
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Vet. Sci. , 28 January 2025
Sec. Veterinary Humanities and Social Sciences
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1456605
Background: To promote gender mainstreaming in future AMR research projects and policy implementation within livestock and other systems, researchers need to embrace gender-responsive research methodologies. Ignoring gender considerations can lead to unsustainable interventions and exacerbate existing equity gaps. Incorporating gender analysis is crucial for identifying data collection needs and opportunities to develop gender-responsive research programs and policies.
Objectives: We have developed a conceptual framework and a set of research questions designed to enhance the gender-responsiveness of AMR research in livestock systems.
Methods: A narrative review previously identified three key entry points for gender dynamic impacting AMR in agricultural systems: gendered antimicrobial resistance exposure, gendered antimicrobial use and gendered outcomes of antimicrobial resistance infections. This information was then analyzed using a health system gender framework. Combining these insights, we developed a comprehensive list of research questions.
Results: We developed comprehensive list of gender-related questions. Given the limited understanding of how gender dynamics and norms influence AMR, we have primarily proposed qualitative, exploratory questions. These questions are categorized into two types: integrated and strategic. Integrated questions offer a deeper understanding of gender dynamics and norms in livestock systems with the aim of improving them, while strategic questions focus on gender-related issues in livestock as entry points, highlighting some of the mechanisms behind these gender issues to progress towards gender equality.
Conclusion: As gender-analysis in livestock research gains prominence, there is an increasing expectation for AMR researchers to integrate gender considerations into their work. This framework provides a starting point for researchers aiming to enhance gender inclusivity and considerations in AMR research within livestock systems. The next phase of our project will involve applying this framework in the field, where a real-life application will enable its validation and further refinement.
Antimicrobial resistance (AMR) is a growing public health threat, which like most public health issues, is inequitably distributed across countries, populations, and economic regions (1–4), and is influenced by social, economic, and cultural factors, including gender dynamics and norms (5). A recent study estimated that in 2019, bacterial AMR contributed to almost 5 million deaths (6) and in 2020, the World Health Organization (WHO) estimated that AMR has caused at least one-third as many deaths as COVID-19 (7).
In livestock systems, gender norms are known to result in inequitable impacts (8). We define gender norms as the spoken and unspoken rules that establish what is appropriate for a woman or a man (based also on their other individual characteristics such as age, ethnicity, and religion) to do, believe, say, access, own, benefit, and claim in various livestock systems and contexts (9). Gender norms shape gender dynamics, which in turn influence the intra-household division of labor, access to assets and decision-making, among other impacts. These arrangements may influence how different household members are exposed to pathogens. Gender dynamics shape: (1) who does what in the household livestock management affecting, for example, the extent to which people are exposed to resistant pathogens; (2) who has decision-making power in livestock-related activities influencing, for example, who decides to utilize antimicrobials or alternative remedies, with women often less able to purchase antibiotics and vaccines for animals they own or manage (10); (3) who can use and access resources [affecting for example, who has money to purchase antimicrobials or who accesses information about available alternatives and support services such as veterinarians (9, 11, 12)]; and (4) who can take advantage of opportunities (for example, attend a training session on AMR) or access new technologies (e.g., genetically modified stock) which might reduce antibiotic use. These differences in roles and opportunities ultimately shape the ways in which resistant infections impact the lives of women and men (13) (Figure 1).
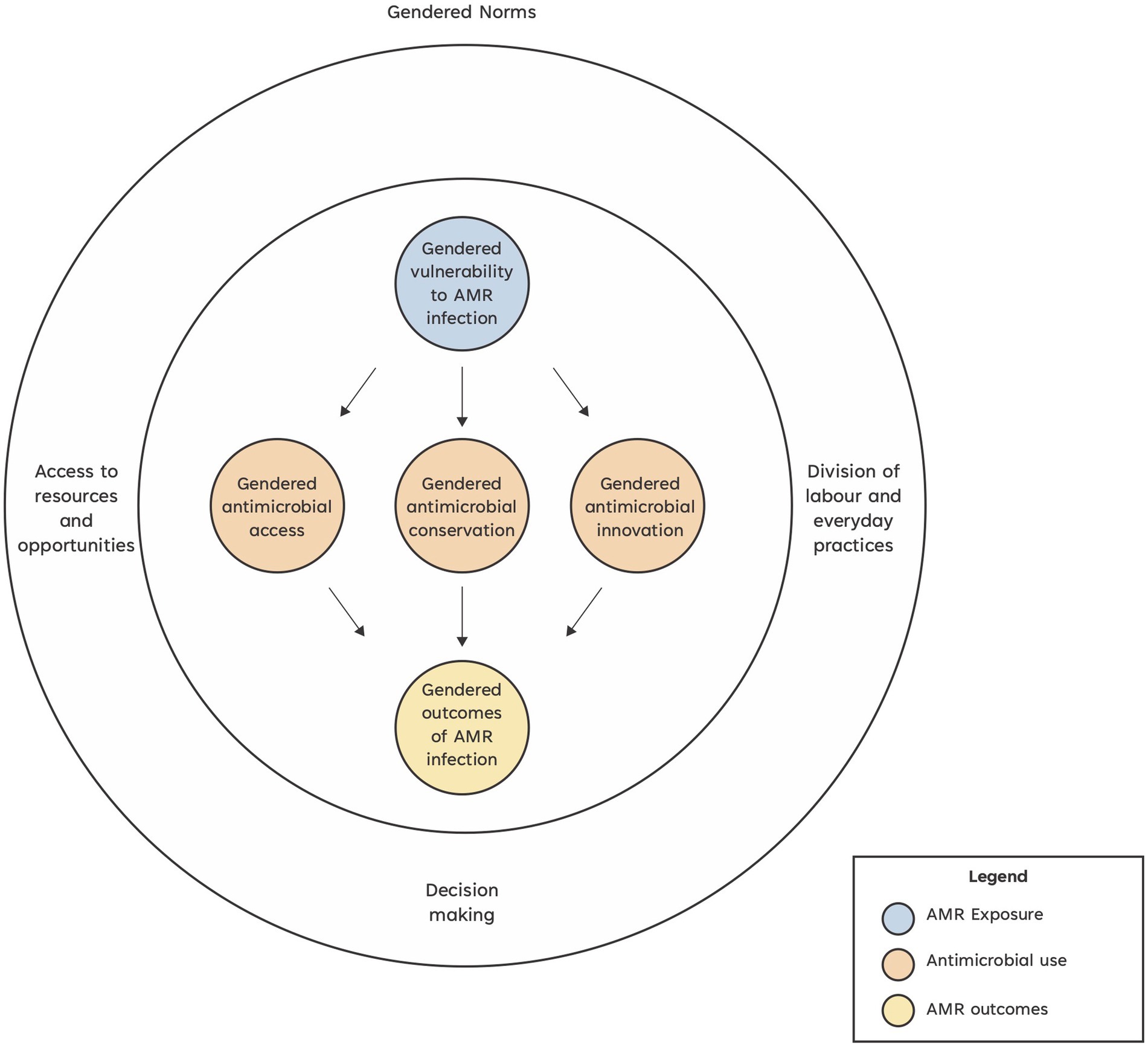
Figure 1. Conceptual framework of how gender issues might impact antimicrobial resistance in livestock systems.
In addition to shaping who is impacted by AMR, and how they experience these impacts, gender dynamics and norms also impact the effectiveness of interventions to reduce AMR. To reach the appropriate household member these interventions need to incorporate a gender lens and be tailored to the activities and roles they undertake, and to the needs and opportunities they may have regarding access to animal health services, medicines, and information. Not only will gender-responsive AMR research reduce AMR impacts, but it will also make interventions more effective, ultimately benefiting whole communities. Economic analyses have shown that empowering women producers can bring economic benefits to households and communities; with increased control over agricultural income resulting in higher spending on food and education (14).
To build gender-responsive AMR research programs and policies that address underlying power structures and norms that may disadvantage some social groups (often women and girls)—and progress towards equitable AMR interventions, more evidence is needed to identify the research gaps related to gender issues and AMR in livestock systems (15). Available data is rarely disaggregated by gender (16) thereby overlooking quantitative assessments of existing gender-based disadvantage and of gendered needs and opportunities. There has also been a limited focus on gender dynamics that lead to disadvantages related to AMR, particularly outside the human health context; few National Action Plans on AMR include gender-related provisions or consider gender as a driver of inequity in AMR-related outcomes. Recently, gender has been identified as a research priority in the One Health Priority Research Agenda on AMR which calls for better consideration of gender as a priority, cross-cutting challenge with the potential to impact all other research priorities (17).
To address this evidence gap around AMR and gender dynamics in livestock systems, we have created a conceptual framework and list of research questions which can be used to improve the gender-responsiveness of AMR research in livestock systems. We previously completed a narrative review which identified three key entry points for gender dynamic impacts on AMR in agricultural systems: gendered antimicrobial resistance exposure, gendered antimicrobial use, and gendered outcomes of antimicrobial resistance infections (18). We then applied Morgan et al.’s health system analysis gender framework (8) to the information derived from this narrative review to develop a novel conceptual framework exploring the role of gender and the impact of gender dynamics on AMR in livestock systems. Morgan et al. argue that gender as a driver of inequity in health systems can be understood by how power is constituted and negotiated (8). They assert that gender power relations can be understood by asking who has what (access to resources and access to opportunities); who does what (the division of labor and everyday practices); who decides (decision-making) and how values are defined (social and gender norms, ideologies, beliefs, and perceptions). Since gender and social norms ultimately affect access to resources and opportunities, division of labor and everyday practices, we incorporated this gender lens as an overarching element of our Conceptual framework of how gender issues might impact AMR in livestock systems (Figure 1). We define gender analysis as an analysis of the gender identities and power dynamics that affect the ways in which women and men are positioned in society and the distribution of resources, opportunities, constraints, and power in each context. Such analysis needs to consider how other individual characteristics (e.g., age, ethnicity, caste, religion, marital status etc.) affect the commonalities and differences between and among women and men. It also needs to appreciate the systemic and relational, as well as the individual dimensions at play.
We defined AMR exposure as exposure to antimicrobial resistant pathogens during livestock production; antimicrobial use as access to antimicrobials, use of antimicrobial alternatives such as vaccines and adoption of innovations like novel antimicrobials or technologies; and AMR outcomes as the consequences of AMR infections within livestock systems including productivity, economic, health, and nutritional impacts.
For each of the three key gender entry points in livestock systems in our conceptual framework (AMR exposure, antimicrobial use (AMU) and AMR outcomes) we developed a list of integrated and strategic research questions. Integrated questions are those with a bioscience entry-point (19). They allow a deep understanding of gender dynamics and norms in livestock systems with the goal of improving the gender-responsiveness of bioscience questions and interventions. An example of an integrated question would be “who is authorized to use antimicrobials in livestock production?” On the other hand, strategic questions are those that have a gender-related question as entry point with the goal of progressing towards gender equity in livestock systems. An example of a strategic question would be “which livestock producers are willing but unable to use antibiotics, and what are the barriers that need to be overcome?” Strategic questions are key to identify gendered aspirations which in turn are necessary to ensure interventions do not just reproduce current (inequitable) gendered roles - thereby reproducing gender-based disadvantage - but rather, leverage interventions to move towards more gender equitable arrangements. Our research questions are the first step to conceptualizing how gender dynamics and norms impact livestock systems and to identify potential entry points to address gender issues and AMR related outcomes in these systems.
The influence of gender on AMR exposure (20, 21), patterns of antimicrobial use (22, 23), and outcomes from AMR infections (24) is well-documented in human health. These three aspects of AMR – exposure, antimicrobial use, and outcomes—can also be gendered in the context of livestock systems. These dimensions are also interlinked: not only do gender inequities drive gendered AMR exposure, antimicrobial use, and AMR policy outcomes, but gendered AMR exposures can also result in gendered antimicrobial use which in turn may drive gendered AMR policy outcomes. As such, these three aspects should be key considerations in any research conducted in this domain.
Gendered AMR exposure refers to inequitable and unequal risk faced by people of different genders to antimicrobial resistant pathogens in livestock production, which arise directly through contact with animals and their waste, or indirectly, through activities required for production such as disinfection of farm equipment or the farm environment, preparing forage and feeds. Gender norms that influence labor division and daily practices can influence such exposure to resistant pathogens. For example, in Cambodia, women and children, typically responsible for managing animal manure and slaughter products, exhibit higher rates of AMR enteric pathogens (25).
In livestock systems, antimicrobial use is influenced by several factors including access to antimicrobials (which we have called “access”), use of antimicrobial alternatives such as vaccines (which we refer to as “conservation”), and adoption of innovations like novel antimicrobials or technologies that decrease the need for antimicrobials or reduce disease incidence (which we have termed “innovation”) (26). Access to antimicrobials, alternatives, and innovations (AAIs) is greatly shaped by gender dynamics and norms, which vary across different local contexts. It is dependent, for example, on a person’s ability to access information on available AAIs, and to then obtain AAIs from animal health service providers or drug sellers. Gender norms and dynamics strongly affect who accesses information and public spaces (such as agrovets). Gender norms can obstruct access to agricultural extension officers, who provide advice on disease treatment and antimicrobial use. For example, women farmers in Ghana were less likely to seek advisory services since most extension officers are men and socio-cultural traditions often prevent them from meeting with them on their own (27, 28). AAIs need to be affordable, also, to be obtained. Affordability is influenced by gender dynamics and norms around who in the household controls income (10). For instance, women farmers have reported having limited authority over financial decisions regarding the animals they manage (29) which may restrict their ability to purchase antimicrobials. A person’s ability to utilize the obtained AAIs to treat animal diseases is influenced, in turn, by gender dynamics around, for example, who takes decisions on livestock and who oversees treating the livestock.
Antimicrobial conservation may be impacted, also by gendered differences in education or training on prophylactic health products or antimicrobial alternatives like vaccines or prebiotics in animal production (30, 31). For example, among pig and chicken farmers in Cambodia, men were more likely to be aware of AMR and demonstrated more appropriate use of antimicrobials (32). Gendered use of novel antimicrobials or antimicrobial reducing innovations and technologies (e.g., improved genetic stock) is also well documented (33, 34).
The consequences of AMR infections within livestock systems may be experienced differently based on gender; these AMR outcomes can affect productivity, economic stability, health, and nutrition in different ways. Studies indicate that in Sub-Saharan Africa, diseases increase the caregiving responsibilities of women, leading to greater household vulnerability to food insecurity. For example, a study in Tanzania showed that a woman spent 45% less time on farming activities when their husbands were ill (35). Additionally, it has been reported that in cases where households experienced the death of a male head, women lost their livestock assets to other relatives in 33% of the instances, further highlighting the gendered impacts of AMR on socio-economic outcomes (36). Health emergencies and disease outbreaks, which may be further exacerbated by AMR, can affect genders differently through nutrition or economic impacts. For example, women producers are more likely to raise poultry flocks for feeding their immediately family, which is why poultry disease outbreaks may be more likely to impact children’s nutrition (37).
Within this framework, beyond the three entry points, we categorized gender-related questions into two types: integrated and strategic. Given how little is known about how gender dynamics and norms shape AMR, we propose qualitative, exploratory questions that can help highlight the most relevant issues in each community and some of the mechanisms behind these issues (including social and gender dynamics which can be explored through the ‘how’ and ‘why’ questions included in the table). To assess the relevance of these issues the qualitative findings of these questions can be used to help shape quantitative surveys. Integrating these insights with gender-disaggregated quantitative data will be the next step to enhancing and refining this framework.
In our questions we use ‘who in the household’ as means to identify (1) which gender group is involved in a specific activity; and (2) account for other individual characteristics beyond gender that may shape involvement in the household (e.g., age, marital status, and education level). We also use ‘who in the community’ to explore gender groups intersected by other social markers that may create diverse groups in a community (e.g., ethnicity, wealth etc.) and shape their interaction with AMR (38). Finally, we refer to ‘gender groups’ to include women, men, non-binary, and intersex. We acknowledge that the experiences of the last two groups are particularly unexplored and overlooked in the context of AMR. While we recognize the need to conduct more research on these two groups, we also are aware of the complexity (and risks) associated to identifying gender groups beyond ‘women and men’ in many cultures (39). The safety of individuals must be a priority in all research which engages with any gender groups, but especially in contexts where gender groups might face stigma, discrimination or persecution (40).
Using our conceptual framework (Figure 1) and gender dimension questions (Table 1), researchers working in AMR can identify gender-related questions pertinent to their programs. While these guiding questions were developed to pinpoint potential gender-related issues affecting AMR research, incorporating the unique context specificities of each research program will facilitate the generation of more tailored questions. To give an example, Table 1 question ‘Who should be able to use, access and afford antimicrobials prescribed or dispensed from animal health service (AHS)’ could be rephrased based on the local system to prescribe or dispense animal drugs. The question ‘What norms will they face?’ can be replaced by norms that are known to affect antimicrobial use in a given context. Exploration of such norms may in fact, need a separate study, given the complexity of the topic (43). This approach encourages deeper investigation and identification of relevant gender considerations.

Table 1. Gender dimension questions related to antimicrobial resistance research in livestock systems.
By targeting livestock systems, this framework addresses a critical knowledge gap. However, further efforts are required to integrate gender responsive approaches to AMR across the One Health sectors (see Galiè et al., 2024; (19)). The three entry points for gender and AMR in livestock systems discussed here are intended as starting point for further research. Additionally, the guiding questions provided are examples meant to highlight various ways gender can impact livestock systems, rather than an exhaustive list. Researchers utilizing this framework should also consider context specific questions to their production system, considering factors such as size, intensity, and species, to ensure a comprehensive understanding of gender dynamics. Similarly, age, culture, ethnicity and other intersectional factors also interact with gender; and need to be considering when applying this framework (Box 1 and Box 2).
BOX 1. Examples of how context might impact framework applications
Example 1: In low intensity cattle production systems in Tanzania, both men and women are actively involved in animal health management and have similar levels of disease knowledge (12). In this context, a research project focused on vaccine use might consider questions such as:
Why do cattle producers of different genders choose one vaccination over another? Are there gendered risks that influencing this selection? What opportunities exist to enhance vaccine uptake among cattle producers of different genders?
Example 2: In contrast, low intensity poultry systems in Tanzania are predominantly managed by women (41). Here, the same research project might explore questions like:
Which women poultry producers are interested in using vaccines but face barriers in doing so?
Who controls access to vaccine-related information, and what are the reasons behind these information gaps? How can access to vaccine information be improved among women poultry producers?
BOX 2. Example of how intersectionality can impact framework applications
Example 1: A study involving a dairy cooperative in India found that caste, as a significant intersectional factor, interacts with gender to shape power dynamics in this region (42). The research revealed that, while gender norms showed some flexibility, caste norms remained more rigid, affecting the empowerment opportunities available to women from different castes within the cooperative. A study looking at antibiotic use in these producers, key questions might include:
What intangible constraints, such as community or cooperative hostility toward unconventional practices, may limit antibiotic use among producers from different castes? What specific barriers need to be addressed to enable equitable antimicrobial access and use across caste groups?
Our next step is to implement this framework in the field. A real-life example will not only refine the framework but also reveal any limitations that may need adjustment to enhance its practical use. This will provide researchers with a concrete example of how to adapt the framework to different contexts and production systems effectively.
As gender-analysis in livestock research gain prominence, there is a growing expectation for AMR researchers to integrate gender considerations in their work. This framework offers a starting point for researchers aiming to enhance gender inclusivity and considerations in AMR research and policy development within livestock systems. To advance gender mainstreaming in future AMR research projects and policy implementation in livestock and other systems, researchers need to embrace gender-responsive research methodologies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
FE: Conceptualization, Writing – original draft, Writing – review & editing. AG: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. SR: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Canadian Institutes of Health Research [#149542], the Social Sciences & Humanities Research Council [#895-2022-1015], and the Wellcome Trust [222422/Z/21/Z]. Arshnee Moodley and Alessandra Galiè were funded by the CGIAR One Health initiative “Protecting Human Health through a One Health Approach” which is supported by contributors to the CGIAR Trust Fund (https://www.cgiar.org/funders/) and the SAPLING Initiative “Sustainable Animal Productivity for Livelihoods, Nutrition and Gender inclusion. The funding bodies were not involved in the study design, data collection, analysis, interpretation, or writing.
This paper was a collaboration between the AMR Policy Accelerator at the Global Strategy Lab (GSL) and the International Livestock Research Institute (ILRI) (and the One Health and SAPLING Initiatives) and we thank both teams for their comments. We would like to thank Sofía Guitérrez for her help with figure design and Arne Ruckert for his feedback and assistance with designing the conceptual framework.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ansari, M, Meraj, KK, Ghadi, R, Date, T, Chaudhari, D, Khan, R, et al. Socioeconomic impact of antimicrobial resistance and their integrated mitigation by one health approach In: N Akhtar, KS Singh, and D Goyal, editors. Emerging modalities in mitigation of antimicrobial resistance. Cham: Springer International Publishing (2022). 135–56.
2. Minssen, T, Outterson, K, Van Katwyk, SR, Pedro Henrique, D, Batista, CIR, Chandler, FC, et al. Social, cultural and economic aspects of antimicrobial resistance. Bull World Health Organ. (2020) 98:823–823A. doi: 10.2471/BLT.20.275875
3. Rochford, C, Sridhar, D, Woods, N, Saleh, Z, Hartenstein, L, Ahlawat, H, et al. Global governance of antimicrobial resistance. Lancet. (2018) 391:1976–8. doi: 10.1016/S0140-6736(18)31117-6
4. Wilson, LA, Rogers Van Katwyk, S, Fafard, P, Viens, AM, and Hoffman, SJ. Lessons learned from COVID-19 for the post-antibiotic future. Glob Health. (2020) 16:94. doi: 10.1186/s12992-020-00623-x
5. Gautron, JMC, Thanh, GT, Barasa, V, and Voltolina, G. Using intersectionality to study gender and antimicrobial resistance in low-and middle-income countries. Health Policy Plan. (2023) 38:1017–32. doi: 10.1093/heapol/czad054
6. Murray, CJL, Ikuta, KS, Sharara, F, Swetschinski, L, Aguilar, GR, Gray, A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
7. WHO Infection prevention and control during health care when novel coronavirus (ncov) infection is suspected. (2022). Available at: https://www.who.int/publications-detail-redirect/10665-331495 (Accessed September 27, 2022).
8. Morgan, R, George, A, Ssali, S, Hawkins, K, Molyneux, S, and Theobald, S. How to do (or not to do)… gender analysis in health systems research. Health Policy Plan. (2016) 31:1069–78. doi: 10.1093/heapol/czw037
9. Galiè, A, Pyburn, R, Baltenweck, I, and Quintero, S. Institutional and theoretical learning on gender analysis through the CGIAR research program on livestock. Kenya: ILRI (2022).
10. Heffernan, C., Thomson, K., and Nielsen, L. (2008). Livestock vaccine adoption among poor farmers in Bolivia: Remembering innovation diffusion theory. Vaccine. 26:2433–42. doi: 10.1016/j.vaccine.2008.02.045
11. Galiè, A, Teufel, N, Korir, L, Baltenweck, I, Webb Girard, A, Dominguez-Salas, P, et al. The Women’s empowerment in livestock index. Soc Indic Res. (2019) 142:799–825. doi: 10.1007/s11205-018-1934-z
12. Galiè, A, Distefano, F, Kangogo, D, Mattioli, RC, Wieland, B, and Baltenweck, I. Gendered perspectives on smallholder cattle production and health Management in Three Sites in Tanzania. J Gender Agric Food Sec. (2017) 3:43–65. doi: 10.22004/ag.econ.293403
13. Smith, MJ, and Wattles, BA. Appropriate antibiotic prescribing—the safer and less expensive choice. JAMA Netw Open. (2022) 5:e2214160. doi: 10.1001/jamanetworkopen.2022.14160
14. Anderson, CL, Reynolds, TW, Biscaye, P, Patwardhan, V, and Schmidt, C. Economic benefits of empowering women in agriculture: assumptions and evidence. J Dev Stud. (2021) 57:193–208. doi: 10.1080/00220388.2020.1769071
15. Gough, Brendan, and Novikova, Irina. (2020). WHO Gender Responsive Assessment Scale: Criteria for Assessing Programmes and Policies. Text. WHO Regional Office for Europe. 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK559709/table/ch2.t1/ (Accessed 202, March 28).
16. Allwood, G. Gender mainstreaming and policy coherence for development: unintended gender consequences and EU policy. Womens Stud Int Forum. (2013) 39:42–52. doi: 10.1016/j.wsif.2013.01.008
17. WHO A one health priority research agenda for antimicrobial resistance. (n.d.). Available at: https://www.who.int/publications-detail-redirect/9789240075924 (Accessed February 7, 2024).
18. Emdin, F., and Rogers Van Katwky, S. “Gender and Agriculture: Implications for Antimicrobial Resistance Policy.; (2022) Poster presentation presented at the ISVEE, Halifax, Canada.
19. Galiè, A, McLeod, A, Campbell, ZA, Ngwili, N, Terfa, ZG, and Thomas, LF. Gender considerations in One Health: A framework for researchers. Front Public Health. (2024). 12:1345273. doi: 10.3389/fpubh.2024.1345273
20. Patel, SJ, Patel, MD, Patel, JH, Patel, AS, and Gelani, RN. (n.d). Role of women gender in livestock sector: a review. Journal of Livestock Science (ISSN online 2277-6214).7:92–6.
21. Weeratunge, N., and Snyder, K. Gleaner, fisher, trader, processor: understanding gendered employment in fisheries and aquaculture. Fish and Fisheries. (2010) 11:405–20.
22. Bertakis, K. D. (2009). “The influence of gender on the doctor–patient interaction.” Patient education and counseling, EACH 2008- international conference on communication in healthcare.
23. Wertheim, HFL, Chuc, NTK, Punpuing, S, Khan, WA, Gyapong, M, Asante, KP, et al. Community-level antibiotic access and use (ABACUS) in low-and middle-income countries: finding targets for social interventions to improve appropriate antimicrobial use – an observational multi-Centre study. Wellcome Open Res. (2017) 2:58. doi: 10.12688/wellcomeopenres.11985.1
24. Maraka, M, Akala, HM, Amolo, AS, Juma, D, Omariba, D, Cheruiyot, A, et al. A seven-year surveillance of epidemiology of malaria reveals travel and gender are the key drivers of dispersion of drug resistant genotypes in Kenya. PeerJ. (2020) 8:e8082. doi: 10.7717/peerj.8082
25. Atterby, C, Osbjer, K, Tepper, V, Rajala, E, Hernandez, J, Seng, S, et al. Carriage of Carbapenemase-and extended-Spectrum Cephalosporinase-producing Escherichia Coli and Klebsiella Pneumoniae in humans and livestock in rural Cambodia; gender and age differences and detection of blaOXA-48 in humans. Zoonoses Public Health. (2019) 66:603–17. doi: 10.1111/zph.12612
26. Hoffman, SJ, Savulescu, J, Giubilini, A, Kirchhelle, C, Van Katwyk, SR, Weldon, I, et al. Governing the global antimicrobial commons: introduction to special issue. Health Care Anal. (2020) 31:1–8. doi: 10.1007/s10728-019-00388-4
27. Ankrah, DA, Freeman, CY, and Afful, A. Gendered access to productive resources–evidence from small holder farmers in Awutu Senya West District of Ghana. Scient Afr. (2020) 10:e00604. doi: 10.1016/j.sciaf.2020.e00604
28. Galiè, A, Najjar, D, Petesch, P, Badstue, L, and Farnworth, CR. Livestock innovations, social norms, and Women’s empowerment in the global south. Sustain For. (2022) 14:3741. doi: 10.3390/su14073741
29. Kinati, W, Baker, D, Temple, EC, Najjar, D, and Mulema, AA. Empowerment resources, decision-making and gender attitudes: which matter Most to livestock keepers in the mixed and livestock-based Systems in Ethiopia? CABI Agric Biosci. (2022) 3:49. doi: 10.1186/s43170-022-00114-6
30. Omondi, I, Galiè, A, Teufel, N, Loriba, A, Kariuki, E, and Baltenweck, I. Women’s empowerment and livestock vaccination: evidence from Peste des Petits ruminants vaccination interventions in northern Ghana. Animals. (2022) 12:717. doi: 10.3390/ani12060717
31. Wang, W, Jin, J, He, R, and Gong, H. Gender differences in pesticide use knowledge, risk awareness and practices in Chinese farmers. Sci Total Environ. (2017) 591:22–8. doi: 10.1016/j.scitotenv.2017.03.053
32. Ström, G, Boqvist, S, Albihn, A, Fernström, L-L, Andersson Djurfeldt, A, Sokerya, S, et al. Antimicrobials in small-scale urban pig farming in a lower middle-income country - arbitrary use and high resistance levels. Antimicrob Resist Infect Control. (2018) 7:35. doi: 10.1186/s13756-018-0328-y
33. Ragasa, C, Berhane, G, Tadesse, F, and Taffesse, AS. Gender differences in access to extension services and agricultural productivity. J Agric Educ Ext. (2013) 19:437–68. doi: 10.1080/1389224X.2013.817343
34. Rola-Rubzen, MF, Paris, T, Hawkins, J, and Sapkota, B. Improving gender participation in agricultural technology adoption in Asia: from rhetoric to practical action. Appl Econ Perspect Policy. (2020) 42:113–25. doi: 10.1002/aepp.13011
35. Mutangadura, G, Mukurazita, D, and Jackson, H. A review of household and community responses to the HIV/AIDSepidemic in the rural areas of sub-Saharan Africa. Geneva: UNAIDS (1999). Available: https://www.unaids.org/sites/default/files/media_asset/una99-39_en_0.pdf
36. UNFPA Women and HIV/AIDS: Confronting the Crisis. (n.d.). Available at: https://www.unfpa.org/news/women-and-hivaids-confronting-crisis (Accessed February 7, 2024).
37. Ahmed, S, Begum, M, Afia Khatun, MR, Gofur, MTA, Azad, AK, and Haque, TS. Family poultry (FP) as a tool for improving gender equity and Women’s empowerment in developing countries: evidence from Bangladesh. Eur J Agric Food Sci. (2021) 3:37–44. doi: 10.24018/ejfood.2021.3.2.251
38. Tavenner, K, Crane, TA, Bullock, R, and Galiè, A. Intersectionality in gender and agriculture: toward an applied research design. Gend Technol Dev. (2022) 26:385–403. doi: 10.1080/09718524.2022.2140383
39. Ritz, SA, and Greaves, L. We need more-nuanced approaches to exploring sex and gender in research. Nature. (2024) 629:34–6. doi: 10.1038/d41586-024-01204-3
40. Littlejohn, T, Poteat, T, and Beyrer, C. Sexual and gender minorities, public health, and ethics In: AC Mastroianni, JP Kahn, and NE Kass, editors. The Oxford handbook of public health ethics. Oxford: Oxford University Press (2019)
41. Galiè, A, Teufel, N, Girard, AW, Baltenweck, I, Dominguez-Salas, P, Price, MJ, et al. Women’s empowerment, food security and nutrition of pastoral communities in Tanzania. Glob Food Sec. (2019) 23:125–34. doi: 10.1016/j.gfs.2019.04.005
42. Farnworth, CR, Bharati, P, and Galiè, A. Empowering women, challenging caste? The experience of a dairy cooperative in India. Front Sustain Food Syst. (2023) 7:405. doi: 10.3389/fsufs.2023.1114405
Keywords: gender, antimicrobial resistance, livestock, framework, perspective
Citation: Emdin F, Galiè A, Moodley A and Rogers Van Katwyk S (2025) Gender and antimicrobial resistance: a conceptual framework for researchers working in livestock systems. Front. Vet. Sci. 11:1456605. doi: 10.3389/fvets.2024.1456605
Received: 28 June 2024; Accepted: 16 December 2024;
Published: 28 January 2025.
Edited by:
Jens Andre Hammerl, Bundesinstitut für Risikobewertung, GermanyReviewed by:
Flavie Luce Goutard, Institut National de la Recherche Agronomique (INRA), FranceCopyright © 2025 Emdin, Galiè, Moodley and Rogers Van Katwyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiona Emdin, ZmlvbmEuZW1kaW5AZ2xvYmFsc3RyYXRlZ3lsYWIub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.