
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 24 December 2024
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1505151
This article is part of the Research TopicAdvancements in Synthetic Microbiomes for Enhancing Animal HealthView all 9 articles
The purpose of this study was to investigate the effects of dietary supplementation of compound probiotics on the performance, egg quality, biochemical parameters and intestinal morphology of laying hens. A total of 180 healthy 200-day-old Hyline Brown laying hens with similar initial laying rate (87.5% ± 0.2%) were randomly divided into the control group and the treatment group. Each group included 6 replicates and each replicate included 15 laying hens. The control group was provided a basal diet, while the treatment group received the basal diet supplemented with compound probiotics. The experiment lasted for 52 days. The study indicated the following outcomes: (1) The laying rate (LR) and average egg weight (AEW) of laying hens in the treatment group were significantly higher than those of the control group (p < 0.05), whereas the feed-to-egg ratio (F/E) was significantly lower (p < 0.05); (2) The yolk weight (YW), egg shape index (ESI) and albumen height (AH) were significantly higher (p < 0.05), whereas the eggshell percentage (EP) was significantly lower (p < 0.05) after the dietary supplementation of compound probiotics; (3) The treatment group significantly decreased in total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), immunoglobulin A (IgA), and immunoglobulin G (IgG) levels in serum compared to the CON group (p < 0.05). Additionally, serum levels of total protein (TP), globulin (GLB), albumin (ALB), high-density lipoprotein (HDL), alkaline phosphatase (ALP), and total antioxidant capacity (T-AOC) were significantly higher in the treatment group (p < 0.05); (4) The supplementation of compound probiotics to laying hen diets led to a significant reduction in crypt depth (CD) and the ratio of villus height to crypt depth (V/C) in the jejunum compared to the CON group (p < 0.05). In conclusion, the supplementation of compound probiotics can regulate the body metabolism and improve the intestinal morphology, thus enhancing the antioxidant capacity and immune function of the body, which in turn improves the performance and egg quality of laying hens.
The misuse of antibiotics has led to the widespread of resistant bacteria, posing a threat to human health, animal welfare, animal production and environmental health (1). Since July 2020, China has imposed a total ban on the addition of antibiotics to animal feeds, resulting in an urgent need to find alternatives to antibiotics to ensure the growth and health of commercial poultry. In recent years, feed additives such as plant extracts (2), probiotics (3), acidifiers (4), and antimicrobial peptides (5) have been widely used in animal husbandry in China to control pathogenic bacterial infections and improve animal growth performance.
In particular, probiotics are widely used as a potential green alternative to antibiotics due to their effective probiotics effects. As non-pathogenic organisms, probiotics are able to positively influence livestock performance and health indicators by improving blood biochemical parameters, intestinal flora, immunity, intestinal integrity and digestive enzyme activity (6, 7). The main modes of action of probiotics include the following: 1. Competition with pathogenic bacteria for attachment sites in the epithelium of the digestive tract, and when the beneficial bacteria in probiotics occupy these binding sites, a protective physical barrier is formed; 2. The ability of probiotics to produce antimicrobial substances, such as bacteriocins, to inhibit the activity of pathogenic bacteria; 3. Anaerobic bacteria are present in probiotics, and in the gut microbiota, anaerobic bacteria are the dominant flora, and these bacteria promote a low oxygen tension environment in the gut, thereby inhibiting the growth of pathogens and creating an unfavorable environment for their survival and reproduction (8–10).
The addition of probiotics to laying hen diets has been shown to improve hen performance parameters including egg production, feed factor and egg quality, disease resistance and animal welfare (11–15). It has been shown that Bacillus subtilis can produce a variety of extracellular enzymes such as α-amylase, β-amylase, cellulase, protease, etc., which enhances intestinal digestibility, nutrient absorption and immune function (16, 17). Macit et al. (18) reported that the addition of probiotics to feed increased the monounsaturated fatty acid content of egg yolks and improved feed factor and yolk color. Park et al. (19) demonstrated that the addition of probiotics significantly increased egg production, eggshell thickness and nutrient digestibility, and reduced ammonia emissions in laying hens. It was indicated that the addition of yeast cultures could improve egg quality and enhance feed digestibility (20). Compound probiotics consist of many different types of probiotics, and the common strains are Bacillus, Lactobacillus plantarum and Saccharomyces cerevisiae. By combining various probiotics in appropriate proportions, it can enhance the growth of livestock and poultry, improve antioxidant capacity and regulate immunity (21). Studies have shown that the supplementation of compound probiotics to the basal diets can improve the performance of laying hens and enhance egg quality (22). In addition, it improves the immune system by increasing the production of anti-inflammatory cytokines, decreasing intestinal permeability and inhibiting oxidative stress (23). In view of this, we hypothesized that the supplementation of compound probiotics to the diets of laying hens is effective. By evaluating the effects of compound probiotics on laying performance, egg quality, serum biochemical indexes, antioxidant capacity, immune function and intestinal morphology of laying hens, we can provide theoretical basis for the application of compound probiotics in laying hens.
The Institute of Feed Research of the Chinese Academy of Agricultural Science’s Animal Care and Use Committee granted a license for the research based on an ethical review (Statement no. IFR-CAAS20240105, Beijing, China). A total of 180 healthy 200-day-old Hyline Brown laying hens with similar initial laying rate (87.5% ± 0.2%) were randomly divided into the control group (CON) and the treatment group (PRO). Each group included 6 replicates and each replicate included 15 laying hens. The CON received a basal diet, while the PRO received a basal diet supplemented with 0.5 g/kg of compound probiotics. And the compound probiotics were supplied into the basal diet as a powder. The compound probiotics were first mixed with the premixes and then with the other ingredients to make feed mixing uniformity higher. The basic corn-soybean meal diets were formulated to meet or exceed the nutritional requirements for laying hens calculated according to The National Research Council (NRC, 1994) recommended (Table 1). The compound probiotics were consisted of Bacillus subtilis (1 × 109 CFU/g), Lactobacillus plantarum M8 (1 × 109 CFU/g) and Saccharomyces cerevisiae (1 × 109 CFU/g), an were mixed in a ratio of 1: 1:1 to form compound probiotics preparation. Saccharomyces cerevisiae were purchased from Angel Yeast. Co, the dosage of probiotics should follow the company’s commercial recommendations. While Bacillus subtilis and Lactobacillus plantarum M8 were screened and preserved in our laboratory, and Lactobacillus plantarum M8 was screened with antioxidant capacity as an index and the method of Duz et al. (24) was referred to and slightly modified, and its antioxidant capacity was shown in Table 2.
The experiment was carried out in Dongying, Shandong Province, Lanhai Animal Husbandry Base for a total of 52d. All laying hens were raised in an environmentally controlled facilities with stereoscopic cages. Adjust test conditions such as temperature, humidity and light to meet Hyline Brown laying hens management guidelines, during the trial, they were given an unlimited supply of food and enough of clean water to drink. No medications or antibiotics were administered.
Throughout the experiment, the amount of eggs production, egg weight and feed consumption were all precisely recorded every day, and the remaining feed weight was weighed once a week, and the laying rate (LR), average egg weight (AEW), average daily feed intake (ADFI) and feed-to-egg ratio (F/E) were calculated with the unit of repetition. The calculation formula is as follows:
Two eggs were chosen at random from each replication after the experiment to assess the quality of the eggs. Egg analyzer (Orka Food Technology Ltd., Israel) was used to measure yolk color (YC), albumen height (AH), and Haugh unit (HU). To determine the egg shape index (ESI), the length and width of the egg are measured using an electronic digital caliper. The ESI is calculated by the formula index = egg length/ egg width. The egg force reader (Orka Food Technology Ltd., Israel) was used to test the eggshell strength (ES). The thickness of the eggshell was measured at the blunt, sharp and equator of the eggshell using the electronic digital caliper and the mean value obtained was the eggshell thickness (ET). To determine the yolk percentage (YP), albumen percentage (AP), and eggshell percentage (EP), an electronic balance was used to measure the egg weight (EW), yolk weight (YW), and egg shell weight (ESW). The formulas are as follows.
Two hens were removed from each replication at the conclusion of the experiment, and about 3–5 mL of blood was extracted from the wing vein. The serum was obtained by centrifuging the blood samples for 15 min at 4°C at 3000 × g, which was stored in a cryogenic refrigerator at −20°C for the subsequent determination of the relevant indexes. A fully automated biochemical analyzer (AU5800, American Beckman Coulter Co., Ltd., United States) was used to measure serum biochemical parameters, including total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), globulin (GLB), albumin (ALB), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and alkaline phosphatase (ALP).
Serum samples stored in −20°C refrigerator were taken to determine serum total antioxidant capacity (T-AOC) and malondialdehyde (MDA) content and immunoglobulin A (IgA), immunoglobulin G (IgG) and immunoglobulin M (IgM) according to the instructions of the kit produced by Nanjing Jianjian Bioengineering Institute (Nanjing, China).
One hen of approximately average weight was randomly selected for slaughter in each replicate. The segments of the duodenum, jejunum, and ileum were taken, each approximately 2–3 cm in length, were rinsed with PBS solution and then fixed in a 10% paraformaldehyde solution. After 12 h, the solution was replaced and left to fix for over 24 h. The samples were embeded with the paraffin, then dewaxed after section, and stained with hematoxylin and eosin stains (H&E). The villus height (VH) and crypt depth (CD) were measured using a micrometer, and the villus height to crypt depth ratio (V/C) was calculated.
The results of indicators were all tested by t-test using the SPSS 22.0 software (SPSS Inc., Chicago, IL, United States). Statistically significant effects were further analyzed and means were compared by Duncan’s multiple range test. If p < 0.05, the difference was significant. All data in this study were presented as mean ± standard error of mean (SEM), and the graphs were generated by GraphPad Prism software 8.0 (GraphPad Inc., San Diego, United States).
Table 3 demonstrates that when compound probiotics were supplemented into the diet, LR and AEW were clearly raised (p < 0.05) and F/E was significantly lowered (p < 0.05) in comparison to CON. Nonetheless, there was no statistically significant difference (p > 0.05) in the ADFI of laying hens between PRO and CON.
As shown in Table 4, with the supplementation with compound probiotics, YW, ESI, and AH of were significantly higher (p < 0.05) than CON, but the EP was significantly lower (p < 0.05). While between CON and PRO, there was no discernible difference in HU (p > 0.05).
Figure 1 shows that the serum levels of TC, TG, ALT and AST significantly decreased (p < 0.05) in the in PRO. However, the serum levels of TP, GLB, ALB, HDL, and ALP significantly raised in PRO (p < 0.05).
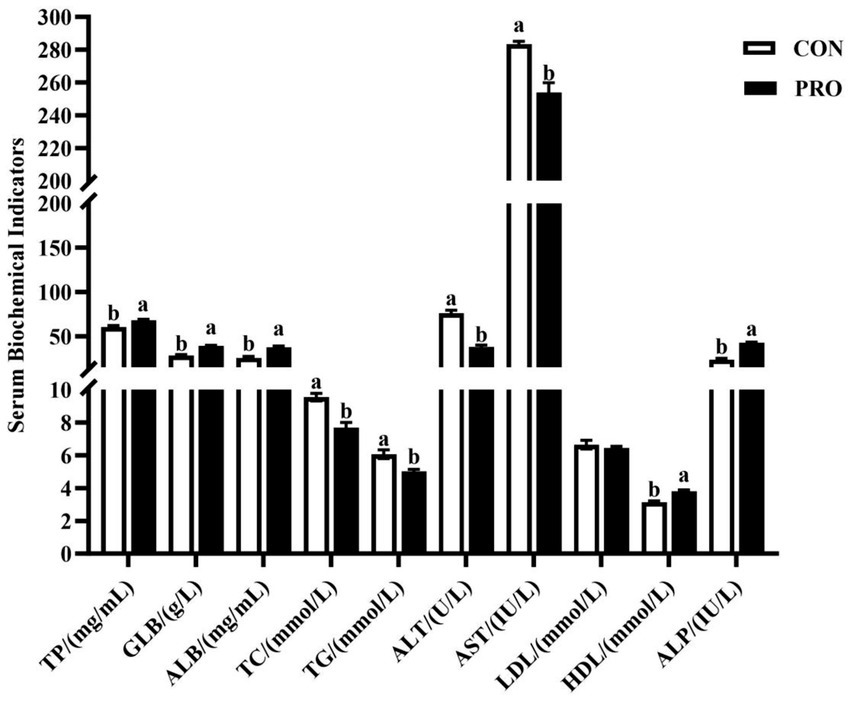
Figure 1. Effects of supplementation of compound probiotics on serum biochemical indicators of laying hens. TC, total cholesterol; TG, triglyceride; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; GLB, globulin; ALB, albumin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALP, alkaline phosphatase; CON, the control group; PRO, the treatment group. No letter or the same letter superscript indicates a nonsignificant difference (p > 0.05), whereas different small letters superscript indicates a significant difference (p < 0.05).
As depicted in Figure 2, compared to CON, PRO had significantly less MDA (p < 0.05) and the T-AOC of PRO was significantly increased (p < 0.05). And Figure 3 demonstrates that while there was no significant difference in the IgM content between the CON and PRO (p > 0.05), the IgA and IgG levels in the serum of laying hens in PRO were considerably greater than those in CON (p < 0.05).

Figure 2. Effects of supplementation of compound probiotics on antioxidant capacity of laying hens. MDA, malondialdehyde; T-AOC, total antioxidant capacity; CON, the control group; PRO, the treatment group. No letter or the same letter superscript indicates a nonsignificant difference (p > 0.05), whereas different small letters superscript indicates a significant difference (p < 0.05).
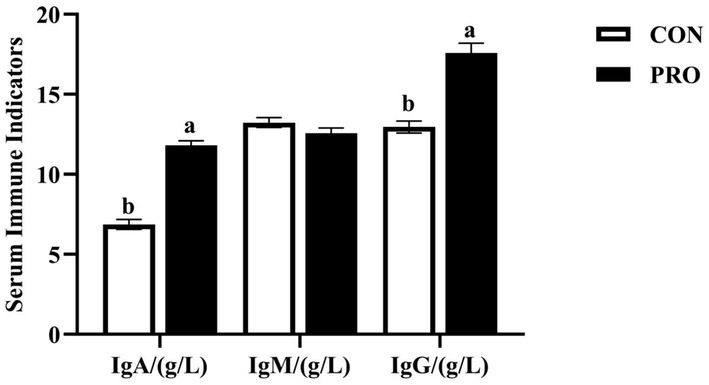
Figure 3. Effects of supplementation of compound probiotics on immune function of laying hens. IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; CON. the control group; PRO, the treatment group. No letter or the same letter superscript indicates a nonsignificant difference (p > 0.05), whereas different small letters superscript indicates a significant difference (p < 0.05).
Figure 4 presents that intestinal morphology of the duodenum in PRO did not differ substantially from that in CON (p > 0.05). However, compared to CON, the CD of the ileum and jejunum in PRO significantly decreased (p < 0.05) while the V/C of the jejunum in PRO significantly increased (p < 0.05).
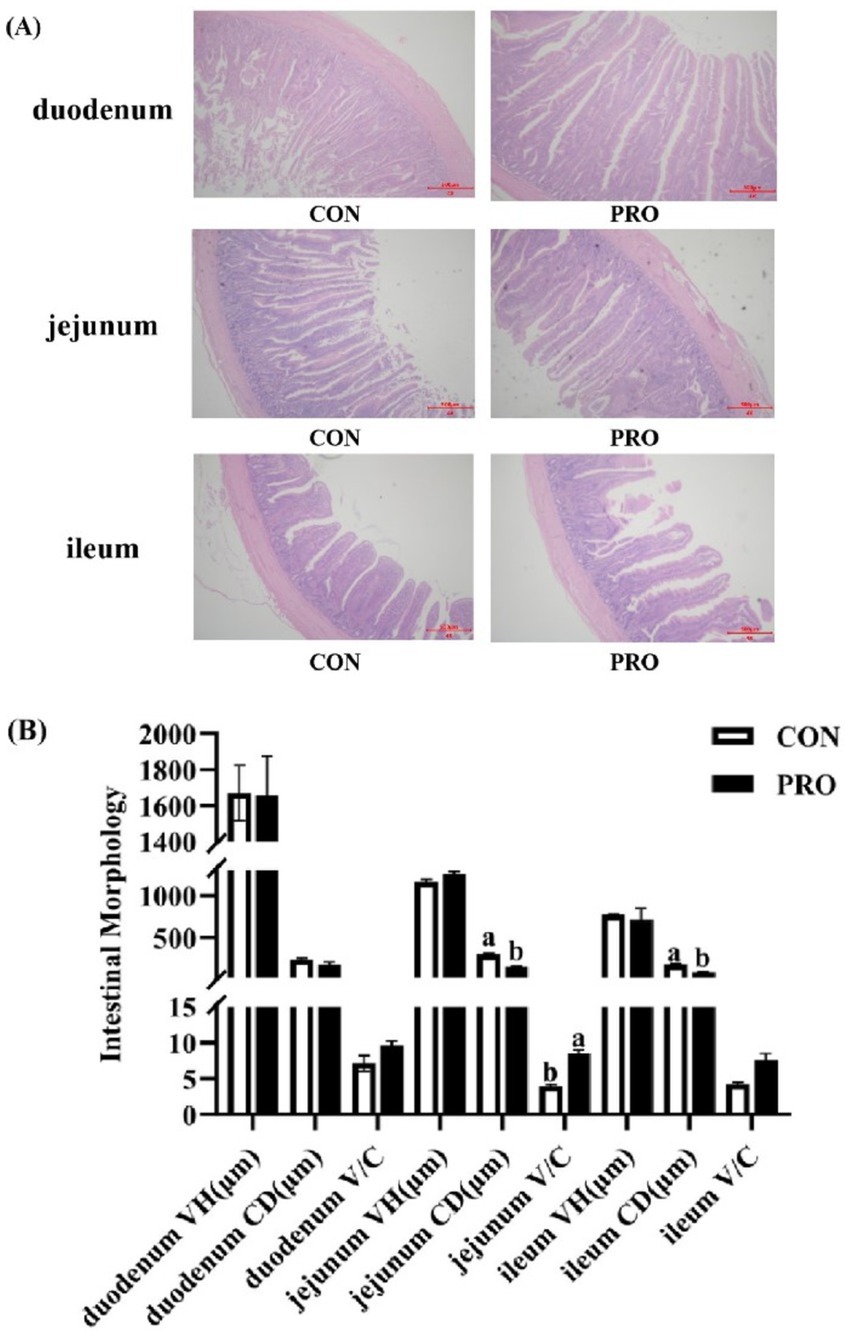
Figure 4. Effects of supplementation of compound probiotics on intestinal morphology of laying hens. (A) The morphology of duodenum, jejunum, ileum of the CON group and treatment group; (B) The VH, CD, V/C of duodenum, jejunum, ileum of the CON group and treatment group. VH, villus height; CD, crypt depth; V/C, villus height to crypt depth ratio; CON, the control group; PRO, the treatment group. No letter or the same letter superscript indicates a nonsignificant difference (p > 0.05), whereas different small letters superscript indicates a significant difference (p < 0.05).
In the context of intensive farming, the production performance and egg quality of laying hens are key indicators that affect the economic benefit of laying hens. And LR, AEW and F/E are key indicators for evaluating laying hens; production performance. As one of the most promising new feed additives after the ban on antibiotics, probiotics have a specific impact on the production performance of livestock and poultry after entering the intestine (25). Abdelqader et al. (26) reported that the dietary supplementation of Bacillus subtilis (2.3 × 108 CFU/g) resulted in higher egg production and feed conversion ratio in hens. Previous studies showed that the addition of Bacillus subtilis culture (500 mg/kg) to laying hens can improve egg production and feed conversion rates (27). After the supplementation of a mixed culture of L. acidophilus and L. casei, L. acidophilus, or B. subtilis to the diet, egg production significantly increased (28, 29). The results of the present study were similar to those of the above studies in that the dietary supplementation of compound probiotics increased the LR and AEW while the F/E was reduced, which shows that compound probiotics as feed additives can improve the laying performance. This may be due to the fact that probiotics, after entering the intestinal tract, accelerate the utilization of feed nutrients by enhancing fiber digestion and enzyme activity. In contrast, some studies showed that the addition of 2.0 g/t of probiotics consisting of Bacillus subtilis and Bacillus amyloliquefaciens to the diet did not have a significant improvement on egg production and egg weight (30). Davis and Anderson (31) also found no significant increase in egg production after the supplementation of hens with products containing mixtures of bacteria such as Lactobacillus and Bacillus. These results differed from the results of the present study, which may be due to the fact that the effectiveness of probiotics application is limited by a number of factors such as microbial species composition, habitability, supplemental administration dose, method and frequency of application, dietary composition, age of the birds and environmental stress factors (32).
The quality of the eggshell is an important factor affecting the shelf-life of eggs, and about 10 to 15 per cent of eggs are damaged due to eggshell quality problems during egg collection, storage and transport. For example, the egg shape index directly determines the selection of breeding eggs and is positively correlated with hatching rate (33). And the eggs damaged due to weak eggshell quality account for 6–10% of all eggs produced worldwide, which causes huge economic losses (34). The study showed that the dietary supplementation with a mixture of Lactobacillus cultures resulted in a significant improvement in eggshell quality (35). However, in this study, the supplementation of compound probiotics did not significantly improve EW, ET and other indicators that can measure eggshell quality, which may be related to the amount of probiotics added, age and heritability of laying hens (36). Carvalho et al. and Ramasamy et al. showed that the supplementation of Saccharomyces boulardii (0.05 g/kg) and Lactobacillus acidophilus (0.1 g/kg) to the diet of laying hens did not improve eggshell quality (35, 36), this is in agreement with the results of the present study, indicating that the supplementation of compound probiotics had no adverse effect on the eggshell quality of the eggs. Moreover, in this study, the egg shape index of the eggs was also significantly improved by the supplementation of compound probiotics, which indicated that compound probiotics had an effect on calcium deposition in the eggshell, and also showed that the supplementation of compound probiotics could improve the eggshell quality of the eggs to a certain extent. The results of Cao et al. (37) showed a significant increase in egg shape index after the dietary supplementation of compound probiotics (1 g/kg), which is consistent with the results of the present study. Yolk weight and albumen weight were significantly and positively correlated with egg weight. Eggs with higher weights tended to have greater yolk quality compared to eggs with lower weights (38). In the conditions of this study, egg weight and yolk weight were significantly increased in the PRO group, indicating that the supplementation of compound probiotics can improve the nutritional value of eggs. AH is one of the important indicators used to evaluate the freshness of eggs (7). The result of Zhang et al. (39) showed that the addition of 5.6 mL of Lactobacillus plantarum (3 × 1,010 CFU/mL) per gram of basal diet significantly increased albumen height. Liu et al. (20) showed that the supplementation of yeast cultures (2 g/kg) to the diet significantly increased the AH of eggs. The results of the above study are in agreement with the results of the present study, where the dietary supplementation of compound probiotics significantly increased the albumen height, which may be attributed to the fact that the compound probiotics facilitated the synthesis and transport of proteins, which resulted in an enhancement in AH (40), and this result also demonstrates that the supplementation of compound probiotics improves the freshness of the eggs and thus effectively improves the egg quality.
In serum, immunoglobulins play an important role in the immune function of the body, among which IgA, IgG and IgM are commonly used indicators to evaluate the immune status of the body (37). IgG has the role of neutralizing bacterial toxins and antimicrobial antitoxins, meanwhile, it has the highest content of all the immunoglobulins in the organism, and is an important antibody in the humoral immunity. IgM is capable of dissolving pathogenic bacteria. Probiotics have been shown to modulate serum systemic antibody responses to antigens and are involved in the development of immunity. Ahmed et al. demonstrated that the addition of Bacillus amyloliquefaciens to diets altered the immune response of broilers, with a linear improvement in serum heavy IgG and IgA levels (41). Previous studies on probiotics showed that 1.0 × 109 CFU/day of Bacillus subtilis BS1 or 4.0 × 109 CFU/ m2 of Bacillus amyloliquefaciens improves and diversifies the status of serum IgG, IgM, and IgA in broilers and mice and modulates the immune state (42, 43). The above studies were similar to the results of the present study, the supplementation of compound probiotics resulted in a significant increase of levels of IgA and IgG in serum, which improved the body’s immune defense mechanism to a certain extent. Probiotics have an important effect on the immune system of poultry against invading pathogens (44) and can stimulate innate and adaptive immunity by regulating the expression of toll-like receptors, activating dendritic cells and natural killer cells, increasing the response of t-helper cells, and inducing the production of cytokines and secretion of immunoglobulins, such as IgM, IgG and IgA (45).
Oxidative stress is an imbalance between oxidation and antioxidation in the body, which generates a variety of reactive oxygen species (ROS), damaging proteins, nucleic acids and lipids, leading to tissue damage and diseases (46). T-AOC is an important comprehensive indicator of antioxidant capacity (47). Antioxidant enzymes (SOD, CAT, GSH and GSH-Px) are the first line of ROS elimination, whereas MDA is the end product of lipid peroxidation and is often used as a biomarker to assess oxidative stress (48). There is a close relationship between the antioxidant capacity of the body defense system and the health degree. Superoxide dismutase can clear superoxide anion radical (O2−·) disproportionation to generate oxygen and hydrogen peroxide, then catalase can promote the decomposition of H2O2 into molecular oxygenated water to remove hydrogen peroxide in the body, thus protecting cells from damage (49). Qin et al. (50) showed that dietary supplementation with 300 mg/kg of probiotics did not increase the activity of antioxidant enzymes in the serum of laying hens, which is consistent with the results of the present study. However, the results of the present study showed that the MDA content decreased after the dietary supplementation of compound probiotics, which may be attributed to the ability of probiotics to secrete antioxidant peptides, which act as antioxidants with the ability to scavenge free radicals, such as ROS, and thus reduce the generation of lipid peroxides (51). The results of Xiang et al. showed that dietary supplementation of probiotics (1 g/kg) significantly reduced serum MDA levels, which is consistent with the results of the present study. Furthermore, the results of the present study also showed that the total antioxidant capacity (T-AOC) was significantly higher in the PRO group, the results of Fu et al. (52) similarly showed a significant increase in total antioxidant capacity with the dietary supplementation of probiotics. Thus, the improvement in serum T-AOC indicates that supplementation with compound probiotics improves antioxidant capacity and enhances innate immunity (53).
The content of TP, ALB and GLB in serum reflects the digestion, absorption and metabolism of feed proteins (54). The results of this study showed that the contents of TP, ALB and GLB were significantly increased after the supplementation of compound probiotics, indicating that the protein metabolism in the animal’s organism was vigorous, and it was able to better intake and utilize the feed proteins, which led to the reduction of the FER. Albumin is mainly synthesized by the liver, and has the role of protecting globulin and transporting metabolites in the organism, and the improvement in ALB content in this experiment may be attributed to the fact that the compound probiotics inhibited the growth of pathogens, which reduced the degradation of proteins, and improved the utilization rate of proteins in the feed (55). In addition, Abdel-Moneim et al. (56) showed that the supplementation of probiotics was able to enhance mucosal immunity and nutrient absorption by the animal organism, thereby, increased the level of immunoglobulin in the serum. Therefore, the results of this study indicate that the supplementation of compound probiotics of the basal diet can promote the ability of the organism to protein intake and utilization, improve the nutritional level of the organism, and thus enhance the immunity of the organism.
Probiotics have been shown to influence cholesterol metabolism by reducing cholesterol levels (57). According to our study, the serum cholesterol level of laying hens in the PRO group was significantly lower than that of the CON group. Loh et al. (58) reported that the dietary supplementation with 0.6% Lactobacillus plantarum reduced blood cholesterol levels in Pengging ducks. Qiu et al. (59) showed that dietary supplementation with low doses of probiotics reduced serum triglyceride concentrations in broilers. The above results are in agreement with the present study, which suggests that the dietary supplementation of compound probiotics can positively affect lipid metabolism and regulate triglyceride levels, thereby improving health status. Probiotics increase serum lipase activity, and in the presence of lipase, fat in the body is broken down by lipase into small molecules of fatty acids and glycerol, and excess fat is oxidatively broken down and converted into adenine nucleosides for direct consumption (60). And this may also be one of the reasons that the supplementation of compound probiotics can improve the lipid metabolism in laying hens. HDL regulates cholesterol levels and prevents cholesterol from accumulating in cells, and sterols are shed from membranes at the same rate as cholesterol synthesized by the liver to maintain a balance (61). The function of HDL is to transport unused surplus cholesterol to the liver. The remaining cholesterol will be used as a component in the production of steroid hormones and bile salts, while the remaining inactive cholesterol will be excreted from the body (62). In the study, the serum level of HDL was higher in laying hens in the PRO group. Mohamed et al. showed a significant enhancement in serum HDL levels with the supplementation of probiotics (500 mg/kg) to the diet, which is in agreement with the result of the present study (63). This indicates that the dietary supplementation of compound probiotics can regulate the level of lipid metabolism to a certain degree, which improves the health of the organism and enhances the laying hens’ performance of laying hens. ALT and AST levels are commonly used to reflect the health of the liver in animals, and elevated levels of both can indicate different degrees of liver damage (64). The results of the present study showed a significant reduction in the levels of AST and ALT by the dietary supplementation with compound probiotics. The study conducted by Chen (65) showed that feeding hens a diet containing Lactobacillus cultures greatly reduced the levels of ALT and AST. Liu et al. (20) showed that the addition of yeast cultures showed a significant reduction in AST levels, which is a positive sign of liver function. The results of our experiment are basically consistent with the above studies, suggesting that the supplementation of compound probiotics in the diet enhanced the repair and regeneration ability of the liver, thus playing a protective role for the liver.
The intestinal tract is the main region of the organism for nutrient absorption, and the integrity of its barrier structure and function is a prerequisite for the diet to be fully digested and absorbed. Additionally, the primary indices for assessing the intestinal barrier’s structural and functional integrity are VH, CD, and V/C, which represent the intestinal tract’s capacity for absorption and digestion (66). VH and cell number are positively correlated, and the increase of VH expands the absorptive area of the small intestine, so the more mature epithelial cells and the shallower the CD, the better the intestinal digestion and absorption of nutrients (67). The V/C ratio is a measure of the digestive and absorptive capacity of the small intestine, and an improvement in the V/C ratio indicates an improvement in the intestinal mucosa and a greater intestinal capacity for digestion and absorption of nutrients (68). The supplementation of probiotics to the diet of laying hens significantly increased jejunal and ileal VH (69). The supplementation of yeast polysaccharides to the diet significantly increased VH of jejunum and ileum (70). The results of this experiment showed that the supplementation of compound probiotics to the diet of laying hens significantly decreased the CD of jejunum and ileum, while the V/C of jejunum was significantly increased, which may be attributed to the fact that the supplementation of compound probiotics could provide the nutrient requirements for the cell division and proliferation of intestinal tissues of the livestock and poultry, promote the development of the intestinal epithelial cells, and enhance the absorption ability of intestinal mucosa. Simultaneously, the experiment’s findings point to a possible connection between improved intestinal morphology and nutrient absorption function and the ability of compound probiotics to enhance laying efficiency and egg quality.
The results of this study showed that the dietary supplementation of compound probiotics of laying hens was able to regulate lipid metabolism pathways, reduce the degree of lipid peroxidation in the body, and thus improve the total antioxidant capacity of the body. In addition, in this study, the compound probiotics was able to regulate protein synthesis and improve intestinal morphology, which improved the immune performance of laying hens, and ultimately had a positive impact on laying rate and egg quality of laying hens. At the same time, this study also found that the supplementation of compound probiotics could reduce the F/E and improve the economic benefit of laying hens. In conclusion, compound probiotics have great potential in the field of ‘anti-antibiotic’ feed additives for laying hens and are worthy of further research.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was approved by the Institute of Feed Research of the Chinese Academy of Agricultural Science’s Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
YW: Conceptualization, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. CZ: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XC: Conceptualization, Data curation, Formal analysis, Software, Writing – review & editing. AZ: Data curation, Formal analysis, Resources, Writing – review & editing. GL: Funding acquisition, Project administration, Resources, Writing – review & editing. YR: Data curation, Project administration, Supervision, Writing – review & editing. ZC: Data curation, Funding acquisition, Project administration, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Supported by Science and Technology Innovation 2030-Major Project (2023ZD0406307).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Alagawany, M, Abd El-Hack, ME, Arif, M, and Ashour, EA. Individual and combined effects of crude protein, methionine, and probiotic levels on laying hen productive performance and nitrogen pollution in the manure. Environ Sci Pollut Res. (2016) 23:22906–13. doi: 10.1007/s11356-016-7511-6
2. Varmuzova, K, Matulova, ME, Gerzova, L, Cejkova, D, Gardan-Salmon, D, Panhéleux, M, et al. Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella Enteritidis infection. Poult Sci. (2015) 94:2049–58. doi: 10.3382/ps/pev190
3. Gao, PF, Ma, C, Sun, Z, Wang, LF, Huang, S, Su, XQ, et al. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. (2017) 5:91. doi: 10.1186/s40168-017-0315-1
4. Gao, CQ, Shi, HQ, Xie, WY, Zhao, LH, Zhang, JY, Ji, C, et al. Dietary supplementation with acidifiers improves the growth performance, meat quality and intestinal health of broiler chickens. Animal Nutrit. (2021) 7:762–9. doi: 10.1016/j.aninu.2021.01.005
5. Silveira, RF, Roque-Borda, CA, and Vicente, EF. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: an overview. Animal Nutrit. (2021) 7:896–904. doi: 10.1016/j.aninu.2021.01.004
6. Obianwuna, UE, Qiu, K, Chang, XY, Zhang, HJ, Wang, J, Qi, GH, et al. Enhancing egg production and quality by the supplementation of probiotic strains (clostridium and Brevibacillus) via improved amino acid digestibility, intestinal health, immune response, and antioxidant activity. Front Microbiol. (2022) 13:241. doi: 10.3389/fmicb.2022.987241
7. Xiang, QH, Wang, C, Zhang, H, Lai, W, Wei, HK, and Peng, J. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals. (2019) 9:1110. doi: 10.3390/ani9121110
8. Duggan, C, Gannon, J, and Walker, WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. (2002) 75:789–808. doi: 10.1093/ajcn/75.5.789
9. Stahl, CH, Callaway, TR, Lincoln, LM, Lonergan, SM, and Genovese, KJ. Inhibitory activities of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob Agents Chemother. (2004) 48:3119–21. doi: 10.1128/AAC.48.8.3119-3121.2004
10. Wu, X, Vallance, BA, Boyer, L, Bergstrom, KSB, Walker, J, and Madsen, K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. American J Physiology-Gastrointestinal Liver Physiol. (2008) 294:G295–306. doi: 10.1152/ajpgi.00173.2007
11. Bindari, YR, and Gerber, PF. Centennial review: factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult Sci. (2022) 101:101612. doi: 10.1016/j.psj.2021.101612
12. Jiang, S, Hu, JY, and Cheng, HW. The impact of probiotic Bacillus subtilis on injurious behavior in laying hens. Animals. (2022) 12:870. doi: 10.3390/ani12070870
13. Khan, S, Moore, RJ, Stanley, D, and Chousalkar, KK. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol. (2020) 86:e00600–20. doi: 10.1128/AEM.00600-20
14. Smith, JM. A review of avian probiotics. J Avian Med Surg. (2014) 28:87–94. doi: 10.1647/2012-031
15. Villageliu, DN, and Lyte, M. Microbial endocrinology: why the intersection of microbiology and neurobiology matters to poultry health. Poult Sci. (2017) 96:2501–8. doi: 10.3382/ps/pex148
16. Kaczmarek, SA, Rogiewicz, A, Mogielnicka, M, Rutkowski, A, Jones, RO, and Slominski, BA. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult Sci. (2014) 93:1745–53. doi: 10.3382/ps.2013-03739
17. Stefanello, C, Vieira, SL, Rios, HV, Simoes, CT, Ferzola, PH, Sorbara, JOB, et al. Effects of energy, α-amylase, and β-xylanase on growth performance of broiler chickens. Anim Feed Sci Technol. (2017) 225:205–12. doi: 10.1016/j.anifeedsci.2017.01.019
18. Macit, M, Karaoglu, M, Celebi, S, Esenbuga, N, Yoruk, MA, and Kaya, A. Effects of supplementation of dietary humate, probiotic, and their combination on performance, egg quality, and yolk fatty acid composition of laying hens. Trop Anim Health Prod. (2021) 53:63. doi: 10.1007/s11250-020-02546-6
19. Park, JW, Jeong, JS, Lee, SI, and Kim, IH. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in Isa brown laying hens. Poult Sci. (2016) 95:2829–35. doi: 10.3382/ps/pew241
20. Liu, YC, Cheng, X, Zhen, WR, Zeng, D, Qu, LJ, Wang, Z, et al. Yeast culture improves egg quality and reproductive performance of aged breeder layers by regulating gut microbes. Front Microbiol. (2021) 12:633276. doi: 10.3389/fmicb.2021.633276
21. Gao, J, Wang, R, Liu, JX, Wang, WL, Chen, Y, and Cai, WT. Effects of novel microecologics combined with traditional Chinese medicine and probiotics on growth performance and health of broilers. Poult Sci. (2022) 101:101412. doi: 10.1016/j.psj.2021.101412
22. Zhang, JL, Xie, QM, Ji, J, Yang, WH, Wu, YB, Li, C, et al. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult Sci. (2012) 91:2755–60. doi: 10.3382/ps.2012-02339
23. Paszti-Gere, E, Szeker, K, Csibrik-Nemeth, E, Csizinszky, R, Marosi, A, Palocz, O, et al. Metabolites of lactobacillus plantarum 2142 prevent oxidative stress-induced overexpression of Proinflammatory cytokines in Ipec-J2 cell line. Inflammation. (2012) 35:1487–99. doi: 10.1007/s10753-012-9462-5
24. Düz, M, Dogan, YN, and Dogan, I. Antioxidant activitiy of Lactobacillus plantarum, lactobacillus sake and Lactobacillus curvatus strains isolated from fermented Turkish Sucuk Anais Da Academia Brasileira De Ciencias (2020). 92:e20200105. doi: 10.1590/0001-3765202020200105
25. Liu, X, Ma, ZH, Wang, YF, Li, L, Jia, H, and Zhang, LH. Compound probiotics can improve intestinal health by affecting the gut microbiota of broilers. J Anim Sci. (2023) 101:skad388. doi: 10.1093/jas/skad388
26. Abdelqader, A, Al-Fataftah, AR, and Das, G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim Feed Sci Technol. (2013) 179:103–11. doi: 10.1016/j.anifeedsci.2012.11.003
27. Xu, CL, Ji, C, Ma, Q, Hao, K, Jin, ZY, and Li, K. Effects of a dried Bacillus subtilis culture on egg quality. Poult Sci. (2006) 85:364–8. doi: 10.1093/ps/85.2.364
28. Forte, C, Acuti, G, Manuali, E, Proietti, PC, Pavone, S, Trabalza-Marinucci, M, et al. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult Sci. (2016) 95:2528–35. doi: 10.3382/ps/pew164
29. Rehman, A, Arif, M, Sajjad, N, Al-Ghadi, MQ, Alagawany, M, El-Hack, MEA, et al. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult Sci. (2020) 99:6946–53. doi: 10.1016/j.psj.2020.09.043
30. Khan, S, and Chousalkar, KK. Salmonella Typhimurium infection disrupts but continuous feeding of bacillus based probiotic restores gut microbiota in infected hens. J Animal Sci Biotechnol. (2020) 11:29. doi: 10.1186/s40104-020-0433-7
31. Davis, GS, and Anderson, KE. The effects of feeding the direct-fed microbial, PrimaLac, on growth parameters and egg production in single comb white Leghorn hens. Poult Sci. (2002) 81:755–9. doi: 10.1093/ps/81.6.755
32. Mikulski, D, Jankowski, J, Naczmanski, J, Mikulska, M, and Demey, V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult Sci. (2012) 91:2691–700. doi: 10.3382/ps.2012-02370
33. Narushin, VG, and Romanov, MN. Egg physical characteristics and hatchability. Worlds Poultry Sci J. (2002) 58:297–303. doi: 10.1079/WPS20020023
34. Hamilton, RMG, and Bryden, WL. Relationship between egg shell breakage and laying hen housing systems - an overview. Worlds Poultry Sci J. (2021) 77:249–66. doi: 10.1080/00439339.2021.1878480
35. Ramasamy, K, Abdullah, N, Jalaludin, S, Wong, M, and Ho, YW. Effects of lactobacillus cultures on performance of laying hens, and total cholesterol, lipid and fatty acid composition of egg yolk. J Sci Food Agric. (2009) 89:482–6. doi: 10.1002/jsfa.3477
36. Carvalho, CL, Andretta, I, Galli, GM, Stefanello, TB, Camargo, NDT, Mendes, RE, et al. Dietary supplementation with β-mannanase and probiotics as a strategy to improve laying hen performance and egg quality. Front Vet Sci. (2023) 10:1229485. doi: 10.3389/fvets.2023.1229485
37. Cao, Y, Xun, MY, Ren, SM, and Wang, J. Effects of dietary organic acids and probiotics on laying performance, egg quality, serum antioxidants and expressions of reproductive genes of laying ducks in the late phase of production. Poult Sci. (2022) 101:102189. doi: 10.1016/j.psj.2022.102189
38. Shim, MY, Song, E, Billard, L, Aggrey, SE, Pesti, GM, and Sodsee, P. Effects of balanced dietary protein levels on egg production and egg quality parameters of individual commercial layers. Poult Sci. (2013) 92:2687–96. doi: 10.3382/ps.2012-02569
39. Zhang, GQ, Yang, N, Liu, ZY, Chen, XY, Li, MJ, Fu, TY, et al. Genome-assisted probiotic characterization and application of Lactiplantibacillus plantarum 18 as a candidate probiotic for laying hen production. Microorganisms. (2023) 11:2373. doi: 10.3390/microorganisms11102373
40. Wang, XC, Wang, XH, Wang, J, Wang, H, Zhang, HJ, Wu, SG, et al. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-line Brown hens during the late laying period. J Anim Sci. (2018) 96:225–35. doi: 10.1093/jas/skx0007
41. Ahmed, ST, Islam, MM, Mun, HS, Sim, HJ, Kim, YJ, and Yang, CJ. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult Sci. (2014) 93:1963–71. doi: 10.3382/ps.2013-03718
42. Li, AY, Wang, YP, Li, ZX, Qamar, H, Mehmood, K, Zhang, LH, et al. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Factories. (2019) 18:112. doi: 10.1186/s12934-019-1161-6
43. Luan, SJ, Sun, YB, Wang, Y, Sa, RN, and Zhang, HF. Bacillus amyloliquefaciens spray improves the growth performance, immune status, and respiratory mucosal barrier in broiler chickens. Poult Sci. (2019) 98:1403–9. doi: 10.3382/ps/pey478
44. Azad, MA, Sarker, M, and Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int. (2018) 2018:8063647. doi: 10.1155/2018/8063647
45. Tsai, YT, Cheng, PC, and Pan, TM. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol. (2012) 96:853–62. doi: 10.1007/s00253-012-4407-3
46. Vézina, F, and Williams, TD. Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol. (2005) 19:119–28. doi: 10.1111/j.0269-8463.2005.00942.x
47. Zhang, SH, Wu, ZH, Heng, JH, Song, HQ, Tian, M, Chen, F, et al. Combined yeast culture and organic selenium supplementation during late gestation and lactation improve preweaning piglet performance by enhancing the antioxidant capacity and milk content in nutrient-restricted sows. Animal Nutrit. (2020) 6:160–7. doi: 10.1016/j.aninu.2020.01.004
48. Geret, F, Serafim, A, and Bebianno, MJ. Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussatus? Ecotoxicology. (2003) 12:417–26. doi: 10.1023/A:1026108306755
49. Zhao, K, Huo, B, and Shen, XY. Studies on antioxidant capacity in selenium-deprived the Choko yak in the Shouqu prairie. Biol Trace Elem Res. (2021) 199:3297–302. doi: 10.1007/s12011-020-02461-9
50. Qin, M, Wang, ZG, Liang, MZ, Sha, YF, Liu, MX, Liu, JW, et al. Effects of dietary supplementation with tea polyphenols and probiotics on laying performance, biochemical parameters intestinal morphology and microflora of laying hens. Int J Biol Macromol. (2024) 256:128368. doi: 10.1016/j.ijbiomac.2023.128368
51. Rahman, MS, Choi, YH, Choi, YS, Alam, MB, Lee, SH, and Yoo, JC. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. (2018) 239:502–10. doi: 10.1016/j.foodchem.2017.06.106
52. Fu, RQ, Chen, DW, Tian, G, Zheng, P, Mao, XB, Yu, J, et al. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Animal Nutrit. (2019) 5:366–72. doi: 10.1016/j.aninu.2019.06.003
53. Min, YN, Li, LL, Liu, SK, Zhang, J, Gao, YP, and Liu, FZ. Effects of dietary distillers dried grains with solubles (Ddgs) on growth performance, oxidative stress, and immune function in broiler chickens. J Appl Poult Res. (2015) 24:23–9. doi: 10.3382/japr/pfv002
54. Guo, JR, Dong, XF, Liu, S, and Tong, JM. Effects of long-term Bacillus subtilis Cgmcc 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult Sci. (2017) 96:1280–9. doi: 10.3382/ps/pew389
55. Ahmat, M, Cheng, JH, Abbas, Z, Cheng, Q, Fan, Z, Ahmad, B, et al. Effects of Bacillus amyloliquefaciens Lfb112 on growth performance, carcass traits, immune, and serum biochemical response in broiler chickens. Antibiotics-Basel. (2021) 10:1427. doi: 10.3390/antibiotics10111427
56. Abdel-Moneim, AME, Selim, DA, Basuony, HA, Sabic, EM, Saleh, AA, and Ebeid, TA. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop Anim Health Prod. (2020) 52:671–80. doi: 10.1007/s11250-019-02055-1
57. Lim, YH, Foo, HL, Loh, TC, Mohamad, R, and Abdullah, N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J Animal Sci Biotechnol. (2019) 10:15. doi: 10.1186/s40104-019-0323-z
58. Loh, TC, Choe, DW, Foo, HL, Sazili, AQ, and Bejo, MH. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC Vet Res. (2014) 10:149. doi: 10.1186/1746-6148-10-149
59. Qiu, K, Li, CL, Wang, J, Qi, GH, Gao, J, Zhang, HJ, et al. Effects of dietary supplementation with Bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front Nutrit. (2021) 8:786878. doi: 10.3389/fnut.2021.786878
60. Alaqil, AA, Abbas, AO, El-Beltagi, HS, Abd El-Atty, HK, Mehaisen, GMK, and Moustafa, ES. Dietary supplementation of probiotic Lactobacillus acidophilus modulates cholesterol levels, immune response, and productive performance of laying hens. Animals. (2020) 10:1588. doi: 10.3390/ani10091588
61. Okrathok, S, and Khempaka, S. Modified-dietary fiber from cassava pulp reduces abdominal fat and meat cholesterol contents without affecting growth performance of broiler chickens. J Appl Poult Res. (2020) 29:229–39. doi: 10.1016/j.japr.2019.10.009
62. Yusuf, D, Nuraida, L, Dewanti-Hariyadi, R, and Hunaefi, D. In vitro characterization of lactic acid bacteria from Indonesian kefir grains as probiotics with cholesterol-lowering effect. J Microbiol Biotechnol. (2020) 30:726–32. doi: 10.4014/jmb.1910.10028
63. Mohamed, TM, Sun, WZ, Bumbie, GZ, Dosoky, WM, Rao, ZB, Hu, P, et al. Effect of dietary supplementation of Bacillus subtilis on growth performance, organ weight, digestive enzyme activities, and serum biochemical indices in broiler. Animals. (2022) 12:12. doi: 10.3390/ani12121558
64. Yousefi, M, Shivazad, M, and Hafhdoost, IS. The effect of dietary factors on induction of fatty liver-hemorrhagic syndrome and its diagnosis methods with use of serum and liver parameters in laying hens. Poult Sci. (2004) 83:168–8.
65. Chen, CY, Chen, SW, and Wang, HT. Effect of supplementation of yeast with bacteriocin and lactobacillus culture on growth performance, cecal fermentation, microbiota composition, and blood characteristics in broiler chickens. Asian Australas J Anim Sci. (2017) 30:211–20. doi: 10.5713/ajas.16.0203
66. Metzler-Zebell, BU, Lawlor, PG, Magowan, E, Mccormack, UM, Curiao, T, Hollmann, M, et al. Finishing pigs that are divergent in feed efficiency show small differences in intestinal functionality and structure. PLoS One. (2017) 12:e0174917. doi: 10.1371/journal.pone.0174917
67. Xiao, D, Wang, ZH, Dai, XX, Hu, YW, Zhong, MY, Xiong, L, et al. Effects of Bacillus methylotrophicus Sy200 supplementation on growth performance, antioxidant status, intestinal morphology, and immune function in broiler chickens. Probiotics and Antimicrobial Proteins. (2023) 15:925–40. doi: 10.1007/s12602-022-09924-6
68. Liu, W, Liu, J, Li, DP, Han, HX, Yan, HX, Sun, Y, et al. Effect of Lactobacillus salivarius Snk-6 on egg quality, intestinal morphology, and cecal microbial community of laying hens. Poult Sci. (2024) 103:103224. doi: 10.1016/J.PSJ.2023.103224
69. Lee, KW, Lee, SH, Lillehoj, HS, Li, GX, Jang, SI, Babu, US, et al. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult Sci. (2010) 89:203–16. doi: 10.3382/ps.2009-00418
Keywords: compound probiotics, laying hens, egg quality, biochemical parameters, intestinal morphology and technology
Citation: Wang Y, Zhang C, Chen X, Zheng A, Liu G, Ren Y and Chen Z (2024) Dietary supplementation of compound probiotics to improve performance, egg quality, biochemical parameters and intestinal morphology of laying hens. Front. Vet. Sci. 11:1505151. doi: 10.3389/fvets.2024.1505151
Received: 02 October 2024; Accepted: 09 December 2024;
Published: 24 December 2024.
Edited by:
Jianmin Chai, Foshan University, ChinaReviewed by:
Jose L. Vicente-Salvador, Novozymes, United StatesCopyright © 2024 Wang, Zhang, Chen, Zheng, Liu, Ren and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Ren, cmFuZWUxOTc0QDE2My5jb20=, Zhimin Chen, Y2hlbnpoaW1pbkBjYWFzLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.