
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 21 February 2025
Sec. Veterinary Epidemiology and Economics
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1494103
 Catherine Ollagnier1*
Catherine Ollagnier1* Maria-Rita Mellino2
Maria-Rita Mellino2 Nicolas Pradervand3
Nicolas Pradervand3 Marco Tretola4
Marco Tretola4 Sebastien Dubois5
Sebastien Dubois5 Stephane Durosoy6
Stephane Durosoy6 Olivier Desrues7
Olivier Desrues7 Johana Bellon1
Johana Bellon1Most antimicrobials used in pig production are prescribed to treat post-weaning diarrhea (PWD), which constitutes a major health issue in pig production. With the spread of multidrug-resistant pathogens, finding solutions to diminish the severity of PWD without antibiotics has become increasingly critical. Potentiated forms of zinc oxide (ZnO) and plant-based bioactive compounds like tannins have been shown to alleviate the severity of diarrhea, thus reducing the need for antibiotic treatment. The aim of this project was to test whether a potentiated form of ZnO (pZnO), alone (study 1) or in combination with tannin-rich extract (study 2), can be used in starter diets for weaned piglets infected by enterotoxigenic Escherichia coli (ETEC) to reduce PWD severity. At 26 ± 1.6 days of age (average body weight 7.8 ± 1.02 kg), 160 piglets (study 1, n = 72; study 2, n = 88) were randomly and equally assigned to four dietary treatments (study 1 = 18; study 2 = 22 pigs/group) and orally infected 4 days after weaning with a solution containing 1010 ETEC F4. Study 1 compared the effect of 150 mg Zn/kg pZnO (pZnO-150) and 300 mg Zn/kg pZnO (pZnO-300) to a negative control (C) and a positive control, 3,000 mg Zn/kg ZnO (C-3000). In study 2, a combination of 7.5 g/kg tannin extract (quebracho and chestnut) and 150 mg Zn/kg pZnO (TAN+pZnO-150) was compared to pZnO-150 and 7.5 g/kg tannin-rich extract (TAN) alone and to a negative control (C). The fecal score, bodyweight, daily food intake per pen, fecal F4 ETEC and Zn levels were analyzed. The small intestine content was sampled 9 days after infection to analyze the number of antimicrobial resistance genes. Regardless of the inclusion level, TAN+pZnO-150, TAN and pZnO led to a reduction in antibiotic treatment (p < 0.05), but only TAN and TAN+pZnO-150 reduced the fecal score (p < 0.05). C-3000 improved the average daily gain (p < 0.05). Tannin-rich extract and potentiated zinc oxide (pZnO) in starter diets effectively reduce the need for antibiotics in ETEC-challenged piglets. Traditional high-dose ZnO improved growth rates, but lower-dose alternatives with tannins provided health benefits without high zinc levels. These findings highlight sustainable dietary strategies to manage post-weaning diarrhea, supporting reduced antibiotic use in pig production.
The way antibiotics (AB) are used in animal production has changed in the last 20 years due to the emerging threat of bacteria that are resistant to antibiotics. European Union first banned AB as feed additives for growth promotion in 2003 (1) and then prohibited their use as a prophylactic treatment for groups of animals in 2018 (2). Throughout Europe, governments are asking for prudent use of AB, and this approach has been fruitful. In Denmark, the AB use in animals decreased by 14% between 2013 and 2018 (3). Many European countries continue to establish action plans to further reduce, refine and replace the use of AB. In this context, management and breeding practices are changing, with alternatives to AB being explored.
In pig production, the majority of AB are used during the post-weaning period. In a study on 227 farms in Switzerland (4), 421 kg of active AB ingredients were used for pigs, 49.4% of which were used for weaners. The post-weaning period is critical for pigs. During this transition period, they are separated from their mothers and regrouped in a new environment, with a new drinking and feeding system. Their digestive tract has to adapt to a new diet composition. All these changes generate stress and lead to an intestinal dysbiosis that can cause diarrhea (5). Post-weaning diarrhea (PWD) is defined as a diarrhea occurring within 14 days after weaning (6). This digestive disorder does not always have an infectious component, but it may be complicated by enterotoxigenic Escherichia coli (ETEC) infection (7). ETEC are pathogenic E. coli that adhere to the small intestine mucosae, with F4 and F18 being the two main types of fimbriae. After adhesion to the mucosa, ETEC F4 secrete two types of toxins: heat stable (STa and STb) and labile toxins (LT).
High doses of zinc oxide (ZnO) were found to be an effective solution to replace AB use for digestive disorders in pigs. ZnO doses higher than 2,500 mg Zn/kg feed reduce diarrhea and improve feed intake, bodyweight (BW) gain and feed conversion rate (8). However, due to the non-volatile and non-degradable physicochemical properties of Zn, a continuous field application of manure from animals treated with a high dose of ZnO leads to a gradual and continuous accumulation of Zn in soil, water and sediments. A field study in Denmark reported an increase of >45% in Zn concentrations in soil from 1998 to 2014 (9). Zn might also promote the spread of antimicrobial resistance (10–12). Indeed, the level of Zn in liquid pig manure correlates with the level of antimicrobial resistance (13). Ultimately, the European Union decided in 2017 to ban the use of high doses of ZnO as veterinary medicinal product (14) because the benefits of ZnO in terms of preventing diarrhea in pigs did not outweigh the risks for the environment. Anticipating that the European ban on high doses of ZnO will become effective in 2022, new formulations of ZnO have been explored to provide a therapeutic effect at doses accepted by the regulatory authorities. Previous studies have demonstrated that a potentiated form of ZnO (pZnO) could improve zootechnical performance and reduce the severity of diarrhea when given to weaning pigs (15–18).
Plant polyphenolic compounds, such as those in plant extracts, have great value as a natural source of bioactive compounds to alleviate digestive disorders in pigs (19). In the polyphenol group, tannins are the most important class and are mainly classified into two subgroups: hydrolysable and condensed tannins (20). Tannins extracted from chestnut trees (Castanea sativa Mill.) and quebracho trees (Schinopsis spp.) contain hydrolysable and condensed tannins, respectively. Those tannin extracts have been tested in pigs for their antioxidant, anti-inflammatory and antibacterial activities, both on conventional and ETEC-infected weaners (21–26).
This study evaluated the efficacy of a potentiated form of ZnO with or without a chestnut and quebracho tannin-rich extract in reducing the severity of PWD in an enterotoxigenic Escherichia coli infection model. The effects of these dietary treatments on health status, growth performance, excretion of pathogens, zinc and antimicrobial resistance genes were assessed.
Two studies were carried out to test the efficacy of two ZnO sources supplemented at different inclusion levels with or without tannin extracts in reducing PWD after an ETEC F4 challenge. In study 1, four starter diets were prepared: the negative control (C) and positive control (C-3000) diets were supplemented with 150 and 3,000 mg/kg ZnO (zinc oxide, Millenis SAS, France), respectively, whereas the pZnO-150 and pZnO-300 diets were supplemented with 150 or 300 mg/kg pZnO (HiZox®, Animine, Annecy, France), respectively. The HiZox® formulation contained 94% ZnO. In study 2, we used two diets in addition to the C and pZnO-150 diets: the TAN diet was supplemented with 7.5 g/kg of a tannin extract containing chestnut and quebracho tannins (Silvafeed®Nutri P, Silvateam, Italy), and the TAN+pZnO-150 diet was supplemented with 7.5 g/kg of the tannin extract plus 150 mg/kg of pZnO. The Silvafeed®Nutri P product contained a minimum of 75% tannins, as measured by the ISO 14088 standard. The standard starter diet was formulated in accordance with Swiss feeding guidelines (27) for weaned pigs (Supplementary Table S1). The doses of pZnO and tannins riche extract were selected based on literature (18, 19). Both experiments were approved by the Swiss cantonal veterinary office under the application numbers 29,361 and 31,400, respectively. The model of infection with ETEC F4 had been previously validated (22).
Female and castrated male Swiss Large White piglets, originating from the Agroscope sows' herd, were weaned at 26 (±1.6) days (mean ± standard deviation) with an average bodyweight of 7.8 ± 1.02 kg. A total of 72 piglets participated in study 1, while study 2 included 88 piglets, resulting in 18 and 22 piglets per group, respectively. All piglets were determined to be sensitive to ETEC F4, using a previously described method (28). Briefly, an ear punch (3 mm in diameter) was collected from 420 piglets before weaning. After DNA extraction, PCR was carried out using primers coding for flanking markers of F4acR localized near the MUC13 gene. Piglets having at least one copy of the genetic marker for ETEC F4 receptor were considered sensitive to ETEC F4 infection.
For each study, piglets within each litter were randomly allocated to one of four dietary treatments based on their body weight. Animals were housed in pens (2.6 m2) in groups of three to four animals, all belonging to the same dietary treatment group. Fresh feed was offered once a day, and leftovers were weighed daily. Water was available ad libitum, and an electrolyte solution was provided to limit the risk of dehydration.
Four days after weaning, all piglets were infected (d0) with an oral solution containing 1010 CFU ETEC F4. Trained staff monitored the piglets' health at least once a day. Nine (±1) days after infection, all piglets, except 4 piglets per group in study 2, were euthanized by electronarcosis followed by exsanguination, and samples of intestinal content and jejunum mucosa were collected.
A total of three farrowing series were necessary to achieve the requested number of piglets for each study. There were two sudden deaths in study 1, one in group C and one in C-3000. The reasons for these deaths are unknown, as these pigs did not have high fecal scores the day before. Two pigs (one in group C and one in group pZnO-150, study 1) had to be removed due to arthritis and panaris, respectively. One pZnO-150 pig in study 2 had to be euthanized by an overdose of pentobarbital (Esconarkon®, Streuli Tiergesundheit AG, Uznach, Switzerland) due to acute pneumonia on d0.
The fecal score was monitored for each pig on d −4, −1, 0, 1, 2, 3, 6 and 7, with d0 being the day of infection. To evaluate the fecal score, a cotton swab was introduced into piglet's rectum to collect a small amount of feces. The consistency of the feces was scored on a scale ranging from 1 to 4. Normal molted feces were given a score of 1 and watery diarrhea a score of 4. Liquid diarrhea was scored as 3, and creamy feces was given a score of 2. In addition, a VAS (Visual Analogic Score) was used in study 1 to assess the severity of the diarrhea. A VAS is a psychometric response scale used to measure subjective characteristics (29, 30). The VAS consisted of a 40-mm-long horizontal line with numeric descriptors (1–4) every 10 mm to represent fecal score class. An observer marked the point on the line that best reflects the observed severity of the diarrhea. Compared to fecal scores, VAS enabled the observer to characterize the severity of the diarrhea as a continuous process, thus capturing the quality of the feces in more detail. In study 1, feces were collected directly from the rectum of each pig to assess the Zn concentration on d −1, 0, 1, 2, 3, 6 and 7.
To ensure animal welfare, piglets with severe ETEC infection clinical signs, i.e., watery diarrhea for more than five consecutive days, or watery diarrhea and apathy, were removed from the study. These pigs are described as “rescue treated” as they were treated with AB (sulfamide trimethoprim, 15 mg/kg BW Borgal® 24%, MSD AH, Switzerland) for five consecutive days to alleviate the clinical signs.
On the day of euthanasia, 100–200 mg of mucosa were scraped from the middle jejunum of piglets and immediately frozen in liquid nitrogen. Samples were kept at −80°C until further analysis. For IL-6 and IL-8 measurements, mucosa was homogenized by sonication in 20 mmol/L Tris base, 137 nmol/L NaCl, 1% NP-40 (Nonidet P-40) and 10% glycerol, supplemented with a cocktail of protease inhibitors (Complete TM, Sigma, Buchs, Switzerland). After 5 min of centrifugation at 800 g at 4°C, the supernatant was recovered and used to determine IL-6 and IL-8 concentrations using specific pig IL-6 and IL-8 ELISA kits (Abcam, Cambridge, United Kingdom) following the manufacturer's procedure. The results were expressed as ng of IL/mg of protein used in the assay.
The concentrations in tight junction proteins (i.e., occludin and claudin-1, claudin-5 and claudin-7) were assessed by western blots. The proteins were extracted by three freeze–thaw cycles from 100 to 200 mg of mucosa with CelLytic MT buffer (Sigma, Buchs, Switzerland), supplemented with a cocktail of protease inhibitors (Complete TM, Sigma, Buchs, Switzerland), using the freeze–thaw lysis method. Samples were then centrifuged for 10 min, 12,000 × g at 4°C. Proteins (20–30 μg) were separated on an 8% acrylamide SDS-PAGE gel and further transferred to polyvinylidene difluoride membranes (Fisher Scientific, Reinach, Switzerland), as described previously (31). Hybridizations were performed overnight at 4°C in PBS (phosphate buffered saline) supplemented with 0.1% Tween-20 and 5% BSA (bovine serum albumin). The following primary antibodies were used at the indicated dilutions: rabbit anti-occludin 1:1000 (Abcam, Cambridge, United Kingdom), rabbit anti-claudin-1 1:1000 (Abcam, Cambridge, United Kingdom), rabbit anti-claudin-7 1:500 (Fisher Scientific, Reinach, Switzerland) and mouse anti-claudin-5 1:200 (Fisher Scientific, Reinach, Switzerland). After washing, blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or mouse IgG secondary antibodies diluted 1:1000 in PBS supplemented with 0.1% Tween-20 and 5% non-fat milk for 1 h at room temperature. Protein bands were further detected using chemiluminescence with a Quantum kit (Witec, Luzern, Switzerland). The intensity of the tight junction proteins signals was normalized to the signal of vinculin.
Piglets' oxidative status was determined in serum using commercial kits as instructed by the manufacturer. Serum was obtained from blood directly sampled during bleeding on the day of euthanasia. Blood was collected using blood collection tubes with serum clot activator (Vacuette; Greiner Bio-One GmbH), which were stored upside down at room temperature for 1 h before processing. The Vacuette serum tubes were then centrifuged for 15 min at 3,000 g and subsequently for 2 min at 4,000 g. Two aliquots of serum were stored at −20°C in Eppendorf tubes. The MDA equivalent content was assessed using the Lipid Peroxydation Assay kit (Sigma, Buchs, Switzerland). Total antioxidant status (TAS) was assessed using the Antioxidant Assay kit (Sigma, Buchs, Switzerland). Superoxide dismutase (SOD) activity was assessed using the Superoxide Dismutase colorimetric activity kit (Invitrogen, Waltham MA, USA). Finally, lactate dehydrogenase (LDH) activity was assessed using the human LDH SCE Humazym test (Human Diagnostics, Wiesbaden, Deutschland).
The infective strain was resistant to rifampicine (rif) and harbored the F4 fimbriae (K88ac+), the heat-labile toxin (LT+) and the heat-stable toxin (STb+), as determined by PCR (11). The amount of F4 ETEC excreted was measured by counting the number of colony-forming units per culture on an E. coli-selective medium (Eosin Methylene Blue Agar plates) containing rifampin (50 μg/mL).
Measurements were performed on feces samples collected on d0, 1, 2, 3 and 6. On the day of euthanasia, the contents of the small intestine were collected from 6 pigs per group in study 1 and subjected to resistome analysis. qPCR was performed on 89 bacterial resistance genes (Qiagen microbial PCR array, Qiagen AG, Hombrechtikon, Switzerland) following the manufacturer's recommendations. In brief, DNA was extracted from each sample (QIAamp kit, Qiagen AG, Hombrechtikon, Switzerland) and mixed with MicrobialqPCR mastermix (included in Qiagen microbial PCR array kit) and then aliquoted to each well of the plate containing a pre-dispensed gene-specific primer and hydrolysis probe set. The array can simultaneously target a broad-spectrum profile of 89 genes from all major classes of antibioresistance genes (Supplementary Table S2), including aminoglycoside, β-lactam, erythromycin, quinolone and fluoroquinolone, macrolide-lincosamide-streptogramin_b, tetracycline and vancomycin.
Each diet was analyzed in triplicate to determine its chemical composition, including crude protein, crude fiber and fat content. Feces collected on d0, 1, 2, 3 and 6 (study 1) were pooled together, homogenized and analyzed for Zn to compare the excretion of Zn relative to the quantity in the diet in the pZnO-150, pZnO-300, C and C-3000 groups. In brief, all samples were freeze-dried before analyses (Christ Delta 2-24, Kühner AG, Birsfelden, Switzerland). After being ground to pass a 1-mm screen (Brabender rotary mill; Brabender GmbH & Co. KG, Duisburg, Germany), feed samples were analyzed for dry matter content by heating at 105°C for 3 h followed by incineration at 550°C until a stable mass was reached to determine the ash content according to ISO 5984_2002 (prepASH, Precisa Gravimetrics AG, Dietikon, Switzerland).
The tannin content of the tannin-rich extract was determined by the ISO 14088 method. Zn content was analyzed according to EN 15510:2008 by ICP-OES (ICP-OES 5800, Agilent Technologies, Switzerland) after microwave digestion. Samples were dissolved in a glass tube (5 mL HNO3 65% + 3 mL H2O ASTM Class I) using a microwave digester (UltraClave MLS, Leutkirch, Germany) at 235°C for 60 min (1000 W). If necessary, samples were diluted with HNO3 2% prior to analysis.
All analyses were performed with R studio (v3.6.3) using the nlme, MASS, FSA, ordinal, car, emmeans and multicomp packages. Fecal scores were analyzed with a repeated two-way ordinal regression, with dietary treatment, study day and farrowing series as fixed effects. All other variables were analyzed with a linear mixed effect model, with dietary treatment, study day (if appropriate) and farrowing series as fixed effects. Interactions between dietary treatment, study day and farrowing series were always tested and only retained in the model if they were significant. In general, the models were reduced by stepwise exclusion of non-significant (P > 0.05) interactions and factors (except dietary treatment and study day). Least-squares means of the response variables and Tukey–Kramer pairwise comparisons were computed if the dietary treatment was significant, and differences were considered significant if P < 0.05 (a tendency was considered if P < 0.10). Homoscedasticity and normality of the residues were checked for each model. In case of interaction, the contrast of the parameter of interest was always calculated for fixed values of the interactive parameter using the emmeans package.
Feed intake was calculated per day as the amount of feed disappearance in the pen and divided by the number of animals, and the pen was used as the experimental unit. Average daily feed intake (ADFI) was calculated as the average daily feed intake of a pen for a specific period. Diarrhea was defined as a fecal score above 2, and the duration of diarrhea was calculated as the sum of days in diarrhea for each pig. The daily prevalence of diarrhea was calculated as the percentage of pigs in a dietary treatment having a fecal score greater than 2. Feed efficiency was calculated as the sum of total feed used per pen divided by the total weight gain of the pen.
For all animals removed from the study, missing values were attributed to all subsequent measurements after the removal date. Exceptions were made for “rescue-treated” animals, where the last fecal score and VAS measured were carried over to all subsequent measurements, to penalize ineffective treatment, as done in clinical trials for drug development (32).
A total of 12 pigs were “rescue treated” with AB due to severe ETEC infection symptoms. Nine pigs were in the C group, with five and four pigs in studies 1 and 2, respectively. In contrast, the other treatment groups (pZnO-150, pZnO-300, C-3000, TAN and TAN + pZnO-150) each had either one pig or no pigs that needed rescue treatment, p = 0.05 (Table 1).
Infection on d0 led to an increased fecal score in each dietary treatment on d1 (p < 0.0001), except for C-3000 (Figure 1). In study 1, group C had higher fecal scores than group C-3000 on d-1, 0, 1, 2, 3, 6 and 7 (p < 0.0001). Regardless of the day, there was no difference in fecal scores between C and the two other experimental treatments (pZnO-150 and pZnO-300). Starting on d1, pigs in the C-3000 group had lower fecal scores than those in the pZnO-150 or pZnO-300 groups (p < 0.01). The visual analogic score (VAS) followed the same pattern as the fecal scores (Supplementary Figure S1). In study 2, TAN and TAN+pZnO-150 pigs had lower fecal scores than C starting on d1 (p < 0.05).
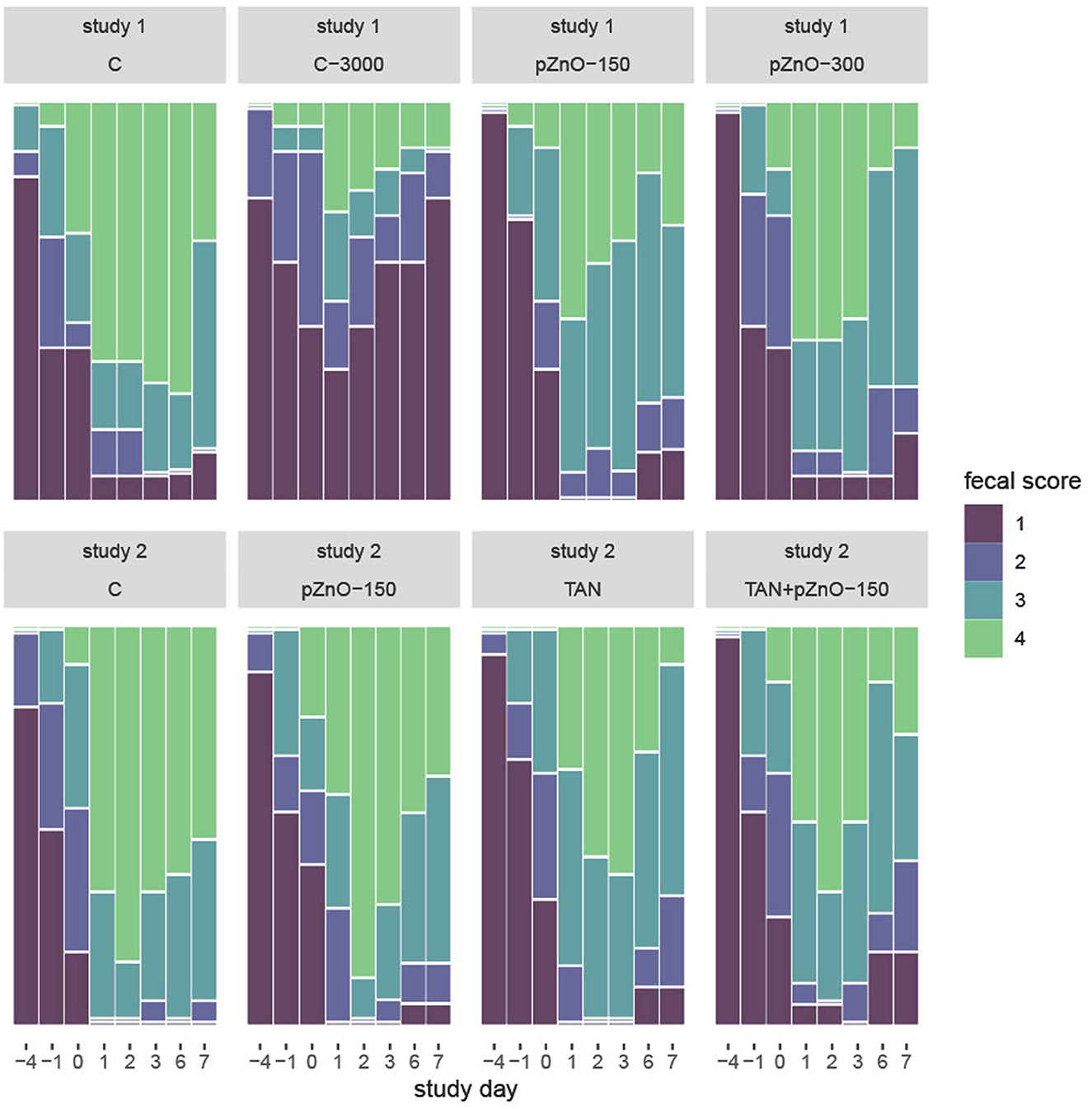
Figure 1. Mosaic plot of fecal score in relation to study day in studies 1 and 2. Fecal score was evaluated on a scale of 1–4. Normal molted feces were given a score of 1, watery diarrhea a score of 4. C: control group with standard starter diet and 150 mg/kg Zn of conventional ZnO (maximal Zn concentration allowed in EU); C-3000: positive control group with standard starter diet with 3,000 mg/kg Zn of conventional ZnO; pZnO-150 and pZnO-300: starter diet with 150 and 300 mg/kg Zn of potentiated ZnO, respectively; TAN: starter diet with 7.5 g/kg of tannins extract and 150 mg/kg Zn of conventional ZnO; TAN+pZnO-150: starter diet with 7.5 g/kg of tannins extract and 150 mg/kg Zn of potentiated ZnO.
The duration of diarrhea was 1.6 days on average in group C-3000 and over 4 days in the C, pZnO-150 and pZnO-300 groups (p < 0.0001; Table 1). The average daily prevalence of diarrhea, expressed as the percent of pigs with diarrhea within the experimental treatment, was 19% in C-3000 compared to > 50% in the other groups in study 1 (p < 0.001). In study 2, there was no effect of the groups on the duration (5 days, on average) or daily prevalence of diarrhea (>72% in all groups).
In study 1, C-3000 pigs grew faster (p < 0.05) from d4 to d7 than pigs in the other experimental treatments, which resulted in a greater bodyweight on day 7 (p = 0.02; Table 2). Improved growth was associated with a greater average daily feed intake (ADFI) after ETEC infection (Figure 2; p < 0.05). There was a significant interaction between experimental treatments and time for the variables BW and ADFI. In study 2, BW, average daily gain (ADG), ADFI and feed efficiency (FE) were not affected by the dietary treatments (Table 2). Regardless of the study, pigs ingested only a small amount of feed the day of weaning (d −4), and the consumption slightly increased until d −1 before dropping again for all dietary treatments after infection at d0. In study 1, the C-3000 pigs ate 0.10 kg more on d7 than the rest of the pigs (p < 0.01). In study 2, the feed consumption on d7 in dietary treatments TAN and TAN+pZnO-150 was 0.05 kg higher than in the other dietary treatments (p>0.05). There was no difference in FE among the dietary treatments.
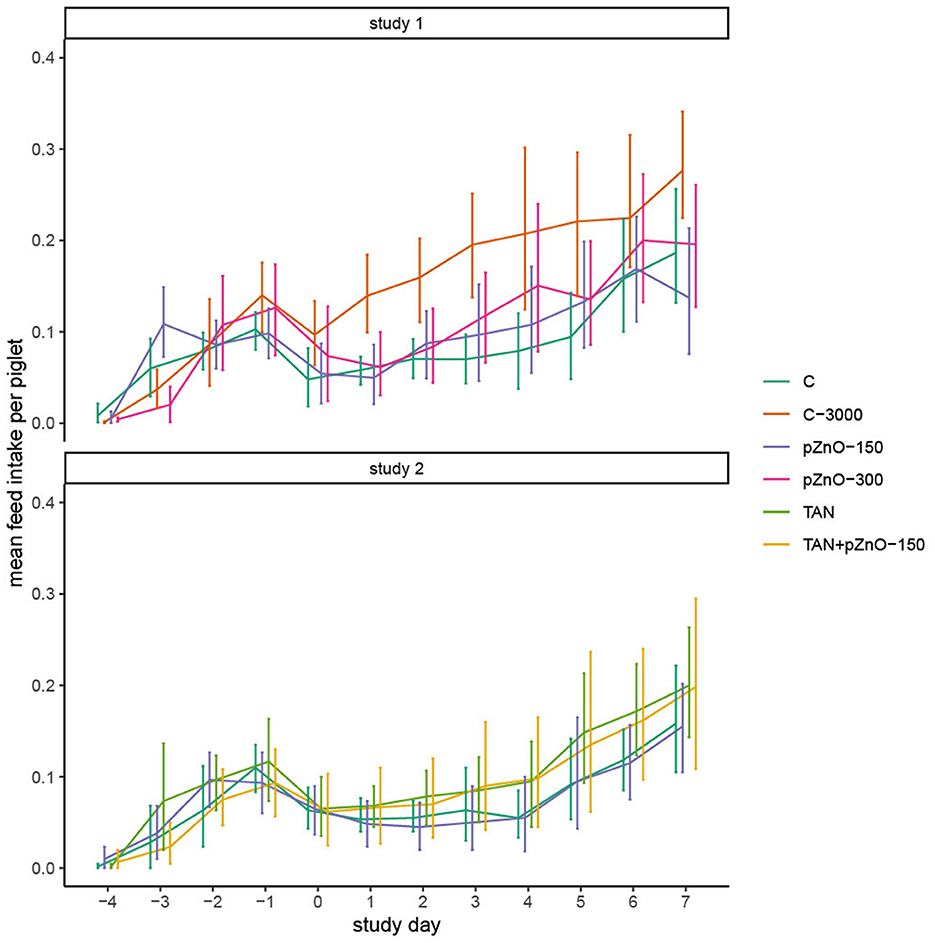
Figure 2. Mean daily feed intake calculated per piglet and per pen. The daily feed intake was measured per pen and divided by the number of pigs in the pen, as the number of pigs per pen was not always the same (mortality). The pigs ate little on weaning day, and their consumption increased slightly until d-1, before dropping again after infection.
In both studies, the concentrations of tight junction proteins occludin, claudin 1 and claudin 5 in the small intestine were not affected by the dietary treatments (Figure 3; Table 3). In contrast, the concentration of claudin 7 was greater in C-3000 pigs than in pZnO-150 pigs in study 1, and it was greater in pZnO-150 and TAN pigs than in TAN + pZnO-150 pigs in study 2. The concentrations of interleukin 6 (IL-6) and interleukin 8 (IL-8) were not affected by the dietary treatment in either study (Table 3). In study 2, the Malondialdehyde (MDA) concentrations were higher in the TAN than in the C and pZnO-150 groups (p < 0.05; Table 3). In the pZnO-150 group, TAS concentrations were lower than in the TAN + pZnO-150 group (p < 0.05).
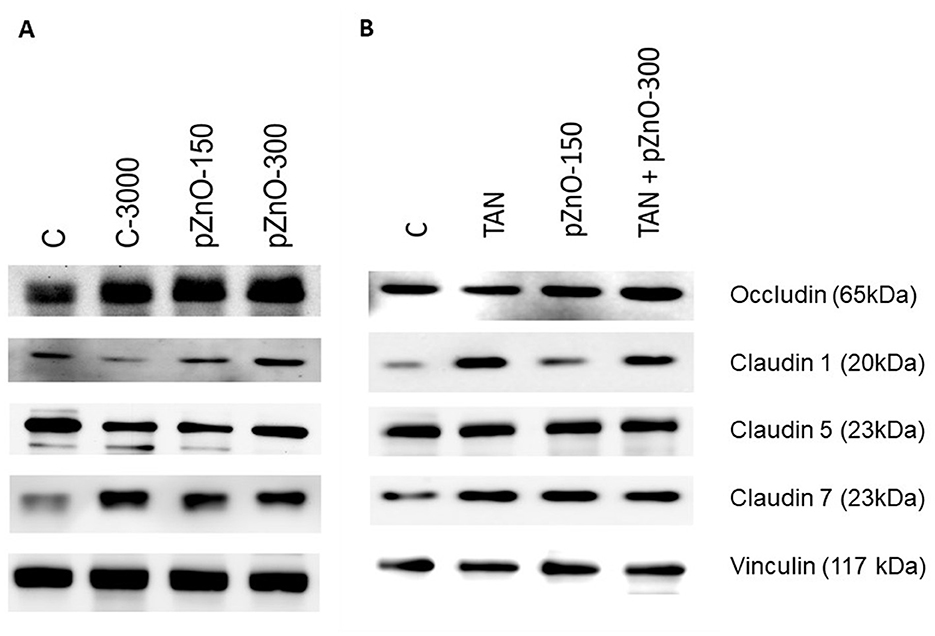
Figure 3. Representative protein bands for the tight junction proteins. Piglets were euthanized on d9 (±1), and small intestine mucosa was scraped for western blot (tight junction proteins) and interleukin analyses. (A) study 1: C: control group with standard starter diet and 150 mg/kg Zn of conventional ZnO (maximal Zn concentration allowed in EU); C-3000: positive control group with standard starter diet with 3,000 mg/kg Zn of conventional ZnO; pZnO-150 and pZnO-300: starter diet with 150 and 300 mg/kg Zn of potentiated ZnO, respectively; (B) study 2: TAN: starter diet with 7.5 g/kg of tannins extract and 150 mg/kg Zn of conventional ZnO; TAN+pZnO-150: starter diet with 7.5 g/kg of tannins extract and 150 mg/kg Zn of potentiated ZnO.
The C-3000 pigs excreted 12,312 mg Zn/kg dry mater of feces, which was higher (p < 0.0001, Table 4) than the fecal Zn excretion in the C, pZnO-150 and pZnO-300 groups (1,028 mg Zn/kg dry mater of feces, on average). The excretion of ETEC F4 was the lowest in the C-3000 pigs and the highest in the pZnO-150 pigs (p < 0.05), with intermediate excretion levels for the pZnO-300 and C pigs in study 1 (Table 4). There was no dietary treatment effect on the ETEC F4 excretion level in study 2.
In study 1, C-3000 dietary treatment led to a 13-fold increase in the number of resistance genes compared to C-group (p < 0.001), whereas pZnO-150 and pZnO-300 resulted in a net decrease (p < 0.001; Table 4).
The high dose of ZnO led to a reduction of AB treatment, lower fecal score and increased feed intake, resulting in higher ADG but not higher FE. The high dose of ZnO also resulted in a high Zn excretion and a greater number of copies of resistance genes in the intestinal microbiota compared to the control group. The potentiated source of ZnO in the 150 and 300 mg Zn/kg diets reduced the severity of infection, leading to a lower number of AB treatments. A mix of hydrolysable and condensed tannin extract given at 7.5 g/kg diet with or without a pZnO (150 mg Zn/kg) also contributed to reducing the fecal score and the number of AB treatments, without affecting feed intake, ADG or FE.
The effects of pZnO on the ADG and feed intake of post-weaning pigs vary across studies. Compared to the current study, Peng et al. (17) and Wang et al. (15) reported that starter diets supplemented with 200–500 mg/kg pZnO increased the ADG and feed intake of weaned piglets that were not subjected to an ETEC challenge. Trevisi et al. (18) reported that supplementing starter diets with 150–300 mg/kg pZnO increased average daily gain (ADG) in ETEC-challenged F18/4-susceptible piglets within 2 weeks after weaning, though the increase in feed intake was not statistically significant. Compared to the present study, Trevisi et al. (18) used a markedly lower infection load of 107 ETEC. This might explain the difference in the impact of the pZnO on the growth performance traits. Additionally, in the present study, feed intake was not measured at the individual level but rather calculated as the average individual feed intake per pen. This pen-level assessment may be less accurate than individual measurements.
There was no reduction in the prevalence of diarrhea using a low dose of pZnO in the present study, which is in agreement with Wang et al. (15), who reported no reduction in the prevalence of diarrhea when pZnO was added to a starter diet at 110 or 220 mg/kg. On the contrary, in the study of Peng et al. (17), the prevalence of diarrhea was reduced by more than 4% (p < 0.01) when piglets were fed a diet supplemented with 200 or 500 mg/kg pZnO.
The high dose of ZnO performed as expected (17, 18, 33, 34): diarrhea severity reduced, and the piglets became healthier, ate more and grew faster. Therefore, the present model was validated per se, with a positive control group performing much better than the negative control group.
The effects of tannins in the present study are consistent with the analysis of Caprarulo et al. (19), who reported that tannins given in low doses (<1%) did not significantly improve animals' overall performances traits after weaning, although their growth performance tended to improve numerically. Indeed, quebracho tannins given at doses up to 0.3% had no clear effect on ADG, feed intake or FE, but they reduced fecal scores when the piglets were weaned without artificial infection (25). Even at the 1% inclusion level, chestnut tannin extract improves only numerically ADG and feed intake when tested using the same in-house infection model (23), but it did reduce the severity and duration of diarrhea. It is only when given at 2% inclusion level that chestnut extract markedly increased weight gain and feed intake while limiting the severity and duration of diarrhea (22).
Although some performances were numerically improved, there was no evidence of synergies between tannins and pZnO relative to feed intake, ADG or fecal score. These results are consistent with the observations of Liu et al. (35), who tested a high dose of ZnO (2,000 mg/kg Zn, conventional source) with or without the addition 0.1% of tannin extract (containing 0.75% of chestnut tannins) in a conventional weaning process, namely without artificial infection. Only the diarrhea incidence rate was reduced by ZnO and tannins, but the combination of the two did not have greater efficacy than either treatment separately.
The source and dose of zinc, with or without tannins, had very little impact on the inflammation status of the piglets, as demonstrated by the absence of changes in IL-6 and IL-8 levels compared to the control group. However, tissues for IL-6 and IL-8 measurements were sampled 9 (±1) days after infection, which may be too late to detect acute inflammation. The effect of ZnO on the inflammation status of pigs remains controversial. Indeed, results of in vitro studies (36, 37) tend to indicate that ZnO reduces the inflammatory response by reducing the IL-8 and tumor necrosis factor alpha (TNF-α) serum levels of ETEC-infected intestinal cells. In contrast, the results of in vivo studies are less consistent. Pei et al. (34) reported an increase in IL-6 and TNFα serum levels in non-infected pigs fed with nano ZnO (150 and 300 mg/kg) or with a high dose of conventional ZnO (3,000 mg/kg) when compared to a basal diet. In agreement with the present results, Wang et al. (15) found that neither the source (pZnO or ZnO) nor the inclusion level (pZnO at 110 or 220 mg/kg Zn or ZnO at 2,400 mg/kg Zn) affected the mRNA levels of pro-inflammatory cytokines like TNF-α, interferon gamma and interleukin-1 beta in piglets weaned without artificial infection.
Improvements in the intestinal barrier due to ZnO administration have been reported in several studies: high doses of ZnO (>2,000 mg/kg Zn) increased the gene expression of occludin and claudin 1 (15, 38) and protein levels of occludin (38) in conventionally weaned piglets. In the present study, an increase in claudin 7 intestinal protein expression was only observed in C-3000 pigs. In study 2, TAN+pZnO pigs had higher claudin 1 intestinal protein expression levels than the pigs in the other treatment groups, but they had lower claudin 7 intestinal protein expression than TAN and pZnO-150 pigs. As previously described, both TAN (39) and ZnO (15, 38) enhance tight junction protein expression. However, the low dose of TAN (0.75%) and pZnO (150 mg/kg Zn), alone, may not have been sufficient to trigger greater protein expression of the tight junction proteins.
TAN and pZnO inconsistently affected the oxidative status of the animals in this study. The MDA levels were higher in the TAN groups than in the C-150 and pZnO-150 groups, indicating higher oxidative stress, whereas tannins are known for their antioxidative properties (35, 40). In addition, the total antioxidant status was lower in the pZnO group than in the TAN+pZnO group, which is not consistent with the aforementioned results. These inconsistencies make it difficult to interpret those results.
High doses of ZnO have been shown to increase the occurrence of zinc-resistant bacteria in the pig gut microbiota and to play a significant role in the co-selection of methicillin-resistant Staphylococcus aureus (MRSA) (41). Recent studies have suggested that feeding ZnO at high concentrations during weaning increases the proportion of multidrug-resistant E. coli in the gut of the piglets (11, 42). We also observed an increase in the number of copies of antimicrobial resistance genes in the C-3000 group compared to the C group. However, our approach aimed at detecting resistance genes in all bacteria from the small intestine rather than in one specific bacteria. The exact reasons for this co-selection of antibiotic resistance genes are unknown. Possible mechanisms include physiological coupling, such as the interaction of heavy metals with efflux pumps, and genetic coupling, such as co-resistance or the association of antimicrobial and heavy metal resistance genes on mobile genetic elements (41). The interaction of heavy metals with the bacterial conjugation system is another possible mechanism of action that is more supported by recent studies (10, 11, 42). Unfortunately, the present study did not investigate the heavy metal resistance genes, which limits the interpretation of the present results. Bacteria under stress may also have a higher conjugation rate, which would facilitate the exchange of genetic material that contains antimicrobial resistance genes (10).
The pharmacological dose of ZnO (2,500 mg Zn/kg diet) improved growth performance traits and alleviated PWD symptoms but increased Zn excretion and antimicrobial resistance genes. Tannin-rich extract and pZnO alleviated PWD, leading to a decrease in AB usage. Furthermore, tannins reduced the fecal scores.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Swiss cantonal veterinary office under the application numbers 29361 and 31400. The study was conducted in accordance with the local legislation and institutional requirements.
CO: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft. M-RM: Formal analysis, Investigation, Writing – review & editing. NP: Investigation, Writing – review & editing. MT: Writing – review & editing. SDub: Writing – review & editing. SDur: Writing – review & editing. OD: Writing – review & editing. JB: Formal analysis, Investigation, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from Animine and Silvateam. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. Animine and Silvateam provided support in the form of providing material (zinc oxide and tannin-rich extract, respectively). The rest of the study expenses was covered by Agroscope. Open access funding by Agroscope.
The authors express their gratitude to Olivier Desrues and Stephane Durosoy for their support throughout this study. They also extend their thanks to Guy Maikoff and his team for their assistance during the animal phase of the research.
SDur and OD report an affiliation (employment) to a commercial company, Animine and Silvateam, respectively.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1494103/full#supplementary-material
1. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition (2003). Available at: https://eur-lex.europa.eu/eli/reg/2003/1831/oj/eng
2. Regulation (ec) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency (2004). Available at: https://eur-lex.europa.eu/eli/reg/2004/726/oj/eng
3. DANMAP. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Copenhagen (2018).
4. Echtermann T, Muentener C, Sidler X, Kümmerlen D. Antimicrobial drug consumption on swiss pig farms: a comparison of Swiss and European defined daily and course doses in the field. Front Vet Sci. (2019) 6:240. doi: 10.3389/fvets.2019.00240
5. Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
6. Eriksen EØ, Kudirkiene E, Christensen AE, Agerlin MV, Weber NR, Nødtvedt A, et al. Post-weaning diarrhea in pigs weaned without medicinal zinc: risk factors, pathogen dynamics, and association to growth rate. Porcine Health Manage. (2021) 7:54. doi: 10.1186/s40813-021-00232-z
7. Eriksen EØ, Kudirkiene E, Barington K, Goecke NB, Blirup-Plum SA, Nielsen JP, et al. An observational field study of porcine post-weaning diarrhea: clinical and microbiological findings, and fecal pH-measurements as a potential diagnostic tool. Porcine Health Manage. (2023) 9:33. doi: 10.1186/s40813-023-00325-x
8. Debski B. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol J Vet Sci. (2016) 19:917–24. doi: 10.1515/pjvs-2016-0113
9. Jensen J, Kyvsgaard NC, Battisti A, Baptiste KE. Environmental and public health related risk of veterinary zinc in pig production - Using Denmark as an example. Environ Int. (2018) 114:181–90. doi: 10.1016/j.envint.2018.02.007
10. Bednorz C, Oelgeschlager K, Kinnemann B, Hartmann S, Neumann K, Pieper R, et al. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int J Med Microbiol. (2013) 303:396–403. doi: 10.1016/j.ijmm.2013.06.004
11. Ciesinski L, Guenther S, Pieper R, Kalisch M, Bednorz C, Wieler LH. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE. (2018) 13:e0191660. doi: 10.1371/journal.pone.0191660
12. Slifierz MJ, Friendship R, Weese JS. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant Staphylococcus aureus in pigs: a randomized controlled trial. Zoonoses Public Health. (2015) 62:301–8. doi: 10.1111/zph.12150
13. Hölzel CS, Müller C, Harms KS, Mikolajewski S, Schäfer S, Schwaiger K, et al. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ Res. (2012) 113:21–7. doi: 10.1016/j.envres.2012.01.002
14. EMEA. Questions and answers on veterinary medicinal products containing zinc oxide to be administered orally to food-producing species. London (2017).
15. Wang W, Noten N, Degroote J, Romeo A, Vermeir P, Michiels J. Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J Anim Physiol Anim Nutr. (2019) 103:231–41. 2018. doi: 10.1111/jpn.12999
16. Morales J, Cordero G, Pineiro C, Durosoy S. Zinc oxide at low supplementation level improves productive performance and health status of piglets. J Anim Sci. (2012) 9:436–8. doi: 10.2527/jas.53833
17. Peng P, Chen J, Yao K, Yin Y, Long L, Fang R. The effects of dietary supplementation with porous zinc oxide on growth performance, intestinal microbiota, morphology, and permeability in weaned piglets. Animal Sci J. (2019) 90:1220–8. doi: 10.1111/asj.13228
18. Trevisi P, Durosoy S, Gherpelli Y, Motta V, Colombo M, Bosi P, editors. Effect of zinc oxide sources on growth performance and health of Escherichia coli K88-challenged susceptible weaning pigs. Trace Elements Man Animals. (2014) 3:511–20. doi: 10.3920/9789086867998_076
19. Caprarulo V, Giromini C, Rossi L. Review: Chestnut and quebracho tannins in pig nutrition: the effects on performance and intestinal health. Animal. (2021) 15:100064. doi: 10.1016/j.animal.2020.100064
20. Girard M, Bee G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. animal. (2019) 14:1–13. doi: 10.1017/S1751731119002143
21. Reggi S, Giromini C. Dell'Anno M, Baldi A, Rebucci R, Rossi L. In vitro digestion of chestnut and quebracho tannin extracts: antimicrobial effect, antioxidant capacity and cytomodulatory activity in swine intestinal IPEC-J2. Cells Animals. (2020) 10:195. doi: 10.3390/ani10020195
22. Girard M, Hu D, Pradervand N, Neuenschwander S, Bee G. Chestnut extract but not sodium salicylate decreases the severity of diarrhea and enterotoxigenic Escherichia coli F4 shedding in artificially infected piglets. PLoS ONE. (2020) 15:e0214267. doi: 10.1371/journal.pone.0214267
23. Girard M, Thanner S, Pradervand N, Hu D, Ollagnier C, Bee G. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: efficacy on an experimental ETEC F4 model. PLoS ONE. (2018) 13:e0197878. doi: 10.1371/journal.pone.0197878
24. Caprarulo V, Hejna M, Giromini C, Liu Y, Dell'Anno M, Sotira S, et al. Evaluation of dietary administration of chestnut and quebracho tannins on growth, serum metabolites and fecal parameters of weaned piglets. Animals. (2020) 10:11945. doi: 10.3390/ani10111945
25. Ma M, Chambers JK, Uchida K, Ikeda M, Watanabe M, Goda Y, et al. Effects of supplementation with a quebracho tannin product as an alternative to antibiotics on growth performance, diarrhea, and overall health in early-weaned piglets. Animals. (2021) 11:3316. doi: 10.3390/ani11113316
26. Miragoli F, Patrone V, Prandini A, Sigolo S. Dell'Anno M, Rossi L, et al. A mixture of quebracho and chestnut tannins drives butyrate-producing bacteria populations shift in the gut microbiota of weaned piglets. PLoS ONE. (2021) 16:e0250874. doi: 10.1371/journal.pone.0250874
27. Agroscope. Apports recomandés en énergie, en matière azotée, en acides aminés et en macro-éléments. (2022). Available at: https://ira.agroscope.ch/fr-CH/publication/39986
28. Hu D, Rampoldi A, Bratus-Neuenschwander A, Hofer A, Bertschinger HU, Vogeli P, et al. Effective genetic markers for identifying the Escherichia coli F4ac receptor status of pigs. Anim Genet. (2019) 50:136–42. doi: 10.1111/age.12770
29. Srithunyarat T, Höglund OV, Hagman R, Olsson U, Stridsberg M, Lagerstedt A-S, et al. Catestatin, vasostatin, cortisol, temperature, heart rate, respiratory rate, scores of the short form of the Glasgow composite measure pain scale and visual analog scale for stress and pain behavior in dogs before and after ovariohysterectomy. BMC Res Notes. (2016) 9:381. doi: 10.1186/s13104-016-2193-1
30. Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. (1974) 47:211–8. doi: 10.1111/j.2044-8341.1974.tb02285.x
31. Biolley C, Tretola M, Bee G, Jud C, Silacci P. Punicalagin increases glutamate absorption in differentiated Caco-2 cells by a mechanism involving gene expression regulation of an EAAT3 transporter. Food Funct. (2019) 10:5333–8. doi: 10.1039/C9FO00191C
32. Christensen SE, Cooper SA, Mack RJ, McCallum SW, Du W, Freyer A, et al. Randomized double-blind controlled trial of intravenous meloxicam in the treatment of pain following dental impaction surgery. J Clin Pharmacol. (2018) 58:593–605. doi: 10.1002/jcph.1058
33. Sun YB, Xia T, Wu H, Zhang WJ, Zhu YH, Xue JX, et al. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim Feed Sci Technol. (2019) 258:114312. doi: 10.1016/j.anifeedsci.2019.114312
34. Pei X, Xiao Z, Liu L, Wang G, Tao W, Wang M, et al. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J Sci Food Agric. (2019) 99:1366–74. doi: 10.1002/jsfa.9312
35. Liu H, Hu J, Mahfuz S, Piao X. Effects of hydrolysable tannins as zinc oxide substitutes on antioxidant status, immune function, intestinal morphology, and digestive enzyme activities in weaned piglets. Animals. (2020) 10:757. doi: 10.3390/ani10050757
36. Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J Nutr. (2003) 133:4077–82. doi: 10.1093/jn/133.12.4077
37. Wang Y, Couture OP, Qu L, Uthe JJ, Bearson SMD, Kuhar D, et al. Analysis of porcine transcriptional response to salmonella enterica serovar choleraesuis suggests novel targets of NFkappaB are activated in the mesenteric lymph node. BMC Genomics. (2008) 9:437. doi: 10.1186/1471-2164-9-437
38. Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. (2009) 102:687–93. doi: 10.1017/S0007114509289033
39. Tretola M, Bee G, Silacci P. Gallic acid affects intestinal-epithelial-cell integrity and selected amino-acid uptake in porcine in vitro and ex vivo permeability models. Br J Nutr. (2021) 126:492–500. doi: 10.1017/S0007114520004328
40. Puiggros F, Llópiz N, Ardévol A, Bladé C, Arola L, Salvadó MJ. Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J Agric Food Chem. (2005) 53:6080–6. doi: 10.1021/jf050343m
41. EFSA. Scientific Opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. (2014) 12:3668. doi: 10.2903/j.efsa.2014.3668
Keywords: Escherichia coli, post-weaning diarrhea, resistome, tannins, zinc oxide
Citation: Ollagnier C, Mellino M-R, Pradervand N, Tretola M, Dubois S, Durosoy S, Desrues O and Bellon J (2025) Feed supplementation with potentiated zinc and/or tannin-rich extracts reduces ETEC infection severity and antimicrobial resistance genes in pig. Front. Vet. Sci. 12:1494103. doi: 10.3389/fvets.2025.1494103
Received: 25 September 2024; Accepted: 20 January 2025;
Published: 21 February 2025.
Edited by:
Roswitha Merle, Free University of Berlin, GermanyReviewed by:
Yu Pi, Chinese Academy of Agricultural Sciences, ChinaCopyright © 2025 Ollagnier, Mellino, Pradervand, Tretola, Dubois, Durosoy, Desrues and Bellon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Ollagnier, Y2F0aGVyaW5lLm9sbGFnbmllckBhZ3Jvc2NvcGUuYWRtaW4uY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.