- 1Department of Animal Science, Hunan Agriculture University, Changsha, China
- 2Key Laboratory of Agro-Ecological Processes in Subtropical Region, Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process, Hunan Research Center of Livestock and Poultry Sciences, South Central Experimental Station of Animal Nutrition and Feed Science in the Ministry of Agriculture, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
- 3School of Life Science and Engineering, Southwest University of Science and Technology, Mianyang, China
Introduction: Soybean meal is an excellent protein source and is widely used in pig feed. However, the Americas account for more than 80% of global soybean production, so European and Asia swine production largely depends on soybean imports. The use of safe and functional unconventional feed sources can effectively alleviate worldwide protein shortage problems.
Methods: Here, we formulated a low-protein soybean-free diet (LPNS) for growing and fattening pigs using rice, potatoes, tea, and other unconventional feed sources. Thirty-six healthy Daweizi pigs (average body weight 23.60 ± 1.34 kg) were raised under the same conditions and randomly assigned to two dietary treatments: (1) Con group, corn-soybean base meal; (2) LPNS group. When the average weight of pigs in the group reached 85 kg, two pigs per pen were randomly selected and euthanized for collection of the colonic digesta and carcass traits and for meat quality determination.
Results: Compared with the corn-soybean based diet, the LPNS diet decreased the average daily gain (ADG) and feed conversion ratio (FCR) of Daweizi pigs but had a lower cost per kilogram of gain. In addition, the LPNS diet significantly increased leanness and decreased the fat-skin rate and bone rate of Daweizi pigs. The cooking loss of meat decreased, and unsaturated fatty acids such as C22:6 and n−3 PUFA significantly increased in the LPNS group. Moreover, the purine content in the meat substantially decreased with the LPNS diet. The 16S rDNA analysis revealed that the LPNS diet greatly modified the composition of the colonic microbiota community, with a decrease in the Firmicutes/Bacteroidetes ratio and an increase in the abundance of Lactobacillus spp.
Discussion: The use of functional herbs along with a low-protein diet helped to regulate fat and purine metabolism in fatty-type pigs. The LPNS diet formulated with unconventional-feed sources not only helps reduce the feed cost in swine production but also improves the carcass traits and meat quality of pigs, which is more suitable for small-scale pig farming.
1 Introduction
Soybean meal is an excellent protein source and is widely used in pig feed. However, the Americas account for more than 80% of global soybean production, so European and Asia swine production largely depends on soybean imports (1). The use of alternative feedstuffs as substitutes for soybeans has been investigated worldwide to alleviate protein shortage problems and reduce feed costs in swine production (2–4).
The Daweizi pig is a fatty-type pig breed in China and is characterized by tender meat and tolerance to rough feeding (5). However, compared with lean-type pigs, such as Duroc × Landrace × Large White pigs, Daweizi pigs have a lower growth rate and feed efficiency, a higher fat percentage, and a lower lean mass rate (6). Moreover, like most fat-type Chinese domestic pigs, the nutritional requirements and feeding practices of Daweizi pigs differ from those of lean-type pigs. The current nutritional standards for lean-type pigs refer mainly to NRC 2012, but the recommended dietary protein levels are not applicable for fatty-type pigs. For most fatty-type pigs, appropriately reducing dietary protein level and using non-conventional protein sources can not only reduce the cost of feed but also help to regulate the health of pigs (7, 8). For example, dietary partially replaced with cassava residue can regulate the lipid metabolism and antioxidant capacity of Huanjiang Mini-pigs (a Chinese fatty-type pig breed) (9). However, few studies have used soybean-free meals for fatty-type pig production.
The food-feed competition is a global challenge. Use of food-not feed resources, such as biofuel co-produces, food waste, and other safe-edible by-products, not only decrease the feed costs but also avoid cost associated with disposal (10). Corn is a cereal crop that is most commonly cultivated worldwide. It is usually used as a sustenance source for humans and livestock, as well as to manufacture ethanol and citric acid (11). Corn residue after extraction of citric acid by fermentation (RCC meal) is non-toxic and cost-effective. RCC meal contains more than 25% crude protein and is rich in amino acids (Supplementary Table S1). It’s usually used as a protein feed resource in China (12). In addition, nearly 30% of the world’s rice production is attributed to China (13). Rice bran, a nutrient-rich byproduct of rice production, contains 17.5% crude protein, antioxidants, vitamins, and minerals and is widely used as animal feed (14). Furthermore, the province of Hunan, where Daweizi pigs originated, is abundant in citrus and tea. Black tea consumption has numerous health benefits, such as cholesterol reduction, cardiovascular protection, and antioxidation (15). Dried citrus peel (tangerine peel) is a traditional Chinese medicine with antioxidant and anti-inflammatory effects (16). In this study, we developed a low-protein, soybean-free diet (LPNS diet) that included RCC meal, rice bran, black tea powder, tangerine peel powder, and other unconventional feedstuffs. The effects of the LPNS diet on the growth performance, carcass traits, meat quality, and intestinal microbiota of Daweizi pigs were investigated.
2 Materials and methods
2.1 Preparation of the low-protein soybean-free diet
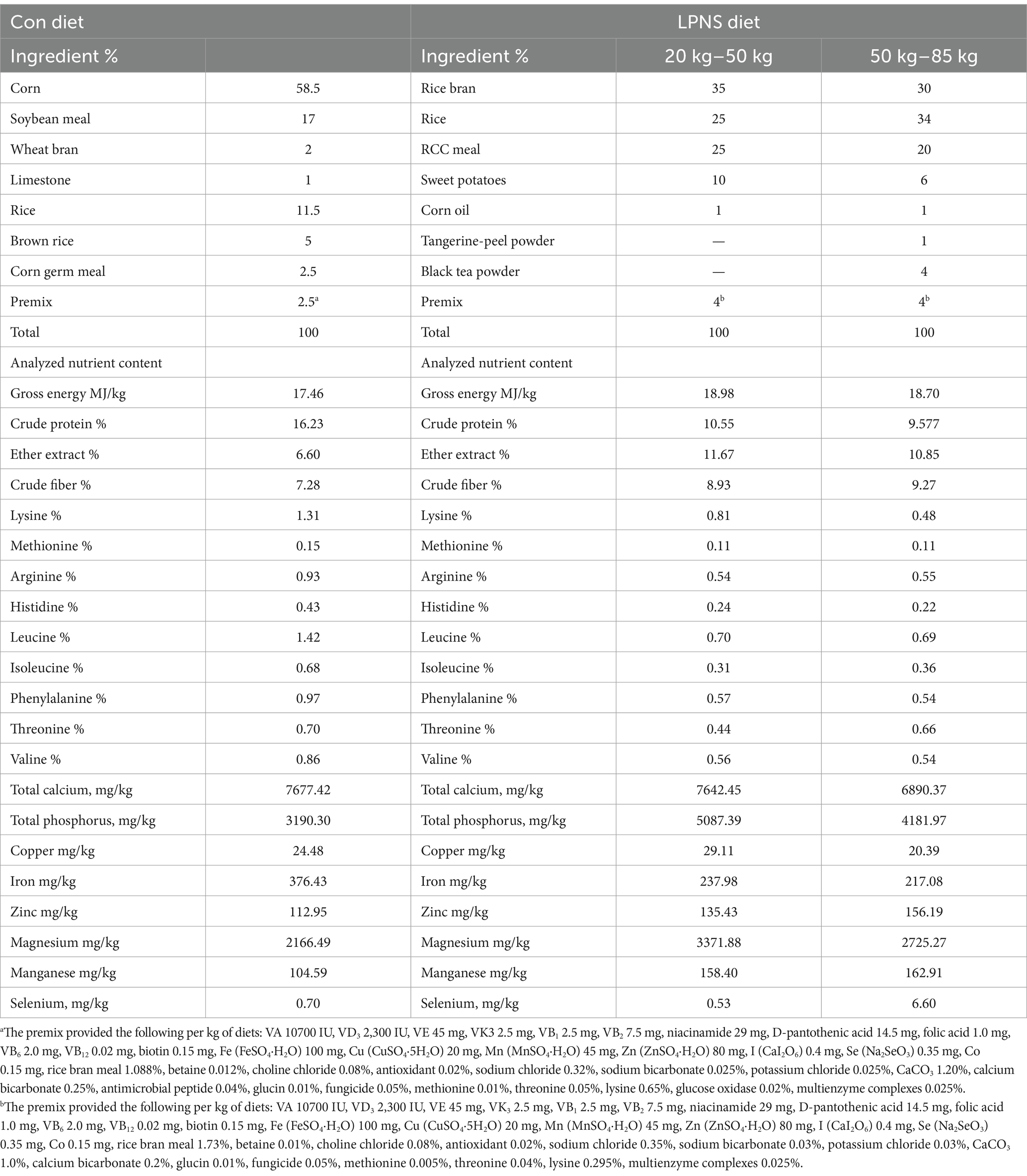
Rice, rice bran, RCC meal, sweet potatoes, black tea powder, and tangerine-peel powder were initially mixed and steamed for 1 h. The ingredients were subsequently cooled and dried to a moisture content of approximately 10%, then pulverized and blended. Finally, the mixture was thoroughly blended with soybean oil and premix and stored in a dry and cool place to be used within 3 days. The nutritional levels and ingredient components are shown in Table 1.
2.2 Animals and treatments
The animal experiment was reviewed and approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (IACUC # 202302).
Thirty-six healthy Daweizi pigs (average body weight 23.60 ± 1.34 kg) were raised under the same conditions at Hunan Tianfu Agriculture & Animal Husbandry Ecology Co., Ltd., Changsha, China. The pigs were randomly assigned to two dietary treatments, with four replicates per group and four pigs per replicate. Each pen consisted of a concrete wallow (4.0 m × 2.0 m) and free access to feed and water. The dietary treatment groups were as follows: (1) Con group, which was fed with a conventional diet based on corn and soybean meal; and (2) LPNS group, which was fed a with the low-protein, soybean-free diet. The nutritional level of the conventional diet (see Table 1) met the National Research Council (2012) nutrient recommendation (17). The crude protein and some amino acids contents in the LPNS diet (20–50 kg and 50–85 kg) are lower than those in the Pig Nutrient Requirements of China (GB/T 39235-2020) (18). When the average weight of pigs in the group reached 50 kg and 85 kg (the finishing weight), the number of feeding days was recorded, and feed intake, body weight gain and feed efficiency were calculated with a pen as the experimental unit. After that, two pigs per pen were randomly selected with a 12 h fast. Then, the selected pigs were electrical stunning with 250 V before being slaughtered. The colonic digesta and longissimus thoracis (LT) muscle were collected for further analysis.
2.3 Assessment of carcass traits
The carcass traits of the Daweizi pigs were determined according to previous study (19). Briefly, the carcass sides were processed into primal cuts, and the half-carcass weight was recorded (the head, hair, hooves, tail, and internal organs were not included). The mean backfat thickness was measured with a ruler at three sites in the right dorsal midline of the right carcass: the shoulder, the last rib, and the lumbar-sacral junction. The carcass oblique length and the carcass straight length were also measured. The outline of the loin eye area (LEA) of the longissimus thoracis (LT) muscle was traced with an acetate film between the last 3rd and 4th ribs, perpendicular to the dorsal midline, and the area of LEA was subsequently calculated via planimetry. The skin with the subcutaneous fat, lean, and bone of the left carcass was separated from the right carcass and weighed.
2.4 Assessment of meat quality
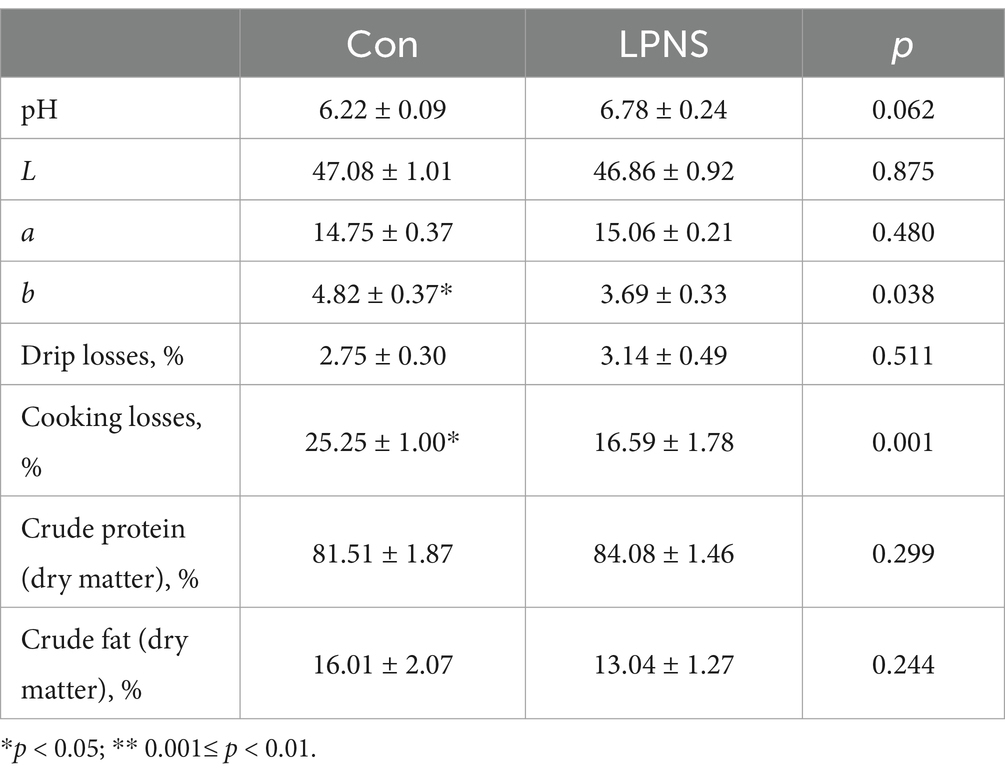
The LT muscle at the junction of the waist and sacrum was collected and separated into several portions. Half was stored in a refrigerator at 2–4°C for meat quality determination, and the other half was stored at −80°C for further analysis. The pH value and color (L*a*b*) of the LT muscle at 24 h after sampling were determined by using a hand-held pH meter and a colorimeter. Meat color traits (a*, redness, and b*, yellowness, L*, lightness) were measured with a CR-410 hand-held chromameter (Kinica Minolta Sensing Inc., Osaka, Japan). The marbling score was determined by a five-member trained sensory panel using visual standard cards. The shear force was determined via a Warner–Bratzler shear force device (TA.XT Plus, Stable Micro Systems, Godalming, United Kingdom). The cooking loss and drip loss of meat were measured as previously described (20).
2.5 Determination of fatty acid composition
The extraction of fatty acids from LT muscle was conducted according to a previous study (20). The determination of the muscular fatty acid composition was performed on a GC/MS system consisting of a 7890B GC/5977A mass selective detector (Agilent Technologies, Inc. United States). The saturated fatty acid ratio (SFA), monounsaturated fatty acid ratio (MUFA), polyunsaturated fatty acids ratio (PUFA), ∑n−3, ∑n−6, and ∑n−6/∑n−3 were calculated according to a previous study (21).
2.6 Determination of the purine concentration
The concentrations of xanthine, adenine, guanine, hypoxanthine, and the purine metabolites creatinine, adenosine, and guanosine in the LT muscle were determined (22). Briefly, the samples were minced thoroughly, and 0.200 g ± 0.005 g was weighted. Three milliliters of 10% (v/v) perchloric acid was added to each meat sample and mixed. The mixture was subsequently incubated at 99°C for 60 min. After cooling, the pH of the mixture was adjusted to 7.0 ± 0.1 with 1 mol/L KOH, and the volume was adjusted to 10 mL. After shaking well, the supernatant was obtained by centrifugation (6,000 r/min, 10 min) and filtered through a 0.22 μm filter for HPLC analysis.
2.7 The colonic microbiota composition
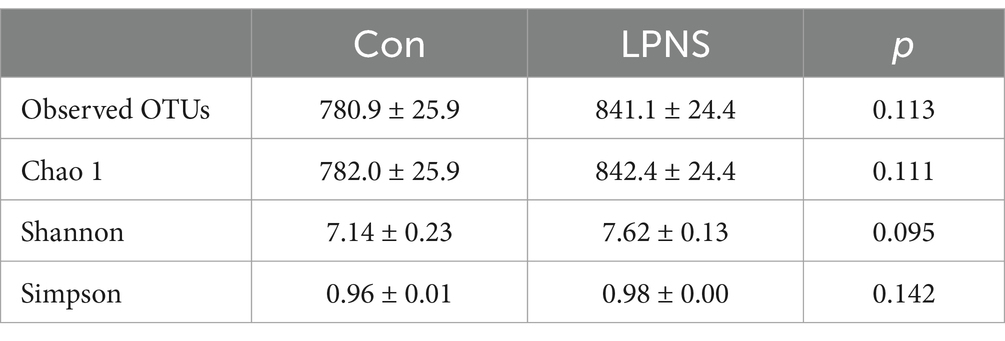
In accordance with the instruction manual, microbial DNA was isolated from the colonic digesta via a genomic DNA extraction kit (TIANGEN, Beijing, China). The isolated DNA was separated via 1% agarose gel electrophoresis, and its concentration was quantified via a Nanodrop 2000. The 16S rDNA was amplified via PCR using primers for the V3–V4 region (341F: 5′-CCTAYGGGRBGCASCAG-3′; 806R: 5′-GGACTACHVGGGGTWTCTAAT-3′). After being purified, the PCR products were sequenced on the Illumina HiSeq platform (Novogene Bioinformatics Technology Co., Ltd., Beijing, China). The α-diversity, β-diversity, and principal coordinate analyses (PCoAs) of the colonic microbiota were analyzed (23).
2.8 Statistical analysis
The pen was used as an experimental unit for body weight, ADG, and FCR analysis. Individual pigs constituted the experimental unit for the analysis of carcass traits, meat quality, fatty acid and purine concentrations, and the colonic microbiota. Two-tailed independent-sample t-tests (SPSS 25.0 software) were performed to analyze differences across groups. Differences were considered significant at p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
3 Results
3.1 Growth performance
The growth performance of the pigs is presented in Table 2. Compared with the Con group, the LPNS group had a significantly greater FCR value at the 20–50 kg stage and a significantly lower ADG at the 50–85 kg stage (p < 0.05). Moreover, the ADG of the LPNS group was significantly lower than that of the Con group at all stages (p < 0.05), and the time to slaughter was prolonged.
3.2 Carcass traits and meat quality
The result for carcass traits and meat quality are shown in Tables 3, 4. Compared with the Con diet, the LPNS diet significantly increased leanness and decreased the skin fat rate and bone rate of the Daweizi pigs (p < 0.05). In addition, the LPNS group had a lower b* value and cooking loss than the Con group (p < 0.05).
3.3 Fatty acid profile
The fatty acid profile of LT muscle is presented in Table 5. Compared with the Con diet, the LPNS diet significantly increased the concentrations of C18:2n6, C20:3n3 and C22:6 in the LT muscle (p < 0.05). The concentrations of C18:3n3, C20:4n6 and C20:5n3 were elevated (0.05 < p < 0.10). The concentration of PUFA in pork was greater in the LPNS group than in the Con group, as were the concentrations of total n−3 and total n−6 (p < 0.05).

Table 5. The effect of LPNS on the fatty acid profile of longissimus dorsi muscle of Daweizi pigs (% of total fatty acids).
3.4 Purine profile
The purine profile of the LT muscle is presented in Table 6. Compared with the Con diet, the LPNS diet significantly reduced the contents of xanthine, hypoxanthine and guanine in the meat (p < 0.05). The total purine content in the LPNS group was also significantly lower (p < 0.05), with a value approximately half that of the Con group. The contents of purine metabolites, such as creatinine and guanosine, were significantly lower in the LPNS group than in the Con group (p < 0.05).

Table 6. The effect of LPNS on the purine profile of longissimus dorsi muscle of Daweizi pigs (μg/g).
3.5 Colonic microbiota
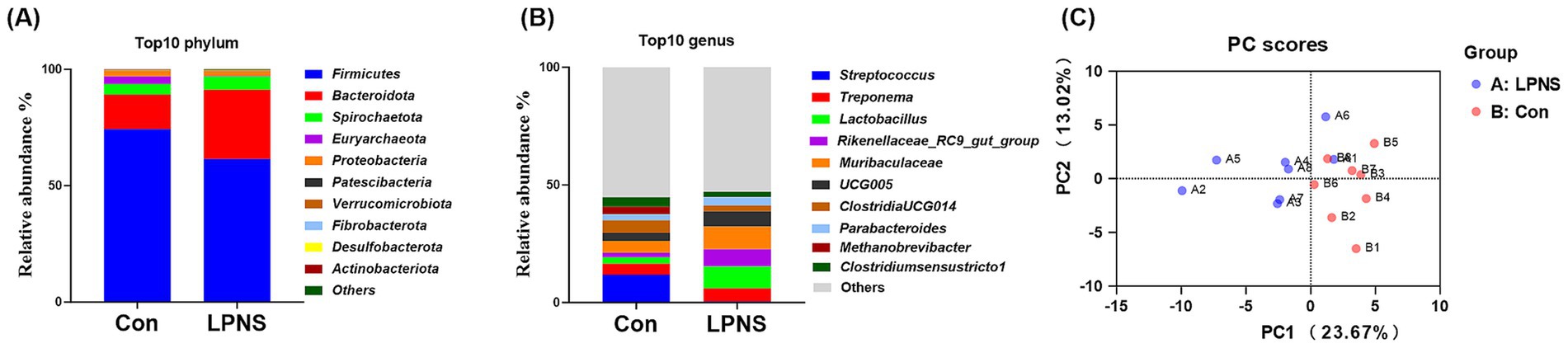
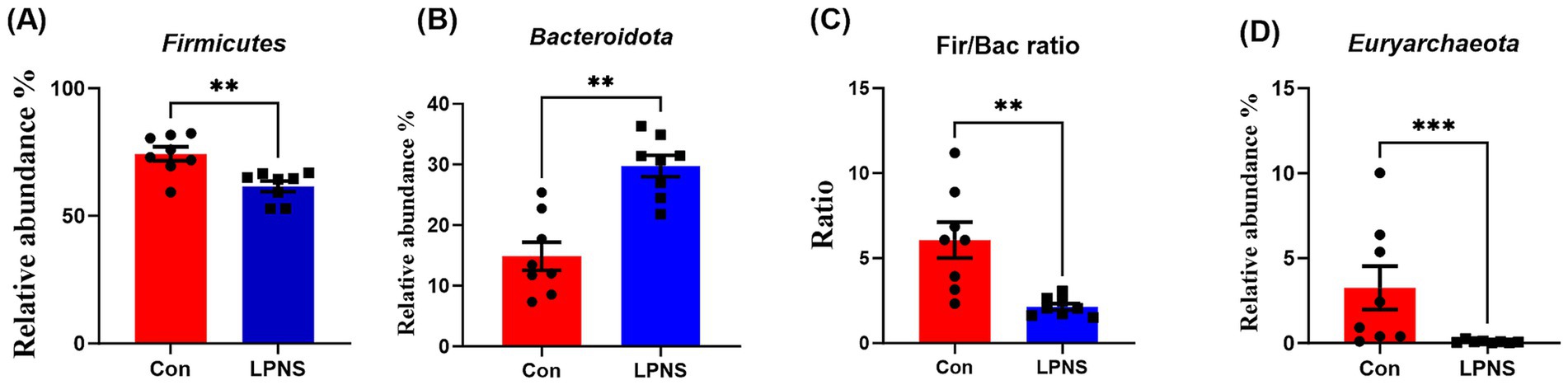
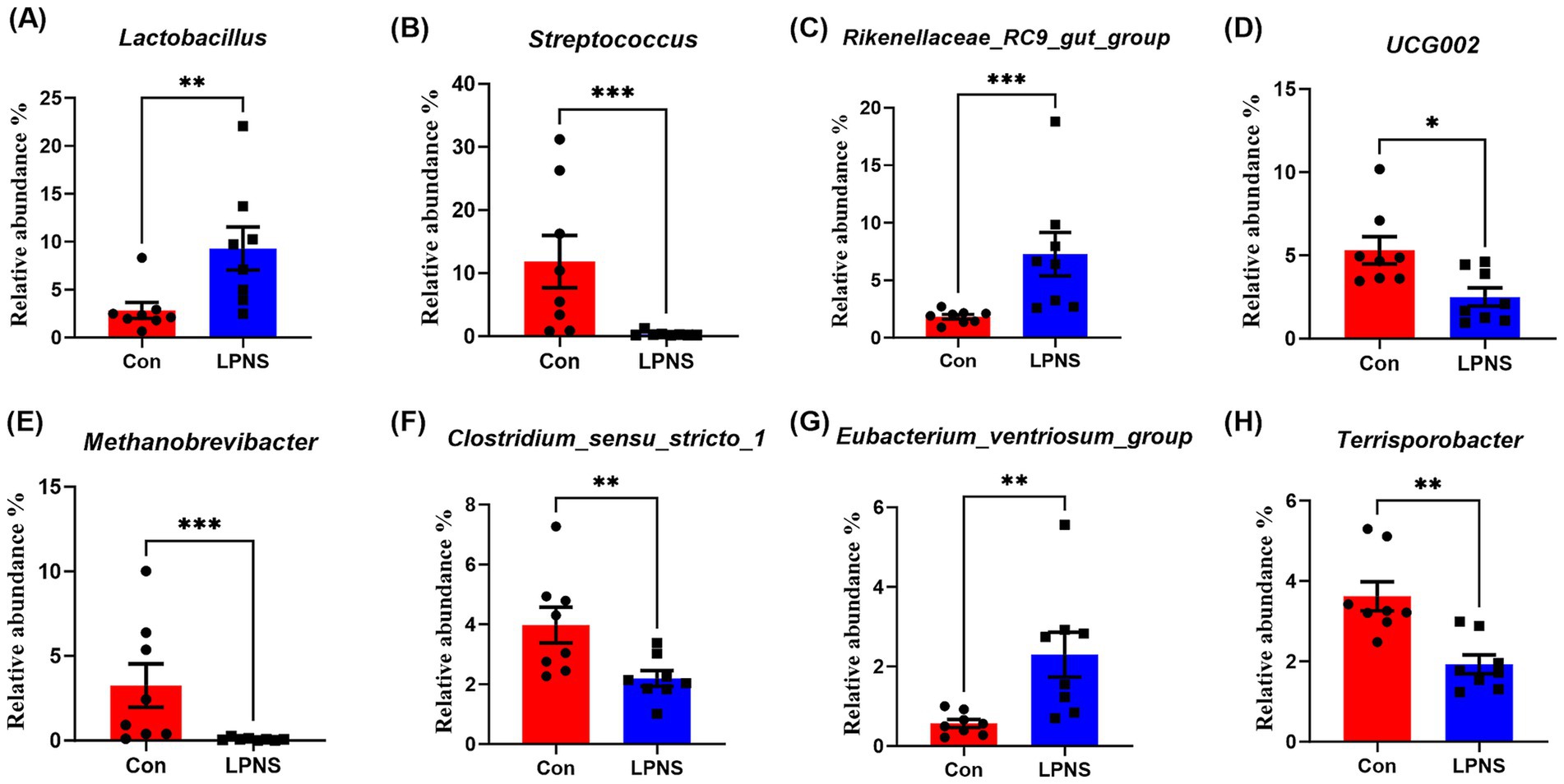
An average of 811 OTUs were detected from the colonic microbiota. The number of observed species and the Chao 1, Shannon and Simpson indices did not differ significantly between the groups (Table 7). The top 10 phyla accounted for approximately 90% of the total phyla; the dominant phylum was Firmicutes, followed by Bacteroidetes, which accounted for more than 80% of the total phyla (Figure 1A). The top 10 genera are presented in Figure 1B. The most abundant genus in the Con group was Streptococcus, whereas the most abundant genus in the LPNS group was Lactobacillus. The principal component analysis (PCA) plots revealed a difference in clustering between the two groups (Figure 1C).
The differences in the relative abundance of the microbiota at the phylum and genus levels were analyzed and are shown in Figures 2, 3. Compared with the Con diet, the LPNS diet significantly decreased the Firmicutes and Euryarchaeota levels and the Fir/Bac ratio at the phylum level (p < 0.05). The Bacteroidota level in the LPNS group was greater than that in the Con group. At the genus level, the relative abundances of Lactobacillus, Rikenellaceae_RC9_gut_group and Eubacterium_ventriosum_group were significantly greater in the LPNS group than in the Con group (p < 0.05). The relative abundances of Streptococcus, UCG0002, Methanobrevibacter, Clostridi-um_sensu_stricto_1, and Terrisporobacter were significantly lower than those in the Con group (p < 0.05).
4 Discussion
The advantages and disadvantages of reducing dietary protein with free amino acid supplementation have drawn great attention in recent years. Growth performance is an important indicator for evaluating low-protein diets. During the growing and finishing stages, a dietary crude protein reduction of 3% or less of the NRC-recommended amount does not affect growth performance (19, 24). However, if the reduction is more than 3%, e.g., 6% or more, the growth performance of pigs is significantly lower than that of pigs fed a high-protein diet (25–27). Unlike Long White pigs or Large White pigs, Daweizi pigs have rough feeding resistance and are better adapted to very-low-protein diets, as they lived on leaves, roots, and berries in the wild before being domesticated (5). Therefore, we designed a very-low-protein diets in the present study. The CP content of the LPNS diet was approximately 10%, which was 3% lower than the Pig Nutrient Requirements of China and 6% lower than the NRC 2012. The CP content of the Con diet was approximately 16%, which was 3% greater than the Pig Nutrient Requirements of China. After being fed the LPNS diet, the ADG of the pigs in the growing stage did not differ from that of the Con group, but the ADG of the pigs in the fattening stage decreased. Studies have shown that the concentration of branched-chain amino acids in a low-protein diet is a limiting factor affecting the growth performance of fattening pigs (28); therefore, we hypothesized that increasing the content of branched-chain amino acids in the LPNS diet may help improve the growth performance of Daweizi pigs.
The use of a low-protein diet has caused great concern, in part, because of its effect on fat deposition. Many studies have shown that feeding with an LP diet increases the amount of dietary energy available for fat deposition (29, 30). Supplementation with functional amino acids, plants, probiotics, etc., may help reduce excess fat deposits in the body. Tea polyphenols, polysaccharides, and other functional substances can accelerate the rate of body metabolism and promote fat decomposition (31, 32). Tangerine peel powder, a traditional Chinese medicine, has been proven to lower blood cholesterol (33). In the present study, the LPNS diet did not lead to fatter carcasses but rather to leaner carcasses, suggesting that supplementation of black tea and tangerine peel in the low-protein diet may help to decrease fat deposition caused by the low-protein diet. This may also be related to the different breeds of pigs, as fat pigs were used in this study, whereas all the pigs reported to have been used in other studies were lean pigs. Since this study used fatty-type pigs, while all other reports used lean-type pigs, the pig breeds may also have contributed to the differential results.
In addition to growth performance, meat quality is a key indicator of farming efficiency (34). Tea powder is reported to significantly improve the meat color and increase the water retention (increased moisture content) of meat (35–37). In this study, the LPNS diet, which contains 2% black tea powder, significantly improved the meat quality by reducing the yellowness value and cooking losses. Meat is an important source of unsaturated fatty acids for humans. The fatty acid composition of pork can be influenced by feed components. For example, pigs cannot synthesize linoleic acid, and its content in tissues is highly correlated with dietary intake (38). The LPNS diet contains a large proportion of rice and sweet potatoes, both of which are rich in linoleic acid, so the linoleic acid content in meat is significantly elevated (39). On the other hand, linoleic acid is a metabolic precursor of n−3 PUFAs, including C20:5 and C22:6 (40). In this study, we found that the contents of C20:5, C22:6, and n−3 PUFAs in meat were significantly elevated in the LPNS group. PUFA n−3 intake is very important for reducing the risk of cardiovascular disease in humans. However, PUFA n−3 intake in adults is very low, and increasing the level of PUFA n−3 in meat is essential to help consumers meet the minimum nutritional requirements (40). High-protein diets are usually high in purines, and soybeans are a purine-rich food. Low-protein diets can reduce purine intake (41). After consumption of the LPNS diet, the meat of the Daweizi pigs had a very low purine content, without affecting the fat and protein levels, making it a high-quality meat. Nowadays there are a lot of people suffering from hyperuricosuria and they need to reduce the purine intake. So the pork from the LPNS group may meet the dietary needs of patients with hyperuricosuria.
The type and amount of feed ingredients can affect the composition of gut microorganisms and thus the metabolism of pigs (42). In the present study, the intestinal Fir/Bac ratio was significantly reduced after LPNS treatment, and this change was associated with a low body fat percentage. Moreover, the LPNS group showed a significant reduction in the abundance of intestinal Euryarchaeota, one of the few archaea known to colonize humans and animals, which contain many methanogens. Methanogens are hydrogenotrophic groups involved in interspecies hydrogen transfer. H2 utilization and transfer between bacteria and methanogens can increase energy absorption in the gut, which is associated with obesity and is clinically harmful to the host (43). In addition, many Lactobacillus species have been shown to degrade purines (44, 45). The ability of LPNS to reduce purines in meat may be related not only to the low-protein diet but also to the increased number of Lactobacillus in the gut. The relative abundances of both the Rikenellaceae RC9 gut group and the Eubacterium ventriosum group were significantly elevated in the colon, and both alleviated intestinal inflammation and increased fiber utilization in the diet (46, 47). LPNS diets significantly reduced intestinal Clostridium_sensu_stricto_1 and Streptococcus, which are thought to be positively correlated with the expression of intestinal proinflammatory factors (48, 49). These results suggest that LPNS diets are more helpful in maintaining intestinal microecological homeostasis and promoting intestinal health.
5 Conclusion
We designed a low-protein soybean-free (LPNS) diet. Compared with the corn-soybean meal-type diet, the LPNS diet significantly improved the carcass traits and meat quality of Daweizi pigs, although it affected the feed-to-weight ratio. The use of functional herbs along with a low-protein diet helped to regulate fat and purine metabolism in fatty-type pigs. The use of other alternative feed sources that could be grown locally for fatty-type local pig breeds, which are cost-effective and suitable for small-scale pig farming, is suggested.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number: BioProject PRJNA1171598.
Ethics statement
The animal studies were approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
HF: Formal analysis, Methodology, Writing – original draft. TY: Investigation, Methodology, Writing – original draft. HN: Methodology, Supervision, Writing – review & editing. JLi: Investigation, Methodology, Writing – review & editing. FL: Methodology, Writing – review & editing. JLiu: Writing – review & editing, Software. YY: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Scientific and Technological Project of Changsha City (kh2201234).
Acknowledgments
The authors would like to thank Hunan Tianfu Agriculture & Animal Husbandry Ecology Co., Ltd. for providing the animals and research facilities. The authors sincerely thank Faping Wu for her hard work and professional contribution in the animal experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1516198/full#supplementary-material
References
1. Rotundo, JL, Marshall, R, McCormick, R, Truong, SK, Styles, D, Gerde, JA, et al. European soybean to benefit people and the environment. Sci Rep. (2024) 14:7612. doi: 10.1038/s41598-024-57522-z
2. Thornton, P, Gurney-Smith, H, and Wollenberg, E. Alternative sources of protein for food and feed. Curr Opin Environ Sustain. (2023) 62:101277. doi: 10.1016/j.cosust.2023.101277
3. Md Nasir, NAN, Kamaruddin, SA, Zakarya, IA, and Aminul Islam, AKM. Sustainable alternative animal feeds: recent advances and future perspective of using azolla as animal feed in livestock, poultry and fish nutrition. Sustain Chem Pharm. (2022) 25:100581. doi: 10.1016/j.scp.2021.100581
4. Mordenti, AL, Martelli, G, Brogna, N, Nannoni, E, Vignola, G, Zaghini, G, et al. Effects of a soybean-free diet supplied to Italian heavy pigs on fattening performance, and meat and dry-cured ham quality. Ital J Anim Sci. (2012) 11:7. doi: 10.4081/ijas.2012.e80
5. Xu, D, He, CQ, Li, QH, He, J, and Ma, HM. The complete mitochondrial genome of the Daweizi pig. Mitochondrial DNA. (2015) 26:640–1. doi: 10.3109/19401736.2013.836514
6. Chen, C, Chang, YT, Deng, Y, Cui, QM, Liu, YY, Li, HL, et al. Comprehensive analysis of miRNAs, lncRNAs and mRNAs profiles in backfat tissue between Daweizi and Yorkshire pigs. Anim Biosci. (2023) 36:404–16. doi: 10.5713/ab.22.0165
7. Wang, J, Zhao, SM, Song, XL, Pan, HB, Li, WZ, Zhang, YY, et al. Low protein diet up-regulate intramuscular lipogenic gene expression and down-regulate lipolytic gene expression in growth-finishing pigs. Livest Sci. (2012) 148:119–28. doi: 10.1016/j.livsci.2012.05.018
8. Parrini, S, Aquilani, C, Pugliese, C, Bozzi, R, and Sirtori, F. Soybean replacement by alternative protein sources in pig nutrition and its effect on meat quality. Animals. (2023) 13:494. doi: 10.3390/ani13030494
9. Azad, MAK, Jiang, H, Ni, H, Liu, Y, Huang, P, Fang, J, et al. Diets partially replaced with cassava residue modulate antioxidant capacity, lipid metabolism, and gut barrier function of Huanjiang Mini-pigs. Front Vet Sci. (2022) 9:902328. doi: 10.3389/fvets.2022.902328
10. Makkar, HPS. Review: feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal. (2018) 12:1744–54. doi: 10.1017/S175173111700324X
11. Jiao, Y, Chen, H-D, Han, H, and Chang, Y. Development and utilization of corn processing by-products: a review. Foods. (2022) 11:3709. doi: 10.3390/foods11223709
12. Wei, B, Wang, Y, and Han, S. Effects of feed with different proportions of corn citric acid residure on weight gain of fatterning pig. China Swine Ind. (2021) 16:59–63. doi: 10.16174/j.cnki.115435.2021.01.012
13. Nie, L, and Peng, S. Rice production in China In: BS Chauhan, K Jabran, and G Mahajan, editors. Rice production worldwide. Cham: Springer (2017). 33–52.
14. Manzoor, A, Pandey, VK, Dar, AH, Fayaz, U, Dash, KK, Shams, R, et al. Rice bran: nutritional, phytochemical, and pharmacological profile and its contribution to human health promotion. Food Chem Adv. (2023) 2:100296. doi: 10.1016/j.focha.2023.100296
15. Pan, M-H, Lai, C-S, Wang, H, Lo, C-Y, Ho, C-T, and Li, S. Black tea in chemo-prevention of cancer and other human diseases. Food Sci Human Wellness. (2013) 2:12–21. doi: 10.1016/j.fshw.2013.03.004
16. Rafiq, S, Kaul, R, Sofi, SA, Bashir, N, Nazir, F, and Ahmad, NG. Citrus peel as a source of functional ingredient: a review. J Saudi Soc Agric Sci. (2018) 17:351–8. doi: 10.1016/j.jssas.2016.07.006
17. NRC. Nutrient requirements of Swine. 11th ed. Washington, DC: The National Academies Press (2012).
18. GB/T 39235-2020. (2020). Nutrient requirements of swine. National Standards Press of China Press, Beijing.
19. Wang, YM, Yu, HT, Zhou, JY, Zeng, XF, Wang, G, Cai, S, et al. Effects of feeding growing-finishing pigs with low crude protein diets on growth performance, carcass characteristics, meat quality and nutrient digestibility in different areas of China. Anim Feed Sci Technol. (2019) 256:114256. doi: 10.1016/j.anifeedsci.2019.114256
20. Wang, YD, Chen, JY, Ji, YL, Lin, X, and Zhao, YR. Effect of betaine diet on growth performance, carcass quality and fat deposition in finishing Ningxiang pigs. Animals. (2021) 11:10. doi: 10.3390/ani11123408
21. Tejeda, JF, Hernández-Matamoros, A, Paniagua, M, and González, E. Effect of free-range and low-protein concentrated diets on growth performance, carcass traits, and meat composition of Iberian pig. Animals. (2020) 10:13. doi: 10.3390/ani10020273
22. Fan, H, Yang, FQ, and Li, SP. Determination of purine and pyrimidine bases in natural and cultured Cordyceps using optimum acid hydrolysis followed by high performance liquid chromatography. J Pharm Biomed Anal. (2007) 45:141–4. doi: 10.1016/j.jpba.2007.02.032
23. Liang, J, Zeng, Y, Hu, H, Yin, YL, and Zhou, XH. Prevotella copri improves selenium deposition and meat quality in the longissimus dorsi muscle of fattening pigs. Probiotics Antimicrob Proteins. (2024). doi: 10.1007/s12602-024-10340-1 online ahead of print.
24. Wang, D, Chen, G, Li, W, Chai, M, Zhang, H, and Su, Y. Effects of low protein diet on production performance and intestinal microbial composition in pigs. Vet Sci. (2023) 10:655. doi: 10.3390/vetsci10110655
25. Wu, LT, Zhang, XX, Tang, ZR, Li, YX, Li, TJ, Xu, QQ, et al. Low-protein diets decrease porcine nitrogen excretion but with restrictive effects on amino acid utilization. J Agric Food Chem. (2018) 66:8262–71. doi: 10.1021/acs.jafc.8b03299
26. Wykes, LJ, Fiorotto, M, Burrin, DG, DelRosario, M, Frazer, ME, Pond, WG, et al. Chronic low protein intake reduces tissue protein synthesis in a pig model of protein malnutrition. J Nutr. (1996) 126:1481–8. doi: 10.1093/jn/126.5.1481
27. Spring, S, Premathilake, H, Bradway, C, Shili, C, DeSilva, U, Carter, S, et al. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci Rep. (2020) 10:15859. doi: 10.1038/s41598-020-72816-8
28. Habibi, M, Shili, CN, Sutton, J, and Pezeshki, A. Dietary supplementation of branched-chain amino acids higher than recommended levels in nursery pigs fed with moderately low protein diet: effects on growth performance. J Anim Sci. (2019) 97:82. doi: 10.1093/jas/skz258.169
29. Pezeshki, A, and Chelikani, PK. Low protein diets and energy balance: mechanisms of action on energy intake and expenditure. Front Nutr. (2021) 8:655833. doi: 10.3389/fnut.2021.655833
30. Zapata, RC, Singh, A, Pezeshki, A, Avirineni, BS, Patra, S, and Chelikani, PK. Low-protein diets with fixed carbohydrate content promote hyperphagia and sympathetically mediated increase in energy expenditure. Mol Nutr Food Res. (2019) 63:e1900088. doi: 10.1002/mnfr.201900088
31. George, G, Sridhar, SNC, and Paul, AT. Investigation of synergistic potential of green tea polyphenols and orlistat combinations using pancreatic lipase assay-based synergy directed fractionation strategy. S Afr J Bot. (2020) 135:50–7. doi: 10.1016/j.sajb.2020.08.009
32. Nakamura, M, Miura, S, Takagaki, A, and Nanjo, F. Hypolipidemic effects of crude green tea polysaccharide on rats, and structural features of tea polysaccharides isolated from the crude polysaccharide. Int J Food Sci Nutr. (2017) 68:321–30. doi: 10.1080/09637486.2016.1232376
33. Bok, SH, Lee, SH, Park, YB, Bae, KH, Son, KH, Jeong, TS, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. (1999) 129:1182–5. doi: 10.1093/jn/129.6.1182
34. Gao, G, Gao, N, Li, S, Kuang, W, Zhu, L, Jiang, W, et al. Genome-wide association study of meat quality traits in a three-way crossbred commercial pig population. Front Genet. (2021) 12:614087. doi: 10.3389/fgene.2021.614087
35. Chen, X, Zhu, W, Liu, X, Li, T, Geng, Z, and Wan, X. The growth performance, meat quality, and gut bacteria of broilers raised with or without antibiotics and green tea powder. J Appl Poult Res. (2019) 28:712–21. doi: 10.3382/japr/pfz023
36. Yan, ZM, Zhong, YZ, Yin, YJ, Duan, YH, Wang, WL, Zhang, LY, et al. Effects of dietary tea powder on the growth performance, carcass traits, and meat quality of Tibetan pig × Bama miniature pigs. Animals. (2021) 11:14. doi: 10.3390/ani11113225
37. Li, L, Sun, XH, Luo, JY, Chen, T, Xi, QY, Zhang, YL, et al. Effects of herbal tea residue on growth performance, meat quality, muscle metabolome, and rumen microbiota characteristics in finishing steers. Front Microbiol. (2022) 12:821293. doi: 10.3389/fmicb.2021.821293
38. Gol, S, González-Prendes, R, Bosch, L, Tor, M, Reixach, J, Pena, RN, et al. Linoleic acid metabolic pathway allows for an efficient increase of intramuscular fat content in pigs. J Anim Sci Biotechnol. (2019) 10:33. doi: 10.1186/s40104-019-0343-8
39. Mu, T-H, and Zhang, M. Chapter 6—sweet potato lipids In: T-H Mu and J Singh, editors. Sweet potato. New York, NY: Academic Press (2019). 149–75.
40. Bartkovsky, M, Sopková, D, Andrejcáková, Z, Vlcková, R, Semjon, B, Marcincák, S, et al. Effect of concentration of flaxseed (Linum usitatissimum) and duration of administration on fatty acid profile, and oxidative stability of pork meat. Animals. (2022) 12:13. doi: 10.3390/ani12091087
41. Villegas, R, Xiang, YB, Elasy, T, Xu, WH, Cai, H, Cai, Q, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men’s Health Study. Nutr Metab Cardiovasc Dis. (2012) 22:409–16. doi: 10.1016/j.numecd.2010.07.012
42. Li, P, Qu, R, Li, M, Sheng, P, Jin, L, Huang, X, et al. Impacts of food additives on gut microbiota and host health. Food Res Int. (2024) 196:114998. doi: 10.1016/j.foodres.2024.114998
43. Horz, HP, and Conrads, G. The discussion goes on: what is the role of Euryarchaeota in humans? Archaea. (2010) 2010:967271. doi: 10.1155/2010/967271
44. Li, M, Yang, DB, Mei, L, Yuan, L, Xie, A, and Yuan, JL. Screening and characterization of purine nucleoside degrading lactic acid Bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PLoS One. (2014) 9:e105577. doi: 10.1371/journal.pone.0105577
45. Yamada, N, Saito, C, Murayama-Chiba, Y, Kano, H, Asami, Y, and Itoh, H. Lactobacillus gasseri PA-3 utilizes the purines GMP and guanosine and decreases their absorption in rats. Nucleosides Nucleotides Nucleic Acids. (2018) 37:307–15. doi: 10.1080/15257770.2018.1454949
46. Mukherjee, A, Lordan, C, Ross, RP, and Cotter, PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. (2020) 12:28. doi: 10.1080/19490976.2020.1802866
47. Cheng, XD, Du, X, Liang, YP, Degen, AA, Wu, XK, Ji, KX, et al. Effect of grape pomace supplement on growth performance, gastrointestinal microbiota, and methane production in Tan lambs. Front Microbiol. (2023) 14:1264840. doi: 10.3389/fmicb.2023.1264840
48. Abdulamir, AS, Hafidh, RR, and Abu, BF. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. (2010) 9:249. doi: 10.1186/1476-4598-9-249
Keywords: fatty-type pig, low protein diet, meat quality, gut microbiota, carcass traits
Citation: Fu H, Yang T, Ni H, Li J, Liu F, Liu J and Yin Y (2025) A low-protein soybean-free diet improves carcass traits and meat quality and modulates the colonic microbiota in Daweizi pigs. Front. Vet. Sci. 11:1516198. doi: 10.3389/fvets.2024.1516198
Edited by:
Valiollah Palangi, Ege University, TürkiyeReviewed by:
Bao Yi, Chinese Academy of Agricultural Sciences (CAAS), ChinaYushan Jia, Inner Mongolia Agricultural University, China
Copyright © 2025 Fu, Yang, Ni, Li, Liu, Liu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengjia Ni, bmloZW5namlhQGlzYS5hYy5jbg==; Yulong Yin, eWlueXVsb25nQGlzYS5hYy5jbg==
†These authors have contributed equally to this work
 Haohua Fu
Haohua Fu Taoming Yang
Taoming Yang Hengjia Ni
Hengjia Ni Jing Li
Jing Li Fenfen Liu
Fenfen Liu Jingbo Liu
Jingbo Liu Yulong Yin
Yulong Yin