- Infectious Bacterial Diseases of Livestock Research Unit, National Animal Disease Center, Agricultural Research Service, United States Department of Agriculture, Ames, IA, United States
Introduction: Brucellosis is endemic in bison and elk in Yellowstone National Park and surrounding areas.
Methods: A comparative study was conducted using data from naive (n = 82 and 67, respectively) and Brucella abortus strain RB51 (RB51) vaccinated (n-99 and 29, respectively) bison and elk experimentally challenged with virulent B. abortus strain during pregnancy.
Results: The incidence of abortion, fetal infection, uterine or mammary infection, or infection in maternal tissues after experimental challenge was greater (p < 0.05) in naïve and vaccinated bison when compared to similar groups in elk. Vaccinated bison had lower (p < 0.002) abortion rates and recovery of Brucella from fetal or uterine/mammary tissues when compared to naïve bison. Vaccinated elk had reduced (p < 0.01) rates of maternal infection, but rates of abortion and fetal or uterine/mammary infection did not differ (p > 0.05) from naïve elk. Naïve and vaccinated bison had greater (p < 0.05) Brucella colonization in placentomes, and parotid and supramammary lymphatic tissues when compared to elk. In elk or bison that aborted, mean colonization in placentome tissues were typically more than 5 logs higher than in animals that did not abort.
Discussion: The results of our study suggest differences in disease pathogenesis between these two wildlife reservoirs of B. abortus.
Introduction
Although elimination of Brucella abortus from cattle was essentially achieved in most of the United States by the early 2000s, the maintenance of the disease in wildlife reservoirs in the states of Idaho, Montana, and Wyoming has impaired the total eradication of brucellosis from cattle. In those states, free-ranging bison (Bison bison) and elk (Cervus elaphus nelsoni) have been known for many years to be infected with brucellosis (1917 and 1930, respectively) (1, 2). Management of the two species for brucellosis control differs as free-ranging bison predominantly reside within Yellowstone National Park and elk are more widely distributed. It should also be noted that elk populations in the area are greater and more widespread than bison, with disease control measures impacted by the congregation of elk on 22 states and 1 federal winter feed grounds (3). Both wildlife reservoirs in this area maintain high seroprevalence for brucellosis (historically approximately 35% for female elk on feed grounds and 50–60% for bison in Yellowstone National Park) (3–5), and disease prevalence in elk appears to be expanding outward at a rate of 5–8 km/year (6–8). Historical and epidemiologic data suggest that initial infection in these wildlife hosts resulted from lateral transmission of brucellosis from domestic livestock.
During winter months, the migration of bison out of Yellowstone National Park and the movement of elk to lower elevations can lead to interactions with domestic livestock, resulting in the transmission of brucellosis. In the areas surrounding Yellowstone National Park, state and federal agencies maintain vaccination policies and serologic testing for all animals grazing in areas with high brucellosis seroprevalence in wildlife. Despite expensive control measures, disease transmission from wildlife reservoirs has resulted in the identification of approximately 2.5 infected cattle herds each year in the region for the last decade. At the present time, all new infected cattle herds have been epidemiologically linked to the transmission of disease from free-ranging elk (9, 10). Despite efforts to prevent wildlife-cattle interactions during feeding during winter months, epidemiologic implications that elk are the source of infection may suggest that current management practices to maintain temporal and spatial segregation between domestic livestock and bison are effective.
Billions of dollars have been spent on the brucellosis eradication program in the United States since its inception in the 1930s as a drought recovery program (11, 12). Current disease control efforts in the Greater Yellowstone Area are expensive and require long-term commitments as they primarily target domestic livestock and do not reduce disease prevalence in wildlife reservoirs. Improving control of brucellosis in the area will require initiating efforts to reduce disease prevalence in free-ranging wildlife reservoirs. We have previously demonstrated phenotypic differences in susceptibility to brucellosis between bison and cattle (13), and also reported that elk fail to develop cellular immune responses after brucellosis vaccination (14). To provide basic data needed for the development of successful intervention strategies, we conducted a meta-analysis of data from multiple challenge studies to document phenotypic differences between naïve and B. abortus strain RB51-vaccinated bison and elk after experimental infection with B. abortus strain 2308 during gestation. These data demonstrate immunologic and pathophysiologic differences between the two species, emphasizing the need for infectious disease research to be conducted in the species of interest. Some of these data have been previously presented in other manuscripts (15–19).
Materials and methods
Vaccination
For vaccination, elk and bison were vaccinated intramuscularly with 1–6 × 1010 colony-forming units (CFU) of B. abortus strain RB51 (RB51) between 4 and 12 months of age. For this study, data from naïve or single-vaccinated bison and elk from independent efficacy studies (17 and 8, respectively) were compared [(15–18, 20–22) and unpublished data].
Breeding and experimental challenge
Bison and elk heifers were obtained from brucellosis-free herds and housed at the National Animal Disease Center in Ames, IA, during the study. All animals were pasture-bred at approximately 2 1/2 years of age. Pregnancy status and stage of gestation in bison were determined by rectal palpation. In elk, pregnancy was confirmed by serologic measurement of pregnancy-specific protein B. Pregnant bison and elk were transferred to a biolevel 3 containment facility 2 to 3 weeks prior to experimental challenge where they were housed during the study. Bison and elk were experimentally infected with 107 CFUs of B. abortus strain 2308 administered bilaterally to conjunctival surfaces (50 μL per eye) at an estimated 12 weeks prior to parturition. Data were also included from two studies in which additional pregnant elk were experimentally challenged with 108 CFUs of B. abortus strain 2308 at a similar time in gestation. Concentrations of viable bacteria within each challenge were determined by serial dilution in saline and standard plate counts.
Bison and elk were euthanized within 1 week of the anticipated parturition date or within 72 h of abortion or parturition by intravenous injection of pentobarbital sodium. Fetal viability was assessed in both elk and bison by calf mobility and demonstration of milk in the stomach at necropsy. Maternal samples obtained at necropsy for bacteriologic evaluation using sterile forceps and a scalpel included lymphatic tissues (bronchial, hepatic, internal iliac, mandibular, mesenteric, parotid, prescapular, retropharyngeal, and supramammary lymph nodes), samples of milk and mammary tissue from all four quarters, maternal placentome, spleen, liver, lung, and vaginal and conjunctival swabs. Fetal samples included: rectal swab, lung, liver, spleen, bronchial lymph node, and abomasal contents. Fetal and maternal blood was also obtained at necropsy for bacteriologic evaluation.
Abortion was defined as the premature birth of a Brucella-infected, non-viable fetus at any time after experimental challenge with B. abortus strain 2308. Fetal infection was defined as bacteriologic recovery of the challenge strain from any fetal sample. Mammary/uterine infection was defined as the bacteriologic recovery of the 2308 challenge strain from milk, mammary gland, supramammary lymph node, placentome, vaginal swab, or any fetal sample. Evaluation of uterine/mammary infection allows assessment of potential transmission as transfer of infection occurs laterally through fluids or tissues associated with the abortion/parturition process (most common) or vertically through milk to the offspring (less common). Maternal infection was defined as the recovery of the 2308 challenge strain from any maternal sample. The time between experimental challenge and parturition was calculated for both vaccinated and naïve bison and elk.
Bacterial culture
For bacterial culture, the tissues were macerated in 0.85% NaCl (saline) using a tissue grinder and placed on tryptose agar containing 5% bovine serum or Kuzdas and Morse agar as previously described (17, 23). The swabs were directly plated on the media. Following incubation at 37o C and 5% CO2, B. abortus was identified by colony morphology and growth characteristics (24), and isolates were confirmed by a polymerase chain reaction procedure using primers specific for identification of B. abortus omp2a (25).
In 6 of the elk and 12 of the bison studies, colonization (CFU/gm) of B. abortus in four target tissues (supramammary, parotid, and prescapular lymph nodes and placentomes) was determined by obtaining a cross-section of the tissue, weighing the sample (approximately 1 gm), macerating the sample in a tissue grinder, preparing serial dilutions in saline, and placing aliquots in duplicate on media. Dilutions of the tissue homogenate extended to 10−9. Estimated concentrations of bacteria within tissues were determined by performing standard plate counts.
Statistical analysis
Bacteriologic data are presented as mean ± SEM. Colonization (CFU/gm) data were converted to a logarithmic scale for analysis. Any values of zero were converted to 1 prior to logarithmic conversion. Colonization data and time between challenge and parturition were compared using the generalized linear model (SAS Institute Inc., Cary, NC, United States) and presented as mean ± SEM. Fisher’s exact test was used to compare the incidence of abortion and infection between naïve or vaccinated bison and elk following experimental challenge.
Results
For the study, data were analyzed from 82 naïve and 99 RB51-vaccianted bison and 67 naïve and 29 vaccinated elk. Of the 67 naïve elk, 52 were experimentally challenged with 10 7 CFU and 15 were infected with 108 CFU of B. abortus strain 2308. Statistical analysis demonstrated that pregnant elk challenged at approximately 12 weeks prior to parturition with either 107 or 108 CFU of strain 2308 did not differ in rates of abortion (p = 0.09), fetal infection (p = 0.95), uterine/mammary infection (p = 0.78), or maternal infection (p = 0.19) (Table 1). In addition, the time between experimental challenge and parturition did not differ (p > 0.05) between elk infected with high or low challenge dosages. As there were no statistical differences between these two groups, both groups were combined for subsequent statistical analyses.

Table 1. Abortion and infection rates after conjunctival infection of naïve elk with two dosages of B. abortus strain 2308 during gestation.
Species differences in susceptibility to abortion and infection and differences in vaccine efficacy
When abortions and infections were compared between naïve bison and elk, naïve bison had greater (p < −0.001) rates of abortion, fetal infection, uterine/mammary infection, and material infection after experimental challenge with B. abortus strain 2308 during gestation (Table 2). Similarly, RB51-vaccinated bison had higher abortion rates (p < 0.01) and greater rates of infection (p < 0.025) in fetal, uterine/mammary, or maternal tissues than vaccinated elk. In vaccinated bison, there was a reduction in abortion rates (p < 0.002) and recovery of Brucella from fetal or uterine/mammary tissues (p < 0.001) as compared to experimentally infected naïve bison. Vaccinated elk had reduced (p < 0.01) rates of maternal infection, but rates of abortion and fetal or uterine/mammary infection did not differ (p > 0.05) from naïve elk after experimental challenge.
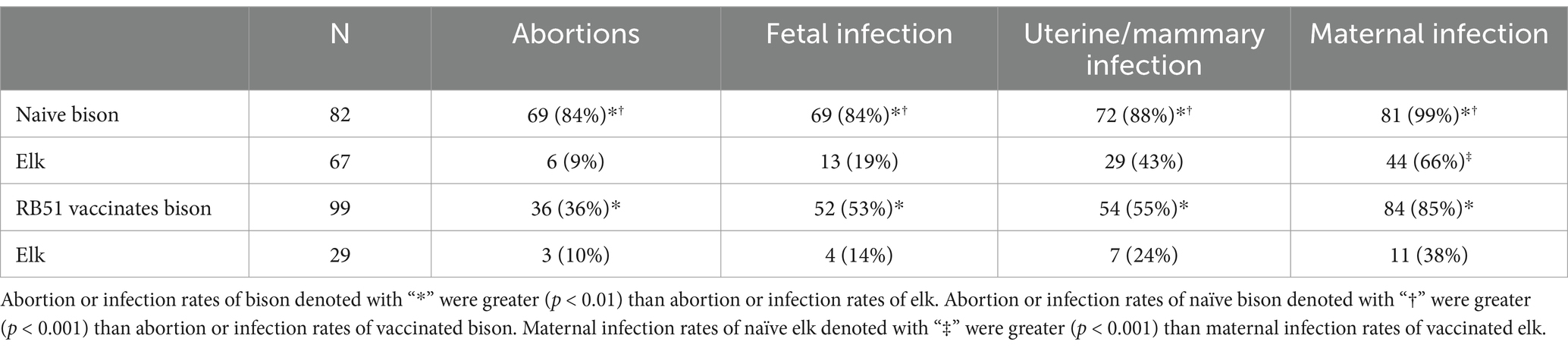
Table 2. Abortion and infection rates after mid-gestational challenge of naïve or RB51 vaccinated bison and elk with Brucella abortus strain 2308 during gestation.
Lack of species differences in gestation length after experimental infection
The time between challenge and parturition in naïve and vaccinated elk did not differ in animals that delivered full-term calves (111.6 ± 3.7 and 123.3 ± 3.5 days, respectively). Similarly, no differences (p > 0.05) between challenge and parturition were detected for full-term calves from naïve and vaccinated bison (75.3 ± 7.2 and 85.6 ± 2.7 days). Recognizing the low percentage of elk that aborted, there was a shorter time (p < 0.05) between experimental challenge and abortion as compared to full-term calves in naïve (81.0 ± 20 and 112.3 ± 3.5 days, respectively) and vaccinated elk (77.7 ± 1.4 and 92.8 ± 14.8 days, respectively). The time between experimental challenge and parturition did not differ (p > 0.05) between naïve and vaccinated bison that aborted (46.4 ± 1.4 and 51.4 ± 2.1 days, respectively), but the length of time in both groups was shorter (p < 0.05) when compared to animals delivering full-term calves.
Species differences and vaccine effects on Brucella colonization
In naïve bison and elk, colonization in placentome and parotid, prescapular, and supramammary lymph nodes was greater (p < 0.05) in animals that aborted as compared to animals that delivered full-term calves (Table 3). In naïve bison and elk that delivered full-term calves, colonization in parotid, prescapular lymph node and placentome was greater (p < 0.05) in bison. Recognizing the low number of elk that aborted, only colonization in the placentome differed (p < 0.05) between naïve bison and elk after abortion. In vaccinated animals, bison had higher colonization (p < 0.05) in placentome, parotid, and supramammary tissues than elk after delivery of a full-term calf. In vaccinated animals that aborted, colonization in placentomes of bison was greater (p < 0.05) than mean bacterial numbers recovered from elk samples. This observation may have been influenced by the low number of vaccinated elk that aborted. Of epidemiologic importance is the approximately 5-log reduction in both naive and vaccinated bison between animals that delivered full-term calves as compared to those that aborted. A similar difference in placentome colonization was noted between full-term and aborting naïve elk. In RB51-vaccinated elk, differences in placental colonization between full-term and aborting elk were only 2 logs but, as discussed previously, may have been influenced by low numbers of vaccinated elk that aborted in our studies.
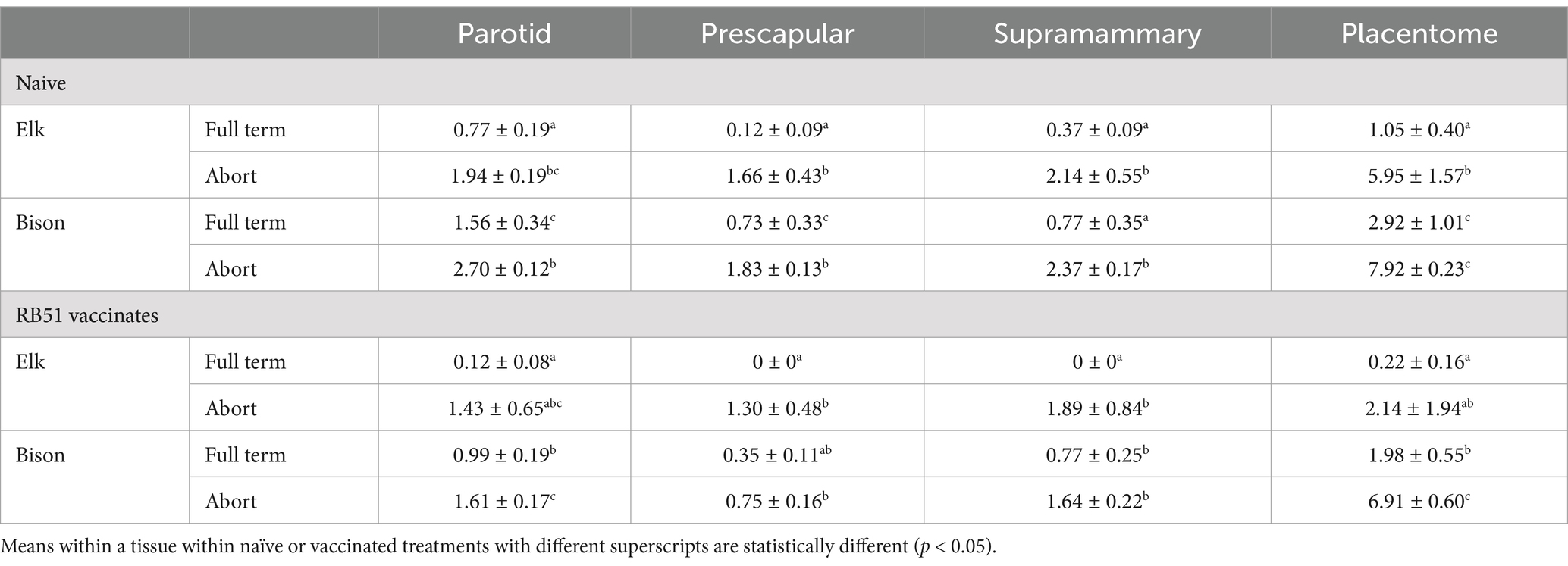
Table 3. Colonization (CFU/gm) of target tissues (lymph nodes and placentomes) after mid-gestational challenge of naïve or RB51-vaccinated bison and elk with Brucella abortus strain 2308 during gestation.
Discussion
In a similar manner as noted previously when comparing cattle and bison, our data suggest differences in the pathophysiology of brucellosis between bison and elk after experimental infection. In general, bison abort at a higher rate and have higher infection rates in fetal, uterine/mammary, and maternal tissues when compared to elk. Vaccination of bison with RB51 reduces abortion rates after experimental infection when compared to naïve animals, but similar efficacy by vaccination was not noted in elk. As naïve elk had a low abortion rate after experimental challenge, no difference was detected in fetal wastage between vaccinated and naïve elk after experimental challenge. However, it should be noted that almost 70% of naïve elk had Brucella recovered from tissues at parturition, suggesting a high infection rate after experimental challenge. The higher abortion and infection rates in bison indicate that the species is more susceptible to brucellosis and its adverse effects on reproduction when compared to elk.
Abortion rates in elk in the current study were comparable to estimates of 6% fetal losses (CI: 2–15%) in seropositive elk under field conditions (26). The same study estimated that 8% (CI: 4–19%) of seropositive elk shed B. abortus after abortion or live birth under field conditions, which is lower than data from the current study. As the field study was conducted in seropositive elk greater than 2 years of age and primarily relied on culture data from an expelled vaginal implant transmitter, differences between the two studies could have been influenced by the acute nature and more extensive sampling in the experimental challenge studies, the length of time between initial infection and parturition, and other factors. Others have estimated a 31% reduction in pregnancies in 2-year-old elk and a 7% reduction in pregnancies in elk 3 to 9 years of age in a longitudinal study in seropositive elk on Wyoming feed grounds (27). Overall, experimental and field data suggest that abortion rates from brucellosis in elk are considerably less than those observed in bison and cattle.
The epidemiologic characteristics of brucellosis in the chronically infected Yellowstone and Grand Teton National Park bison herds have been evaluated. Although reports documenting abortions have been limited in the Yellowstone herd (28, 29), the herd has high seroprevalence (50–60%) with approximately half (46%) of seropositive bison found to be culture positive for B. abortus (28, 30). Although the population is smaller, higher seroprevalence (77%) has been reported in the Grand Teton herd as compared to the Yellowstone herd (31), but published data on abortion rates are lacking. Although recoveries from maternal tissues of bison were higher in our experimental studies, the higher rate may reflect evaluation of infection during more acute stages of disease. As exposure to virulent B. abortus field strains would be anticipated to induce immunologic responses comparable to responses after vaccination, it would be of interest to compare abortion rates in these chronically infected herds to vaccine efficacy data obtained under experimental conditions.
We observed that colonization (CFU/gm) in target tissues is somewhat similar between the two species but frequently is higher in bison when compared to the paired treatment in elk. The four tissues chosen for evaluation were selected because they represent lymphatic tissues at the site of experimental challenge (parotid lymph node), localization in reproductive tissues (placentome), mammary gland infection (supramammary lymph node), or a lymph node not associated with a preferred localization site for Brucella (prescapular lymph node). The abortion event is the most significant route of transmission of brucellosis for ruminants, and higher numbers of bacteria in the uterine environment are associated with greater capability for lateral transmission of brucellosis from expelled fluids, placental tissues, or fetal tissues. As a minimum infectious dosage of B. abortus is estimated to be between 103 and 104 CFU (32), the high concentrations (sometimes up to 10 logs of Brucella per gm) detected in placentomes at abortion in bison and elk demonstrate significant capability for lateral transmission. Our data demonstrated that vaccination reduces abortions by approximately 42% and average bacterial colonization increases by 5 logs of bacteria within placentomes of bison that abort. The combination of reduced abortions and reproductive colonization would be anticipated to reduce disease transmission under field conditions.
Our data suggested that co-localization in both fetal and reproductive tissues is common, but it is extremely rare to find bison or elk with B. abortus colonization only within mammary tissues after experimental challenge during gestation. Although vertical transmission appears to be of lesser epidemiologic importance, brucellosis can be transmitted to calves through infected milk. It has been postulated that oral exposure of brucellosis to calves can lead to latent infections. In cattle, it was estimated that 2.5% of heifer calves from seropositive dams are at risk for developing and transmitting brucellosis (33, 34). Other studies in cattle have reported that 1.3–5.4% of calves nursing from infected cows develop persistent seropositive responses (34, 35). Although data are lacking in elk and greater characterization is needed in bison, seroconversion was observed in 12% of calves from a Brucella-infected bison herd that were seronegative when first quarantined (36). Currently, the epidemiologic importance of transmission of brucellosis through milk to elk or bison calves under field conditions requires further characterization.
We have previously reported that bison take up to 22 weeks to clear the attenuated B. abortus RB51 vaccine strain (20, 37). We have recovered the vaccine strain from elk as late as 44 weeks after inoculation (Olsen, unpublished data), and others have recovered RB51 from elk at 34 weeks (19). Unpublished data from murine models in our laboratory indicated that in vivo clearance of virulent strains of B. abortus is longer than the approximately 8 to 12 weeks required to clear attenuated vaccine strains (38). Therefore, the recovery of B. abortus from a high percentage of non-aborting bison and elk at parturition in the present study may reflect that temporal requirements for in vivo clearance exceed those allowed by the experimental challenge model (target of 12 to 14 weeks between experimental challenge and parturition). Therefore, data on infection rates at necropsy in the current study should be interpreted in the context of species-specific temporal limitations for clearance of Brucella.
Previous studies in elk have demonstrated that humoral responses predominate after brucellosis or tuberculosis vaccination and antigen-specific cellular immune responses are lacking (14, 19, 39). Other studies have also reported that abortion rates did not differ between RB51 vaccinates and naïve control elk after experimental challenge (40, 41). Others have reported differences in disease pathogenesis between bison and elk. In comparative studies on susceptibility to foot and mouth disease (FMDV), elk demonstrated milder clinical signs and did not transmit disease to contact elk in contrast to more severe clinical disease in bison (42). These data highlight the fact that differences in disease pathogenesis may require species-specific intervention strategies.
Overall, our data suggest significant differences between bison and elk in the pathogenesis of brucellosis and species differences in vaccine protection that may impact disease control. In concurrence with others, our data suggested that abortion events caused by brucellosis in elk are less common when compared to bison and cattle. The low rate of abortions in elk would initially appear to be contradictory to epidemiologic data that almost exclusively link them to the transmission of disease to cattle herds in the Greater Yellowstone Area. The presence of higher populations of elk with greater potential for interactions with cattle in the GYA area is more likely to be responsible for the correlation of elk with brucellosis transmission. However, both wildlife reservoirs can transmit disease (10, 43) and if given equal access to cattle herds, bison would appear to be a greater risk for brucellosis transmission due to their higher abortion rates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by National Animal Disease Center Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SO: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. PB: Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. EP: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1519453/full#supplementary-material
References
1. Tunnicliff, EA, and Marsh, H. Bang's disease in Bison and elk in the Yellowstone National Park and on the National Bison Range. J Am Vet Med Assoc. (1935) 86:745–52.
2. Mohler, JR. Abortion disease In: Agriculture USDo. Washington, D.C: U.S. Department of Agriculture (1917). 105–6.
3. Clause, D, Kilpatrick, S, Dean, R, and Smith, B. Brucellosis-Feedground-habitat program: an integrated management approach to brucellosis in elk in Wyoming In: Brucellosis in elk and Bison in the greater Yellowstone area. Jackson, WY: Greater Yellowstone Interagency Brucellosis Committee (2002)
4. Cheville, NF, McCollough, DR, and Paulson, LR. Brucellosis in the greater Yellowstone area In: Science NAo. Washington, D.C.: National Academy Press (1996)
5. Pac, HL, and Frey, K. Some population characteristics of the northern Yellowstone Bison herd during the winter of 1988-1989 In: Montana Department of Fish W, and parks. Bozeman, MT: Montana Department of Fish, Wildlife, and Parks (1991). 5–11.
6. Brennan, A, Cross, PC, Portacci, K, Scurlock, BM, and Edwards, WH. Shifting brucellosis risk in livestock coincides with spreading Seroprevalence in elk. PLoS One. (2017) 12:e0178780. doi: 10.1371/journal.pone.0178780
7. Rayl, ND, Merkle, JA, Proffitt, KM, Almberg, ES, Jones, JD, Gude, JA, et al. Elk migration influences the risk of disease spillover in the greater Yellowstone ecosystem. J Anim Ecol. (2021) 90:1264–75. doi: 10.1111/1365-2656.13452
8. Kamath, P, Foster, J, Drees, K, Luikart, G, Quance, CR, Anderson, N, et al. Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nat Commun. (2016) 7:11448. doi: 10.1038/ncomms11448
9. O'Brien, M, Beja-Pereira, A, Anderson, N, Ceballos, R, Edwards, W, Harris, B, et al. Brucellosis transmission between wildlife and livestock in the greater Yellowstone ecosystem: inferences form DNA genotyping. J Wildl Dis. (2017) 53:339–43. doi: 10.7589/2015-12-329
10. Rhyan, JC, Nol, P, Quance, CR, Gertonson, A, Belfrage, J, Harris, L, et al. Transmission of brucellosis from elk to cattle and Bison, greater Yellowstone area, U.S.a., 2002-2012. Emerg Infect Dis. (2013) 19:1992–5. doi: 10.3201/eid1912.130167
11. Sriranganathan, N, Seleem, MN, Olsen, SC, Samartino, LE, Whatmore, AM, Bricker, B, et al. Brucella In: K VNaC, editor. Genome mapping and genomics in animal-associated microbes. Berlin: Springer (2009). 1–64.
12. Ragan, VE. The brucellosis eradication program in the United States In: Brucellosis in elk and bison in the greater Yellowstone area. Jackson, WY: Wyoming Game and Fish Department (2002)
13. Olsen, S, and Johnson, C. Comparison of abortion and infection after experimental challenge of pregnant Bison and cattle with Brucella Abortus strain 2308. Clin Vaccine Immunol. (2011) 18:2075–8. doi: 10.1128/CVI.05383-11
14. Olsen, S, Fach, S, Palmer, M, Sacco, R, Stoffregen, W, and Waters, WR. Immune responses of elk to initial and booster vaccinations with Brucella Abortus strain Rb51 or 19. Clin Vaccine Immunol. (2006) 13:1098. doi: 10.1128/CVI.00213-06
15. Olsen, S, Boggiatto, P, and Kainipe, C. Immune responses and clinical effects of experimental challenge of elk with Brucella Abortus strain 2308. Vet Immunol Immunopathol. (2020) 227:110086. doi: 10.1016/j.vetimm.2020.110086
16. Olsen, S, Boyle, S, Schurig, G, and Siriranganthan, N. Immune responses and protection against experimental challenge after vaccination of Bison with Brucella Abortus strain Rb51 or Rb51 overexpressing superoxide dismutase and glycosyltransferase genes. Clin Vaccine Immunol. (2009) 16:535–40. doi: 10.1128/CVI.00419-08
17. Olsen, S, Jensen, A, Stoffregen, W, and Palmer, MV. Efficacy of Calfhood vaccination with Brucella Abortus strain Rb51 in protecting Bison against brucellosis. Res Vet Sci. (2003) 74:17–22. doi: 10.1016/s0034-5288(02)00146-7
18. Olsen, S, and Johnson, C. Efficacy of dart or booster vaccination with strain Rb51 in protecting Bison against Experimetnal Brucella Abortus challenge. Clin Vaccine Immunol. (2012) 19:886–90. doi: 10.1128/CVI.00107-12
19. Olsen, S, Kreeger, T, and Palmer, M. Immune responses of elk to vaccination with Brucella Abortus strain Rb51. J Wild Dis. (2002) 38:746–51. doi: 10.7589/0090-3558-38.4.746
20. Olsen, SC, Cheville, NF, Kunkle, RA, Palmer, MV, and Jensen, AE. Bacterial survival, lymph node pathology, and serological Resposnes of Bison (Bison Bison) vaccinated with Brucella Abortus strain Rb51 or strain 19. J Wildlife Dis. (1997) 33:146–51. doi: 10.7589/0090-3558-33.1.146
21. Olsen, S, McGill, J, Sacco, R, and Hennager, S. Immune responses of Bison and efficacy after booster vaccination with Brucella Abortus strain Rb51. Clin Vaccine Immunol. (2015) 22:440–7. doi: 10.1128/CVI.00746-14
22. Nol, P, Olsen, S, Rhyan, J, Sriranganathan, N, McCollum, M, Hennager, S, et al. Vaccination of elk (Cervus Canadensii) with Brucella Abortus strain Rb51 overexpressing superoxide dismutase and glycosyltransferase genes does not induce adequate protection against experimental Brucella Abortus challenge. Front Cell Infect Microbiol. (2016) 6:10. doi: 10.3389/fcimb.2016.00010
23. Olsen, S. Immune Repsonses and efficacy after administration of a commercial Brucella Abortus strain Rb51 vaccine to cattle. Vet Ther. (2000) 3:183–91.
24. Alton, GG, Jones, LM, Angus, RD, and Verger, JM. Techniques for the brucellosis laboratory. Paris, Fr: Institut National De La Recherche Agronomique (1988).
25. Lee, IK, Olsen, SC, and Bolin, CA. Effects of exogenous recombinant Interleukin-12 on immune responses and protection against Brucella Abortus in a murine model. Can J Vet Res. (2001) 65:223–8.
26. Jones, JDPKM, Ramsey, JM, Almberg, ES, and Anderson, NJ. Reproductive fate of brucellosis-seropositive elk (Cervus Canadensis): implications for disease transmission risk. J Wildlife Dis. (2024) 60:52–63. doi: 10.7589/JWD-D-22-00123
27. Cotterill, GGCP, Middleton, AD, Rogerson, JD, and Scurlock, BM. Hidden cost of disease in a free-ranging ungulate: brucellosis reduces mid-winter pregnancy in elk. Ecol Evol. (2018) 8:10733–42. doi: 10.1002/ece3.4521
28. Rhyan, JCAK, Roffe, T, Ewalt, D, Hennager, S, Gidlewski, T, Olsen, S, et al. Pathogenesis and epidemiology of brucellosis in Yellowstone Bison: serologic and culture results from adult females and their progeny. J Wildl Dis. (2009) 45:729–39. doi: 10.7589/0090-3558-45.3.729
29. Rhyan, JCQWJ, Stackhouse, LL, Henderson, JJ, Ewalt, DR, Payeur, JB, Johnson, M, et al. Abortion caused by Brucella Abortus Biovar 1 in a free-ranging Bison (Bison Bison) from Yellowstone National Park. J Wildl Dis. (1994) 30:445–6. doi: 10.7589/0090-3558-30.3.445
30. Roffe, TJRJC, Aune, K, Philo, LM, Ewalt, DR, and Gidlewski, T. Brucellosis in Yellowstone National Park Bison: quantitative serology and infection. J Wildl Manag. (1999) 63:1132–7. doi: 10.2307/3802831
31. Williams, ESTET, Anderson, SL, and Herriges, JD. Brucellosis in free-ranging Bison (Bison Bison) from Teton County, Wyoming. J Wildlife Dis. (1993) 29:118–22. doi: 10.7589/0090-3558-29.1.118
32. Teske, SS, Huang, Y, Tamrakar, SB, Bartrand, TA, Weir, MH, and Haas, CN. Animal and human dose-response models for Brucella species. Risk Anal. (2011) 31:1576–96. doi: 10.1111/j.1539-6924.2011.01602.x
33. Wilesmith, JW. The persistence of Brucella Abortus infection in calves; a retrospective study of heavily infected herds. Vet Rec. (1978) 103:149–53. doi: 10.1136/vr.103.8.149
34. Crawford, RP, Huber, JD, and Sanders, RB. Brucellosis in heifers weaned from seropositive dams. J Am Vet Med Assoc. (1986) 189:547–9.
35. Ray, WC, Brown, RR, Stringfellow, DA, Schnurrenberger, PR, Scanlan, CM, and Swann, AI. Bovine brucellosis: an investigation of latency in progeny of culture-positive cows. J Am Vet Med Assoc. (1988) 192:182–6.
36. Clarke, PRFRK, Rhyan, JC, McCollum, MP, Nol, P, and Aune, K. Feasibility of quarantine procedures for Bison (Bison Bison) calves from Yellowsotne National Park for conservation of brucellosis-free Bison. J Am Vet Med Assoc. (2014) 244:588–91. doi: 10.2460/javma.244.5.588
37. Roffe, TJ, Olsen, SC, Gidlewski, T, Jensen, AE, Palmer, MV, and Huber, R. Biosafety of parenteral Brucella Abortus Rb51 vaccine in Bison calves. J Wildlife Manag. (1999) 63:950–5. doi: 10.2307/3802809
38. Stevens, MG, Olsen, SC, Pugh, GW, and Brees, D. Comparison of immune responses and resistance to brucellosis in mice vaccinated with Brucella Abortus 19 or Rb51. Infect Immun. (1995) 63:264–70. doi: 10.1128/iai.63.1.264-270.1995
39. Waters, WRPMV, Olsen, SC, Sacco, RE, and Whipple, DL. Immune responses of elk to Mycobacterium Bovis Bacillus Calmette Guerin vaccination. Vaccine. (2003) 21:1518–26. doi: 10.1016/S0264-410X(02)00678-3
40. Kreeger, T, Cook, W, Edwards, W, Elzer, P, and Olsen, S. Brucella Abortus strain Rb51 vaccination in elk. Ii. Failure of high dosage to prevent abortion. J Wild Dis. (2002) 38:27–31. doi: 10.7589/0090-3558-38.1.27
41. Cook, WEWES, Thorne, ET, Kreeger, TJ, Stout, G, Bardsley, K, Edwards, H, et al. Brucella Abortus strain Rb51 vaccination in elk. I. Efficacy of reduced dosage. J Wildl Dis. (2002) 38:18–26. doi: 10.7589/0090-3558-38.1.18
42. Rhyan, JDM, Wang, H, Ward, G, Gidlewski, T, McCollum, M, Metwally, S, et al. Foot-and-mouth disease in north American Bison (Bison Bison) and elk (Cervus Elaphus Nelsoni): susceptibility, intra- and interspecies transmission, clinical signs and lesions. J Wildlife Dis. (2008) 44:269–79. doi: 10.7589/0090-3558-44.2.269
Keywords: bison (Bison bison), elk (Cervus canadensis), Brucella abortus 2308, Brucella , pathogenesis
Citation: Olsen SC, Boggiatto PM and Putz EJ (2025) Comparison of bison and elk susceptibility to experimental challenge with Brucella abortus strain 2308. Front. Vet. Sci. 11:1519453. doi: 10.3389/fvets.2024.1519453
Edited by:
Orhan Sahin, Iowa State University, United StatesReviewed by:
Sara Savic, Scientific Veterinary Institute Novi Sad, SerbiaHan Sang Yoo, Seoul National University, Republic of Korea
Copyright © 2025 Olsen, Boggiatto and Putz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. C. Olsen, U3RldmVuLm9sc2VuQHVzZGEuZ292
 S. C. Olsen
S. C. Olsen P. M. Boggiatto
P. M. Boggiatto E. J. Putz
E. J. Putz