- 1Department of Poultry Science, University of Georgia, Athens, GA, United States
- 2Paklihawa Campus, Institute of Agriculture and Animal Science, Tribhuvan University, Bhairahawa, Nepal
- 3Department of Health Management, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PE, Canada
- 4Faculty of Animal Science, Veterinary Science and Fisheries, Agriculture and Forestry University, Bharatpur, Nepal
- 5Department of Food Hygiene and Environmental Health, Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland
- 6Department of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Oyo, Nigeria
- 7Department of Veterinary Services, Kwara State Ministry of Agriculture and Rural Development, Ilorin, Kwara, Nigeria
Interactions between humans and livestock could increase the risk of zoonotic disease transmission. In addition, limited knowledge of zoonoses and foodborne diseases among livestock farmers could heighten the risks of foodborne illness and outbreaks of zoonotic diseases. This study evaluated the awareness of zoonotic diseases and preventive practices for zoonotic and foodborne diseases among livestock farmers of the Chitwan, Rupandehi, and Tanahun districts of Nepal by conducting a cross-sectional survey of 280 livestock farmers. They were recruited using the purposive sampling method from October to December 2022. Descriptive statistics revealed that most (72.1%; n = 202/280) livestock farmers were aware of zoonosis. None of the farmers knew about the zoonotic nature of leptospirosis. Two-thirds of pig farmers (67%; n = 12/18) were aware of zoonotic transmission of swine flu, and more than half of the poultry (58%; 50/86) farmers knew about zoonotic avian influenza. The majority of the farmers who had dogs (83%) and cats (89.4%) in their homes or farms knew that rabies can be transmitted to humans from dogs or cats. The multivariable logistic regression analysis revealed that farmers from the Rupandehi district (aOR: 5.56; 95% CI: 2.18–14.22) and Chitwan (aOR: 6.52; 95% CI: 2.46–17.25) had a higher odds of having good preventive practices than those from Tanahun. Also, farmers who had no sickness in the past 6 months after consumption of animal products were three times (aOR: 2.98; 95% CI: 1.48–6.01) more likely to have better practices. Furthermore, secondary education (aOR: 3.64; 95% CI: 1.41–9.44) was a significant positive predictor of good zoonotic diseases and food safety preventive practices. Our study underscores the necessity to enhance Nepalese livestock farmers’ awareness and practices regarding zoonotic and foodborne diseases. It emphasizes the importance of understanding risks, effective behavioral change strategies, and engaging farmers in developing zoonotic disease and foodborne illness prevention programs.
1 Introduction
Zoonotic diseases, originating from animals and transmitted to humans, pose a significant global health threat. Approximately 60% of all infectious diseases are zoonotic, and up to 75% of newly emerging diseases have zoonotic origins (1). Recent pandemics, such as the COVID-19 and Mpox, are believed to have originated from animal reservoirs before human-to-human transmission (2–4). Human activities such as intensified agriculture and animal domestication have increased the risk of zoonotic diseases (5, 6). These zoonotic diseases whether endemic, epidemic, or pandemic, have far-reaching consequences for public health and the global economy (7).
Nepal is an agricultural country in South Asia where people interact closely with domestic animals and pets. Farming remains largely uncommercialized, with crop cultivation and livestock rearing being an essential activity for many Nepalese families. Many families grow crops and raise livestock for household needs, often relying on traditional farming methods, potentially increasing the risk of zoonotic disease (8). Livestock farming is increasingly recognized as a key driver for poverty reduction, ensuring food security and sustainable livelihoods (9). Farmers involved in livestock production are at elevated risk of zoonotic disease acquisition due to the varied nature and intensity of their animal interactions (10). The care and handling of animals, close contact with cattle or other animals, and consuming raw, uncooked, and tainted animal products are primary sources of human infections (11).
Additionally, farmers’ poor personal hygiene, lack of basic knowledge and practice regarding zoonosis, and disregard for biosecurity measures may contribute to the transmission of zoonotic and foodborne diseases (12). Similarly, farmers may be exposed to zoonotic infection from uterine discharge, an aborted fetus, and an infected placenta when they help the animals during parturition and neonatal care (13). Ethnicity, culture, and tradition can contribute to the vulnerability of specific farming populations to zoonotic diseases, as illustrated by practices such as consuming raw yak blood on special occasions in the Himalayan region and drinking cow urine in certain Hindu rituals. Other predisposing factors include the consumption of unpasteurized milk and undercooked meat is common in different ethnic groups of Nepal and increases the risk of foodborne illness caused by pathogens such as Escherichia coli, Salmonella, Campylobacter, Staphylococcus, and Mycobacterium (14–18).
In Nepal, where livestock holds significant economic, social, and cultural importance, frequent human-livestock interactions increase the risk of zoonotic infections (19). Diseases like avian influenza (20, 21), swine flu (22), anthrax (23), leptospirosis (24), zoonotic trematodes (25), helminths, and protozoal infections have been reported in both livestock and humans. However, the human disease burden and the ecology of these pathogens remain poorly understood, especially concerning their response to changing climates and land use (26). Additionally, although Brucella spp. and Mycobacterium bovis are common in livestock, their prevalence in humans is under-researched due to limited awareness and diagnostic capabilities (26). Six zoonotic diseases- Taeniasis/Cysticercosis/Neurocysticercosis, Leptospirosis, Hydatidosis, Brucellosis, Toxoplasmosis, and Avian Influenza– were designated as significant threats. In April 2021, the Nepalese Government updated the list to include Influenza (Avian and Seasonal), Rabies, Coronavirus (SARS-CoV and MERS-CoV, SARS-CoV2), Leptospirosis, Brucellosis, Salmonellosis, Leishmaniasis, Zoonotic Tuberculosis, Cestode (Cysticercosis/Hydatidosis) and Toxoplasmosis (27). Rabies has been reported to kill around 500 animals and up to 32 persons in recent years (28, 29) and recent rabies outbreaks in various domestic animals across western Nepal underscore the significance of our research (30).
Investigating the awareness of livestock farmers on zoonotic diseases can offer valuable insights into their knowledge of transmission routes and preventive measures. Evaluating the practices employed by livestock farmers concerning zoonotic diseases and food safety can identify gaps and shortcomings in current farming practices, hygiene measures, and disease surveillance systems. However, very few studies conducted in Nepal assessed awareness of zoonotic disease and food safety practices among livestock farmers (26, 31). Thus, this study was conducted to determine the zoonotic disease awareness and food safety preventive practices among livestock farmers in Nepal. Chitwan district has a high number of livestock farmers and is one of Nepal’s leading milk producers. Similarly Rupandehi district also have a high number of livestock farmers and border trade with India. Tanahun district was selected due to its geographical and economical difference with Chitwan and Rupandehi districts. This study can aid in customizing educational initiatives and interventions to promote safer farming practices, thereby mitigating the risk of zoonotic diseases, ensuring the health of both humans and animals and bolstering the sustainability of the livestock industry.
2 Methodology
2.1 Study design and study area
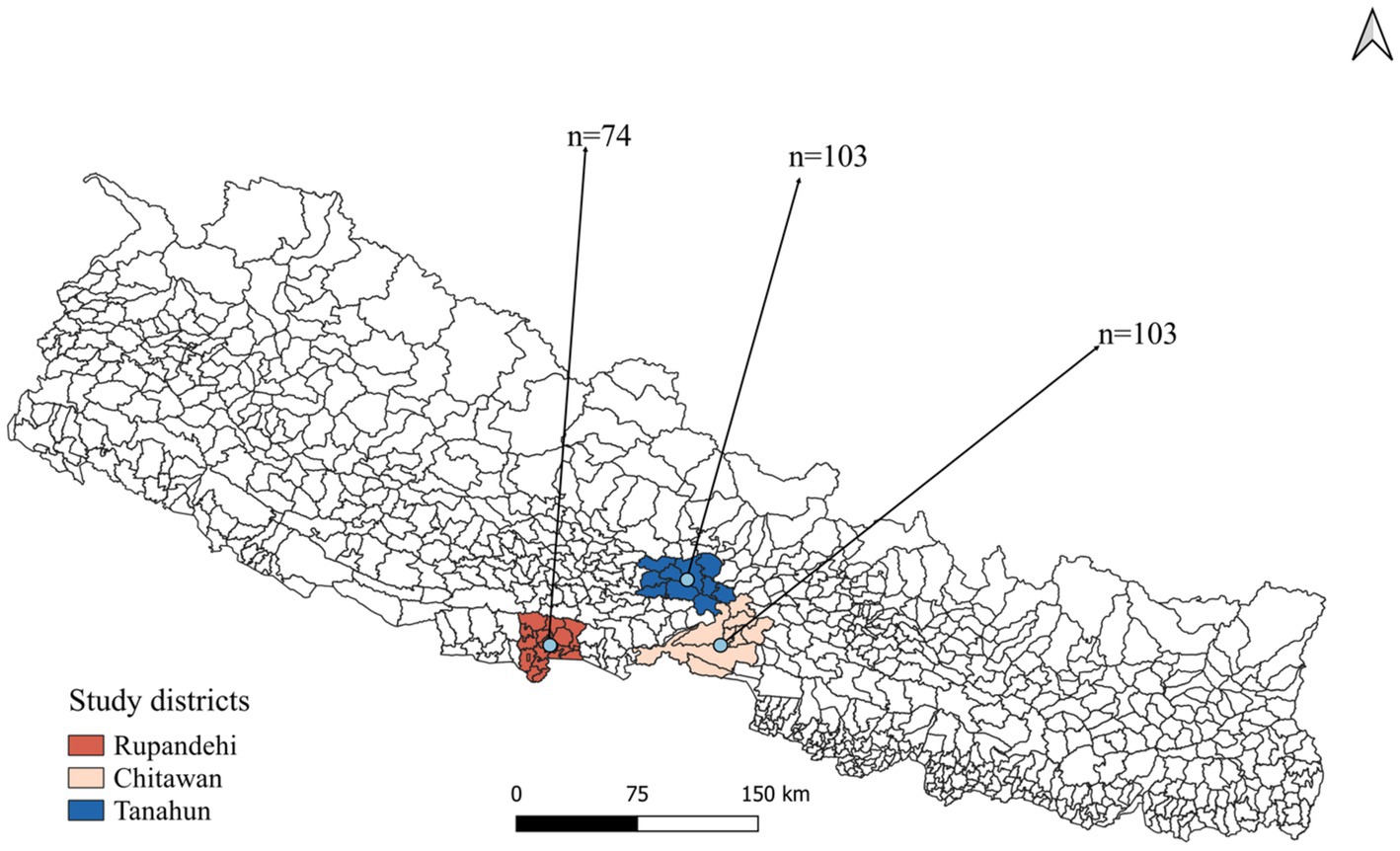
A cross-sectional study was designed to investigate livestock farmers’ awareness and practices toward zoonotic diseases and food safety in Rupandehi district from Lumbini province, Chitwan district from Bagmati province, and Tanahun district from Gandaki province of Nepal (Figure 1). From each district, more than 70 farmers were interviewed from October to December 2022. In this study, “farmer” refers to individuals engaged in farming or animal husbandry as either their primary or secondary occupation.
2.2 Study participants, sample size and sampling
Being a Nepalese citizen of age 18 years or above, residing within the country, and currently rearing livestock were used as the inclusion criteria for this study. Purposive sampling was used to enroll at least 70 farmers from each district. The primary data was collected using structured questionnaires to livestock farmers through face-to-face interviews. The objective and purpose of the study were initially described to the respondents during the interview, and the interviewer obtained their oral consent to participate in the study. The survey response was only obtained from those who provided consent and participated voluntarily in the study. To mitigate potential biases such as response bias, where participants may provide socially desirable answers, and recall bias, where participants may have difficulty accurately remembering past events, standardized questions, objective measures, short recall periods, cross-validation, and interviewer training were implemented.
2.3 Questionnaire design
The questionnaires were created based on a comprehensive literature assessment (26, 31) and the researcher’s knowledge. To improve its quality, the questionnaire was pre-tested among 20 farmers, but their responses were not included in the study. The questionnaire had sections for demographic information, types of animals reared, awareness of zoonotic diseases, and preventive practices for zoonotic diseases and food safety. The questionnaire was initially prepared in English and translated into Nepali for face-to-face interviews. The questionnaire was administered using the KoboToolbox and can be accessed here https://ee.kobotoolbox.org/x/OOYhnCfH.
2.4 Data analysis
2.4.1 Data processing and descriptive analysis
The obtained responses from the online survey were imported into Stata software (version 15.1). The data were then examined for duplicate entries and inconsistencies. Frequencies and percentages were calculated for the demographics of the farmers and their awareness of zoonotic diseases. A graphical bar chart was used to display the status of livestock ownership within the study population. Additionally, the collected responses regarding farmers’ awareness levels of various zoonotic diseases and their disease prevention and control practices were presented in tabular format, showing the frequency of each response.
2.4.2 Univariable and multivariable logistic regression
A univariable and multivariable logistic regression analysis was conducted to identify the association between demographic variables and practice levels. A scoring scale was devised since we needed a single practice-level variable representing the overall zoonotic disease and food safety practice standards. Eight practice questions (Table 3) were employed in developing this scale. A correct response was assigned a score of 1, while an incorrect response was given a score of 0. Each participant could achieve a score from 0 to 8 using this scale. Additionally, as followed by Subedi et al. (32), the threshold of 75% was established to convert the practice score into a binary scoring system, facilitating straightforward comparison and interpretation. Consequently, a score below six was categorized as “poor,” whereas a score of 6 or higher was classified as “good.” The independent demographic variables like farmer’s age, gender, education status, major occupation, farming experience, farmer illness status in the past 6 months, and herd size were considered for the univariable logistic regression model.
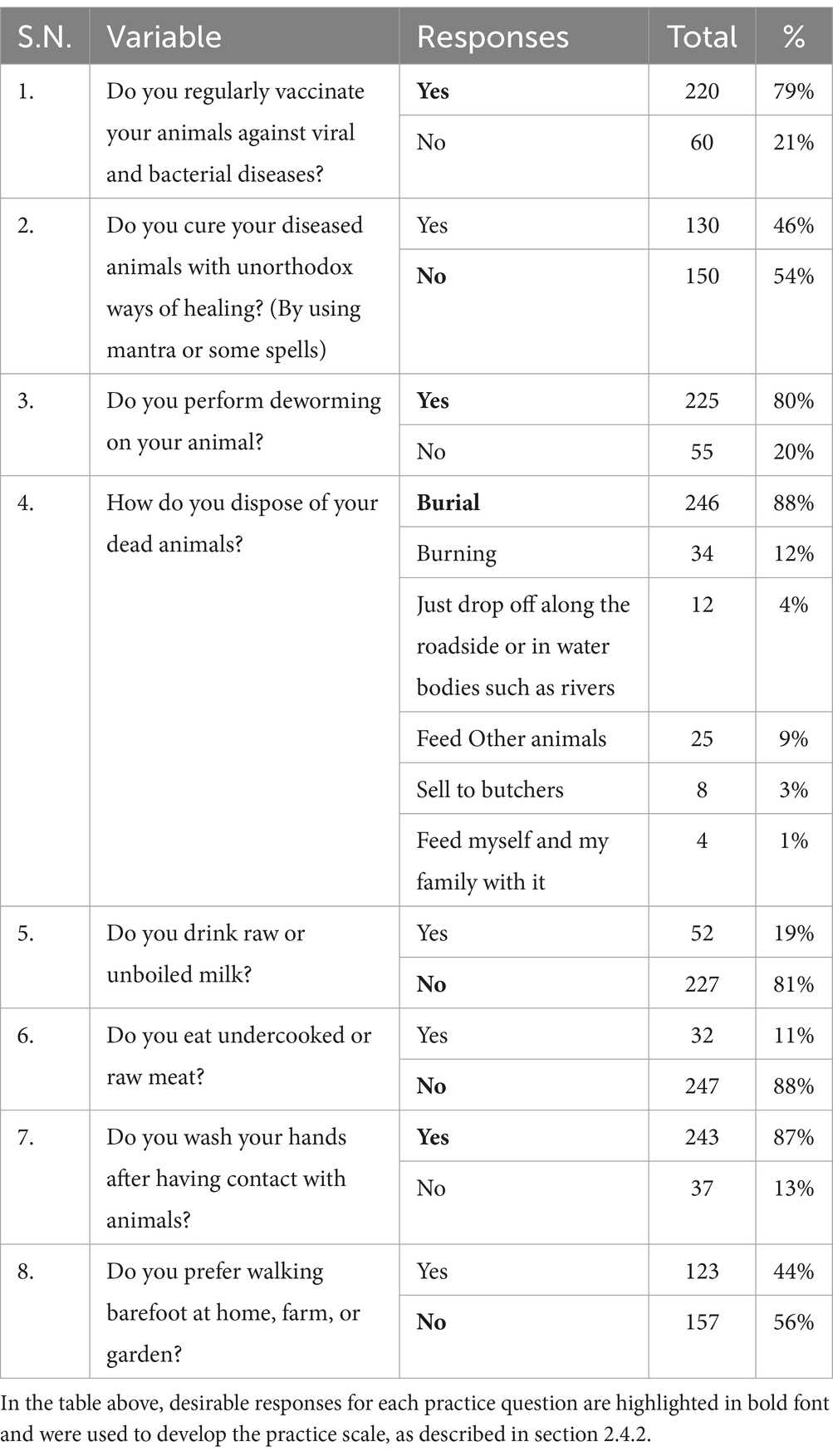
Table 3. Frequency table for the preventive practice of zoonotic diseases and food safety among Nepalese livestock farmers.
2.4.2.1 The equation for univariable logistic regression
In the Equation 1, the term “logit” refers to the natural logarithm of the odds of an event occurring. Here, p represents the probability of obtaining a good practice level, β0 is the intercept, and β1 is the coefficient of the independent demographic variable x. Independent variables (x) include demographic factors such as age, gender, education, district, experience, and occupation, as well as herd size and past illness history. Variables with a p-value <0.2 in the univariable logistic regression model were considered for inclusion in the multivariable logistic regression model.
2.4.2.2 Equation for multivariable logistic regression
In Equation 2, β0 is the intercept, and β1 to βk represents the coefficients of each variable (x1 to xk) included in the model. Collinearity among the independent variables was assessed in the multivariable model using the variance inflation factor (VIF) utilizing the “collin” command in Stata. Interactions between independent variables were examined, and significant interactions were incorporated into the final model. The goodness of fit of the final multivariable model was evaluated using the Hosmer-Lemeshow test. The coefficients of the logistic models were exponentiated and presented as odds ratio (OR) in the result section. Both the univariable and multivariable model results are presented in the same table. Coefficients are presented as odds ratios (OR) for the univariable logistic model and as adjusted odds ratios (aOR) for variables that met the criteria for the multivariable logistic model.
3 Results
3.1 Demographic description of participants
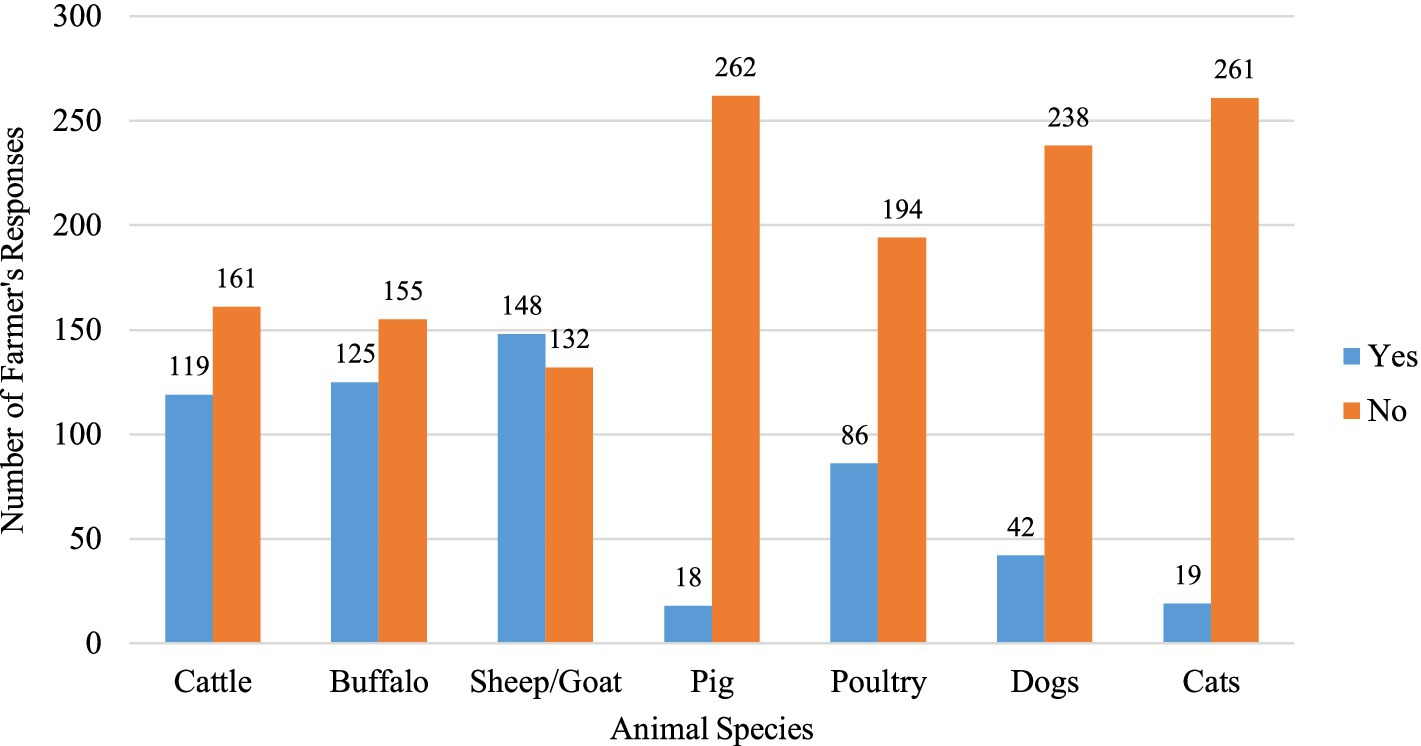
A total of 147 male and 133 female farmers participated in the study (Table 1). Among the participants, 38% were in the 50–59 age group, while 35% were aged between 30 and 39. Regarding the educational status of farmers, only 3.2% possessed tertiary education, while 38.9% had primary, and 32.9% lacked formal education. There were 103 farmers each from the Chitwan and Tanahun districts and 74 from the Rupandehi district. Livestock rearing was the primary occupation for the majority (53.6%) of the farmers, and about 10% had more than 30 years of farming experience. Family sizes varied, with around half (49.3%) of the farmers having five or fewer family members. One-fourth of the participants reported falling ill after recent contact with or consumption of animals or animal products. Approximately half (52.8%) of the farmers reported owning goats or sheep, while 42.5% owned cattle and 44.6% owned buffalo. A smaller percentage, 6.4%, mentioned owning pigs, and 30.7% had reared poultry. Additionally, 15% of the farmers reported having dogs, and 6.8% mentioned having cats on their farms or houses (Figure 2).
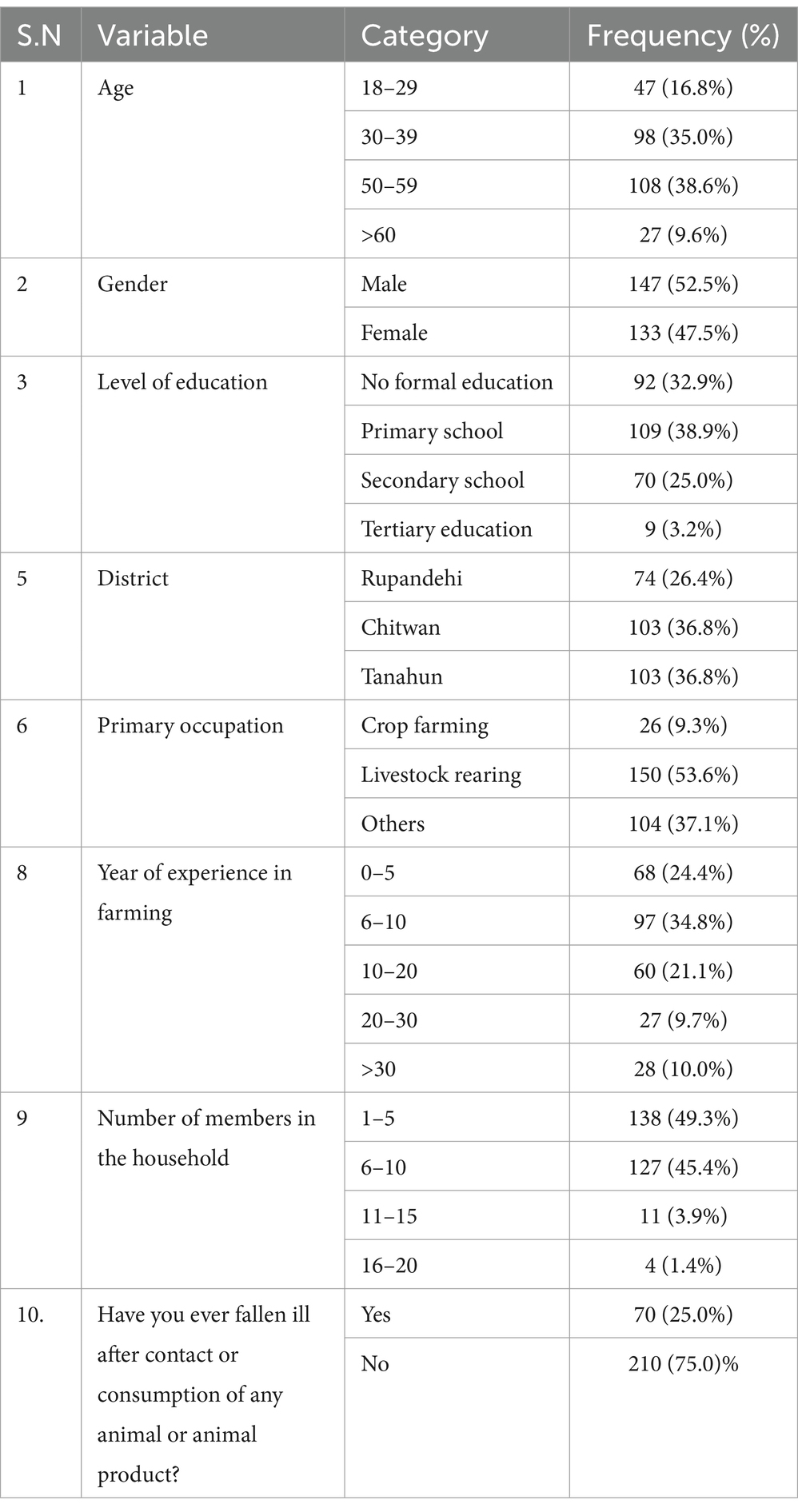
Table 1. Frequency table for demographic variables in awareness study of zoonotic diseases among livestock farmers of Nepal.
3.2 Awareness of zoonotic diseases
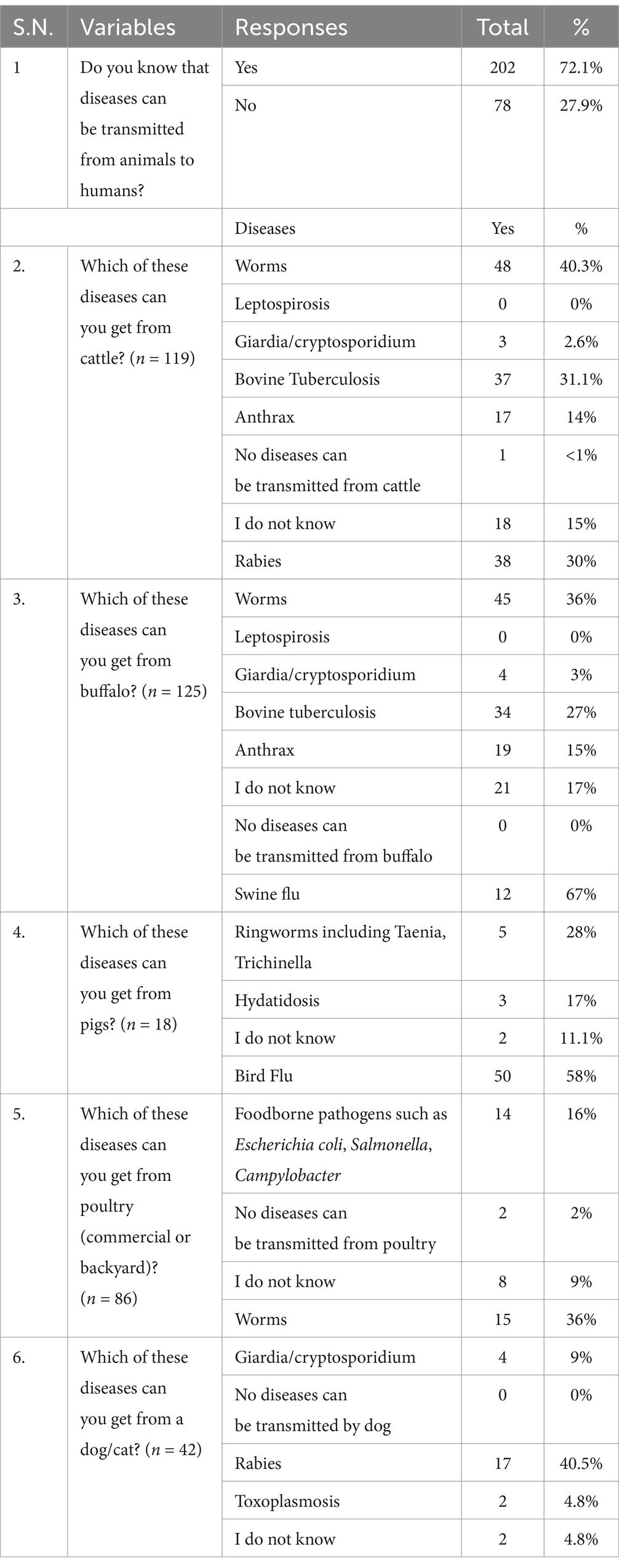
Out of 280 surveyed farmers, the majority (72.1%) knew that diseases could be transmitted from animals to humans (Table 2). However, among the 119 cattle and 125 buffalo farmers, only 31.9 and 30%, respectively, knew that cattle or buffalo can also be infected with rabies. None of the cattle and buffalo farmers knew the zoonotic nature of the leptospirosis. Similarly, only 31.1% of cattle and 27% of buffalo farmers knew bovine tuberculosis was a zoonotic disease. However, a significant proportion, 67% of pig farmers and 58% of poultry farmers were aware of the zoonotic nature of swine flu and bird flu, respectively. Only 16% of the poultry farmers were aware of the foodborne pathogens such as Escherichia coli, Salmonella, and Campylobacter. On a different note, most of farmers with dogs and cats were aware of the transmission of rabies to human from these animals.
3.3 Farmer’s disease prevention and containment practices
The majority of farmers in our study reported regular vaccination (79%) and deworming (80%) of their animals, respectively (Table 3). Almost half of the farmers (46%, n = 130/280) mentioned using unorthodox healing methods, such as mantras or spells, to treat their animals. While the majority of farmers mentioned burial and burning as methods for disposing of dead animals, a small percentage reported consuming dead animals (1%) and selling them to butchers (3%). Among the farmers surveyed, 11% reported consuming undercooked or raw meat, and 19% mentioned consuming raw or unpasteurized milk. While most farmers (87%) reported washing their hands after contact with animals, 44% preferred walking barefoot at home, on the farm, or in the garden.
3.4 Effect of sociodemographic factors on zoonotic diseases and food safety preventive practices among farmers
The results of univariable and multivariable logistic regression analysis are presented in Table 4. In the univariable logistic model, farmers’, education status, district, occupation, history of illness, and the herd size were significantly associated with good disease prevention and containment practices. However, in the multivariable logistic model, only the farmers’ district, and history of illness were significant, and education level was only marginally significant with the positive practice level (p = 0.045). Farmers from Rupandehi (OR: 5.56; 95% CI: 2.18–14.22) and Chitwan (aOR: 6.52; 95% CI: 2.46–17.25) had higher odds of having good preventive practices than farmers of Tanahun district. Farmers who were not sick within the last 6 months after contact with or consumption of any animal or animal product were three times more likely to have better practices of zoonotic disease and food safety than the farmers who were ill (aOR: 2.98; 95% CI: 1.48–6.01). Similarly, farmers with secondary education (aOR: 3.64; 95% CI: 1.41–9.44) were more likely to have good preventive practices than farmers with no formal education. Finally, farmers who were aware of the possibility of zoonotic disease transmission from their animals were more likely (OR: 4.2; 95% CI: 1.78, 9.92; p < 0.001) to have good practices of disease prevention.
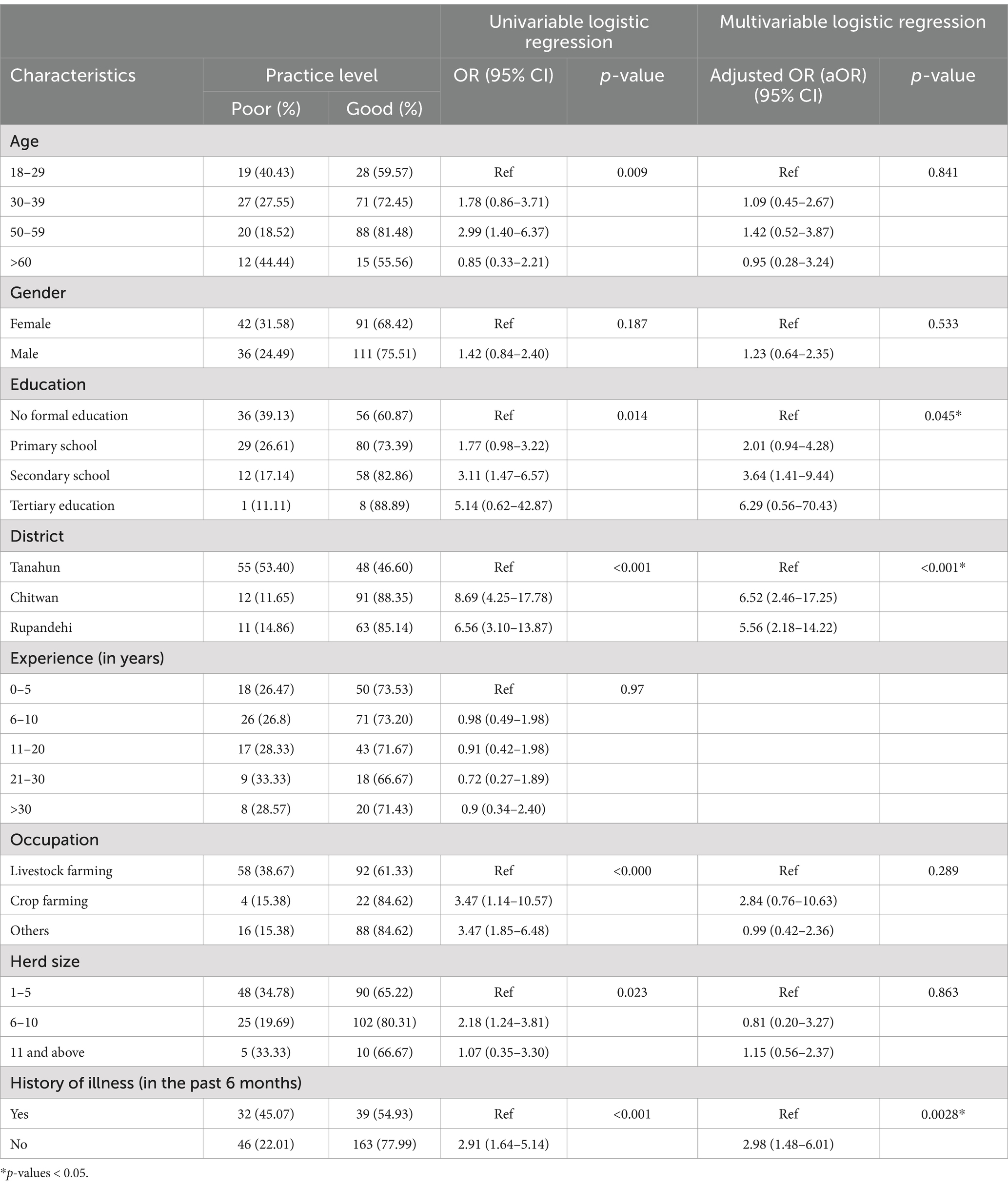
Table 4. Univariable and multivariate logistic regression model of the preventive practice of zoonotic diseases and food safety among Nepalese livestock farmers.
4 Discussion
The agricultural sector in Nepal provides livelihoods for roughly 66% of the population, with the livestock industry representing a key economic component, accounting for approximately 11.5% of the total GDP and 25.7% of the agricultural GDP (33). Agricultural workers are more vulnerable to several zoonotic diseases due to their frequent direct contact with animals, increased exposure to environmental pathogens, and higher risk of encountering disease vectors in their work settings (34). Lack of knowledge about disease transmission from animals to humans and their prevention strategies can lead to higher exposures to multiple zoonotic diseases. Identifying the awareness status of zoonotic diseases and their prevention strategies can help design effective awareness and targeted intervention strategies, particularly in lower middle-income countries like Nepal. This study aimed to assess livestock farmers’ awareness and practices related to zoonotic diseases and food safety.
Our findings indicate that approximately 72% of livestock farmers knew zoonotic disease transmission from animals to humans. This figure is notably higher than those reported in previous studies in Nepal (45% by Kelly et al.) (26) and in Ethiopia (45.1%) (35). Education level was a significant predictor of preventive practice adoption, with tertiary and secondary educated farmers showing markedly better practices than those without formal education, a finding corroborated by earlier work on rabies knowledge in Nepal (36). Moreover, a regional effect was observed, with farmers in the more commercialized Terai region (Rupandehi and Chitwan) demonstrating a greater propensity for adopting preventative measures compared to farmers in the less developed hilly region (Tanahun). These geographical disparities are likely linked to variations in the commercialization and advancement of the agricultural and livestock sectors across the different districts. Interestingly, farmers who did not get sick after coming into contact with animals or animal products in the past 6 months were more likely to follow good practices than those who had been ill.
Public health data from the Department of Health Services in Nepal indicated that about half of the population are at high risk of rabies exposure, with an additional quarter at moderate risk (13). In our study, only 31.9% of cattle farmers and 30% of buffalo farmers were cognizant of the risk of rabies transmission from infected cattle or buffalo. However, a majority of farmers who owned dogs and cats demonstrated awareness of rabies transmission from these animals. The perception of dogs and cats as major sources of rabies is reinforced by the higher incidence of rabies outbreaks in these populations and the targeted focus of rabies control efforts (37, 38). Data from the Far Western region of Nepal, although not within the current study area, illustrates the breadth of rabies presence in the country; over a one-year period (27 November 2022 to 22 November 2023), rabies was confirmed in 41 dogs, 26 bovines (comprising 15 cattle and 11 buffalo), 12 goats, three jackals, and one cat (30). Consequently, while public awareness campaigns often target companion animals, it is imperative that farmers are educated on the susceptibility of livestock to rabies to ensure comprehensive risk mitigation strategies.
Furthermore, a significant proportion of pig farmers (67%) and poultry farmers (58%) demonstrated awareness of zoonotic nature of swine flu and bird flu, respectively. These findings align with Bagale et al. (31), who also identified avian influenza, rabies, and swine flu as the most recognized zoonotic diseases, while simultaneously noting a lack of knowledge regarding bovine tuberculosis, neurocysticercosis, and brucellosis. Notably, no cattle and buffalo farmers in our study were aware of the potential for leptospirosis transmission to humans. This finding is consistent with a study conducted in the Rupandehi district, also part of our research area, where none of farmers had knowledge of leptospirosis (24). Similarly, Kelly et al. (26) reported a significant lack of awareness concerning livestock-associated zoonoses such as leptospirosis, brucellosis, anthrax, and tuberculosis in Nepal. In our study, awareness of zoonotic nature of anthrax was limited to approximately 15% of the farmers. This proportion is higher than that reported in prior study in Nepal (4%), but lower than that found in Turkey (62.8%) (39). The level of awareness of common zoonotic diseases is likely to be influenced by several factors including lifestyle, disease burden, access to animal health care services, and the educational level of individuals.
Bovine tuberculosis (bTB), also known as zoonotic TB, primarily transmits to humans through the ingestion of raw meat, unpasteurized dairy products, and occupational exposure to cattle (40, 41). Nepal reported 70,000 cases of human tuberculosis in 2022, resulting in 18,000 fatalities (42). A study in Chitwan, Nepal, documented that 15% of animals in 60 households with human tuberculosis cases had bTB, and human patients were engaged in livestock-related activities such as feeding and milking (43). Despite bTB being endemic in Nepalese cattle populations, only 35% of cattle and buffalo farmers in our study were aware of its zoonotic potential. This finding contrasts with Kelly et al. (26), who reported a lower awareness (14%) among farmers across three districts of Nepal. In our study, 11 and 19% of farmers reported consuming undercooked or raw meat and raw or unboiled milk (unpasteurized), respectively, indicating a potential route of zoonotic transmission as well as increased risk of foodborne illness. Multiple studies have underscored the heightened risk of human tuberculosis associated with cattle exposure (44, 45). A study conducted in Nepal demonstrated that individuals with a history of exposure to sick cattle, consumption of raw dairy products, or cattle-rearing at homes had a fourfold increased likelihood of developing tuberculosis compared to those without these exposures (46). The necessity of a collaborative One Health approach, encompassing animal, human, and environmental health sectors, is paramount to mitigating the burden of tuberculosis in Nepal.
The 2019 recording of the first human death in Nepal due to avian influenza (H5N1) emphasized the existing zoonotic risk for poultry farmers and workers, as well as their heightened vulnerability (20, 21). In our study, approximately 60% of surveyed farmers were aware of zoonotic nature of bird flu, possibly attributable to its endemicity in Nepal (47). Similar awareness level was observed in previous study, where over 60% of respondents acknowledged a personal risk of avian influenza infection and expressed related concerns (48). These findings suggest that while awareness regarding avian influenza as a zoonotic disease is relatively widespread in Nepal, the frequent recurrence of outbreaks signifies a critical deficiency in the practical application of preventive measures among farmers and workers.
Our findings indicate a low level of awareness regarding toxoplasmosis as a zoonotic disease transmissible from cats, with only 10.5% of participants demonstrating knowledge of this disease. Given the potential for increased Toxoplasma gondii transmission through interaction with cats, particularly free-roaming, this lack of awareness is a significant public health concern (49). Notably, T. gondii is recognized as a major etiological agent in abortion among pregnant women (50).
In contrast to the low awareness of zoonotic risks, a substantial majority (80%) of farmers in our study reported routinely vaccinating their livestock. Unlike our result, Bagale et al. (31) reported that approximately two-thirds of respondents administered prophylactic vaccination to cattle. These discrepancies may reflect the diverse agro-ecological zones and varying access to veterinary services in different regions of Nepal. As highlighted by Kelly et al. (26), routine immunization against common diseases like foot and mouth disease is more prevalent in regions with intensive dairy production. In other areas, government-led mass vaccination campaigns are primarily reactive, initiated in response to outbreaks, rather than proactively implemented. Regarding other preventative measures, our survey revealed that 80% of farmers reported deworming their livestock. These results are consistent with observations from a study in the Bagmati province of Nepal, where the majority of farmers reported engaging in regular vaccination (63%) and deworming (59%) practices (51).
The majority of farmers in our study mentioned burial and burning as methods for disposing of dead animals, and a small percentage reported consuming dead animals (1%) and selling them to butchers (3%). Recently, a study conducted in Nepal by Bagale et al. (31) found that 12% of respondents disposed of dead animals in nearby rivers, a notably higher proportion than our finding of 4% who reported discarding carcasses on roads or in water bodies. These practices, including the consumption or sell of deceased animals and improper disposal in environment, poses a substantial threat to food safety and the potential spread of waterborne diseases.
The consumption of unpasteurized milk and raw meat is well-established as a risk factor for the transmission of zoonotic pathogens including Brucella, Mycobacterium bovis, Salmonella, and Campylobacter, as well as parasites such as tapeworms (44, 52–54). In our study, 11% of the farmers reported consuming undercooked or raw meat, and 19% mentioned consuming raw or unboiled milk. These findings necessitate targeted public health interventions, including campaigns to raise awareness of the risks associated with consuming unpasteurized milk and raw meat, to mitigate foodborne and zoonotic disease transmission.
Consistent with prior research in Nepal, approximately 90% of farmers in our study reported practicing handwashing after animal contact. This aligns with findings from the Chitwan, Tanahun, and Gorkha districts (26) and Manang, Tanahun, and Nawalpur districts (31). However 44% farmers in this study prefer walking barefoot at home, farm, or garden. These findings highlight a discrepancy between hand hygiene and footwear practices, underscoring the need for comprehensive hygiene education programs to mitigate the dual risks of farmer and public health hazards from contaminated products.
This cross-sectional study provides valuable insights into Nepali livestock farmers’ knowledge and practices regarding foodborne illness, though it captures only a single point in time. Future research should extend and replicate this study with a longitudinal approach to monitor changes over time and include qualitative methods to explore the social (access to veterinary care), economic, cultural (culture of eating raw meat and milk), and environmental factors (climate change, regular floods and landslides) influencing the adoption of zoonotic disease prevention practices. Given the limited awareness, particularly among illiterate and rural farmers, there is a pressing need for targeted training and outreach programs. Implementing these programs through a One Health approach could improve livestock health, enhance community well-being, and contribute to poverty reduction and national economic growth.
We acknowledge that this study has some limitations. Firstly, data collection relied on personal interviews, which may have led to socially desirable responses, potentially impacting response accuracy. Secondly, the use of purposive sampling could introduce bias, as participants may refer to others with similar characteristics or opinions. Finally, the participants of the study were limited to specific districts of Nepal, so the observed awareness and practices may not be representative of the entire country. Hence, the findings should be interpreted with caution.
5 Conclusion
Our study emphasizes the importance of enhancing the knowledge of Nepalese livestock farmers on zoonoses and food safety. It advocates for adopting both existing and new health practices to mitigate the risk of zoonotic pathogen transmission and improve food safety. Particularly, it is paramount to address the limited awareness observed among less educated and rural farmers. This highlights the need for on-site training and outreach initiatives tailored to diverse educational backgrounds and regions. Bridging the gap between awareness and practice will entail further investments in disease surveillance and collaborative efforts with farmers to develop tailored training programs focused on effective zoonotic and foodborne illness prevention and control measures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Nepal Veterinary Council (NVC) (Ref. No.: 197/2078.79). Following the guidelines of the 2013 World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects, we ensured that participation was voluntary, informed consent was obtained from the participants, and they could withdraw from the survey anytime.
Author contributions
DS: Conceptualization, Data curation, Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review & editing. AD: Data curation, Project administration, Visualization, Writing – original draft, Writing – review & editing. SJ: Formal analysis, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. SP: Data curation, Funding acquisition, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. GR: Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. AT: Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. AA-M: Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors like to acknowledge all the farmers who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Woolhouse, MEJ, and Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. (2005) 11:1842–7. doi: 10.3201/eid1112.050997
2. Zhu, N, Zhang, D, Wang, W, Li, X, Yang, B, Song, J, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. (2019) 382:727–33. doi: 10.1056/nejmoa2001017
3. Andersen, KG, Rambaut, A, Lipkin, WI, Holmes, EC, and Garry, RF. The proximal origin of SARS-CoV-2. Nat Med. (2020) 26:450–2. doi: 10.1038/s41591-020-0820-9
4. Poudel, U, Subedi, D, Pantha, S, and Dhakal, S. Animal coronaviruses and coronavirus disease 2019: lesson for one health approach. Open Vet J. (2020) 10:239–51. doi: 10.4314/ovj.v10i3.1
5. Beran, GW. Disease and destiny-mystery and mastery. Prev Vet Med. (2008) 86:198–207. doi: 10.1016/j.prevetmed.2008.05.001
6. Pearce-Duvet, JMC. The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc. (2006) 81:369–82. doi: 10.1017/S1464793106007020
7. Salyer, SJ, Silver, R, Simone, K, and Behravesh, CB. Prioritizing zoonoses for global health capacity building—themes from one health zoonotic disease workshops in 7 countries, 2014–2016. Emerg Infect Dis. (2017) 23:S55–64. doi: 10.3201/eid2313.170418
8. Bagale, KB, and Adhikari, R. Risk of Zoonoses among livestock farmers in Nepal, journal of. Health Promot. (2019) 7:99–110. doi: 10.3126/jhp.v7i0.25520
9. The World Bank, Moving towards sustainability: the livestock sector and the World Bank, the World Bank (2021). Available at: https://www.worldbank.org/en/topic/agriculture/brief/moving-towards-sustainability-the-livestock-sector-and-the-world-bank.
10. Klous, G, Huss, A, Heederik, DJJ, and Coutinho, RA. Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health. (2016) 2:65–76. doi: 10.1016/j.onehlt.2016.03.001
11. Mpatswenumugabo, J.P., Mukasafari, M.A., Ndahetuye, J.B., and Mpatswenumugabo, J.P.M., A systematic review on milk consumption and associated bacterial zoonoses in east Africa, (2021) 1–25. doi: 10.1093/jambio/lxad080
12. Cediel, N, Conte, V, Tomassone, L, Tiberti, D, Guiso, P, Romero, J, et al. Risk perception about zoonoses in immigrants and Italian workers in northwestern Italy. Rev Saude Publica. (2012) 46:850–7. doi: 10.1590/S0034-89102012000500012
13. Alemayehu, G, Mamo, G, Desta, H, Alemu, B, and Wieland, B. Knowledge, attitude, and practices to zoonotic disease risks from livestock birth products among smallholder communities in Ethiopia. One Health. (2021) 12:100223. doi: 10.1016/J.ONEHLT.2021.100223
14. Bhandari, S, Subedi, D, Tiwari, BB, Shrestha, P, Shah, S, and Al-Mustapha, AI. Prevalence and risk factors for multidrug-resistant Escherichia coli isolated from subclinical mastitis in the western Chitwan region of Nepal. J Dairy Sci. (2021) 104:12765–72. doi: 10.3168/jds.2020-19480
15. Ranabhat, G, Subedi, D, Karki, J, Paudel, R, Luitel, H, and Bhattarai, RK. Molecular detection of avian pathogenic Escherichia coli (APEC) in broiler meat from retail meat shop. Heliyon. (2024) 10:e35661. doi: 10.1016/j.heliyon.2024.e35661
16. Tiwari, BB, Subedi, D, and Bhandari, S. Prevalence and risk factors of staphylococcal subclinical mastitis in dairy animals of Chitwan, journal of pure and applied microbiology Tiwari et al. J Pure Appl Microbiol. (2022) 16:1392–403. doi: 10.22207/JPAM.16.2.67
17. Gautam, A, Bastola, S, Lamsal, K, Kaphle, K, Shrestha, P, Shah, S, et al. Prevalence and risk factors of multidrug resistant (MDR) Escherichia coli isolated from Milk of small scale dairy buffaloes in Rupandehi, Nepal. Zoonot Dis. (2024) 4:174–86. doi: 10.3390/ZOONOTICDIS4030016
18. Subedi, D, Paudel, M, Poudel, S, and Koirala, N. Food safety in developing countries: common foodborne and waterborne illnesses, regulations, organizational structure, and challenges of food safety in the context of Nepal. Food Front. (2024):517. doi: 10.1002/FFT2.517
19. Gautam, A, Upadhayay, P, Ghimre, D, Khanal, A, Gaire, A, and Kaphle, K. Prioritised zoonotic diseases of Nepal: a review. Int J Appl Sci Biotechnol. (2021) 9:1–15. doi: 10.3126/IJASBT.V9I1.34967
20. Deepak, S, and Kaphle, K. Highly pathogenic avian influenza in Nepal. Int J Grad Res Rev. (2019) 5:194–203.
21. Subedi, D, Haris, M, Farhan, R, Niraula, A, Shrestha, P, Chandran, D, et al. Muhammad Ahmad, avian influenza in low and middle-income countries (LMICs): outbreaks, vaccination challenges and economic impact. Pak Vet J. (2024) 44:9–17. doi: 10.29261/pakvetj/2024.139
22. Adhikari, BR, Shakya, G, Upadhyay, BP, Prakash Kc, K, Shrestha, SD, and Dhungana, GR. Outbreak of pandemic influenza A/H1N1 2009 in Nepal. Virol J. (2011) 8:1–8. doi: 10.1186/1743-422X-8-133
23. Subedi, D, Pantha, S, Jyoti, S, Gautam, B, Kaphle, K, Yadav, RK, et al. Anthrax in humans, animals, and the environment and the one health strategies for Anthrax control. Pathogens. (2024) 13:773. doi: 10.3390/PATHOGENS13090773
24. Gompo, TR, Jyoti, S, Pandit, S, Sapkota, RC, and Pandey, A. Sero-prevalence and risk factors of leptospirosis in commercial cattle herds of Rupandehi district, Nepal. BioRxiv. (2020). doi: 10.1101/2020.07.29.226464
25. Subedi, D, Subedi, S, Gautam, A, Bhandari, S, and Kandel, M. Cysticercosis in Nepal: a review. Nepalese Vet J. (2020) 37, 148–62. doi: 10.3126/NVJ.V37I37.55526
26. Kelly, TR, Bunn, DA, Joshi, NP, Grooms, D, Devkota, D, Devkota, NR, et al. Awareness and practices relating to zoonotic diseases among smallholder farmers in Nepal. EcoHealth. (2018) 15:656–69. doi: 10.1007/s10393-018-1343-4
27. DOHS/MOHP, Annual Report. (2022), Available at: http://moh.bagamati.gov.np/uploads/documents/w61klqll-343-1698832842.pdf
28. Pantha, S, Subedi, D, Poudel, U, Subedi, S, Kaphle, K, and Dhakal, S. Review of rabies in Nepal. One Health. (2020) 10:100155. doi: 10.1016/J.ONEHLT.2020.100155
29. Acharya, KP, Subedi, D, and Wilson, RT. Rabies control in South Asia requires a one health approach. One Health. (2021) 12:100215. doi: 10.1016/J.ONEHLT.2021.100215
30. Thakur, S, Joshi, NP, Chand, B, and Neupane, L. Unprecedented rabies outbreak in Nepal’s far Western region: a call for urgent action. IJID One Health. (2024) 4:100027. doi: 10.1016/J.IJIDOH.2024.100027
31. Bagale, KB, Adhikari, R, and Acharya, D. Regional variation in knowledge and practice regarding common zoonoses among livestock farmers of selective districts in Nepal. PLoS Negl Trop Dis. (2023) 17:e0011082–17. doi: 10.1371/journal.pntd.0011082
32. Subedi, D, Jyoti, S, Thapa, B, Paudel, S, Shrestha, P, Sapkota, D, et al. Knowledge, attitude, and practice of antibiotic use and resistance among poultry farmers in Nepal. Antibiotics. (2023) 12:1369. doi: 10.3390/antibiotics12091369
33. Poudel, U, Dahal, U, Upadhyaya, N, Chaudhari, S, and Dhakal, S. Livestock and poultry production in Nepal and current status of vaccine development. Vaccine. (2020) 8:322. doi: 10.3390/VACCINES8020322
34. Shah, HA, Huxley, P, Elmes, J, and Murray, KA. Agricultural land-uses consistently exacerbate infectious disease risks in Southeast Asia. Nat Commun. (2019) 10:4299. doi: 10.1038/s41467-019-12333-z
35. Abunna, F, Gebresenbet, G, and Megersa, B. Assessment of knowledge, attitude and practices (KAP) of farmers about transmission of zoonotic diseases in Ada’a district, Oromia, Ethiopia. Heliyon. (2024) 10:e25713. doi: 10.1016/j.heliyon.2024.e25713
36. Subedi, S, Adhikari, K, Regmi, D, Sharma, HK, Bolakhe, N, Kandel, M, et al. Assessment of community knowledge and practices towards rabies prevention: a cross-sectional survey in Bharatpur, Chitwan, Nepal. Zoonot Dis. (2023) 3:203–14. doi: 10.3390/ZOONOTICDIS3030017
37. Pal, P., Yawongsa, A., Bhatta, R., Shimoda, H., and Rukkwamsuk, T., Animal rabies epidemiology in Nepal from 2005 to 2017, (2021), 190–195. doi: 10.14202/IJOH.2021.190-195
38. Acharya, KP, Kwon, R, Cho, SH, and Yon, DK. Rabies control in Nepal: a missed opportunity. Front Vet Sci. (2023) 10:1184371. doi: 10.3389/fvets.2023.1184371
39. Özlü, H, Atasever, M, and Atasever, MA. Knowledge, attitude, and practices of cattle farmers regarding zoonotic diseases in Erzurum, Turkey, austral. J Vet Sci. (2020) 52:79–85. doi: 10.4067/S0719-81322020000300079
40. Teppawar, RN, Chaudhari, SP, Moon, SL, Shinde, SV, Khan, WA, and Patil, AR. Zoonotic tuberculosis: a concern and strategies to combat In: Basic biology and applications of actinobacteria, IntechOpen, Ed. S. Enany. (2018). 23–38.
41. Ayele, WY, Neill, SD, Zinsstag, J, Weiss, MG, and Pavlik, I. Bovine tuberculosis: an old disease but a new threat to. Int J Tuberc Lung Dis. (2004) 8:924–37.
42. National Tuberculosis Control Center. Tuberculosis profile 2079/80 (2022/23). Kathmandu: (2023).
43. Pandey, G, Dhakal, S, Sadaula, A, KC, G, Subedi, S, Pandey, K, et al. Status of tuberculosis in bovine animals raised by tuberculosis infected patients in Western Chitwan, Nepal. Int J. Infect Microbiol. (2012) 1:49–53. doi: 10.3126/ijim.v1i2.7407
44. Fetene, T, Kebede, N, and Alem, G. Tuberculosis infection in animal and human populations in three districts of Western Gojam, Ethiopia. Zoonoses Public Health. (2011) 58:47–53. doi: 10.1111/j.1863-2378.2009.01265.x
45. Meisner, J, Curtis, K, Graham, TW, Apamaku, MB, Manhart, LE, and Rabinowitz, PM. Cattle - associated risk factors for human tuberculosis in rural livestock – keeping communities, Uganda. Zoonoses Public Health. (2019) 66:73–82. doi: 10.1111/zph.12530
46. Gompo, TR, Shrestha, A, Ranjit, E, Gautam, B, Ale, K, Shrestha, S, et al. Risk factors of tuberculosis in human and its association with cattle TB in Nepal: a one health approach. One Health. (2020) 10:100156. doi: 10.1016/J.ONEHLT.2020.100156
47. Chowdhury, S, Hossain, ME, Ghosh, PK, Ghosh, S, Hossain, MB, Beard, C, et al. The pattern of highly pathogenic avian influenza H5N1 outbreaks in South Asia. Trop Med Infect Dis. (2019) 4:138. doi: 10.3390/TROPICALMED4040138
48. Neupane, D, Khanal, V, Ghimire, K, Aro, AR, and Leppin, A. Knowledge, attitudes and practices related to avian influenza among poultry workers in Nepal: a cross sectional study. BMC Infect Dis. (2012) 12:1–7. doi: 10.1186/1471-2334-12-76/COMMENTS
49. Gerhold, RW, and Jessup, DA. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health. (2013) 60:189–95. doi: 10.1111/j.1863-2378.2012.01522.x
50. Kheirandish, F, Ezatpour, B, Fallahi, S, Tarahi, MJ, Hosseini, P, Rouzbahani, AK, et al. Toxoplasma serology status and risk of miscarriage, a case-control study among women with a history of spontaneous abortion. Int J Fertil Steril. (2019) 13:184–9. doi: 10.22074/ijfs.2019.5740
51. Kharel, S, and Dahal, S. Study of livestock production system and management practices adopted by small ruminants farmers in Bagmati Province, Nepal, Nepalese journal of. Agric Sci. (2023) 25:259–75.
52. Hundal, JS, Sodhi, SS, Gupta, A, Singh, J, and Chahal, US. Awareness, knowledge, and risks of zoonotic diseases among livestock farmers in Punjab. Vet World. (2016) 9:186–91. doi: 10.14202/vetworld.2016.186-191
53. Kochar, DK, Gupta, BK, Gupta, A, Kalla, A, Nayak, KC, and Purohit, SK. Hospital-based case series of 175 cases of serologically confirmed brucellosis in Bikaner. J Assoc Physicians India. (2007) 55:271–5.
Keywords: food safety, livestock farmers, Nepal, preventive practice, risk, zoonosis
Citation: Subedi D, Dhakal A, Jyoti S, Paudel S, Ranabhat G, Tiwari A and Al-Mustapha AI (2025) Zoonotic diseases awareness and food safety practices among livestock farmers in Nepal. Front. Vet. Sci. 11:1514953. doi: 10.3389/fvets.2024.1514953
Edited by:
Windell L. Rivera, University of the Philippines Diliman, PhilippinesReviewed by:
Ana Cláudia Coelho, University of Trás-os-Montes and Alto Douro, PortugalJay Prakash Yadav, Guru Angad Dev Veterinary and Animal Sciences University, India
Copyright © 2025 Subedi, Dhakal, Jyoti, Paudel, Ranabhat, Tiwari and Al-Mustapha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepak Subedi, c3ViZWRpZWVwdTI2QGdtYWlsLmNvbQ==; Ahmad I. Al-Mustapha, YWhtYWQuYWwtbXVzdGFwaGFAaGVsc2lua2kuZmk=
 Deepak Subedi
Deepak Subedi Alok Dhakal
Alok Dhakal Sumit Jyoti
Sumit Jyoti Sanjay Paudel
Sanjay Paudel Ganesh Ranabhat
Ganesh Ranabhat Ananda Tiwari
Ananda Tiwari Ahmad I. Al-Mustapha
Ahmad I. Al-Mustapha