
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 07 January 2025
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1505801
This article is part of the Research Topic Natural Compounds/Products and Livestock Productivity: Enhancing Antioxidant Levels, Gut Health, Mitigating Greenhouse Gas Emissions, and Disease Control View all 24 articles
Maternal nutritional status plays a crucial role in embryonic development and has persistent effects on postnatal chicks. Vitamin C (VC) plays an important role in embryonic and postnatal development involved in nutri-epigenetics. The present study was conducted to investigate the effects of in ovo feeding (IOF) of VC on embryonic development, egg hatching time, and chick rectal temperature. Trial 1 was conducted under normal incubation conditions without the IOF procedure and was designed to analyze the characteristics of embryonic development and establish the scoring standards for yolk absorption and the rupture of the shell membrane. The results showed that the relative weight of the embryo and residual yolk and the organ indexes were reliable indicators of embryonic development. Yolk absorption was scored 0, 1, 2, 3, and 4, with a higher score indicating more complete absorption. In addition, the rupture of the shell membrane was divided into two cases: YES and NO. Trial 2 included three groups, control (CON), normal saline (NS), and vitamin C (VC), and was designed to detect the effects of IOF of VC on the indicators in trial 1, as well as the plasma biochemical indicators. At embryonic age 11 (E11), each egg in the CON group was non-injected, each egg in the NS group was injected with 0.1 mL of sterile normal saline, and each egg in the VC group was injected with 0.1 mL of sterile normal saline containing 3 mg vitamin C. The whole day of E21 was evenly divided into three time periods: early (incubation hours 480–488), middle (incubation hours 488–496), and late (incubation hours 496–504). Among the CON, NS, and VC groups, the percentages of the early-hatched chicks (egg hatching time) were 29.31, 12.00, and 33.90%, respectively. The proportions of early and middle hatched chicks in these groups were 51.72, 42.00, and 38.27%, respectively. The rectal temperature of chicks was lower (p < 0.05) in the VC group than in the CON and NS groups. Compared to the NS group, the plasma biochemical indicators in the VC group showed significantly lower levels of alkaline phosphatase (ALP), total protein (TP), albumin (ALB), GLB, total bilirubin (TBIL), TBA, uric acid (UA), high-density lipoprotein cholesterol (HDL-C), and corticosterone (CORT) (p < 0.05). Additionally, alanine aminotransferase (ALT) had an increasing trend (p = 0.059) in the VC group. In conclusion, our data demonstrated that VC accelerated the hatching process and reduced chicks’ rectal temperature, which may be related to the improvement of liver function and changes in metabolism, as indicated by blood biochemical indicators.
The developmental origins of health and disease (DOHaD) theory suggests that factors present during the gestational and early-postnatal period (nutrition, climate, stress, toxins, and exercise) can significantly influence adult health and likelihood of developing chronic diseases later in life (1). Growing evidence has shown that an impoverished in utero environment may cause many diseases after birth, such as cardiovascular disease, metabolic syndrome, obesity, and diabetes (2). As with livestock animals, the abovementioned effects directly reduce production performance and lead to economic losses. Malnutrition during incubation (achieved by removing quantities of albumen from the egg) can lead to low hatch rates in chicks and decreased post-hatch body weight up to 7 days of age (3). Using Arbor Acres broilers as an example, the incubation period accounts for up to one-third of the entire life cycle, and this proportion is expected to increase with the development of breeding, which will lead to the embryonic environment playing a more prominent role in the production performance of broilers.
Nutrition is an important factor affecting embryonic development and also serves as an intervention strategy that neutralizes the negative effects of other factors on embryonic development (4). Prenatal epigenetic diets, including vitamin C (VC) from fruits, resveratrol from grapes, and isothiocyanates from broccoli, help protect against environmental pollution and improve fetal and offspring health outcomes (5). Embryos derive nutrients from the mother through the placenta in mammals, while avian embryos only rely on the nutrients deposited in the eggs. If there is insufficient nutrient deposition in breeder eggs, it may directly affect embryonic development and the offspring’s production performance. Our previous studies reported that the endogenous synthetic ability of VC was weak during embryonic development, and VC supply was mainly from endogenous absorption from the yolk and albumen (6). However, dietary VC supplementation failed to increase VC deposition in the yolk and albumen (7). In addition, stress during the breeding process reduced the synthesis capacity of VC, and VC was preferentially used to eliminate stress at the same time (8), leading to less deposition in breeder eggs.
VC plays an important role in embryonic or postnatal development, and vitamin C supplementation during pregnancy improves placental function (9). Some studies have shown that in ovo feeding (IOF) of VC has positive effects on growth performance in postnatal broilers (10), suggesting that VC might improve embryonic development in broilers based on the DOHaD theory. In addition, the elimination and reconstruction of DNA methylation occur during embryonic development in broilers (11), and VC can act as a cofactor for related enzymes involved in DNA and histone demethylation (12).
The hatching process is closely related to embryonic development and is also reflected in the rupture of the shell membrane (or shell), yolk absorption, and hatching time (13). In addition, rectal temperature is a reliable indicator for evaluating a chick’s stress resistance both inside and outside of the egg (14). In this study, we investigated the effects of IOF of VC on embryonic development, the hatching process, and the rectal temperature of newly hatched chicks. We also explored the potential underlying mechanisms.
The animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Southwest University of Science and Technology (Permit Number: L2023013).
At embryonic age 11 (E11), the eggs were candled, and the unfertilized eggs and dead embryos were removed. At E19, all the eggs were transferred to hatching baskets. All the eggs, sourced from Arbor Acres broiler breeder flocks, were purchased from the Xianyang Dacheng Poultry Industry Co. Ltd. The fertilized eggs were collected from the same flock at 27 weeks of age for trial 1 and 31 weeks of age for trial 2. All the eggs were incubated in an automatic incubator, and the hatching program was set to chicken mode.
A total of 300 disinfected eggs were randomly divided into 10 replicates, with 30 eggs per replicate. Each egg was weighed, and its weight was recorded on the eggshell. At E19, each egg was put into a mesh bag. At E11, E13, E15, E17, E19, E20, E21, and on the postnatal 1st day (D1), one egg or chick per replicate was selected for the measurement of embryonic or chick weight and organ indexes. Every 2 h, starting from E19.5 to E20.5, one egg per replicate was selected for the standard establishment of yolk absorption and shell membrane rupture scores. The eggs were incubated using an automatic incubator (9TV-2A, Beijing Blue Sky Electronic Technology Co., Ltd.). The incubation procedures were as follows: (1) E1–E6: temperature 38°C, humidity 60%; (2) E7–E12: temperature 37.8°C, humidity 55%; (3) E13–E18: temperature 37.6°C, humidity 60%; and (4) E19–E21: temperature 37.2°C, humidity 70%. The eggs were turned every 2 h before E19 and not turned after E19.
Two incubators (A and B) were used in trial 2. Three groups were set up in both incubators A and B: control (CON), normal saline (NS), and vitamin C (VC) groups. At E11, each egg in the CON group was non-injected, each egg in the NS group was injected with 0.1 mL of sterile normal saline, and each egg in the VC group was with injected 0.1 mL of sterile normal saline containing 3 mg of vitamin C. The injection site was the yolk sac. The egg injection method is based on a previous publication (15). A total of 450 disinfected eggs were randomly divided into three groups with 10 replicates in incubator A, while a total of 210 disinfected eggs were randomly divided into three groups without replicates in incubator B. At E13, E15, E17, E19, E20, E21, and on D1, one egg or chick per replicate was selected for the measurement of embryonic or chick weight and organ indexes in incubator A. At the end of E20, two eggs per replicate were selected for yolk absorption and shell membrane rupture scoring in incubator A. After hatching, two chicks per replicate were selected for the measurement of rectal temperature and then one chick per replicate was selected for plasma collection in incubator A. All the eggs were used for analyzing the hatching time in incubator B.
After the embryo or chick was slaughtered, the weight of the embryo or chick, residual yolk, liver, heart, lung, and gallbladder was measured. The weight of the embryo or chick and residual yolk was expressed as the ratio of each to the egg weight. The organ index was expressed as the ratio of the organ weight to the embryonic weight, excluding the residual yolk.
To the best of our knowledge, this was the first time a standard for yolk absorption and shell membrane rupture scoring was established. The egg was opened from the air chamber, and the status of the rupture of the shell membrane was photographed. Then, the embryo was immediately slaughtered and placed in a Petri dish, and the status of yolk absorption was photographed. In the end, a visual scoring standard was established. At a certain time, all the eggs to be evaluated were removed from the incubator, and the evaluation was completed within 30 min.
After the feathers of the newly hatched chicks were dry, the rectal temperature was measured. The plasma biochemical parameters included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), globulin (GLO), total bilirubin (TBIL), blood urea nitrogen (BUN), uric acid (UA), creatinine (CRE), glucose (GLU), total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). They were detected using an automated biochemical analyzer (Hitachi, Tokyo, Japan). The plasma samples were sent to the laboratory department of Mianyang Central Hospital (Mianyang, China) to measure the levels of thyroxin (T4), free thyroid hormone (fT4), triiodothyronine (T3), and free triiodothyronine hormone (fT3) using electro-chemiluminescence immunoassay. The plasma corticosterone (CORT) level was measured using the Chicken Corticosterone ELISA Kit (Shanghai Enzyme Biotechnology Co. Ltd., Shanghai, China).
As shown in Figure 1A, the entire day of E21 was evenly divided into three time periods: early (incubation hours 480–488), middle (incubation hours 488–496), and late (incubation hours 496–504). The hatched chicks in each group were counted individually during the early, middle, and late periods. These periods were calculated as a percentage of the total number of the hatched chicks for each group.
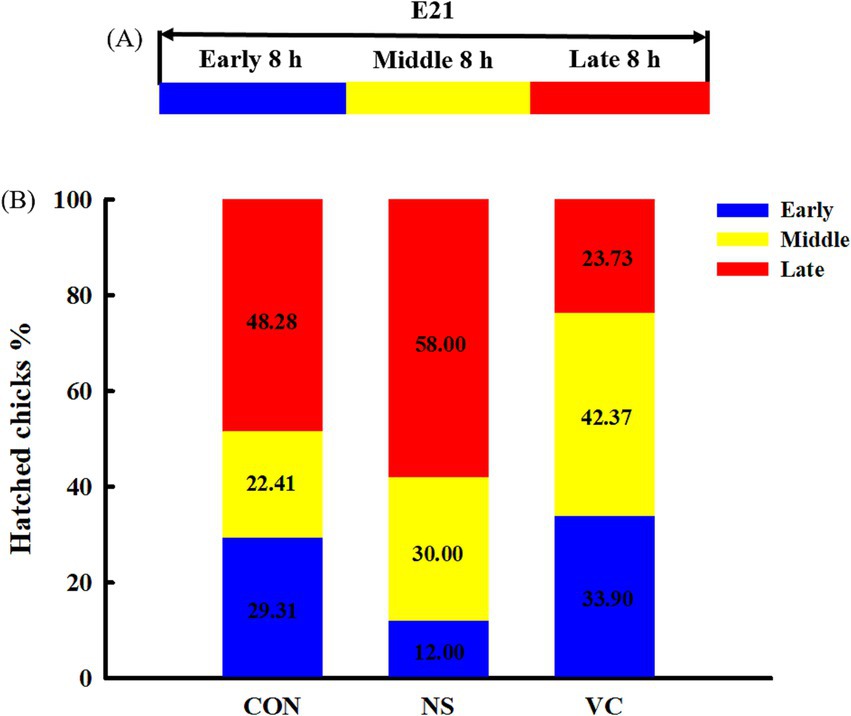
Figure 1. Three stages of analyzing hatchability (A) and effects of in ovo feeding of vitamin C on egg hatching time (B).
The data on the embryonic or chick weight, organ indexes, and chick rectal temperature were analyzed using one-way ANOVA, and the data on the plasma parameters were analyzed using an independent sample t-test with SPSS 21.0 (SPSS Inc., Chicago, IL, United States). Statistical significance was considered at p < 0.05 and trends at p < 0.1. All other data were compared in absolute value.
As shown in Figure 2A, the relative weight of the embryo or chick gradually increased from E11 to D1 (p < 0.05). The relative weight of the residual yolk gradually decreased from E20 to D1 (p < 0.05, Figure 2B). As shown in Figures 2C,F, the liver and gallbladder indexes both reached the maximum on D1 (o < 0.05). The liver index showed no significant difference at E15, E17, E19, E20, and E21 (p > 0.05, Figure 2C), and the gallbladder index showed no significant difference at E19, E20, and E21 (p > 0.05, Figure 2F). As shown in Figure 2D, the heart index showed no significant difference at E11, E13, E15, and E17 and at E20, E21, and D1 (p > 0.05), but the heart index was significantly higher at E11, E13, E15, and E17 than at E21 and on D1 (p < 0.05). As shown in Figure 2E, the lung index showed no significant difference at E11, E13, and E15 and at E17, E19, E20, E21, and D1 (p > 0.05), and the lung index at E11, E13, and E15 was significantly higher than that at E17, E19, E20, E21, and D1 (p < 0.05).
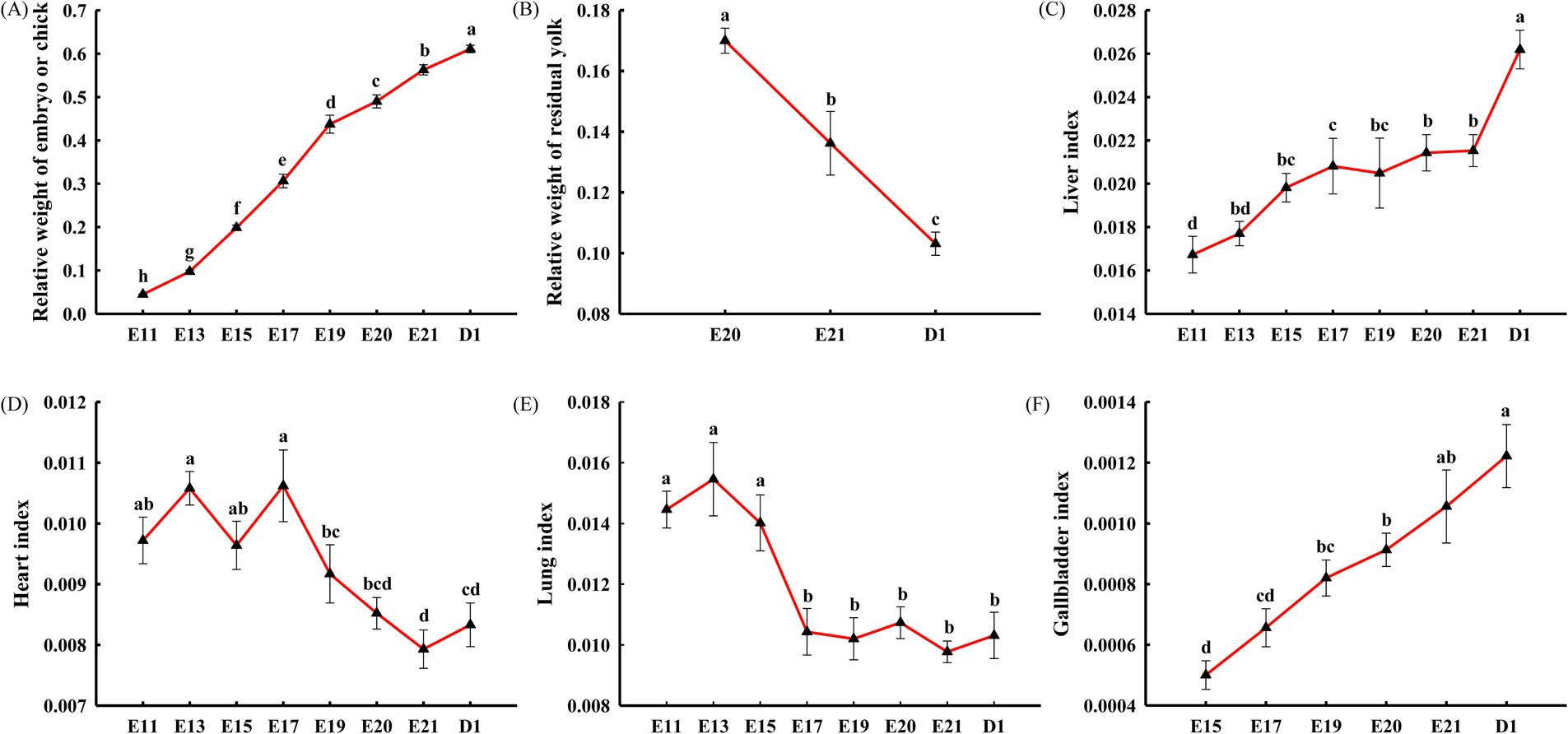
Figure 2. Embryonic development characteristics during incubation in the broiler chickens, including the relative weight of the embryo or chick (A), the relative weight of the residual yolk (B), the liver index (C), the heart index (D), the lung index (E), and the gallbladder index (F). Each triangle represents the mean ± SEM (n = 10). Triangles with different letters are significantly different (p < 0.05). The relative weight of the embryo or chick refers to the ratio of the weight of the embryo or chick, excluding the residual yolk, to the initial egg weight. The relative weight of the residual yolk refers to the ratio of the weight of the residual yolk to the initial egg weight. The organ index refers to the ratio of the organ weight to the embryonic or chick’s weight, excluding the residual yolk.
As shown in Figure 3A, yolk absorption was divided into five representative cases, which were scored 0, 1, 2, 3, and 4. The higher the score, the more complete the yolk absorption. As shown in Figure 4A, the rupture of the shell membrane was divided into two cases: YES and NO. YES meant that the shell membrane had been ruptured, while NO meant the opposite.
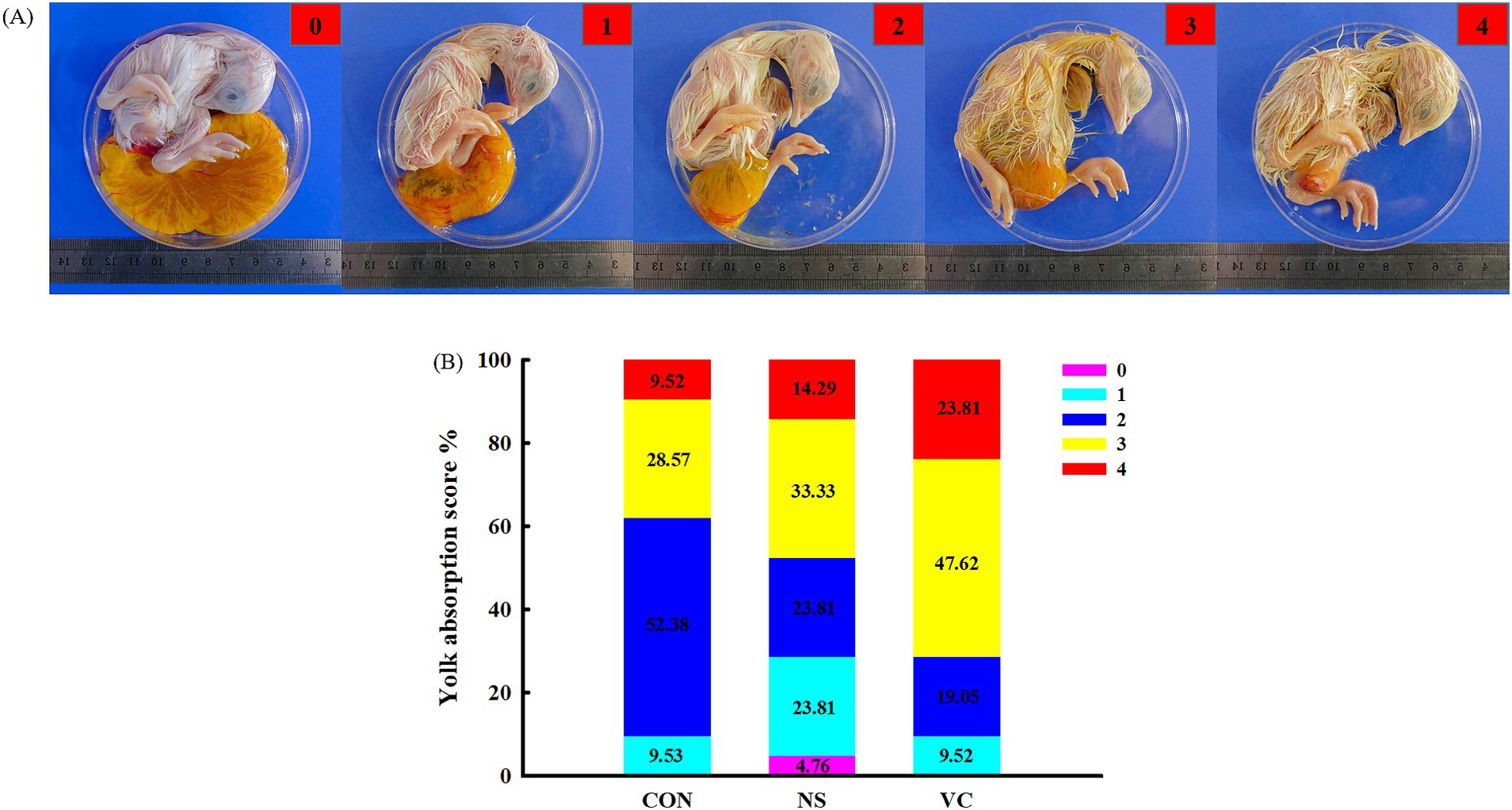
Figure 3. Standard of the yolk absorption score (A) and effects of in ovo feeding of vitamin C on the yolk absorption score (B).
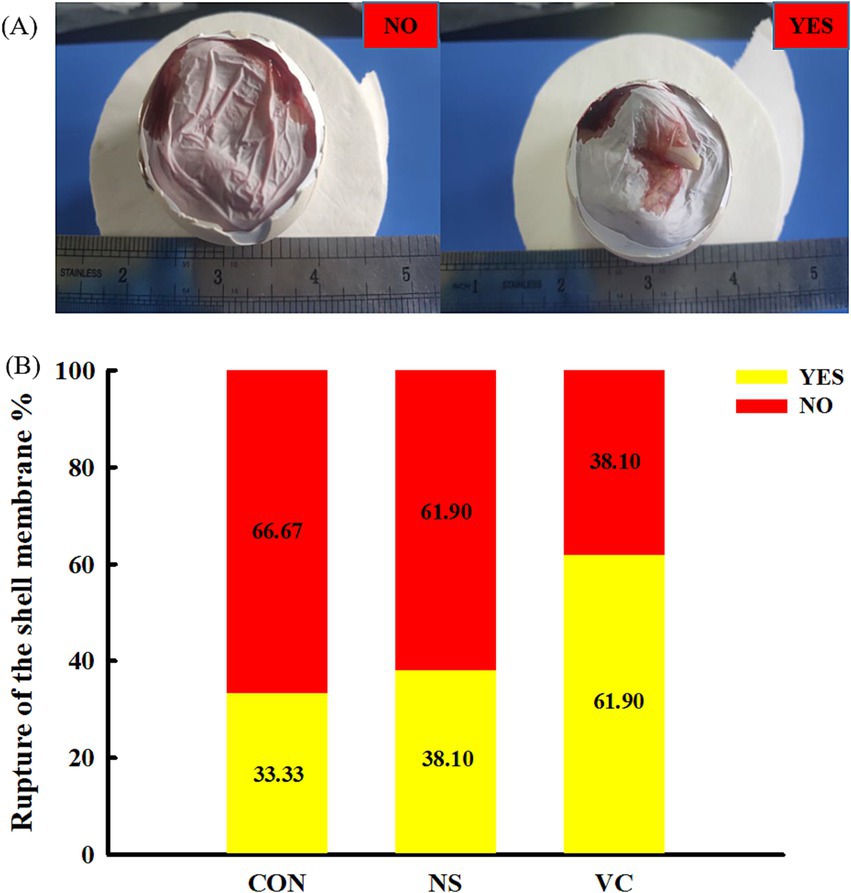
Figure 4. Standard of the rupture of the shell membrane score (A) and effects of in ovo feeding of vitamin C on the yolk absorption score (B).
As shown in Table 1, IOF of VC had no significant influence on the relative weight of the embryo or chick at E13, E15, E17, E19, E20, E21, and on D1 and the relative weight of the residual yolk at E20, E21 and on D1 (p > 0.05). As shown in Table 2, IOF of VC had no significant influence on the organ indexes of the heart, liver, lung, and gallbladder (except at E13) at E13, E15, E17, E19, E20, E21, and on D1 (p > 0.05).
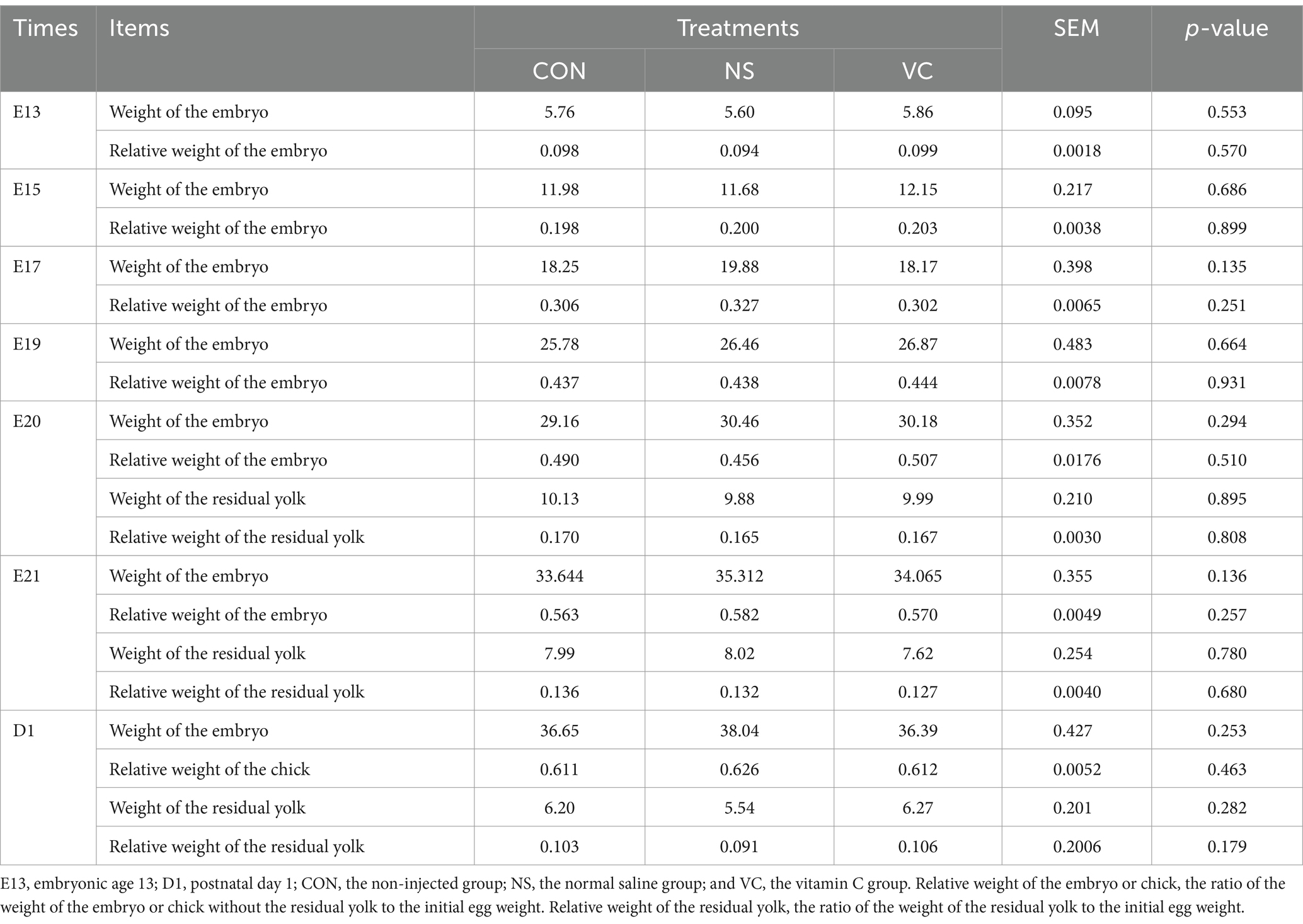
Table 1. Effects of in ovo feeding of vitamin C at E11 on the relative weight of the embryo or chick and the residual yolk during incubation in broiler chickens.
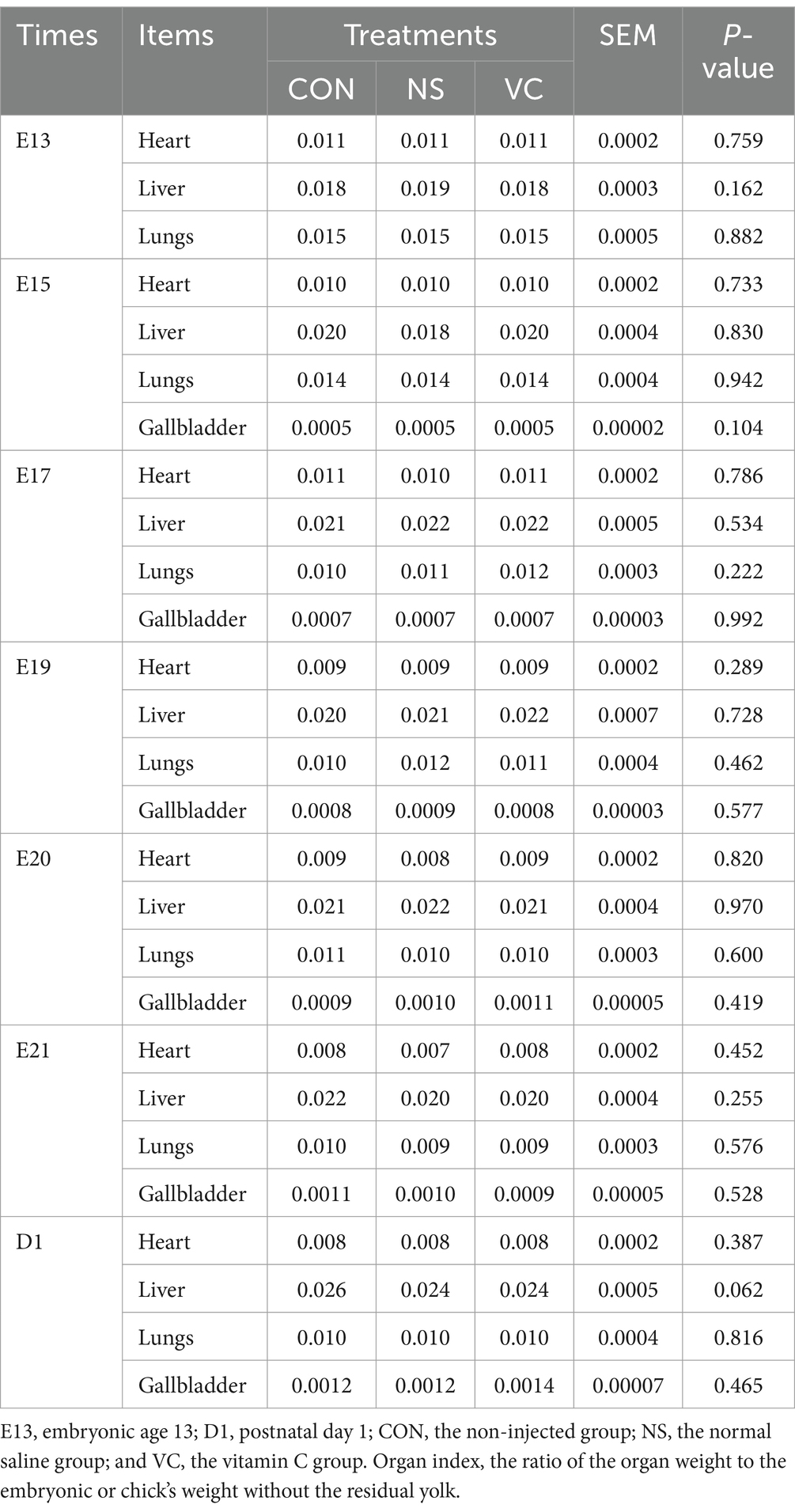
Table 2. Effects of in ovo feeding of vitamin C at E11 on the embryonic or chick organ indexes of the heart, liver, lungs, and gallbladder during incubation in broiler chickens.
As shown in Figure 3B, the proportions of a score of 4 among the CON, NS, and VC groups were 9.52, 14.29, and 23.81%, respectively; the proportions of scores 3 and 4 among the CON, NS, and VC groups were 38.09, 47.62, and 71.43%, respectively; and the proportions of scores 0, 1, and 2 among the CON, NS, and VC groups were 61.91, 52.38, and 28.57%, respectively. As shown in Figure 4B, the proportions of YES among the CON, NS, and VC groups were 33.33, 38.10, and 61.90%, respectively, while the proportions of NO among the CON, NS, and VC groups were 66.67, 61.90, and 38.10%, respectively.
As shown in Figure 1B, the percentages of the early hatched chicks among the CON, NS, and VC groups were 29.31, 12.00, and 33.90%, respectively; the proportions of the early and middle hatched chicks among the CON, NS, and VC groups were 51.72, 42.00, and 38.27%, respectively; and the proportions of the late hatched chicks among the CON, NS, and VC groups were 48.28, 58.00, and 23.73%, respectively. As shown in Figure 5, the chicks’ rectal temperature was lower in the VC group than in the CON and NS groups (p < 0.05).
As shown in Table 3, in the VC group, the biochemical indicators of ALP, TP, ALB, GLB, TBIL, TBA, UA, and HDL-C were lower (p < 0.05) and ALT showed an increasing trend (p = 0.059), compared to the NS group,. In the VC group, the hormone level of CORT was lower (p < 0.05) and T4 showed a decreasing trend (p = 0.096), compared to the NS group,
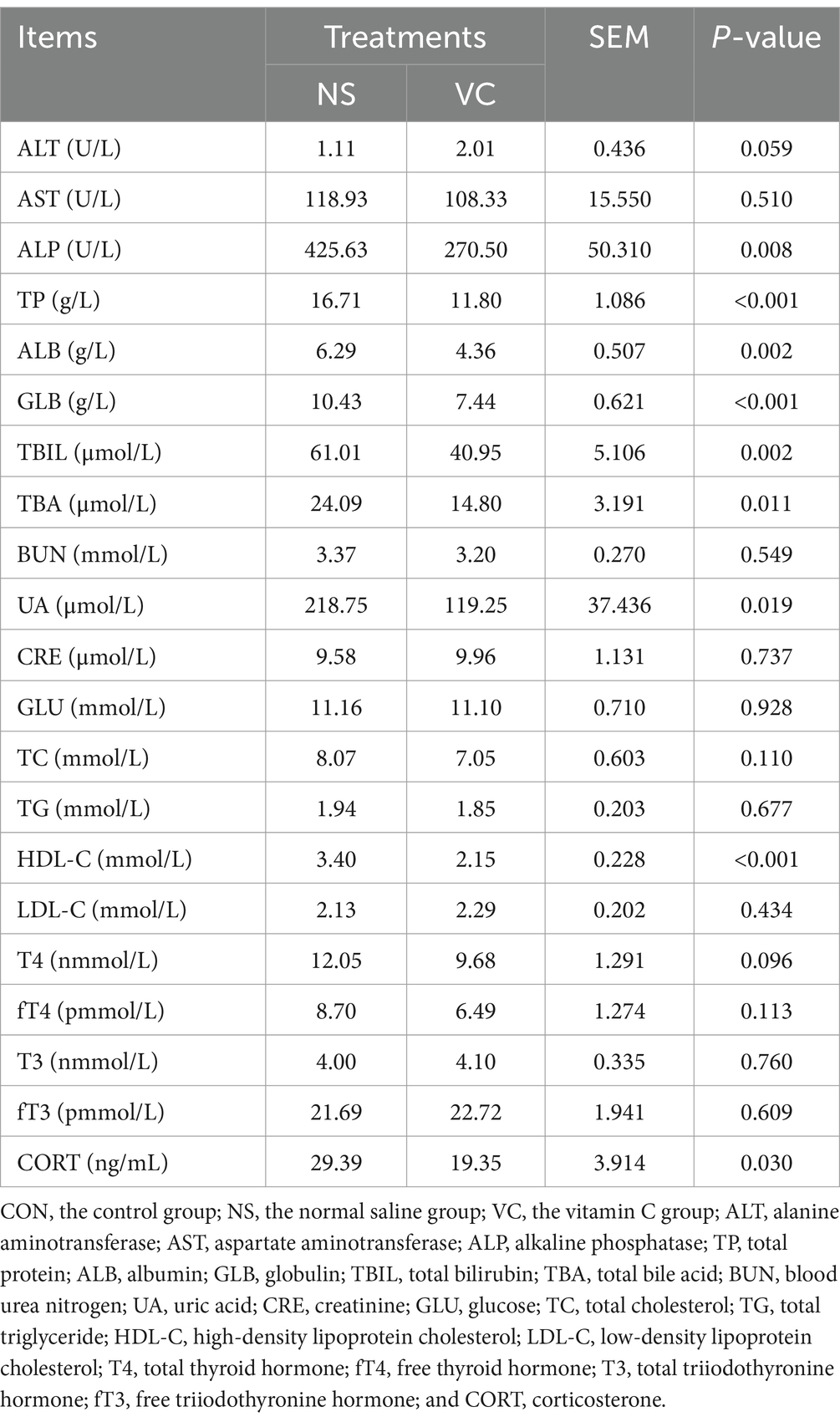
Table 3. Effects of in ovo feeding of vitamin C at E11 on plasma parameters in the newly hatched broiler chicks.
During a short period of 21 days, a fertilized egg eventually turns into a viable chick through a series of developmental procedures, including access to oxygen, diverse energy sources, and the accommodation of metabolic patterns (16). The heart, lungs, and chorioallantoic membrane are the basis for ensuring oxygen supply and gas exchange, while the liver is the center of metabolism and the basis for accommodating metabolic patterns. In addition, bile in the gallbladder improves the utilization of lipids, which are the major energy-supplying substances from the yolk. Interestingly, a synchronized progression is observed in the organ indexes of the heart, liver, lungs, and gallbladder, as well as in the relative weight of the embryo (or chick) and residual yolk, which may together reflect embryonic developmental characteristics.
The chorioallantoic membrane forms from E4, gradually develops blood vessels, and attains mature gas exchange capacity by E11-12 (17). Meanwhile, the paired primordia of the heart begin to fuse and develop from E2 (13), and the heart adapts to mature chorioallantoic respiration by E11-12. Nevertheless, the lungs develop capillaries by E18, and pulmonary respiration occurs by E19, gradually replacing chorioallantoic respiration (18). At the same time, the heart is forced to adapt to the transition from chorioallantoic respiration to pulmonary respiration. In summary, E18 is a key time for the transition from chorioallantoic respiration to pulmonary respiration, as well as for cardiac adaptive changes. Therefore, the turning points of the heart and lung indexes were observed at E17 and E15, respectively, indicating that the transition from chorioallantoic respiration to pulmonary respiration forced adaptive changes in the heart.
The liver is structurally completed by E14 and then fully functions as the metabolic center (16), which were synchronized with the increase and stability of the liver index from E11 to E15 and from E15 to E21, respectively, in this study. From E21 to D1, a significant increase in the liver index was attributed to the rapid deposition of lipids in the liver (19). The gallbladder formed at E9 and then grew rapidly, which was consistent with the increase in the gallbladder index from E11 to D1. Bile, secreted by the gallbladder, is beneficial for the liver to utilize lipids from the yolk, which was supported by the relative weight of the residual yolk from E20 to D1. A shift in the growth rate was observed at E13, which may be related to the structural completion of the liver and bile secretion from the gallbladder, both of which together ensure the utilization of lipids as the main energy supply.
Changes in the organ indexes (the heart, liver, lungs, and gallbladder) and the relative weight of the embryo or remaining yolk are synchronized with changes in important physiological functions during embryonic development and can be used as indicators to reflect the hatching characteristics of broiler chickens. Recently, the incubation period accounts for more than 50% of an Arbor Acres broiler’s productive life. Therefore, it is necessary to encourage positive effects on broiler growth performance during the early growth period (20). In this study, IOF of VC had no harmful effects on the relative weight of the embryo (or chick), residual yolk, and organ indexes. In previous studies, it was found that IOF of VC had no significant influence on the weight of D1 chicks, which was the same as our results (7).
At E19, the structural development of the chicken embryo is complete. Before hatching, the embryo must complete hatching behaviors such as absorbing the remaining yolk sac into the abdominal cavity, pecking the shell membrane, and pecking the shell. Early or delayed occurrence of the above behaviors will affect the quality of chicks and the hatching time. Therefore, the hatching time and the score for yolk absorption and the rupture of the shell membrane were selected to comprehensively evaluate the effects of IOF of VC on the hatching process in broilers. In this study, the rupture of the shell membrane and yolk absorption occurred earlier, and the hatching time was correspondingly advanced in the VC group, indicating that VC accelerated the hatching process of preparation for emergence. The hatching muscle, a pair of muscles located at the back of the head, assists in the movement of the embryonic head and mouth (13) and is responsible for puncturing the shell membrane and swallowing amniotic fluid through vigorous contractions (21). The swallowing of amniotic fluid directly facilitates intestinal development (intestinal morphology, digestive enzyme activities, and nutrient transporter expression) and provides the foundation for digestion and absorption of the remaining yolk (22), which is in favor of yolk absorption. We speculated that VC may promote energy reserves in the hatching muscle to accelerate the hatching process, which needs to be further investigated.
Producing chicks with strong anti-stress ability is crucial for the poultry industry. VC has been used to mitigate heat stress by improving production performance through antioxidant modulation in chickens (23). Rectal temperature is a reliable indicator for evaluating anti-stress ability (14, 24), as are stress-related hormones, including CORT (25). CORT is a sensitive indicator in broiler chickens that reflects their physiological condition under stress. To a certain extent, environmental changes before and after hatching are stressful to newly hatched chicks. In this study, IOF of VC reduced the chicks’ rectal temperature and plasma CORT levels, indicating that the chicks in the VC group possessed stronger anti-stress ability. Our previous research showed that IOF of VC could regulate embryonic endogenous protection (heat shock protein) and metabolism, which may reduce the risk associated with overheating in chicken embryos (26). In a previous study, IOF of VC reduced the rectal temperature of newly hatched chicks, but the study lacked a non-injected group (7). This study addressed the shortcomings and further confirmed the results. In addition, some studies have shown that dietary VC could reduce rectal temperature and improve heat tolerance in poultry under heat stress (27).
During the preparation for emergence, dramatic physiological and metabolic changes occur. Any intervention can markedly affect the embryonic hatching process and postnatal performance. In this study, the CON group was set up to confirm the effects of IOF of VC on embryonic development, the hatching process, and the chicks’ rectal temperature, while the NS group served as the strictly biological control group for the VC group. Therefore, the plasma biochemical indicators and hormone levels, sensitively reflecting embryonic physiology and metabolism, were only detected between the NS and VC groups.
It is noteworthy that the liver of newly hatched chicks was rich in lipids (28). Lipids in plasma are mainly derived from egg yolk, which is mainly composed of TG, phospholipids, and cholesterol. IOF of VC decreased the plasma HDL-C levels, implying a reduced transport of cholesterol from the blood to the liver. In addition, IOF of VC decreased the plasma levels of ALP, TBIL, and TBA, indicating that the function of the liver was in better status in the VC group. In summary, IOF of VC may be conducive for the liver to manage the physiological characteristics of rich lipids. The decreased level of plasma UA indicated that VC reduced protein catabolism. Combined with the decreased levels of plasma TP, ALB, and GLB, we speculated that VC promoted protein anabolism. Plasma GLU is mainly regulated by liver glycogen, which is derived from gluconeogenesis from glycogenic amino acids and glycerol during late incubation (29). Based on the reduced protein catabolism, we speculated that VC may promote gluconeogenesis from glycerol in the liver, which may be related to improved liver function.
Thyroid hormones including T3, fT3, T4, and fT4 regulate physiology and metabolism in birds. In addition, thyroid hormones vary dramatically during late incubation and after hatching (30). These hormones play an important role in the thermoregulation of broiler embryos and broiler chickens, involving metabolic heat production (31). In this study, IOF of VC showed a decreasing trend in the plasma T4 level, indicating that the D1 chicks had lower metabolic heat production, which was supported by the lower rectal temperature in the VC group.
The rupture of the shell membrane and yolk absorption occurred earlier, and correspondingly, the hatching time was advanced in IOF of VC (3 mg/egg), indicating that VC accelerated the hatching process. In addition, IOF of VC improved the anti-stress ability of the D1 chicks, as evidenced by lower rectal temperatures and plasma CORT levels. These effects may be related to the regulation of body energy metabolism.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by Institutional Animal Care and Use Committee of Southwest University of Science and Technology. The study was conducted in accordance with the local legislation and institutional requirements.
SD: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. JZ: Data curation, Software, Writing – original draft. XA: Data curation, Writing – review & editing. YZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Sichuan Science and Technology Program (2024NSFSC1175). The animal experimental procedures was approved by the Institutional Animal Care and Use Committee of Southwest University of Science and Technology (Permit Number: L2023013).
SD, JZ, and XA were employed by Techlex Food Co., Ltd. YZ was employed by Shanxi Dayu Bioengineering Co., Ltd.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ganguly, E, Hula, N, Spaans, F, Cooke, CLM, and Davidge, ST. Placenta-targeted treatment strategies: an opportunity to impact fetal development and improve offspring health later in life. Pharmacol Res. (2020) 157:104836. doi: 10.1016/j.phrs.2020.104836
2. Suzuki, K. The developing world of DOHaD. J Dev Orig Health Dis. (2018) 9:266–9. doi: 10.1017/S2040174417000691
3. Everaert, N, Métayer-Coustard, S, Willemsen, H, Han, H, Song, Z, Ansari, Z, et al. The effect of albumen removal before incubation (embryonic protein under-nutrition) on the post-hatch performance, regulators of protein translation activation and proteolysis in neonatal broilers. Br J Nutr. (2013) 110:265–74. doi: 10.1017/S000711451200503X
4. Peebles, ED. In ovo applications in poultry: a review. Poult Sci. (2018) 97:2322–38. doi: 10.3382/ps/pey081
5. Li, S, Chen, M, Li, Y, and Tollefsbol, T. Prenatal epigenetics diets play protective roles against environmental pollution. Clin Epigenetics. (2019) 11:82. doi: 10.1186/s13148-019-0659-4
6. Zhu, Y, Zhao, J, Wang, C, Zhang, F, Huang, X, Ren, Z, et al. Exploring the effectiveness of in ovo feeding of vitamin C based on the embryonic vitamin C synthesis and absorption in broiler chickens. J Animal Sci Biotechnol. (2021) 12:86. doi: 10.1186/s40104-021-00607-w
7. Zhu, Y, Guo, W, Zhao, J, Qin, K, Yan, J, Huang, X, et al. Alterations on vitamin C synthesis and transportation and egg deposition induced by dietary vitamin C supplementation in Hy-line Brown layer model. Anim Nutrit. (2021) 7:973–80. doi: 10.1016/j.aninu.2021.01.006
8. Delos Reyes, JB, Kim, JH, Han, GP, Won, SY, and Kil, DY. Effects of dietary supplementation of vitamin C on productive performance, egg quality, tibia characteristics and antioxidant status of laying hens. Livest Sci. (2021) 248:104502. doi: 10.1016/j.livsci.2021.104502
9. Shorey-Kendrick, LE, McEvoy, CT, O’Sullivan, SM, Milner, K, Vuylsteke, B, Tepper, RS, et al. Vitamin C supplementation improves placental function and alters placental gene expression in smokers. Sci Rep. (2024) 14:25486. doi: 10.1038/s41598-024-73005-7
10. Zhu, Y, Li, S, Duan, Y, Ren, Z, Yang, X, and Yang, X. Effects of. Br J Nutr. (2020) 124:903–11. doi: 10.1017/S000711452000210X
11. Li, S, Zhu, Y, Zhi, L, Han, X, Shen, J, Liu, Y, et al. DNA methylation variation trends during the embryonic development of chicken. PLoS One. (2016) 11:e0159230. doi: 10.1371/journal.pone.0159230
12. Young, JI, Züchner, S, and Wang, G. Regulation of the Epigenome by vitamin C. Annu Rev Nutr. (2015) 35:545–64. doi: 10.1146/annurev-nutr-071714-034228
13. Hamburger, V, and Hamilton, HL. A series of normal stages in the development of the chick embryo. Dev Dyn. (1992) 195:231–72. doi: 10.1002/aja.1001950404
14. Chen, XY, Wei, PP, Xu, SY, Geng, ZY, and Jiang, RS. Rectal temperature as an indicator for heat tolerance in chickens. Anim Sci J. (2013) 84:737–9. doi: 10.1111/asj.12064
15. Zhu, YF, Li, SZ, Sun, QZ, and Yang, XJ. Effect of in ovo feeding of vitamin C on antioxidation and immune function of broiler chickens. Animal. (2019) 13:1927–33. doi: 10.1017/S1751731118003531
16. Moran, ET. Nutrition of the developing embryo and Hatchling1. Poult Sci. (2007) 86:1043–9. doi: 10.1093/ps/86.5.1043
17. Ackerman, RA, and Rahn, H. In vivo O2 and water vapor permeability of the hen’s eggshell during early development. Respir Physiol. (1981) 45:1–8. doi: 10.1016/0034-5687(81)90044-x
18. Theodosiou, NA. The atlas of chick development, second edition. Vitro Cell Dev Biol Anim. (2006) 42:281–1. doi: 10.1290/050601.1
19. Liu, Y, Liu, X, Zhou, J, Ren, Z, Yang, X, Cao, Y, et al. Folic acid perfusion administration reduced abdominal fat deposition in starter arbor acres broilers. Poult Sci. (2019) 98:6816–25. doi: 10.3382/ps/pez413
20. Tarhan, M, and Peşmen, G. Effects of in ovo injection of glucose and vitamin C on hatching performance in broiler fertile eggs. J Radiat Res Appl Sci. (2023) 16:100690. doi: 10.1016/j.jrras.2023.100690
21. John, TM, George, JC, and Moran, ET. Pre-and post-hatch ultrastructural and metabolic changes in the hatching muscle of Turkey embryos from antibiotic and glucose treated eggs. Cytobios. (1987) 49:197–210.
22. Uni, Z, Tako, E, Gal-Garber, O, and Sklan, D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult Sci. (2003) 82:1747–54. doi: 10.1093/ps/82.11.1747
23. Gouda, A, Amer, SA, Gabr, S, and Tolba, SA. Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop Anim Health Prod. (2020) 52:2987–96. doi: 10.1007/s11250-020-02316-4
24. Goel, A, Ncho, CM, Gupta, V, and Choi, Y-H. Embryonic modulation through thermal manipulation and in ovo feeding to develop heat tolerance in chickens. Anim Nutrit. (2023) 13:150–9. doi: 10.1016/j.aninu.2023.01.005
25. Zaboli, G-R, Rahimi, S, Shariatmadari, F, Torshizi, MAK, Baghbanzadeh, A, and Mehri, M. Thermal manipulation during pre and post-hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult Sci. (2017) 96:478–85. doi: 10.3382/ps/pew344
26. Zhu, YF, Bodinga, MB, Zhou, JH, Zhu, LQ, Cao, YL, Ren, ZZ, et al. Effects of in ovo injection of vitamin C on heat shock protein and metabolic genes expression. Animal. (2020) 14:360–7. doi: 10.1017/S1751731119002088
27. Minka, NS, and Ayo, JO. Behavioral and rectal temperature responses of black Harco pullets administered vitamins C and E and transported by road during the hot-dry season. J Vet Behav. (2010) 5:134–44. doi: 10.1016/j.jveb.2009.12.022
28. Liu, Y, Zhou, J, Musa, BB, Khawar, H, Yang, X, Cao, Y, et al. Developmental changes in hepatic lipid metabolism of chicks during the embryonic periods and the first week of posthatch. Poult Sci. (2020) 99:1655–62. doi: 10.1016/j.psj.2019.11.004
29. Dal Pont, GC, Goes, EC, Araujo, RA, Oliveira, SG, Rocha, C, and Maiorka, A. Glycerol inoculation in eggs of young broiler breeders at different embryonic periods. Poult Sci. (2019) 98:3989–93. doi: 10.3382/ps/pez141
30. Lu, JW, McMurtry, JP, and Coon, CN. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in Chick embryos and hatched chicks. Poult Sci. (2007) 86:673–83. doi: 10.1093/ps/86.4.673
Keywords: vitamin C, in ovo feeding, embryonic development, hatching time, rectal temperature
Citation: Du S, Zhou J, Ao X and Zhu Y (2025) Effects of in ovo feeding of vitamin C on embryonic development, hatching process, and chick rectal temperature of broiler embryos. Front. Vet. Sci. 11:1505801. doi: 10.3389/fvets.2024.1505801
Received: 03 October 2024; Accepted: 05 December 2024;
Published: 07 January 2025.
Edited by:
Valiollah Palangi, Ege University, TürkiyeReviewed by:
Ilias Giannenas, Aristotle University of Thessaloniki, GreeceCopyright © 2025 Du, Zhou, Ao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufei Zhu, OTgwNjIzMDg2QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.