- 1Department of Animal Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, New Valley University, El-Kharga, Egypt
- 2Department of Animal Wealth Development, Faculty of Veterinary Medicine, Zagazig University, Sharkia, Egypt
- 3Department of Pathology, Faculty of Veterinary Medicine, Benha University, Toukh, Egypt
- 4Department of Food Hygiene, Safety and Technology, Faculty of Veterinary Medicine, New Valley University, El Kharga, Egypt
- 5Department of Animal Production, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
- 6Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
- 7Poultry Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 8Department of Veterinary Medicine, University of Bari Aldo Moro, Valenzano, Italy
- 9Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Abu Dhabi, United Arab Emirates
- 10Animal Production Department, National Research Centre, Giza, Egypt
- 11Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, New Valley University, El Kharga, Egypt
Introduction: This study examined the influence of Spirulina platensis, ochratoxin A (OTA), and their combination on growth, antioxidant status, liver and kidney functions, immunity, and carcass traits of broiler chickens.
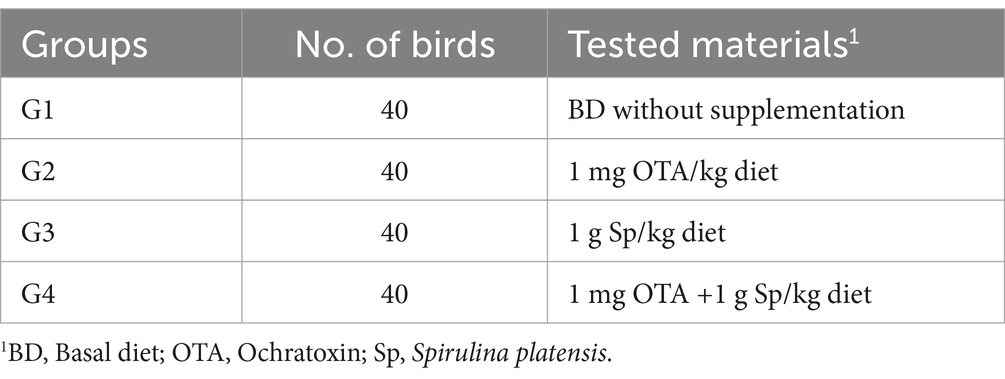
Methods: 160 unsexed 1-day broiler chicks were divided into four treatment groups, each consisting of 4 replications of 10 chicks. The duration of this study was six weeks, during which the experimental groups were organized as follows: G1 consumed a basal diet (control), G2 consumed a basal diet treated with OTA at a level of 1 mg/kg of diet, G3 consumed a basal diet treated with Spirulina platensis at a level of 1 g/kg of diet, G4 consumed a basal diet treated with OTA at a level of 1 mg/kg of diet and Spirulina platensis at a level of 1 g/kg diet.
Results and discussion: The results illustrated that OTA-contaminated feed resulted in a significant elevation in total cholesterol, triglyceride, low- and very low density lipoprotein, and malondialdehyde, along with a significant reduction in antioxidant status and immunological response. On the other hand, Spirulina supplementation significantly enhanced performance performance (body weight, body weight gain and feed conversion ratio). Lipid profile was significantly decreased by Spirulina supplementation. Antioxidant activity (superoxide dismutase, catalase, total antioxidant capacity, and glutathione peroxidase) of broilers exposed to OTA was significantly increased by Spirulina supplementation. Finally, supplementing Spirulina platensis in broiler chickens fed on OTA contaminated diet attenuated the harmful effects of OTA, while improving the growth performance, antioxidant activity, lipid profile, and immune response of broiler chickens.
Introduction
The prevalence of mycotoxins is a general problem that has negative effects on animals and humans (1). Several researchers have established that ochratoxin A (OTA) is one of the mycotoxins in the livestock and agriculture sectors. OTA is a mycotoxin commonly found in feed ingredients, particularly grains, and harms poultry health and productivity (1). Several research works have provided evidence of the beneficial effects of nutritional supplements with vitamins and electrolytes (2–6), probiotics, prebiotics (7–10), phytogenics and organic acids (11–13). Additionally, various feed additives and management practices are also included (14–18) in decreasing the detrimental effects of environmental stress on broiler chicken performance. The impact of Spirulina platensis supplementation on various physiological characteristics in broilers exposed to ochratoxin A (OTA) stress is a topic of significant interest within the realm of animal nutrition and health (19). Multiple research investigations have reported the influence of Spirulina platensis supplementation on broiler chickens under different stressors and conditions (20, 21). Mirzaie et al. (21) explored the influence of dietary supplementation with Spirulina on the immune system, lipid profile, antioxidant status, and performance traits of broiler chickens raised in hot environments, proposing that the addition of Spirulina could relieve the negative effects produced by elevated environmental temperature on a biochemical level. Furthermore, studies have shown that dietary supplementation of Spirulina platensis can benefit animal health (22, 23). For instance, Fries-Craft et al. (20) demonstrated that algae-based feed ingredients can protect the gastrointestinal tract health and modulate the immunity responses in poultry. Additionally, Eldesoky et al. (24) found that Spirulina platensis could improve testis injuries and sperm quality in rats exposed to mercuric chloride. Research by Park et al. (23) has demonstrated that including 1.0% Spirulina powder in the diet can be an alternative to enhance broiler chicken production. Studies by Qureshi et al. (25) have also shown the positive influence of Spirulina supplementation on the immune system, antioxidant status, and antibody formation in broiler chickens. Further, El-Shall et al. (26) have emphasized the ability of Spirulina platensis as a poultry feed supplement to enhance immunological regulation and growth performance, particularly in high ambient temperature environments. Additionally, Elbaz et al. (27) emphasized the beneficial effects of Spirulina platensis in mitigating the detrimental impacts due to heat stress on broiler chickens. Furthermore, Khalilnia et al. (28) indicated that including Spirulina platensis microalgae in broiler diets can lead to positive immune responses by increasing serum levels of immunoglobulins and phagocytic activity. Understanding the complex relationship between dietary Spirulina platensis supplementation and OTA-induced stress in broilers requires a thorough investigation covering various aspects of avian physiology. Key indicators of the broiler’s ability to utilize dietary nutrients and combat stressors include parameters such as feed consumption, body weight gain, and feed conversion ratio (29). The assessment of antioxidant status offers insights into cellular defense mechanisms against oxidative damage induced by OTA, with biomarkers such as superoxide dismutase (SOD) level, the activity of catalase (CAT), and glutathione peroxidase (GPx) activity reflecting antioxidant capacity. Liver and kidney function tests provide valuable information on the hepatic and renal health of broilers, which may be compromised under OTA-induced toxicity (21, 30). Evaluation of carcass traits, including carcass yield, meat quality attributes, and organ weights, reflects the overall performance and marketability of broiler chickens (31). Research has shown that OTA exerts its harmful effects by suppressing mitochondrial function, causing increased oxidative stress, and hindering protein synthesis.
By examining the impact of dietary Spirulina platensis supplementation on a wide range of physiological parameters in broilers exposed to OTA stress, researchers aim to uncover the potential mechanisms behind its protective effects and optimize its inclusion levels in poultry diets (21). It is hypothesized that the dietary addition of microalgae such as Spirulina platensis is expected to benefit poultry. The beneficial impacts of Spirulina platensis as a feed supplement has been previously studied separately and in the absence of natural pollutants such as OTA. Therefore, this study attempted to investigate the impact of Spirulina platensis on mitigating the adverse effects of OTA and enhancing growth efficiency, antioxidant capacity, liver and renal function, immune systems, and serum biochemical indicators in broilers.
Materials and methods
Production of ochratoxin A and Spirulina
The strain Aspergillus ochraceus (CGMCC 3.4412) was employed for the production of ochratoxin A. This particular strain was obtained from the Central Laboratory of Residues of Agricultural Products, located at the Agriculture Pesticides Residues Centre in Dokki, Egypt. The process of synthesizing OTA involved culturing the fungal strain in a liquid medium (containing 2% yeast extract and 20% sugar) for 8 days (12, 29). The concentration of ochratoxin A in the media was quantified using the stated technique in the AOAC (32) publication. The Spirulina platensis powder was purchased from Harraz Co, a local supplier based in Egypt.
Housing and birds
The trial was carried out in the poultry farm at the Faculty of Veterinary Medicine, Nutrition and Clinical Nutrition Department, New Valley University, New Valley, Egypt. The facility, including drinkers and feeders, were cleaned and disinfected before the trial. The experimental conditions included maintaining a consistent temperature range of 22–25°C, controlling air humidity levels between 55 and 65%, and ensuring adequate ventilation. Broiler chicks (Ross 308) were 1 day old and showed similar mean body weights (40.82 ± 0.70 g) in all groups. Birds were obtained from a private hatchery and housed at a stocking density of 10 chicks in each cage. The experiment adheres to the regulations set by the New Valley University Ethics Committee for the utilization of experimental animals. Throughout the experiment, all groups had the same managerial and environmental conditions.
Experimental design, diets, and treatments
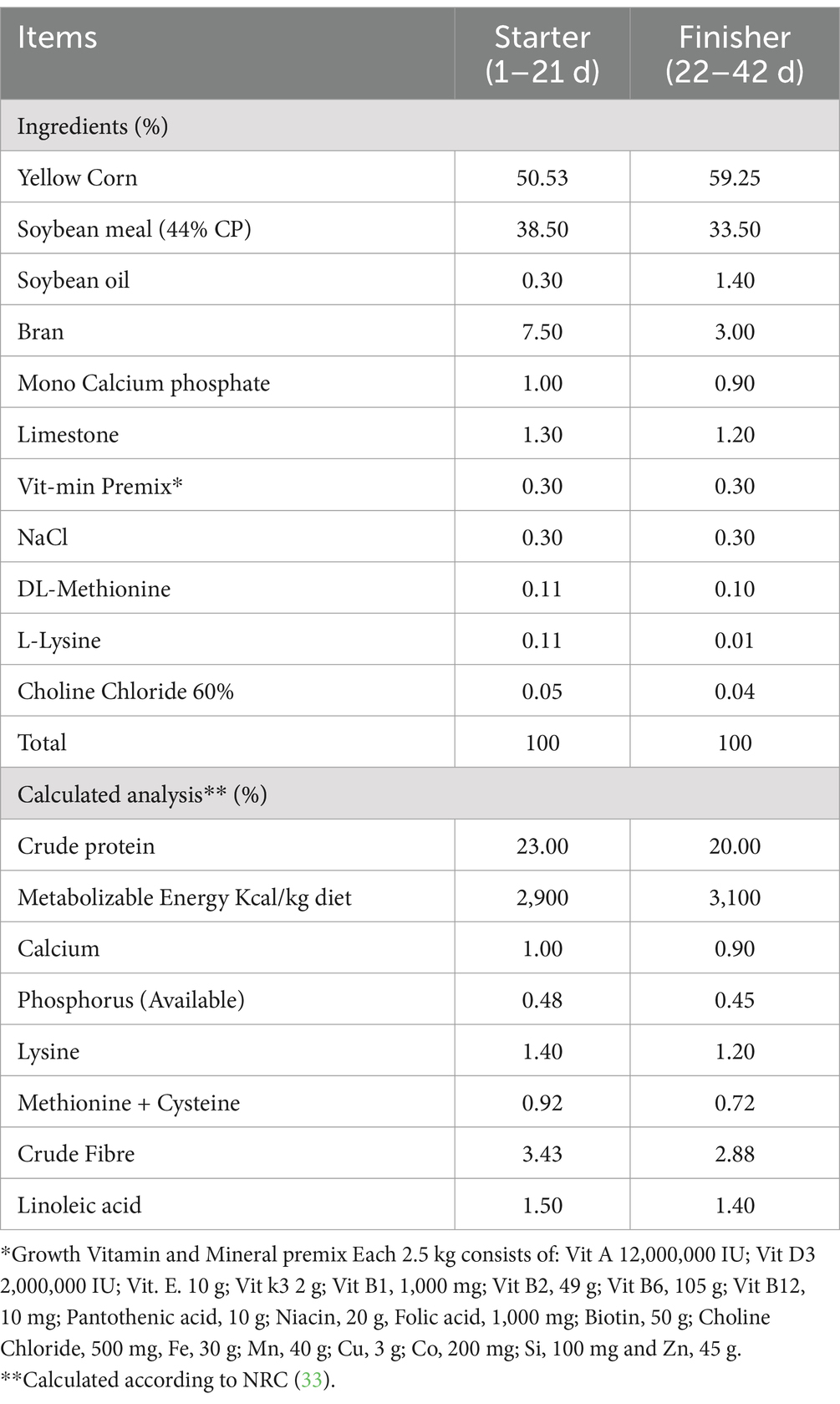
The trial method used in this study was a randomized complete block design. One hundred sixty unsexed 1-day broiler chicks were assigned into four treatment groups, each contains four replicates of 10 chicks. The study lasted for 6 weeks (1–42 d). The experimental design is summarized in Table 1. All birds were reread under the same conditions and in clean environments and was provided with diets that were nutritionally balanced in order to supply their nutritional needs, as stated in NRC (33) (Table 2). Feed and drink were provided in ad libtum.
Growth performance
All of the birds were measured for body weight (BW) at 0, 2, 4, and 6 weeks of age. Additionally, body weight gain (BWG) was measured throughout the duration of the experiment. In addition, the feed intake was constantly assessed in a replicated method throughout the study periods to calculate the feed conversion ratio (FCR = g of feed intake/g of body gain).
Carcass traits
After the study, a total of 10 broiler chicks per group were selected randomly. These birds were subsequently weighed and manually slaughtered for the purpose of conducting carcass traits. The carcass weight, giblets, gizzard, heart, liver, and intestine were measured and stated as a percentage of the total weight at slaughter. Additionally, the dressing percentage was determined.
Blood parameters
At the end of the study, birds were euthanized, and blood samples were collected using aseptic techniques into sterile tubes. The samples were let to coagulate and were then subjected to centrifugation at 4,000 rpm for a duration of 10 min. The obtained serums were stored at −20°C until they were prepared for examination. The spectrophotometric measurement of multiple parameters was performed using kits imported by Biodiagnostic Company (Giza, Egypt) and Mybiosource.com (San Diego, CA, USA). These parameters included the levels of total protein (Catalog No.: MBS165636), albumin (Catalog No.: MBS2881881), aspartate transaminase (AST) (Catalog No.: MBS740867), alanine transaminase (ALT) (Catalog No.: MBS266858), lactate dehydrogenase (LDH) (Catalog No.: MBS263022), triglycerides (Catalog No.: MBS1601908), total cholesterol (Catalog No.: MBS1601900), high-density lipoprotein (HDL) (Catalog No.: MBS040311), and low-density lipoprotein (LDL) (Catalog No.: MBS269281). The determination of serum globulin levels involved the subtraction of albumin levels from the total serum protein levels. The concentrations of immunoglobulins G (IgG), M [IgM], and A [IgA] were detected in plasma samples using available kits (Catalog No.: MBS260043, MBS706158 and MBS564152, respectively) provided by Mybiosource.com (San Diego, CA, USA). The activity of glutathione peroxidase (GPx) was detected in plasma by the kit available kits with catalog number MBS1604302 (Mybiosource.com, San Diego, CA, USA). The activity of superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (TAC), was detected in plasma samples using available kits provided by Bio-diagnostic, Egypt, with the following catalog numbers (SD 25 21, MD 25 29, and TA 25 13, respectively) and a spectrophotometer manufactured by Shimadzu, Japan, in conjunction with other laboratory instruments according to Elbarbary et al. (34).
Histopathological alteration
Bursa and thymus were extracted and stored in 10% neutral buffered formalin for 72 h for histological analysis. Following fixation, the samples underwent dehydration using increasing concentrations of ethanol, subsequently, the specimens were purified using xylene and subsequently encased in paraffin. Tissue sections measuring five micrometers in thickness were cut using a microtome. Hematoxylin and eosin (H&E) staining was applied to the slides and analyzed by a Leika DM500 light microscope to observe histological alterations (35).
Immunohistochemical staining to PCNA
The immunohistochemistry technique used for proliferating cell nuclear antigen (PCNA) was performed according to (36). The sections of tissue underwent deparaffinization and rehydration. Following the wash with PBS (0.1 M, pH 7.2–7.4), the sections of tissue were exposed to a solution of 3.0% hydrogen peroxide in PBS at room temperature for 10 min. After being rinsed with PBS, the sections were treated with normal goat sera for 30 min to prevent nonspecific antibody binding. The sections were exposed to the rabbit anti-PCNA polyclonal antibody (bs-0754R, Bioss, Beijing, China) for 20 h at a temperature of 4°C. The antibody was diluted to a working concentration of 1:100. Following three consecutive washes in PBS, the sample was treated with a secondary antibody, specifically biotinylated goat anti-rabbit IgG, and then with streptavidin-biotin complex (SA1020, Boster, Wuhan, China). Afterward, the slices were gently stained with hematoxylin and immersed in 100% ethyl alcohol and xylene for 3 min before being covered with a coverslip. For the negative controls, the identical procedure was followed, with the exception that PBS was used instead of the primary antibody. The stained slices were photographed with a Leika DM500 digital camera.
Statistical analysis
SAS (SAS Institute Inc., 2001) was used for the statistical analysis. A one-way ANOVA was utilized to analyze the performance, carcasses, serum components, and oxidative state using the post-hoc Newman–Keuls test (with the diet as the fixed factor). At p < 0.05, the significance was determined.
Results
Growth performance
The influence of dietary Spirulina supplement on body weight and body weight gain of broilers exposed to ochratoxin A illustrated in Table 3. The finding displayed a significant (p = 0.049) improvement in body weight and body weight gain at age 42 due to Spirulina supplementation, where G3 achieved the best results. While, ochratoxicosis significantly decreased body weight and body weight gain at the end of the experiment (42 days of age). Moreover, the influence of dietary Spirulina supplement on feed consumption and feed conversion ratio of birds exposed to ochratoxin A illustrated in Table 4. The result revealed a significant decline in feed intake in the ochratoxin-treated group (G2) (p = 0.001) during the (1–42) period. Furthermore, the result illustrated a significant improvement in FCR (p = 0.029) due to Spirulina supplementation during the period (1–42) and G3 revealed the best result (1.50).
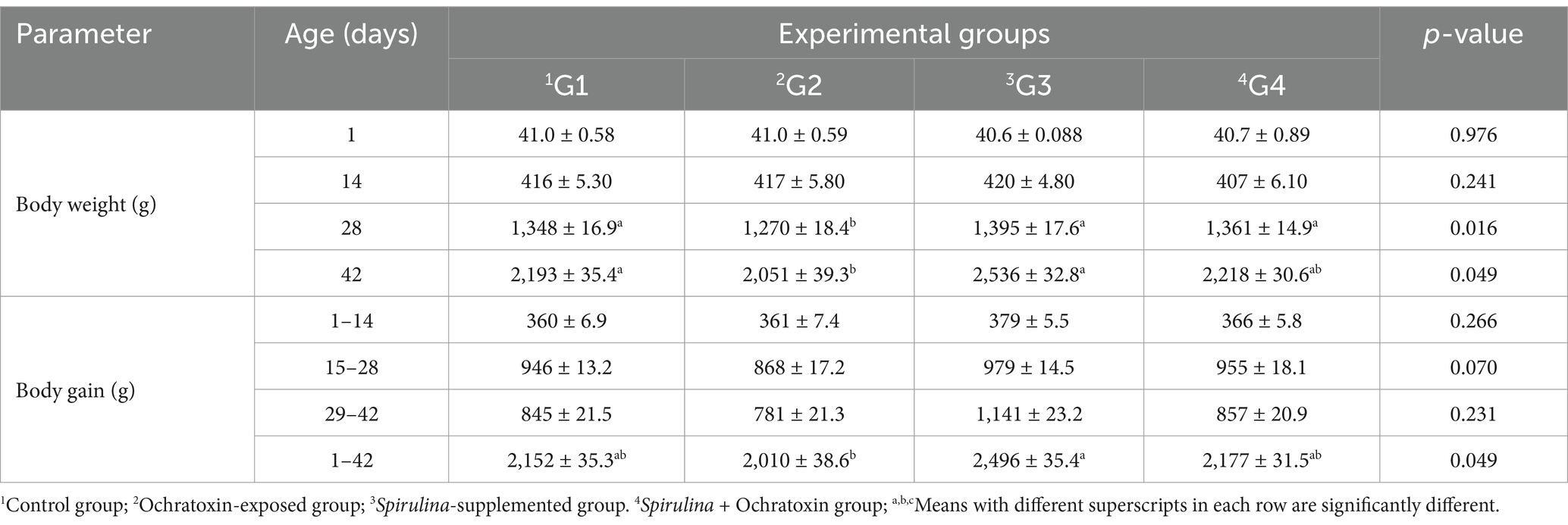
Table 3. Effect of dietary Spirulina supplement on body weight and body weight gain of broilers exposed to ochratoxin A.
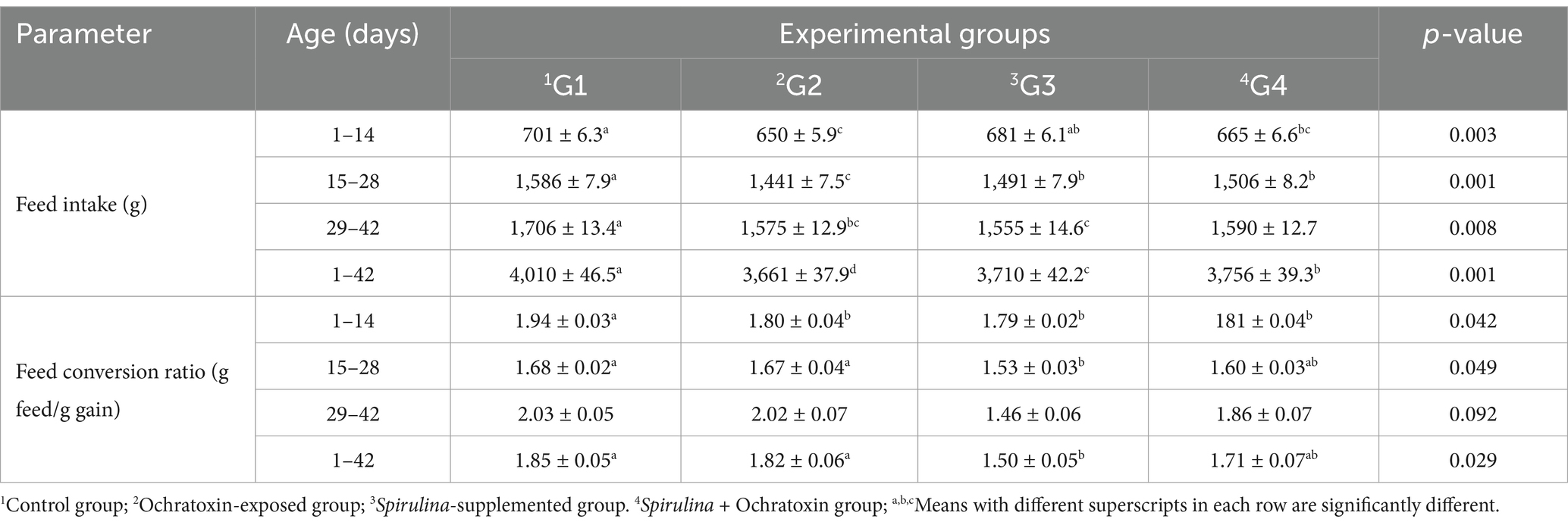
Table 4. Effect of dietary Spirulina supplement on feed intake and feed conversion ratio of broilers exposed to ochratoxin A.
Measurements of carcass
The effect of dietary Spirulina supplement on carcass traits of broilers exposed to ochratoxin A demonstrated in Table 5. The results illustrated a significant variation in carcass traits and the Spirulina-treated group presented a significant improvement (p = 0.048, p = 0.002) in carcass weight and dressing percentage (2,001 g, 79.2%). Moreover, revealed a significant decrease (p = 0.003) in abdominal fat percentage (1.27%). While, G2 revealed a significant reduction in carcass weight and dressing percentage (1,547 g, and 75.3%) respectively relative to control and other groups.
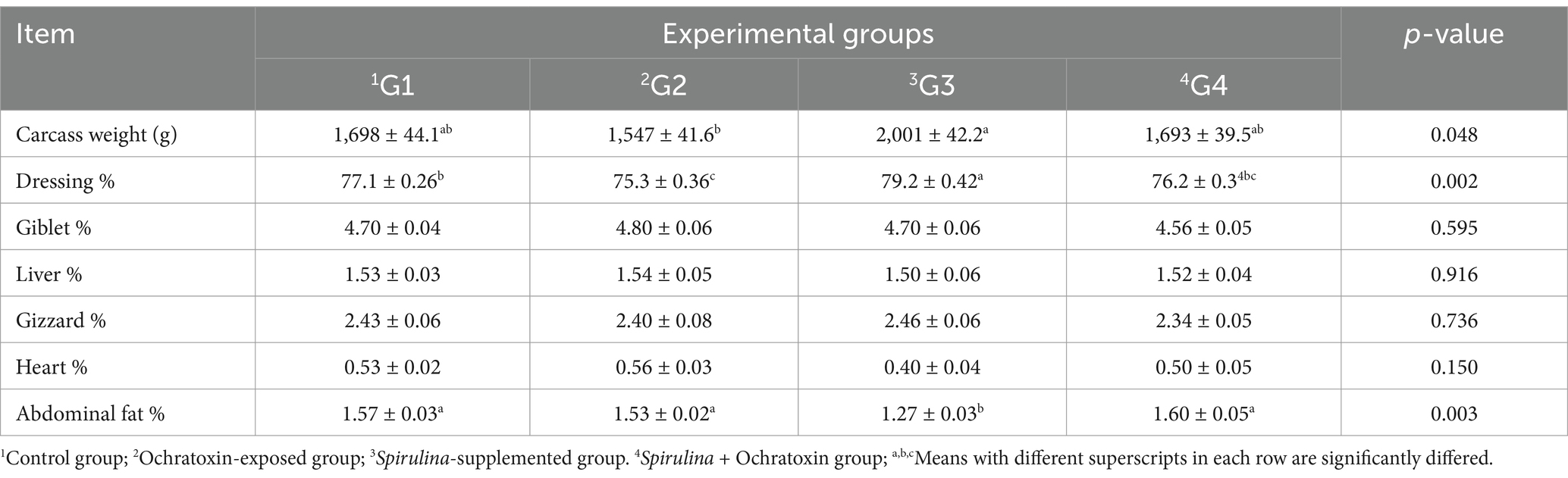
Table 5. Effect of dietary Spirulina supplement on carcass traits of broilers exposed to ochratoxin A.
Serum biochemical parameters
The influence of Spirulina supplementation on the blood biochemical indices of broilers exposed to ochratoxin A was illustrated in Table 6. The result showed significant differences in liver and kidney function tests and G2 that was treated with Ochratoxin revealed a significant elevation (p = 0.001, p = 0.003) in liver enzymes ALT and AST (37.64 and 172.0 IU/L). Moreover, ochratoxicosis significantly increases (p = 0.001, p = 0.008) uric acid and creatinine levels (6.45 and 1.85 mg/dL, respectively) in the blood. G3 that was supplemented with Spirulina revealed a significant decrease (p = 0.001, p = 0.006, p = 0.008, p = 0.004) in cholesterol, triglycerides, LDL, and VLDL (178.2, 137.3, 71.17, and 34.69 mg/dL, respectively). While G2 that was treated with ochratoxin illustrated a significant elevation in cholesterol, triglycerides, LDL, and VLDL (357.8, 215.5, 124.7, and 65.43 mg/dL, respectively).
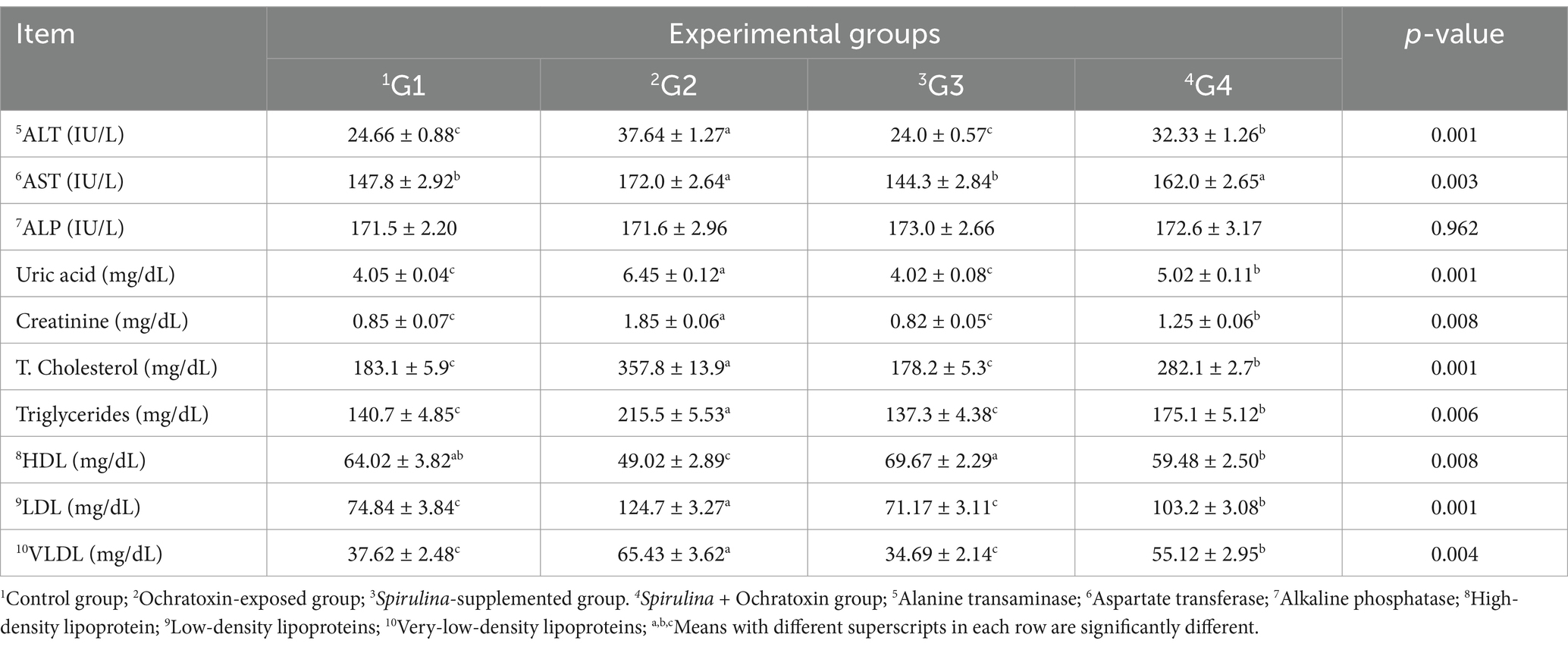
Table 6. Effect of dietary Spirulina supplement on blood biochemical indices of broilers exposed to ochratoxin A.
Antioxidant status
The influence of Spirulina supplementation on the antioxidant status of birds exposed to ochratoxin A was illustrated in Table 7. The finding presented significant variation in antioxidant activity and G3 that was supplemented with spirulina provided a significant decline (p = 0.001) in MDA (14.56 nmol/g) relative to the control. While G2 treated with ochratoxin illustrated the increased level of MDA (30.6 nmol/g). Moreover, G3 presented a significant increase (p = 0.015, p = 0.006, p = 0.036, p = 0.045) in SOD, GPx, CAT and TAC (439.5 u/g, 85.11 u/g, 38.96 nmol/g, and 141.2 nmol/g) respectively. Whereas the group supplemented with ochratoxin presented decreased levels of SOD, GPx, CAT, and TAC (279.4 u/g, 48.41 u/g, 25.20 nmol/g, and 108.1 nmol/g) respectively.
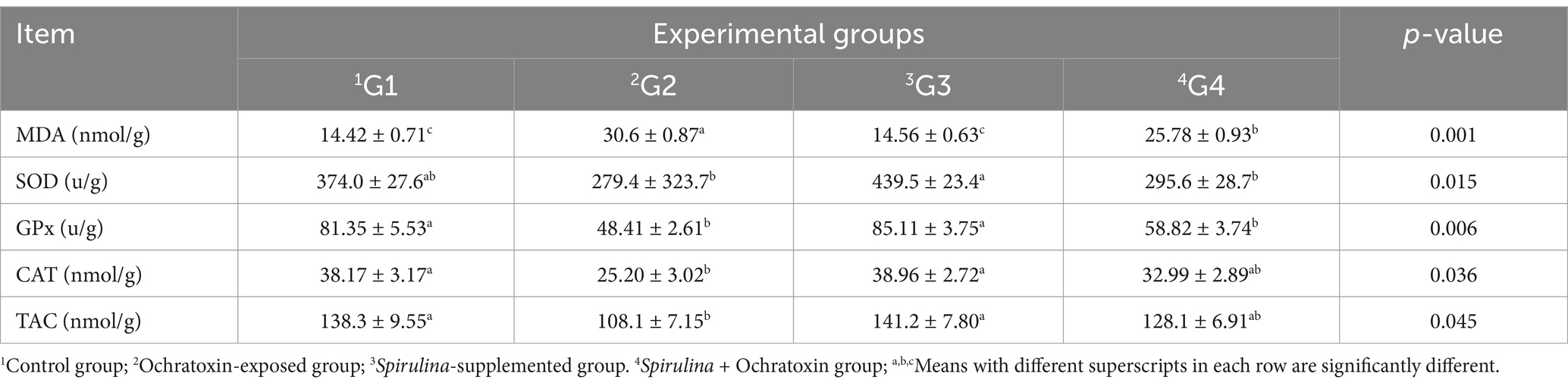
Table 7. Effect of dietary Spirulina supplement on antioxidant activity of broilers exposed to ochratoxin A.
Immunological indices
Effect of dietary Spirulina supplement on some immunological indices of broilers exposed to ochratoxin A illustrated in Table 8. The finding revealed a nonsignificant variation (p = 0.081) in phagocytic activity and group 3 supplemented with Spirulina showed an increased level of phagocytic activity (41.1) while group 2 supplemented with ochratoxin revealed decreased levels of phagocytic activity (24.94) relative to control and other treatments. Moreover, group 3 treated with Spirulina illustrated a significant (p = 0.003) elevation of the phagocytic index (0.50) while group 2 supplemented with ochratoxin revealed significantly decreased levels of the phagocytic index (0.20) relative to control and other treatments.
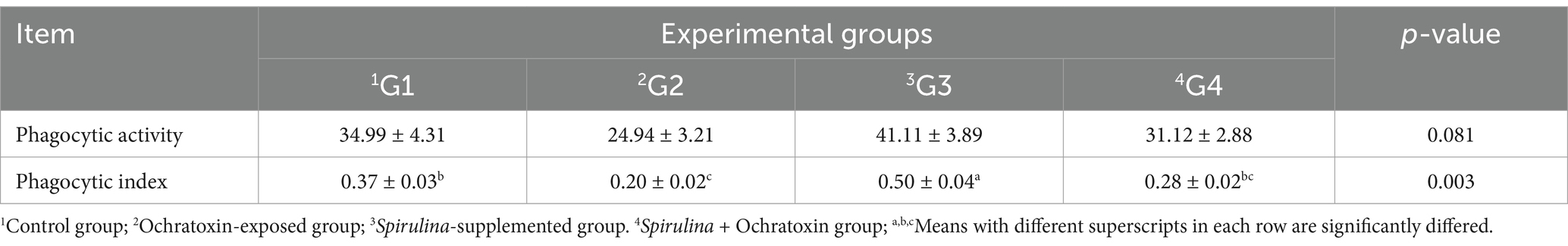
Table 8. Effect of dietary Spirulina supplement on some immunological indices of broilers exposed to ochratoxin A.
Pathological and immunohistochemical staining
Bursa Fabricius
The control group showed normal folds each fold contains tightly packed lymphoid follicles (arrowheads) divided by connective tissue (CT) and covered by pseudostratified columnar epithelium. Compared to the control group, OTA fed group illustrated a loss of typical bursal architecture, cortical layer thinning, and medullary lymphoid depletion with lymphocytolysis. Some interfollicular edema was noticed. There was extensive loss of the covering epithelium with exposure to the underlying connective tissue. There were no alterations in SP fed group. Prominent amelioration of these changes in the OTA + SP group by the absence of edema and mild depletion of lymphoid follicles (Figures 1A–D). Regarding immunohistochemical reaction to PCNA, no reactions were detected in bursal tissues for the control and SP group. Strong positive reactions for PCNA in lymphoid follicles and follicle-associated epithelium were seen in the OTA group. Only mild reactions in lymphoid follicles and follicle-associated epithelium of the OTA + SP group were revealed (Figures 1E–H).
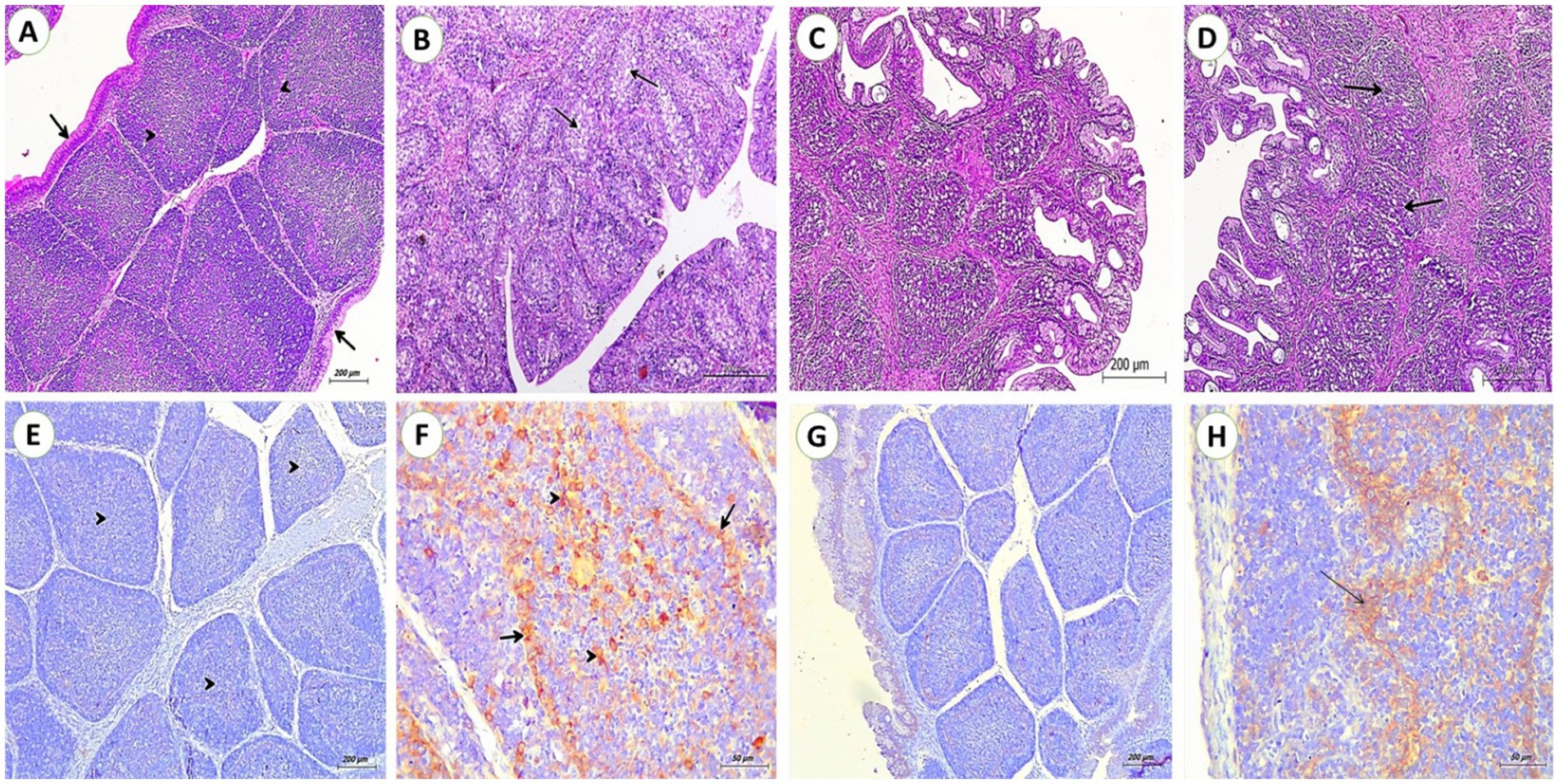
Figure 1. Bursa from broilers experimentally fed ochratoxin. (A,C) From control and SP groups showed folds Each fold contains tightly packed lymphoid follicles (arrow heads) separated by connective tissue and covered by pseudostratified columnar epithelium (arrow) (H&E, scale bar: 200). (B) From OTA group showed depletion of lymphoid follicles and extensive loss of the covering epithelium with exposing for the underlaying connective tissue. (H&E, scale bar: 200). (D) From OTA + SP group showed mild depletion of lymphoid follicles (H&E, scale bar: 200). (E,G) From control and SP groups showed no positive reaction for PCNA (IHC stain, scale bar: 200). (F) From OTA group showed a strong positive reaction for PCNA in lymphoid follicles (arrows heads) and follicle-associated epithelium (arrows) (IHC stain, scale bar: 50). (H) Mild positive reaction for PCNA in lymphoid follicles and follicle-associated epithelium of OTA + SP group (arrows) (IHC stain, scale bar: 50).
Thymus
Both control and SP groups showed normal tissue architecture, connective tissue capsule, and septa partially separate the cortex, ending at the cortico-medullary junction. This area contains a high concentration of lymphocytes, resulting in a deep basophilic staining of the cortex. Relative to the control group, the OTA group showed severe tissue alterations; thymic lobular atrophy, nuclear fragmentation or even lysis, extensive hemorrhages, and necrosis all over the cortex and medulla. For the OTA + SP group, most microscopic changes were improved, only congestion and focal depletion of lymphocyte populations were detected (Figures 2A–D). Regarding immunohistochemical reaction to PCNA, no reactions were detected in thymus tissues for the control and SP group. A strong positive reaction for PCNA in lymphocyte populations was seen in the OTA group. Only mild reactions in lymphocyte populations of the OTA + SP group were detected (Figures 2E–H).
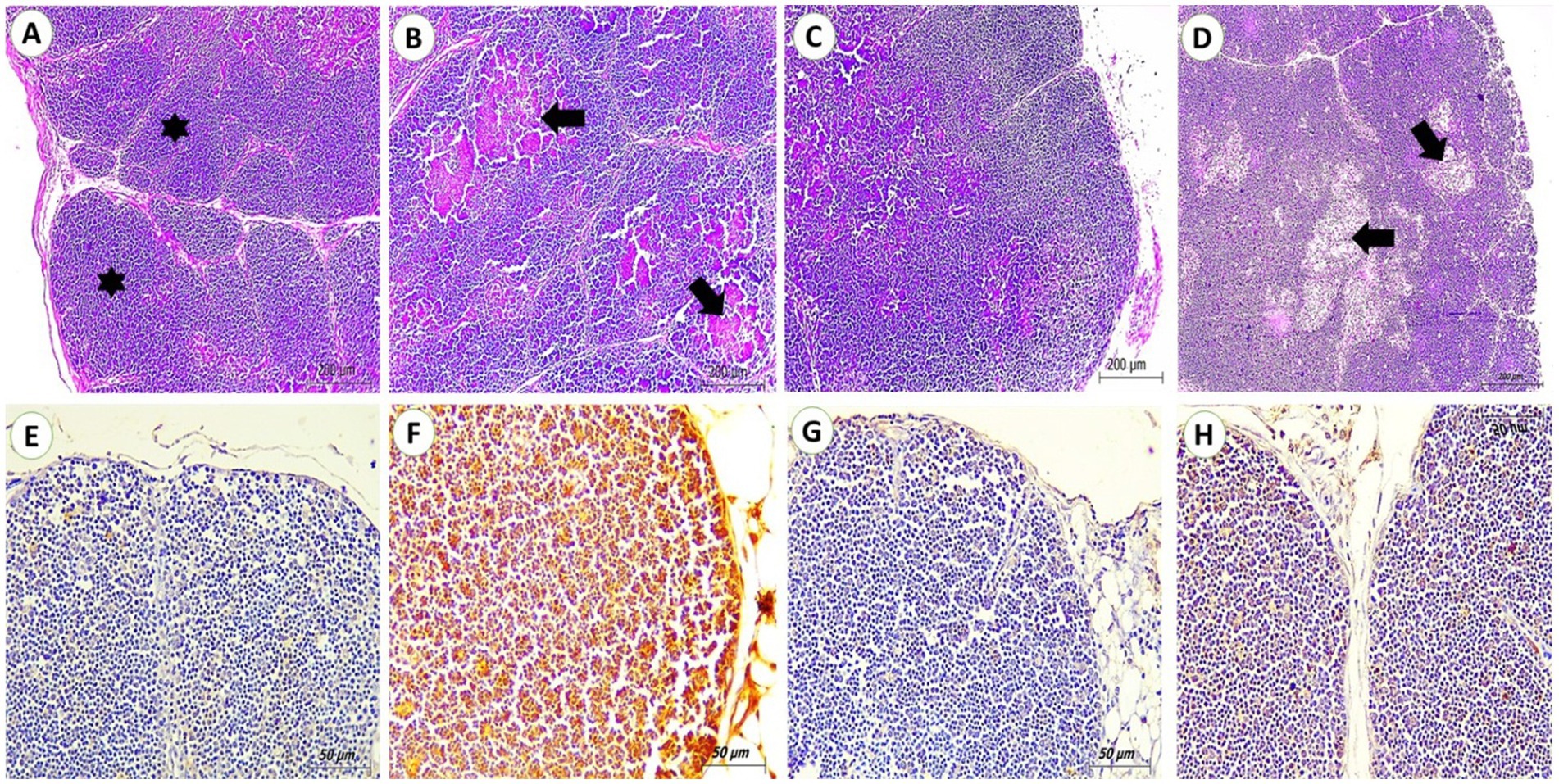
Figure 2. Thymus from broilers experimentally fed ochratoxin. (A,C) From control and SP groups showed connective tissue capsule and Septa from the capsule incompletely divide the cortex and end at the cortico-medullary junction with the dense lymphocyte population (stars) giving the cortex deep basophilic staining. (H&E, scale bar: 200). (B) From OTA group showed extensive hemorrhages and necrosis all over the cortex and medulla (arrows). (H&E, scale bar: 200). (D) From OTA + SP group showed focal depletion of lymphocyte populations (arrows) (H&E, scale bar: 200). (E,G) From control and SP groups showed no positive reaction for PCNA (IHC stain, scale bar: 50). (F) From OTA group showed a strong positive reaction for PCNA in lymphocyte populations (IHC stain, scale bar: 50). (H) Mild positive reaction for PCNA in lymphocyte populations of OTA + SP group (arrows) (IHC stain, scale bar: 50).
Discussion
Research has shown that OTA poisoning can negatively impact the gastrointestinal system of hens, resulting in reduced food absorption and thus hindering their normal growth. Broiler hens that were given ochratoxin A (OTA) at concentrations of 20 or 50 μg/kg body weight (BW) experienced a reduction in both their overall body weight and weight gain (37, 38). The results of this study elaborated that supplementing Spirulina to the diet could successfully decrease the negative effect of OTA toxicity on broiler performance. The beneficial impacts of Spirulina are most likely due to its abundant concentration of antioxidants, vitamins, and other bioactive substances (39). These substances can counteract the harmful effects of oxidative stress and tissue damage produced by exposure to OTA. These data show that when chicks are fed a diet treated with up to 1 g/kg diet SP, their growth performance improves. These findings concur with those of Abou-Zeid et al. (40), who discovered that birds given a diet with 2 g of Spirulina per kg had superior body weight means. Spirulina enhances the assimilation of minerals, safeguards against diarrhea, and optimizes the process of nutrient digestion. The beneficial effects of adding Spirulina to the diet of broiler chickens can be recognized due to its high levels of amino acid digestibility and metabolizable energy (39). This is particularly important considering the negative effect of heat stress on the structure of the intestines and the amount of feed consumed (41).
The findings of our research revealed that supplementing Spirulina in the diet might effectively alleviate the negative impacts of OTA stress on broiler carcass characteristics. The findings of this trail align with the findings of Abou-Zeid et al. (40), who found that birds who were given a meal containing 2 g of Spirulina per kg showed a notable variation in carcass and abdominal fat %. However, there was no significant variation in liver, heart, or gizzard percentage across the different groups.
The outcomes of this trial indicate that adding Spirulina to the diet can successfully mitigate the adverse impacts of OTA stress on blood parameters, and antioxidant levels. Consistent with the findings of the current investigation by Pestana et al. (42), there was a significant elevation in total protein concentration (p ≤ 0.001) relative to the other groups of birds. The improved digestibility of protein seen in diets supplemented with Spirulina may be attributed to enhanced absorption, leading to increased development in broiler chickens (23). These findings agree with the results of former studies applied by Fathi (43) and Opoola et al. (44). They observed that chickens receiving diets containing Spirulina at doses of 6, 12, and 18 g/kg had significantly increased levels of globulin, glucose, and total protein relative to those on a control diet.
The scientists proposed that the elevated levels of serum protein, globulin, and albumin could be attributed to the superior protein content and the amount of Spirulina platensis, which is abundant in phycocyanin and polyunsaturated fatty acids (45). The plasma lipids profile revealed that the levels of plasma cholesterol and total lipids were reduced in all supplemented groups relative to the control group. Abdel-Hady and EI-Ghalid (46) noticed similar findings when they discovered that treating a broiler diet with 3 and 6% Spirulina led to a substantial reduction in serum concentration of total lipid, triglyceride, cholesterol, and low-density lipoprotein in both experimental groups.
The inclusion of Spirulina in the diet of birds resulted in a reduction in their blood lipid profile. The concentration of high-density lipoprotein (HDL) in broilers increased significantly (p ≤ 0.05) when they were given 1 g of Spirulina. Spirulina has been found to reduce cholesterol concentration in the blood by affecting the metabolism of lipoproteins and increasing the activity levels of lipoprotein enzymes. This hypocholesterolemic effect is achieved by lowering both plasma and liver cholesterol levels through the increased action of lipoprotein lipase and hepatic triglyceride lipase (45). The liver is considered as the primary metabolic organ in the body (47). It demonstrates the hepatoprotective impacts of Spirulina, which can be attributed to its antioxidative and anti-inflammatory properties (48). The concentration of AST and ALT showed a significant decrease in the Spirulina groups. These results align with the results reported by Abaza et al. (49) and Jamil et al. (48), they showed that the activity of ALT and AST decreased significantly in all treatment groups that were supplemented with Spirulina. Similarly, Zeweil et al. (50) found that supplementing chickens with Spirulina at levels of 0.5 and 1 g/kg in their diet decreased the adverse impacts of heat stress on ALT and AST levels. There was also a slight reduction in plasma ALP and activities of liver enzymes, but these levels stayed within the normal range observed in the present study, suggesting normal liver function. The supplemented groups exhibited a reduction in ALP, ALT, and AST concentration. The finding by Abdel-Daim et al. (51) illustrated that Spirulina has a hepatoprotective effect, which can restore the normal concentration of liver enzymes and improve liver health. The rise in serum ALP, ALT, and AST activity has been linked to physiological stressful conditions (52) such as ochratoxicosis. This contradicts the results of Sugiharto et al. (53), who revealed that administering supplements containing 1% Spirulina platensis for seven, 21, and 35 days had no significant influence on AST and ALT levels. The conflicting outcomes of the research may be attributed, to some part, to the variation in the nutritional and functional characteristics of the Spirulina platensis utilized. Moreover, Spirulina supplementation decreased the adverse effect of OTA on the kidney function tests and decreased the uric acid and creatinine in the blood relative to other groups and controls. on the other hand, our findings agree with Li et al. (54) who applied a study to investigate the influence of adding OTA to the feed of birds at a concentration of 50 g/kg. They noticed that the presence of OTA resulted in elevated MDA concentration in the kidneys, whereas the TAC was decreased. In addition, the concentration of SOD, CAT, and GPx was significantly reduced. The results indicate that OTA induces the production of reactive oxygen species, resulting in oxidative stress in the kidneys of birds (55).
The findings demonstrated that the addition of Spirulina effectively alleviated the adverse effects of ochratoxin on some immunological parameters in broiler chickens, and boosted phagocytic activity and phagocytic index relative to control and other groups. Our results align with (25, 56, 57) who reported that Spirulina has demonstrated a distinct effect on monocytes and natural killer (NK) cells, which are vital constituents of the innate immune system. The supplementation of Spirulina has been shown to improve the phagocytic response of macrophages and the action of natural killer (NK) cells in both chickens and humans.
The results of this study illustrated that supplementing Spirulina to the broiler’s diet can successfully mitigate the adverse effect of OTA toxicosis on broiler pathological changes of lymphoid organs such as the bursa and thymus. Lymphocyte depletion, necrosis, and hemorrhage were observed in the OTA group. Chickens with underdeveloped bursal follicles are known to have heightened susceptibility to bacterial (11, 58) and viral (59) infections. Because lymphoid organs have an important role in humoral immunological responses. Therefore, these histological abnormalities have the potential to negatively impact the humoral immune function in hens following exposure to OTA. Our finding illustrated that OTA caused the rise of PCNA proteins in the bursa and thymus, that essential for replication. It acts as a scaffold to recruit proteins involved in DNA replications. Since PCNA requires ubiquitination to carry out its biological role, the increase in PCNA levels cannot definitively be attributed to its enhanced functionalities (36).
Conclusion
In conclusion, the administration of Spirulina (1 g/kg diet) in broiler chickens fed on ochratoxin A (1 mg/kg diet) contaminated diet or as a standalone supplement illustrated a decrease in the adverse impacts of ochratoxin A, additionally improving the growth performance, antioxidant activity, liver and kidney function, immune response, of broiler chicken. Using Spirulina up to 1 g/kg of diet can be beneficial and a good strategy in improving health, performance and solving the OTA problem in poultry farms. From our results, the future of Spirulina as a functional supplement looks promising due to its good benefits. However, before this novel additive can be widely used in poultry diets, large-scale commercial production of Spirulina for the feed sector must be established.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The animal study was approved by the ethical statement conformed to the guidelines set up by the Ethical Committee of the Egyptian Research for the Use and Care of Laboratory Animals, as established by New Valley University (NVREC/02/3/5/2024/17). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AS: Conceptualization, Investigation, Software, Writing – original draft. ME-T: Data curation, Formal analysis, Writing – original draft. MMo: Data curation, Methodology, Software, Writing – original draft. RZ: Data curation, Formal analysis, Project administration, Writing – original draft. MAz: Funding acquisition, Resources, Writing – original draft. OE: Writing – original draft, Writing – review & editing. MAl: Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. AE: Writing – original draft, Writing – review & editing. MMa: Writing – original draft, Writing – review & editing. AF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This work was supported by the Researchers Supporting Project (RSPD2025R731), King Saud University (Riyadh, Saudi Arabia).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tahir, MA, Abbas, A, Muneeb, M, Bilal, RM, Hussain, K, Abdel-Moneim, A-ME, et al. Ochratoxicosis in poultry: occurrence, environmental factors, pathological alterations and amelioration strategies. Worlds Poult Sci J. (2022) 78:727–49. doi: 10.1080/00439339.2022.2090887
2. Abd El-Hack, ME, Alagawany, M, Salah, AS, Abdel-Latif, MA, and Farghly, MF. Effects of dietary supplementation of zinc oxide and zinc methionine on layer performance, egg quality, and blood serum indices. Biol Trace Elem Res. (2018) 184:456–62. doi: 10.1007/s12011-017-1190-0
3. Farghly, MF, Ahmad, EA, Alagawany, M, Abd El-Hack, ME, Ali, RA, Elnesr, SS, et al. Use of some nutritional supplements in drinking water of growing turkeys during 1st month of age and their effect on performance, meat quality, blood profile, and antioxidant status. J Anim Physiol Anim Nutr. (2018) 102:1625–33. doi: 10.1111/jpn.12988
4. Aboubakr, M, Elmahdy, AM, Taima, S, Emam, MA, Farag, A, Alkafafy, M, et al. Protective effects of N acetylcysteine and vitamin E against acrylamide-induced neurotoxicity in rats. Pak Vet J. (2023) 43:262–8. doi: 10.29261/pakvetj/2023.027
5. Mevliyaoğulları, E, Karslı, MA, and Mert, B. Utilizing surplus bread as an ingredient in dog food: evaluating baking and extrusion processing on physicochemical properties and in vitro digestibility performance. J Cereal Sci. (2023) 113:103741. doi: 10.1016/j.jcs.2023.103741
6. Mevliyaoğulları, E, Demirci, M, and Karsli, MA. Stock selection and nutrition in canaries. J Erciyes Univ Faculty Vet Med. (2021) 18:122–8. doi: 10.32707/ercivet.95320
7. Atalla, S, Youssef, MA, Ebraheem, EM, El-Diasty, M, and Rizk, MA. Effect of prebiotic and spirulina on blood gas parameters and acute phase proteins in dairy cattle with sub-acute ruminal acidosis. Int J Vet Sci. (2023) 12:24–30. doi: 10.47278/journal.ijvs/2022.149
8. Mehmood, A, Nawaz, M, Rabbani, M, and Mushtaq, MH. Probiotic effect of Limosilactobacillus fermentum on growth performance and competitive exclusion of Salmonella gallinarum in poultry. Pak Vet J. (2023) 43:659–64. doi: 10.29261/pakvetj/2023.103
9. Rashid, S, Tahir, S, Akhtar, T, Ashraf, R, Altaf, S, and Qamar, W. Bacillus-based probiotics: an antibiotic alternative for the treatment of salmonellosis in poultry. Pak Vet J. (2023) 43:167–73. doi: 10.29261/pakvetj/2023.017
10. Salah, AS, Ahmed-Farid, OA, and El-Tarabany, MS. Carcass yields, muscle amino acid and fatty acid profiles, and antioxidant indices of broilers supplemented with synbiotic and/or organic acids. J Anim Physiol Anim Nutr. (2019) 103:41–52. doi: 10.1111/jpn.12994
11. Abu El Hammed, W, Soufy, H, El-Shemy, A, Fotouh, A, Nasr, SM, and Dessouky, MI. Prophylactic effect of oregano in chickens experimentally infected with avian pathogenic Escherichia coli O27 with special reference to hematology, serum biochemistry, and histopathology of vital organs. Egypt J Chem. (2022) 65:269–82. doi: 10.21608/EJCHEM.2021.102510.4754
12. Mohamed, RS, Attia, AI, El-Mekkawy, MM, Ismail, FS, Salah, AS, Nicotra, M, et al. Leverage of Matricaria chamomilla L. oil supplementation over Ochratoxin A in growing quails. J Food Quality. (2024) 2024:7254066. doi: 10.1155/2024/7254066
13. Reda, FM, Alagawany, M, Alsolami, AM, Mahmoud, HK, Salah, AS, Momenah, MA, et al. The incorporation of sumac seed powder (Rhus coriaria L.) into the diet of quail breeders as a novel feed additive. Poult Sci. (2024) 103:103593. doi: 10.1016/j.psj.2024.103593
14. Ahmed-Farid, OA, Salah, AS, Nassan, MA, and El-Tarabany, MS. Performance, carcass yield, muscle amino acid profile, and levels of brain neurotransmitters in aged laying hens fed diets supplemented with guanidinoacetic acid. Animals. (2021) 11:3091. doi: 10.3390/ani11113091
15. AL-Ruwad, SH, Attia, AI, Monem, UMA, Abdel-Maksoud, A, Thagfan, FA, Alqahtani, HA, et al. Dietary supplementation with copper nanoparticles (CuNP) enhances broiler performance by improving growth, immunity, digestive enzymes and gut microbiota. Poult. Sci. (2024) 103:104026. doi: 10.1016/j.psj.2024.104026
16. Demirci, M, and Mevliyaoğulları, E. Comparative nutritional analysis of domestic and imported commercial canary egg food and mixed seeds based diets. Brazil J Poultry Sci. (2023) 25:1–10. doi: 10.1590/1806-9061-2023-1809
17. El-Tarabany, MS, Nassan, MA, and Salah, AS. Royal jelly improves the morphology of the reproductive tract, internal egg quality, and blood biochemical parameters in laying hens at the late stage of production. Animals. (2021) 11:1861. doi: 10.3390/ani11071861
18. Reda, FM, Alagawany, M, Salah, AS, Mahmoud, MA, Azzam, MM, Di Cerbo, A, et al. Biological selenium nanoparticles in quail nutrition: biosynthesis and its impact on performance, carcass, blood chemistry, and cecal microbiota. Biol Trace Elem Res. (2023) 202:4191–202. doi: 10.1007/s12011-023-03996-3
19. Khatoon, A, Nawaz, MY, Mehboob, G, Saleemi, MK, Gul, ST, Abbas, RZ, et al. Unraveling the combined deleterious effects of ochratoxin a and atrazine upon broiler’s health: toxicopathological, serum biochemical and immunological perspectives. Toxicon. (2023) 236:107327. doi: 10.1016/j.toxicon.2023.107327
20. Fries-Craft, K, Meyer, MM, and Bobeck, EA. Algae-based feed ingredient protects intestinal health during Eimeria challenge and alters systemic immune responses with differential outcomes observed during acute feed restriction. Poult Sci. (2021) 100:101369. doi: 10.1016/j.psj.2021.101369
21. Mirzaie, S, Zirak-Khattab, F, Hosseini, SA, and Donyaei-Darian, H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian Australas J Anim Sci. (2018) 31:556–63. doi: 10.5713/ajas.17.0483
22. Abd Elzaher, HA, Ibrahim, ZA, Ahmed, SA, Salah, AS, Osman, A, Swelum, AA, et al. Growth, carcass criteria, and blood biochemical parameters of growing quails fed Arthrospira platensis as a feed additive. Poult Sci. (2023) 102:103205. doi: 10.1016/j.psj.2023.103205
23. Park, JH, Lee, SI, and Kim, IH. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult Sci. (2018) 97:2451–9. doi: 10.3382/ps/pey093
24. El-Desoky, GE, Bashandy, SA, Alhazza, IM, Al-Othman, ZA, Aboul-Soud, MA, and Yusuf, K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS One. (2013) 8:59177. doi: 10.1371/journal.pone.0059177
25. Qureshi, MA, Garlich, JD, and Kidd, MT. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharm Immunot. (1996) 18:465–76. doi: 10.3109/08923979609052748
26. El-Shall, NA, Jiang, S, Farag, MR, Azzam, M, Al-Abdullatif, AA, Alhotan, R, et al. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front Immunol. (2023) 14:1072787. doi: 10.3389/fimmu.2023.1072787
27. Elbaz, AM, Ahmed, AM, Abdel-Maqsoud, A, Badran, AM, and Abdel-Moneim, AME. Potential ameliorative role of Spirulina platensis in powdered or extract forms against cyclic heat stress in broiler chickens. Environ Sci Pollut R. (2022) 29:45578–88. doi: 10.1007/s11356-022-19115-z
28. Khalilnia, F, Mottaghitalab, M, Mohiti, M, and Seighalani, R. Effects of dietary supplementation of probiotic and Spirulina platensis microalgae powder on growth performance immune response, carcass characteristics, gastrointestinal microflora and meat quality in broilers chick. Vet Med Sci. (2023) 9:1666–74. doi: 10.1002/vms3.1154
29. Mohamed, RS, Alagawany, M, Attia, AI, Ismail, FS, Salah, AS, Di Cerbo, A, et al. The role of chamomile oil against ochratoxin A in quail breeders: productive and reproductive performances, egg quality, and blood metabolites. Poult Sci. (2024) 103:103440. doi: 10.1016/j.psj.2024.103440
30. Abo-Aziza, FA, Zaki, AKA, Adel, RM, and Fotouh, A. Amelioration of aflatoxin acute hepatitis rat model by bone marrow mesenchymal stem cells and their hepatogenic differentiation. Vet World. (2022) 15:1347–64. doi: 10.14202/vetworld.2022.1347-1364
31. Barkallah, M, Ben Atitallah, A, Hentati, F, Dammak, M, Hadrich, B, Fendri, I, et al. Effect of Spirulina platensis biomass with high polysaccharides content on quality attributes of common carp (Cyprinus carpio) and common Barbel (Barbus barbus) fish burgers. Appl Sci. (2019) 9:2197. doi: 10.3390/app9112197
32. AOAC. Official methods of analysis. 15th ed. Washington DC: Association of Official Analytical Chemists (1990).
33. National Research Council and Subcommittee on Poultry Nutrition. Nutrient requirements of poultry: 1994. Washington: National Academies Press (1994).
34. Elbarbary, NK, Abdelmotilib, NM, Gomaa, RA, Elnoamany, F, Fotouh, A, Noseer, EA, et al. Impact of thawing techniques on the microstructure, microbiological analysis, and antioxidants activity of Lates niloticus and Mormyrus kannume fish fillets. Egypt J Aquat Res. (2023) 49:530–6. doi: 10.1016/j.ejar.2023.10.004
35. Fotouh, A, Abdel-Maguid, DS, Abdelhaseib, M, Zaki, RS, and Darweish, M. Pathological and pharmacovigilance monitoring as toxicological imputations of azithromycin and its residues in broilers. Vet World. (2024) 17:1271–80. doi: 10.14202/vetworld.2024.1271-1280
36. Hu, P, Zuo, Z, and Li, H. The molecular mechanism of cell cycle arrest in the Bursa of Fabricius in chick exposed to aflatoxin B1. Sci Rep. (2018) 8:1770. doi: 10.1038/s41598-018-20164-z
37. El Cafsi, I, Bjeoui, S, Rabeh, I, Nechi, S, Chelbi, E, El Cafsi, M, et al. Effects of ochratoxin A on membrane phospholipids of the intestine of broiler chickens, practical consequences. Animal. (2020) 14:933–41. doi: 10.1017/S1751731119002593
38. Solcan, C, Pavel, G, Floristean, V, Chiriac, I, Şlencu, B, and Solcan, G. Effect of ochratoxin A on the intestinal mucosa and mucosa-associated lymphoid tissues in broiler chickens. Acta Vet Hung. (2015) 63:30–48. doi: 10.1556/avet.2015.004
39. Lestingi, A, Alagawany, M, Di Cerbo, A, Crescenzo, G, and Zizzadoro, C. Spirulina (Arthrospira platensis) used as functional feed supplement or alternative protein source: a review of the effects of different dietary inclusion levels on production performance, health status, and meat quality of broiler chickens. Life. (2024) 14:1537. doi: 10.3390/life14121537
40. Abou-Zeid, AE, El-Damarawy, SZ, Mariey, YA, and El-Mansy, MM. Effect of using spirulina platensis and/or chlorella vulgaris algae as feed additives on productive performance of broiler chicks. J Anim Poult. (2015) 6:623–34. doi: 10.21608/jappmu.2015.52940
41. Levine, R, Horst, G, Tonda, R, Lumpkins, B, and Mathis, G. Evaluation of the effects of feeding dried algae containing beta-1,3-glucan on broilers challenged with eimeria. Poult Sci. (2018) 97:3494–500. doi: 10.3382/ps/pey227
42. Pestana, JM, Puerta, B, Santos, H, Madeira, MS, Alfaia, CM, Lopes, PA, et al. Impact of dietary incorporation of spirulina (Arthrosira plattensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult Sci. (2020) 99:2519–32. doi: 10.1016/j.psj.2019.11.069
43. Fathi, MA. Effect of dietary supplementation of algae meal (Spirulina platensis) as growth promoter on performance of broiler chickens. EPSJ. (2018) 38:375–89.
44. Opoola, E, Makinde, OJ, and Lawal, AN. Effect of Spirulina platensis supplementation on performance, haematological and serum biochemical profiles of broiler chickens reared under tropical environment. Niger J Sci. (2019) 21:352–60.
45. Hassanein, HAM, Arafa, MM, Warda, MAA, and Abd-Elall, AA. Effect of using spirulina platensis and chlorella vulgaris as feed additives on growing rabbit performance. Egypt J Rabbit Sci. (2014) 24:413–31. doi: 10.21608/ejrs.2014.47489
46. Abd El-Hady, AM, and El-Ghalid, OAH. Spirulina platensis algae (SPA): a novel poultry feed additive. Effect of SPA supplementation in broiler chicken diets on productive performance, lipid profile and calcium-phosphorus metabolism. Worlds Poult Sci J. (2018) 1-7:7498.
47. Hatipoglu, D, Ates, MB, Bulut, A, Gezgic, B, and Dik, B. Efficacy of resatorvid and alpha-lipoic acid in ameliorating gentamicin-induced liver injury: insights from rat model study. Pak Vet J. (2024) 44:931–8. doi: 10.29261/pakvetj/2024.222
48. Jamil, AR, Akanda, MR, Rahman, MM, Hossain, MA, and Islam, MS. Prebiotic competence of spirulina on the production performance of broiler chickens. J Adv Vet Anim Res. (2015) 2:304–9. doi: 10.5455/javar.2015.b94
49. Abaza, I, Helal, AM, and Sherif, EY. Antioxidant status, growth and some physiological performances of the Gimmizah male chickens supplemented by dietary Spirulina platensis under chronic heat stress. JDEA. (2021) 1:35–49. doi: 10.21608/JDEA.2021.53384.1004
50. Zeweil, H, Abaza, IM, Zahran, SM, Ahmed, MH, Aboul-Fla, MH, and Asmaa, AS. Effect of spirulina platensis as dietary supplement on some biological traits for chickens under heat stress conditions. Asian J Biomed Pharm Sci. (2019) 6:8–12.
51. Abdel-Daim, MM, Farouk, SM, Madkour, FF, and Azab, SS. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharm Immunot. (2015) 37:126–39. doi: 10.3109/08923973.2014.998368
52. Karabekir, SC, Gultekın, B, Ayan, IC, Savas, HB, Cuce, G, and Kalkan, S. Protective effect of astaxanthin on histopathologic changes induced by bisphenol a in the liver of rats. Pak Vet J. (2024) 44:244–51. doi: 10.29261/pakvetj/2024.178
53. Sugiharto, S, Yudiarti, T, Isroli, I, and Widiastuti, E. Effect of feeding duration of spirulina platensis on growth performance, haematological parameters, intestinal microbial population and carcass traits of broiler chicks. S Afr J Anim Sci. (2018) 48:98–107. doi: 10.4314/sajas.v48i1.12
54. Li, K, Cao, Z, Guo, Y, Tong, C, Yang, S, Long, M, et al. Selenium yeast alleviates Ochratoxin A-induced apoptosis and oxidative stress via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in the kidneys of chickens. Oxidative Med Cell Longev. (2020) 2020:1–12. doi: 10.1155/2020/4048706
55. Kövesi, B, Cserháti, M, Erdélyi, M, Zándoki, E, Mézes, M, and Balogh, K. Long-term effects of ochratoxin A on the glutathione redox system and its regulation in chicken. Antioxidants. (2019) 8:178. doi: 10.3390/antiox8060178
56. Al-Batshan, HA, Al-Mufarrej, SI, Al-Homaidan, AA, and Qureshi, MA. Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharm Immunot. (2001) 23:281–9. doi: 10.1081/IPH-100103866
57. Hirahashi, T, Matsumoto, M, Hazeki, K, Saeki, Y, Ui, M, and Seya, T. Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int J Immunopharmacol. (2002) 2:423–34. doi: 10.1016/S1567-5769(01)00166-7
58. Soufy, H, Gab-Allah, MS, Tantawy, AA, Fotouh, A, and Nasr, SM. Pathogenesis of experimental Salmonella Gallinarum infection (fowl typhoid) in broiler chicks. Egypt J Vet Sci. (2016) 47:1–15. doi: 10.21608/ejvs.2016.1098
Keywords: broilers, OTA, Spirulina platensis, amelioration, mitigation, growth, blood
Citation: Salah AS, El-Tarabany MS, Mostafa M, Zaki RS, Azzam MM, El Euony OI, Alagawany M, Lestingi A, Elolimy AA, Madkour M and Fotouh A (2025) Impact of dietary Spirulina on performance, antioxidant status, carcass traits and pathological alteration in broilers exposed to ochratoxin A stress. Front. Vet. Sci. 11:1532353. doi: 10.3389/fvets.2024.1532353
Edited by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeReviewed by:
Aisha Khatoon, University of Agriculture, Faisalabad, PakistanKarima El-Naggar, Alexandria University, Egypt
Ercan Mevliyaoğullari, Middle East Technical University, Türkiye
Copyright © 2025 Salah, El-Tarabany, Mostafa, Zaki, Azzam, El Euony, Alagawany, Lestingi, Elolimy, Madkour and Fotouh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed A. Elolimy, ZWxvbGlteUB1YWV1LmFjLmFl
 Ayman S. Salah1
Ayman S. Salah1 Mahmoud S. El-Tarabany
Mahmoud S. El-Tarabany Mahmoud M. Azzam
Mahmoud M. Azzam Mahmoud Alagawany
Mahmoud Alagawany Ahmed A. Elolimy
Ahmed A. Elolimy Mahmoud Madkour
Mahmoud Madkour