and Economics
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1517373
 Omima Adam1*
Omima Adam1* Omolade A. Oladele2*
Omolade A. Oladele2* Tadesse Mihret Yimam3*
Tadesse Mihret Yimam3* Belayneh Getachew4
Belayneh Getachew4 Getaw Deresse4
Getaw Deresse4 Kenaw Birhanu4
Kenaw Birhanu4 Abinet Legesse4
Abinet Legesse4 Takele Abayneh Tefera4
Takele Abayneh Tefera4 Molalegne Bitew5*
Molalegne Bitew5*Introduction: Infectious laryngotracheitis (ILT) is a highly contagious upper respiratory tract disease of chickens caused by a Gallid herpesvirus 1 (GaHV-1). The current study was to establish molecular evidence of Infectious laryngotracheitis virus (ILTV) in the Amhara region, Ethiopia, and determine its seroprevalence in areas of high chicken population and assess the risk factors associated with the disease.
Methods: Serological study was conducted on 385 serum samples collected from commercial and backyard chickens in the study area, and the presence of antibodies against ILTV was determined by indirect ELISA. In addition, oropharyngeal swab samples were collected from chickens suspected of ILT infection and inoculated into embryonated chicken eggs through the Chorioallantoic membrane (CAM) route for isolation of the virus. Isolates were confirmed using polymerase chain reaction (PCR) upon amplification of ICP4 gene. Furthermore, potential factors were recorded, and their association with the virus seropositivity assessed.
Results: The overall seroprevalence of ILT in the study area was 19.4%. A significant difference (P < 0.05) among districts, and between commercial (14.2%) and backyard (22.9%) production systems was observed (P < 0.05). Significantly higher seroprevalence was observed in layers compared to broilers and dual-purpose chickens however, there were no significant differences in prevalence based on age and sex. Of all (n = 27) tested oropharyngeal swab samples, four were positive for ILTV by PCR targeting a 688 bp region of ICP4 gene. Three of the PCR positive cases were from backyard chickens, while one was from commercial chicken farms. Based on oropharyngeal samples tested using PCR, a quarter of the samples were positive for ILT.
Discussion: The result confirms the presence of ILT infection in the Amhara region of Ethiopia using serological and molecular methods. The study shows chickens shed the virus potentially spreading the infection to other birds. Vaccination strategy, strict biosecurity measures, rapid diagnosis, and detection of latent carriers are recommended to control and eradicate the disease. Further studies on clinical cases and the molecular characterization of the target gene are needed to identify circulating strains.
Infectious laryngotracheitis is a respiratory disease of chickens, which is found all over the world, caused by ILTV, also known as gallid herpesvirus 1(GaHV-1) (1), classified under the genus Iltovirus, in the subfamily Alphaherpesvirinae. It is a double-stranded DNA virus that is enveloped, non-segmented, and linear (2). May and Tittsler (41), were the first to characterize infectious laryngotracheitis as a distinct disease entity; they recorded an outbreak on a farm in Rhode Island in 1923. However, several of the early workers pointed out that the disease had most likely been present in North American chickens for some years before 1925 (3). Infectious laryngotracheitis is on the Office International des Epizooties' (OIE) List B. The virus is one of the most prevalent diseases seen among chickens with contagious respiratory infections (4).
Gasping, depression, nasal discharge, conjunctivitis, and the secretion of bloody mucus are major symptoms associated with the acute form of the disease in chickens (5). ILTV also causes coughing, decreased egg production in layers, and mortality (4). Postmortem in dead chickens show hemorrhages and mucus plugs in the trachea (6). The severity of clinical symptoms is determined by the virulence of a particular strain or isolate, with mortality rates ranging from 0% to 70% (7). The virus can establish lifelong latent infections in the central nervous system, particularly within the trigeminal ganglion, after a 6–12-day incubation period following natural infection by ocular or respiratory routes. Although difficult to detect, sporadic reactivations can result in productive virus replication in the respiratory system, leading to shedding and transmission to susceptible animals (1). Infectious laryngotracheitis virus mostly found in chickens, but it's also been shown in pheasants and turkeys that were experimentally infected (8).
Chickens can be infected through the upper respiratory tract and ocular routes (9). Sanitary barriers and hygienic procedures play a vital impact in the severity of viral diseases in afflicted farms (10). ILT's high contagiousness is owing to the virus's ease of transmission and propagation, which is aided by sick chickens fomites, lack of biosecurity measures, animal movement, and inappropriate disposal of contaminated litter (11). Transmission occurs commonly from acute cases. Clinically inapparent infection can persist for long periods with intermittent re-excretion of the virus, serving as a potential source of disease transmission. Studies have shown that recombination between two or more varying strains can produce highly virulent and transmissible agents (12). Vaccination using live attenuated or recombinant viral vector vaccines is the most effective way to prevent ILT infection (13). Vaccination with two types of ILTV live attenuated vaccines, the chicken embryo origin (CEO), which is attenuated by sequential passages in embryonated eggs, and the tissue-culture origin (TCO), which is generated by sequential passages in tissue culture, is commonly used. A study showed that, live attenuated vaccines, particularly the CEO, can spread from bird to bird in close proximity and is able to revert to virulence after a few passages (14).
ILTV is found throughout the world, and several epidemiological investigations to detect circulating ILTVs undertaken in various countries (9). The virus has been identified in several nations where intensive chicken production is practiced, including North America, South America, Europe, China, Southeast Asia, and Australia. Laboratory diagnosis is essential to distinguish ILT from other diseases with similar clinical signs and lesions such as infectious bronchitis, Newcastle disease, avian influenza, infectious coryza, and mycoplasmas (15).
Only a few studies on ILT have been undertaken in Ethiopia, with the majority of them taking place in commercial and backyard chicken flocks. Previous reports by (10) and (16) indicated a higher seroprevalence of ILTV in chickens (without detecting the virus) in South and Central Ethiopia (10, 16), and another recent study has for the first time confirmed the virus in diseased chickens in the open markets of Addis Ababa, two genes were sequenced and the analysis showed that vaccine-like strains of ILTV were circulating (17). Indicated that the diseases possibly cause a substantial productive loss in the country, and more studies using a combination of molecular and serological techniques are needed to cover more regions of the country, create awareness about the disease, and make recommendations on prevention and future research. Therefore, the objective of this study is to determine seroprevalence, detect ILTV using molecular methods, and assess risk factors associated with infectious laryngotracheitis in chicken production systems in Amhara Region, Ethiopia.
This research was carried out in Central Gondar Zone in the Amhara National Regional State (ANRS), Ethiopia. The ANRS is one of the largest states in Ethiopia, holding a huge poultry population. Among the different administrative zones of the ANRS, the Central Gondar City is geographically located between coordinates 12.3°-13.38° N latitudes and 35.5°-38.3° E longitudes, and has an altitude ranging between 1,750 and 2,600 m above sea level. It has an average annual rainfall of 1047.6 mm, mean maximum temperature of 27.4°C, and mean minimum temperature of 14.7°C and relative humidity of 45% (7).
The study area is a remarkable source of livestock, which contribute to the country's GDP with about 737,713 sheep, 1,479,366 goats, 3,234,012 cattle, 392,546 donkeys, 12,252 mules 39,178 horses and 3,310,498, chickens are kept in this area (18). The samples were collected from four districts of Gondar city (Gondar, Maksegneit, West Dembya, and East Dembya).
Commercial and backyard chickens in Amhara region, Ethiopia were sampled from February to July 2022 in a cross-sectional study design. Districts and commercial chicken farms were selected purposely. Households with backyard chicken and individual chickens for blood sampling were selected haphazardly mimicking random sampling. Clinically ill animals with signs of nasal discharge, dyspnea and coughing were selectively sampled for viral detection, and blood samples were obtained from apparently healthy chickens. It was possible to collect a total of 27 oropharyngeal swabs from diseased chickens. For the sero-epidemiology, 385 blood samples were collected using (19) formula taking an expected prevalence of 50% and absolute desired precision of 5% at 95% confidence level (19).
The chickens in this study were of existing exotic (Bovance and Sasso) and local breeds (Backyards chickens). Specimens were collected from backyard and commercial flocks located in four different districts in Central Gondar zone, Ethiopia (Gondar, Maksegnit, West Dembya, and East Dembya) (Table 1). The examined flocks were at 1–13 months age whose purpose ranged widely from egg production and meat production to dual purpose, and all flocks were not vaccinated against ILTV. During samples collection, potential risk factors such as flock history (sex, age, type of production, and breed) were noted. The informed consent was obtained from owners of the chickens to collect the samples.
A total of 385 blood samples (2–3 mL) were collected from backyard and commercial chickens (Table 2) from the wing vein using 5 mL syringes and 21-gauge needles. The blood samples were kept at room temperature overnight to produce serum. Serum samples were decanted into cryovial tubes and transported using an icebox to the National Veterinary Institute (NVI), Ethiopia, where they were kept at −20°C.
Twenty-seven oropharyngeal swab samples were collected from backyard and commercial flocks (1–3 samples from each flock) in the study area which display nasal discharge, dyspnea and coughing. Sterile cotton-tipped swabs were introduced into the mouth of each bird and gently swabbing the oropharynx. The samples were placed directly into sterile labeled cryovial tubes containing a 1.5 mL virus transport medium and transported on ice to the Virology Laboratory, NVI, Ethiopia, where they were stored at −80°C. A total of 27 samples, preserved in sterile PBS Phosphate- Buffered Saline (PBS) containing antibiotics and antifungal drug, were collected. A 10% of sample suspension made using PBS was centrifuged at 4,500 rpm for 10 min and the supernatant was used for virus isolation and DNA extraction.
Indirect Enzyme linked Immunosorbent Assay (ELISA) technique was carried out using commercial Indirect ELISA kit (iELISA) (IDvet Screen® ILT Indirect, 310 rue Louis Pasteur, 34790 Grabels, France) to detect the specific antibodies for GaHV-1 following the instructions of the manufacturer. After the microtiter plate was placed in the ELISA reader, the intensity of the color produced by the reaction was measured photometrically at 450 nm wavelength, then the values of sample optical density (OD sample) and the values of positive control optical density (ODPC) were noted. The test was considered valid when the mean OD value of the positive control was greater than 0.250 and when the ratio of the mean values of the positive and negative control was greater than 3 (ODpc/ODnc > 3). Test positivity or negativity was based on the formula indicated below:
Where S/P: sample to positive ratio, ODS: sample optical density, ODNC: optical density of the negative controls, ODPC: optical density of the positive controls. Sample to positive (S/P) ratios of ≤ 0.3 and > 0.3 were interpreted as negative and positive, respectively.
The result was considered positive when the sample S/P ratio is greater than 0.3 (Titer > 611 per microliter). If the S/P ratio of the sample is less than 0.3 (Titer < 611 per microliter) it was interpreted as negative.
The swab samples (n = 27) in 10% PBS (pH 7.2) solution was centrifuged at low speed to remove debris. Aliquots of 0.2 and 0.3 mL of the supernatant fluid was inoculated on the dropped Chorioallantoic membrane (CAM) of at least two, 9–11 day-old embryonated chicken eggs of specific pathogen free (SPF) chicken. The eggs were sealed with paraffin wax, incubated at 37°C and candled daily for 4–7 days. During incubation, eggs with dead embryos were chilled at 4°C. After the last day of incubation, eggs with live embryos were chilled at 4°C overnight. The CAMs were harvested and homogenized in PBS. Homogenized CAM samples were re-passaged three times in SPF embryos. (20) explained the difficult of isolation of low virulent ILTV, also (21) described the limited propagation of ILTV isolated from mild cases. Therefore, three consecutive passages were performed for ILTV negative samples.
As stated earlier, swab samples (n = 27) were used for DNA extraction and virus isolation. DNA was extracted using QIAGEN kit (QIAGEN® DNA mini columns kit, QIAGEN, Germany) as instructed by the manufacturer. DNA was stored at −20°C for PCR amplification of targeted ICP4 gene. Following the isolation of the virus in chicken embryonated eggs, further DNA extraction and molecular detection were carried out.
Conventional PCR was performed according to the method described by (22) using a pair of forward primers, ICP4-1F (5′-ACTGATAGCTTTTCGTACAGCACG-3′) and reverse primer, ICP4-1R (5′ CATCGGGACATTCTCCAGGTAGCA-3′) that amplify 688 bp fragments of the ILTV ICP4 gene (GenBank accession number: NC_006623) (23). The 18.5 μL amplification reaction containing 3 μL of RNase free water, 5 μL of dNTP mix (2 mM each of dATP, dCTP, dGTP and dTTP), 2 μL each of the primers, 1.5 μL (2.5 units) Dream TaqTM DNA polymerase, 5 μL of 10 × Taq DNA polymerase buffer was mixed with 5 μl extracted DNA to run the PCR.
The following PCR condition was applied: initial denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 15 s (denaturation), 60°C for 45 s (annealing) and 72°C for 150 s (extension), and for final extension incubated at 72°C for 3 min. In each series of PCR amplification a sterile distilled water, instead of extracted DNA, was included as a negative control.
PCR products were visualized by agarose gel (1.5%) electrophoresis using PRONASAFE dye. Loading dye (4 μL) was added into 20 μL PCR product, then 10 μL of the mixtures was loaded into each well. A 10 μL of molecular ladder (GelPilot DNA molecular weight marker, QIAGEN, Germany) starting at 100 bp, negative, and positive controls were added in addition to test sample. Electrophoresis was run for 80 min at 120 V. Then the result is read using UV light. The expected product is about 688 bp for ILTV ICP4 gene (22).
Data generated from the study were arranged, coded, and entered into an Excel spreadsheet (Microsoft® excel 2016, Microsoft Corporate, USA). The data were checked for errors of entry and then imported to STATA16 (Stata Corp., College Station, TX, USA) for descriptive and further statistical analyses, Pearson's Chi-square was used to analyze differences in seroprevalence and assess association between risk factors and ILT infection. Statistical significance was determined at p < 0.05.
The overall prevalence of ILTV in the four districts of Central Gondar Zone (Gondar, Maksegnit, West dembya, and East Dembya) was 19.4% (Table 3). The highest seroprevalence recorded was 31.6% in the West Dembya area, while the lowest was 12.2% in the Maksegnit area, with significant differences (p < 0.05). The mean antibody titer was 764.5 ± 1920.63. The highest mean antibody titers for Infectious Laryngotracheitis (ILT) in backyard chickens were recorded at 990.19, while commercial chickens had a highest titer of 426.0168. Overall seroprevalence was 22.9% (53 out of 231) in backyard poultry systems and 14.2% (22 out of 154) in commercial poultry production systems. Chi square analysis revealed the prevalence was significantly different (p < 0.05) between commercial and backyard production systems (Table 4). Local breeds had higher seropositivity rates compared to commercial chickens. Layers were significantly more seropositive (p < 0.05) compared to broilers and dual-purpose chickens. On the other hand, the seroprevalence was 16.2% and 20.9% for males and females, respectively. However, there were no significant differences in prevalence based on age and sex (Table 5).
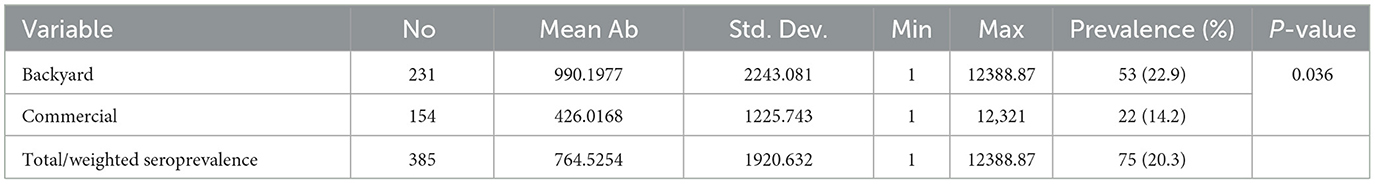
Table 4. Mean infectious laryngotracheitis virus antibody titers (ELISA) in backyard and commercial chickens in Central Gondar Zone, Ethiopia.
Out of 27 oropharyngeal samples, four samples (14.8) were found to be positive for ILT virus ICP4 gene, in which two samples were collected from West Dembya (2/8, 25%), one sample from Gondar city (1/6, 16.6%) and one sample was from East Dembya (1/7, 14.2%) (Table 6). Three of the positive oropharyngeal swabs were from backyard chickens (3/13, 23.4%), while one sample was from commercial chicken farms (1/14, 7.1%) (Table 7). Similarly, three positive samples were detected in broiler chickens (3/7, 42%), and one was from layer chickens (1/13, 7%) (Table 8).
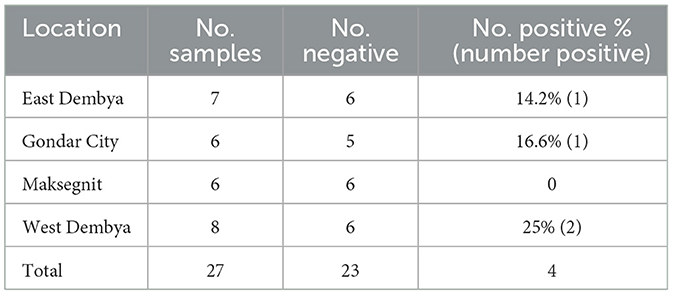
Table 6. Molecular detection of infectious laryngotracheitis virus in four districts in Central Gondar Zone, Ethiopia.
Initially, only 3 samples (pre-inoculated sample suspension) tested positive using PCR, but the detection rate increased to 4 following culture in embryonated chicken egg. These four oropharyngeal swab samples were positive for ICP4 gene of ILTV. The positive band also appeared in the reference strain used as positive control while no band was detected in the negative control (Figure 1).
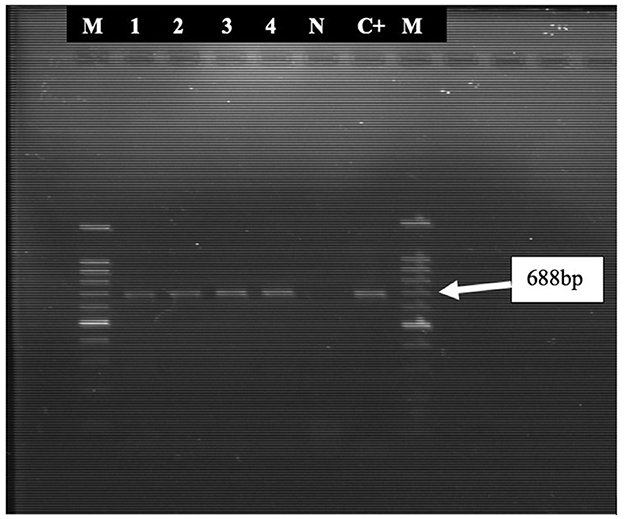
Figure 1. PCR amplified products (688 bp fragments) of the ICP4 gene of ILTV. Lane 1, 2, 3, and 4 were positive for the 688-bp fragment. M = 100-bp DNA ladder; N, Negative control; C+, Positive control (ILTV reference strain).
In total, 385 samples were collected and tested against ILTV using an indirect ELISA test. An overall seroprevalence of 19.4% was obtained. A positive serology result indicates that ILTV is circulating in Amhara Region since there is no vaccination against ILTV provided. Ethiopia has adopted a non-vaccination policy against ILTV (10). Thus, the findings of this investigation point to either a previous or active ILT infection throughout poultry facilities across a number of districts. It might result from ILTV infection of the field type or from the latent virus reactivating in birds that have recovered from previous infection. ILTVis latent in the trigeminal ganglia of recovered birds until its reactivation by stress, which leads to its excretion and further lateral spread (24).
The first report of Infectious Laryngotracheitis was published in 2019, in central and south Ethiopia based on serological tests. The overall prevalence was higher in backyard poultry (34.4%) than commercial poultry production (13.3%) (10). This was followed by a report in which the highest percentage of prevalence 54.7% in Ada'a district of Bishoftu city in Ethiopia (16). The finding is consistent with reports from (25), Baksi et al. (26), and Langeroudi et al. (14) who demonstrated overall seroprevalence of 17.33% in Chittagong district in Bangladesh, 26.77% in India, and 13% in Iran respectively. However, the seroprevalence found in this study was lower than a previous report of 59.1% from the same region (17) and others' around the globe (11, 27). Conversely, our findings are higher than those reported by Bhuiyan et al. (28) (0.4%), Ana et al. (29) (0.194%), and Shittu et al. (30) (1.2%). Variations in seroprevalences can be attributed to differences in chicken breed, flock age, flock density, biosecurity practice, vaccination status, and test sensitivity and specificity used (31, 32). In this study, seropositivity of ILTV infection was higher in backyard poultry (22.9%) than commercial poultry production (14.2%). The lack of biosecurity measures observed during sample collection from backyard poultry may have increased the risk of exposure in these chickens, potentially contributing to the observed difference. Several other studies support this finding. Several other studies have corroborated this finding (7, 10, 11). Also breed effects on seroprevalence were observed for ILTV. The higher seropositivity of local chickens for ILTV compared to exotic chickens may be associated with local breeds being able to survive better than exotic breeds after ILTV infection.
Up to now, no studies had been conducted on the molecular detection of ILTV in Amhara region. This is the first documentation of ILT infection in Amhara region using PCR. But the first molecular evidence of ILTV in Ethiopia came from a study that confirmed the viral genome from two diseased chickens as part of an investigation of respiratory pathogens of chickens in Central Ethiopia (7). In the present study, clinical signs observed include respiratory signs such as mucoid to hemorrhagic nasal discharge, dyspnea, conjunctivitis, lacrimation, a high rate of morbidity, and low rate of mortality. Our observations agree with those reported by Salhis et al. (11), Ponnusamy et al. (33), Gowthaman et al. (31), García and Spatz (34), and Menendez et al. (35).
Although clinical findings observed during sample collection can help in diagnosing suspected ILT cases, laboratory confirmation is essential (24). Hence, confirming a mild infection of ILT requires laboratory tests that detect the presence of the virus (29). PCR is a method of choice to confirm ILTV infection in chickens with clinical signs suggestive of ILT disease (36). In the present study, the detection of the virus from the oropharyngeal swabs by PCR confirmed the results.
According to PCR results, three swab samples showed positive results out of 27 samples that were collected. To confirm the result, all the samples were inoculated into the CAM of embryonated chicken eggs, followed by virus detection in four samples by PCR. This suggests that the load of the virus in some samples was very low before isolation in embryonated chicken eggs. The positive results by ELISA test and PCR confirmation, which were consistent with ILT's characteristic symptoms that were observed during sample collection, showed that ILTV is circulating in both commercial and backyard chicken, with synchronous higher seroprevalence in Ethiopian backyard chicken. The primer in this study targeted the ICP4 gene of ILTV. The ICP4 is an ~4,386 nucleotide transcriptional protein that regulates ILTV gene expression (37), specifically involved in regulation of gene expression early in infection and is commonly used in epidemiologic studies for characterization of circulating virus strains (38). The gene could serve as a good target for RFLP analysis (22). The PCR results obtained in this study were in agreement with previous reports using the ICP4 gene (39) including Fagbohun et al. (40) in Nigeria, and Rojs et al. (23) in Slovenia. The findings on backyard chickens in this study are unbiased, as samples were taken from unvaccinated chickens, eliminating false-positive results despite rumors of commercial chicken vaccination (34).
Although the vaccine is not officially permitted to be administered in Ethiopia, there is ongoing debate on its use due to rumors that some commercial poultry farms were vaccinating their flocks against the disease (16). Several recent studies have confirmed that partial sequencing and analysis of the ICP4 gene can successfully differentiate between vaccine and field ILT strains (35). PCR-RFLP, sequencing of PCR products for strain differentiation is regionally dependent. Partial sequencing of the ICP4 has been used successfully in both Africa and the Middle East (35). The prevention, control, and source of ILTV infection in chicken might be better understood with additional studies using large sample size and molecular methods.
The lack of hygiene and biosecurity measures that were seen during sample collection in local chickens may be the reason that infections are more prevalent in the local chickens than in commercial flocks. Also the ability of ILTV to persist in a latent form beyond recovery and to reactivate when the host is under stress highlights the significance of avoiding co-infections to reduce host stress. Co-infection treatment is vital to ILT management and should not be neglected (37).
However, mixed respiratory infections are challenging to identify because typically only one pathogen is required for a diagnosis (11). An essential component of ILT management is co-infection prevention. Additionally, it is important to consider the possibility of pathogen exposure and transfer from small flocks to commercial flocks.
Ana Garrido et al. (29) observed that all birds are susceptible to infection, but clinical disease affects layers more frequently. Our findings support this observation because laying chickens made up the majority of positive farms. They may have a longer productive life than broilers, which could account for higher infection. On the other hand, protected flocks must have a high average antibody titer from the baseline arising from vaccination, with no specific clinical indications (11). In the present study, the differences in the seroprevalence of ILT among districts were statistically significant.
ILTV strains can spread from flocks that have been continuously infected to birds that are not vaccinated (36). Hence, the authors suggest the source of the ILT infection in this investigation was introduced by carrier birds that are reservoirs, and unvaccinated stressed flocks. Modified live vaccines must be used in these situations, even though they can induce latent infection and spread quickly to nearby flocks. It is necessary to establish continuous epidemiological surveillance to evaluate the incidence and prevalence of the disease using conventional methods like isolation in embryonated chicken eggs (ECE), PCR, PCR-RFLP, and sequencing of PCR products. In order to avoid disease and lessen its economic impact, proper biosecurity procedures and good hygiene are essential.
• The present study confirms the presence of ILTV infection in commercial and backyard chickens in Amhara region, Ethiopia, using serological and molecular methods. The study showed that the backyard and commercial chickens in Amhara region, Ethiopia, shed the virus with the potential of spreading the infection to other birds, owing to low herd immunity to ILTV. The result emphasizes the need to implement a control program of ILT based on vaccination strategy using recombinant viral vector vaccine and standard biosecurity measures in order to avoid further ILT outbreaks and reduce the risk of widespread virus transmission.
• Furthermore, to identify the circulating strains and define their temporal and geographic distribution, particularly among backyard chickens, further extensive research is critically needed.
• The potential virulence of ILTV strains, prevention, control, and the source of ILT infection could all be better understood with additional research on more samples and molecular characterization.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The animal studies were approved by the Ethics Committee of the National Veterinary Institute of Ethiopia (Ref. No 1000/N4). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
OA: Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. OO: Supervision, Writing – review & editing. TY: Investigation, Methodology, Writing – review & editing. BG: Project administration, Supervision, Writing – review & editing. GD: Methodology, Writing – review & editing. KB: Methodology, Writing – review & editing. AL: Methodology, Writing – review & editing. TT: Project administration, Writing – review & editing. MB: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to highly acknowledge commercial and backyard chicken owners for their consent to participate in the study and permit the collection of samples. The sample collection would not be successful without the help of kind professionals in the field. Great thanks to Pan African University and university of Ibadan. The authors also thank the NVI for allowing their laboratories and facilities. Moreover, we received a great support from laboratory technicians of the NVI during laboratory work, which we are grateful for.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1517373/full#supplementary-material
1. Walter F, Jutta V, Dorothee H, Jens PT. Thomas CM. Review article Molecular biology of avian infectious laryngotracheitis virus. Vet Res. (2007) 38:261–79. doi: 10.1051/vetres:200657
2. Bouska CK. Studies of Immunological and Molecular Biological Techniques with Infectious Laryngotracheitis Virus of Chickens. Dr Thesis. (1955).
3. Jordan FTW. A review of the literature on infectious laryngotracheitis (ILT). Avian Dis. (1966) 10:1. doi: 10.2307/1588203
4. Kalin R, Turan T, Işidan H. Molecular detection and characterization of infectious laryngotracheitis virus in backyard chickens in Turkey. Kocatepe Vet J. (2020) 13:332–9. doi: 10.30607/kvj.777721
5. OIE (Office International des Epizootics). Avian infectious laryngotracheitis. In: OIE Terrestrial Manual, Chapter 10.3. (2008). Available at: https://www.woah.org/app/uploads/2023/11/en-cast-toc-2-2008.pdf
6. Ali M, Giasuddin M. Prevalence and molecular detection of infectious laryngotracheitis virus in chickens in selected areas of Bangladesh. Bangladesh J Livest Res. (2021) 2021:113–7. doi: 10.3329/bjlr.v27i1.55175
7. Birhan M, Temesgen M, Shite A, Berhane N, Bitew M, Gelaye E, et al. Seroprevalence and associated risk factors of infectious bronchitis virus in chicken in Northwest Ethiopia. Sci World J. (2021) 2021:3890. doi: 10.1155/2021/4553890
8. Wilks CR, May JT. Differentiation of infectious laryngotracheitis virus strains using restriction endonucleases. Avian Dis. (2015) 26:718–31. doi: 10.2307/1589858
9. Craig MI, Rojas MF, Ploeg CA Van Der, Olivera V, Craig MI. Molecular characterization and cluster analysis of field isolates of avian infectious laryngotracheitis virus from argentina. (2017) 4:1–7. doi: 10.3389/fvets.2017.00212
10. Tesfaye A, Sahle M, Sori T, Kassa T, Garoma A, Koran T, et al. Infectious laryngotracheitis virus in commercial and backyard chicken production systems in central and South Ethiopia (first report) ILT in Ethiopian poultry production. J Appl Poult Res. (2019) 28:1324–9. doi: 10.3382/japr/pfz100
11. Salhi O. Indicators and risk factors of infectious laryngotracheitis in layer hen flocks in Algeria. Vet World. (2021) 14:182–9. doi: 10.14202/vetworld.2021.182-189
13. Beltrán G, Williams SM, Zavala G, Guy JS, García M. The route of inoculation dictates the replication patterns of the infectious laryngotracheitis virus (ILTV) pathogenic strain and chicken embryo origin (CEO) vaccine. Avian Pathol. (2017) 46:585–93. doi: 10.1080/03079457.2017.1331029
14. Langeroudi G, Hosseini H, Fallah M, Aghaeean L, Dizaji R, Ziafati Z, et al. Original article serological survey of infectious laryngotracheitis in broiler flocks, Iran, 2018. Iran J Virol. (2020) 14:1–5.
15. Spalding M. Diseases of poultry, 12th edition. Vol. 45. J Wildlife Dis. (2009) 2009:251–256. doi: 10.7589/0090-3558-45.1.251
16. Roba YT. Seroprevalence of infectious laryngotracheitis disease in backyard chickens in villages of Ada' a district, Oromia, Ethiopia: first report. (2020). doi: 10.1007/s11250-020-02334-2
17. Tekelemariam TH, Walkden-Brown S, Atire FA, Tefera DA, Alemayehu DH, Gerber PF. Detection of chicken respiratory pathogens in live markets of Addis Ababa, Ethiopia, and epidemiological implications. Vet Sci. (2022) 9:1–10. doi: 10.3390/vetsci9090503
18. CSA. Federal Democratic Republic of Ethiopia Central Statistical Agency Agricultural Sample Survey 2020/21 [2013 E.C.]: Report On Livestock and Livestock Characteristics (Private Peasant Holdings). Cent Stat Agency (CSA), Addis Ababa, Ethiop. (2021).
20. Hughes CS, Williams RA, Gaskell RM, Jordan FTW, Bradbury JM, Bennett M, et al. Latency and reactivation of infectious laryngotracheitis vaccine virus. Arch Virol. (1991) 121:213–8. doi: 10.1007/BF01316755
21. Sellers HS, García M, Glisson JR, Brown TP, Sander JS, Guy JS. Mild infectious laryngotracheitis in broilers in the Southeast. Avian Dis. (2004) 48:430–6. doi: 10.1637/7129
22. Chacón JL, Ferreira AJP. Differentiation of field isolates and vaccine strains of infectious laryngotracheitis virus by DNA sequencing. Vaccine. (2009) 27:6731–8. doi: 10.1016/j.vaccine.2009.08.083
23. Rojs OZ, Dovc A, Krapez U, Zlabravec Z, Racnik J, Slavec B, et al. Detection of Laryngotracheitis Virus in Poultry Flocks with Respiratory Disorders in Slovenia. Basel (2021).
24. Hughes CS, Gaskell RM, Jones RC, Bradbury JM, Jordan FTW. Effects of certain stress factors on the re-excretion of infectious laryngotracheitis virus from latently infected carrier birds. Res Vet Sci. (1989) 46:274–6. doi: 10.1016/S0034-5288(18)31158-5
25. Uddin MI. Seroepidemiology of infectious laryngotracheitis (ILT) in the commercial layer farms of Chittagong District, Bangladesh. Adv Anim Vet Sci. (2014) 2:316–20. doi: 10.14737/journal.aavs/2014/2.6.316.320
26. Baksi S, Savaliya BF, Rao N, Panchal M. Sero-prevalence of infectious laryngotracheitis of poultry in India. Indian J Poult Sci. (2016) 51:234. doi: 10.5958/0974-8180.2016.00036.2
27. Aras Z, Yavuz O, Sanioǧlu Gölen G. Occurrence of infectious laryngotracheitis outbreaks in commercial layer hens detected by ELISA. J. Immunoass. Immunochem. (2018) 39:190–5. doi: 10.1080/15321819.2018.1428991
28. Bhuiyan Z, Ali M, Moula M, Bary M, Arefin N, Giasuddin M, et al. Seroprevalence of major avian respiratory diseases in broiler and sonali chicken in selected areas of Bangladesh. J Adv Vet Anim Res. (2019) 6:561. doi: 10.5455/javar.2019.f383
29. Garrido A, Barrionuevo M, Santiana Jara I, Sandoval P, Alfonso P, Barrera M. Serologic and molecular survey of avian infectious laryngotracheitis in ecuador. Ecuador es Calid Rev Científica Ecuatoriana. (2016) 3:43–51. doi: 10.36331/revista.v3i1.18
30. Shittu I, Sulaiman LK, Gado DA, Egbuji AN, Ndahi MD, Pam E, et al. Sero-epizootiological investigation of infectious laryngotracheitis infection in commercial poultry of Plateau State, north central Nigeria. J Immunoass Immunochem. (2016) 37:368–75. doi: 10.1080/15321819.2016.1151439
31. Gowthaman V, Kumar S, Koul M, Dave U, Murthy TRGK, Munuswamy P, et al. Infectious laryngotracheitis: etiology, epidemiology, pathobiology, and advances in diagnosis and control–a comprehensive review. Vet Q. (2020) 40:140–61. doi: 10.1080/01652176.2020.1759845
32. Adair BM, Todd D, McKillop ER, Burns K. Comparison of serological tests for detection of antibodies to infectious laryngotracheitis virus. Avian Pathol. (1985) 14:461–9. doi: 10.1080/03079458508436249
33. Ponnusamy P, Sukumar K, Raja A, Saravanan S, Srinivasan P. Histopathological and molecular confirmation of infectious laryngotracheitis virus in desi chicken. Pharma Innovat J. (2021) 10:2852–6.
34. García M, Spatz S. Infectious Laryngotracheitis. Hoboken, NJ: Wiley (2020). doi: 10.1002/9781119371199.ch5
35. Menendez KR, García M, Spatz S, Tablante NL. Molecular epidemiology of infectious laryngotracheitis: a review. Avian Pathol. (2014) 43:108–17. doi: 10.1080/03079457.2014.886004
36. Magouz A. Isolation and molecular characterization of infectious laryngotracheitis virus from naturally infected layer chicken flocks in Egypt. Glob Vet. (2015) 14:929–34. doi: 10.5829/idosi.gv.2015.14.06.95215
37. Blakey J, Stoute S, Crossley B, Mete A. Retrospective analysis of infectious laryngotracheitis in backyard chicken flocks in California, 2007–2017, and determination of strain origin by partial ICP4 sequencing. J Vet Diagnostic Investig. (2019) 31:350–8. doi: 10.1177/1040638719843574
38. Bayoumi M, El-Saied M, Amer H, Bastami M, Sakr EE, El-Mahdy M. Molecular characterization and genetic diversity of the infectious laryngotracheitis virus strains circulating in Egypt during the outbreaks of 2018 and 2019. Arch Virol. (2020) 165:661–70. doi: 10.1007/s00705-019-04522-4
39. Creelan JL, Calvert VM, Graham DA, McCullough SJ. Rapid detection and characterization from field cases of infectious laryngotracheitis virus by real-time polymerase chain reaction and restriction fragment length polymorphism. Avian Pathol. (2006) 35:173–9. doi: 10.1080/03079450600598244
40. Fagbohun O. Molecular Detection and Characterization of Infectious Laryngotracheitis Virus in Apparently Healthy Commercial and Backyard Chickens in Ibadan, Nigeria. Makhanda/Grahamstown (2019).
Keywords: chicken, ILT virus, ICP4 gene, ELISA, PCR, isolation, seroprevalence, Amhara
Citation: Adam O, Oladele OA, Yimam TM, Getachew B, Deresse G, Birhanu K, Legesse A, Tefera TA and Bitew M (2025) Serological and molecular detection of infectious laryngotracheitis virus in chickens in Central Gondar Zone, Ethiopia. Front. Vet. Sci. 12:1517373. doi: 10.3389/fvets.2025.1517373
Received: 11 November 2024; Accepted: 11 February 2025;
Published: 03 March 2025.
Edited by:
Paolo Mulatti, Experimental Zooprophylactic Institute of the Venezie (IZSVe), ItalyReviewed by:
Alessio Bortolami, Experimental Zooprophylactic Institute of the Venezie (IZSVe), ItalyCopyright © 2025 Adam, Oladele, Yimam, Getachew, Deresse, Birhanu, Legesse, Tefera and Bitew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omima Adam, T21pbWFhZGFtMTk5NEBnbWFpbC5jb20=; Omolade A. Oladele, bGFkZS5vbGFkZWxlQGdtYWlsLmNvbQ==; Tadesse Mihret Yimam, bWlocmV0YWRlc3NlOEBnbWFpbC5jb20=; Molalegne Bitew, TW9sYWxlZ25lMjNAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.