
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 05 February 2025
Sec. Veterinary Infectious Diseases
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1453150
This article is part of the Research Topic The application of new technologies such as new vaccines, therapeutic cytokines and antibodies, and antiviral drugs in the prevention and treatment of animal infectious diseases View all 15 articles
Duck enteritis virus (DEV), the pathogen of duck viral enteritis, belongs to the α-herpesvirus subfamily. Like other herpesviruses, it has a large genome with multiple non-coding and non-essential regions for viral replication. It is suitable as a live virus vector for inserting and expressing antigenic genes from other pathogens to develop multivalent vaccines. With the advancement of molecular biology research and experimental technology, genetic modification of the DEV genome has matured, leading to the successful construction of recombinant DEV live vector vaccines. These vaccines have demonstrated the ability to resist DEV and other pathogens, showing potential as recombinant viral vaccine vectors and playing a crucial role in the development of new avian vaccines. This article provides an overview of the progress of research on recombinant vaccines using DEV as the vector. It includes the biological characteristics of DEV and its advantages and limitations as a vaccine vector, methods for constructing recombinant DEV, the technical platform for efficiently building recombinant DEV, factors affecting the immune protection efficacy of recombinant DEV, and the application of recombinant DEV in vaccine development. Aiming to provide a reference for the development of duck enteritis virus vector-based vaccines.
Vaccination and biosecurity stand as the foremost strategies for preventing and controlling infectious diseases, with vaccine immunization serving as a cornerstone in this endeavor. Although widely employed, live and inactivated vaccines come with inherent limitations, including the potential for virulence reversion in live vaccines, shorter immunization duration, and high production costs for inactivated vaccines. Poultry farming contends with a multifaceted infectious disease landscape, necessitating the prevention and control of a diverse array of diseases. This reality leads to challenges such as cumbersome vaccination procedures, high transportation costs for vaccines, contraindications between different vaccines, and stress-induced production losses in eggs or meat. Recent advancements in molecular biology technology have paved the way for the development of genetically engineered vaccines, offering a potential alternative to traditional vaccines. Genetically engineered vaccines encompass a variety of types, including subunit vaccines, vector vaccines, nucleic acid vaccines, gene-deficient live vaccines, and protein-engineered vaccines, among others. Among them, recombinant viral vector vaccine is a new type of vaccine that uses live virus as a carrier to carry and express antigenic genes of other pathogens. The exogenous genes integrated into the genome of the vector virus can continuously deliver exogenous antigenic proteins with the proliferation of the vector virus in cells, thereby inducing an immune response in the body.
Live viral vector vaccines have been shown to be effective in controlling infectious diseases, and a number of recombinant vector vaccines have been licensed, such as VECTORMUNE® HVT ND and VECTORMUNE® HVT AI from Ceva Animal Health Corporation, and VAXXITEK®HVT + IBD + ND, VAXXITEK®HVT + IBD, NEWXXITEK™HVT + ND from Merial-Boehringer Ingelheim, and so on (1, 2). By incorporating antigenic genes from multiple pathogens into viral vectors, a multi-plex polyvalent live vector vaccine can be constructed. Such a vaccine has the potential to prevent several infectious diseases with a single immunization, thereby reducing vaccine costs, simplifying the immunization process, and alleviating immunization pressures. Consequently, utilizing live vaccine vectors for expressing antigenicity genes of multiple pathogens represents a crucial direction for future vaccine research and development. Viral vectors suitable for live-vector vaccines include poxviruses (3–5), adenoviruses (6, 7), retroviruses (8), and herpesviruses (9–11), among which herpesviruses have become one of the popular vectors due to their infectiousness, large genome, and mature gene manipulation technology (12). This paper reviews the research progress of recombinant viral live-vector vaccines utilizing DEV as a vector, aiming to offer insights into the development of duck enteritis virus vector vaccines.
Duck enteritis virus (DEV), also known as duck plague virus (DPV), is a significant pathogen that poses a serious threat to the waterfowl industry, which mainly infects birds of the order Anseriformes, causing duck viral enteritis (DVE) and induces acute, febrile, and septic infectious diseases (13). DEV belongs to the genus Mardivirus, family Herpesviridae, and subfamily Alpha-herpesvirinae (13). Its genome consists of double-stranded DNA with a molecular weight of about 160 kilobases (kb), including a long unique region (UL), a short unique region (US), an internal repetitive sequence (IRS), and a terminal repetitive sequence (TRS) in the arrangement UL-IRS-US-TRS (14), as shown in Figure 1. Being enveloped, the virus is sensitive to ether and chloroform.
The DEV genome consists of 67 open reading frames (ORFs) encoding non-structural and structural proteins, of which the non-structural proteins are mainly involved in regulating the processes of viral genome replication, transcription, and assembly, while the structural proteins mainly include capsid proteins, cortical proteins and envelope glycoproteins, which are involved in constituting and assembling the viral capsid, cortex and envelope, as well as in regulating the basic biological functions of the virus. Among them, envelope glycoproteins mainly include gB (UL27), gC (UL44), gD (US6), gE (US8), gG (US4), gH (UL22), gI (US7), gK (UL53), gL (UL1), which are the main genes affecting the basic biological functions of DEV such as DEV entry into the host cell, viral transmission, viral replication, viral assembly, and viral virulence (14). In addition to the envelope glycoprotein gene, there were significant sequence differences between strong and weak strains of DEV in the UL2, UL12, UL41, UL47, and US10 genes, suggesting that these five genes may be related to viral virulence (15). UL41, US3, UL28, UL53, UL24, and UL48 of DEV can block IFN-β activation and inhibit host innate immune response, which is thought to be associated with DEV immune escape (16–18).
DEV replicates primarily in the epithelial cells of the gastrointestinal mucosa and then spreads to the bursa, thymus, spleen and liver, where it replicates abundantly in epithelial cells and lymphoid cells. DEV levels in systemic tissues and organs correlate strongly with disease progression (19). DEV infection causes apoptosis and necrosis of lymphoid tissues, leading to immunosuppression and an increased risk of secondary bacterial infections. DEV also has a strong preference for vascular endothelial cells, and its replication in vascular endothelial cells of small blood vessels, small veins and capillaries leads to their destruction, which in turn causes severe hemorrhage, inflammatory responses and progressive degeneration of parenchymal organs. In addition, DEV can establish a latent infection in the trigeminal ganglia, which can be reactivated under the right conditions, leading to viral shedding and transmission (20). This reactivation may be due to immunosuppression in flocks due to stress from any cause. Latency and reactivation of DEV are significant factors contributing to outbreaks within both domestic and migratory waterfowl populations (13).
Constructing a recombinant DEV vector vaccine poses two primary challenges: first, inserting the target gene into the viral vector genome and stabilizing its presence; second, ensuring that the inserted foreign gene can be expressed normally. The advancements in molecular biology and gene editing technologies have facilitated the manipulation of the duck enteritis virus genome, including gene deletion and insertion, to a mature level. Currently, there are four main methods for constructing recombinant duck enteritis virus as follows.
Intracellular homologous recombination represents a traditional approach for the generation of recombinant DEV. This method involves the insertion of an exogenous gene expression cassette into a specific region of the viral genome by utilizing a transfer vector equipped with homologous sequences flanking the insertion site. Subsequently, the repair and integration of the genome occur through the host cell’s homologous recombination mechanism. In addition to the exogenous gene expression cassette, the transfer vector typically incorporates a screening marker, such as the Escherichia coli (E. coli) guanine phosphoribosyl transferase (gpt) gene, β-galactosidase (LacZ) gene, or enhanced green fluorescent protein (EGFP) gene. These markers facilitate the subsequent screening and purification stages of the recombinant DEV. The construction strategy is illustrated in Figure 2, including one-step and two-step methods. One-step method: insert the exogenous gene into the DEV genome together with the screening marker to obtain the recombinant DEV; two-step method: insert the screening marker into the DEV genome first, and then replace the screening marker with the exogenous gene to obtain the recombinant DEV that expresses only the exogenous gene.
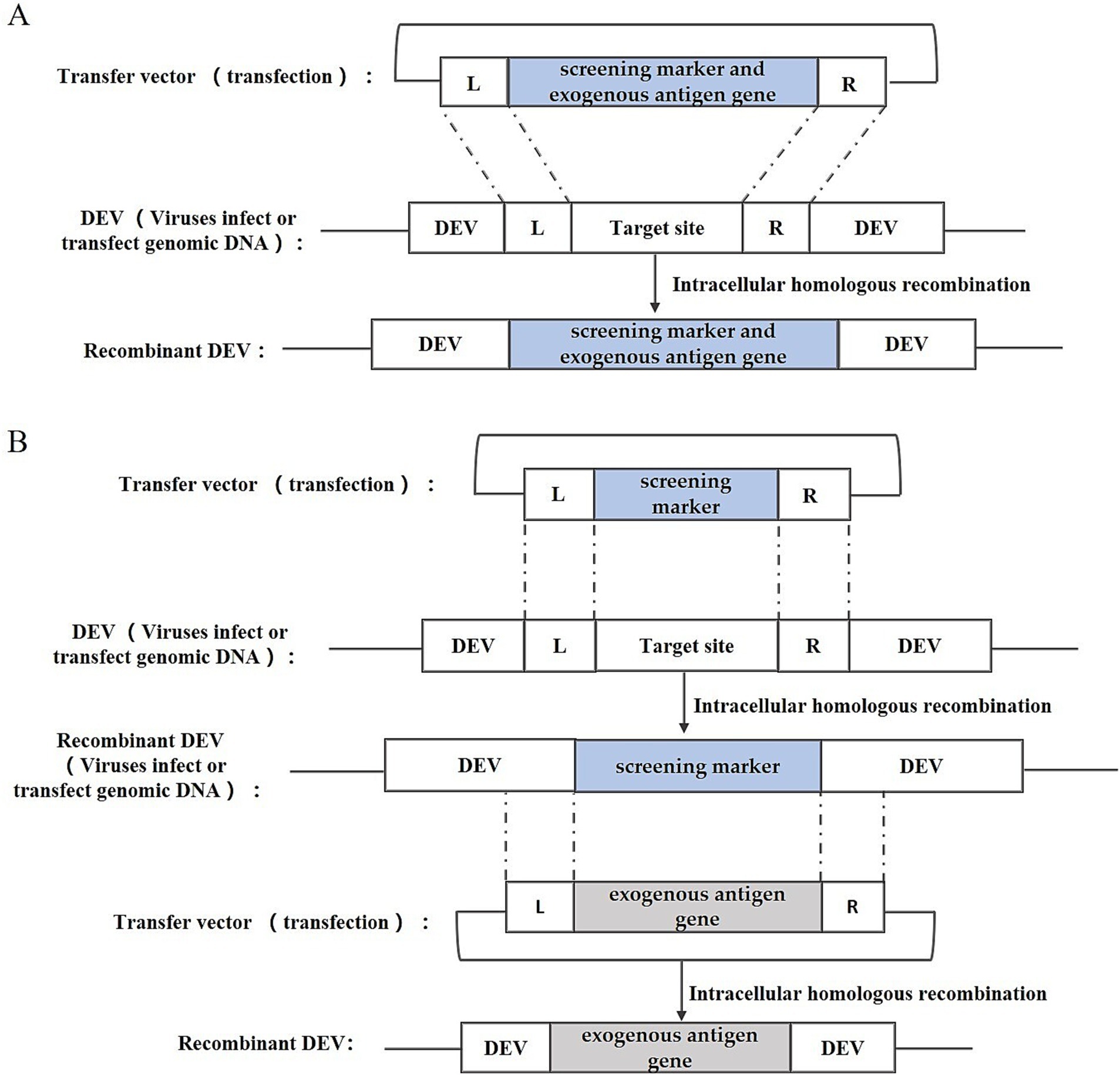
Figure 2. Recombinant DEV construction strategy based on intracellular homologous recombination. (A) One-step method; (B) Two-step method; L and R represent the left and right homologous arm sequences, respectively.
Liu et al. (21) utilized this method to fuse the E. coli gpt gene with the haemagglutinin (HA) gene of the highly pathogenic avian influenza virus (HPAIV) H5N1, and by linking it with the US2 gene and the gIgE gene of DEV, successfully integrated the gpt gene and the HA gene of H5N1 HPAIV into the DEV genome, thereby constructing two strains of rDEVs, which underwent subsequent screening and purification via the MPA/xanthine/hypoxanthine screening medium. Liu et al. (22) employed this method to insert the N, S, or S1 genes of infectious bronchitis virus (IBV) into the US10 locus of the DEV genome, initially recombining the EGFP reporter gene into DEV’s US10 gene and purifying the resultant rDEV-EGFP through screening for green fluorescent plaques, followed by crafting transfer vectors harboring IBV’s N, S, or S1 genes, and finally substituting the EGFP gene with IBV antigenic genes through a second round of homologous recombination, selecting the desired recombinant viruses based on fluorescence. Sun et al. (23) inserted the HA gene of the avian influenza virus H9N2 into the UL2 region of DEV by the same construction strategy.
This method is straightforward, involving only the creation of a transfer plasmid for recombinant virus recovery, and was widely adopted in its early stages. However, this approach has significant drawbacks. The likelihood of natural recombination in cells is very low, making it difficult to produce recombinant viruses.
Bacterial artificial chromosome technology is an efficient tool for genetic manipulation, particularly beneficial for studying viruses with large genomes like DEV. BAC, a low-copy plasmid based on the E. coli F-factor, is known for its large capacity, genetic stability, and straightforward manipulation. The inclusion of bacterial antibiotic resistance genes on the BAC vector aids in screening for E. coli containing the BAC-virus genome. Once DEV-BAC is successfully constructed, the DEV genome can be replicated in E. coli as a plasmid, manipulated using established Red homologous recombination techniques, and recombinant DEV can be rescued by transfecting BAC-DEV into host cells. The construction strategy is depicted in Figure 3. Initially, a transfer vector was constructed, incorporating the screening marker, BAC plasmid, and homologous arm sequences flanking the insertion site. Subsequently, DEV-BAC was generated through homologous recombination. The exogenous gene (EG) was then introduced into DEV-BAC using either Red/ET recombineering or mating-assisted genetically integrated cloning (MAGIC) in E. coli, yielding DEV-BAC-EG. Finally, the BAC sequence was excised using Cre/LoxP recombination or CRISPR/Cas9.
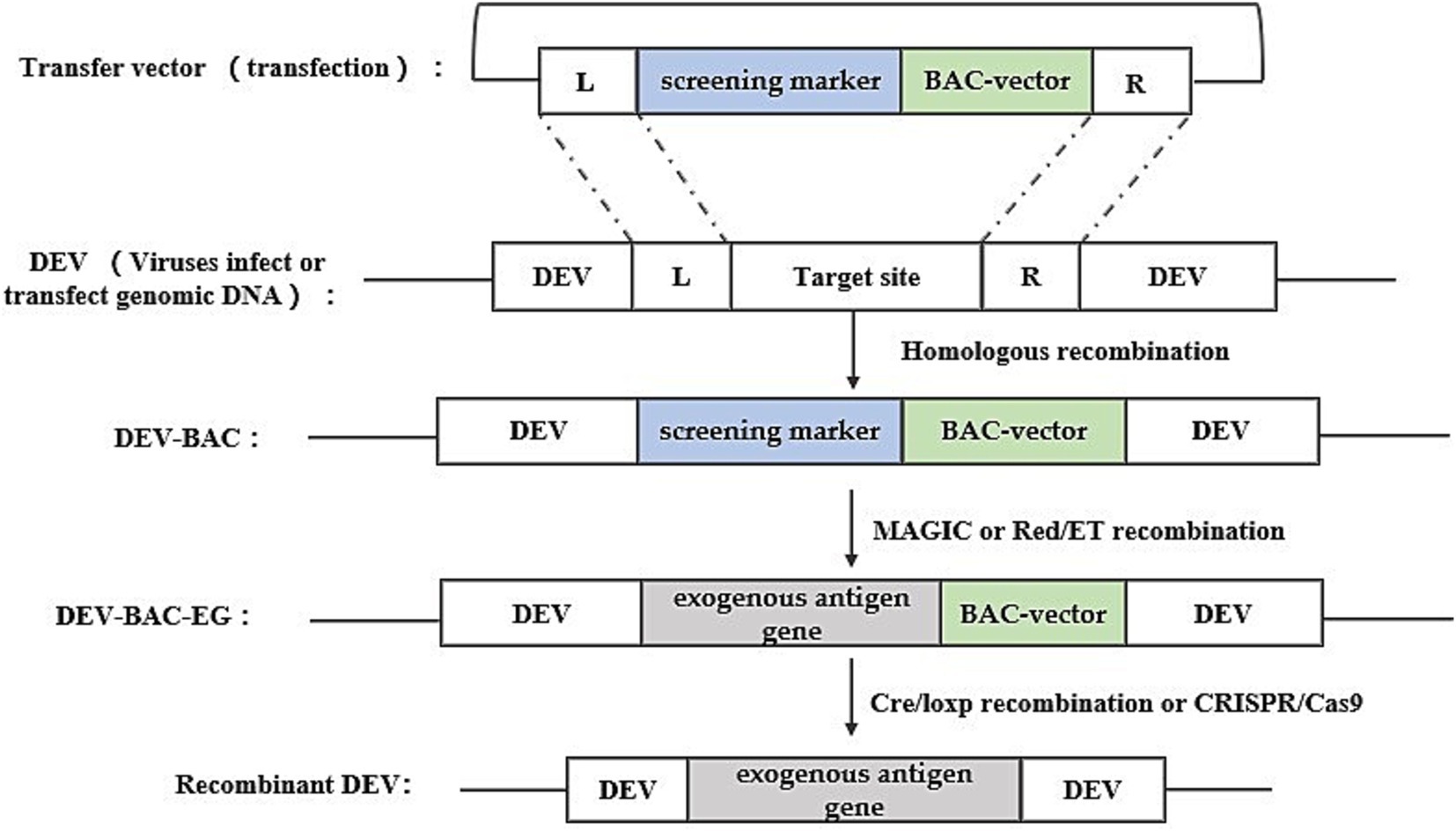
Figure 3. Recombinant DEV construction strategy based on bacterial artificial chromosome technology. Obtaining DEV-BAC as well as recombinant DEV was performed in avian cells, while obtaining DEV-BAC-EG was performed in E. coli. L and R represent the left and right homologous arm sequences, respectively.
Liu et al. (24) used this method with a two-step RedE/T recombination approach to insert seven variations of the duck tembusu virus (DTMUV) E gene into the US7/US8 intergenic region of the DEV genome, creating seven distinct pDEV-E BAC clones, which were subsequently utilized to generate the recombinant virus rDEV-E in chicken embryonic fibroblasts (CEFs) through a calcium phosphate precipitation approach. Similarly, Wang et al. (25) inserted the HA gene of H5N1 AIV into a non-coding region of the DEV genome between UL55 and LORF11.
BAC is a widely used platform for herpesvirus genetic manipulation, with numerous successful applications in developing gene deletion vaccines and recombinant live viral vector vaccines, particularly for herpesviruses like MDV and PRV (26, 27). Although BAC offers higher efficiency than traditional homologous recombination, creating recombinant BAC constructs remains labor-intensive and time-consuming.
The Fosmid multi-fragment rescue system represents a relatively novel approach for constructing recombinant duck enteritis virus. Its fundamental principle involves the random fragmentation of the DEV genome into multiple segments with overlapping ends through physical or enzymatic cleavage. These fragments are subsequently cloned into Fosmid vectors. Different combinations of Fosmid plasmid DNAs covering the entire DEV genome are then co-transfected into host cells after enzymatic linearization, aiming to identify Fosmid plasmid combinations capable of rescuing infectious recombinant DEV, thus completing the construction of the reverse genetic platform. Exogenous genes can be inserted into specific sites within the Fosmid plasmid using conventional genetic engineering techniques. Subsequently, the Fosmid plasmid containing the exogenous gene and other plasmids in the combination are co-transfected into host cells after enzymatic linearization, and the success of the recombinant viruses can be assessed through the observation of cell lesions. The construction strategy is illustrated in Figure 4.
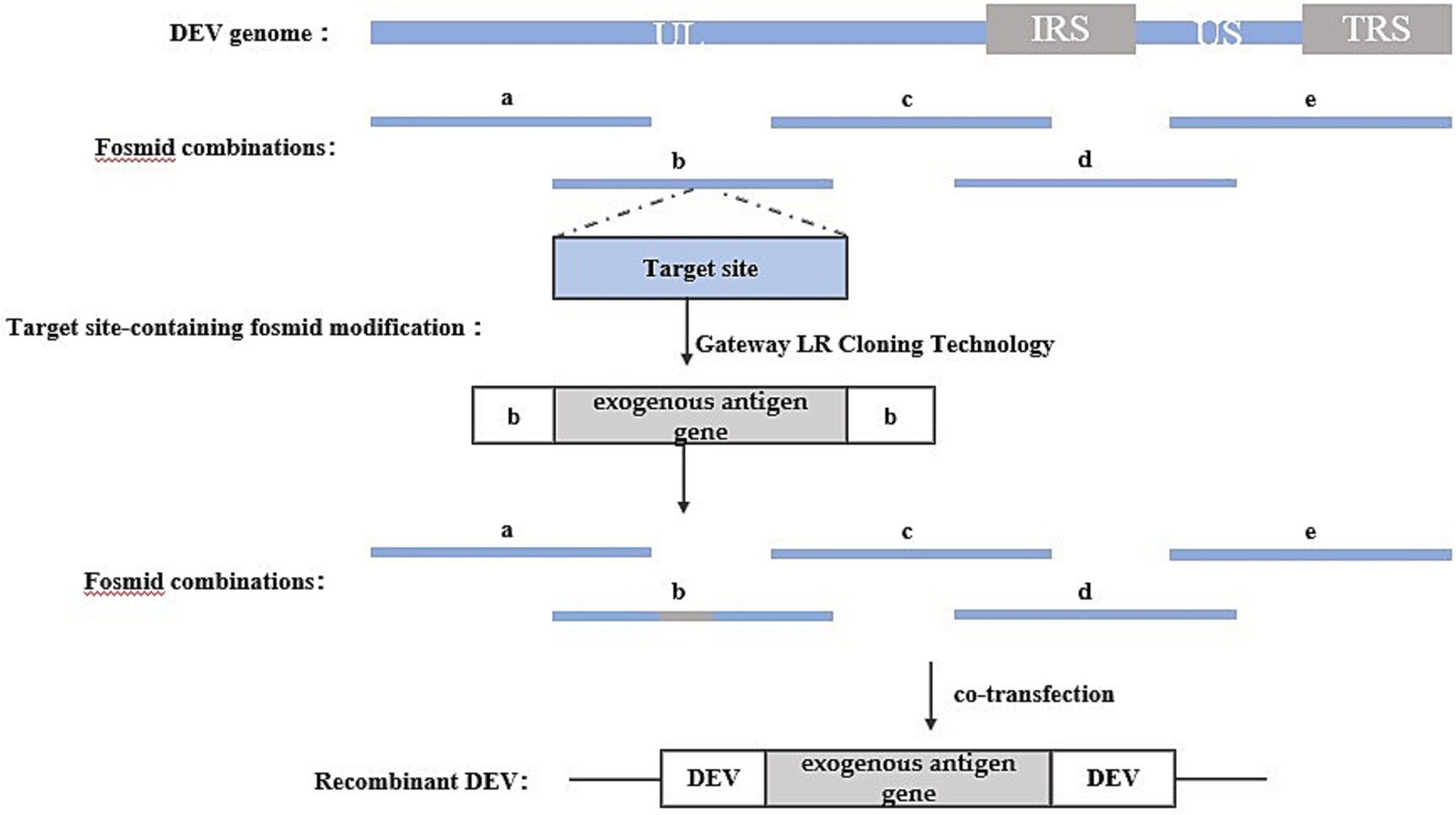
Figure 4. Recombinant DEV construction strategy based on Fosmid multi-fragment rescue system. Segments a, b, c, d, and e represent combinations of Fosmid plasmids that rescue infectious recombinant DEVs.
For instance, Zhao, et al. (28) utilized this method to insert the HA gene of H5 and H7 AIV into various loci of the DEV genome, such as UL41, US7/US8, SORF3/US2, US7/US8, and US8/US1. Similarly, Yang et al. (29) employed this method to insert the P1 and 3C genes of serotype 3 duck hepatitis A virus (DHAV-3) into UL26/UL27 and US7/8 loci.
One advantage of this method is that the rescued recombinant virus does not require purification, and recombinant viruses carrying exogenous genes can be obtained directly. However, a notable drawback lies in the necessity to construct numerous Fosmid vector plasmids to establish the reverse genetic manipulation platform. Additionally, screening for plasmid combinations with high recombination efficiencies is required. Failure to identify such combinations can lead to low intracellular homologous recombination efficiency, resulting in difficulties in rescuing recombinant viruses.
CRISPR/Cas9 gene editing technology, developed in recent years, represents a powerful tool for directly editing the genomes of herpes viruses. With its straightforward operation and high efficiency, this technology has found successful applications across various herpes viruses. The CRISPR/Cas9 gene editing system stems from an acquired immune system initially discovered in certain bacteria and archaea. Leveraging its working principle, this system has been harnessed for gene editing purposes. The Cas9 protein within the system exhibits nuclease activity, capable of binding to guide RNA (gRNA) and navigating to the target site under gRNA guidance. Upon reaching the target site, Cas9 cleaves the DNA, inducing a double-strand break (DSB) (30, 31), and the cleavage and breakage sites are generally in the PAM (Protospacer Adjacent Motif). The cut and break site is usually between the first three and four bases of the PAM (Protospacer Adjacent Motif) sequence, and the cell will activate the repair system to repair the break after the DSB is generated. The DSB repair system in eukaryotic cells consists of non-homologous end joining (NHEJ) and homology-directed repair (HDR) (32).NHEJ repair occurs at all stages of the cell life cycle, and can cause random base insertions and deletions, resulting in target gene shift mutations and the inability to encode the corresponding proteins, thus realizing the silencing and knockdown of target genes; In contrast, HDR repair predominantly occurs during the G2 and S phases of the cell cycle. It facilitates precise repair of DNA double-strand breaks by utilizing another homologous chromosome as a template. This repair mechanism can be utilized in cell lines and eukaryotic organisms with artificial templates, enabling targeted gene knockout, phenotype modification, and introduction of exogenous or reporter genes. This process represents a crucial mechanism for repairing DNA double-strand damage through homologous recombination (33).
Studies have shown that based on these two repair methods, the construction of corresponding donor plasmids and appropriate screening methods can knock-in exogenous genes into the herpesvirus genome and construct recombinant herpesviruses expressing exogenous genes (34, 35). In the rescue of recombinant viruses, both repair-based exogenous gene knock-in strategies entail the construction of sgRNA-Cas9 plasmids targeting the cleavage site sequences and donor plasmids containing the exogenous genes. The HDR-based knock in strategy requires donor plasmids containing homology arm sequences on both sides of the insertion site, whereas the NHEJ-based knock-in strategy does not necessitate homology arm sequences on either side of the insertion site. However, the NHEJ-based knock-in strategy requires an additional sgRNA-Cas9 plasmid to linearize the donor plasmid. The construction strategy is illustrated in Figure 5.
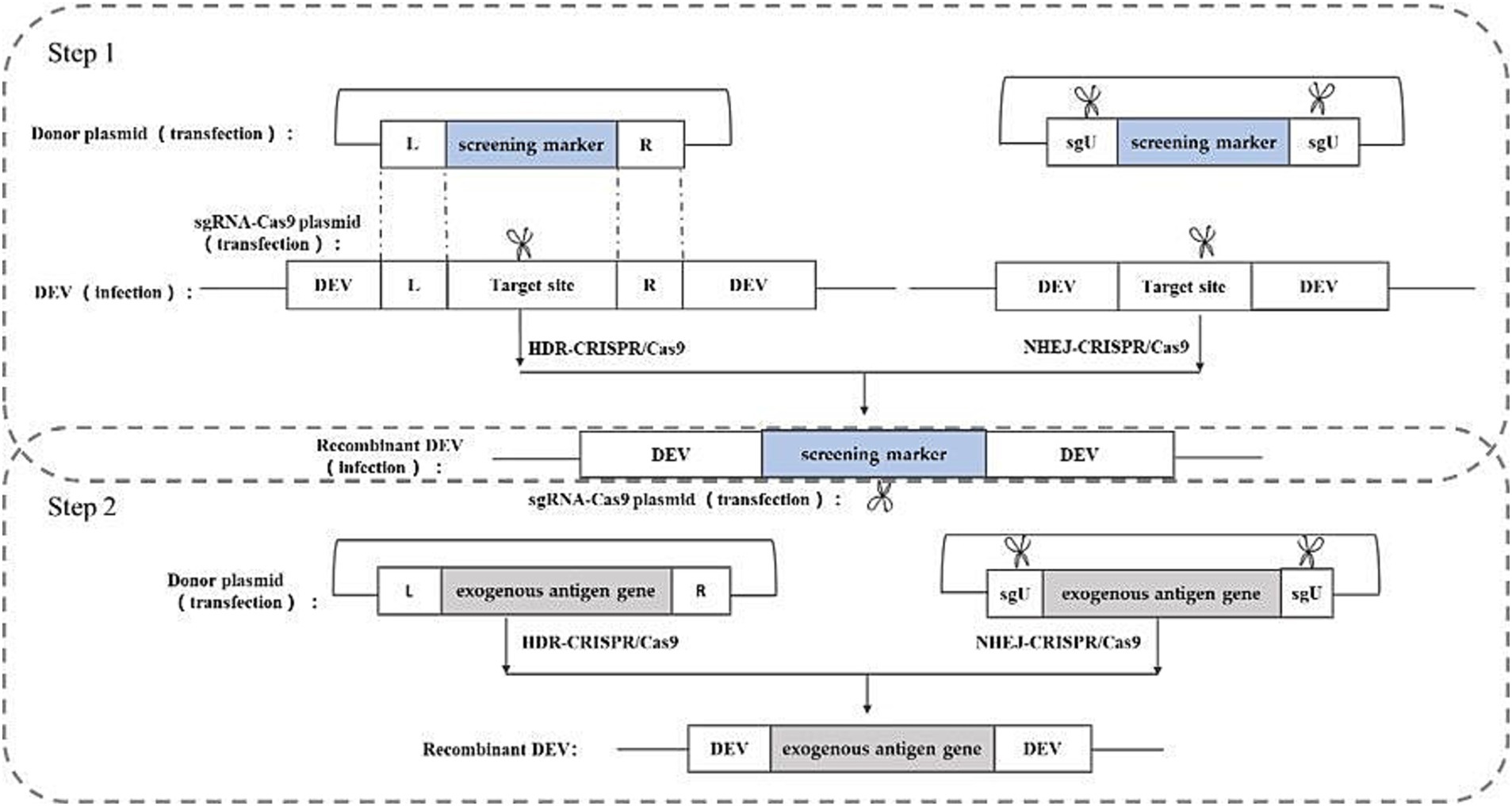
Figure 5. Recombinant DEV construction strategy based on CRISPR/Cas9 gene editing technology. sgU is a scrambled sequence with no homology to human, chicken, pig, prokaryotic, or viral genome sequences. L and R represent the left and right homologous arm sequences, respectively.
Chang et al. (36) utilized the NHEJ-CRISPR/Cas9 coupled with the Cre-Lox System to insert the HA gene of H5N1 into the UL26/UL27 region of the DEV genome. Similarly, Apinda et al. (37) employed the same strategy to insert the ompH gene of Pasteurella multocida into the UL55/LORF11 and UL44/44.5 loci. This represents the first application of the CRISPR/Cas9 system to insert highly immunogenic genes from bacteria into the DEV genome, facilitating the rapid and efficient construction of a multivalent vector vaccine. This underscores the substantial potential of duck enteritis virus as a vector. While most current applications of CRISPR/Cas9 gene editing rely on NHEJ repair due to its high efficiency, HDR repair offers greater accuracy. Numerous successful cases of HDR repair-based CRISPR/Cas9 have been reported in constructing other herpesvirus vector vaccine (34, 38, 39).
The advantages of this method are the higher efficiency of rescuing recombinant viruses and the simplicity of the method, and the disadvantages are the presence of off-targeting, which may lead to unintended insertions or deletions.
Table 1 outlines the key points and the advantages and disadvantages of four methods for constructing recombinant DEV. Each method has its specific applications and limitations. The choice of method depends on the conditions of the laboratory and the specific requirements of the task. In practical applications, researchers need to weigh these pros and cons and select the method that best suits their research objectives.
DEV serves as a highly promising viral vector capable of delivering antigenic genes from one or more pathogens, thereby constructing polyvalent vaccines that significantly broaden the scope of vaccine protection. Two main strategies exist for constructing polyvalent recombinant DEV vectored vaccines that are similar to those used to construct other herpesvirus vectored vaccines.
The first strategy employs a polycistron expression scheme, which utilizes elements such as an internal ribosome entry site (IRES) or a self-shearing polypeptide 2A (2A) to link multiple antigenic genes to form a eukaryotic expression cassette, which is then inserted into the DEV genome. Gergen et al. (40) have employed this strategy and successfully constructed vaccines expressing the Newcastle disease virus (NDV) fusion (F) gene and the infectious laryngotracheitis virus (ILTV) gD and gI genes in a recombinant turkey herpesvirus (HVT). This recombinant virus provided excellent protection against all three pathogens, ILTV, NDV, and Marek’s disease virus (MDV), after a single inoculation via the in-ovo or SC route.
Another strategy is to insert different antigenic genes into different loci of the DEV genome, respectively. Following this strategy, Zhao et al. (28) constructed a recombinant duck enteritis virus named rDEV-dH5/H7. The virus carried the hemagglutinin genes of two H5 viruses and one H7 virus. Animal studies showed that rDEV-dH5/H7 behaved similarly to its parental DEV viruses in inducing neutralizing antibody responses and protection against lethal DEV attacks. More importantly, rDEV-dH5/H7 was able to induce potent and long-lasting hemagglutinin-inhibiting antibodies against different H5 and H7 viruses, thus providing comprehensive protection against homologous and heterologous highly pathogenic H5 and H7 influenza virus attacks in ducks. In addition, this strategy was also used by Tang et al. to successfully generate a triple-inserted recombinant HVT-VP2-gDgI-HA vaccine by inserting the gD-gI gene of ILTV and the hemagglutinin expression cassette of the H9N2 AIV into different loci of the recombinant HVT-IBDV VP2 viral genome (41).
As a member of the herpesviridae family, DEV is considered an ideal live viral vector for the development of multivalent vaccines. DEV possesses a large genome that includes several non-essential regions for replication, such as US2, SORF3/US2, SORF3, US7/US8, UL2, UL27/UL26, UL41, and SORF11/UL55. The presence of these regions facilitates the insertion of foreign genes, thereby allowing for customized genetic engineering of the virus.
Within the host, DEV demonstrates rapid replication capabilities and can stimulate a comprehensive immune response within hours after vaccination, including humoral immunity and cellular immunity. It is noteworthy that the intensity and effectiveness of this immune response seem to be unaffected by the presence of maternal antibodies in the host. Furthermore, when used as a vaccine, DEV can establish persistent infection in the host, thereby providing long-term and stable immune protection. Zou et al. (42) inserted the (HA) gene of H5NI into the DEV vaccine strain C-KCE to obtain the recombinant virus C-KCE-HA. After a single immunization, Hemagglutination Inhibiting Antibodies were detected in the serum of immunized ducks, and the number of IFN γ-secreting cells in the splenocytes increased significantly.
In terms of safety, the natural host range of DEV is limited to ducks and does not pose a threat to other livestock or humans, which ensures the safety of its use as a live viral vector vaccine. Although recombinant DEV does not infect chickens, its transient replication in chickens is sufficient to express and present foreign antigenic proteins, effectively stimulating the host to produce an immune response. Based on these characteristics, DEV has been widely used in the development of vaccines against a variety of avian pathogens, including but not limited to avian influenza virus (28, 43), Newcastle disease viruses (NDV) (44), infectious bronchitis viruses (22), duck Tembusu viruses (24), Duck hepatitis A virus (29, 45), and even Pasteurella multocida (46), etc., providing an important means of disease prevention and control for the poultry industry. Table 2 details research data on various recombinant DEV vector vaccines that are critical to understanding the efficacy and potential applications of recombinant DEV vector vaccines harboring different pathogen antigen genes.
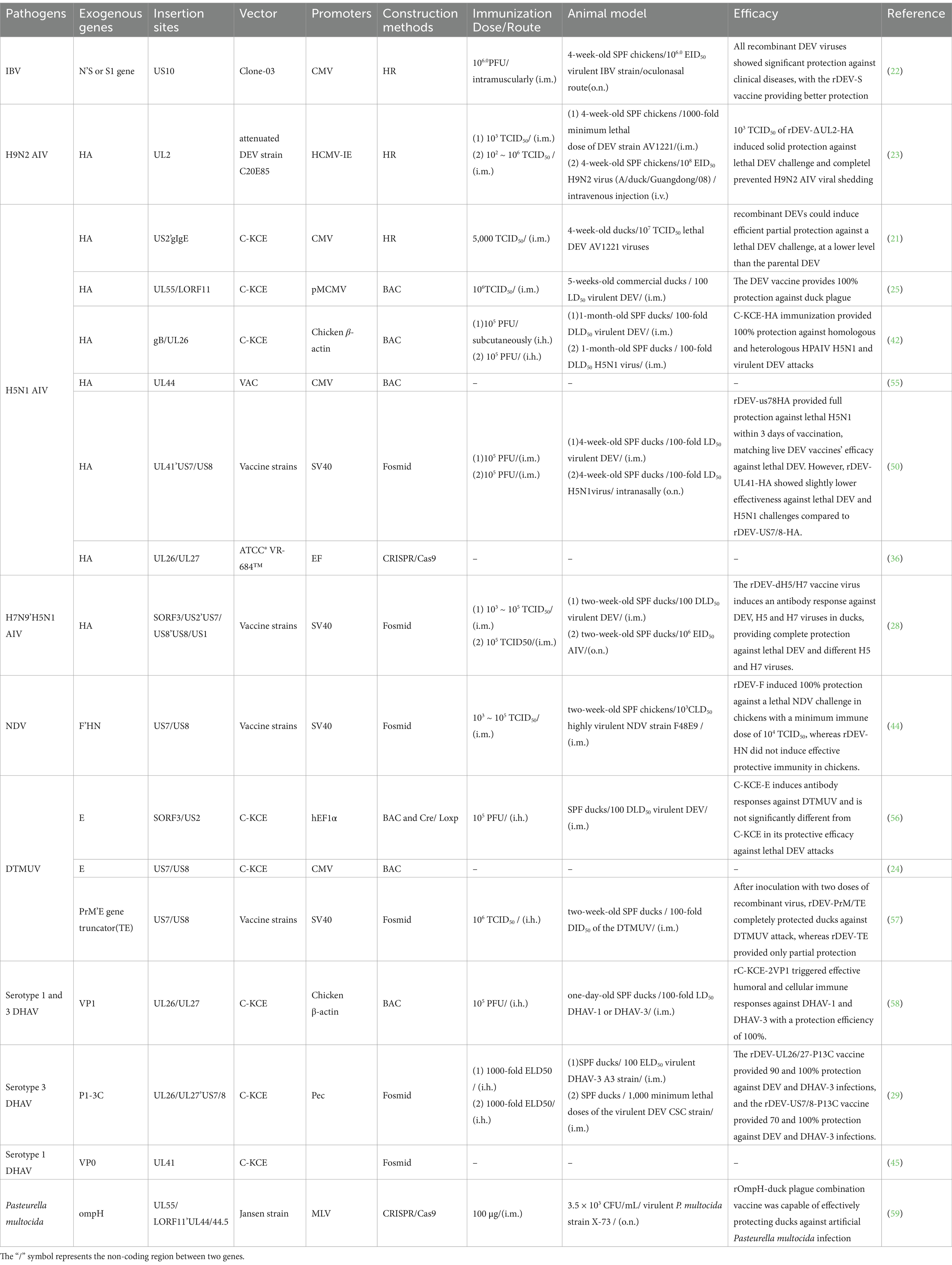
Table 2. Development and evaluation of recombinant DEV viral vector vaccines as immunogens for birds.
DEV serves as a promising vector for developing recombinant vaccines, offering numerous advantages, yet it is not without its limitations. Primarily replicating in Anseriformes, DEV vectors ensure a degree of safety while also restricting their applicability across diverse avian species and hosts. As a herpesvirus, DEV presents significant challenges in constructing reverse genetics systems due to its large genome. Rescuing recombinant DEV is notably complex and laborious compared to other commonly used viral vectors such as adenoviruses and Newcastle disease virus (47, 48). Genetic stability is another concern; recombinant viral vector vaccines may undergo genetic mutations or recombinations after multiple passages, potentially affecting the vaccine’s stability and efficacy. In addition, large-scale vaccine production requires specific cell lines or avian embryos. Most DEV strains, especially vaccine strains, can only be grown in homologous primary cell culture systems of avian origin, and the lack of commercial cell lines further complicates the production of vector vaccines (49).
The selection of vector virus strains plays a crucial role in determining the effectiveness and safety of recombinant DEV. To ensure safety, most studies have used a weak strain of DEV, such as C-KCE or VAC, which minimizes the risk of adverse reactions associated with vaccination. These strains exhibit reduced virulence compared to wild-type DEV strains, thereby reducing the likelihood of causing disease in vaccinated animals.
To ensure the normal expression of exogenous genes and maximize the expression efficiency of the inserted exogenous genes, according to the principle of eukaryotic gene expression regulation, the inserted exogenous genes must contain expression regulatory elements, so that they can exist independently in the genome of the virus, so most of the exogenous genes inserted in the current study are in the form of expression cassettes. Additionally, to trigger both cellular and humoral immunity, the inserted genes are typically full-length or partial sequences of key immunogenic proteins from other pathogens, such as the HA gene from AIV, the F and HN genes from NDV, the N and S genes from IBV, the E gene from DTMUV, and the ompH gene from Bartonella multocida.
However, some foreign genes exhibit low expression post-insertion, which can hinder immune response stimulation. This issue can be addressed through codon optimization, gene truncation, or the addition of signal peptides that enhance transcription and translation. For instance, Liu et al. (24) inserted various forms of the DTMUV E gene into the DEV genome, including origin E gene (E-ori), truncated E451-ori gene, codonoptimized E-dk gene optimized referring to duck’s codon bias, as well as the truncated E451-ch and E451-dk, Etpa-ori and Etpa-451-ori, which contain prefxing chick TPA signal peptide genes. Seven recombinant viral strains (rDEV-E) were constructed, and Western blot analysis revealed the presence of E or E451 proteins in infected CEFs, with the highest expression levels observed in CEFs infected with rDEV-E451-dk.
The site of exogenous gene insertion is another critical factor influencing the immune response elicited by recombinant duck enteritis virus. The DEV genome contains numerous replication non-essential regions suitable for exogenous gene insertion. Many studies have reported successful insertion and expression of exogenous genes in various regions of the DEV genome without compromising viral replication. These regions include US2, US10, US7/US8, UL26/UL27, UL44-44.5, UL41, UL55/LORF11, UL55-LORF11, and others, with a preference for non-coding regions between two open reading frames.
Multiple studies have evaluated the influence of different insertion sites on the immunogenicity and protective efficacy of recombinant DEV. For example, one study compared the immunogenicity and protective effects of two recombinant strains, with the HA gene of H5N1 inserted into the US2 and gIgE regions. The results showed no significant difference in antibody production or protection against DEV between the two strains, although both demonstrated lower efficacy than the parental DEV strain (21).
In another investigation, the HA gene was inserted into the UL41 and US7/US8 loci, yielding two recombinant viruses, rDEV-UL41HA and rDEV-US7/8HA. Both were found to be immunogenic and provided robust protection against a lethal DEV challenge. Importantly, rDEV-US7/8HA rapidly induced antibodies against the H5N1 virus and granted complete protection against the H5N1 virus challenge (50). Similarly, the DHAV-3 P1 and 3C genes were inserted into the non-coding regions between DEV’s UL26/UL27 and US7/8, resulting in the creation of rDEV-UL26/27-P13C and rDEV-US7/8-P13C. Both recombinant viruses triggered rapid immune responses and offered full protection against the DHAV-3 challenge. Furthermore, recombinant DEV expressing DHAV-3 antigens provided strong protection against lethal DEV challenge, with rDEV-UL26/27-P13C showing superior protection over rDEV-US7/8-P13C (29).
Although these studies suggest that different insertion sites may have some impact on the protective efficacy of recombinant DEV, the differences observed were not significant. However, the precise mechanisms underlying the effects of insertion sites on exogenous protein expression and the protective efficacy of recombinant viruses remain unclear. Therefore, further systematic studies are needed to elucidate these mechanisms and identify the optimal insertion sites for exogenous genes.
In Table 2, we have comprehensively summarized the relevant information on the recombinant DEV studied so far and their immune effects. Through the data presented, we can clearly observe the construction strategies of various recombinant DEV, such as the DEV strains used as vectors, the promoters, the insertion sites of foreign genes, the gene fragments utilized, as well as their immune effects in experimental animals or actual breeding environments. These data provide crucial reference information for further optimizing the development of recombinant duck enteritis virus vaccines.
The development of recombinant DEV vector vaccines not only provides a new strategy for the prevention and control of duck viral enteritis but also opens up new avenues for the integrated prevention and control of other poultry diseases. This new type of vaccine can achieve immune protection against multiple pathogens through a single vaccination, greatly simplifying the traditional immunization process, reducing the labor intensity and economic costs in the poultry farming industry, and alleviating the stress on animals caused by multiple vaccinations.
Looking back at the past three decades, the field of recombinant virus-vectored vaccines has undergone extensive research and evaluation. Yet, only a handful of cases have successfully transitioned to commercialization. Most of these commercialized vaccines are based on vectors such as the herpesvirus of turkeys and fowlpox virus, effectively addressing various poultry diseases including influenza (51), laryngotracheitis (52), Newcastle disease (53), and infectious bursal disease (54). Meanwhile, recombinant DEV vector vaccines, as rising stars, have rapidly become a research focus over the past decade. Researchers have leveraged cutting-edge biotechnologies such as Bacterial Artificial Chromosome, Fosmid rescue systems, and CRISPR/Cas9, significantly accelerating the development of recombinant DEV vaccine candidates, enhancing vaccine construction efficiency, and achieving greater precision and flexibility in vaccine design.
Even though recombinant DEV vaccines have demonstrated robust protective efficacy, their commercialization process lags behind that of other well-established viral vector vaccines, and there are no avian vaccine products based on this vector currently available on the market. Nevertheless, the research value of DEV as a highly promising vaccine vector cannot be overlooked. Future research should focus on optimizing insertion sites and promoters to enhance the expression efficiency and antigen presentation of foreign genes; delving into the correlation between the genetic stability and protective efficacy of recombinant DEV vaccines; analyzing the potential impact of foreign genes on the immunogenicity of the parental DEV virus; identifying the optimal balance between foreign gene expression levels and immune response capabilities; and ensuring the safety of recombinant DEV vaccines throughout production and administration et al.
Notably, studies on recombinant DEV vector vaccines for avian influenza prevention and control are particularly abundant (21, 23, 25, 50). Given that waterfowl serve as significant reservoirs and cross-regional transmission vectors for avian influenza viruses, their low immunization coverage poses a considerable challenge to avian influenza control. The avian influenza recombinant DEV vector live vaccine, with its advantage of “one shot, two diseases prevented, “rapid induction of protective effects, and long-lasting immunity, offers dual lifelong protection against avian influenza and duck virus enteritis in ducks and potentially a broader range of poultry species, representing a significant milestone toward the long-term control and potential eradication of avian influenza.
Looking ahead, the focus of scientific research should be on further optimizing the live recombinant DEV vector vaccine, ultimately achieving the industrialization and application of research achievements. This not only represents a pivotal breakthrough in the development of the poultry vaccine industry but also serves as a crucial safeguard for promoting the healthy and sustainable development of the poultry farming industry. This review, by outlining the construction methods of the recombinant live duck enteritis virus vector vaccine and the key factors influencing its immunological effects, aims to provide valuable references for researchers in related fields, working together to drive the in-depth development of research on the recombinant DEV vaccine.
W-FJ: Writing – original draft, Writing – review & editing. A-PW: Writing – review & editing. ZW: Writing – review & editing. X-NL: Writing – review & editing. Y-TC: Writing – review & editing. S-YZ: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the project of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (no. 24KJB230003), the Agricultural Project of Taizhou Science and Technology Support Program (no. TN202412), the project of the Jiangsu Agri-animal Husbandry Vocational College (no. NSF2023CB04), the Science and Technology Innovation Team Project of Jiangsu Agri-Animal Husbandry Vocational College (no. NSF2023TC02).
We express our gratitude to the members of the Waterfowl Infectious Diseases Division and the Jiangsu Key Laboratory for High-Tech Research and Development of Veterinary Biopharmaceuticals.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Romanutti, C, Keller, L, and Zanetti, FA. Current status of virus-vectored vaccines against pathogens that affect poultry. Vaccine. (2020) 38:6990–7001. doi: 10.1016/j.vaccine.2020.09.013
2. Vannucci, L, Lai, M, Chiuppesi, F, Ceccherini-Nelli, L, and Pistello, M. Viral vectors: a look back and ahead on gene transfer technology. New Microbiol. (2013) 36:1–22.
3. García-Arriaza, J, Cepeda, V, Hallengärd, D, Sorzano, C, Kümmerer, BM, Liljeström, P, et al. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J Virol. (2014) 88:3527–47. doi: 10.1128/jvi.03418-13
4. Paoletti, E. Poxvirus recombinant vaccines. Ann N Y Acad Sci. (1990) 590:309–25. doi: 10.1111/j.1749-6632.1990.tb42239.x
5. Skinner, MA, Laidlaw, SM, Eldaghayes, I, Kaiser, P, and Cottingham, MG. Fowlpox virus as a recombinant vaccine vector for use in mammals and poultry. Expert Rev Vaccines. (2005) 4:63–76. doi: 10.1586/14760584.4.1.63
6. Majhen, D. Human adenovirus type 26 basic biology and its usage as vaccine vector. Rev Med Virol. (2022) 32:e2338. doi: 10.1002/rmv.2338
7. Gebre, MS, Brito, LA, Tostanoski, LH, Edwards, DK, Carfi, A, and Barouch, DH. Novel approaches for vaccine development. Cell. (2021) 184:1589–603. doi: 10.1016/j.cell.2021.02.030
8. Palù, G, Parolin, C, Takeuchi, Y, and Pizzato, M. Progress with retroviral gene vectors. Rev Med Virol. (2000) 10:185–202. doi: 10.1002/(SICI)1099-1654(200005/06)10:3<185::AID-RMV285>3.0.CO;2-8
9. Zhou, M, Abid, M, Cao, S, and Zhu, S. Recombinant pseudorabies virus usage in vaccine development against swine infectious disease. Viruses. (2023) 15:15. doi: 10.3390/v15020370
10. Jia, W, Zhang, X, Wang, H, Teng, Q, Xue, J, and Zhang, G. Construction and immune efficacy of a recombinant Turkey herpesvirus vaccine strain expressing fusion protein of genotype VII Newcastle disease virus. Vet Microbiol. (2022) 268:109429. doi: 10.1016/j.vetmic.2022.109429
11. Liu, Y, Li, K, Gao, Y, Gao, L, Zhong, L, Zhang, Y, et al. Recombinant Marek's disease virus as a vector-based vaccine against avian Leukosis virus subgroup J in chicken. Viruses. (2016) 8:8. doi: 10.3390/v8110301
12. De Groot, AS, Einck, L, Moise, L, Chambers, M, Ballantyne, J, Malone, RW, et al. Making vaccines "on demand": a potential solution for emerging pathogens and biodefense? Hum Vaccin Immunother. (2013) 9:1877–84. doi: 10.4161/hv.25611
13. Dhama, K, Kumar, N, Saminathan, M, Tiwari, R, Karthik, K, Kumar, MA, et al. Duck virus enteritis (duck plague) - a comprehensive update. Vet Q. (2017) 37:57–80. doi: 10.1080/01652176.2017.1298885
14. Li, Y, Huang, B, Ma, X, Wu, J, Li, F, Ai, W, et al. Molecular characterization of the genome of duck enteritis virus. Virology. (2009) 391:151–61. doi: 10.1016/j.virol.2009.06.018
15. Wang, J, Höper, D, Beer, M, and Osterrieder, N. Complete genome sequence of virulent duck enteritis virus (DEV) strain 2085 and comparison with genome sequences of virulent and attenuated DEV strains. Virus Res. (2011) 160:316–25. doi: 10.1016/j.virusres.2011.07.004
16. Li, Y, Wang, M, Cheng, A, Jia, R, Yang, Q, Chen, S, et al. Duck enteritis virus VP16 antagonizes IFN-β-mediated antiviral innate immunity. J Immunol Res. (2020) 2020:9630452–13. doi: 10.1155/2020/9630452
17. Liu, R, Gao, L, Yang, F, Li, X, Liu, C, Qi, X, et al. Duck enteritis virus protein kinase US3 inhibits DNA sensing signaling by phosphorylating interferon regulatory factor 7. Microbiol Spect. (2022) 10:e0229922. doi: 10.1128/spectrum.02299-22
18. Gao, L, Liu, R, Yang, F, Li, X, Liu, C, Qi, X, et al. Duck enteritis virus inhibits the cGAS-STING DNA-sensing pathway to evade the innate immune response. J Virol. (2022) 96:e0157822. doi: 10.1128/jvi.01578-22
19. Qi, X, Yang, X, Chen, A, Wang, M, Zhu, D, and Jia, R. The pathogenesis of duck virus enteritis in experimentally infected ducks: a quantitative time-course study using TaqMan polymerase chain reaction. Avian Pathol. (2008) 37:307–10. doi: 10.1080/03079450802043775
20. Shawky, S, and Schat, KA. Latency sites and reactivation of duck enteritis virus. Avian Dis. (2002) 46:308–13. doi: 10.1637/0005-2086(2002)046[0308:Lsarod]2.0.Co;2
21. Liu, X, Wei, S, Liu, Y, Fu, P, Gao, M, Mu, X, et al. Recombinant duck enteritis virus expressing the HA gene from goose H5 subtype avian influenza virus. Vaccine. (2013) 31:5953–9. doi: 10.1016/j.vaccine.2013.10.035
22. Li, H, Wang, Y, Han, Z, Wang, Y, Liang, S, Jiang, L, et al. Recombinant duck enteritis viruses expressing major structural proteins of the infectious bronchitis virus provide protection against infectious bronchitis in chickens. Antivir Res. (2016) 130:19–26. doi: 10.1016/j.antiviral.2016.03.003
23. Sun, Y, Yang, C, Li, J, Li, L, Cao, M, Li, Q, et al. Construction of a recombinant duck enteritis virus vaccine expressing hemagglutinin of H9N2 avian influenza virus and evaluation of its efficacy in ducks. Arch Virol. (2017) 162:171–9. doi: 10.1007/s00705-016-3077-3
24. Chen, L, Yu, B, Hua, J, Ni, Z, Ye, W, Yun, T, et al. Optimized expression of duck Tembusu virus E gene delivered by a vectored duck enteritis virus in vitro. Mol Biotechnol. (2019) 61:783–90. doi: 10.1007/s12033-019-00206-1
25. Wang, J, Ge, A, Xu, M, Wang, Z, Qiao, Y, Gu, Y, et al. Construction of a recombinant duck enteritis virus (DEV) expressing hemagglutinin of H5N1 avian influenza virus based on an infectious clone of DEV vaccine strain and evaluation of its efficacy in ducks and chickens. Virol J. (2015) 12:126. doi: 10.1186/s12985-015-0354-9
26. Yan, S, Huang, B, Bai, X, Zhou, Y, Guo, L, Wang, T, et al. Construction and immunogenicity of a recombinant pseudorabies virus variant with TK/gI/gE/11k/28k deletion. Front Vet Sci. (2021) 8:797611. doi: 10.3389/fvets.2021.797611
27. Adler, H, Messerle, M, and Koszinowski, UH. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev Med Virol. (2003) 13:111–21. doi: 10.1002/rmv.380
28. Zhao, Y, Chen, P, Hu, Y, Liu, J, Jiang, Y, Zeng, X, et al. Recombinant duck enteritis virus bearing the hemagglutinin genes of H5 and H7 influenza viruses is an ideal multivalent live vaccine in ducks. Emerg Microbes Infect. (2024) 13:2284301. doi: 10.1080/22221751.2023.2284301
29. Yang, F, Liu, P, Li, X, Liu, R, Gao, L, Cui, H, et al. Recombinant duck enteritis virus-vectored bivalent vaccine effectively protects against duck hepatitis a virus infection in ducks. Front Microbiol. (2021) 12:813010. doi: 10.3389/fmicb.2021.813010
30. Zuo, Z, and Liu, J. Allosteric regulation of CRISPR-Cas9 for DNA-targeting and cleavage. Curr Opin Struct Biol. (2020) 62:166–74. doi: 10.1016/j.sbi.2020.01.013
31. Singh, D, Sternberg, SH, Fei, J, Doudna, JA, and Ha, T. Real-time observation of DNA recognition and rejection by the RNA-guided endonuclease Cas9. Nat Commun. (2016) 7:12778. doi: 10.1038/ncomms12778
32. Ma, Y, Zhang, L, and Huang, X. Genome modification by CRISPR/Cas9. FEBS J. (2014) 281:5186–93. doi: 10.1111/febs.13110
33. Lino, CA, Harper, JC, Carney, JP, and Timlin, JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. (2018) 25:1234–57. doi: 10.1080/10717544.2018.1474964
34. Gong, Y, Bi, Y, Li, Z, Li, Y, Yao, Y, Long, Q, et al. High-efficiency nonhomologous insertion of a foreign gene into the herpes simplex virus genome. J Gen Virol. (2020) 101:982–96. doi: 10.1099/jgv.0.001451
35. Tang, N, Zhang, Y, Pedrera, M, Chang, P, Baigent, S, Moffat, K, et al. A simple and rapid approach to develop recombinant avian herpesvirus vectored vaccines using CRISPR/Cas9 system. Vaccine. (2018) 36:716–22. doi: 10.1016/j.vaccine.2017.12.025
36. Chang, P, Yao, Y, Tang, N, Sadeyen, JR, Sealy, J, Clements, A, et al. The application of NHEJ-CRISPR/Cas9 and Cre-lox system in the generation of bivalent duck enteritis virus vaccine against avian influenza virus. Viruses. (2018) 10:10. doi: 10.3390/v10020081
37. Apinda, N, Yao, Y, Zhang, Y, Reddy, V, Chang, P, Nair, V, et al. CRISPR/Cas9 editing of duck enteritis virus genome for the construction of a recombinant vaccine vector expressing ompH gene of Pasteurella multocida in two novel insertion sites. Vaccines (Basel). (2022) 10:686. doi: 10.3390/vaccines10050686
38. Chang, P, Ameen, F, Sealy, JE, Sadeyen, JR, Bhat, S, Li, Y, et al. Application of HDR-CRISPR/Cas9 and erythrocyte binding for rapid generation of recombinant Turkey herpesvirus-vectored avian influenza virus vaccines. Vaccines (Basel). (2019) 7:192. doi: 10.3390/vaccines7040192
39. Luo, C, Wang, Q, Guo, R, Zhang, J, Zhang, J, Zhang, R, et al. A novel pseudorabies virus vaccine developed using HDR-CRISPR/Cas9 induces strong humoral and cellular immune response in mice. Virus Res. (2022) 322:198937. doi: 10.1016/j.virusres.2022.198937
40. Gergen, L, Cook, S, Ledesma, B, Cress, W, Higuchi, D, Counts, D, et al. A double recombinant herpes virus of turkeys for the protection of chickens against Newcastle, infectious laryngotracheitis and Marek's diseases. Avian Pathol. (2019) 48:45–56. doi: 10.1080/03079457.2018.1546376
41. Tang, N, Zhang, Y, Sadigh, Y, Moffat, K, Shen, Z, Nair, V, et al. Generation of a triple insert live avian herpesvirus vectored vaccine using CRISPR/Cas9-based gene editing. Vaccine. (2020) 8:8. doi: 10.3390/vaccines8010097
42. Zou, Z, Hu, Y, Liu, Z, Zhong, W, Cao, H, Chen, H, et al. Efficient strategy for constructing duck enteritis virus-based live attenuated vaccine against homologous and heterologous H5N1 avian influenza virus and duck enteritis virus infection. Vet Res. (2015) 46:42. doi: 10.1186/s13567-015-0174-3
43. Liu, J, Chen, P, Jiang, Y, Deng, G, Shi, J, Wu, L, et al. Recombinant duck enteritis virus works as a single-dose vaccine in broilers providing rapid protection against H5N1 influenza infection. Antivir Res. (2013) 97:329–33. doi: 10.1016/j.antiviral.2012.12.015
44. Ding, L, Chen, P, Bao, X, Li, A, Jiang, Y, Hu, Y, et al. Recombinant duck enteritis viruses expressing the Newcastle disease virus (NDV) F gene protects chickens from lethal NDV challenge. Vet Microbiol. (2019) 232:146–50. doi: 10.1016/j.vetmic.2019.04.022
45. Niu, Y, Liu, B, Sun, C, Zhao, L, and Chen, H. Construction of the recombinant duck enteritis virus delivering capsid protein VP0 of the duck hepatitis a virus. Vet Microbiol. (2020) 249:108837. doi: 10.1016/j.vetmic.2020.108837
46. Apinda, N, Muenthaisong, A, Chomjit, P, Sangkakam, K, Nambooppha, B, Rittipornlertrak, A, et al. Simultaneous protective immune responses of ducks against duck plague and fowl cholera by recombinant duck enteritis virus vector expressing Pasteurella multocida OmpH gene. Vaccine. (2022) 10:10. doi: 10.3390/vaccines10081358
47. Watanabe, M, Nishikawaji, Y, Kawakami, H, and Kosai, KI. Adenovirus biology, recombinant adenovirus, and adenovirus usage in gene therapy. Viruses. (2021) 13:13. doi: 10.3390/v13122502
48. He, J, An, Y, Qi, J, Cui, L, Yang, K, Liu, M, et al. The recombinant Newcastle disease virus Anhinga strain expressing human TRAIL exhibit antitumor effects on a glioma nude mice model. J Med Virol. (2021) 93:3890–8. doi: 10.1002/jmv.26419
49. Aravind, S, Kamble, NM, Gaikwad, SS, Shukla, SK, Dey, S, and Mohan, CM. Adaptation and growth kinetics study of an Indian isolate of virulent duck enteritis virus in Vero cells. Microb Pathog. (2015) 78:14–9. doi: 10.1016/j.micpath.2014.11.008
50. Liu, J, Chen, P, Jiang, Y, Wu, L, Zeng, X, Tian, G, et al. A duck enteritis virus-vectored bivalent live vaccine provides fast and complete protection against H5N1 avian influenza virus infection in ducks. J Virol. (2011) 85:10989–98. doi: 10.1128/jvi.05420-11
51. Rauw, F, Palya, V, Gardin, Y, Tatar-Kis, T, Dorsey, KM, Lambrecht, B, et al. Efficacy of rHVT-AI vector vaccine in broilers with passive immunity against challenge with two antigenically divergent Egyptian clade 2.2.1 HPAI H5N1 strains. Avian Dis. (2012) 56:913–22. doi: 10.1637/10172-041012-Reg.1.1
52. Johnson, DI, Vagnozzi, A, Dorea, F, Riblet, SM, Mundt, A, Zavala, G, et al. Protection against infectious laryngotracheitis by in ovo vaccination with commercially available viral vector recombinant vaccines. Avian Dis. (2010) 54:1251–9. doi: 10.1637/9401-052310-Reg.1
53. Morgan, RW, Gelb, J Jr, Schreurs, CS, Lütticken, D, Rosenberger, JK, and Sondermeijer, PJ. Protection of chickens from Newcastle and Marek's diseases with a recombinant herpesvirus of turkeys vaccine expressing the Newcastle disease virus fusion protein. Avian Dis. (1992) 36:858–70. doi: 10.2307/1591544
54. Gelb, J Jr, Jackwood, DJ, Brannick, EM, and Ladman, BS. Efficacy of recombinant HVT-IBD vaccines administered to broiler chicks from a single breeder flock at 30 and 60 weeks of age. Avian Dis. (2016) 60:603–12. doi: 10.1637/11344-120815-Reg.1
55. Wang, J, and Osterrieder, N. Generation of an infectious clone of duck enteritis virus (DEV) and of a vectored DEV expressing hemagglutinin of H5N1 avian influenza virus. Virus Res. (2011) 159:23–31. doi: 10.1016/j.virusres.2011.04.013
56. Zou, Z, Liu, Z, and Jin, M. Efficient strategy to generate a vectored duck enteritis virus delivering envelope of duck Tembusu virus. Viruses. (2014) 6:2428–43. doi: 10.3390/v6062428
57. Chen, P, Liu, J, Jiang, Y, Zhao, Y, Li, Q, Wu, L, et al. The vaccine efficacy of recombinant duck enteritis virus expressing secreted E with or without PrM proteins of duck tembusu virus. Vaccine. (2014) 32:5271–7. doi: 10.1016/j.vaccine.2014.07.082
58. Zou, Z, Ma, J, Huang, K, Chen, H, Liu, Z, and Jin, M. Live attenuated vaccine based on duck enteritis virus against duck hepatitis a virus types 1 and 3. Front Microbiol. (2016) 7:1613. doi: 10.3389/fmicb.2016.01613
59. Apinda, N, Nambooppha, B, Rittipornlertrak, A, Tankaew, P, Punyapornwithaya, V, Nair, V, et al. Protection against fowl cholera in ducks immunized with a combination vaccine containing live attenuated duck enteritis virus and recombinant outer membrane protein H of Pasteurella multocida. Avian Pathol. (2020) 49:221–9. doi: 10.1080/03079457.2019.1711020
Keywords: duck enteritis virus, recombinant vector vaccine, genetic modification, waterfowl infectious disease, immune protection efficacy, avian vaccines
Citation: Jia W-F, Wang A-P, Wu Z, Lei X-N, Cheng Y-T and Zhu S-Y (2025) Current status of recombinant duck enteritis virus vector vaccine research. Front. Vet. Sci. 12:1453150. doi: 10.3389/fvets.2025.1453150
Received: 22 June 2024; Accepted: 24 January 2025;
Published: 05 February 2025.
Edited by:
Guoxin Li, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Pavulraj Selvaraj, Louisiana State University, United StatesCopyright © 2025 Jia, Wang, Wu, Lei, Cheng and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan-Yuan Zhu, enN5QGpzYWh2Yy5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.