- Istituto Zooprofilattico Sperimentale Delle Venezie, Legnaro, Italy
Stray cats potentially act as reservoir for zoonotic agents, posing a risk of exposure to humans and domestic cats. The most prevalent Chlamydiaceae species in cats is Chlamydia (C.) felis, which is frequently associated with conjunctivitis and/or upper respiratory disease. The zoonotic potential of C. felis is believed to be relatively low, although exposure is possible through handling infected cats, by contact with their aerosol, and via fomites. Infection is more frequent in conditions of overcrowding, stress, poor hygiene and impairment of the immune system. For this reason, stray cats appear to be particularly susceptible to this pathogen. Aim of the study was to identify the molecular occurrence of Chlamydiaceae in stray and colony cats. Between May 2021 and June 2022, in seven provinces of northeastern Italy, veterinary services officers collected oropharyngeal swabs from 379 stray and colony cats. The samples were screened for Chlamydiaceae by real-time PCR targeting a 23S gene fragment. Positive samples were further analyzed either by a C. felis-specific qPCR or by amplification and sequencing of a 16S rRNA gene fragment. Overall, 7.7% of the cats tested positive for Chlamydia spp., and all were identified as C. felis. Among the positive individuals, only one exhibited respiratory symptoms. The analysis of anamnestic data revealed a significantly higher frequency of C. felis in male intact cats during the spring season, suggesting a potential behavioral aspect of this infection. Although the zoonotic risk of this Chlamydia species is low, it would be prudent to exercise caution when handling stray cats.
1 Introduction
The domestic cat (Felis catus) is one of the most common companion animals worldwide (1). Cats interact with humans in varying degrees, and could be classified as pets, stray/feral cats, or colony cats (2). Pet cats receive sustenance, shelter and medical care from their owner(s), whereas stray/feral cats move freely within urban/rural environments and may choose whether to socialize to people or not. If they are provided with food at one or more locations, they can become part of a ‘colony’, and eventually be colony cats (3).
Given that approximately three-quarters of emerging infectious diseases originate from animals (4), it is crucial to understand and monitor animal pathogens with zoonotic potential. The large populations of stray and colony cats commonly found in rural and urban areas need to be screened to identify the possible presence of such pathogens. Furthermore, these animals may come in contact with humans, free-roaming pets and other urban and peri-urban animals, emphasizing the need for a comprehensive surveillance program (5).
Chlamydia are obligate intracellular eubacterial pathogens that cause disease in humans and animals (6). The genus includes several species that are of significant public health concern, among which the human pathogens C. trachomatis, and C. pneumoniae, as well as zoonotic species such as C. psittaci, C. abortus, C. caviae, and C. felis (6).
C. felis infection is common in domestic cats and an important cause of feline acute and chronic conjunctivitis. The symptoms include eye discharge, conjunctival chemosis, blepharospasm, and redness of the nictitating membrane. Some cats may exhibit fever, lethargy, anorexia, sneezing, and nasal discharge (7). The infection often resolves spontaneously; however, in the event of an inadequate or absent treatment, or in case of a compromised immune system, the infection can result in chronic conjunctivitis. The duration of ocular shedding has been observed to last up to 60 days; however, intermittent shedding up to 8 months has also been documented in experimental cats, indicating the potential for an asymptomatic carrier state (7).
The zoonotic potential of C. felis is considered to be rather low, with rare reports of human conjunctivitis linked to infected cats (8–11). The human infection with C. felis typically does not result in serious illness, with only an HIV-positive patient whose infection was traced to a personal pet kitten (12) that presented chronic conjunctivitis and general malaise. Although the risk due to C. felis seems to be minimal, cat owners and professionals working with cats might be infected, and caution is advised when handling infected cats (11). Moreover, the presence of other zoonotic Chlamydia were reported in cats: C. psittaci was associated with a fatal outcome in a domestic cat (13), while C. pneumoniae was identified in cats with conjunctivitis (14).
The impact of C. felis and other respiratory pathogens in cats has been globally studied, revealing significant geographic and population-based variations. The prevalence of C. felis in domestic cats has been reported to range from 0 to 10% in clinically healthy animals and from 5.6 to 30.9% in cats with conjunctivitis (15–17). Several studies indicate that stray cats are more likely to be infected by C. felis than domestic cats (18–20). For instance, in 2010 a Slovakian study reported positivity rates of 30.9% in domestic cats and 65.78% in stray cats (19). This was confirmed in a more recent study in the same country, that reported high positivity rates in the population of stray cats (35.7%) and shelter cats (31%), whereas only 10% of domestic free-roaming cats resulted positive (17). In Switzerland C. felis positivity rates were 19.1% in stray cats and 11.6% in pets, with significant associations with conjunctivitis (18). In China, recent studies reported overall seroprevalence rates of 5.9% in cats and 12.1% in dogs, with seroprevalence rates of 3.9% in household cats and 14.3% in stray cats (21). Another Chinese study detected C. felis in 11.6% of stray cats (22). Research in Japan identified C. felis in 59.1% of cats with conjunctivitis (20), making it the most common pathogen associated with feline respiratory diseases in the country.
Overall, in stray cats the prevalence may reach 35.7%, while in subgroups of cats with conjunctivitis it can raise up to 65.8% (18, 23–28).
In Italy, data on the prevalence of Chlamydiaceae in stray cats remain scarce (29, 30) and suggest that C. felis is widely distributed among domestic and colony cats. The present study aimed to assess the prevalence of Chlamydiaceae in stray cats in northeastern Italy, and to identify the circulating chlamydial species. By filling this knowledge gap, our findings will contribute to a better understanding of Chlamydia epidemiology in unmanaged feline populations.
2 Materials and methods
2.1 Sample population
Between May 2021 and June 2022, oropharyngeal swabs were collected from 389 stray and colony cats in seven provinces of northeastern Italy (Bozen and Trento in the Trentino-Alto Adige region and Padua, Rovigo, Venice, Vicenza, and Verona in the Veneto region). Sampling was carried out under anesthesia by veterinarians in the context of “trap–neutered–return” (TNR) programs, in compliance with the Guide for the Care and Use of Laboratory Animals and Directive 2010/63/EU for animal experiments (national law: D.L. 26/2014). The study received the approval of the Ethical Committee of the Istituto Zooprofilattico Sperimentale delle Venezie—IZSVe (CE_IZSVE 8/2020). Dry swabs were maintained at +4°C and conveyed to the laboratory within 24 h. Upon arrival, they were stored at −80°C until further processing.
Oropharyngeal swabs were chosen over nasal or conjunctival swabs, typically more specific for C. felis, to optimize sampling for multiple diagnostic purposes as part of a broader research project funded by the Italian Ministry of Health (RC IZSVE 12/19). This approach enabled concurrent testing for pathogens such as Capnocytophaga spp., SARS-CoV-2, and Influenza A virus. Some results from this research have already been published (31, 32), while others are currently under submission.
The dataset included information on the sex, castration status, age, clinical symptoms, and geographical locations of the stray cats enrolled, along with the date and province of sample collection. Cats were categorized into four age groups (33): kittens (up to 1 year old), young adults (1 to 6 years old), mature adults (7 to 10 years old), and seniors (older than 10 years). To assess any correlation between infection and seasonality, samples were assigned to a specific seasons based on the collection date: spring (from March to May), summer (from June to August), autumn (from September to November), and winter (from December to February).
2.2 Molecular investigation
The collected oropharyngeal swabs were cut into sterile microtubes filled with 1 mL of 1X phosphate-buffered saline (PBS), mixed by vortexing, and stored at -80°C. DNA extraction was performed using the KingFisher™ Flex Purification System instrument (Life Technologies, Carlsbad, CA, United States) and the ID Gene® Mag Universal Extraction Kit (IDvet, Grabels, France), according to the manufacturer’s instructions. A pretreatment step was performed before extraction, consisting of the addition of 20 μL of Proteinase K (QIAGEN, Hilden, Germany), 250 μL of the Lysis buffer provided by the kit, and 100 μL of the sample, incubated for 10 min at 70°C. Every DNA extraction included a negative control [nuclease-free water (Merk Life Science S.r.l., Darmstadt, Germany)]. DNA was stored at -80°C until further processing.
The eluted DNAs were screened with a Chlamydiaceae family-specific real-time PCR assay targeting a 111 bp fragment of the 23S rRNA gene (34). A universal heterologous control, Intype IC-DNA (Indical Bioscience GmbH, Leipzig, Germany), was added to each DNA with a ratio of 1:10 of the total elution volume and co-amplified (35), in order to validate each negative result (36). C. felis DNA and DNase-RNase free water were used as positive and negative controls for the reaction mix in each reaction run.
All Chlamydiaceae-positive samples (cut-off Ct ≤ 40) were further analyzed using a species-specific real-time PCR targeting a 78 bp fragment of the outer membrane protein of C. felis (37). Negative samples to the species-specific real-time PCR were further characterized by sequencing a 278 bp portion of the 16S rRNA gene (38).
Samples with a Ct > 35.0 in the Chlamydiaceae family-specific real-time PCR and negative in both species-specific assays were classified as negative. This decision was made taking into account possible cross-reactions with related microorganisms (36).
2.3 Statistical analysis
Data were analyzed using the open-source software R (version 4.3.3). The outcome variable was defined as the infection status of the animals. The prevalence of positive animals was calculated for each demographic and clinical data, along with the 95% confidence interval (95% CI). Statistical significance was assessed using Pearson’s χ2 test.
Generalized Additive Models (GAMs) were employed to evaluate the associations between predictor variables and the outcome variable. The analysis was conducted using the ‘mgcv’ package with a binomial error distribution (logit-link function). Odds ratios were calculated for the following predictors: sampling site, sampling season, sex (male vs female), castration status, age, and clinical symptoms. Statistical significance was determined at a p-value threshold of <0.05.
3 Results
3.1 Molecular investigation results
Out of the initial 389 oropharyngeal swabs collected, 10 were considered unsuitable for analyses due to the insufficient sample volume. For this reason, 379 samples were examined, 34 of which tested positive in the real-time PCR to screen for the presence of Chlamydiaceae DNA, with threshold cycle (Ct) values ranging between 25.1 to 40.0 (mean Ct = 34.7). These samples were further analyzed using the species-specific real-time PCR assay for C. felis.
Among the 34 samples, 27 tested positive for C. felis through the species-specific molecular assay. The remaining seven samples underwent additional testing with the end-point PCR targeting a 16S fragment and sequencing. Only two of the seven samples were successfully amplified and sequenced, and sequence analysis confirmed 100% identity with C. felis sequences present in the GenBank database (https://www.ncbi.nlm.nih.gov/ accessed on 11/07/23).
The remaining five samples resulted positive only in the initial family-specific molecular assay with Ct values above the cutoff of Ct = 35.0 (Ct ranging between 39.0 and 40.0), while they turned out to be negative in both typing assays. These five samples were classified as negative for Chlamydia spp. due to the possibility of cross-reactions or non-specific amplifications, as recommended by diagnostic guidelines.
3.2 Prevalence and risk factor analysis
Out of the 379 stray and colony cats tested, 29 were confirmed positive for the presence of Chlamydiaceae DNA, with all the animals infected with C. felis. The prevalence in the population tested totaled to 7.7% (CI95% 5.2–10.8). Epidemiological information, C. felis infection status and results of Pearson’s χ2 test are summarized in Table 1. The analysis revealed that sex, sterilization status and sampling season were statistically significant (p < 0.05) and associated with the infection status of the animal.
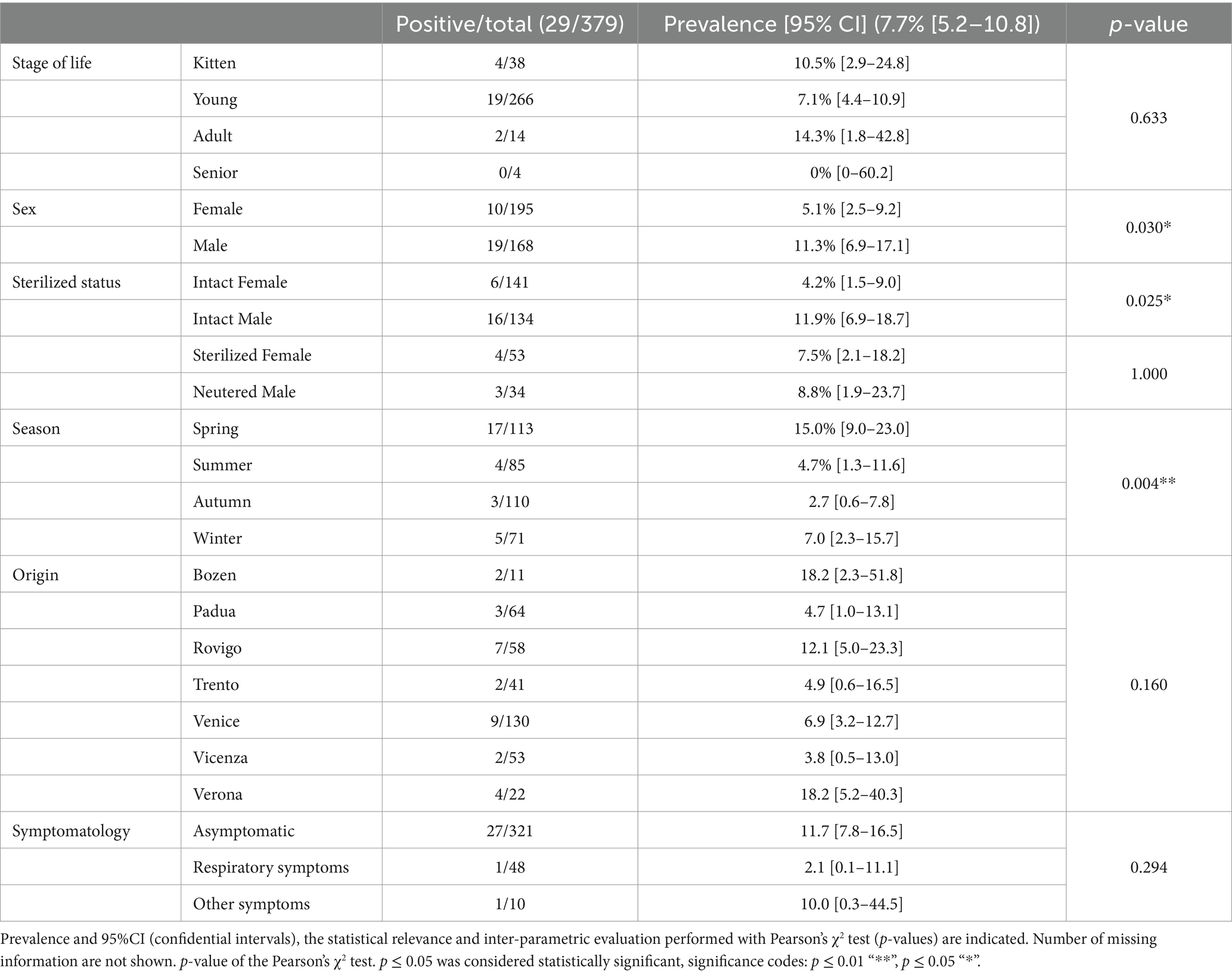
Table 1. Demographic and clinical information, and C. felis infection status of the enrolled animals.
GAMs (Table 2) were used to analyze the risk factor and results further supported these findings. The sampling site, stage of life, and symptomatology did not exhibit statistically significant associations with Chlamydiacea infection (p > 0.05). Meanwhile, the sex of the animals, and the season of collection emerged as key predictors of the infection with C. felis. Male cats were found to have more than double the odds of being infected compared to females (p = 0.035). When both the sex and sterilization status of the animals were taken into consideration, intact males had 3 times higher odds of infection than intact females (p = 0.020). Sterilized animals did not show a statistically significant association with the infection status. Seasonal variation in infection was also notable. Cats sampled in summer (OR = 0.279, p = 0.027) and autumn (OR = 0.158, p = 0.004) had significantly lower odds of infection compared to animals sampled in spring. Using autumn as the baseline, the odds of infection in spring were approximately 6.50 times higher (p = 0.004).
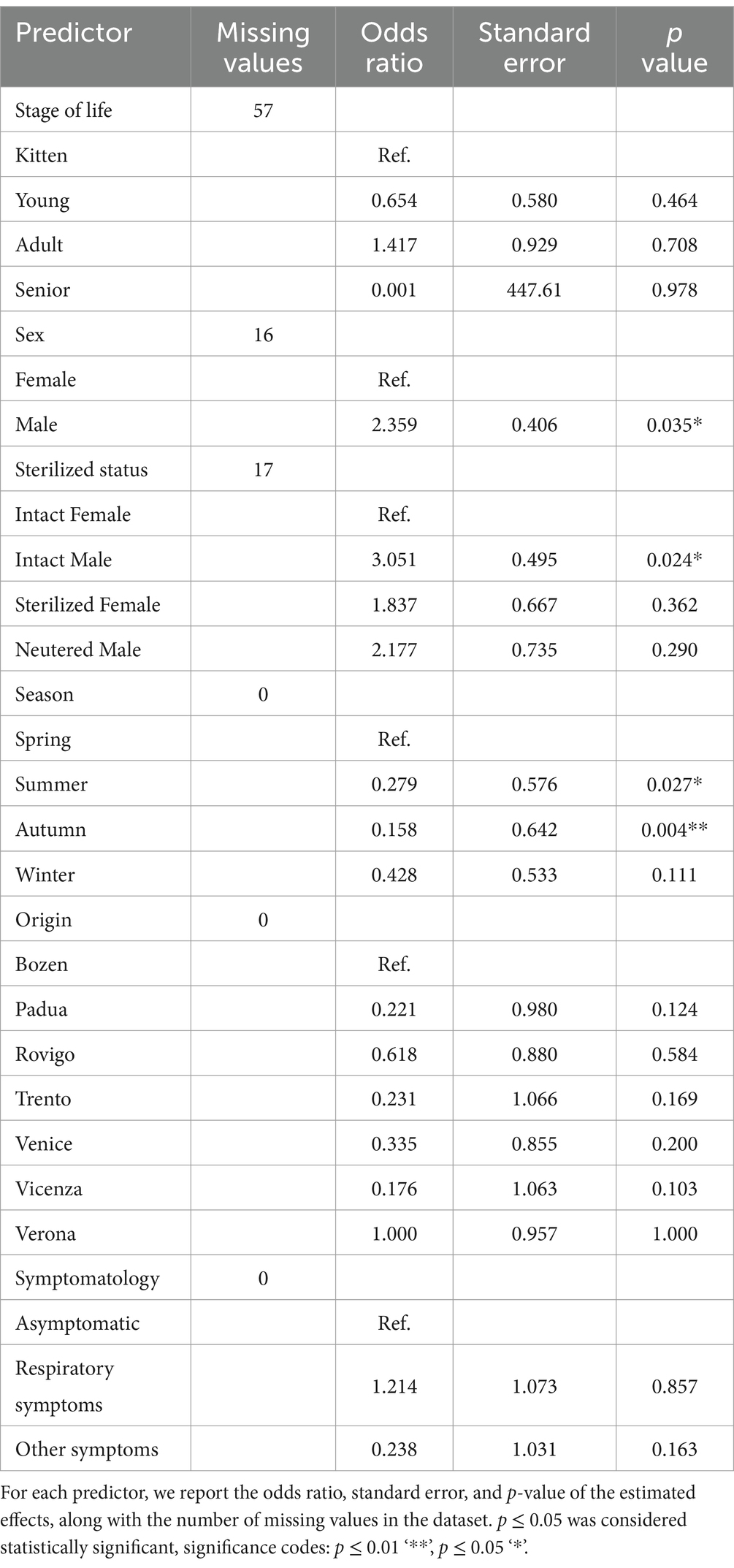
Table 2. Results of the Generalized Additive Models (GAM) analyzing the relationship between the positivity to C. felis and the predictor variables.
4 Discussion
Several field studies have reported that the prevalence of chlamydial infection in pet cats ranges from 0 to 10% in healthy animals and from 5.6 to 30.9% in cats with conjunctivitis (15–17). The stray cat population exhibits higher positivity rates, ranging from 24.4 to 35.7% (17, 18, 20), with subgroups exhibiting conjunctivitis showing positive rates up to 65.8% (19). A previous Italian study used immunofluorescence assays to detect C. felis (29) both in cat colonies (prevalence of 68.4%) and owned cats (prevalence of 22.7%), reporting the widespread circulation of C. felis in the two cat populations, particularly elevated in enclosed cat colonies. However, the diagnostic value of serology for C. felis is limited given the high degree of cross-reactivity of antibodies against different Chlamydia species, and the persistence of the antibody response after infection. Moreover, the intracellular habitat of the pathogen and the predominantly localized epithelial infection may result in restricted antibody production (11). For these reasons, the direct detection of antigens or DNA provides the ultimate proof of a current infection, rather than the presence of specific antibodies. Consequently, the gold standard for diagnosing a C. felis-induced conjunctivitis is through the use of flocked swabs or cytology brushes collected from the conjunctiva, followed by molecular analysis of the samples (18). A previous Italian study by Rampazzo et al. (30) using a molecular assay reported 20% prevalence of C. felis in pet cats with conjunctivitis, and no asymptomatic positive cases detected. However, the investigation did not include stray animals.
While real-time PCR remains the gold standard for Chlamydia detection, infections caused by a variety of pathogens as in the case of feline upper respiratory disease (39), end-point or real-time PCR requires individual assays for each organism. In contrast, methods such as metagenomics can potentially identify all pathogens present in a sample simultaneously. Clinical metagenomics is a newer technique that employs targeted sequencing to reveal all pathogens of interest in clinical samples, while also providing their genomic information (40). A targeted metagenomics assay has recently been developed for feline upper respiratory disease (41). However, its sensitivity for detecting C. felis was lower compared to real-time PCR assay. This may stem from the assay design, or from the target employed. Indeed, the real-time PCR method used targeted all Chlamydia, whereas the target of the clinical metagenomics assay focused on a specific C. felis sequence. Given that cats can be infected with other Chlamydia species (13, 14), an approach with a Chlamydiaceae-specific assay to initially screen the samples, it is important to ensure a broader detection.
In our study, colony and stray cats in northeastern Italy showed a 7.7% (CI95% 5.2–10.8) prevalence of Chlamydiaceae infection, a percentage comparable to the one reported in previous surveys involving asymptomatic cats in Switzerland (18). All the infected animals were positive with C. felis, which is the most common Chlamydia species found in cats and a significant cause of feline acute and chronic conjunctivitis (7). The standard treatment for C. felis infection is doxycycline (7), and both live or inactivated vaccines are commercially available. It is important to note that the implementation of treatments, diagnostics, and prevention strategies may be challenging or ineffective in shelter or colony environments (42).
A limitation of this study is the choice to analyze oropharyngeal swabs rather than nasal/conjunctival swabs, which are considered more specific for C. felis infection. This could have negatively conditioned the estimated prevalence. However, a previous study did not find a significant difference in the molecular detection of C. felis from ocular, oropharyngeal, nasal and tongue swabs (43). Additionally, C. felis has already been detected using owner-collected buccal swabs for the analysis (16). For this reasons, we decided to optimize the collection process for multiple diagnoses within the same research project while minimizing the stress for the cats, considering the oropharyngeal swab as the best compromise. Another limitation of this study is that real-time PCR results, particularly those with high Ct values, only confirm the presence of C. felis DNA in the investigated tissue, without providing information on the pathogen viability. The step of cultivation in cell culture necessary to confirm the viability of C. felis is challenging and has a low sensitivity. Therefore, the presented data do not allow us to conclude whether the sampled cats were infectious and able to pose a risk to humans and other cats.
Statistical analysis revealed a higher risk of infection with C. felis in intact male cats and during the spring season, suggesting that behavior may influence the spread of infection. Intact male cats tend to roam more widely than females, frequently engage in fights with other tomcats to defend their territory (44), and have close contacts with female cats during the mating period, which is more common between February and June (45). This typical behavior of tomcats leads to a close contact with multiple cats, potentially increasing the risk of infection with pathogens, including C. felis. However, previous studies have yielded conflicting results regarding the predisposition of sex, with only one study reporting a significantly higher prevalence in male cats (46). It has been reported that the chlamydial occurrence in cats is typically higher in spring and summer, and more common in younger cats (47). In contrast, a recent study in Shanghai (22) found that C. felis infection in urban asymptomatic cats had a peak detection in summer and winter, with the highest positivity rates in winter, and a statistically significant difference among the seasons. Our findings corroborate that chlamydial infection have a seasonal influence; however, spring seems to be the season with the higher prevalence of infected cats. The lack of statistical significance regarding age in our study may reflect the sampling bias toward younger cats in TNR programs, which comprised 72% of the study population. The presence of symptoms, particularly conjunctivitis, have been associated with C. felis infection in various studies (18–20). Contrary to expectations, clinical symptoms were not associated with infection in our study. Only one of the positive cats presented respiratory symptoms, and no positive cats displayed signs of conjunctivitis. These findings highlight the need for further targeted sampling of symptomatic cats, to gain a deeper understanding of the prevalence of C. felis infection among the symptomatic feline population in Italy.
Although documented cases of zoonotic transmission of C. felis are uncommon, cats have a relatively high prevalence of C. felis and their proximity to humans may pose a risk. Moreover, there are reports of infection in dogs (21), and the ubiquity of cats and dogs as well as their close interactions with humans may facilitate the dissemination of C. felis. Indeed, sero-epidemiological studies have detected a high prevalence of antibodies against C. felis in humans: 1.7% of the general population and 8.8% of small animals veterinarians in Japan (20), and a prevalence of 7.6% in the Italian population (48). Infection with C. felis in humans typically does not result in severe disease in non-immunocompromised individuals, and it is likely that the infection is frequently overlooked or undiagnosed. Nevertheless, cat owners and professionals working with cats should pay attention, particularly when handling animals exhibiting symptoms (11).
5 Conclusions
In conclusion, our study demonstrates the circulation of C. felis in the stray cat populations of northeastern Italy, including asymptomatic individuals. This underscores the potential risk of exposure to this pathogen for humans and free-roaming domestic cats. Although the zoonotic risk is low, precautions should be taken when handling stray and colony cats, particularly among volunteer staff and professionals. Further studies are required to gain deeper understanding of the epidemiology of C. felis. This should include sequence analyses to implement the current knowledge on the strain circulating in Italy, as well as a strategic sampling approach based on the risk factors evidenced in our study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was approved by ethical statements: all samples were collected by veterinarians for diagnostic, therapeutic, or prophylactic purposes. Animal care and procedures adhered to the Guide for the Care and Use of Laboratory Animals and Directive 2010/63/EU for animal experiments (national law: D.L. 26/2014). The study received approval from the ethical committee: CE_IZSVE 8/2020. Participants signed consent forms for the processing of clinical and epidemiological data in research projects. The data is presented in an anonymous and/or aggregated form. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. LC: Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. EM: Data curation, Project administration, Writing – review & editing. MC: Investigation, Methodology, Writing – review & editing. LL: Project administration, Writing – review & editing. AN: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Italian Ministry of Health (Current Research IZSVe 12/19 – grant number B24I19001100001).
Acknowledgments
The authors would like to thank Francesca Ellero for language editing and proofreading.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lynn, WS, Santiago-Ávila, FJ, and Stewart, KL. Outdoor cats: an introduction. Soc Anim. (2022) 30:693–702. doi: 10.1163/15685306-bja10111
2. Crawford, HM, Calver, MC, and Fleming, PA. A case of letting the cat out of the bag—why trap-neuter-return is not an ethical solution for stray cat (Felis Catus) management. Animals. (2019) 1:37. doi: 10.3390/ani9040171
3. Bradshaw, JWS, Horsfield, GF, Allen, JA, and Robinson, IH. Feral cats: their role in the population dynamics of Felis Catus. Appl Anim Behav Sci. (1999) 65:273–83. doi: 10.1016/S0168-1591(99)00086-6
4. Dharmarajan, G, Li, R, Chanda, E, Dean, KR, Dirzo, R, Jakobsen, KS, et al. The animal origin of major human infectious diseases: what can past epidemics teach us about preventing the next pandemic? Zoonoses (Ireland). (2022) 2:1–13. doi: 10.15212/ZOONOSES-2021-0028
5. Taetzsch, SJ, Bertke, AS, and Gruszynski, KR. Zoonotic disease transmission associated with feral cats in a metropolitan area: a geospatial analysis. Zoonoses Public Health. (2018) 65:412–9. doi: 10.1111/zph.12449
6. Borel, N, and Sachse, K. Zoonotic transmission of Chlamydia Spp.: known for 140 years, but still underestimated In: Zoonoses: Infections affecting humans and animals. Ed: Andreas Sing. Cham: Springer International Publishing (2023). 793–819. doi: 10.1007/978-3-030-85877-3_53-1
7. Sykes, JE. Feline Chlamydiosis. Clin Tech Small Anim Pract. (2005) 20:129–34. doi: 10.1053/j.ctsap.2004.12.018
8. Filho, B, Fred, LT, and Hay, R. Woman with red eyes. Ann Emerg Med. (2019) 73:685–7. doi: 10.1016/j.annemergmed.2018.12.017
9. Schachter, J, Bruce Ostler, H, and Meyer, KF. Human infection with the AGENT of feline PNEUMONITIS. Lancet. (1969) 293:1063–5. doi: 10.1016/S0140-6736(69)91703-6
10. Schmeer, N, Jahn, GJ, Bialasiewicz, AA, and Weber, A. The cat as a possible infection source for Chlamydia Psittaci Keratoconjunctivitis in humans. Tierarztliche Praxis. (1987) 15:201–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3617045.
11. Wons, J, Meiller, R, Bergua, A, Bogdan, C, and Geißdörfer, W. Follicular conjunctivitis due to Chlamydia Felis—case report, review of the literature and improved molecular diagnostics. Front Med. (2017) 4:1–6. doi: 10.3389/fmed.2017.00105
12. Hartley, JC, Stevenson, S, Robinson, AJ, Littlewood, JD, Carder, C, Cartledge, J, et al. Conjunctivitis due to Chlamydophila Felis (Chlamydia Psittaci feline Pneumonitis Agent) acquired from a cat: case report with molecular characterization of isolates from the patient and cat. J Infect. (2001) 43:7–11. doi: 10.1016/S0163-4453(01)90845-X
13. Sanderson, H, Vasquez, M, Killion, H, Vance, M, Sondgeroth, K, and Fox, J. Fatal Chlamydia Psittaci infection in a domestic kitten. J Vet Diagn Invest. (2021) 33:101–3. doi: 10.1177/1040638720966960
14. Sibitz, C, Rudnay, EC, Wabnegger, L, Spergser, J, Apfalter, P, and Nell, B. Detection of Chlamydophila Pneumoniae in cats with conjunctivitis. Vet Ophthalmol. (2011) 14:67–74. doi: 10.1111/j.1463-5224.2011.00919.x
15. Cai, Y, Fukushi, H, Koyasu, S, Kuroda, E, Yamaguchi, T, and Hirai, K. An etiological investigation of domestic cats with conjunctivitis and upper respiratory tract disease in Japan. J Vet Med Sci. (2002) 64:215–9. doi: 10.1292/jvms.64.215
16. Chan, I, Dowsey, A, Lait, P, Tasker, S, Blackwell, E, Helps, CR, et al. Prevalence and risk factors for common respiratory pathogens within a cohort of pet cats in the UK. J Small Anim Pract. (2023) 64:552–60. doi: 10.1111/jsap.13623
17. Halánová, M, Petrová, L, Halán, M, Trbolová, A, Babinská, I, and Weissová, T. Impact of way of life and environment on the prevalence of Chlamydia Felis in cats as Potentional sources of infection for humans. Ann Agric Environ Med. (2019) 26:222–6. doi: 10.26444/aaem/100655
18. Bressan, M, Rampazzo, A, Kuratli, J, Marti, H, Pesch, T, and Borel, N. Occurrence of Chlamydiaceae and Chlamydia Felis Pmp9 typing in conjunctival and rectal samples of Swiss stray and pet cats. Pathogens. (2021) 10:951. doi: 10.3390/pathogens10080951
19. Halánová, M, Sulinová, Z, Čisláková, L, Trbolová, A, Páleník, Ľ, Weissová, T, et al. Chlamydophila Felis in cats – are the stray cats dangerous source of infection? Zoonoses Public Health. (2011) 58:519–22. doi: 10.1111/j.1863-2378.2011.01397.x
20. Yan, C, Fukushi, H, Matsudate, H, Ishihara, K, Yasuda, K, Kitagawa, H, et al. Seroepidemiological investigation of feline Chlamydiosis in cats and humans in Japan. Microbiol Immunol. (2000) 44:155–60. doi: 10.1111/j.1348-0421.2000.tb02477.x
21. Wu, SM, Huang, SY, Xu, MJ, Zhou, DH, Song, HQ, and Zhu, XQ. Chlamydia Felis exposure in companion dogs and cats in Lanzhou, China: a public health concern. BMC Vet Res. (2013) 1:5. doi: 10.1186/1746-6148-9-104
22. Yang, D, Houbin, J, Li, X, Shen, H, Ge, F, Yang, X, et al. Epidemiological surveillance of respiratory diseases in urban stray cats in Shanghai. Animals. (2024) 1:10. doi: 10.3390/ani14111562
23. Barimani, M, Mosallanejad, B, Ghorbanpoor, M, and Esmaeilzadeh, S. Molecular detection of Chlamydia Felis in cats in Ahvaz, Iran. Arch Razi Inst. (2019) 74:119–26. doi: 10.22092/ari.2018.116617.1172
24. Kang, Y-H, Cong, W, Qin, S-Y, Shan, X-F, Gao, Y-H, Wang, C-F, et al. First report of toxoplasma Gondii, Dirofilaria Immitis, and Chlamydia Felis infection in stray and companion cats in northeastern and eastern China. Vector-Borne Zoonotic Dis. (2016) 16:654–8. doi: 10.1089/vbz.2016.1993
25. McManus, CM, Levy, JK, Andersen, LA, McGorray, SP, Leutenegger, CM, Gray, LK, et al. Prevalence of upper respiratory pathogens in four management models for unowned cats in the Southeast United States. Vet J. (2014) 201:196–201. doi: 10.1016/j.tvjl.2014.05.015
26. Ravicini, S, Pastor, J, Hawley, J, Brewer, M, Castro-López, J, Beall, M, et al. Prevalence of selected infectious disease agents in stray cats in Catalonia, Spain. J Feline Med Surg Open Rep. (2016) 2:205511691663410. doi: 10.1177/2055116916634109
27. Seki, M.C., Carrasco, A.O.T., Raso, T.F., Sousa, R. L. M., and Pinto, A. A.. (2009). “Molecular survey of Chlamydophila Felis in Brazilian cats.” In World Small Animal Veterinary Association World Congress Proceedings, 1:1–4.
28. Tîrziu, A, Herman, V, Imre, K, Degi, DM, Boldea, M, Florin, V, et al. Occurrence of Chlamydia Spp. in conjunctival samples of stray cats in Timișoara municipality, Western Romania. Microorganisms. (2022) 10:2187. doi: 10.3390/microorganisms10112187
29. Di Francesco, A, Donati, M, Battelli, G, Cevenini, R, and Baldelli, R. Seroepidemiological survey for Chlamydophila Felis among household and feral cats in northern Italy. Vet Rec. (2004) 155:399–400. doi: 10.1136/vr.155.13.399
30. Rampazzo, A, Appino, S, Pregel, P, Tarducci, A, Zini, E, and Biolatti, B. Prevalence of Chlamydophila Felis and feline herpesvirus 1 in cats with conjunctivitis in northern Italy. J Vet Intern Med. (2003) 17:799–807. doi: 10.1111/j.1939-1676.2003.tb02517.x
31. Bellinati, L, Campalto, M, Mazzotta, E, Ceglie, L, Cavicchio, L, Mion, M, et al. One-year surveillance of SARS-CoV-2 exposure in stray cats and kennel dogs from northeastern Italy. Microorganisms. (2022) 11:110. doi: 10.3390/microorganisms11010110
32. Cavicchio, L, Campalto, M, Carrino, M, Lucchese, L, Ceglie, L, Fincato, A, et al. Influenza in feral cat populations: insights from a study in north-East Italy. Front Vet Sci. (2024) 11:1439354. doi: 10.3389/fvets.2024.1439354
33. Quimby, J, Gowland, S, Carney, HC, DePorter, T, Plummer, P, and Westropp, J. 2021 AAHA/AAFP Feline Life Stage Guidelines. J Feline Med Surg. (2021) 23:211–33. doi: 10.1177/1098612X21993657
34. Ehricht, R, Slickers, P, Goellner, S, Hotzel, H, and Sachse, K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes. (2006) 20:60–3. doi: 10.1016/j.mcp.2005.09.003
35. Hoffmann, B, Depner, K, Schirrmeier, H, and Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for Pestiviruses. J Virol Methods. (2006) 136:200–9. doi: 10.1016/j.jviromet.2006.05.020
36. World Organisation for Animal Health. Chapter 3.3.1 avian Chlamydiosis In: Manual of diagnostic tests and vaccines for terrestrial animals (2018). 1–20. Available at: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.01_AVIAN_CHLAMYD.pdf
37. Pantchev, A, Sting, R, Bauerfeind, R, Tyczka, J, and Sachse, K. Detection of all Chlamydophila and Chlamydia Spp. of veterinary interest using species-specific real-time PCR assays. Comp Immunol Microbiol Infect Dis. (2010) 33:473–84. doi: 10.1016/j.cimid.2009.08.002
38. Vicari, Nadia, Riccardo, Santoni, Vigo, PG, and Magnino, Simone. (2004). “A PCR-RFLP assay targeting the 16S ribosomal gene for the diagnosis of animal Chlamydioses.” In 5th meeting of the European Society for Chlamydia Research, Budapest – September 1–4 2004, 297.
39. Kennedy, U, Paterson, MBA, Magalhaes, RS, Callaghan, T, and Clark, N. A scoping review of the evidence on prevalence of feline upper respiratory tract infections and associated risk factors. Vet Sci. (2024) 11:232. doi: 10.3390/vetsci11060232
40. Dulanto Chiang, A, and Dekker, JP. From the pipeline to the bedside: advances and challenges in clinical metagenomics. J Infect Dis. (2020) 221:S331–40. doi: 10.1093/infdis/jiz151
41. Kattoor, JJ, Mlalazi-Oyinloye, M, Nemser, SM, and Wilkes, RP. Development of a targeted NGS assay for the detection of respiratory pathogens including SARS-CoV-2 in felines. Pathogens. (2024) 13:1–10. doi: 10.3390/pathogens13040335
42. Lewin, AC, Hicks, SK, and Carter, RT. A review of evidence-based Management of Infectious Ocular Surface Disease in shelter-housed domestic cats. Vet Ophthalmol. (2023) 26:47–58. doi: 10.1111/vop.13063
43. Schulz, C, Hartmann, K, Mueller, RS, Helps, C, and Schulz, BS. Sampling sites for detection of feline Herpesvirus-1, feline Calicivirus and Chlamydia Felis in cats with feline upper respiratory tract disease. J Feline Med Surg. (2015) 17:1012–9. doi: 10.1177/1098612X15569615
44. Johnson, AK. Normal feline reproduction: the tom. J Feline Med Surg. (2022) 24:212–20. doi: 10.1177/1098612X221079707
45. Ng, TT, Fascetti, AJ, and Larsen, JA. Reproduction of domestic cats in laboratories, catteries, and feral colonies: a review. Top Companion Anim Med. (2023) 55:100780. doi: 10.1016/j.tcam.2023.100780
46. Wills, JM, Gruffydd-Jones, TJ, Richmond, SJ, Gaskell, RM, and Bourne, FJ. Effect of vaccination on feline Chlamydia Psittaci infection. Infect Immun. (1987) 55:2653–7. doi: 10.1128/iai.55.11.2653-2657.1987
47. Sykes, JE, Anderson, GA, Studdert, VP, and Browning, GF. Prevalence of feline Chlamydia Psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. J Vet Int Med. (1999) 13:153–62. doi: 10.1111/j.1939-1676.1999.tb02172.x
48. Francesco, ADi, Donati, M, Mazzeo, C, Battelli, G, Piva, S, Cevenini, R, et al. (2006). “Feline Chlamydiosis: a Seroepidemiological investigation of human beings with and without contact with cats.” Vet Rec, 159: 778–779. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17142626.
Keywords: Chlamydiaceae, Chlamydia felis, zoonosis, stray cats, conjunctivitis
Citation: Bellinati L, Ceglie L, Mazzotta E, Campalto M, Lucchese L and Natale A (2025) One-year surveillance of Chlamydia spp. infection in stray cats from northeastern Italy. Front. Vet. Sci. 12:1502642. doi: 10.3389/fvets.2025.1502642
Edited by:
Roswitha Merle, Free University of Berlin, GermanyReviewed by:
Jobin Jose Kattoor, University of Pittsburgh, United StatesJanos Degi, University of Life Sciences King Mihai I Timișoara, Romania
Copyright © 2025 Bellinati, Ceglie, Mazzotta, Campalto, Lucchese and Natale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Bellinati, bGJlbGxpbmF0aUBpenN2ZW5lemllLml0
 Laura Bellinati
Laura Bellinati Letizia Ceglie
Letizia Ceglie Elisa Mazzotta
Elisa Mazzotta Mery Campalto
Mery Campalto Laura Lucchese
Laura Lucchese Alda Natale
Alda Natale