- 1College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, China
- 2Key Laboratory of Clinical Diagnosis and Treatment of Animal Diseases, Ministry of Agriculture, National Animal Medicine Experimental Teaching Center, Hohhot, China
- 3National Center of Technology Innovation for Dairy, Hohhot, China
- 4Zhongnong Dong Jun Animal Diagnosis Technology (Beijing) Co., Ltd., Beijing, China
- 5College of Pharmacy Heze University, Heze, China
- 6Rui Pu Agricultural Technology Co., Ltd., Hohhot, China
Introduction: The control of parasites infections in livestock is an ongoing concern, with parasites developing resistance to commonly used antiparasitic drugs. The current study investigated in vitro the destructive effect of the fungus Pochonia chlamydosporia on the eggs and oocysts of several equine parasites, as well as assessing the safety of the fungus in mice.
Methods: S. equinus, P. equorum, Anoplocephala spp eggs and Eimeria spp. oocysts were treated with P. chlamydosporia. The prepared preparation was also administered to mice, and the physiological indexes and lesions of major tissues and organs, as well as pathological sections of tissue, were then observed.
Results: P. chlamydosporia exhibited varying degrees of efficacy in the control of S. equinus, P. equorum, Anoplocephala spp eggs and Eimeria spp. oocysts. The acute toxicity test demonstrated that there was no death or toxicity symptom observed in the mice, with no significant difference in clinical observations, such as respiration, mental state, appetite, or feces, between the control and treated mice after the feeding of the biological preparation of P. chlamydosporia.
Discussion: These findings suggested that administration of P. chlamydosporia would be safe to use in livestock and provided a rationale for its potential clinical application, pending further analyses.
1 Introduction
Anthelmintic drugs are widely used to control parasitic diseases of livestock, which can otherwise result in high economic losses as a result of significant morbidity. However, the long-term use of such drugs can result in the development of resistance, rendering them increasingly less effective (1–3). Therefore, researchers have begun to investigate the use of natural enemies for the biological control of parasites (4). Such an approach also helps improve animal food safety, environmental protection, and the sustainable development of animal husbandry.
Nematophagous fungi, including Zygomycotina, Deuteromycotina, Basidomycotina, and Ascomycotina, are natural enemies of parasites and, thus, have an important role in the biological control of both plant and animal parasites (5). They can be classified into four major groups based on the mode of nematode attack: (i) nematode-trapping/predators, (ii) opportunistic or ovicidal, (iii) endoparasites, (iv) toxin-producing fungi and (v) producers of special attack devices (6). They can reproduce in three ways, via aerial mycelia, conidia, and chlamydospores, and spread primarily by mycelial breakage and conidial germination. Fungal conidia are ingested by animals and excreted in the feces, where they can germinate to then kill any parasitic eggs; thus, conidia can be used as food additives for livestock to prevent and control parasitic eggs.
Pochonia chlamydosporia (38) is a ovicidal fungus of the Hypocreales (Clavicipitaceae) belonging to Ascomycota. Pochonia chlamydosporia has shown efficacy in vitro and in vivo for the biocontrol of gastrointestinal parasites in domestic animals by parasitizing eggs and female nematodes (7), while Monacrosporium sinense [belongs to Arthrobotrys, (39)] captures nematode larvae via adhesive networks (8). The combination of M. sinense and P. chlamydosporia demonstrated a 98.90% reduction in the number of bovine nematode infective larvae under in vitro conditions at 25°C (9). In a study by Braga et al. (10), P. chlamydosporia (VC4) was successfully administered orally to horses in the form of pellets. Although available data indicate that P. chlamydosporia kills the eggs of a variety of parasites, there is no detailed report on which eggs of equine parasites are affected and how effectively, or whether the fungus is safe for use in animals. Thus, the current study investigated the impact of P. chlamydosporia on the eggs and oocysts of equine parasites and examined the safety of a lyophilized preparation in mice to explore the possibility of its application for the control of parasites. The results lay the foundation for the clinical application of P. chlamydosporia as an effective and sustainable biological control method for the prevention and control of animal parasitic diseases.
2 Materials and methods
2.1 Materials
2.1.1 Test strains, eggs, and oocysts
A test strain of P. chlamydosporia, ARSEF3539, was obtained from the Veterinary Parasite Laboratory of the Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, China.
A floating method was used for the separation of Strongylus equinus, Parascaris equorum, Anoplocephala spp. eggs and Eimeria spp. oocysts from feces excreted by naturally infected horses. Light microscopy (20×) was used for the morphological analysis of the eggs and oocysts. Eggs and oocysts were collected and washed with distilled water, 3,000 g, for 5 min. They were then sterilized using a 1% NaClO solution and their morphological integrity was analyzed under an optical microscope. Centrifugation was repeated and the intact eggs and oocysts were washed a further three to five times to remove the NaClO solution. A suspension of 1,000 eggs or oocysts/mL was then prepared.
2.1.2 Preparation of culture medium and solution
Potato dextrose agar (PDA) medium (HopeBio) was prepared using water agar (WA) medium, whereby 15 g of agar was added to 1 L of water and mixed, before heating to 121°C for 15 min. Spore eluate was prepared by adding 1 mL Tween-80 to 500 mL of water, and then heating it to 121°C for 15 min.
2.1.3 Preparation of lyophilized biologics
Pochonia chlamydosporia was inoculated into PDA medium until the mycelium covered the surface of the petri dish; this was then cut into 0.5 cm × 0.5 cm squares, and transferred to corn medium for 3 weeks (Figure 1A). The conidia were eluted from the medium with the spore eluate and filtered. The samples were prefrozen for 2 h at −80°C, and then placed in a vacuum freeze dryer. When the samples were dry powder, the conidia were counted (Figure 1B); the chlamydospore count in the lyophilized formulation was recorded as 2.13 × 108 chlamydospores/g (Figure 1C). The dishes were then sealed with sealing film, and stored at 4°C.

Figure 1. Lyophilized biologics of Pochonia chlamydosporia. (A) P. chlamydosporia culture; (B) P. chlamydosporia lyophilized powder; (C) P. chlamydosporia powder culture.
2.2 Methods
2.2.1 Impact of P. chlamydosporia on eggs and oocysts
The conidial suspension was spread evenly on WA medium spread on a dialysis membrane and left to grow mycelium for 3 days. Then 100 μL of each egg or oocyst suspension was added to different WA medium inoculated with P. chlamydosporia, with another 100 μL of each eggs or oocysts suspension being added to WA medium not inoculated with P. chlamydosporia as a control. Each petri dish was stored at 25°C for 7 days, with 10 repetitions for each treatment. After 7 days, the eggs or oocysts were photographed under a light microscope and examined for any signs of infestation; the integrity of the eggs or oocysts was evaluated according to the parameters established by Lýsek (11).
Equation 1 was used to obtain the percentage reduction in the number of eggs or oocysts after treatment (12):
2.2.2 Acute transoral toxicity test in mice
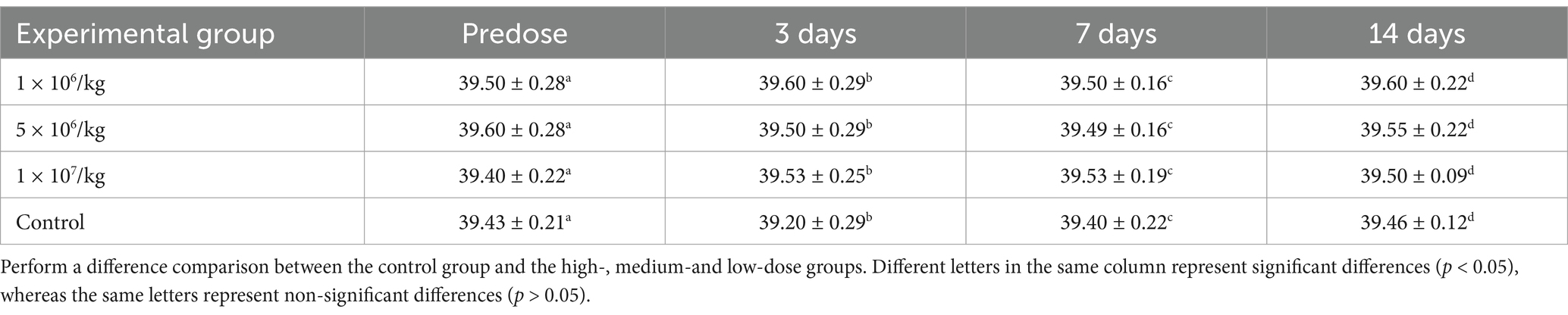
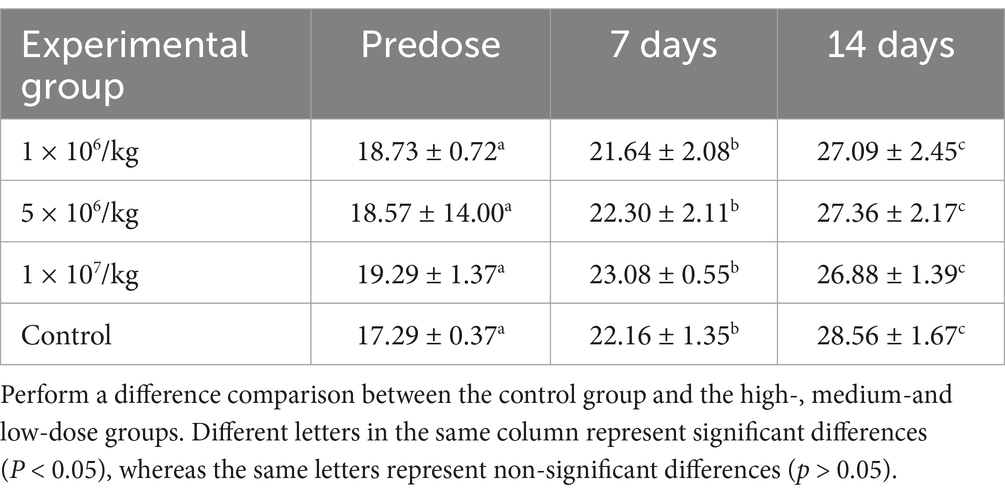
The safety of the P. chlamydosporia inoculum was tested following the procedure of the Chinese National Food Safety Standard Acute Oral Toxicity Test (GB 15193.3–2014). Twenty male and 20 female mice, 6 ~ 8-weeks-old, and each weighing ~20 g were fasted for 6 h before use in the study. The recommended therapeutic dose of P. chlamydosporia was 1 × 106 conidium/kg body weight (b.w.) (13). The mice were randomly divided into four groups: (1) normal dose group (1 × 106 conidium/kg b.w.); (ii) 5 × dose group (5 × 106 conidium/kg b.w.); (iii) 10 × dose group (1 × 107conidium/kg b.w.); and (iv) control group, with 10 mice in each group. The lyophilized preparation of P. chlamydosporia was dissolved in distilled water and the appropriate dose was weighed according to the b.w. and administered orally to ensure that the preparation entered the stomach via the mouth. The control group was given the same stimulus by administering distilled water. Mice were fasted for 2 h after gavage, then housed normally, and observed continuously for 14 days.
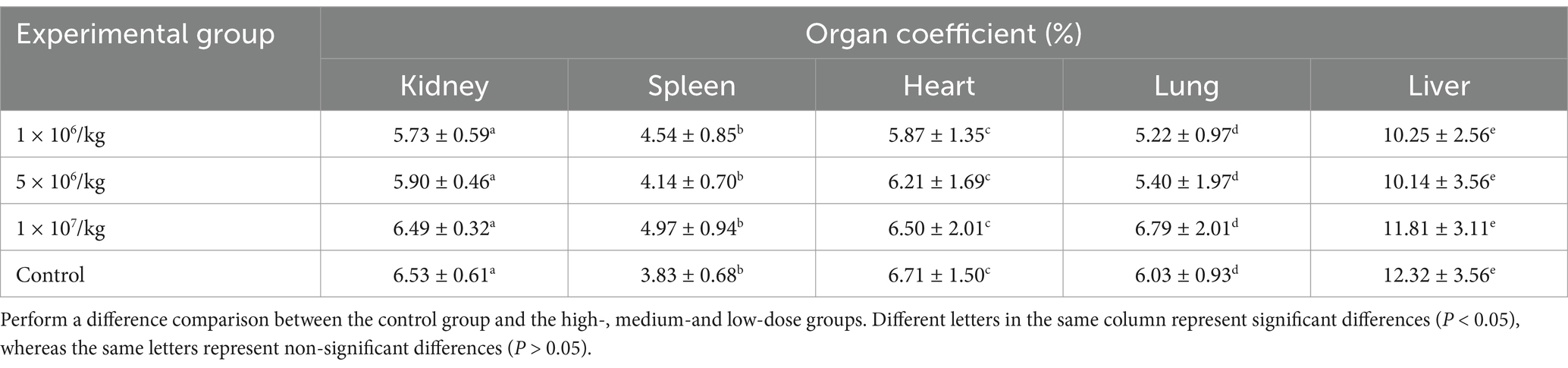
During the test, mice were observed in terms of their mental status. Defecation was observed to see whether the mice had constipation or diarrhea. During the test period, all mice were uniformly fed with rat food 3 times/day. All mice in each group were weighed to determine the effect of the oral biologics on their b.w. Their body temperature was also measured before and 3, 7, and 14 days after inoculation. Blood was collected from each mouse on Day 14 after administration for routine blood and biochemical index examinations detected. At the end of the experiment, all mice were euthanized and the heart, liver, spleen, lungs, and kidneys were removed and weighed, and the organ coefficient was calculated using Equation 2:
Samples of each tissue were taken for fixation, sectioning, and microscopic examination to observe their morphology and color.
2.2.3 Mouse skin sensitization test
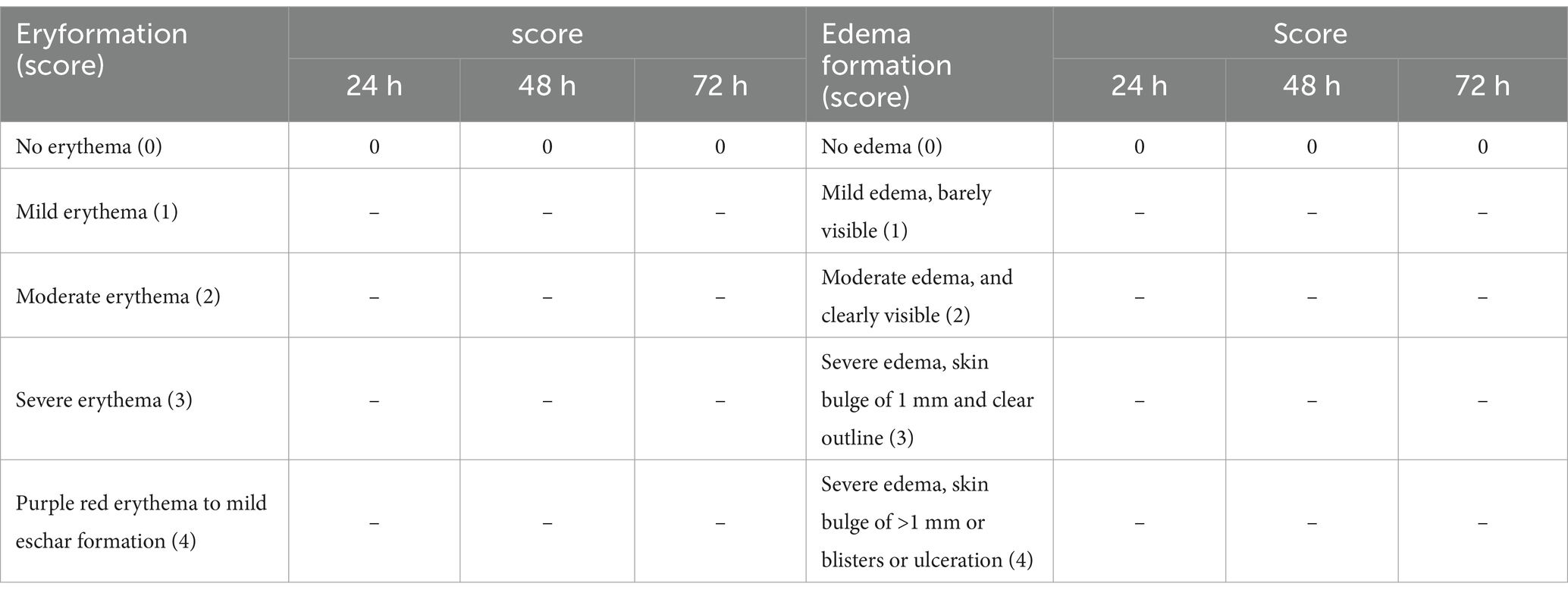
Eight healthy mice, four males and four females, were selected, and the hair on both sides of the spine was shaved off from an area of ~2 cm2 24 h before the test so that the shaved area was determined to be free of abnormalities for 24 h before testing. Next, 0.5 mL of the P. chlamydosporia inoculum at a concentration of 1 × 108/kg was applied to one of the shaved areas on each mouse, which were then covered for 4 h, after which the inoculum was removed. The other patch of shaved skin was used as a control. The intensity of sensitization was judged by comparing the erythema, swelling, and hardness of the local skin according at 24, 48, and 72 h.
2.2.4 Acute eye stimulation test in mice
Eight healthy mice, four males and four females, were selected, and both eyes were examined 24 h before the test. No obvious sign of eye irritation, corneal defects, or conjunctival damage was observed. The animals were divided into a control group and a fungal stimulation group; the latter was treated once by taking 0.1 mL of inoculum (at a concentration of 1 × 108 CFU/kg) and placing it directly into the left eye of the animal. The right eye of each of the test animals comprised the control group. The responses of the conjunctiva, cornea, and iris to the inoculum were examined and recorded 24, 48, and 72 h after treatment, with recovery observed on Day 3 and 7.
2.3 Data processing and analysis
Data were expressed as the mean ± SD and analyzed using GraphPad Prism 7. Data were analyzed for normal distributions using Kolmogorov–Smirnov and Levene’ s tests. If variance was unevenly distributed, equivalent non-parametric tests (primarily Kruskal–Wallis analysis of variance) were used. A p < 0.05 value was considered significant.
3 Results
3.1 Results of the ovicidal activity test
Observations showed that P. chlamydosporia mycelia first aggregated near the eggs or oocysts, with numerous mycelia then penetrating the egg membrane before gradually destroying the internal structure. The mycelia enter the eggs or oocysts via mechanical and enzymatic actions, leading to significant structural changes that eventually deform the eggs or oocysts, causing their death. P. chlamydosporia was found to infect eggs of S. equinus, P. equorum, Anoplocephala spp. and Eimeria spp. oocysts, but at different rates, being slower to infect those of Eimeria spp. oocysts and P. equorum eggs (Table 1 and Figure 2).
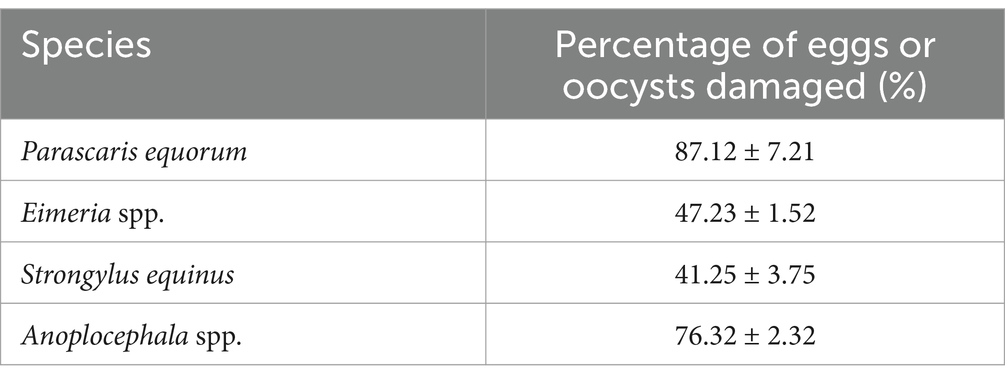
Table 1. Results of infestation test of P. chlamydosporia on eggs of S. equinus, P. equorum, Anoplocephala spp. and Eimeria spp. oocysts.
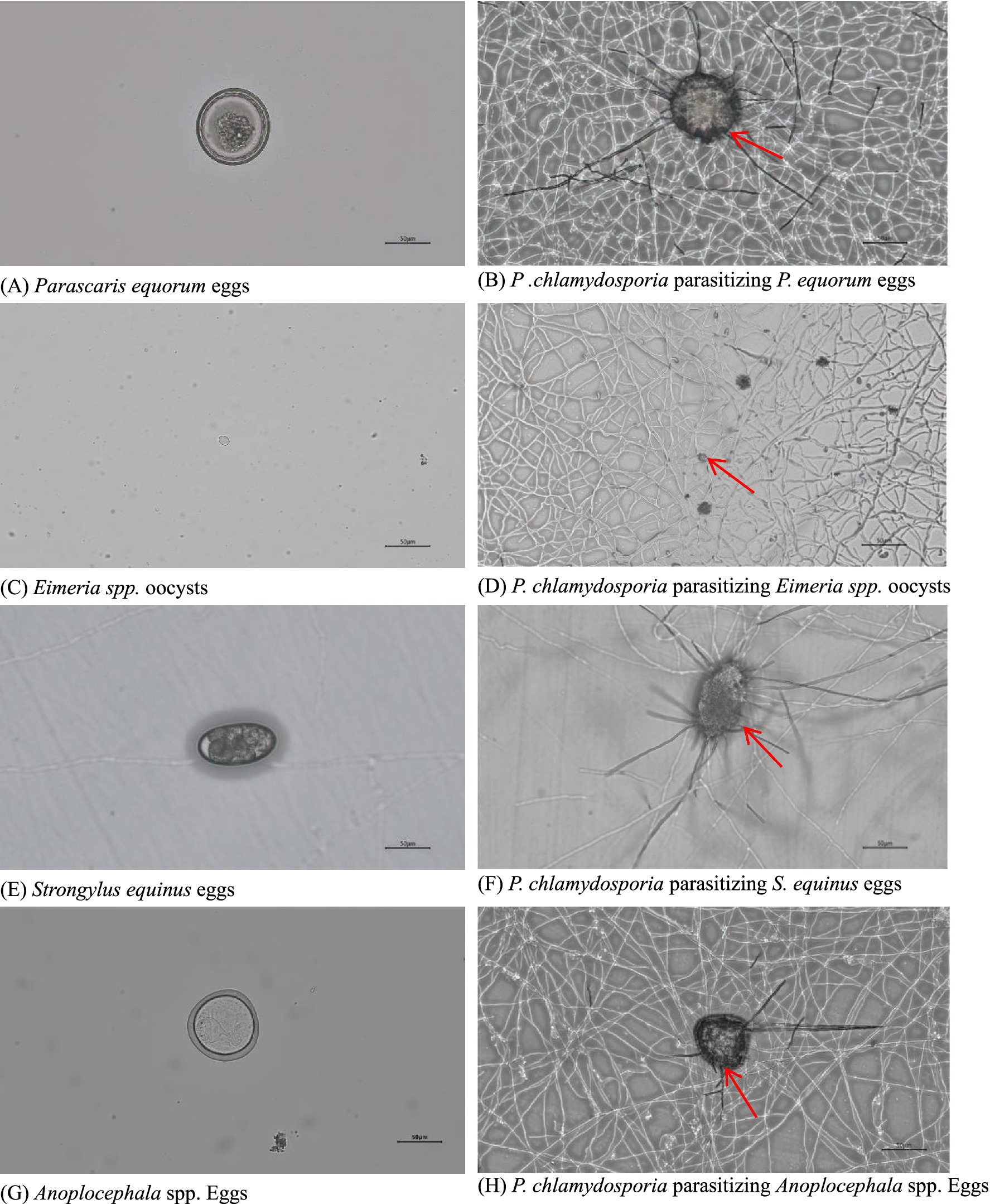
Figure 2. Images of eggs of (A) S. equinus, (C) P. equorum, (E) Eimeria spp., and (G) Anoplocephala spp. and their parasitism by P. chlamydosporia for 7d (B,D,F,H).
3.2 Results of acute transoral toxicity test in mice
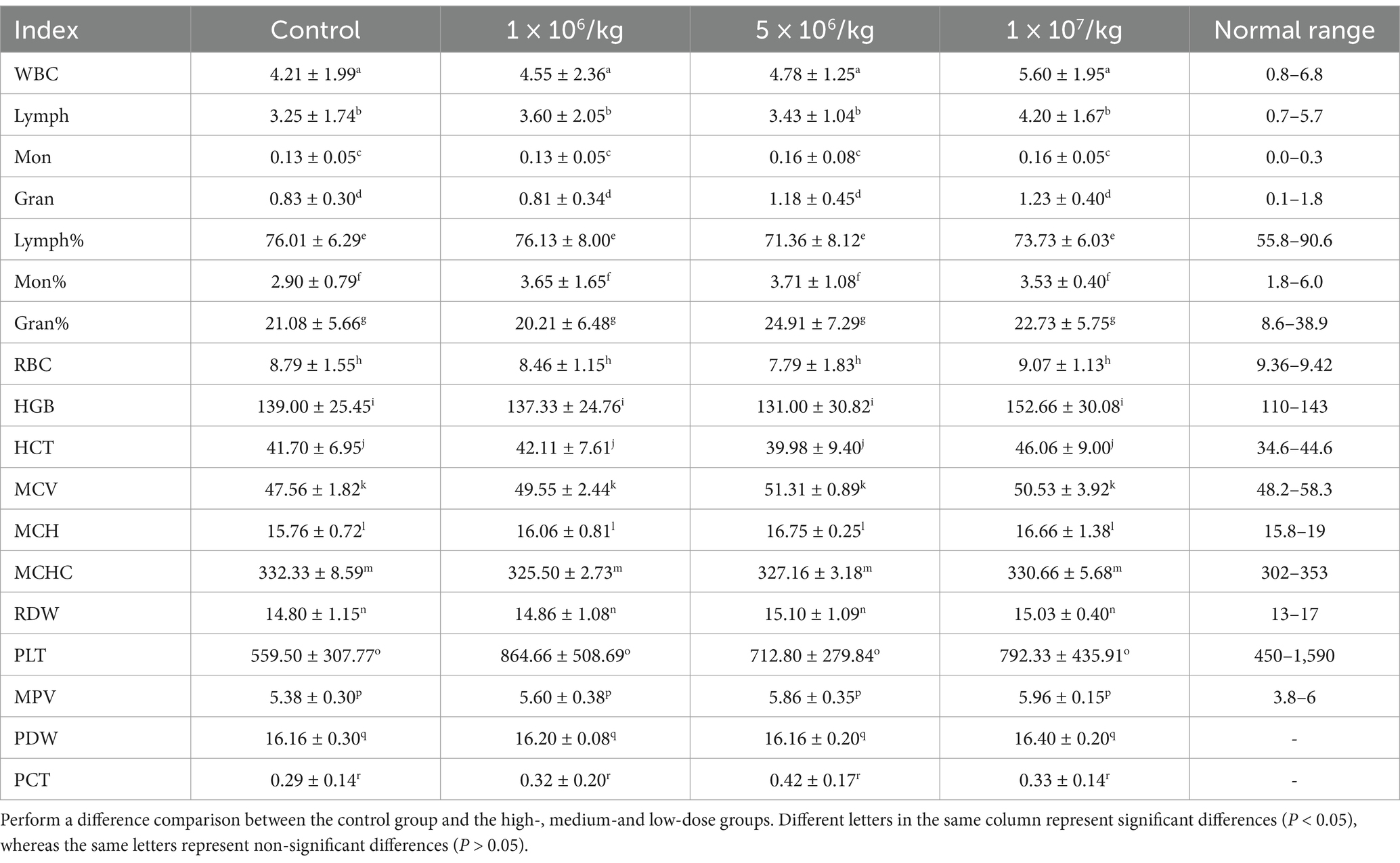
No abnormal clinical observation such as respiration, mental state, appetite, or feces of the experimental or control mice, was made during the test period. The body temperature of each experimental group did not change significantly over the course of the study (Table 2). The b.w. of the mice in each group increased over the course of the study but the changes were not statistically significant (Table 3). There was no significant difference in organ coefficients between the experimental groups (Table 4). Upon autopsy of the mice in the test groups and the control group, no obvious effusion or abnormal content was observed in the thoracic and abdominal cavities, and the location, appearance, and morphology of the tissues and organs were normal; neither was there any change such as dislocation, adhesion, torsion and rupture. All organs were of normal size and texture, with no hemorrhage, scarring, nodular or necrotic change in overall appearance or tissue structure (Figures 3, 4). All blood routine indices and blood biochemical indices were within the normal range in each dose group before and at Day 14 after treatment. There was no significant difference between the groups (Tables 5, 6). The results of the acute oral toxicity test in mice showed that none of the animals had any obvious symptom of poisoning and no death was recorded; LD50 was >1 × 107 chlamydospores/kg b.w., which indicated that the acute oral toxicity was of “low toxicity” level.

Figure 3. Comparison of the morphology of kidneys, heart, lungs, spleen, and liver from mice in the experimental and control groups.
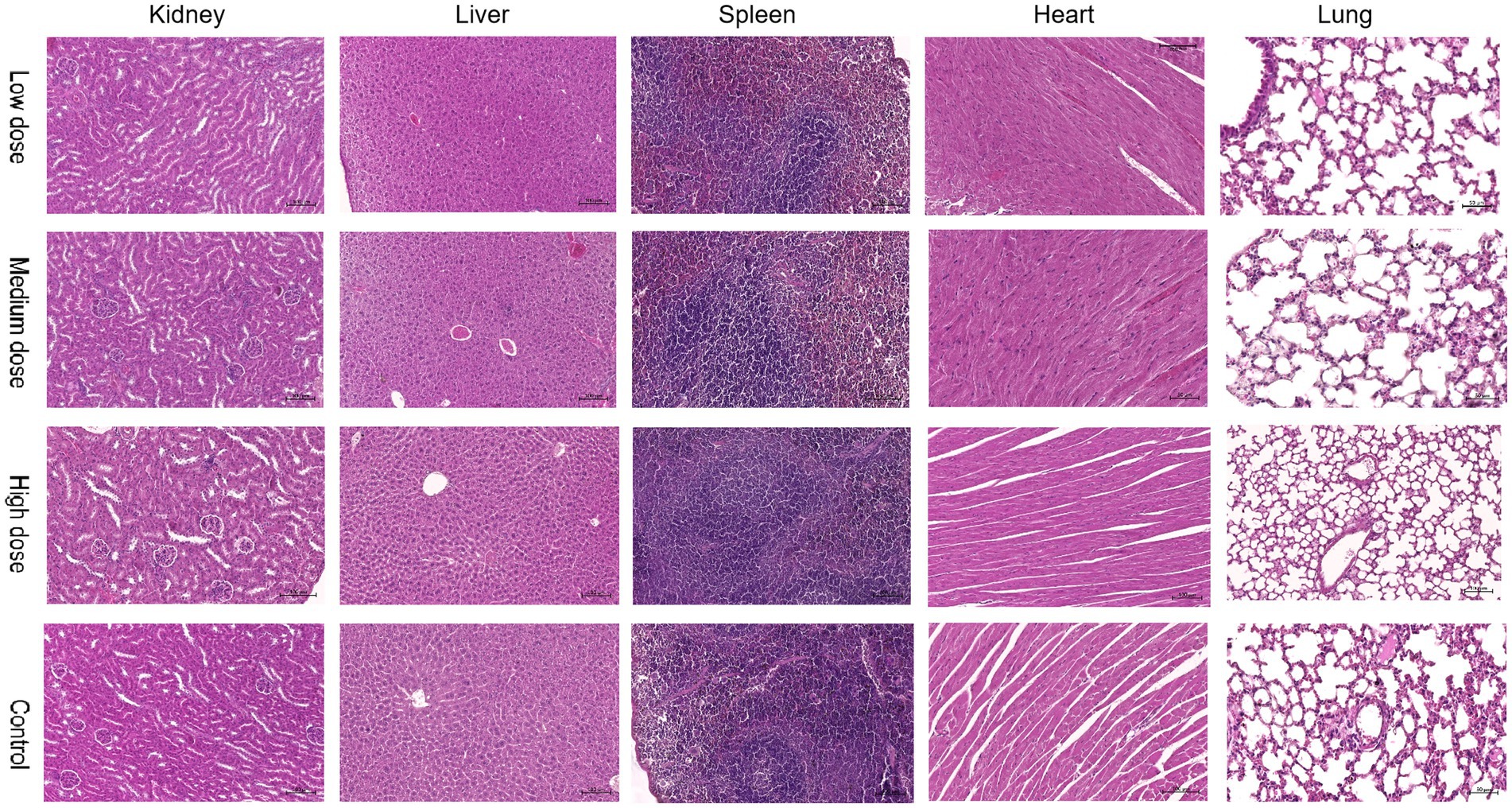
Figure 4. Comparison of pathological images of the kidney, liver, spleen, heart, and lung from mice in the experimental and control groups.

Table 6. Effects of P. chlamydosporia administration on mouse blood biochemical indices (mean ± SD).
3.2.1 Results of the acute skin irritation test
The results of the skin sensitization test showed that no erythema or edema formed in the skin of the treated mice. The results of the acute single skin irritation test were negative, which indicated that the P. chlamydosporia inoculum was “non-irritating” in terms of this single skin irritation test (Table 7).
3.2.2 Results of eye irritation test
The results of the eye irritation test in mice showed no edema or secretion, and no abnormality of cornea and iris, indicating that the P. chlamydosporia inoculum was not irritating to the mice.
4 Discussion
Biological control using P. chlamydosporia is an environmentally friendly control method to support the use of sustainable agriculture and reduce chemical pesticide use. P. chlamydosporia is specialized in attacking the eggs, females, and cysts of animal and plant parasites (14). The use of this type of bioprophylactic fungi for the control of parasites in livestock is widely supported because it leaves no drug residues, the parasites do not develop drug resistance, it does not contaminate the natural environment, and it kills the eggs in feces and the environment before they hatch and infect livestock; such effects greatly advance the control of parasites and pose no harm to livestock (15, 16). So far, P. chlamydosporia strains have been isolated and screened in Brazil, Poland, the UK, Australia and Sweden (17–19) and have been shown to be adapted to, and showed better action when in their natural habitats; researchers have investigated their role in controlling the eggs of Ascaris suum, Ascaridia galli, and Toxocara canis, showing nematicidal efficiencies of up to 94.8% (16, 20–22). The main virulence factors of P. chlamydosporia are serine proteases, chitinases, and various toxins (23, 24). Their mechanism of action is based on a mechanical/enzymatic process, which enables the penetration of their hyphae into helminth eggs, with subsequent internal colonization of these. Thus, morphological changes occur in the eggshell, as well as in the embryo, making it nonviable (10). Nematode infestation by this fungus involves the recognition of females and eggs by the fungus, the subsequent degradation of nematode eggshells by the fungus, and finally, the invasion and dissipation of nematodes by the mycelia (25). Although the ovicidal activity of P. chlamydosporia has been frequently evaluated, it is not known whether it has destructive power against larvae. Vieira et al. specifically studied the ability of P. chlamydosporia to capture larvae and found that it reduced 66.8% of L3 GINs in cattle, but there are few reports in the literature on the larvicidal activity of this fungus (26, 27). Among these degradative enzymes, serine protease and chitinase are key virulence factors in the infestation and lethality of nematodes (28, 29). The extracellular serine protease VCP1 degrades the outer proteins of the eggshell and exposes the inner chitin layer (30).
Although P. chlamydosporia has shown potential for clinical applications, there has been a lack of data regarding its efficacy against equine parasitic eggs and oocysts. Braga et al. (10) reported that a crude enzymatic extract from P. chlamydosporia (VC4) reduced the hatching of cyathostomin eggs, while Braga et al. (31) suggested P. chlamydosporia to be an effective biological control agent of O. equi eggs in natural conditions. The results of Braga et al. indicate that P. chlamydosporia can be used in the control of eggs of gastrointestinal helminth parasites that hatch in a short period of time in the environment. Before this, only helminth eggs with long periods of development in the environment have been studied (14). In our study, regardless of the length of the development time in the environment, the fungus exhibited ovicidal activity against different worm eggs and oocysts after being co-cultured for 7 days. However, there were differences in its effectiveness. Braga et al. also demonstrated that P. chlamydosporia exhibited ovicidal activity on equine eggs following passage through the gastrointestinal tract. The efficacy of this fungus was enhanced when used in conjunction with the nematode-feeding fungus Paecilomyces lilacinus [belongs to Purpureocillium, (40)] which has a distinct mechanism of action (31). Furthermore, the fungus Duddingtonia flagrans also acts as the main predatory fungus of gastrointestinal parasitic nematode larvae. Combined administration of D. flagrans and P. chlamydosporia fungal products reduced the presence of eggs and larvae in pastures, indicating their effectiveness in the strategic control of gastrointestinal parasites in cattle. Another species capable of adhering to the surface of the eggs of certain helminths, penetrating and feeding on their contents, is Mucor circinelloides. It is a filamentous saprophytic fungus with action against trematode eggs (F. hepatica, Calicophoron daubneyi), ascarids (Toxocara canis, Toxascaris leonina, A. suum, Bayliscascaris procyonis), and whipworms (Trichuris spp.) (32). The current results showed that P. chlamydosporia destroyed the S. equinus eggs, Eimeria spp. oocysts, and Anoplocephala spp. eggs, although their effect on P. equorum was not as effective, mainly because of the fast hatching speed of this parasite. In addition, the mycelia of P. chlamydosporia demonstrated a tendency to aggregate close to the parasitic eggs. This phenomenon might be associated with the release of substances by the eggs, which could influence the growth of the mycelia, which then penetrate, and destroy, the eggs (33). Other previous reports indicated the possibility of some eggshell components (such as chitin) acting as stimuli for the fungi can identify them and develop hyphae against them (23, 34).
The current study also used a mouse model to assess the safety of P. chlamydosporia as a biological agent in terms of any possible effects on nontarget organisms. Such studies are vital for the protection of human health and the environment, as well as for the successful commercialization of this fungus as a biopesticide. The lyophilized formulations of P. chlamydosporia at different doses did not produce any toxicity in mice and neither was there any buildup in the feces of the treated mice over the 14-day test period, indicating that solutions of P. chlamydosporia could be safe for use in livestock. In addition, microbiological analysis of mouse feces after 14 days of treatment demonstrated that no conidia of the fungus were identified. This suggested that P. chlamydosporia did not undergo a multiplicative buildup in the body.
The plant parasite killer Rizotec®, which contains P. chlamydosporia, has become an alternative solution for controlling plant parasitic nematodes, showing high parasitism efficiency on eggs, control of juvenile and adult female nematodes, and resulting in a reduction in the number of eggs and female nematodes in sugarcane fields. Studies have investigated the toxicity and ecotoxicity of Rizotec on animals including rats, rabbits, and quail (35, 36), and invertebrates (37), finding no toxic, irritant, pathogenic, or infectious effects, indicating that this compound is safe to use, in agreement with the current study.
Thus, the results of the current study provided a theoretical basis for understanding the process of parasite egg infection by P. chlamydosporia and promoting its application as a biological control agent for the control of gastrointestinal parasites in animals. P. chlamydosporia showed good oocidal effects against the equine parasites tested and had no toxic effects on mice; thus, we suggest that it would be safe to use in livestock.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Animal Experimentation Ethics Committee regulations of the Inner Mongolia Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YM: Data curation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. JL: Data curation, Methodology, Writing – original draft, Writing – review & editing. LJ: Data curation, Methodology, Writing – original draft, Writing – review & editing. ZF: Data curation, Methodology, Writing – original draft, Writing – review & editing. LH: Investigation, Writing – original draft, Writing – review & editing. ZL: Investigation, Writing – original draft, Writing – review & editing. CM: Investigation, Writing – original draft, Writing – review & editing. RW: Conceptualization, Resources, Supervision, Writing – review & editing. HL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this study was provided by Key R&D and achievement transformation projects of Inner Mongolia, China (grant no. 2023YFDZ0048), National Center of Technology Innovation for Dairy (grant no. 2023-JSGG-5), the National Natural Science Foundation of China (grant no. 32160838), Research Project Funding for First-class Disciplines at Inner Mongolia Education Department (grant no. YLXKZX-NND-012), Technology Support Project of Major Innovation Platform (Base) Construction (grant no. KCX2024016), and the Natural Science Foundation of Inner Mongolia, China (grant no. 2023LHMS03022, 2023LHMS03005).
Conflict of interest
JL was employed by Zhongnong Dong Jun Animal Diagnosis Technology (Beijing) Co., Ltd. and HL was employed by Rui Pu Agricultural Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elling, AA. Genomics and molecular genetics of plant nematode interactions. Q Rev Biol. (2012) 87:266. doi: 10.1086/666755
2. Abd-Elgawad, MMM. Understanding molecular plant-nematode interactions to develop alternative approaches for nematode control. Plants. (2022) 11:2141. doi: 10.3390/plants11162141
3. Markéta, Z, Nguyen, LT, Lenka, S, Lucie, RS, and Petra, M. Anthelmintics in the future: current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discov Today. (2019) 25:430–7. doi: 10.1016/j.drudis.2019.12.007
4. Ferreira, SR, Machado, ART, Furtado, LF, Gomes, JHS, Almeida, RM, Oliveira, MT, et al. Ketamine can be produced by Pochonia chlamydosporia: an old molecule and a new anthelmintic? Parasit Vectors. (2020) 13:527. doi: 10.1186/s13071-020-04402-w
5. Vande, VF, Charlier, J, and Claerebout, E. Farmer behavior and gastrointestinal nematodes in ruminant livestock-uptake of sustainable control approaches. Front Vet Sci. (2018) 5:255. doi: 10.3389/fvets.2018.00255
6. Elias, DFSF, Leite, SB, and de Queiroz José, H. Nematophagous fungi: far beyond the endoparasite, predator and ovicidal groups. Agriculture and natural. Resources. (2018) 52:1–8. doi: 10.1016/j.anres.2018.05.010
7. Vieira, ÍS, Oliveira, IC, Campos, AK, and Araújo, JV. Association and predatory capacity of fungi Pochonia chlamydosporia and Arthrobotrys cladodes in the biological control of parasitic helminths of bovines. Parasitology. (2019) 146:1347–51. doi: 10.1017/S003118201900060X
8. Alves, PH, Arujo, JV, Guimarães, MP, Assis, RCL, Sarti, P, and Campos, AK. Aplicação de formulação do fungo predador de nematóides Monacrosporium thaumasium (Drechsler, 1937) no controle de nematóides de bovinos Control of bovine gastrointestinal nematodes using formulation of the nematode-trapping fungus Monacrosporium thaumasium (Drechsler, 1937). Arquivo Brasil Med Vet Zoot. (2003) 55:568–73. doi: 10.1590/S0102-09352003000.500009
9. Oliveira, IC, Vieira, ÍS, Campos, AK, and Araújo, JV. In vitro compatibility and nematicidal activity of Monacrosporium sinense and Pochonia chlamydosporia for biological control of bovine parasitic nematodes. Parasitology. (2021) 148:956–61. doi: 10.1017/S0031182021000652
10. Braga, FR, Araújo, JV, Silva, AR, Carvalho, RO, Araujo, JM, Ferreira, SR, et al. Viability of the nematophagous fungus Pochonia chlamydosporia after passage through the gastrointestinal tract of horses. Vet Parasitol. (2010) 168:264–8. doi: 10.1016/j.vetpar.2009.11.020
11. Lýsek, H, Fassatiová, O, Cuervo, PN, and Lorenzo, HN. Ovicidal fungi in soils of Cuba. Folia Parasitol. (1982) 29:265–70. http://dx.doi.org/.
12. Ribeiro, GB, Almeida, MI, Silva, AR, Araújo, JV, Oliveira, MCM, Santos, FJ, et al. Efficiency of experimental formulation containing Duddingtonia flagrans and Pochonia chlamydosporia against Moniezia expansa eggs. Pathogens. (2023) 12:1028. doi: 10.3390/pathogens12081028
13. Vidal, MLB, VIEIRA, IS, Castro, LS, De Freitas, SG, Silva, AR, and Araujo, JV. Bioverm® (Duddingtonia flagrans) compared to the association of Duddingtonia flagrans and Pochonia chlamydosporia for the biological control of cattle nematodes in Southeast Brazil. Preprints. (2023) 2023:2023071886. doi: 10.20944/preprints202307.1886.v3
14. Ferreira, SR, Araújo, JV, Braga, FR, Araujo, JM, Carvalho, RO, Silva, AR, et al. Ovicidal activity of seven Pochonia chlamydosporia fungal isolates on Ascaris suum eggs. Trop Anim Health Product. (2011) 43:639–42. doi: 10.1007/s11250-010-9744-6
15. Silva, AR, Araújo, JV, Braga, FR, Alves, CDF, and Filho, JDR. Destruction of Anoplocephala perfoliata eggs by the Nematophagous fungus Pochonia chlamydosporia. J Equine Vet. (2010) 30:701–4. doi: 10.1016/j.jevs.2010.11.004
16. Araujo, JM, Araújo, JV, Braga, FR, Oliva, CR, and Rodrigo, FS. Activity of the nematophagous fungi Pochonia chlamydosporia, Duddingtonia flagrans and Monacrosporium thaumasium on egg capsules of Dipylidium caninum. Vet Parasitol. (2009) 166:86–9. doi: 10.1016/j.vetpar.2009.08.003
17. Blaszkowska, J, Kurnatowski, P, Wojcik, A, Goralska, K, and Szwabe, K. In vitro evaluation of the ovistatic and ovicidal effect of the cosmopolitan filamentous fungi isolated from soil on Ascaris suum eggs. Vet Parasitol. (2014) 199:165–71. doi: 10.1016/j.vetpar.2013.10.026
18. Thapa, S, Mejer, H, Thamsborg, SM, Lekfeldt, JDS, Wang, R, Jensen, B, et al. Survival of chicken ascarid eggs exposed to different soil types and fungi. Appl Soil Ecol. (2017) 121:143–51. doi: 10.1016/j.apsoil.2017.10.001
19. Silva, AR, Araújo, JV, Braga, FR, Alves, CDF, and Frassy, LN. In vitro ovicidal activity of the nematophagous fungi Duddingtonia flagrans, Monacrosporium thaumasium and Pochonia chlamydosporia on Trichuris vulpis eggs. Vet Parasitol. (2010) 172:76–9. doi: 10.1016/j.vetpar.2010.04.034
20. Filho, FDSM, Vieira, JN, Aires, BME, Stoll, FE, Silva, NPD, Pötter, L, et al. Fungal ovicidal activity on Toxocara canis eggs. Rev Iberoam Micol. (2013) 30:226–30. doi: 10.1016/j.riam.2012.12.009
21. Thapa, S, Thamsborg, SM, Wang, R, Meyling, NV, Dalgaard, TS, Petersen, HH, et al. Effect of the nematophagous fungus Pochonia chlamydosporia on soil content of ascarid eggs and infection levels in exposed hens. Parasit Vectors. (2018) 11:319. doi: 10.1186/s13071-018-2898-1
22. Yang, J, Tian, B, Liang, L, and Zhang, KQ. Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl Microbiol Biotechnol. (2007) 75:21–31. doi: 10.1007/s00253-007-0881-4
23. Suarez-Fernandez, M, Sambles, C, Lopez-Moya, F, Nueda, MJ, Studholme, DJ, and Lopez-Llorca, LV. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Environ Microbiol. (2021) 23:4980–97. doi: 10.1111/1462-2920.15408
24. Dos Santos, FJ, Altoé, LSC, de Carvalho, LM, de Freitas Soares, FE, Braga, FR, and de Araújo, JV. Nematophagous fungus Pochonia chlamydosporia to control parasitic diseases in animals. Appl Microbiol Biotechnol. (2023) 107:3859–68. doi: 10.1007/s00253-023-12525-0
25. Esteves, I, Peteira, B, Atkins, SD, Magan, N, and Kerry, B. Production of extracellular enzymes by different isolates of Pochonia chlamydosporia. Mycol Res. (2009) 113:867–76. doi: 10.1016/j.mycres.2009.04.005
26. Lopez-Llorca, LV, Olivares-Bernabeu, C, Salinas, J, Jansson, HB, and Kolattukudy, PE. Pre-penetration events in fungal parasitism of nematode eggs. Mycol Res. (2002) 106:499–506. doi: 10.1017/S0953756202005798
27. Vieira, TS, Oliveira, I, Campos, AK, and Araújo, J. Arthrobotrys cladodes and Pochonia chlamydosporia: Nematicidal effects of single and combined use after passage through cattle gastrointestinal tract. Exp Parasitol. (2020) 218:108005. doi: 10.1016/j.exppara.2020.108005
28. Kaczmarek, MB, Struszczyk-Swita, K, Li, XK, Szczęsna-Antczak, M, and Daroch, M. Enzymatic modifications of chitin, chitosan, and Chitooligosaccharides. Front Bioeng Biotechnol. (2019) 7:243. doi: 10.3389/fbioe.2019.00243
29. Segers, R, Butt, TM, Kerry, BR, and Peberdy, JF. The nematophagous fungus Verticillium chlamydosporium produces a chymoelastase-like protease which hydrolyses host nematode proteins in situ. Microbiology. (1994) 140:2715–23. doi: 10.1099/00221287-140-10-2715
30. Braga, FR, Araújo, JV, Carvalho, RO, Silva, AR, Araujo, JM, Soares, FE, et al. Ovicidal action of a crude enzymatic extract of the fungus Pochonia chlamydosporia against cyathostomin eggs. Vet Parasitol. (2010) 172:264–8. doi: 10.1016/j.vetpar.2010.05.011
31. Braga, FR, Araújo, JV, Soares, FE, Tavela, AO, Araujo, JM, Carvalho, RO, et al. Enzymatic analysis and in vitro ovicidal effect of Pochonia chlamydosporia and Paecilomyces lilacinus on Oxyuris equi eggs of horses. Biocontrol Sci Tech. (2012) 22:685–96. doi: 10.1080/09583157.2012.677807
32. Vilela, VLR. Recent advances in the control of helminths of domestic animals by Helminthophagous Fungi. Parasitologia. (2021) 1:18. doi: 10.3390/parasitologia.1030018
33. Escudero, N, Ferreira, SR, Lopez-Moya, F, Naranjo-Ortiz, MA, Marin-Ortiz, AI, Thornton, CR, et al. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol. (2016) 120:572–85. doi: 10.1016/j.funbio.2015.12.005
34. Radwan, MA, Farrag, SAA, Abu-Elamayem, MM, and Ahmed, NS. Extraction, characterization, and nematicidal activity of chitin and chitosan derived from shrimp shell wastes. Biol Fertil Soils. (2012) 48:463–8. doi: 10.1007/s00374-011-0632-7
35. García, L, Bulnes, C, Melchor, G, Vega, E, Ileana, M, Oca, NM, et al. Safety of Pochonia chlamydosporia var catenulata in acute oral and dermal toxicity/pathogenicity evaluations in rats and rabbits. Vet Hum Toxicol. (2004) 46:248–50.
36. García, L, Gleiby, M, Montes, NO, and Hidalgo, L. Estudio de la irritación ocular y dérmica de Pochonia chlamydosporia var. Catenulata Rev Toxicol. (2004) 21:103–7.
37. García, L, Melchor, G, Arévalos, J, and Hidalgo-Díaz, L. Evaluación de la fitotoxicidad de la cepa imi sd 187 de Pochonia chlamydosporia var. catenulata sobre Zea mays L. Rev Prot Vegetal. (2008) 23:38–42.
38. Rincy, MK, Praveena, R, and Eapen, SJ. A modified semi-selective medium for isolation and enumeration of Pochonia chlamydosporia (Goddard) Zare & W. Gams. Journal of Spices & Aromatic Crops. (2021) 30:117–125. doi: 10.25081/josac.2021.v30.i1.6923
39. Xue, YJ, Li, EL, Jing, CX, Ma, L, and Cai, KZ. Isolation, identification and characterization of the nematophagous fungus Arthrobotrys (Monacrosporium) sinense from China. Acta Parasitologica. (2018) 63:325–332. doi: 10.1515/ap-2018-0037
Keywords: biocontrol, P. chlamydosporia , eggs, toxicity, mice, parasites
Citation: Ma Y, Lv J, Jiang L, Fan Z, Hao L, Li Z, Ma C, Wang R and Luo H (2025) In vitro ovicidal studies on egg-parasitic fungus Pochonia chlamydosporia and safety tests on mice. Front. Vet. Sci. 11:1505824. doi: 10.3389/fvets.2024.1505824
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
Eustachio Tarasco, University of Bari Aldo Moro, ItalyAdolfo Paz Silva, Universidade de Santiago de Compostela, Spain
Copyright © 2025 Ma, Lv, Jiang, Fan, Hao, Li, Ma, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Wang, d3IyMDA2QDE2My5jb20=; Hongliang Luo, ODY1NTgzNDgwQHFxLmNvbQ==
 Yuan Ma
Yuan Ma Jinbao Lv4
Jinbao Lv4 Luyao Hao
Luyao Hao Rui Wang
Rui Wang