- 1Department of Emergency and Critical Care, Wheat Ridge Animal Hospital, Wheat Ridge, CO, United States
- 2Veterinary Diagnostic Lab, Kansas State University College of Veterinary Medicine, Manhattan, KS, United States
- 3Ethos Veterinary Health, Woburn, MA, United States
- 4Department of Emergency and Critical Care, North Carolina State University College of Veterinary Medicine, Raleigh, NC, United States
- 5Department of Emergency and Critical Care, Cornell University College of Veterinary Medicine, Ithaca, NY, United States
Introduction: Alongside the United States’ growing landscape of legalized recreational marijuana intended for humans, cases of canine marijuana toxicosis have been on the rise. Most commonly these dogs have mild clinical signs and respond well to supportive therapies. However, patients might still be ataxic, unable to walk, or remain heavily sedated at the time of discharge. Our hypothesis was that flumazenil would improve the level of consciousness, brainstem reflexes, gait, and stance in dogs with marijuana toxicosis.
Methods: Seventeen dogs presenting for marijuana toxicosis were enrolled. MGCS and Canine Marijuana Severity Score (CMSS), were used to assess level of consciousness, brain stem reflexes, gait, and stance. Flumazenil 0.01 mg/kg was administered IV once. Baseline values immediately before flumazenil administration, 5 min, 15 min, and 30 min after flumazenil were recorded. Serum was collected and analyzed for delta-9-THC using ultraperformance liquid chromatography.
Results: There was a significant change in MGCS and CMSS following flumazenil administration (p = 0.0033 and p ≤ 0.001). The median CMSS at baseline was 17 (10–19), at 5 min was 18 (10–21), at 15 min was 18 (12–22), and at 30 min was 19 (14–22). There was a significant difference between the concentration of delta-9-THC and clinical sign score (p = 0.0275).
Discussion: The administration of flumazenil to dog affected by marijuana toxicosis might result in improved gait, stance, and level of consciousness. There might be some discriminative ability of the CMSS to stratify the severity level of canine marijuana toxicosis.
Introduction
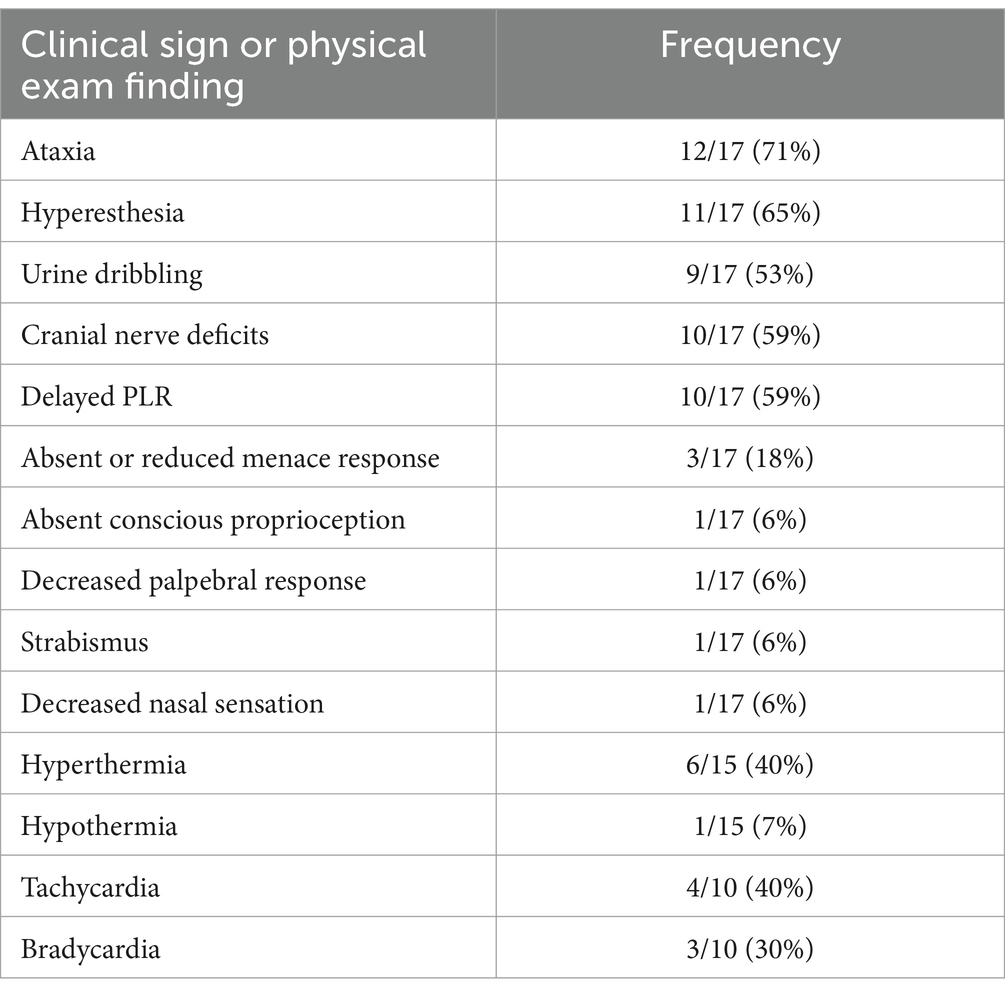
Over the past decade, marijuana intoxications have been more commonly seen in dogs across the United States (1–4). Recreational marijuana use has been legalized in several states, and access to this product has become more available to the public (1). Abnormal neurologic signs are seen in 99% of intoxicated dogs (5), characterized by depressed mentation, ataxia, mydriasis, and hyperesthesia. In addition, hypersalivation, bradycardia, emesis and urine dribbling are commonly reported (6). There can be considerable morbidity in affected cases, with mortality being reported in rare cases. Increased potency of marijuana products has been on the rise over the past several decades, which could be related to the morbidity associated with marijuana intoxication in dogs (7). Treatments are supportive in nature, but in more severely affected animals, treatments could include lipid emulsion or extracorporeal therapy (8).
The main psychoactive compound in marijuana is tetrahydrocannabinol (THC), which primarily acts on cannabinoid receptors 1 and 2 (CB1 and CB2) located in the brain and peripheral nervous system, respectively (6, 9, 10). Dogs have more cannabinoid receptors in their brain compared to humans, which could explain their increased sensitivity to marijuana products (9). The endocannabinoid system has a wide-reaching effect on numerous organ systems. Two well-known endocannabinoids, anandamide (ANA) and 2-arachidonoylglycerol (2-AG), regulate neurologic, immune, and inflammatory functions through their effect on CB1 and CB2 receptors. An in vitro study found that 2-AG might have a unique binding site with the γ-aminobutyric acid type A (GABAA) receptor (11, 12). Agonism of the GABAA receptor is involved in sedation and inhibition of the arousal center which might be involved in sleep cycle and maintenance of anesthesia (13, 14). Flumazenil competitively antagonizes the benzodiazepine binding-site on GABAA (15). In people, flumazenil is associated with reversing the effects of hypnotics, opioids, serotonin syndrome, alcohol intoxication, and other CNS depressants (14, 16–19). A case report described rapid improvement in mentation in two children that were comatose after ingesting marijuana (19). There is a knowledge gap as to whether flumazenil could be a useful adjunct in the management of THC intoxication in dogs.
The primary objective of this study was to evaluate the effect of flumazenil administration in dogs with marijuana toxicosis. We hypothesized that flumazenil administration would result in improved gait scores, brain stem reflexes, stance, and level of consciousness. A secondary objective was to evaluate a novel clinical scoring system in affected dogs, based on evaluation of gait, stance, brain stem reflexes, and level of consciousness.
Materials and methods
Seventeen dogs presented to a private practice referral hospital with suspected or confirmed marijuana toxicosis, and were prospectively enrolled in this pilot study after obtaining client consent. Animal Care and Use Committee approval was obtained prior to initiating the study. Two scoring systems were used simultaneously: the Modified Glasgow Coma Score (MGCS) and a novel marijuana toxicosis scoring system titled the Canine Marijuana Severity Score (CMSS), which was adapted from both the MGCS and the Scale for the Assessment and Rating of Ataxia (Supplementary Table S1) (20, 21). Veterinary technicians and veterinarians were trained on how to use both scoring systems. Technicians, associate veterinarians, and senior emergency veterinarians performed the scoring.
Of the 17 dogs enrolled, 5/17 (29.4%) were toy breeds [(Chihuahua (2), Maltese mix (1), Terrier mix (1), Yorkie mix (1)]. Other breeds represented include the Pembroke Welsh Corgi (1), Australian Cattle Dog mix (1), Newfoundland (1), Goldendoodle (1), Lab mix (1), Pug (1), Cocker spaniel mix (1), Mini Australian Shepherd (1), Boxer mix (1), Shiba Inu (1), Saint Bernard (1) and Labrador Retriever (1). There were 10 males, (8 castrated, 2 intact) and 7 females (6 spayed, 1 intact). About 30% (5/17) were less than 1 year of age with a median age of 3 years (range: 5 months to 12 years). The median weight was 10.5 kg (2.9–58 kg).
Baseline assessment scores were recorded at presentation, and then 3 mL of whole blood was collected via jugular or cephalic venipuncture and immediately placed in a plastic tube without any additive. A single intravenous injection of flumazenil (0.01 mg/kg) was administered over approximately 1 min. The MGCS and CMSS were re-assessed 5, 15, and 30 min after flumazenil administration (Figures 1, 2). In addition, heart rate, urine dribbling, hyperesthesia, vocalization, and vomiting were recorded during the monitoring period. After the 30-min time point, all subsequent treatment interventions provided to clinical cases were at the discretion of the primary clinician.
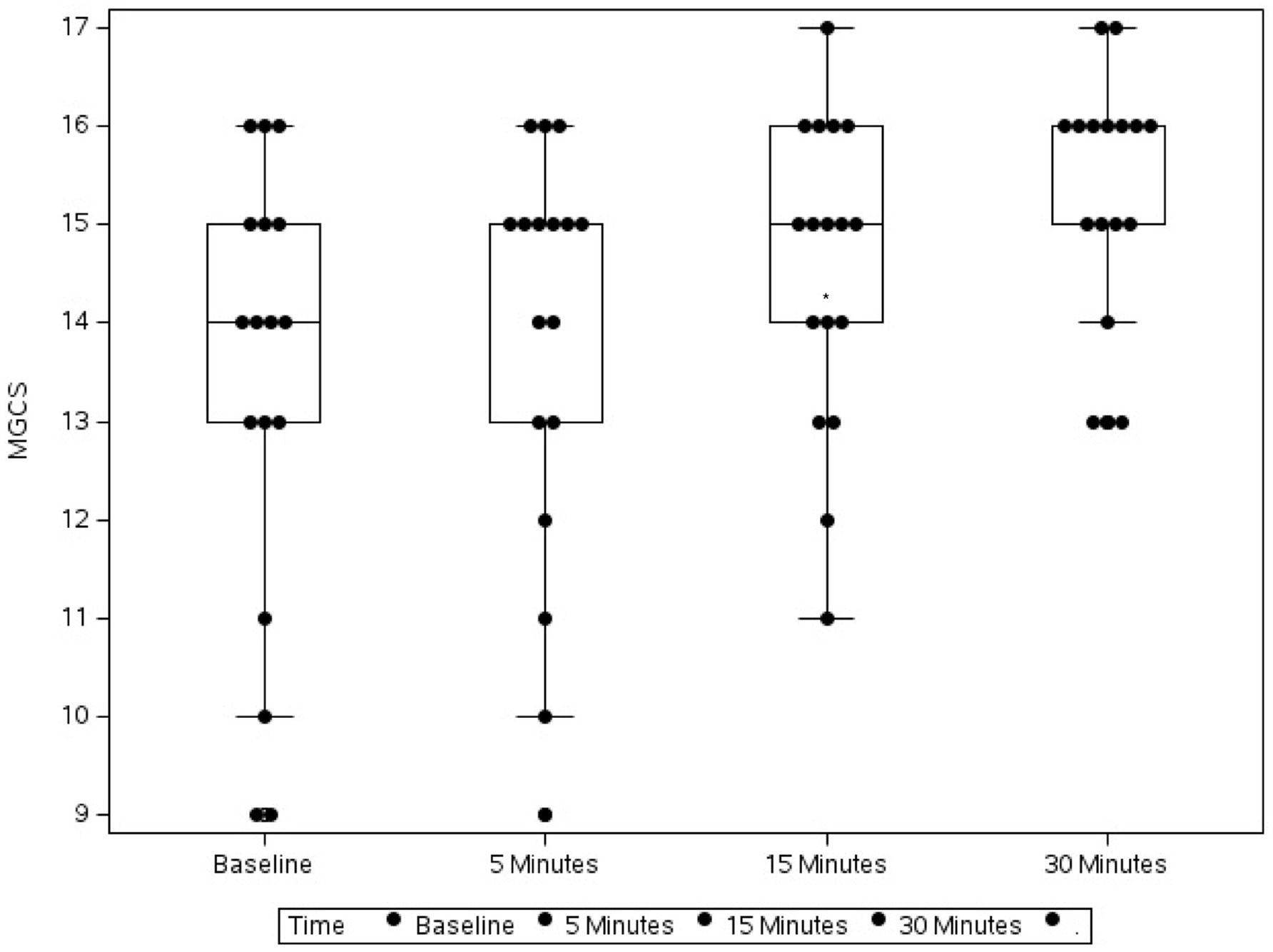
Figure 1. Box plot showing the change in Modified Glasgow Coma Scale (MGCS) in 17 dogs following flumazenil administration at baseline (median 16, range 9–17), 5 min (median 16, range 12–17), 15 min (median 16, range 13–16), and 30 min (median 17, range 13–18) * Indicates statistical significance.
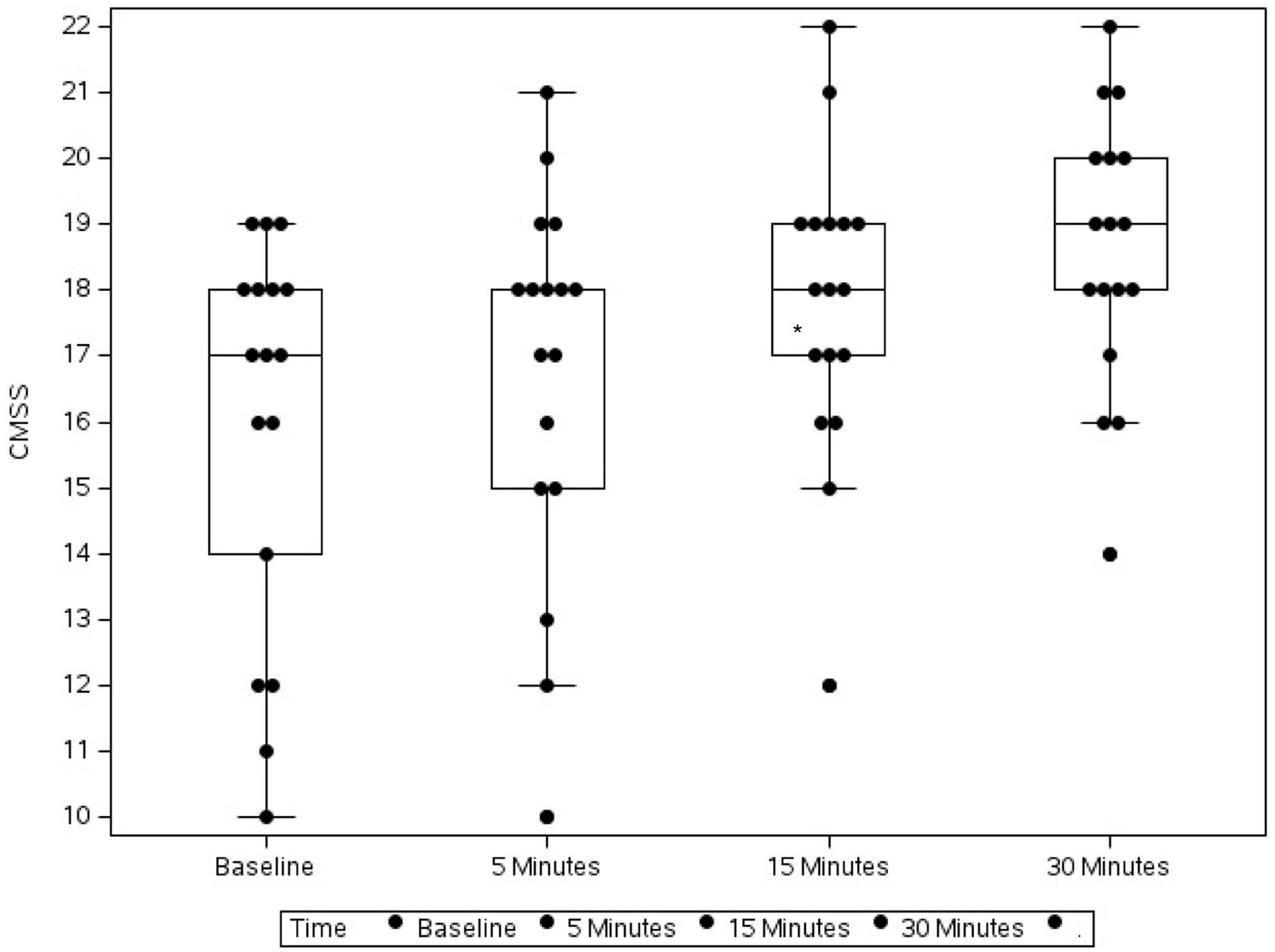
Figure 2. Box plot showing the change in Canine Marijuana Severity Score (CMSS) in 17 dogs following flumazenil administration at baseline (median 17, range 10–19), 5 min (median 18, range 10–21), 15 min (median 18, range 12–22), and 30 min (median 19, range 14–22) * Indicates statistical significance.
The collected blood was immediately centrifuged at 3,400 rpm for 10 min. The serum was then carefully pipetted into separate plastic cryovials and frozen at −20°C or −80°C. Samples collected over the first 2-months were stored in a −20°C freezer. After that time point, the hospital obtained a −80°C freezer and samples were subsequently stored there. After 12 months of sample collection, samples were shipped together overnight on ice to for batch analysis of THC via ultra-performance liquid chromatography electrospray ionization and mass spectrometry (UPLC-ESI-MS/MS) using a previously validated method for canine serum (22).
Statistical methods
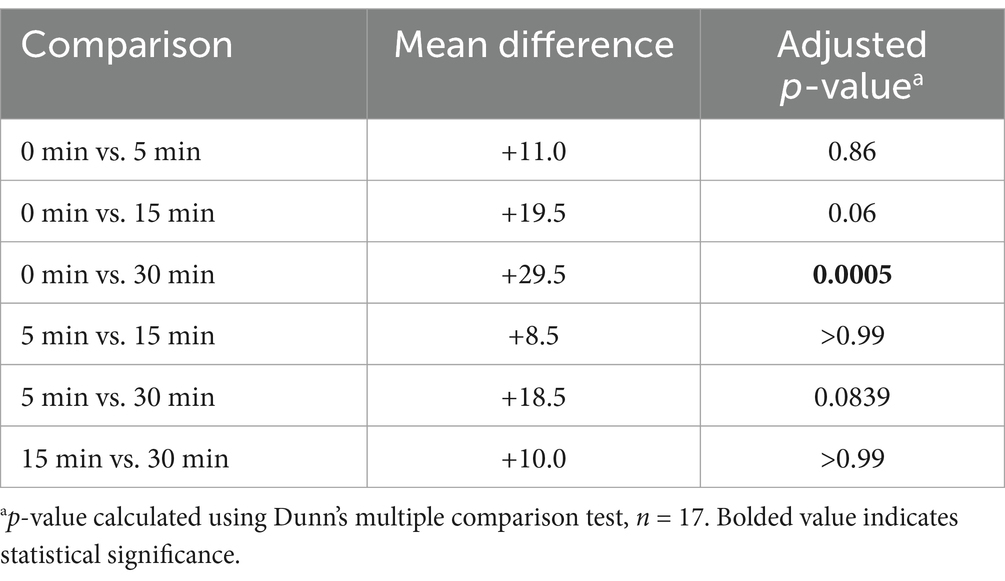
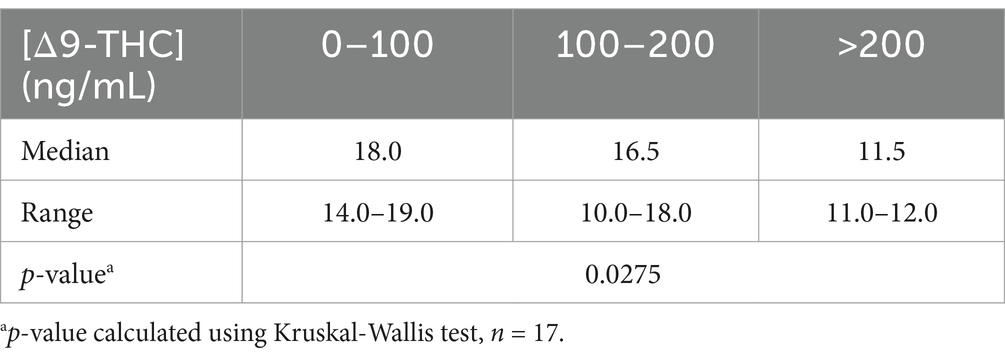
Data were analyzed using statistical software (GraphPad Prism 10.1.2). Continuous variables were assessed for normality using Q-Q plots and Shapiro–Wilk tests. Normality was not met for any of the variables being evaluated, including following log transformations. Change in MGCS and CMSS from baseline to 30 min following flumazenil administration were analyzed using Friedman tests. The change in median CMSS was analyzed between several time points using Dunn’s multiple comparison tests. For analysis, serum THC concentrations were grouped into 0–100 ng/mL, 100–200 ng/mL and >200 ng/mL and were compared to baseline CMSS using a Kruskal-Wallis test. Comparison of patient weights between each of the THC range categories was also calculated using a Kruskal-Wallis test. Significance was determined by a p-value < 0.05.
Results
Of the 17 patients enrolled, 4/17 had confirmed marijuana exposure (2 chocolate-based marijuana products, 1 marijuana plant material and 1 gummy product), and 13/17 were suspected exposures based on the history and the patients’ physical examination. The most common clinical signs were ataxia, hyperesthesia, urine dribbling and cranial nerve deficits (Table 1). Heart rates were recorded in 10/17 patients and rectal temperatures were recorded in 15/17 patients. Patients were more often hyperthermic (rectal temperature 102.5, 6/15) and tachycardic (heart rate > 120 beats per minute) in this group. Only 2/17 (11.8%) patients were treated as inpatients and all others were treated as outpatients. One patient had a history of well-controlled seizures and received phenobarbital and cannabidiol supplements. No other pertinent medical history was noted for any other patient.
Over the 30-min monitoring period there was a significant change in median baseline scores following flumazenil administration for both MGCS and CMSS (Figures 1, 2). The baseline median MGCS was 16 (9–17), at 5 min post-flumazenil was 16 (12–17), at 15 min was 16 (13–16) and at 30 min was 17 (13–18). The median CMSS at baseline was 17 (10–19), at 5 min was 18 (10–21), at 15 min was 18 (12–22), and at 30 min was 19 (14–22). Following Dunn’s multiple comparisons there was a statistically significant improvement in MGCS and CMSS scores from 0 to 30 min (Table 2).
Serum THC was analyzed in all 17 patients. Detectable concentrations of THC were identified in 16 of 17 dogs (94%). The average serum THC concentration in this cohort was 214.93 ng/mL (range: 0–1725.4 ng/mL). There was a significant inverse relationship between the serum concentration of THC and baseline CMSS (Table 3). In patients with serum THC concentrations between 0 and 100 ng/mL the average score was 18, between 100 and 200 ng/mL the average score was 16.5, and >200 ng/mL the average score was 11.5. There were no significant differences when comparing patient weight to each of the THC concentration range categories (p = 0.19). In 4/17 patients, there was no change from baseline CMSS to 30 min after flumazenil administration. Additionally, 1/4 dogs with no change in CMSS following flumazenil also had a decreased menace response in the right eye and absent nasal sensation bilaterally on physical exam. This prompted additional neurologic work up which resulted in the diagnosis of meningoencephalitis of unknown etiology based on MRI and THC toxicosis based on the presence of THC in serum. This patient had a normal CSF tap and diffuse meningeal enhancement on MRI. One patient vomited and and two patients vocalized during the monitoring period. Aside from one patient vomiting, no other adverse effects were recorded. The patient with a previous history of seizures had no reported seizure activity 24 h after discharge.
Discussion
We evaluated the impact of flumazenil administration as an adjunctive treatment for dogs with symptomatic marijuana toxicosis. Symptoms were assessed using an established scoring scheme (MGCS) and a novel score (CMSS). Flumazenil administration was associated with improvements in both scores within 30-min, consistent with reduced severity of neurologic signs. Quantifiable THC concentrations were identified in all but one dog. Dogs with lower CMSS tended to have significantly higher serum THC concentrations. This novel score therefore shows promise as a novel indicator of clinical severity in affected dogs.
Marijuana toxicosis in dogs is common, usually causing significant but self-limiting morbidity in dogs. Fatalities have been reported however and more specific interventions might be necessary to hasten clinical recovery (1). In veterinary medicine, flumazenil is primarily used to reverse paradoxical excitation and prolonged recovery times following benzodiazepine administration (23). Flumazenil is used in human medicine to reverse the effects of several toxicants, including marijuana (14, 16–19). The aim of this study, therefore, was to assess whether flumazenil administration was associated with improvement in clinical signs in dogs with symptomatic THC toxicosis. In our study, 13/17 and 10/17 affected dogs had improved ( 1 change in score from baseline) CMSS and MGCS scores, respectively, within 30-min of flumazenil administration, consistent with less severe neurologic signs (Supplementary Tables S2, S3). MGCS may be a less sensitive scoring system compared to the novel CMSS. The frequency of ataxia in this cohort was 70% and the MGCS does not assess this parameter unlike the CMSS. We did not observe statistical improvement in either score within 5 or 15-min of flumazenil administration. Interestingly, the peak effect of flumazenil occurs 6–10 min after administration in people (15), with an elimination half-life of 40–60 min. In dogs given tiletamine-zolazepam, there is a dose-dependent improvement in the ability to stand and walk 10 min after flumazenil administration (23). It is possible that an additional or higher dose of flumazenil would have achieved a faster clinical response. It is also plausible that the weak GABAA antagonist effect of flumazenil on THC is delayed in comparison to the stronger competitive antagonistic effect seen with flumazenil and benzodiazepines. It is possible that the dogs included in our study might have improved on their own despite the use of flumazenil. However, given the biological half-life of THC is approximately 30 h, spontaneous clinically overt improvements within 30 min are less plausible (6, 9).
Cranial nerve deficits were present in 58% of affected dogs in our study, which appears higher than previous reports in the veterinary literature (4, 8). Delayed PLR in one or both eyes was the most common cranial nerve deficit noted in this cohort. Binagia et al. recently published a large retrospective study of patients with canine marijuana toxicosis, reporting delayed or absent PLR in 1.3% of cases (4). The reason for these differences is not known, although interobserver variation between clinicians performing cranial nerve exams is plausible. The PLR was assessed as normal by the end of the 30-min monitoring period in 6/10 patients suggesting there was an observable change in speed in the PLR response. In humans there appears to be a correlation between higher THC concentrations and more delayed PLR reflexes. The average serum THC concentration in this cohort of dogs was higher than previously reported, which could explain the increased frequency of PLR abnormalities (22).
One dog in this pilot study was diagnosed with both marijuana toxicosis and meningitis of unknown etiology. In humans, there is a possible association between THC and the acute onset of necrotizing encephalitis without evidence of infectious etiology (24). In dogs with steroid-responsive meningitis-arteritis there are increased circulating endocannabinoids, and THC can directly increase the concentration of plasma endocannabinoids (10, 25). While there is literature showing the anti-inflammatory properties of endocannabinoids, both in vivo and in vitro work has shown that AG-2 can play a pro-inflammatory role via the production of reactive oxygen species, promoting white blood cell migration and stimulating pro-inflammatory cytokine release (10). This suggests that the immunomodulatory action of THC could contribute to a pro-inflammatory state that led to the onset of meningoencephalitis in this patient.
There are sparse reports in the human literature stating reversal with flumazenil can cause seizures and cardiac arrhythmias. Reports are often associated with high flumazenil dosing, history of chronic benzodiazepine use, or history of multiple drug co-ingestion (15). In a large observational study in humans, flumazenil administration did not result in seizures or arrhythmias in those patients despite seizure history and multiple drug co-ingestion (18). Other minor side effects of flumazenil in humans are vomiting, salivation, and anxiety (18). In a study where beagles are given flumazenil to reverse tiletamine-zolazepam, seizures, hypersalivation, vocalization, and opisthotonos were reported at doses 8–16 times the dose used in this pilot study (0.01 mg/kg) (23). One dog in this pilot study vomited during the monitoring period but had a history of vomiting prior to presentation, which could be related to prior marijuana ingestion or flumazenil administration. One dog enrolled had a history of well-controlled seizures on phenobarbital and CBD and was seizure-free for more than a year, and no seizure activity was noted during monitoring or in the 24 h after discharge after follow-up with the owner. However, given the small cohort in this study it cannot be determined whether there is an increased risk of seizures when giving flumazenil at standard doses to patients with a history of seizures. It also is prudent to mention that if a dog does have a seizure after flumazenil administration, benzodiazepines may be less effective in terminating the seizure.
In our study, dogs with higher serum THC concentrations had lower CMSS at baseline. This could indicate that these patients were more severely affected; however, we collected serum on presentation and dogs were presented at different time points post-exposure. For three of these dogs, serum was stored in a −20°C freezer until a −80°C freezer was later purchased by the hospital. Resin-based marijuana products with varying levels of THC concentrations showed no statistical difference in THC concentrations over a four-year time period when stored at −20°C (26). Therefore, differences in THC stability between the samples stored in −20°C and −80°C freezer are unlikely.
One patient had a serum THC concentration > 1,500 ng/mL, the highest in this cohort, and concurrently had the lowest CMSS score (11/22). There were only 3 patients with CMSS scores of 12 or less. Those patients, while all having a significant change from baseline CMSS (+2 or greater) across the 30 min, 2/3 had dramatic changes in CMSS from baseline (+5 and + 6). Two of the dogs’ gait were unable to be assessed to due severe impairment (gait contributed 0 to their score) which impacted their overall baseline score. All patients had detectable concentrations of THC in serum except for one patient whose score was unchanged throughout the monitoring period. This patient’s serum was stored at −80°C immediately after collection until shipment for analysis.
Limitations of this study include lack of a control group, small sample size, different scoring assessors, different storage conditions, and the paucity of moderate and severe marijuana toxicosis patients. There was a significant change in CMSS and MGCS after 30 min following flumazenil administration and for some patients this resulted in either mild clinical improvement or no change from baseline. While it appears that flumazenil is well-tolerated in this small cohort of patients, it should still be used with caution depending on the patient factors and exposure scenario.
In conclusion, flumazenil might be considered as an adjunctive treatment for marijuana toxicosis based on our observations of improved neurological symptoms within 30 min of administration in affected dogs. The CMSS scoring system appeared to identify dogs with higher serum THC concentrations and more severe symptoms. Finally, we report on a novel case of concurrent marijuana toxicosis and meningitis of unknown etiology in one dog.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by the ACI Bioscience Animal Care and Use Committee. The protocol/approval number is AUP-21-21. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
AF: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal analysis, Writing – review & editing. SS: Data curation, Methodology, Software, Writing – review & editing. SF: Data curation, Funding acquisition, Writing – review & editing. AL: Data curation, Software, Writing – review & editing. MR: Data curation, Investigation, Writing – review & editing. SM: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Internal funding was provided for laboratory analysis by Kansas State University and Veterinary Diagnostic Laboratory, hospital funding by Wheat Ridge Animal Hospital and by the support of Ethos Discovery.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1516181/full#supplementary-material
Abbreviations
2-AG 2-Arachidonoylglycerol ANA Anandamide CB 1 Cannabinoid receptor 1 CB 2 Cannabinoid receptor 2 CMSS Canine marijuana severity score GABAA receptor γ-aminobutyric acid type A receptor MGCS Modified Glasgow coma scale PLR Pupillary light response THC Delta-9-tetrahydrocannabinol UPLC-ESI-MS/MS Ultra-performance liquid chromatography electrospray ionization and mass spectrometry
References
1. Meola, SD, Tearney, CC, Haas, SA, Hackett, TB, and Mazzaferro, EME. Evaluation of trends in marijuana toxicosis in dogs living in a state with legalized medical marijuana: 125 dogs (2005-2010). J Vet Emerg Crit Care. (2012) 22:690–6. doi: 10.1111/j.1476-4431.2012.00818.x
2. Amissah, RQ, Vogt, NA, Chen, C, Urban, K, and Khokhar, J. Prevalence and characteristics of cannabis-induced toxicoses in pets: results from a survey of veterinarian in North America. PLoS One. (2022) 17:e0261909. doi: 10.1371/journal.pone.0261909
3. Howard-Azzeh, M, Pearl, DL, Swirski, A, Ward, M, Hovdey, R, O’Sullivan, TL, et al. The impact of state cannabis legislation, county-level socioeconomic and dog-level characteristics on reported cannabis poisonings of companion dogs in the USA (2009-2014). PLoS One. (2021) 16:e0250323. doi: 10.1371/journal.pone.0250323
4. Binagia, EM, Gregory, EA, and Yankin, I. Clinical examination findings and electrolyte abnormalities of dogs with marijuana/tetrahydrocannabinol toxicity: 223 cases (January 2017-July 2021). J Am Vet Med Assoc. (2024) 262:1047–54. doi: 10.2460/javma.24.02.0092
5. Janczyk, P, Donaldson, CW, and Gwaltney, S. Two hundred and thirteen cases of marijuana toxicoses in dogs. Vet Hum Toxicol. (2004) 46:19–21.
6. Brutlag, A, and Hommerding, H. Toxicology of marijuana, synthetic cannabinoids and Cannabidiol in dogs and cats. Vet Clin North Am Small Anim Pract. (2018) 48:1087–102. doi: 10.1016/j.cvsm.2018.07.008
7. National Institute on Drug Abuse. Cannabis potency data. Available at: https://nida.nih.gov/research/research-data-measures-resources/cannabis-potency-data (Accessed July 16, 2024).
8. Culler, CA, and Vigani, A. Successful treatment of a severe cannabinoid toxicity using extracorporeal therapy in a dog. J Vet Emerg Crit Care. (2019) 29:674–9. doi: 10.1111/vec.12899
9. Fitzgerald, KT, Bronstein, AC, and Newquist, KL. Marijuana poisoning. Top Companion Anim Med. (2013) 28:8–12. doi: 10.1053/j.tcam.2013.03.004
10. Freundt-Revilla, J, Heinrich, F, Zoerner, A, Gesell, F, Beyerbach, M, Shamir, M, et al. The endocannabinoid system in canine steroid-responsive meningitis-arteritis and intraspinal spirocercosis. PLoS One. (2018) 13:e0187197. doi: 10.1371/journal.pone.0187197
11. Sigel, E, Baur, R, Racz, I, Marazzi, J, Smart, TG, Zimmer, A, et al. The major central endocannabinoid directly acts at GABAA receptors. Proc Natl Acad Sci USA. (2011) 108:18150–5. doi: 10.1073/pnas.1113444108
12. Sethi, BB, Trivedi, JK, Kumar, P, Gulati, A, Agarwal, AK, and Sethi, N. Antianxiety effects of cannabis: involvement of central benzodiazepine receptor. Biol Psychiatry. (1986) 21:3–10. doi: 10.1016/0006-3223(86)90003-x
13. Cylinder, DM, AAJ, v Z, Solt, KM, and van Swinderen, B. Time to wake up! The ongoing search for general anesthetic reversal agents. Anesthesiology. (2024) 140:610–27. doi: 10.1097/ALN.0000000000004846
14. Trotti, LM, Saini, P, Koola, C, LaBarbera, V, Bliwise, DL, and Rye, DB. Flumazenil for the treatment of refractory hypersomnolence: clinical experience with 153 patients. J Clin Sleep Med. (2016) 12:1389–94. doi: 10.5664/jcsm.6196
15. Sivilotti, MLA. Flumazenil, naloxone and the ‘coma cocktail’. Br J Clin Pharmacol. (2015) 81:428–36. doi: 10.1111/bcp/12731
16. Karakosta, A, Andreotti, B, Chapsa, C, Pouliou, A, and Anastasiou, E. Flumazenil expedites recovery from sevoflurane/remifentanil anesthesia when administered to healthy unpremedicated patients. Eur J Anaesthesiol. (2010) 27:955–9. doi: 10.1097/EJA.0b013e3283398ef9
17. Safavynia, SA, Keating, G, Spiegel, I, Fidler, JA, Kreuzer, M, Rye, DB, et al. The effects of GABAA receptor modulation by flumazenil on emergence from general anesthesia. Anesthesiology. (2016) 125:147–58. doi: 10.1097/ALN.0000000000001134
18. Rasimas, JJ, Kivovich, V, Sachdeva, KK, and Donovan, JW. Antagonizing the errors of history: bedside experience with flumazenil. Toxicol Commun. (2020) 4:25–39. doi: 10.1080/24734306.2020.1752551
19. Rubio, F, Quintero, S, Hernandez, A, Fernandez, S, Cozar, L, Lobato, IM, et al. Flumazenil for coma reversal in children after cannabis. Lancet. (1993) 341:1028–9. doi: 10.1016/0140-6736(93)91120-b
20. Platt, SR, Radaelli, ST, and McDonnell, JJ. The prognostic value of the modified Glasgow coma scale in head trauma in dogs. J Vet Intern Med. (2001) 15:581–4. doi: 10.1111/j.1939-1676.2001.tb01594.x
21. Schmitz-Hubsch, T, du Montcel, T, Baliko, L, Berciano, J, Boesch, S, Depondt, C, et al. Scale for the assessment and rating of ataxia, development of a new clinical scale. Neurology. (2006) 66:1717–20. doi: 10.1212/01.wnl.0000219042.60538.92
22. Fitzgerald, AH, Zhang, Y, Fritz, S, Whitehouse, WH, Brabson, T, Pohlman, L, et al. Detecting and quantifying marijuana metabolites in serum and urine of 19 dogs affected by marijuana toxicity. J Vet Diagn Invest. (2021) 33:1002–7. doi: 10.1177/10406387211027227
23. Lee, JY, Son, SJ, Jang, S, Choi, S, and Cho, DW. Antagonistic effect of flumazenil on tiletamine-zolazepam-induced anaesthesia in beagle dogs. Vet Med (Praha). (2018) 63:555–60. doi: 10.17221/65/2018-VETMED
24. Aslam, K, Uldin, H, and Smith, L. Possible toxin-induced acute necrotizing encephalitis (ANE) with secondary vasculopathy and paroxysmal autonomic instability with dystonia (PAID) syndrome. Cureus. (2023) 15:1–6. doi: 10.7759/cureus.50100
25. Thieme, U, Schelling, G, Hauer, D, Greif, R, Dame, T, Laubender, RP, et al. Quantification of anandamide and 2-arachidonoylglycerol plasma levels to examine potential influences of tetrahydrocannabinol application on the endocannabinoid system in humans. Drug Test Anal. (2014) 6:17–23. doi: 10.1002/dta.1561
Keywords: tetrahydrocannabidiol, ultraperformance liquid chromatography, toxicology, marijuana, flumazenil
Citation: Fitzgerald AH, Zhang Y, Stewart S, Fritz SA, Lynch AM, Ramras M and Meola SD (2024) Flumazenil may improve gait and mentation in dogs presenting with marijuana toxicosis. Front. Vet. Sci. 11:1516181. doi: 10.3389/fvets.2024.1516181
Edited by:
Arturo Anadón, Complutense University of Madrid, SpainReviewed by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyMahmoud Kandeel, King Faisal University, Saudi Arabia
Copyright © 2024 Fitzgerald, Zhang, Stewart, Fritz, Lynch, Ramras and Meola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alyson H. Fitzgerald, YWZpdHpnZTVAbmNzdS5lZHU=
 Alyson H. Fitzgerald
Alyson H. Fitzgerald Yuntao Zhang2
Yuntao Zhang2 Samuel Stewart
Samuel Stewart Alex M. Lynch
Alex M. Lynch