- 1Animal Nutrition Institute, Sichuan Agricultural University, Chengdu, China
- 2Key Laboratory of Animal Disease-Resistance Nutrition, China Ministry of Education, China Ministry of Agriculture and Rural Affairs, Key Laboratory of Sichuan Province, Chengdu, China
Maternal dietary supplementation with chitosan oligosaccharide (COS) has been considered as a potential intervention to mitigate the occurrence of intrauterine growth restriction (IUGR) and improve postnatal growth. The present study investigated the effect of COS as a dietary supplement for sows during late gestation and lactation on their productivity, placental function, and the intestinal health of IUGR piglets. From day (d) 85 of late gestation to d 21 of lactation, 30 sows were randomly divided into either a control group (basal diet) or a COS group (basal diet + 100 mg kg−1 COS). At d 21 of lactation, eight normal and eight IUGR littermates from eight litters belong to control sows, as well as eight IUGR littermates from COS sows, were selected for further analysis. The results showed a significant reduction in the number of stillbirths and mummies in COS groups (p < 0.05). Maternal dietary supplementation with COS also significantly up-regulated the expression levels of GLUT1, GLUT3, and VEGFA mRNA in the placenta of IUGR piglets compared to those in control group (p < 0.05). Furthermore, there was a significant decrease in MDA content and a significant increase in GSH content in the placenta of IUGR piglets from COS sows compared to those from control group (p < 0.05). Additionally, the expression levels of MUC2 and occludin mRNA as well as claudin1 protein significantly up-regulated in the jejunum of 21-day-old IUGR piglets from COS sows group compared to those from control group (p < 0.05). Moreover, IL-10 mRNA expression level was significantly increased while MDA content was significantly reduced in the jejunum of 21-day-old IUGR piglets from COS sows group compared to those from control group (p < 0.05). The results indicated that maternal dietary COS supplementation during late gestation effectively reduced the incidence of stillbirths and mummies, potentially linked to enhanced placental function, reduced oxidative stress, and improved immune status. Furthermore, maternal dietary COS supplementation exhibited positive impact on intestinal digestive and absorptive function, intestinal barrier integrity, intestinal antioxidant capacity and immune status in 21-day-old suckling IUGR piglets.
1 Introduction
Intrauterine growth retardation (IUGR) in mammals refers to abnormal fetal growth and organ development in the uterus, resulting in low birth weight and impaired growth and development (1). Various factors, including maternal, fetal, placental or genetic influences, contribute to the formation of IUGR (2). In multiparous animals, we considered neonates whose birth weights did not align with the mean litter weight distribution and were less than twice the standard deviation as IUGR animals (3). The placenta plays a crucial role as a mediator between the mother and fetus. Therefore, oxidative stress, poor developmental status, and impaired nutrient transport of the placenta can collectively contribute to the occurrence of IUGR (4). IUGR adversely impacts neonatal morbidity and mortality, leading to impaired nutrient absorption and abnormalities in body composition, such as reduced muscle mass (5, 6). IUGR has become an issue that cannot be ignored in both nutritional human obstetrics as well as animal production. Recent studies have shown that nutritional interventions in sows can effectively reduce the incidence of IUGR. For instance, Pieszka et al. (7) showed the effectiveness of supplementing sow diets with pancreatic-like enzymes, while Wu et al. (8) found that supplementation with adenosine was also effective in reducing the occurrence of IUGR. However, current IUGR research primarily emphasizes direct maternal effects on the fetus and the characterization of the IUGR placenta. Our aim was to connect the mother, fetus, and placenta, and investigate how IUGR affects the offspring’s gut health.
Chitosan oligosaccharide (COS) is a degradation product of chitosan, typically prepared using physical, chemical, enzymatic, and other methods. COS has been demonstrated to have biological effects such as antioxidant (9), anti-inflammatory (10), antibacterial (11, 12), and anti-tumor activity (13). Study has proved that supplementing COS in the diet of pregnant sows can enhance sows health status, provide an improved growth environment for fetuses and increase the survival rate of newborn piglets (14). Additionally, supplementing sow feed with COS enhanced the antioxidant and anti-inflammatory abilities as well as the nutritional composition of sow milk, thereby improving the growth performance of piglets (15, 16). Furthermore, study has also indicated that COS improves amino acid transport capacity in the placenta and promotes nutrient transport from sow to fetuses (17). Existing studies focusing on reproductive performance (14), milk composition (18), antioxidant capacity (17), and placental capacity of sow with supplementation of COS to their diets have rarely investigated the effects of feeding COS to sows on IUGR piglets.
Therefore, we hypothesized that maternal COS supplementation during late gestation and lactation can improve placental function, reduce the incidence of IUGR, and improve the intestinal health of IUGR piglets. Thus, the aim of this study was to evaluate the effects of maternal supplementation with COS during late gestation and lactation on reproductive performance, placenta function, immune response, and antioxidant capacity of placenta in sows. Furthermore, digestive enzyme activity, intestinal barrier function, cytokine levels and antioxidant indicators were evaluated in piglet jejunum.
2 Materials and methods
2.1 Animal ethics
This experiment was performed in accordance with the Laboratory Animal-Guideline for ethical review of animal welfare of the People’s Republic of China (GB/T 35892-2018) and approved by Sichuan Agricultural University Animal Care and Use Committee.
2.2 Chitosan oligosaccharide
The effective content of COS used in this study is 10% with Maltose dextrin as the carrier, MW <1,000 Da, and degree of deacetylation (DD) is about 95%, which was provided by Zhongke Runxin (Suzhou) Biological Technology Co., Ltd. The amount of COS added was calculated based on the effective content.
2.3 Animals, diets and experimental design
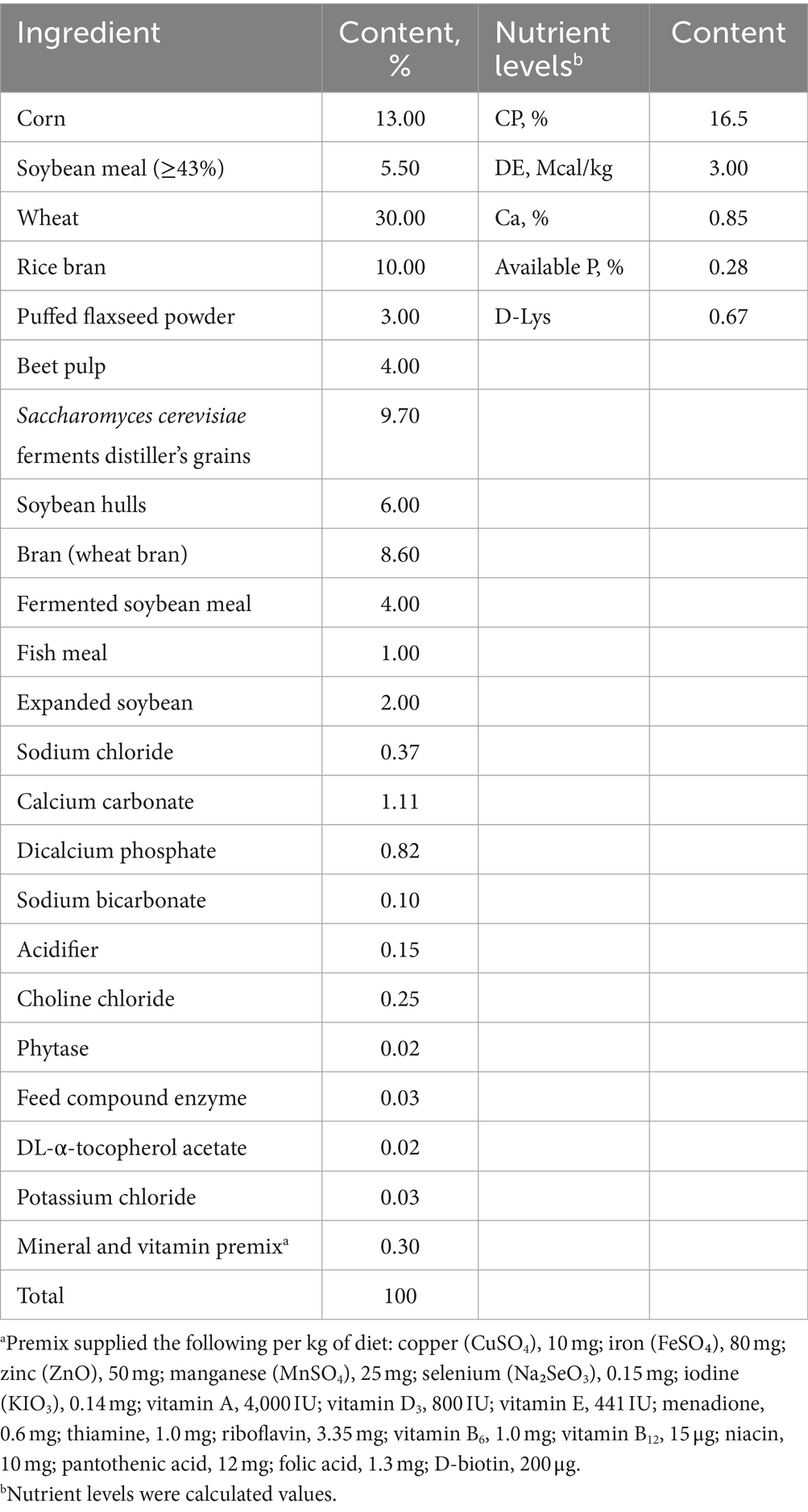
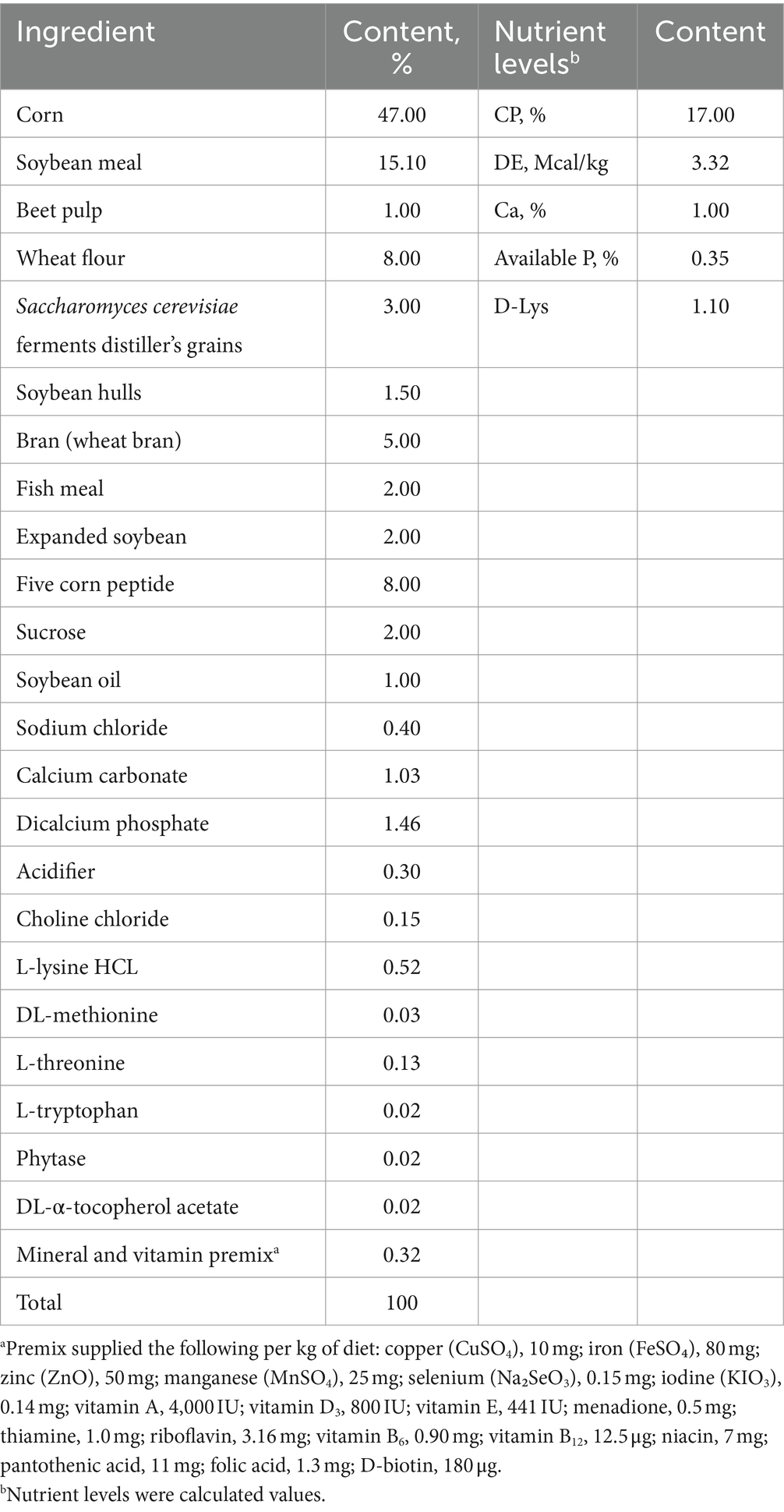
This study was conducted in Zitong Original Breeding Pig Farm, which is a member of Sichuan Mianyang Jinchiyang Agriculture and Animal Husbandry Co., Ltd. A total of 30 sows (Landrace × Yorkshire) with specific criteria for back fat, parity, and expected delivery date close to 85 days of pregnancy, were randomly allocated to 2 groups: control group (control, sows fed basal diet) and COS group (COS, sows fed basal diet + 100 mg kg−1 COS) (14). The basal diets were formulated to meet the nutrient requirements of gestating sows or lactation sows, respectively, as recommended by the National Research Council (NRC, 2012), and their compositions and nutrients levels were shown in Tables 1, 2.
After delivery, the piglets were fostered to other gilts within the same treatment group to ensure each litter included 10–11 piglets, and the number of sows in both COS group and control group was adjusted to 10.
The piglets were breastfed until weaning at day 21. Piglets with a birth weight below 0.9 kg were classified as IUGR piglet. Newborn piglets categorized as NBW weigh 1.50 kg on average, while those categorized as IUGR weigh 0.84 kg. By day 21, their weights have increased to 6.00 kg and 3.17 kg, respectively.
2.4 Sample collection
Based on the birth weight of piglets, the placenta was classified into two categories: those corresponding to normal weight piglets and those corresponding to IUGR piglets. Following the onset of sow delivery, before cutting the umbilical cord as it emerged from the birth canal, three different colored silk threads (white for normal body weight piglet’s placenta in control group, NBW; pink for IUGR piglet’s placenta in control group, IUGR); and (black for IUGR piglet’s placenta in COS group, IUGR + COS) were used to tie off the respective umbilical cords of varying weight piglets. The marked umbilical cord with the silk threads were then retracted back into the sow’s body (the white and pink silk threads are of different materials, making it easier to distinguish them during sample collection). Once all placentas had been discharged by the sows post-delivery, fresh placental samples were collected from a 5 cm distance near the umbilical cord based on their color markings and stored in frozen tubes for subsequent testing after freezing in liquid nitrogen.
When piglets reach 21-day-old, those were anesthetized and euthanized, followed by a rapid opening of the abdominal cavity. The small intestine was then dissected, and tissue samples from the jejunum were promptly frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
2.5 Sow reproduction
Record the changes in backfat of sows at days 85 and 111 of gestation, and day 21 of lactation, as well as the average litter size, live litter size, stillbirth, mummy size and the number of piglets with a birth weight below 1 kg. Calculate the average birth weight, live litter weight, percentage of IUGR, weaned litter size and weight, average weaned weight and daily gain during lactation for piglet, also calculate daily feed intake and backfat loss during lactation for sow. The percentage of IUGR refers to the proportion of piglets with BW <1.0 kg among the total born. Backfat loss was defined as the difference in backfat thickness between farrowing and weaning for each sow.
2.6 Antioxidant status of placenta in sows and jejunum in piglets
The levels of ATP, MDA, glutathione (GSH), and oxidized glutathione (GSSG) in the placenta, as well as the content of MDA, GSH, and GSSG and the activity of CAT, T-AOC, GSH-PX in the jejunum of piglet were determined using the commercial kits provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The total protein content in both the placenta and the piglet’s jejunum was measured using the same kit.
2.7 Placental cytokines concentrations
The levels of placental cytokines IL-1β, IL-10 and TNF-α were quantified using a swine ELISA kit (Beijing 4A Biotech Co., Ltd).
2.8 Activity of digestive enzyme in jejunum of piglets
The activities of trypsin, lipase, maltase, sucrase and lactase in piglet jejunum were determined using the commercial kits provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.9 Quantitative real-time RT-PCR analysis
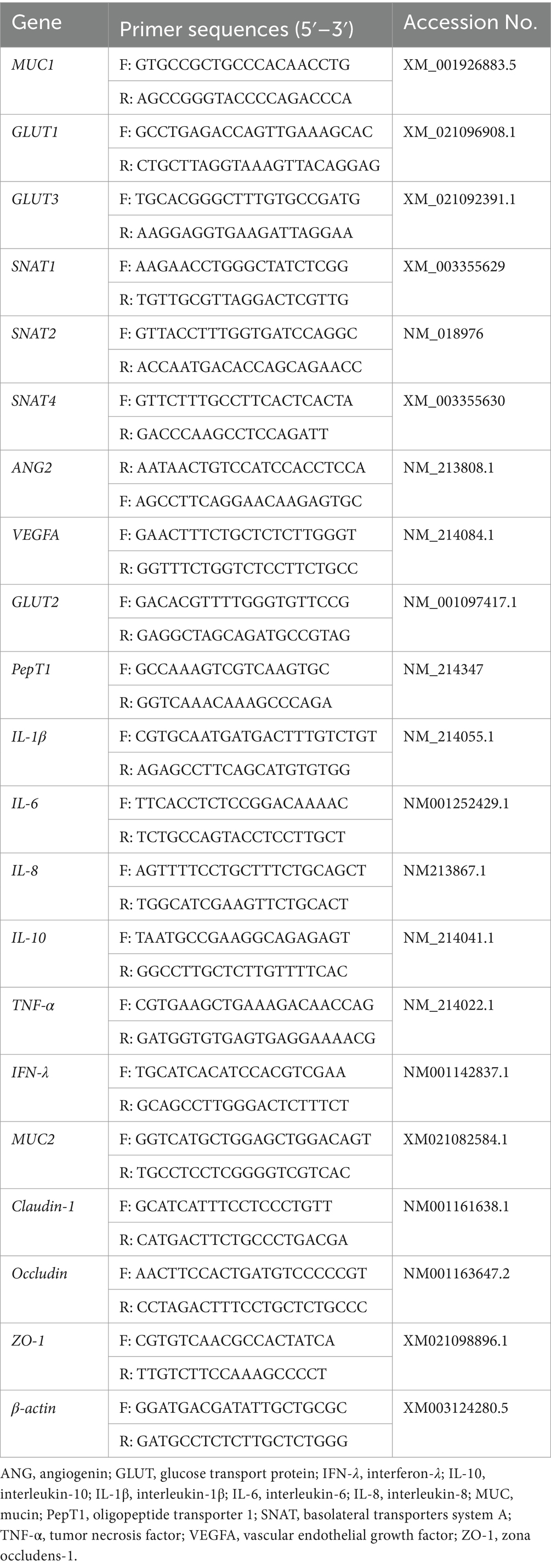
The expression of nutrient transport related genes (GLUT1, GLUT2, SNAT1, SNAT2, SNAT4, PepT1, ANG2, and VEGFA) in placental tissue, intestinal barrier related genes (MUC1, MUC2, claudin1, occludin, and ZO-1) in jejunal tissue, and inflammation related genes (IL-1β, IL-6, IL-8, IL-10, TNF-α and IFN-γ) in jejunal tissue were detected by q-PCR. The gene primer sequences were shown in Table 3. Tissue samples were lysed using TRIzol and homogenized with a homogenizer. Total RNA was extracted from jejunal mucosa using TRIzol reagent (TaKaRa Biotechnology Co, Ltd., Dalian, China) according to the manufacturer’s instructions. The concentration and purity of RNA was analysed spectrophotometrically (Beckman Coulter DU800; Beckman Coulter Inc.), taking into account the ideal absorbance ratio (1.8 A260/280 2.0). Reverse transcription was performed using the PrimeScript™ RT Reagent (TaKaRa, Japan) kit and stored at −20°C for testing. Reference TB Green™ Premix Ex Taq™ II (TaKaRa, Japan) instruction manual was used with QuantStudio5 (Applied Biosystem) detection system for real-time fluorescence quantitative PCR. The cycling conditions were as follows predenaturation at 95°C for 30 s and 40 cycles of denaturation at 95°C for 5 s, annealing for 30 s and extension at 60°C for 34 s.
2.10 Western blotting
The western blot (WB) method was used to detect the expression of claudin1 and occludin protein in jejunal tissue. Jejunal tissue samples were mechanically and ultrasonically crushed, centrifuged, and the supernatant was collected for determination of total protein concentration using the BCA protein assay (Beyotime, Beijing, China). Equal amounts of protein were separated on 10% SDSPAGE gels, and then electrically transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% skimmed milk powder for 1 h at room temperature. Subsequently, they were incubated with the different primary antibody [Abcam (Cambridge, MA, United States)] at 4 degrees for 12 h. Then the PVDF membranes were incubated with corresponding secondary antibodies [Santa Cruz Biotechnology (Santa Cruz, CA, United States)] for 1 h at room temperature, followed by testing the target protein bands using ECL Luminescence Detection Kit (Beyotime, Beijing, China). Image Lab 5.1 was used for exposure photography and strip grayscale value analysis. Antibody concentration of claudin1, occludin, β-actin are 1:1,000.
2.11 Statistical analysis
Statistical differences were analyzed by the independent samples t-test in SPSS 22.0 statistics software (Chicago, IL, United States). Independent sample t-tests were performed for pairwise comparisons between NBW, IUGR, and IUGR + COS. Firstly, the comparison between NBW and IUGR aimed to verify the successful establishment of the IUGR piglet injury model. Secondly, the analysis between IUGR and IUGR + COS was conducted to evaluate the improvement effect of COS supplementation on IUGR piglets. Lastly, the comparison between NBW and IUGR + COS assessed the remaining gap, if any, between IUGR piglets after COS supplementation and normal piglets. The results were expressed as mean ± standard error, with significance indicated by p < 0.05 and a trend indicated by 0.05 ≤ p < 0.10.
3 Results
3.1 Sows’ reproductive performance
The results of sows’ reproductive performance were shown in Table 4. The supplementation of COS in sow diet had no significant effect on the total born, born alive, mean BW and mean litter weight at birth, litter weight and average pig weight at weaning, as well as average daily gain of piglets during lactation (p > 0.05), but significantly reduced the number of stillbirths and mummies (p < 0.05). Furthermore, COS group exhibited a 35.22% reduction in the number of piglets weighing less than 1 kg compared to the control group. After supplementing with COS, the incidence of IUGR decreased by 8.57% compared to the control group.
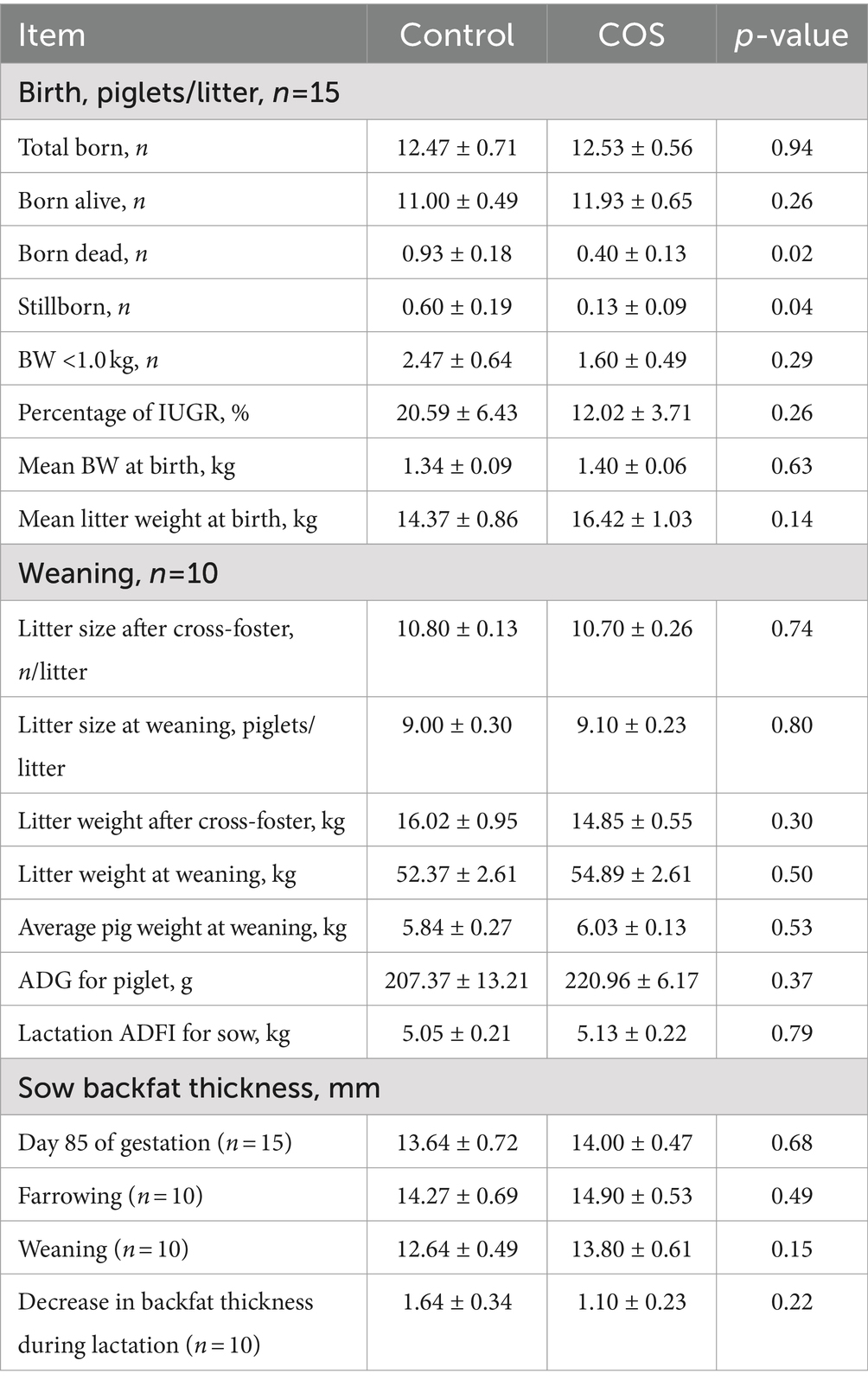
Table 4. Effects of maternal chitosan oligosaccharides supplementation during late gestation and lactation on the reproductive performance of sows.
3.2 Placenta function-related genes expression of sows
As shown in Figure 1, the mRNA expression of glucose transport related genes (GLUT1 and GLUT3), amino acid transport related genes (SNAT1 and SNAT4), and angiogenesis gene VEGFA in the placenta of IUGR piglets (from control sows) were significantly lower than those in the placenta of normal piglets (from control sows) (p < 0.05); However, the expression of GLUT1, GLUT3 and VEGFA mRNA in the placenta of IUGR piglets (from COS sows) was significantly higher than that in the placenta of IUGR piglets (from control sows) (p < 0.05). The expression of ANG2 mRNA in the placenta of IUGR piglets (from COS sows) was significantly lower than that in the placenta of IUGR piglets (from control sows) (p < 0.05).
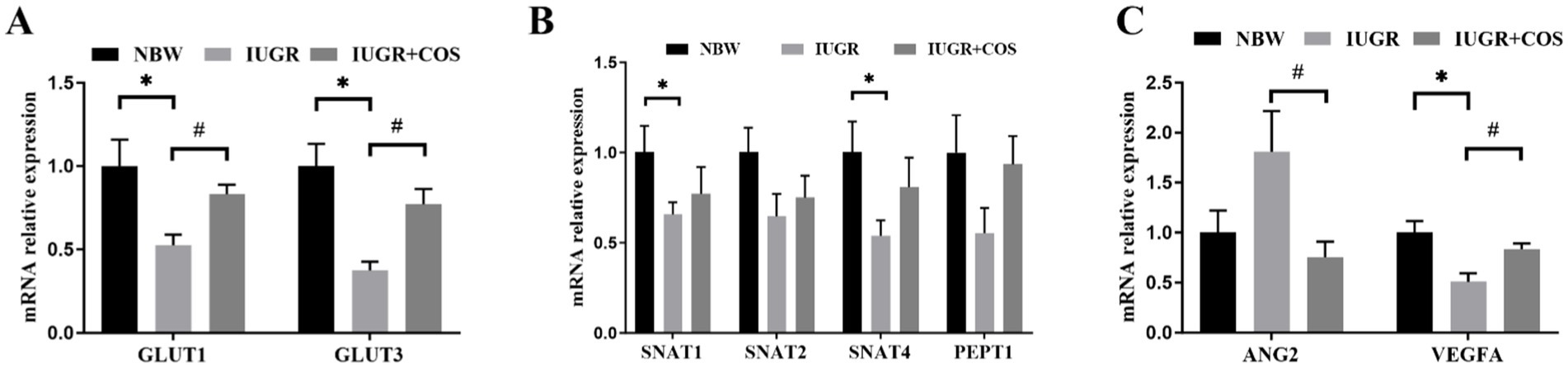
Figure 1. Effects of maternal chitosan oligosaccharides supplementation during late gestation on the placenta function-related genes expression of sows. (A) Glucose transporters: GLUT1 and GLUT3. (B) Amino acid transporters: SNAT1, SNAT2, SNAT4, and PepT1. (C) Angiogenesis: ANG2 and VEGFA. *Means significant difference between NBW vs. IUGR/IUGR + COS. #Means significant difference between IUGR vs. IUGR + COS, p < 0.05, n = 10.
3.3 Placental antioxidant status and cytokine of sows
As shown in Table 5, the levels of ATP, GSH, IL-10 and GSH/GSSG in the placenta of IUGR piglets (from control sows) were significantly lower than those in the placenta of normal body weight piglets (from control sows), whereas the concentrations of MDA and GSSG exhibited an opposite trend (p < 0.05). Furthermore, maternal dietary COS supplementation resulted in a significant decrease in placental MDA levels in IUGR piglets, while there was a significant increase in GSH levels (p < 0.05). Compared to normal sow placenta (NBW), the levels of ATP, GSH, GSH/GSSG, and IL-10 remained significantly lower (p < 0.05), whereas MDA was significantly higher (p < 0.05) after COS supplementation of sow diets (IUGR + COS).
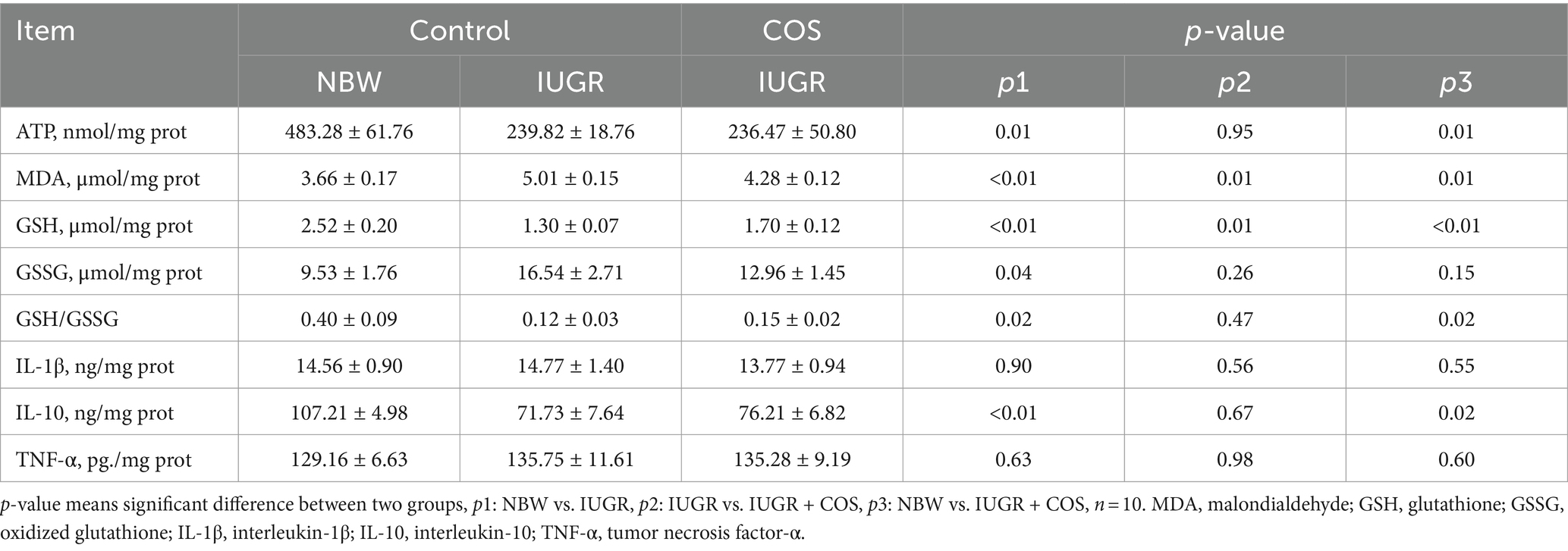
Table 5. Effects of maternal chitosan oligosaccharides supplementation during late gestation on the placenta oxidative parameters and cytokines levels of sows.
3.4 Jejunal digestive enzyme activities of 21-day-old suckling piglets
As shown in Figure 2, the activities of jejunal trypsin, lactase and maltase in 21-day-old IUGR piglets were significantly lower compared to those of normal body weight piglets (p < 0.05). Maternal dietary COS supplementation during late pregnancy and lactation significantly increased the activities of jejunal trypsin and lactase in 21-day-old IUGR piglets (p < 0.05), and significantly reduced sucrase activity (p < 0.05).
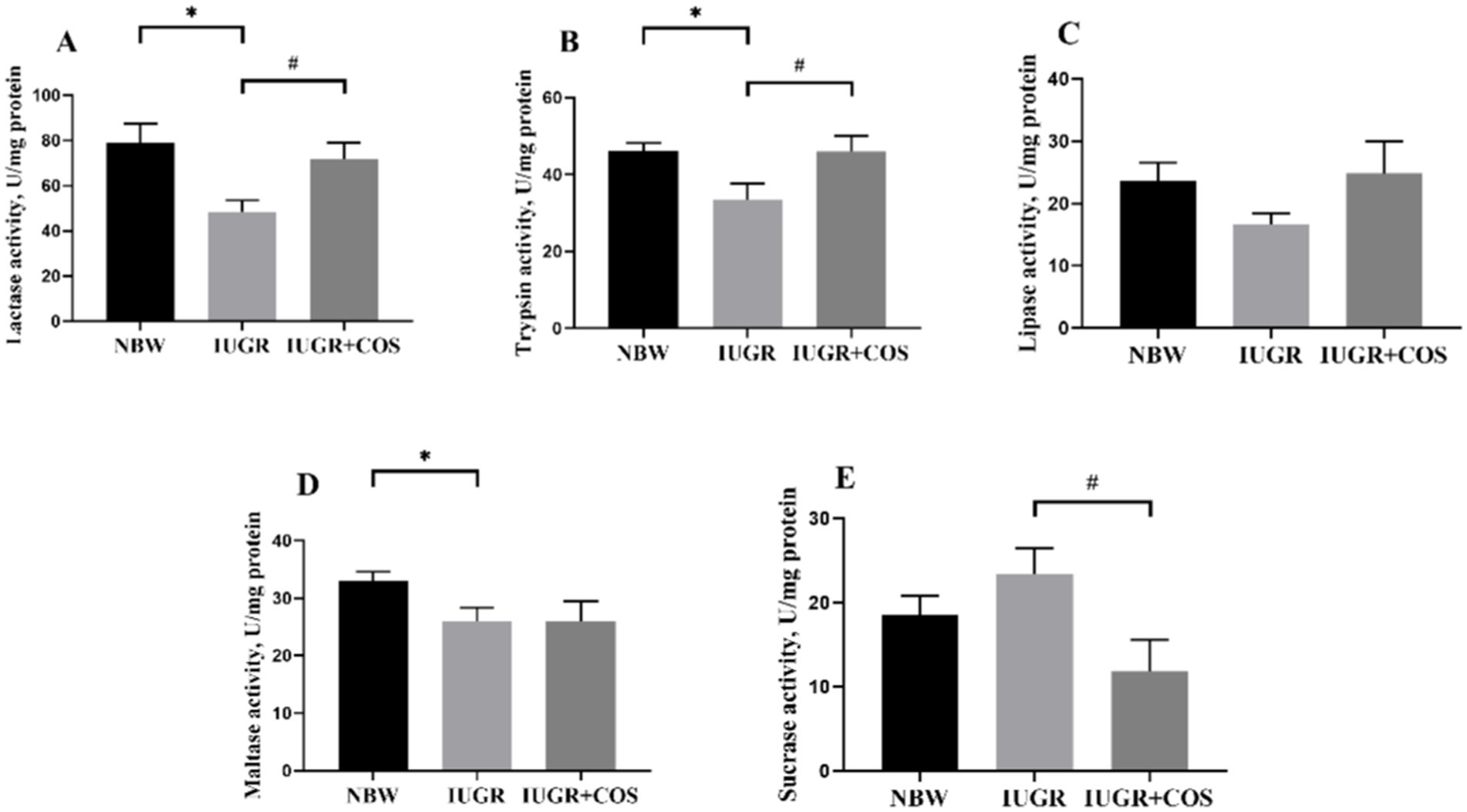
Figure 2. Effects of maternal chitosan oligosaccharides supplementation during late gestation and lactation on the jejunal digestive enzyme activities of 21-day-old suckling piglets. (A) lactase activity; (B) trypsin activity; (C) lipase activity; (D) maltase activity; (E) sucrase activity. *Means significant difference between NBW vs IUGR/IUGR + COS, #Means significant difference between IUGR vs IUGR + COS, p < 0.05, n = 8.
3.5 Jejunal barrier function of 21-day-old suckling piglets
As shown in Figure 3, compared with normal body weight piglets, the expression of MUC1, MUC2, claudin1 mRNA and claudin1 protein in the jejunum of 21-day-old IUGR piglets (from control sows) were significantly reduced (p < 0.05). Supplementing COS to the diet of sows during late gestation and lactation resulted in a significant increase in the expression of MUC2 and occludin mRNA as well as claudin1 protein in the jejunum of 21-day-old IUGR piglets (p < 0.05).

Figure 3. Effects of maternal chitosan oligosaccharides supplementation during late gestation and lactation on the jejunal barrier function of 21-day-old suckling piglets. (A–E) Jejunal barrier-related gene expression levels, n = 8. (F–H) Jejunal barrier-related protein expression levels, n = 4. *Means significant difference between NBW vs. IUGR/IUGR + COS. #Means significant difference between IUGR vs. IUGR + COS, p < 0.05.
3.6 Gene expression levels of cytokines in the jejunum of 21-day-old suckling piglets
As shown in Figure 4, the expression of IL-1β, IL-6, IL-8, and TNF-α mRNA in the jejunum of 21-day-old suckling IUGR piglets was significantly increased compared to normal body weight piglets (p < 0.05), while the expression of IL-10 mRNA was significantly reduced (p < 0.05). Supplementation of COS to the diet of sows during late pregnancy and lactation resulted in a significant increase in the expression of IL-10 mRNA in the jejunum of piglets (p < 0.05), with a tendency to decrease the expression of IL-6 (p = 0.06) and IL-8 (p = 0.08) mRNA compared to IUGR piglet from control sows.
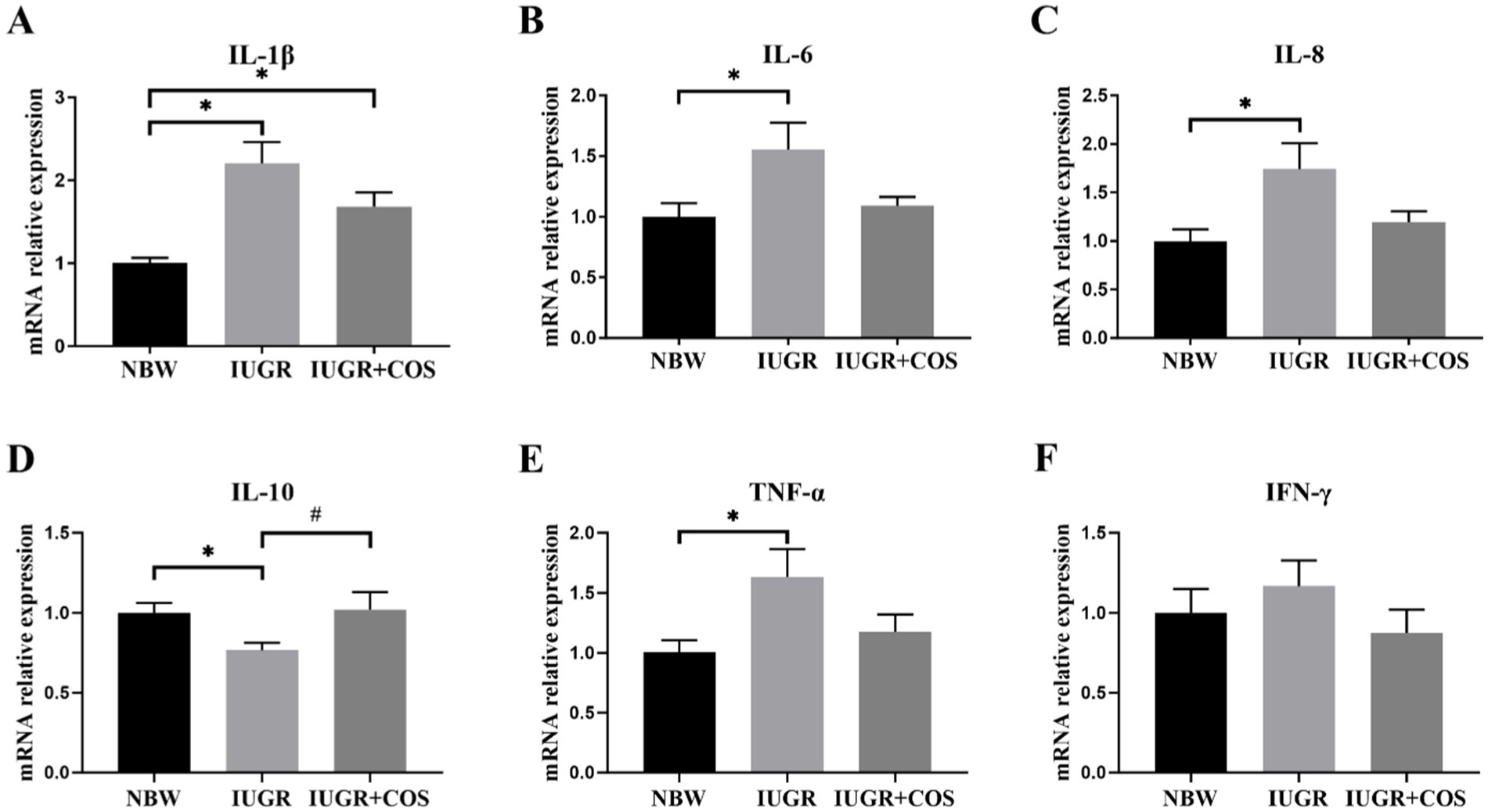
Figure 4. Effects of maternal chitosan oligosaccharides supplementation during late gestation and lactation on the jejunal cytokine gene expression levels of 21-day-old suckling piglets. (A) IL-1β (Interleukin-1β); (B) IL-6 (Interleukin-6); (C) IL-8 (Interleukin-8); (D) IL-10 (Interleukin-10); (E) TNF-α (Tumor necrosis factor-α); (F) IFN-γ (Interferon-γ). *Means significant difference between NBW vs IUGR or NBW vs IUGR + COS, #Means significant difference between IUGR vs IUGR + COS (P3), p < 0.05, n = 8.
3.7 Antioxidant status of jejunum in 21-day-old suckling piglets
The results of the antioxidant status of jejunum in suckling piglets were presented in Table 6. Compared to 21-day-old normal body weight piglets, IUGR piglets (from control sows) exhibited a significant increase in MDA and GSSG levels (p < 0.05), a significant reduction in the GSH/GSSG ratio (p < 0.05), and a tendency to increase CAT level (p = 0.06). The addition of COS to the diet of sows during late pregnancy and lactation significantly reduced the MDA content in the jejunum of IUGR piglets (p < 0.05). However, GSSG remained significantly elevated (p < 0.05) and GSH/GSSG was significantly reduced (p < 0.05) after COS supplementation of the sows’ diets (IUGR + COS), compared with 21-day-old normal body weight piglets.
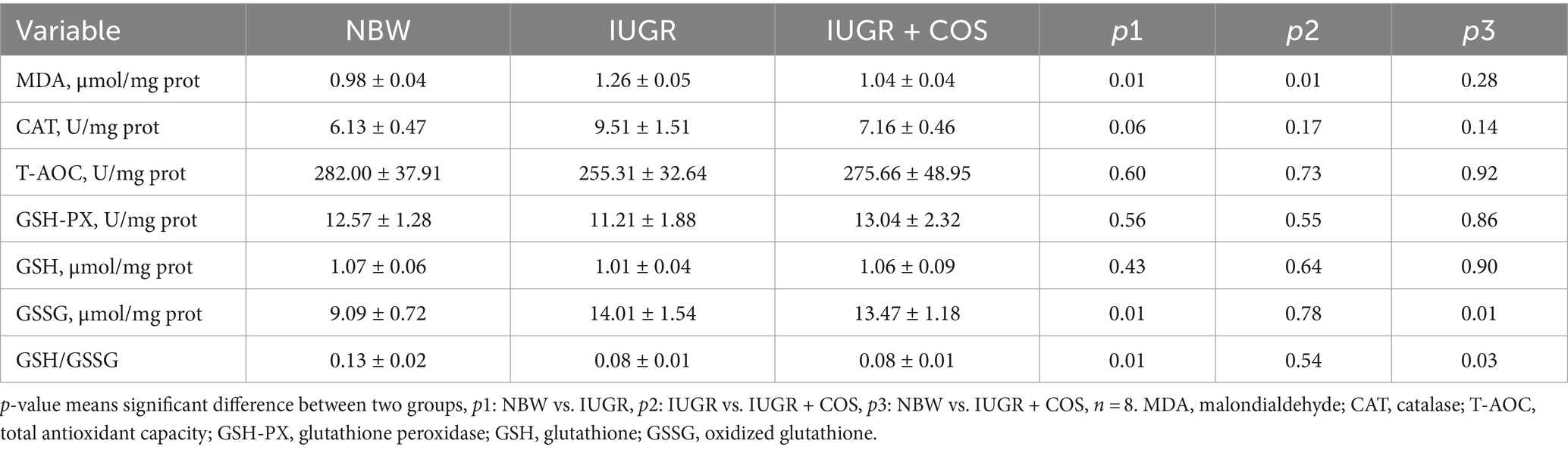
Table 6. Effects of maternal chitosan oligosaccharides supplementation during late gestation and lactation on the antioxidant status of jejunum in 21-day-old suckling piglets.
4 Discussion
The main purpose of this study is to investigate the effect of supplementing COS to the diet of sows during late gestation and lactation on placental function, as well as its effect on intestinal function of IUGR piglets after 21 days of suckling. Previous research has utilized various methods, such as direct nutrient supplementation to piglets post-birth, in order to improve the growth performance and functions of IUGR (19, 20). From another perspective, supplementing nutrients during late gestation and lactation in sows has been demonstrated to improve the reproductive performance of sows and the growth performance of piglets (21, 22). In previous studies, maternal dietary COS supplementation during late gestation and lactation has been shown to significantly increase the average daily gain and weaning weight of piglets, but had no significant effect on the birth weight (16). In addition, it can also improve the born alive (14). Other studies have shown that the supplementation of COS can increase the total number of newborn piglets and live piglets, as well as significantly improve the average live litter weight, however, it has no significant effect on the average daily gain and average weaning weight (18). In this experiment, we found that dietary COS supplementation in sows only reduced the occurrence of stillbirths and mummies, without significant effects on other productive parameters. The results of this study were partially inconsistent with previous findings, possibly due to individual variations among sows and environmental conditions. Additionally, differences in sow management and nutritional control during gestation may also influence reproductive performance. Nevertheless, it is worth noting that our study found a 35.22% reduction in piglets with a birth weight less than 1 kg in the COS group compared to the control group, indicating potential alleviation of IUGR occurrence through maternal COS supplementation during late gestation.
The placenta serves as a crucial interface between the maternal and fetal compartments. It plays an essential role in ensuring the successful progression of pregnancy and the optimal growth and development of the fetus. During gestation, the placenta is responsible for transporting nutrients. Dysregulated nutrient transport can result in intrauterine growth restriction (23). Our Findings indicating impaired placental nutrient transport as a contributing factor to IUGR, COS supplementation during late gestation effectively ameliorated decreased nutrient transport function following placental injury. Previous studies have emphasized the importance of adequate vascularization within the placenta for normal fetal development (24), however, IUGR placentas exhibited significantly poorer vascular development compared to normal placentas (8). In this study, it was found that impaired vascular growth in sow placenta appeared to be another factor contributing to the emergence of IUGR. However, this impaired state could be restored to a certain extent after COS supplementation. It is important to note that placental nutrient transport and vascular development are interdependent, compromised vasculature limits exchange capacity in IUGR placentae (23). The above results indicated that supplementing sows feed with COS during late gestation effectively enhanced placental nutrient transport capacity, improved angiogenesis within the placenta, and reduced the occurrence of IUGR piglets.
Due to the close connection between the mother and the fetus, facilitated by the placenta as a conduit, maternal well-being significantly influences fetal development. In late pregnancy in sows, activities such as fetal growth, mammary gland development, and milk production resulted in elevated levels of reactive oxygen species (ROS), leading to oxidative stress in sows (25). Maternal oxidative stress is associated with an increased risk of IUGR (25). The placenta, an extremely important organ in this process, is highly sensitive to oxidative stress (26). Our study indicating pronounced oxidation in IUGR sows’ placentas and confirming the link between IUGR and oxidative stress. Following COS treatment, effective alleviation of placental oxidative stress. Furthermore, there are reports suggesting that immune cells undergo functional alterations during late gestation, resulting in increased release of inflammatory cytokines which can affect the maternal-fetal health. In this study, we found the same situation in the placenta. This increase in pro-inflammatory cytokines coupled with a decrease in anti-inflammatory cytokines reflects immune damage and inflammation within sow placentas during pregnancy, potentially contributing to adverse outcomes such as IUGR (27). However, following COS supplementation, the inflammatory response was alleviated, to some extent compared to IUGR. Therefore, supplementing COS can effectively alleviate oxidative stress and inflammatory response in the placenta, ultimately reducing the emergence of IUGR piglets.
After parturition, the primary connection between the neonate and the dam is through colostrum and milk, which plays an essential part in neonatal intestinal health. Piglets primarily acquire immunoglobulins by sucking colostrum postnatally, however, IUGR piglets may experience inadequate sucking or exposure to foodborne pathogens due to developmental impairment, resulting in further intestinal damage (6). In this study, changes in jejunal digestive enzyme activity in piglets reflected the addition of COS can effectively improve digestive and absorption function in piglets’ intestines.
The intestinal health of piglets can impact their overall growth, and inadequate development in the mother’s body and insufficient acquisition of colostrum and milk during lactation can have a detrimental effect on the intestinal health of IUGR piglets. This study observed impaired gut barrier, inflammatory response, and oxidative stress in IUGR piglets. Supplementing COS to the diet of sows during lactation can significantly ameliorates intestinal damage in IUGR piglets. Additionally, maternal COS also alleviated the inflammatory response in IUGR suckling piglets. We found that lipid peroxidation occurred in IUGR piglets’ bodies and a change in cellular redox state. However, supplementation of COS to lactating sow diets can inhibit lipid peroxidation. Previous studies have confirmed that supplementing COS to piglet feed can significantly improve the antioxidant defense system function of weaned piglets, protect them from oxidative stress, and improve their immune status (9). In this study, by supplementing COS to lactating sows feed resulted in the transmission of these effect to the piglets, possibly due to some antioxidant and anti-inflammatory components present in mother’s milk, thereby enhancing piglet antioxidant and immune status.
This study primarily focuses on improving IUGR in piglets by supplementing sows with COS through the placenta. By enhancing placental angiogenesis and nutrient transport, we aim to promote healthier fetal development and reduce IUGR incidence. Although this study did not examine the composition and changes of colostrum and regular milk, COS supplementation in sows may help alleviate intestinal damage in IUGR piglets via breast milk. However, research on COS’s impact on milk is limited, and it remains uncertain whether its anti-inflammatory and antioxidant properties extend to milk.
Furthermore, the role of microorganisms cannot be overlooked. After birth, close contact between piglets and their mothers, particularly their initial exposure to sow feces, facilitates the colonization of microorganisms in the piglets’ intestines (28). Consequently, the sow’s diet can also influence the microbial composition of piglets (29). Studies indicate that COS supplementation positively affects host intestinal health and gut microbiota (30). We hypothesize that by improving the sow’s gut microbiota through COS supplementation, beneficial microorganisms can be transmitted to piglets upon contact, thereby enhancing their intestinal health and mitigating the effects of IUGR.
5 Conclusion
In this study, the results demonstrated that supplementing COS in the late gestation of sows can improve reproductive performance, and placental, alleviate oxidative stress in the placenta, and consequently reduce the incidence of IUGR piglets. Continuing to supplement COS during lactation can improve the intestinal barrier function of 21-day-old suckling IUGR piglets, alleviate inflammatory reactions and oxidative stress, thus effectively alleviate the adverse effects of IUGR piglets. In conclusion, supplementing sow feed with COS during late gestation and lactation can effectively reduce the incidence of IUGR piglets and improve their health. This experiment also provided nutritional strategies for pregnant and lactating women to enhance infant health.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of Sichuan Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XW: Writing – original draft, Writing – review & editing. TF: Investigation, Software, Writing – review & editing. DC: Supervision, Writing – review & editing. JP: Formal analysis, Writing – review & editing. GT: Writing – review & editing. JH: Writing – review & editing. PZ: Writing – review & editing. XM: Writing – review & editing. AW: Writing – review & editing. BY: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 31372324) and the Key Research and Development Program of Sichuan Province (2021YFYZ0008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADFI, Average daily feed intake; ADG, Average daily weight gain; ANG, Angiogenin; CAT, Catalase; COS, Chitosan oligosaccharides; DD, Degree of deacetylation; GLUT, Glucose transport protein; GSH, Glutathione; GSH-PX, Glutathione peroxidase; GSSG, Oxidized glutathione; IFN-γ, Interferon-γ; IL-10, Interleukin-10; IL-1β, Interleukin-1β; IL-6, Interleukin-6; IL-8, Interleukin-8; IUGR, Intrauterine growth inhibition; MDA, Malondialdehyde; MUC, Mucin; NBW, Normal body weight; PepT1, Oligopeptide transporter 1; SNAT, Basolateral transporters system A; T-AOC, Total antioxidant capacity; TNF-α, Tumor necrosis factor; VEGFA, Vascular endothelial growth factor; ZO-1, Zona occludens-1.
References
1. Wu, G, Bazer, FW, Wallace, JM, and Spencer, TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. (2006) 84:2316–37. doi: 10.2527/jas.2006-156
2. Sharma, D, Sharma, P, and Shastri, S. Genetic, metabolic and endocrine aspect of intrauterine growth restriction: an update. J Matern Fetal Neonatal Med. (2017) 30:2263–75. doi: 10.1080/14767058.2016.1245285
3. Royston, JP, Flecknell, PA, and Wootton, R. New evidence that the intra-uterine growth-retarded piglet is a member of a discrete subpopulation. Biol Neonate. (1982) 42:100–4. doi: 10.1159/000241582
4. Cui, C, Wu, C, Wang, J, Zheng, X, Ma, Z, Zhu, P, et al. Leucine supplementation during late gestation globally alters placental metabolism and nutrient transport via modulation of the PI3K/AKT/mTOR signaling pathway in sows. Food Funct. (2022) 13:2083–97. doi: 10.1039/D1FO04082K
5. Garite, TJ, Clark, R, and Thorp, JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. (2004) 191:481–7. doi: 10.1016/j.ajog.2004.01.036
6. Wang, J, Chen, L, Li, D, Yin, Y, Wang, X, Li, P, et al. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr. (2008) 138:60–6. doi: 10.1093/jn/138.1.60
7. Pieszka, M, Szczurek, P, Orczewska-Dudek, S, Kamyczek, M, and Pieszka, M. Determining the effect of pancreatic-like enzymes (PLEMs) added to the feed of pregnant sows on fetal size of piglets to minimize IUGR syndrome caused by fetal malnutrition. Animals. (2023) 13:3448. doi: 10.3390/ani13223448
8. Wu, Z, Nie, J, Wu, D, Huang, S, Chen, J, Liang, H, et al. Dietary adenosine supplementation improves placental angiogenesis in IUGR piglets by up-regulating adenosine A2a receptor. Anim Nutr. (2023) 13:282–8. doi: 10.1016/j.aninu.2023.02.003
9. Wan, J, Jiang, F, Xu, Q, Chen, D, Yu, B, Huang, Z, et al. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. (2017) 7:9669–79. doi: 10.1039/C7RA00142H
10. Yang, JW, Tian, G, Chen, DW, Yao, Y, He, J, Zheng, P, et al. Involvement of PKA signalling in anti-inflammatory effects of chitosan oligosaccharides in IPEC-J2 porcine epithelial cells. J Anim Physiol Anim Nutr. (2018) 102:252–9. doi: 10.1111/jpn.12686
11. Marangon, CA, Martins, VCA, Ling, MH, Melo, CC, Plepis, AMG, Meyer, RL, et al. Combination of rhamnolipid and chitosan in nanoparticles boosts their antimicrobial efficacy. ACS Appl Mater Interfaces. (2020) 12:5488–99. doi: 10.1021/acsami.9b19253
12. Abd El-Hack, ME, El-Saadony, MT, Shafi, ME, Zabermawi, NM, Arif, M, Batiha, GE, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int J Biol Macromol. (2020) 164:2726–44. doi: 10.1016/j.ijbiomac.2020.08.153
13. Yu, D, Feng, J, You, H, Zhou, S, Bai, Y, He, J, et al. The microstructure, antibacterial and antitumor activities of chitosan oligosaccharides and derivatives. Mar Drugs. (2022) 20:69. doi: 10.3390/md20010069
14. Wan, J, Yang, K, Xu, Q, Chen, D, Yu, B, Luo, Y, et al. Dietary chitosan oligosaccharide supplementation improves foetal survival and reproductive performance in multiparous sows. RSC Adv. (2016) 6:70715–22. doi: 10.1039/C6RA13294D
15. Duan, X, Tian, G, Chen, D, Yang, J, Zhang, L, Li, B, et al. Effects of diet chitosan oligosaccharide on performance and immune response of sows and their offspring. Livest Sci. (2020) 239:104114. doi: 10.1016/j.livsci.2020.104114
16. Wan, J, Xu, Q, and He, J. Maternal chitosan oligosaccharide supplementation during late gestation and lactation affects offspring growth. Ital J Anim Sci. (2018) 17:994–1000. doi: 10.1080/1828051X.2018.1435313
17. Xie, C, Wu, X, Long, C, Wang, Q, Fan, Z, Li, S, et al. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet Res. (2016) 12:243. doi: 10.1186/s12917-016-0872-8
18. Cheng, LK, Wang, LX, Xu, QS, Huang, LJ, Zhou, DS, Li, Z, et al. Chitooligosaccharide supplementation improves the reproductive performance and milk composition of sows. Livest Sci. (2015) 174:74–81. doi: 10.1016/j.livsci.2015.02.003
19. Niu, Y, He, J, Zhao, Y, Shen, M, Zhang, L, Zhong, X, et al. Effect of curcumin on growth performance, inflammation, insulin level, and lipid metabolism in weaned piglets with IUGR. Animals. (2019) 9:1098. doi: 10.3390/ani9121098
20. Engelsmann, MN, Hansen, CF, Nielsen, MN, Kristensen, AR, and Amdi, C. Glucose Injections at birth, warmth and placing at a nurse sow improve the growth of IUGR piglets. Animals. (2019) 9:519. doi: 10.3390/ani9080519
21. Li, L, Wang, H, Dong, S, and Ma, Y. Supplementation with alpha-glycerol monolaurate during late gestation and lactation enhances sow performance, ameliorates milk composition, and improves growth of suckling piglets. J Anim Sci Biotechnol. (2023) 14:47. doi: 10.1186/s40104-023-00848-x
22. Zhao, Y, Wang, Q, Zhou, P, Li, Z, Zhong, W, Zhuo, Y, et al. Effects of yeast culture supplementation from late gestation to weaning on performance of lactating sows and growth of nursing piglets. Animal. (2022) 16:100526. doi: 10.1016/j.animal.2022.100526
23. Sun, C, Groom, KM, Oyston, C, Chamley, LW, Clark, AR, and James, JL. The placenta in fetal growth restriction: what is going wrong? Placenta. (2020) 96:10–8. doi: 10.1016/j.placenta.2020.05.003
24. Gualdoni, GS, Jacobo, PV, Barril, C, Ventureira, MR, and Cebral, E. Early abnormal placentation and evidence of vascular endothelial growth factor system dysregulation at the feto-maternal interface after periconceptional alcohol consumption. Front Physiol. (2021) 12:815760. doi: 10.3389/fphys.2021.815760
25. Pereira, AC, and Martel, F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol. (2014) 30:301–12. doi: 10.1007/s10565-014-9285-2
26. Al-Gubory, KH, Fowler, PA, and Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. (2010) 42:1634–50. doi: 10.1016/j.biocel.2010.06.001
27. Liu, N, Chen, J, He, Y, Jia, H, Jiang, D, Li, S, et al. Effects of maternal L-proline supplementation on inflammatory cytokines at the placenta and fetus interface of mice. Amino Acids. (2020) 52:587–96. doi: 10.1007/s00726-020-02837-0
28. Boulbria, G, Teixeira-Costa, C, Chevance, C, Grandin, R, Jeusselin, J, Berton, P, et al. Microbial content of non-fermented liquid feed consumed by sows affects the occurrence of neonatal diarrhoea in their piglets: a case-control study. Vet Rec. (2022) 190:e837. doi: 10.1002/vetr.837
29. Dai, F, Lin, T, Jin, M, Huang, X, Wang, L, Ma, J, et al. Bamboo fiber improves piglet growth performance by regulating the microbial composition of lactating sows and their offspring piglets. Front Microbiol. (2024) 15:1411252. doi: 10.3389/fmicb.2024.1411252
Keywords: chitosan oligosaccharide, intrauterine growth retardation, sows, piglets, placenta, intestine
Citation: Wang X, Fang T, Chen D, Pu J, Tian G, He J, Zheng P, Mao X, Wu A and Yu B (2024) Maternal chitosan oligosaccharide supplementation during late gestation and lactation optimizes placental function in sows and intestinal function in 21-day-old IUGR suckling piglets. Front. Vet. Sci. 11:1463707. doi: 10.3389/fvets.2024.1463707
Edited by:
Moyosore Joseph Adegbeye, University of Africa, NigeriaReviewed by:
Yu Pi, Chinese Academy of Agricultural Sciences, ChinaYang Li, Shandong Agricultural University, China
Copyright © 2024 Wang, Fang, Chen, Pu, Tian, He, Zheng, Mao, Wu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Yu, eWJpbmd0aWFuQDE2My5jb20=
†These authors have contributed equally to this work
 Xintao Wang1,2†
Xintao Wang1,2† Daiwen Chen
Daiwen Chen Gang Tian
Gang Tian Jun He
Jun He Ping Zheng
Ping Zheng Xiangbing Mao
Xiangbing Mao Aimin Wu
Aimin Wu Bing Yu
Bing Yu