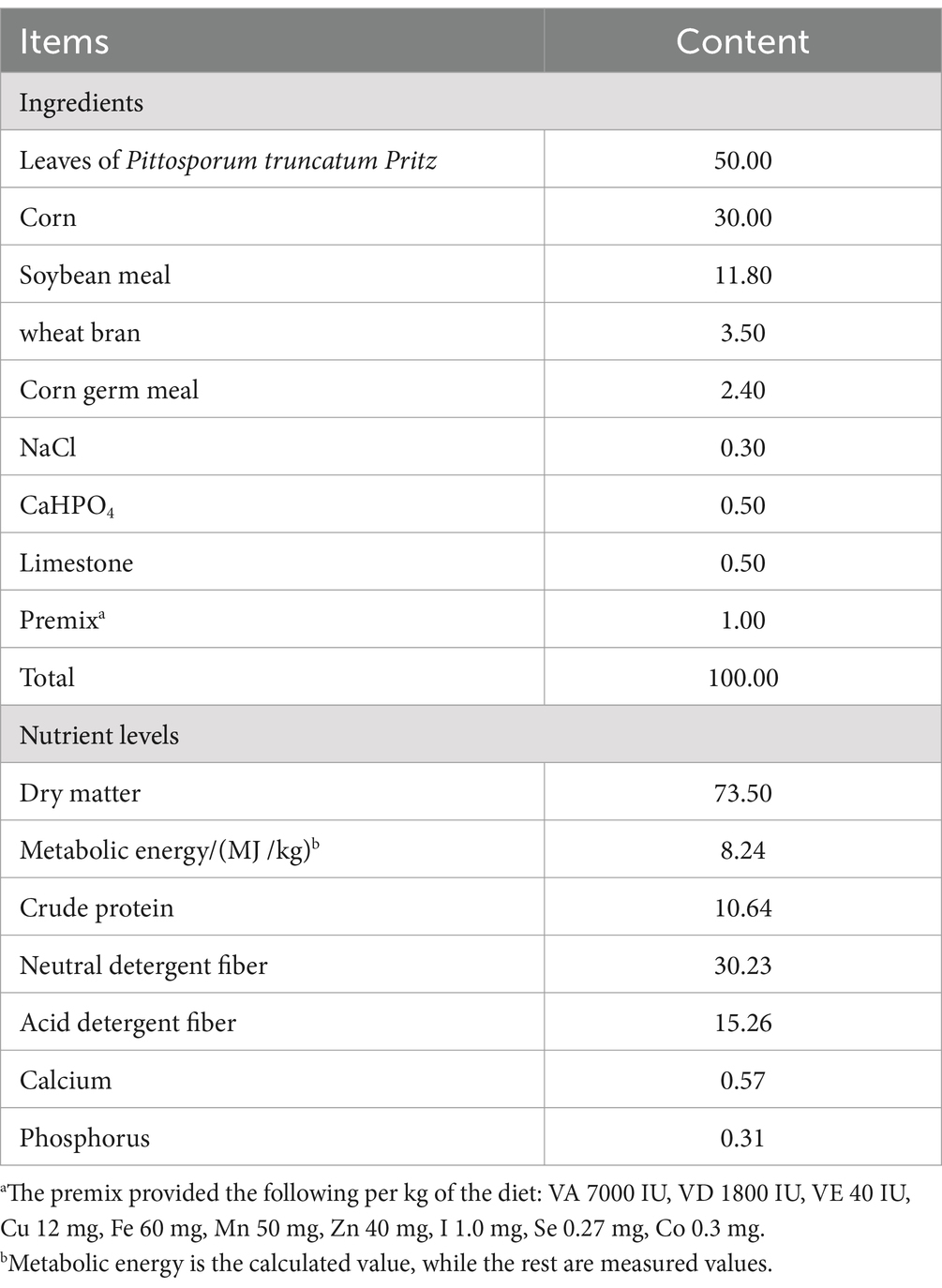
- 1College of Smart Agriculture, Chongqing University of Arts and Sciences, Yongchuan, China
- 2College of Biology and Food Engineering, Chongqing Three Gorges University, Wanzhou, China
- 3Chongqing Institute of Medicinal Plant Cultivation, Nanchuan, China
The Chinese forest musk deer (Moschus berezovskii) is a small ruminant animal with special economic value. It is listed as a National Level I key protected species in China. However, these animals are prone to stress responses in captive environments. Epimedium, a traditional Chinese herb with aphrodisiac and anti-stress properties, may have potential benefits for the health of the captive Chinese forest musk deer, though its efficacy requires further investigation. This study aimed to evaluate the effects of dietary supplementation with Epimedium on the hormone levels, gut microbiota composition, and serum metabolism of the Chinese forest musk deer. The fourteen adult male Chinese forest musk deer with similar initial body weights (7.0 ± 0.3 kg) and an average age of 4.5 years were randomly divided into two groups, each containing seven animals. The control group was fed a standard diet without Epimedium, while the Epimedium group received the standard diet supplemented with 15 g Epimedium /kg DM. The results indicated that the inclusion of Epimedium in the diet increased dry matter intake (DMI) and improved the ratio of feed to gain (F/G), with an increase in fecal testosterone levels (p < 0.05). 16S rDNA sequencing analysis revealed that Epimedium enhanced the richness and diversity of the gut microbiota in the Chinese forest musk deer, increasing the relative abundance of beneficial bacteria such as Firmicutes, while reducing the relative abundance of the potentially pathogenic Proteobacteria (p < 0.05). A widely targeted metabolomics analysis identified 25 differential metabolites between the two groups. Significant alterations were observed in key metabolic pathways related to lipid metabolism, hormone regulation, and antioxidation, such as ovarian steroidogenesis, tyrosine metabolism, and glycerophospholipid metabolism. Furthermore, correlation analysis between gut microbiota and serum differential metabolites showed that the relative abundances of Clostridia_vadinBB60_group and UCG-010 were positively correlated with anserine and 7-ketocholesterol, respectively (p < 0.05). In conclusion, Epimedium positively influenced feed intake and hormone levels in the Chinese forest musk deer by modulating gut microbiota composition and serum metabolism.
1 Introduction
The Chinese forest musk deer (Moschus berezovskii), also known as the dwarf musk deer, is a rare and endangered species primarily found in the mountain forests of Sichuan, Gansu, and Shaanxi provinces in China (1). This animal is highly valued for its unique musk gland, which secretes musk, a substance of significant economic and medicinal importance in fields such as medicine, perfumery, and cosmetics (2, 3). However, the artificial breeding of the Chinese forest musk deer is fraught with considerable challenges. Due to their inherently timid and solitary nature (4), it is particularly difficult to meet their growth and reproductive requirements in captive environments (5). This adversity directly compromises the health of the Chinese forest musk deer and the quality of the musk they produce (6, 7). Compared to their wild counterparts, the captive Chinese forest musk deer are more susceptible to various health issues, including diarrhea, pneumonia, and malnutrition (8, 9). Therefore, the conservation of this rare species and the enhancement of its health status are of paramount importance.
Nutritional supplementation is a critical method for improving animal health and survival capabilities (10). For wildlife, appropriate nutritional interventions can significantly enhance physiological functions and increase resistance to adverse environmental conditions. Epimedium, a traditional Chinese medicinal herb, is extensively utilized in traditional Chinese medicine (TCM) due to its significant bioactive properties, including anti-inflammatory, antioxidant, and hormone-regulating effects. It is particularly recognized for its potential to modulate sexual function and enhance physical strength (11–13). For the Chinese forest musk deer, hormonal levels are critical indicators of health and reproductive capacity (14). Abnormal hormonal levels can result in reproductive disorders, delayed growth and development, and other health issues (15). The primary bioactive compounds in Epimedium, particularly icariin, are believed to effectively promote testosterone and sperm production, thereby improving reproductive function and overall health status (16). Additionally, flavanols extracted from Epimedium have been shown to enhance sexual function by stimulating Leydig cells in the testes, leading to increased secretion of testosterone and other androgens (17).
The gut microbiota is an essential component of animal health, playing critical roles in nutrient absorption, immune regulation, and disease prevention (18, 19). Flavonoids and other bioactive compounds in Epimedium improve digestive absorption and immune status by modulating the composition and function of the gut microbiota (20). Incorporating Epimedium into broiler diets can modulate the abundance of beneficial bacteria, such as Lactobacillus, improve gut microbiota composition, and increase the concentration of metabolites like lactic acid and short-chain fatty acids, thereby enhancing metabolic function (21). Epimedium can also improve the health status of rats by regulating lipid metabolism, energy metabolism, and amino acid metabolism. This helps to reduce the levels of harmful metabolites in the blood while increasing the levels of beneficial metabolites (22). Additionally, the flavonoids in Epimedium can enhance the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) (23). These enzymes play a crucial role in scavenging free radicals and protecting cells from oxidative damage. These characteristics make Epimedium a promising natural feed additive, suitable for use in livestock and poultry farming, offering an effective solution for improving animal health and economic efficiency.
In view of this, the present study aims to explore the potential effects of Epimedium as a dietary supplement on the gut microbiota composition, serum metabolic components, and hormone levels in the Chinese forest musk deer. The results could provide new scientific evidence for enhancing the health and hormonal regulation of the captive Chinese forest musk deer.
2 Materials and methods
2.1 Experimental design
The experiment was conducted at the Chinese forest musk deer breeding base of the Institute of Medicinal Plant Cultivation in Chongqing, China. The fourteen adult male Chinese forest musk deer with similar initial body weights (7.0 ± 0.3 kg) and an average age of 4.5 years were randomly divided into two groups, each containing seven animals. The CK group was fed a standard diet without the addition of Epimedium, while the EPI group received the standard diet supplemented with 15 g Epimedium /kg DM. The composition and nutritional levels of the standard feed are shown in Table 1. All the Chinese forest musk deer were housed individually, and the environmental conditions, including temperature, humidity, and management methods, were kept identical for both groups. One week before the trial, the ventilation equipment was thoroughly inspected to ensure proper functioning, and the pens were meticulously cleaned and disinfected. Daily management during the trial period was conducted according to the protocol, with feeding scheduled at 3:00 PM each day. The pre-trial period lasted 7 d, followed by a formal trial period of 30 d.
2.2 Sample collection
During the trial period, the remaining feed was weighed daily using an electronic scale before feeding to calculate the dry matter intake (DMI). Given that the Chinese forest musk deer are classified as a national first-grade protected animal in China, they are highly sensitive to external environments and prone to stress reactions, which can even lead to death. Growth performance and serum metabolomics analyses require direct manipulation of the animals, including procedures such as weighing and blood collection, which can induce stress in the Chinese forest musk deer. Therefore, in this experiment, we selected only the three Chinese forest musk deer per group for these operations to minimize potential impacts on other individuals and ensure the smooth progression and safety of the experiment. On days 1 and 30 of the trial, the three randomly selected Chinese forest musk deer from each group were weighed after a 12 h fasting period to determine the average daily gain (ADG). On the final day of the trial, the three Chinese forest musk deer from each group were randomly selected for blood collection. The blood samples were placed in coagulation-promoting tubes and centrifuged at 3000 rpm for 10 min at 4°C to collect the serum. All serum samples were stored at −80°C for subsequent analysis. On the same day, fresh fecal samples were collected from all the 14 Chinese forest musk deer, placed in sterile centrifuge tubes, and rapidly frozen in liquid nitrogen. These samples were then stored at −80°C for 16S rDNA sequencing of the gut microbiome.
2.3 Fecal hormone level analysis
The levels of testosterone, estradiol, and progesterone in the fecal were measured using enzyme-linked immunosorbent assay (ELISA) kits. These kits were purchased from Quanzhou Ruixin Biotechnology Co., Ltd.
2.4 Fecal microbiota profiling
The total DNA of fecal bacteria was extracted using the E.Z.N.A. Soil DNA Kit (Omega Bio-tek, Inc., United States). Concentration and quality of the genomic DNA were checked by NanoDrop 2000 spectrophotometer (Thermo Scientific Inc., United States). The V3–V4 regions of the bacterial 16S rDNA gene were amplified using universal primers 338F (5’-ACTCCTACGGGA-GGCAGCAG-3′) and 806R (5’-GGACTACHVGGGTWTCTAAT-3′). The universal primers with barcode sequences were synthesized and the amplification was carried out on an ABI 9700 PCR instrument (Applied Biosystems, Inc., United States). After purifying the PCR amplification products, the concentration was measured, and high-throughput sequencing was performed on the Illumina Novaseq sequencing platform.
Sequencing data were processed using Pear software (24) (version 0.9.6) for sequence assembly, filtering, and chimera removal, resulting in optimized sequences. High-quality sequences were clustered into OTUs using Vsearch software (25) (version 2.7.1) with a sequence similarity threshold of 99%. Species classification for each OTU was determined by aligning the sequences with the Silva138 database (26) using the BLAST algorithm (27). Alpha and Beta diversity analyses were performed based on OTU and abundance data using QIIME2 software (28) (version 2024.2). Species composition bar plots were generated using R project (29) (version 3.6.0) based on species annotation and relative abundance results. LEfSe analysis was conducted using Python software (version 2.7) (30). The sequencing was conducted at Beijing Allwegene Technology Co., Ltd.
2.5 Determination of serum metabolites
The sample stored at −80°C refrigerator was thawed on ice and vortexed for 10 s. 50 μL of serum sample and 300 μL of extraction solution (ACN: Methanol = 1:4, V/V) containing internal standards were added into a 2 mL microcentrifuge tube. The sample was vortexed for 3 min and then centrifuged at 12000 rpm for 10 min (4°C). 200 μL of the supernatant was collected and placed in −20°C for 30 min, and then centrifuged at 12000 rpm for 3 min (4°C). A 180 μL aliquots of supernatant were transferred for LC–MS analysis. The relative concentrations of serum metabolites were analyzed using an LC-ESI-MS/MS system (UPLC, ExionLC AD, https://sciex.com.cn/; MS, QTRAP® System, https://sciex.com/). After the analysis, the raw data obtained were imported into Progenesis QI software (31) (version 3.0) for data preprocessing, which was used for subsequent analysis.
Metabolite information was obtained by annotating the database using HMDB1 and Metlin.2 Partial least squares discriminant analysis (PLS-DA) was performed using R project. For two-group analysis, differential metabolites were determined by Variable Importance in Projection (VIP > 1) and p-value (p-value <0.05, t-test). Identified metabolites were annotated using the KEGG Compound database3, and the annotated metabolites were then mapped to the KEGG Pathway database.4 The analysis was conducted at Beijing Allwegene Technology Co., Ltd.
2.6 Correlation analysis between rumen microbiota and serum metabolite profiles
Correlation analysis was performed between differential microbiota and metabolites. The spearman correlation coefficients between microbiome and metabolite data were calculated using the psych package in the R project. Correlation heatmaps were generated using the heatmap package.
2.7 Statistical analysis
All data were analyzed using independent sample t-tests, and the results are presented as mean ± standard error of the mean (SEM). Experimental data with p < 0.05 were considered statistically significant.
3 Results
3.1 The effects of Epimedium on growth performance and hormone levels in the Chinese forest musk deer
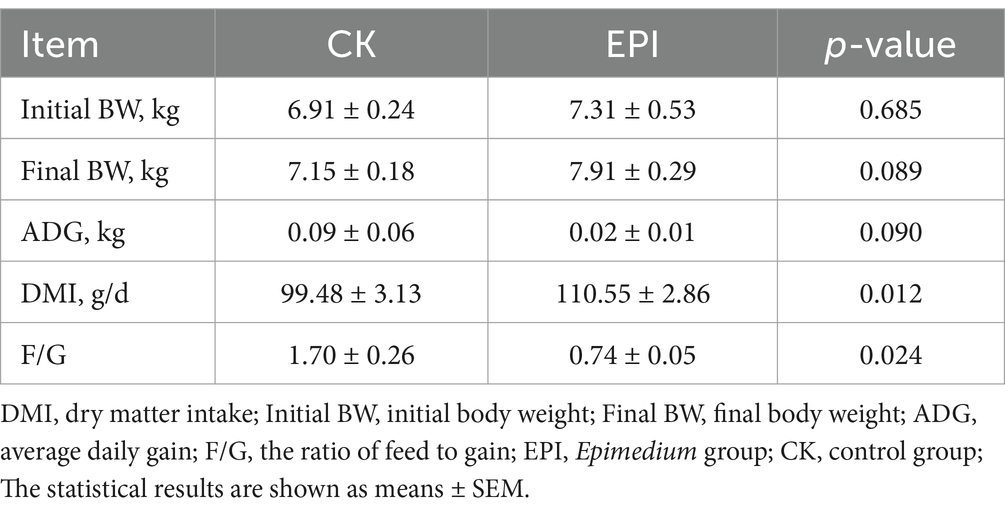
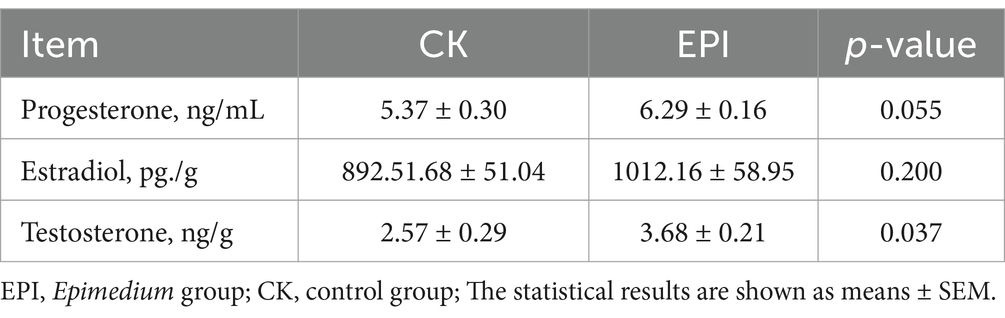
As shown in Table 2, compared to the CK group, the final BW and ADG tended to increase in the EPI group (p < 0.1). The DMI and F/G in the EPI group were higher than those in the CK group (p < 0.05), indicating that dietary supplementation with Epimedium significantly increased the feed intake and feed conversion ratio in the Chinese forest musk deer. As shown in Table 3, Epimedium supplementation increased fecal testosterone levels in the Chinese forest musk deer (p < 0.05). Compared to the CK group, the progesterone levels in the fecal samples of the EPI group showed a trend towards increase (p < 0.1). Although there were slight increases in estradiol levels, these changes were not statistically significant. This suggests that Epimedium can effectively regulate hormone levels in the Chinese forest musk deer.
3.2 Gut microbiome 16S rDNA sequencing analysis
3.2.1 The effects of Epimedium on the abundance and diversity of gut microbiota
As shown in Figure 1A, the EPI group had 1,124 unique operational taxonomic units (OTUs), while the CK group had 908 unique OTUs. Both groups shared 2,919 OTUs. The alpha diversity indices of 14 samples were calculated using the QIIME2 software (Supplementary Table S1). Compared to the CK group, the Chao1 and Observed species indices of the EPI group were slightly higher, but the differences were not statistically significant (Figures 1C,D). Additionally, the Shannon index in the EPI group showed a trend towards increased values, although the change in the PD_whole_tree index was not significant (Figures 1E,F). Beta diversity analysis revealed that the samples from the CK and EPI groups tended to cluster internally, with distinct separation between the groups, indicating significant differences in the gut microbiota composition between the two groups (Figure 1B).
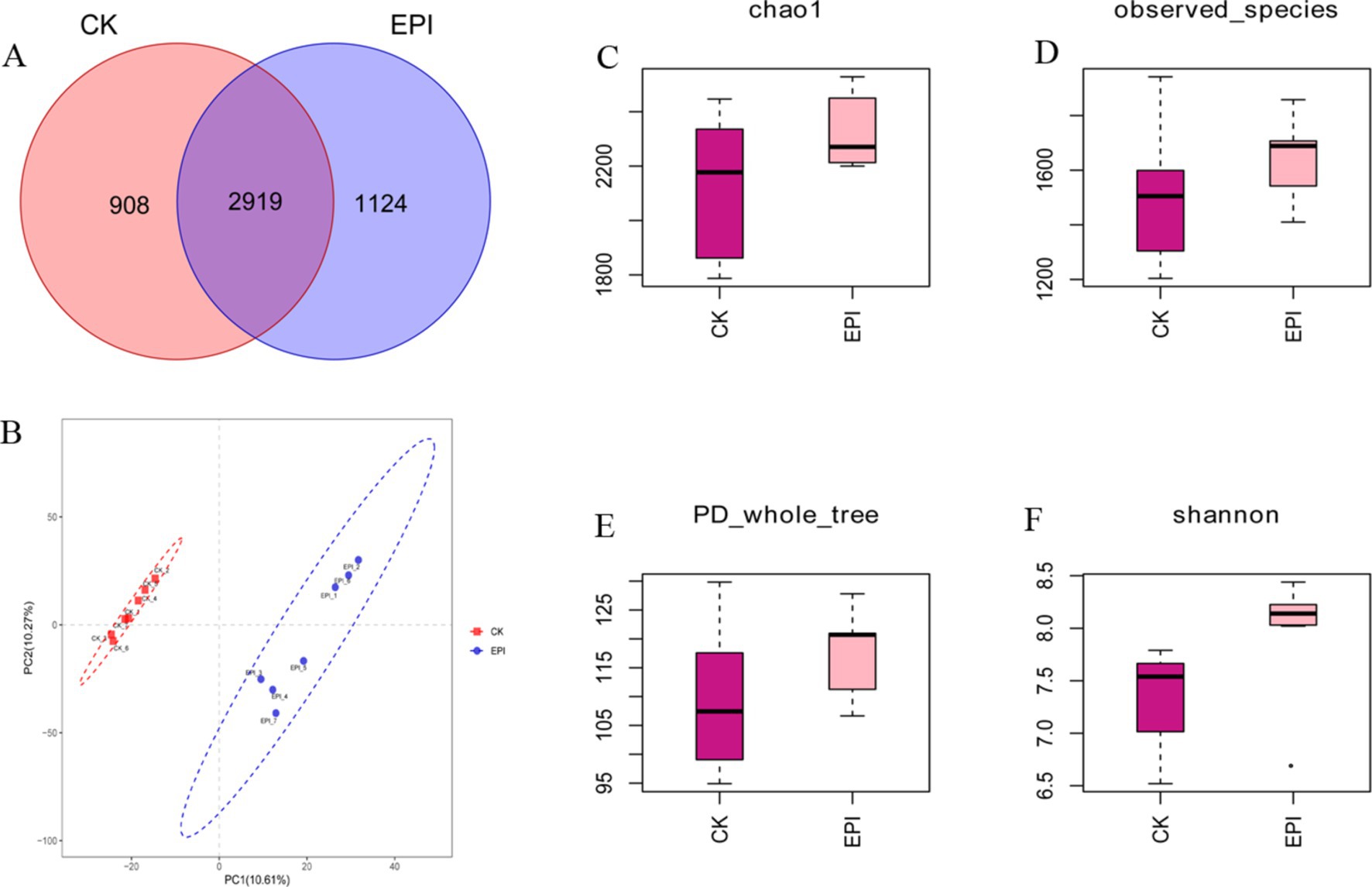
Figure 1. Comparison of gut microbial diversity between the two groups. The Venn diagram shows the number of shared or unique OTUs (A). PLS-DA analysis based on OTUs (B). Box plot of Chao1 index (C). Box plot of observed species index (D). Box plot of PD_whole_tree index (E). Box plot of Shannon index (F). EPI, Epimedium group; CK, control group.
3.2.2 The effects of Epimedium on the gut microbiota composition
A total of 21 phylum, 33 class, 68 order, 117 family, and 258 genus were identified across both groups. As shown in Figure 2A, at the phylum level, the gut microbiota of the Chinese forest musk deer was primarily composed of Bacteroidetes, Firmicutes, and Proteobacteria, with Firmicutes being the dominant phylum. After dietary supplementation with Epimedium, the relative abundance of Firmicutes in the EPI group was higher compared to that in the control group, while the relative abundance of Proteobacteria was lower in the EPI group than in the control group (p < 0.05). Notably, Firmicutes are considered beneficial bacteria, whereas Proteobacteria are viewed as potential pathogens, indicating that Epimedium supplementation may have improved the gut microbiota composition of the Chinese forest musk deer. At the genus level, the relative abundance of Ruminobacter and UCG-005 decreased, while the relative abundance of the beneficial genus Bacteroides increased in the EPI group compared to the CK group (Figure 2B).
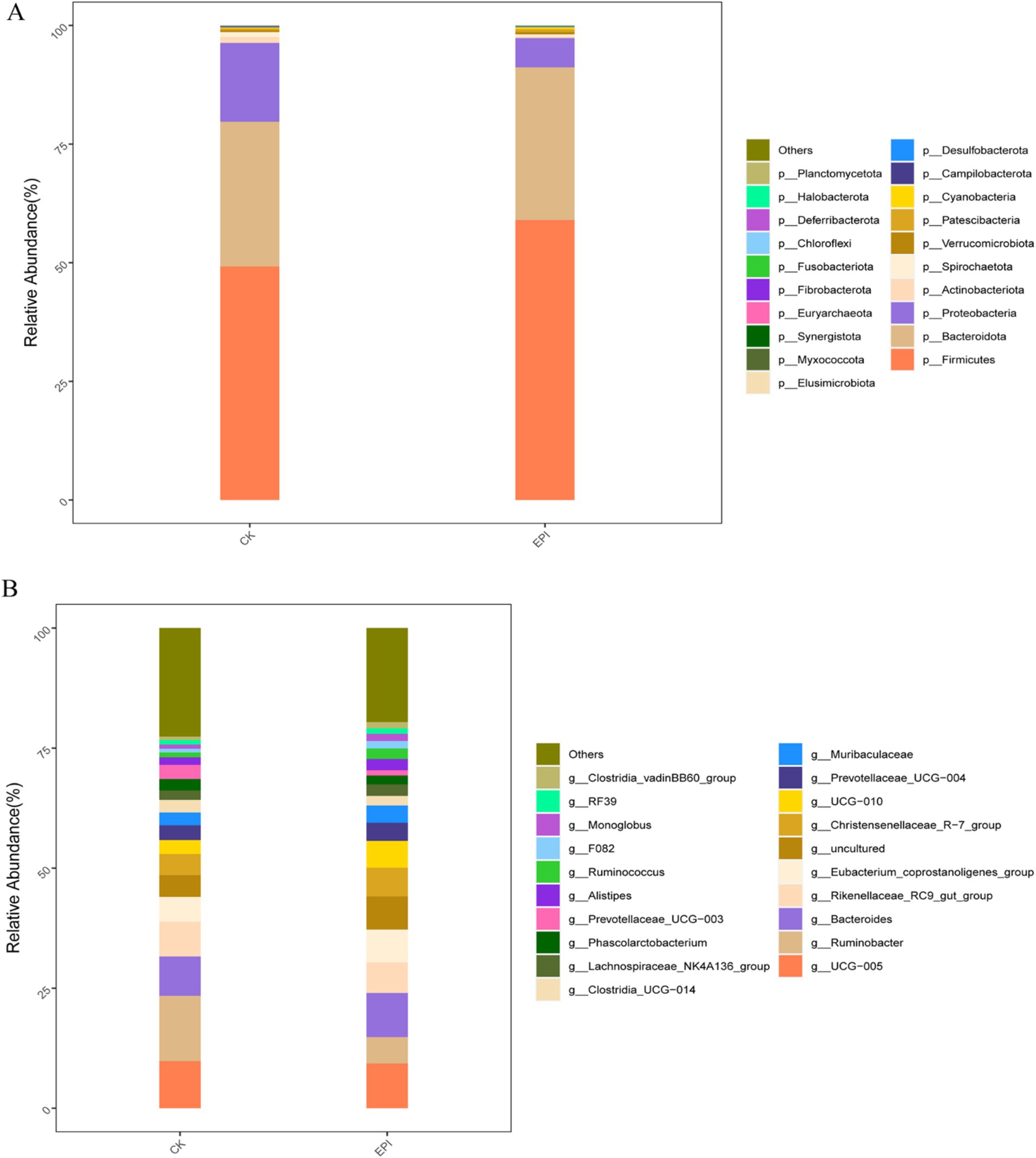
Figure 2. Effects of Epimedium on gut microbial community. Phylum−level (A). Genus−level (B). EPI, Epimedium group; CK, control group.
3.2.3 Gut microbiota differential species analysis
Linear discriminant analysis Effect Size (LEfSe) analysis revealed significantly dominant species in both groups. As shown in Figure 3A, 8 clades were found to have higher abundances in the CK group, while 12 clades were more abundant in the EPI group. The differences in the abundances of various microbial taxa between the CK and EPI groups are depicted in Figure 3B. In the CK group, the most significant difference was observed for the genus Succinivibrio, whereas in the EPI group, the classes Clostridia and Firmicutes, as well as the order Oscillospirales, exhibited notable differences. Among these, Clostridia and Firmicutes showed the greatest intergroup differences, with LDA scores greater than 4. Firmicutes is a critical component of the normal gut microbiota, playing a significant role in maintaining intestinal health and helping to prevent diarrhea and other gastrointestinal diseases.
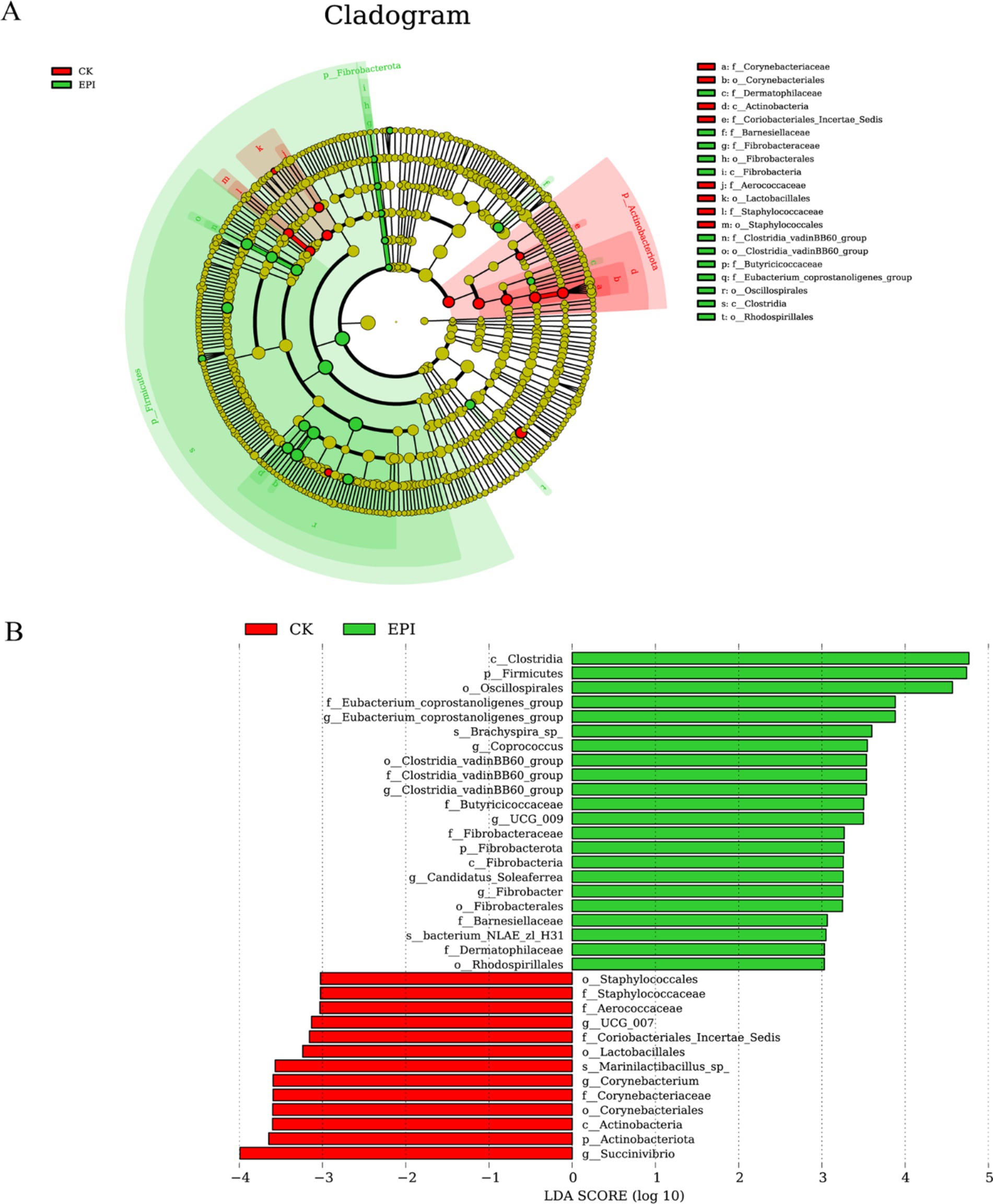
Figure 3. LEfSe cladogram comparing microbial communities between two groups (A). The red represents CK, and the green represents EPI. Significantly different bacterial taxa identified by the linear discriminant analysis effect size (B). Bacteria with an LDA score > 3 are considered to be significantly discriminative. The red represents CK, and the green represents EPI. CK, control group; EPI, Epimedium group.
3.2.4 Prediction of gut microbial functions
Prediction of the metabolic functions of the gut microbiota revealed a relatively high abundance of functional genes associated with thiamine metabolism, nicotinate and nicotinamide metabolism, amino sugar and nucleotide sugar metabolism, and RNA polymerase (Figure 4). Differential analysis using a t-test identified 12 pathways with significant differences in related genes between the two groups. Compared to the CK group, the EPI group exhibited enhanced pathways in gut microbiota, including ubiquinone and other terpenoid-quinone biosynthesis, glutathione metabolism, and nicotinate and nicotinamide metabolism (p < 0.05). Conversely, pathways such as thiamine metabolism, starch and sucrose metabolism, and methane metabolism were significantly reduced in the EPI group (p < 0.05). The functional gene classification of the gut microbiota primarily involved energy metabolism, antioxidant protection, and DNA repair.
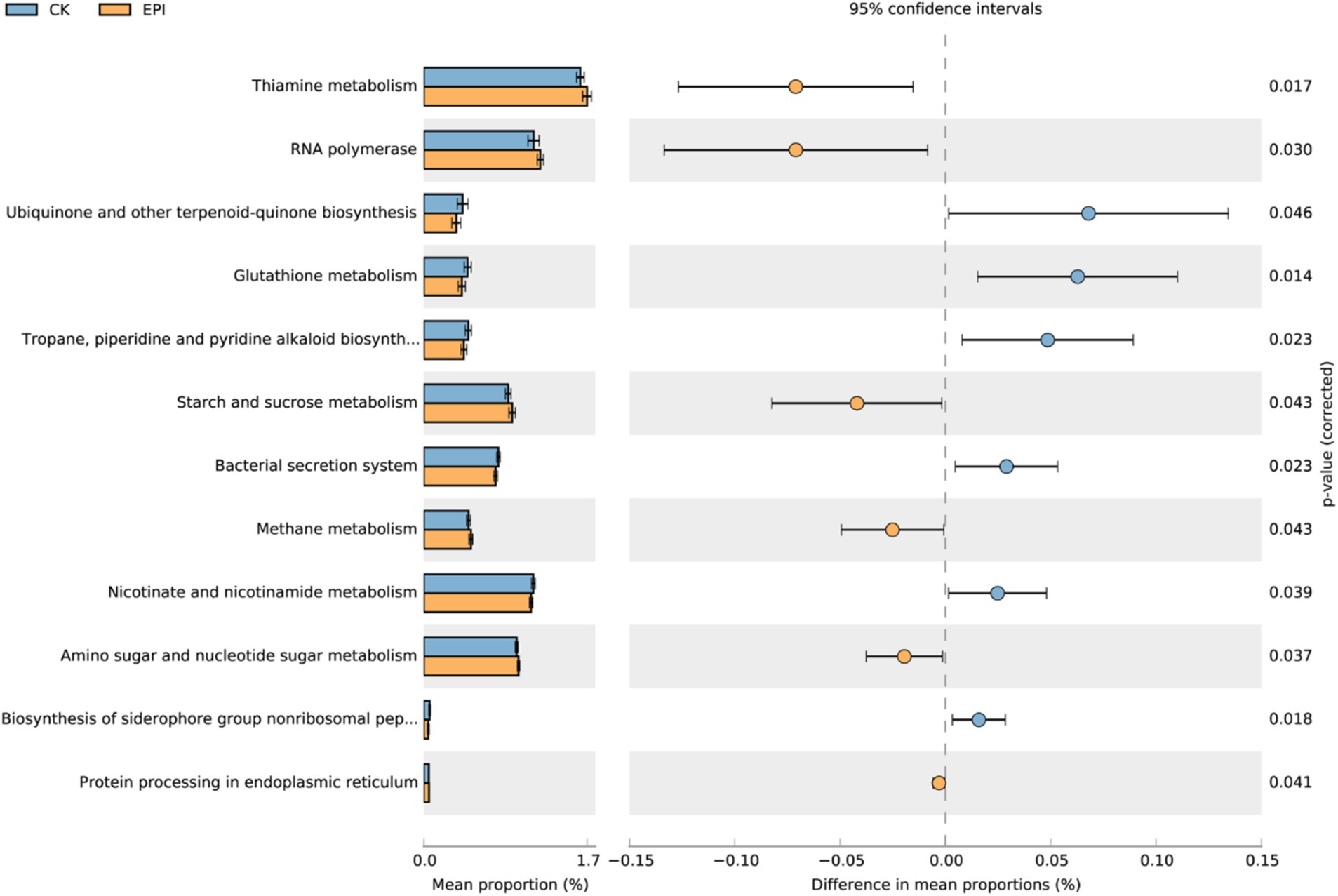
Figure 4. Microbial functional analysis. The blue represents CK, and the yellow represents EPI. CK, control group; EPI, Epimedium group.
3.3 Serum widely targeted metabolomics analysis
3.3.1 Serum metabolite multivariate statistical analysis
As shown in Figure 5A, there is a clear separation of metabolites between the two experimental groups, with a well-defined clustering pattern, indicating significant differences between the metabolites of the EPI group and those of the CK group. To assess the presence of overfitting in the PLS-DA model, permutation tests were conducted for statistical validation of the PLS-DA model. R2Y and Q2 represent the explanatory and predictive abilities within the PLS-DA model, respectively, with values closer to 1 indicating greater stability and reliability of the model. With R2Y = 0.998 and Q2 = 0.632, the model demonstrates good predictive ability and reliable results (Figure 5B).
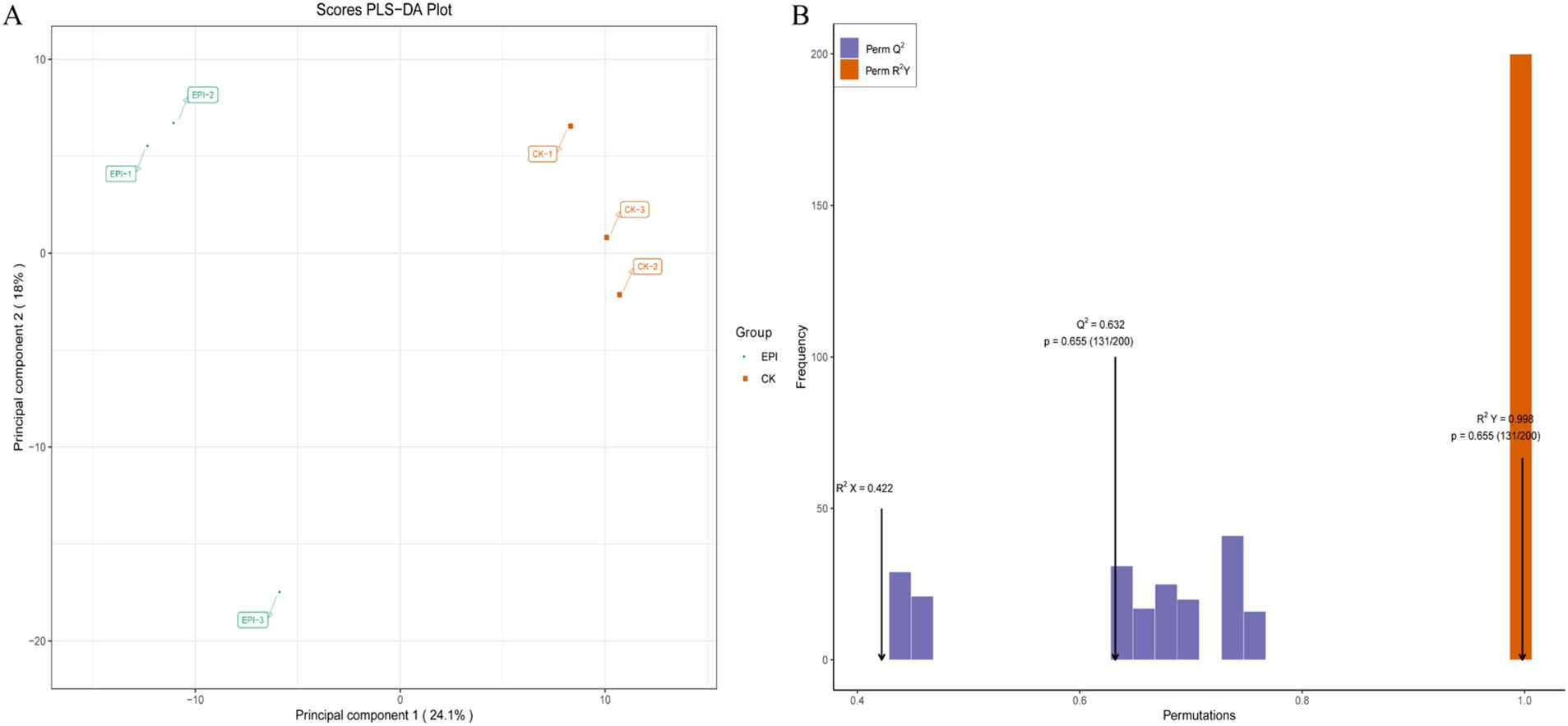
Figure 5. PLS-DA score plot for the CK and EPI groups (A). The yellow represents CK, and the green represents EPI. PLS-DA model validation plot (B). CK, control group; EPI, Epimedium group.
3.3.2 Serum differential metabolites
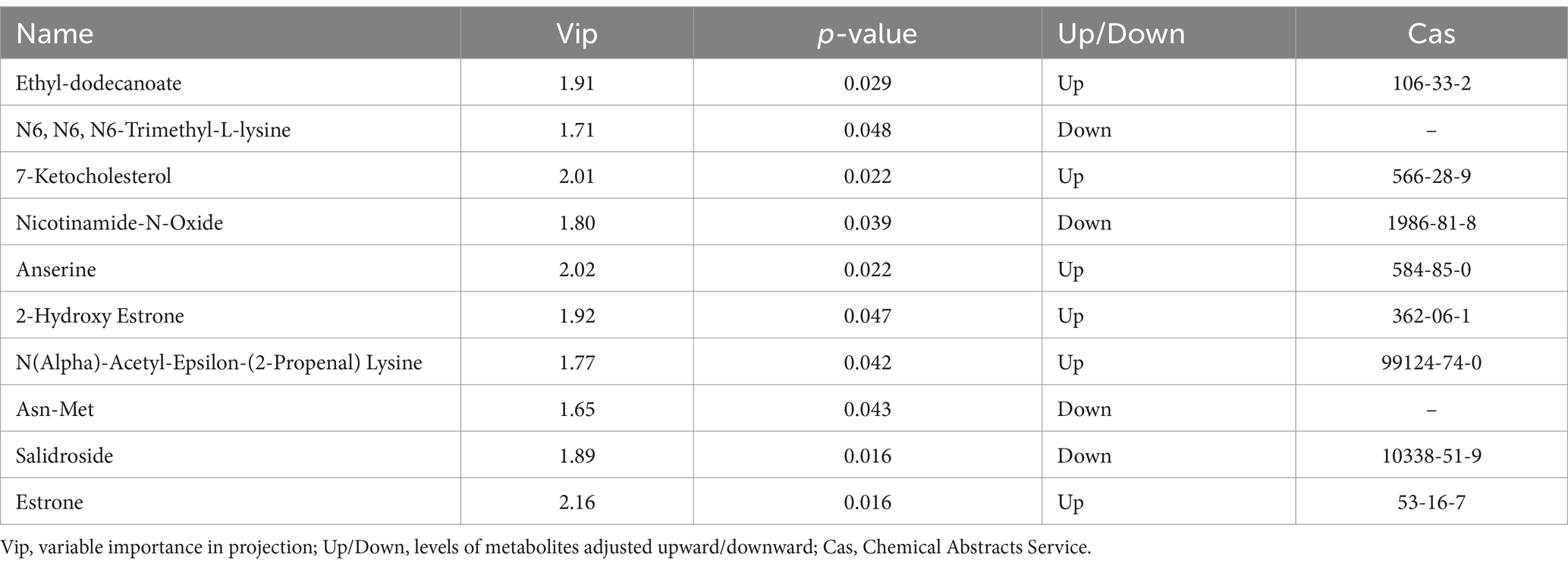
Through a combination of univariate statistical analysis and multivariate statistical analysis methods, we screened for differential metabolites between the groups. The screening criteria used were a p-value <0.05 & VIP ≥ 1. The results of the screening were visualized in a volcano plot, revealing a total of 25 differential metabolites, with 11 metabolites being upregulated and 14 metabolites being downregulated (Figure 6A). In addition, hierarchical clustering analysis of the differential metabolites showed that the serum differential metabolites were distinct between the groups, as shown in Figure 6B. Red indicates an increase in the relative content of substances, while green indicates a decrease in the relative content of substances. The intra-group clustering of the CK group and the EPI group was ideal. Compared with the CK group, the expression levels of anserine, 2-hydroxy estrone, and estrone were upregulated in the EPI group, while the expression levels of nicotinamide-n-oxide, asn-met, and salidroside were downregulated (Table 4). Dietary supplementation with Epimedium led to significant changes in the serum metabolism of the Chinese forest musk deer.
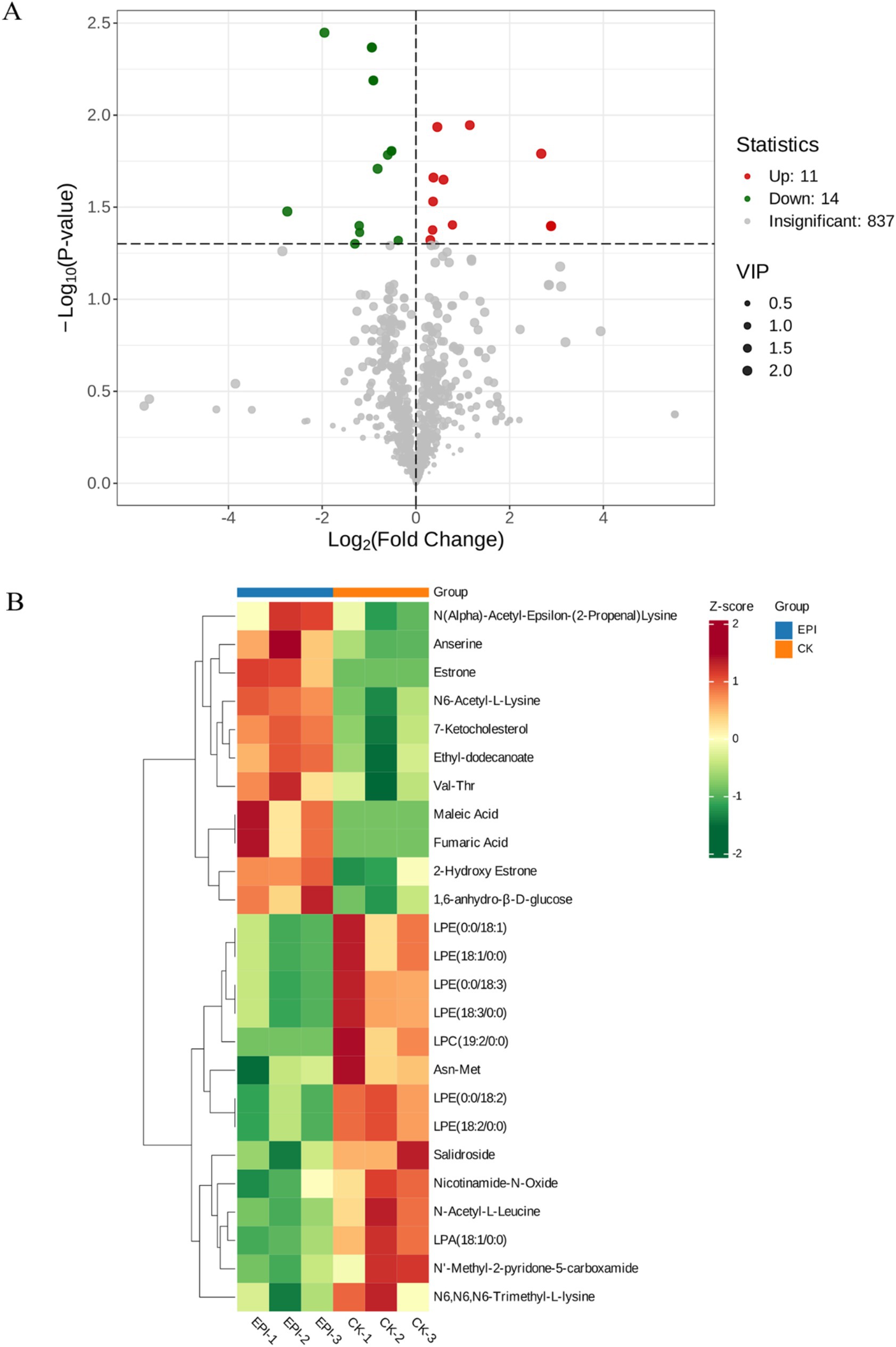
Figure 6. Volcano plot of intergroup differential metabolites (A). The red represents significantly upregulated metabolites, green represents significantly downregulated metabolites, and gray represents non-significantly changed metabolites. The size of the dots represents the VIP value. Heatmap of clustering analysis of gut differential metabolites (B). The color blocks at different positions represent the relative expression levels of the corresponding metabolites at those locations. CK, control group; EPI, Epimedium group.
3.3.3 Serum metabolic pathways
Based on the differential metabolites identified, KEGG pathway enrichment analysis was performed, and the enriched pathways were visually represented in bar charts and bubble plots. The pathways with significantly different metabolisms were selected based on the significance index p-value. The KEGG results showed that the differential metabolites between the CK and EPI groups were annotated into five categories of metabolic pathways, with metabolic pathways and glycerophospholipid metabolism being the most significantly enriched (Figure 7A). Additionally, KEGG pathway enrichment analysis indicated that the major metabolic pathways altered in the EPI group compared to the CK group included glycerophospholipid metabolism, tyrosine metabolism, nicotinate and nicotinamide metabolism, ovarian steroidogenesis, and beta-alanine metabolism (Figure 7B). Among these, glycerophospholipid metabolism and tyrosine metabolism were the most significantly altered pathways, suggesting that the supplementation of Epimedium might have had a notable impact on metabolic pathways related to lipid metabolism and hormone regulation.
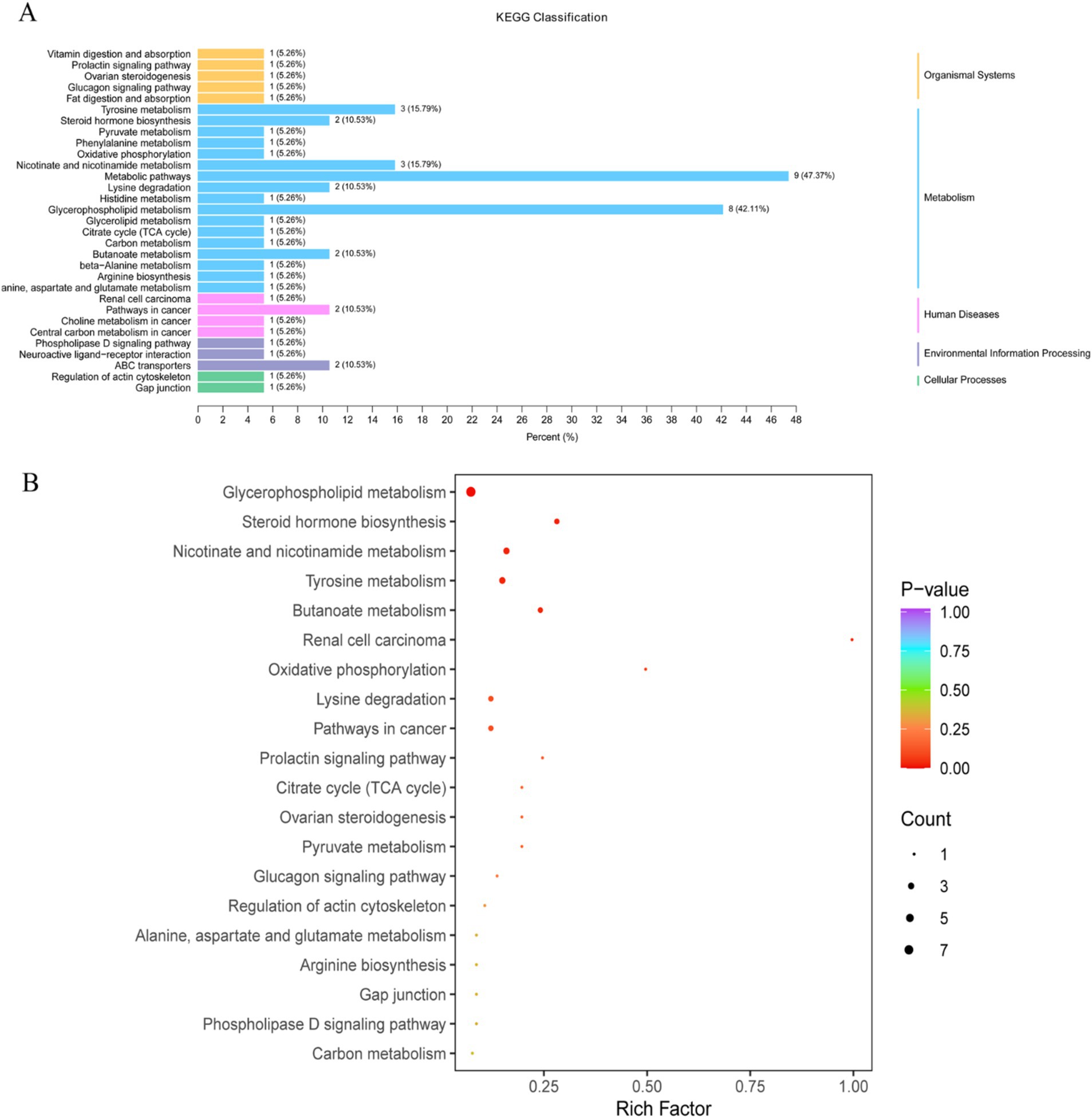
Figure 7. Differential metabolite pathway classification diagram (A). The color represents the p-value of the enrichment analysis, with a deeper red color indicating a more significant degree of enrichment. Enrichment plot of differential metabolite pathways (B). The color of the bubble represents the p-value of the enrichment analysis, with a deeper red color indicating a more significant degree of enrichment. The size of the dots represents the number of differential metabolites enriched in that pathway. CK, control group; EPI, Epimedium group.
3.4 Correlation analysis for differential microbes and metabolites
Through Pearson correlation analysis, it was found that there was a correlation between differential metabolites and differential bacterial genera. As shown in Figure 8, the Clostridia_vadinBB60_group exhibits a positive correlation with anserine and N(alpha)-acetyl-epsilon-(2-propenal) lysine, and a negative correlation with N-acetyl-L-leucine and N-methyl-2-pyridone-5-carboxamide (p < 0.05). Similarly, UCG-010 shows a positive correlation with 7-ketocholesterol and ethyl-dodecanoate, while displaying a negative correlation with N6, N6, N6-trimethyl-l-lysine (p < 0.05).
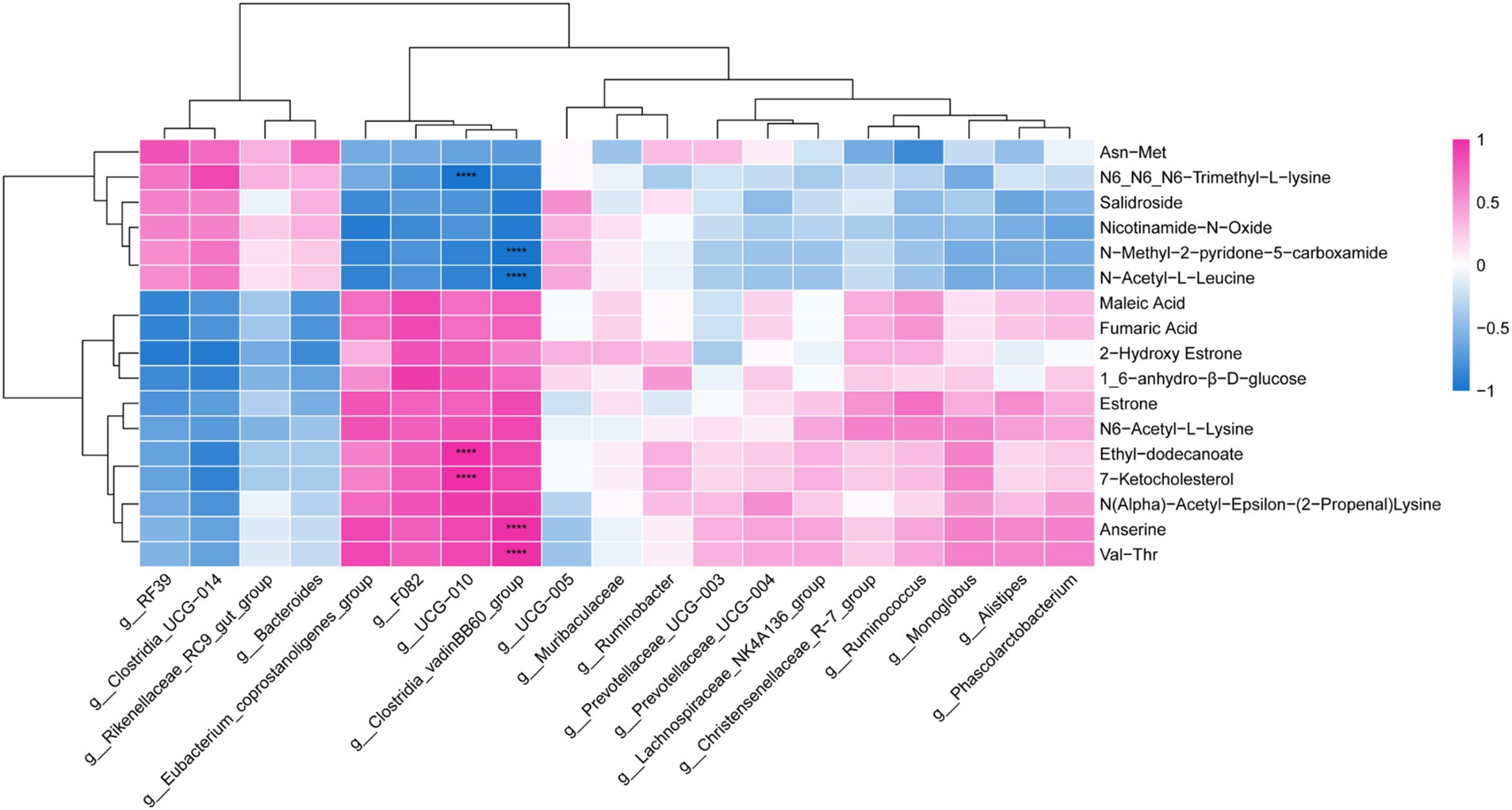
Figure 8. Microbiome-metabolome correlation heatmap. **** p < 0.0001. Red represents a positive correlation, and blue represents a negative correlation.
4 Discussion
Epimedium possesses various bioactive properties, and its appropriate supplementation as a feed additive can effectively enhance feed utilization rates in animals, thereby significantly improving economic benefits (32). In this study, compared to the CK group, dietary supplementation with Epimedium increased feed intake and feed conversion ratio in the experimental group, with a trend towards increased ADG. Zhang et al. (21) found that adding Epimedium to the diet improved intestinal function and gut microbiota in broilers, significantly increasing ADG and enhancing growth performance. This effect is attributed to the flavonoids in Epimedium, which can promote the secretion of digestive enzymes, enhancing the digestion and absorption of nutrients in the feed, thereby improving feed efficiency and promoting animal growth (33, 34). Epimedium leaves can enhance testosterone production in rat Leydig cells by regulating the expression of steroidogenic enzymes (35). Research has shown that polyphenolic compounds in Epimedium significantly elevate levels of reproductive hormones such as testosterone and luteinizing hormone in albino rats, while significantly reducing levels of progesterone and estradiol (36). However, in our study, we found that adding Epimedium to the diet increased fecal testosterone levels, but had no effect on progesterone and estradiol levels. This may be due to the unique digestive system and physiological characteristics of musk deer, which make their metabolic mechanisms significantly different from those of mice. Additionally, the experimental conditions in our study (such as housing environment, diet, sample processing, etc.) may differ from those in mouse experiments. These differences in conditions may have affected the measurement of progesterone levels. These findings suggest that Epimedium has a positive impact on increasing feed intake and regulating hormone levels in the Chinese forest musk deer, thereby effectively maintaining overall health. The yield of musk is closely related to fluctuations in hormone levels (14). Studies have indicated that quercetin can promote musk secretion by regulating the hormone production (such as testosterone and estradiol) in the Chinese forest musk deer (37). Additionally, administering exogenous testosterone during the non-secretory season can also promote musk secretion in the Chinese forest musk deer (38, 39). In this study, the level of testosterone increased after the addition of Epimedium, hypothesizing that Epimedium may increase musk yield by influencing hormone levels. Future studies should consider to provide a more comprehensive assessment of the benefits of Epimedium supplementation in the Chinese forest musk deer.
The diversity of gut microbiota is associated with the host’s health status, and Epimedium can effectively improve animal health by modulating the composition of the gut microbiota (40). In this study, after adding Epimedium to the Chinese forest musk deer diet, the Chao1 index, observed species index, and Shannon index in the EPI group were all higher than in the CK group, but these differences did not reach statistical significance. This may indicate that the addition of Epimedium has a positive effect on the richness and diversity of the Chinese forest musk deer gut microbiota, but this effect was not detected significantly due to the limited sample size or experimental conditions. According to the annotation results, the main bacterial phyla in the gut of the Chinese forest musk deer are Bacteroidetes, Firmicutes, and Proteobacteria, with Firmicutes being the dominant phylum, which is consistent with previous research findings (41, 42). Firmicutes is one of the main microbiota in the gut of ruminants, capable of producing short-chain fatty acids (SCFAs) such as acetic acid, propionic acid, and butyric acid through fermentation (43). These SCFAs play a crucial role in maintaining gut health and amino acid metabolism. Additionally, ecological biotherapy strategies enriched with Firmicutes can be used to prevent or treat colitis, demonstrating potential anti-inflammatory properties (44). In contrast, Proteobacteria are considered potentially harmful microbiota, as their increased relative abundance is associated with various gut diseases and inflammatory bowel diseases (45). They are present in lower amounts in the gut of healthy animals (46). Abnormal growth of Proteobacteria may represent an imbalance in the gut microbiota and could serve as a potential marker for disease risk (47). After adding Epimedium to the Chinese forest musk deer diet, the relative abundance of Firmicutes in the EPI group was higher than in the control group, while the relative abundance of Proteobacteria was lower. This indicates that Epimedium has a positive effect on regulating the composition of the musk deer gut microbiota. At the genus level, the dominant microbiota in the gut is Bacteroides. We found that the relative abundance of Bacteroides in the EPI group was higher than that in the CK group. Studies have demonstrated that an increase in the abundance of Bacteroides species can reduce lipopolysaccharide (LPS) levels and inhibit immune responses, thereby preventing the onset of cardiovascular diseases in humans (48). Bacteroides can effectively degrade polysaccharides, providing essential nutrients for other gut microbiota, which promotes symbiotic relationships and synergistic interactions among gut microbiota (49). In conclusion, we hypothesize that Epimedium has potential benefits in regulating the gut microbiota of the Chinese forest musk deer by increasing the abundance of beneficial bacteria and reducing the abundance of harmful bacteria, thereby maintaining the stability of the gut microbiome composition. Although we minimized the impact of parasites on the gut microbiota of the Chinese forest musk deer through regular health checks and preventive treatment with anti-parasitic drugs, recent studies have shown that parasitic infections can affect the composition and function of the gut microbiota (50). These findings suggest that future research should further investigate the interaction between parasitic infections and the gut microbiota to achieve a more comprehensive understanding of the various factors influencing the health of the Chinese forest musk deer.
In this study, the overall composition of serum metabolites showed differences. A total of 25 differential metabolites were detected in the serum. We found that dietary supplementation with Epimedium may impact metabolic pathways involved in lipid metabolism, antioxidation, and hormone regulation. Epimedium is believed to help increase sex hormone levels and effectively improve sexual function (51). Fluctuations in sex hormone levels can affect the estrous cycle and reproductive capacity of animals (52). Estrone may indirectly influence the behavior of the male Chinese forest musk deer during the breeding season, promoting musk secretion (53). Additionally, sex hormones such as testosterone, estrogen, and cortisol play a significant role in determining the composition of musk (39). In this experiment, compared to the CK group, the levels of estrone and 2-hydroxy estrone in the EPI group increased, which positively impacted the ovarian steroidogenesis and prolactin signaling pathways. Anserine, a natural compound found in animal tissues, has been shown to effectively reduce oxidative stress-induced damage by regulating antioxidant-related signaling pathways such as Keap1-Nrf2 and JNK-Caspase-3, thereby exerting antioxidant effects (54, 55). Chen et al. (56) found that anserine can increase the activity of serum superoxide dismutase and reduce levels of malondialdehyde, alkaline phosphatase, and alanine aminotransferase, thereby alleviating hyperuricemia in rats. In this experiment, anserine levels increased and were enriched in histidine metabolism and beta-alanine metabolism pathways. Histidine metabolism, a critical amino acid metabolic process, plays a vital role in protein synthesis, neurotransmitter function, and the immune system, and has a positive impact on appetite regulation (57, 58). β-Alanine can combine with L-histidine to form carnosine, a compound with antioxidant properties that can neutralize free radicals and reduce oxidative stress-induced damage to muscle cells (59, 60). Glycerophospholipid metabolism involves various enzymes and metabolic pathways that regulate lipid synthesis and degradation, thereby influencing cellular lipid balance and metabolism. The products of glycerophospholipid metabolism can affect the body’s immune response by modulating the synthesis and release of inflammatory mediators (61). In summary, Epimedium may positively influences related metabolic pathways by regulating the production of key metabolites, effectively increasing hormone levels and antioxidant capacity. This contributes to the stabilization of metabolic and physiological functions in the Chinese forest musk deer.
Correlation analysis revealed associations between Clostridia_vadinBB60_group and UCG-010 with different metabolites. We found that Clostridia_vadinBB60_group was positively correlated with anserine. As a beneficial bacterium, Clostridia_vadinBB60_group can effectively degrade complex organic compounds such as cellulose, hemicellulose, and polysaccharides, producing SCFAs like acetate, butyrate, and propionate, which play a crucial role in host energy supply, gut health, and microbial balance (62–64). This is particularly important in ruminant production, where it contributes to improving feed conversion efficiency (65). Dietary supplementation of Epimedium, the level of anserine increased, which may be related to the increased abundance of Clostridia_vadinBB60_group in this study. Further experiments are needed to verify this hypothesis by vitro culture and mechanistic studies. UCG-010 is widely present in the intestines of animals, particularly within the digestive systems of ruminants, where it plays a significant role in the gut microbiota by breaking down complex carbohydrates, thereby effectively promoting nutrient absorption (66, 67). In this study, UCG-010 was found to be positively correlated with 7-ketocholesterol. After adding the diet with Epimedium, the relative abundance of UCG-010 increased, which may affect the metabolism of 7-ketocholesterol, predicting it may affect the metabolism of 7-ketocholesterol. Further research is needed to focus on clarifying their regulation relationship and systematic mechanism.
In this study, only a small number of the Chinese forest musk deer samples (n = 3) were used for growth performance and metabolomics analysis, which is directly related to the rarity and protected status of this species. The limited number of animals included in our study may introduce biases. Specifically, the small sample size may not fully capture the natural variability within the species, potentially affecting the robustness of our statistical analyses. Due to insufficient statistical power, true biological effects may be overlooked. Furthermore, the limited representation of the Chinese forest musk deer population may not adequately reflect the complexity and diversity of their biological systems, leading to an incomplete understanding of the underlying mechanisms. To address these limitations, future studies should aim to replicate our findings with larger and more diverse sample sizes. This would enhance the reliability and validity of the results, allowing for a more comprehensive exploration of the relevant biological pathways. However, given the conservation status and ethical considerations associated with studying the Chinese forest musk deer, alternative approaches such as non-invasive sampling methods or collaborations with multiple research institutions may be necessary to effectively increase the sample size.
5 Conclusion
Dietary supplementation with Epimedium may increase the feed intake and feed conversion ratio of the Chinese forest musk deer, and positively influence their hormone levels, particularly with an observed increase in testosterone levels. Additionally, Epimedium may enhance the richness and diversity of the gut microbiota in the Chinese forest musk deer and altered serum metabolism, including amino acid metabolism, lipid metabolism, and energy metabolism. In summary, appropriate supplementation of Epimedium may contribute to the improvement of hormone levels, gut microbiota, and serum metabolite composition in the Chinese forest musk deer.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1195406.
Ethics statement
The animal study was approved by Ethics Committee of Chongqing University of Arts and Sciences (approval number, CQWLDF0031). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SX: Conceptualization, Data curation, Formal analysis, Writing – original draft. QY: Conceptualization, Data curation, Formal analysis, Writing – original draft. ZY: Methodology, Writing – original draft. MC: Visualization, Writing – original draft. WF: Visualization, Writing – original draft. HG: Methodology, Writing – original draft. XF: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. YW: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the China Postdoctoral Science Foundation in China (2023M740414), the Chongqing Natural Science Foundation in China (CSTB2022NSCQ-MSX1098), the Traditional Chinese Medicine Research Project of Chongqing Science and Technology Bureau and Health Commission in China (2023MSXM181), the Project of Science and Technology Research Program of Chongqing Education Commission in China (KJQN202001312), the Chongqing Basic Research Projects in China (2024jbky-05), the Youth Project of Science and Technology Research Program of Chongqing Education Commission in China (KJQN202201350), and the Tower Foundation Program of Chongqing University of Arts and Sciences in China (R2022YS08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1497115/full#supplementary-material
Footnotes
References
1. Feng, H, Feng, T, Mo, Y, Sun, S, Wang, L, Lu, C, et al. Integrated multi-omics analysis reveals insights into Chinese Forest musk deer (Moschus Berezovskii) genome evolution and musk synthesis. Front cell. Dev Biol. (2023) 11:11. doi: 10.3389/fcell.2023.1156138
2. Liu, K, Xie, L, Deng, M, Zhang, X, Luo, J, and Li, X. Zoology, chemical composition, pharmacology, quality control and future perspective of musk (Moschus): a review. Chin Med. (2021) 16:46. doi: 10.1186/s13020-021-00457-8
3. Gong, R, Song, S, Ai, Y, Wang, S, Dong, X, Ren, Z, et al. Exploring the growing Forest musk deer (Moschus Berezovskii) dietary protein requirement based on gut microbiome. Front Microbiol. (2023) 14:1124163. doi: 10.3389/fmicb.2023.1124163
4. Feng, H, Wang, L, Cao, F, Ma, J, Tang, J, Feng, C, et al. Forest Musk Deer (Moschus Berezovskii) in China: research and protection. J Vertebr Biol. (2023) 72:72. doi: 10.25225/jvb.22067
5. Zhao, Y, Wang, J, Li, Y, Zhou, M, Weladji, RB, Bonoan, JT, et al. Temporal pattern of parturition in captive alpine musk deer (Moschus Sifanicus). Biologia. (2020) 75:259–66. doi: 10.2478/s11756-019-00293-0
6. Qi, W-H, Li, J, Zhang, X-Y, Wang, Z-K, Li, X-X, Yang, C-Z, et al. The reproductive performance of female Forest musk deer (Moschus Berezovskii) in captivity. Theriogenology. (2011) 76:874–81. doi: 10.1016/j.theriogenology.2011.04.018
7. Cai, Y, Yang, J, Wang, J, Yang, Y, Fu, W, Zheng, C, et al. Changes in the population genetic structure of captive Forest musk deer (Moschus Berezovskii) with the increasing number of generation under closed breeding conditions. Animals. (2020) 10:255. doi: 10.3390/ani10020255
8. Tian, Q, Zhou, X, Cheng, J, Luo, Y, Dai, L, Zhao, W, et al. Genome sequence of lung pathogenic Escherichia Coli O78, a chimeric strain isolated from pneumonia Forest musk deer. Genes Genom. (2017) 39:805–15. doi: 10.1007/s13258-017-0545-4
9. Li, Y, Hu, X, Yang, S, Zhou, J, Qi, L, Sun, X, et al. Comparison between the fecal bacterial microbiota of healthy and diarrheic captive musk deer. Front Microbiol. (2018) 9:300. doi: 10.3389/fmicb.2018.00300
10. Montout, L, Poullet, N, and Bambou, J-C. Systematic review of the interaction between nutrition and immunity in livestock: effect of dietary supplementation with synthetic amino acids. Animals. (2021) 11:2813. doi: 10.3390/ani11102813
11. Zhuang, W, Sun, N, Gu, C, Liu, S, Zheng, Y, Wang, H, et al. A literature review on Epimedium, a medicinal plant with promising slow aging properties. Heliyon. (2023) 9:e21226. doi: 10.1016/j.heliyon.2023.e21226
12. Liu, S-P, Li, Y-F, Zhang, D, Li, C-Y, Dai, X-F, Lan, D-F, et al. Pharmacological actions of the bioactive compounds of Epimedium on the male reproductive system: current status and future perspective. Asian J Androl. (2024) 26:1–10. doi: 10.4103/aja20248
13. Bi, Z, Zhang, W, and Yan, X. Anti-inflammatory and Immunoregulatory effects of icariin and Icaritin. Biomed Pharmacother. (2022) 151:151. doi: 10.1016/j.biopha.2022.113180
14. Tang, Z-S, Liu, Y-R, Lv, Y, Duan, J-A, Chen, S-Z, Sun, J, et al. Quality markers of animal medicinal materials: correlative analysis of musk reveals distinct metabolic changes induced by multiple factors. Phytomedicine. (2018) 44:258–69. doi: 10.1016/j.phymed.2018.03.008
15. Xu, Q, Wells, CC, Garman, JH, Asico, L, Escano, CS, and Maric, C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension. (2008) 51:1218–24. doi: 10.1161/hypertensionaha.107.100594
16. Szabo, R, Racz, CP, and Dulf, FV. Bioavailability improvement strategies for icariin and its Derivates: a review. Int J Mol Sci. (2022) 23:7519. doi: 10.3390/ijms23147519
17. Zhang, Y, Zhang, C, Li, Z, Zeng, C, Xue, Z, Li, E, et al. New 8-Prenylated quercetin glycosides from the flowers of Epimedium Acuminatum and their testosterone production-promoting activities. Front Chem. (2022) 10:10. doi: 10.3389/fchem.2022.1014110
18. Lee, W-J, and Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. (2014) 10:416–24. doi: 10.1038/nchembio.1535
19. Chen, S, Luo, S, and Yan, C. Gut microbiota implications for health and welfare in farm animals: a review. Animals. (2022) 12:93. doi: 10.3390/ani12010093
20. Li, B, Xiang, T, Bindawa Isah, M, Chen, C, and Zhang, X. In vitro simulated saliva, gastric, and intestinal digestion followed by Faecal fermentation reveals a potential modulatory activity of Epimedium on human gut microbiota. J Pharm Biomed Anal. (2024) 245:116151. doi: 10.1016/j.jpba.2024.116151
21. Zhang, J, Yu, H, Zhang, H, Zhao, Q, Si, W, Qin, Y, et al. Dietary Epimedium extract supplementation improves intestinal functions and alters gut microbiota in broilers. J Anim Sci Biotechnol. (2023) 14:14. doi: 10.1186/s40104-022-00812-1
22. Pan, S, Chen, A, Han, Z, Wang, Y, Lu, X, and Yang, Y. 1h Nmr-based Metabonomic study on the effects of Epimedium on glucocorticoid-induced osteoporosis. J Chromatogr B. (2016) 1038:118–26. doi: 10.1016/j.jchromb.2016.10.015
23. Yang, X-H, Li, L, Xue, Y-B, Zhou, X-X, and Tang, J-H. Flavonoids from Epimedium Pubescens: extraction and mechanism, antioxidant capacity and effects on cat and Gsh-Px of Drosophila Melanogaster. PeerJ. (2020) 8:e8361. doi: 10.7717/peerj.8361
24. Zhang, J, Kobert, K, Flouri, T, and Stamatakis, A. Pear: a fast and accurate Illumina paired-end read merger. Bioinformatics. (2014) 30:614–20. doi: 10.1093/bioinformatics/btt593
25. Rognes, T, Flouri, T, Nichols, B, Quince, C, and Mahe, F. Vsearch: a versatile open source tool for metagenomics. PeerJ. (2016) 4:e2584. doi: 10.7717/peerj.2584
26. Pruesse, E, Quast, C, Knittel, K, Fuchs, BM, Ludwig, W, Peplies, J, et al. Silva: a comprehensive online resource for quality checked and aligned ribosomal Rna sequence data compatible with arb. Nucleic Acids Res. (2007) 35:7188–96. doi: 10.1093/nar/gkm864
27. Ye, J, McGinnis, S, and Madden, TL. Blast: improvements for better sequence analysis. Nucleic Acids Res. (2006) 34:W6–9. doi: 10.1093/nar/gkl164
28. Hall, M, and Beiko, RG. 16s Rrna gene analysis with Qiime2. Methods Mol Biol. (2018) 1849:113–29. doi: 10.1007/978-1-4939-8728-3_8
29. Copeland, WK, Krishnan, V, Beck, D, Settles, M, Foster, JA, Cho, K-C, et al. Mcagui: microbial community analysis R-graphical user Interface (Gui). Bioinformatics. (2012) 28:2198–9. doi: 10.1093/bioinformatics/bts338
30. Segata, N, Izard, J, Waldron, L, Gevers, D, Miropolsky, L, Garrett, WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. (2011) 12:R60. doi: 10.1186/gb-2011-12-6-r60
31. Liao, J, Zhang, Y, Zhang, W, Zeng, Y, Zhao, J, Zhang, J, et al. Different software processing affects the peak picking and metabolic pathway recognition of metabolomics data. J Chromatogr A. (2023) 1687:1687. doi: 10.1016/j.chroma.2022.463700
32. Zhang, X, Zhou, S, Liang, Y, Xie, G, Zhu, M, Wang, Z, et al. Effects of Astragalus, Epimedium, and Fructus Ligustri Lucidi extractive on antioxidant capacity, production performance, and immune mechanism of breeding pigeons under stress. Poult Sci. (2023) 102:102350. doi: 10.1016/j.psj.2022.102350
33. Guo, Y, Li, Y, Zhang, S, Wu, X, Jiang, L, Zhao, Q, et al. The effect of Total flavonoids of Epimedium on granulosa cell development in laying hens. Poult Sci. (2020) 99:4598–606. doi: 10.1016/j.psj.2020.05.032
34. Liu, J, Yan, P, Li, Y, Yu, J, Huang, Y, Bai, R, et al. Gut microbiota and serum metabolome reveal the mechanism by which Tcm polysaccharides alleviate Salpingitis in laying hens challenged by Bacteria. Poult Sci. (2024) 103:103288. doi: 10.1016/j.psj.2023.103288
35. Sun, X, Li, Y, Li, J, Liang, H, Zhang, J, Chen, X, et al. Bioactive metabolites reveal the therapeutic consistency of Epimedii folium from multi-plant sources for the treatment of kidney-Yang deficiency. J Ethnopharmacol. (2024) 319:319. doi: 10.1016/j.jep.2023.117215
36. Munir, N, Mahmood, Z, Yameen, M, and Mustafa, G. Therapeutic response of Epimedium Gandiflorum's different doses to restore the antioxidant potential and reproductive hormones in male albino rats. Dose-Response. (2020) 18:1559325820959563. doi: 10.1177/1559325820959563
37. Bo, X, Chen, J, Mu, J, Dong, X, Ren, Z, Liu, J, et al. Quercetin promotes the secretion of musk by regulating the hormone level and microbial structure of Forest musk deer. Integr Zool. (2024) 19:596–611. doi: 10.1111/1749-4877.12763
38. Jie, H, Feng, X-L, Zhao, G-J, Zeng, D-J, Zhang, C-L, and Chen, Q. Research Progress on musk secretion mechanism of Forest musk deer. Zhongguo Zhong Yao Za Zhi. (2014) 39:4522–5.
39. Fang, M, Zhang, M, Shi, M, Zhang, T, Qi, L, Yu, J, et al. Sex hormones play roles in determining musk composition during the early stages of musk secretion by musk deer (Moschus Berezovskii). Endocr J. (2018) 65:1111–20. doi: 10.1507/endocrj.EJ18-0211
40. Jin, Q, Cheng, L, Zhu, Y, Zhao, X, Zhang, W, Gao, X, et al. Immune-related effects of compound Astragalus polysaccharide and sulfated Epimedium polysaccharide on newborn piglets. Anim Biotechnol. (2021) 34:508–19. doi: 10.1080/10495398.2021.1979022
41. Jiang, Y, Han, X, Li, M, Feng, N, Yang, P, Zhao, H, et al. Changes in the gut microbiota of Forest musk deer (Moschus Berezovskii) during ex situ conservation. Front Microbiol. (2022) 13:13. doi: 10.3389/fmicb.2022.969593
42. Hu, X, Liu, G, Li, Y, Wei, Y, Lin, S, Liu, S, et al. High-throughput analysis reveals seasonal variation of the gut microbiota composition within Forest musk deer (Moschus Berezovskii). Front Microbiol. (2018) 9:9. doi: 10.3389/fmicb.2018.00009
43. Kapoor, P, Tiwari, A, Sharma, S, Tiwari, V, Sheoran, B, Ali, U, et al. Effect of anthocyanins on gut health markers, Firmicutes-Bacteroidetes ratio and short-chain fatty acids: a systematic review via Meta-analysis. Sci Rep. (2023) 13:1729. doi: 10.1038/s41598-023-28764-0
44. Natividad, JM, Pinto-Sanchez, MI, Galipeau, HJ, Jury, J, Jordana, M, Reinisch, W, et al. Ecobiotherapy rich in Firmicutes decreases susceptibility to colitis in a humanized Gnotobiotic mouse model. Inflamm Bowel Dis. (2015) 21:1883–93. doi: 10.1097/mib.0000000000000422
45. Chen, G, Hu, P, Xu, Z, Peng, C, Wang, Y, Wan, X, et al. The beneficial or detrimental fluoride to gut microbiota depends on its dosages. Ecotoxicol Environ Saf. (2021) 209:111732. doi: 10.1016/j.ecoenv.2020.111732
46. Rizzatti, G, Lopetuso, LR, Gibiino, G, Binda, C, and Gasbarrini, A. Proteobacteria: a common factor in human diseases. Biomed Res Int. (2017) 2017:1–7. doi: 10.1155/2017/9351507
47. Shin, N-R, Whon, TW, and Bae, J-W. Proteobacteria: microbial signature of Dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. doi: 10.1016/j.tibtech.2015.06.011
48. Yoshida, N, Yamashita, T, Kishino, S, Watanabe, H, Sasaki, K, Sasaki, D, et al. A possible beneficial effect of Bacteroides on Faecal lipopolysaccharide activity and cardiovascular diseases. Sci Rep. (2020) 10:13009. doi: 10.1038/s41598-020-69983-z
49. Cheng, J, Hu, J, Geng, F, and Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci Hum Wellness. (2022) 11:1101–10. doi: 10.1016/j.fshw.2022.04.002
50. Deng, L, Chen, S, Meng, W, Zhou, Z, Liu, H, Zhong, Z, et al. Changes in gut microbiota composition associated with the presence of enteric Protist Blastocystis in captive Forest musk deer (Moschus Berezovskii). Microbiol Spectr. (2022) 10:e0226921. doi: 10.1128/spectrum.02269-21
51. Huo, S, Li, Y, Guo, Y, Zhang, S, Li, P, and Gao, P. Improving effects of Epimedium flavonoids on the selected reproductive features in layer hens after forced molting. Poult Sci. (2020) 99:2757–65. doi: 10.1016/j.psj.2019.12.053
52. Wang, Y, Yang, P, Chen, T, Hu, J, An, X, Yao, C, et al. Analysis and comparison of blood metabolome of Forest musk deer in musk secretion and non-secretion periods. Sci Rep. (2024) 14:16980. doi: 10.1038/s41598-024-67981-z
53. Yi, L, Dalai, M, Su, R, Lin, W, Erdenedalai, M, Luvsantseren, B, et al. Whole-genome sequencing of wild Siberian musk deer (Moschus Moschiferus) provides insights into its genetic features. BMC Genomics. (2020) 21:108. doi: 10.1186/s12864-020-6495-2
54. Chen, M, Luo, J, Ji, H, Song, W, Zhang, D, Su, W, et al. The preventive mechanism of anserine on Tert-butyl Hydroperoxide-induced liver injury in L-02 cells via regulating the Keap1-Nrf2 and Jnk-Caspase-3 signaling pathways. Mar Drugs. (2023) 21:477. doi: 10.3390/md21090477
55. Kasamatsu, S, Komae, S, Matsukura, K, Kakihana, Y, Uchida, K, and Ihara, H. 2-Oxo-imidazole-containing dipeptides play a key role in the antioxidant capacity of imidazole-containing dipeptides. Antioxidants. (2021) 10:1434. doi: 10.3390/antiox10091434
56. Chen, M, Ji, H, Song, W, Zhang, D, Su, W, and Liu, S. Anserine beneficial effects in Hyperuricemic rats by inhibiting Xod, regulating uric acid transporter and repairing Hepatorenal injury. Food Funct. (2022) 13:9434–42. doi: 10.1039/d2fo01533a
57. Moro, J, Tome, D, Schmidely, P, Demersay, T-C, and Azzout-Marniche, D. Histidine: a systematic review on metabolism and physiological effects in human and different animal species. Nutrients. (2020) 12:1414. doi: 10.3390/nu12051414
58. Holecek, M. Histidine in health and disease: metabolism, physiological importance, and use as a supplement. Nutrients. (2020) 12:848. doi: 10.3390/nu12030848
59. Kopec, W, Jamroz, D, Wiliczkiewicz, A, Biazik, E, Pudlo, A, Korzeniowska, M, et al. Antioxidative characteristics of chicken breast meat and blood after diet supplementation with carnosine, L-histidine, and Β-alanine. Antioxidants. (2020) 9:1093. doi: 10.3390/antiox9111093
60. Qi, B, Wang, J, Hu, M, Ma, Y, Wu, S, Qi, G, et al. Influences of Beta-alanine and L-histidine supplementation on growth performance, meat quality, carnosine content, and Mrna expression of carnosine-related enzymes in broilers. Animals. (2021) 11:2265. doi: 10.3390/ani11082265
61. Zhu, Q, Wu, Y, Mai, J, Guo, G, Meng, J, Fang, X, et al. Comprehensive metabolic profiling of inflammation indicated key roles of Glycerophospholipid and arginine metabolism in coronary artery disease. Front Immunol. (2022) 13:13. doi: 10.3389/fimmu.2022.829425
62. Xiao, S-S, Mi, J-D, Mei, L, Liang, J, Feng, K-X, Wu, Y-B, et al. Microbial diversity and community variation in the intestines of layer chickens. Animals. (2021) 11:840. doi: 10.3390/ani11030840
63. Harrison, CA, Laubitz, D, Ohland, CL, Midura-Kiela, MT, Patil, K, Besselsen, DG, et al. Microbial Dysbiosis associated with impaired intestinal Na+/H+ exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol. (2018) 11:1329–41. doi: 10.1038/s41385-018-0035-2
64. Zhang, J, Shi, H, Wang, Y, Cao, Z, Yang, H, and Li, S. Effect of limit-fed diets with different forage to concentrate rations on fecal bacterial and archaeal community composition in Holstein heifers. Front Microbiol. (2018) 9:9. doi: 10.3389/fmicb.2018.00976
65. Fonseca, PAS, Lam, S, Chen, Y, Waters, SM, Guan, LL, and Canovas, A. Multi-breed host rumen epithelium transcriptome and microbiome associations and their relationship with beef cattle feed efficiency. Sci Rep. (2023) 13:16209. doi: 10.1038/s41598-023-43097-8
66. Han, X, Lei, X, Yang, X, Shen, J, Zheng, L, Jin, C, et al. A metagenomic insight into the hindgut microbiota and their metabolites for dairy goats fed different rumen degradable starch. Front Microbiol. (2021) 12:12. doi: 10.3389/fmicb.2021.651631
Keywords: Epimedium, Moschus berezovskii, hormone levels, gut microbiota, serum metabolism
Citation: Xie S, Yang Q, Ying Z, Cai M, Fan W, Gao H, Feng X and Wu Y (2025) Dietary supplementation with Epimedium contributes to the improvement of hormone levels, gut microbiota, and serum metabolite composition in the Chinese forest musk deer (Moschus berezovskii). Front. Vet. Sci. 11:1497115. doi: 10.3389/fvets.2024.1497115
Edited by:
Izhar Hyder Qazi, South China Agricultural University, ChinaReviewed by:
Lei Deng, Harvard Medical School, United StatesChao Xu, Jilin Agricultural University, China
Copyright © 2025 Xie, Yang, Ying, Cai, Fan, Gao, Feng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolan Feng, MTM4OTY3Mzc5OTZAMTYzLmNvbQ==; Yongjiang Wu, d3lvbmdqYW5nQDE2My5jb20=
†These authors have contributed equally to this work
 Shan Xie
Shan Xie Qinlin Yang3†
Qinlin Yang3† Wenqiao Fan
Wenqiao Fan Yongjiang Wu
Yongjiang Wu