- 1Usc Petard, Anses, EA 7510, SFR Cap Santé, Université de Reims Champagne-Ardenne, Reims Cedex, France
- 2ANSES, INRAe, ENVA, UMR-BIPAR, Laboratoire de Santé Animale, Maisons-Alfort Cedex, France
Culicoides are vectors that can transmit many different pathogens to mammals — including humans, and domestic and wild animals — and birds. In order to take preventive measures against any vector-borne disease, it is important to gather information on both the host and vector species. Culicoides species are mainly mammalophilic, ornithophilic or ornithophilic/mammalophilic, but females have also been found to occasionally feed on engorged insects. A recent systematic review based on three groups of key words investigated Culicoides on farms, and asserted that 92 species (including four not present species) have been reported among cattle in mainland France and Corsica. We have re-evaluated the presence of Culicoides species in cattle in France using the same data of the review. Our data show that only 18 species are reported among cattle. Furthermore, our research used molecular and indirect investigations to analyse Culicoides species that had been feeding on cattle. Our results demonstrate that 45 species feed on cattle out of 92 species present in France. The paper discusses the relevance of data in the literature when investigating hosts of Culicoides species.
Introduction
Vector-borne diseases, transmitted by a variety of arthropods, cause health problems for humans, livestock and wild animals. Currently, more than 1,400 Culicoides species have been described worldwide (1). Culicoides biting midges have been incriminated in the transmission of viruses, protozoa and filarial worms (2). Their economic impact in Europe is due to their transmission of the bluetongue virus (BTV), epizootic haemorrhagic disease virus (EHDV), Schmallenberg virus (SBV) and African horse sickness virus (AHSV). In order to take preventive measures against a vector-borne disease, it is important to gather information on both the host and vector species. Identifying the feeding patterns of Culicoides biting midges is an essential step in pathogen circulation in order, for example, to break the transmission chain by vaccinating animals at greatest risk of infection (3).
Generally speaking, Culicoides midges have a wide host spectrum and tend to feed on blood opportunistically (4–7); they are classified as mammalophilic, ornithophilic or ornithophilic & mammalophilic species (8–10). Culicoides species are more attracted by cattle than sheep (11), and are found in natural areas and on farms. Indeed, the same species feed on both wild and domestic animals, so a switch is possible and could facilitate the circulation of pathogens between these two communities (12). In this context, an accurate knowledge of Culicoides species present on farms is essential for anticipating diseases. In France, 92 species of Culicoides are reported (13–18). A recent study examining Culicoides species on farms in mainland France and Corsica suggested that 94 species (including two invalid species and four not present species) were present among cattle. The study was based on a systematic review using three groups of key words (19). In this context, do these species play an epidemiological role in the transmission of pathogens among cattle? The first step in answering this question is to prove that there is a relationship between the host and the insect. Culicoides species-host pairing is a key factor in anticipating and understanding the transmission of vector-borne pathogens. Here, we investigate Culicoides species and cattle based on a review in order to research bibliographic references with traditional approach (PCR analysis of blood meals taken from the host by female Culicoides in the field) and indirect investigations (animal baits, direct aspiration, light traps and glue strips). We compare the checklist of Culicoides species reported in France with their cattle preference. Prudhomme et al. (19) reported that all Culicoides species in France are present among cattle and this number of species is superior on other references reported. So, a second objective is to re-evaluate the presence of Culicoides spp. in cattle in France.
Materials and methods
First, in 2012, a list of Culicoides present in France was drawn up by Venail et al. (16). The list of Culicoides species identified 88 species in all, 83 for mainland France and 61 for Corsica.
Four species have since been added to this preliminary list: (i) C. paradoxalis Ramilo and Delécolle, 2013 was identified in mainland France and Corsica (13); (ii) C. boyi Nielsen, Kristensen and Pape, 2015 was reported in mainland France (14) on a farm with cattle (20); (iii) C. bysta Sarvašová and Mathieu, 2017 was reported in mainland France (15) and (iv) C. cryptipulicaris Talavera, Muñoz-Muñoz, Verdún and Pagès, 2017 (13, 17, 18).
The distribution of species is not homogeneous in these four groups: for example Mediterranean littoral and Corsica present the same climate but a species present in Corsica is no necessary present in Mediterranean littoral and vice-versa (i.e., C. corsicus Kremer, Leberre and Beaucournu-Saguez, C. derisor Callot and Kremer, C. heteroclitus Kremer and Callot, C. impunctatus Goetghebuer, C. indistinctus Khalaf, C. jamaicensis Edwards, C. maritimus paucisensillatus Callot, Kremer and Rioux, C. minutissimus (Zetterstedt), C. montanus Shakirzjanova, C. nubeculosus (Meigen), C. paradisionensis Boorman, C. reconditus Campbell and Pelham-Clinton, C. riebi Delécolle, Mathieu and Baldet, C. saevus Kieffer, C. sahariensis Kieffer, C. salinarius Kieffer, C. segnis Campbell and Pelham-Clinton, C. simulator Edwards, C. tauricus Gutsevich, C. vexans (Staeger)). All articles including Culicoides spp. and host preference were selected. All publications have been reading. Our study used a traditional approach (PCR analysis of blood meals taken from the host by female Culicoides) and indirect investigations (animal baits, direct aspiration, light traps and glue strips) in the Palaeartic region to research the feeding preferences of Culicoides spp. The data we found in the literature were from various countries, including the country that first described the type and species in the Palaeartic region (Table 1). The feeding preferences of Culicoides biting midge species present in France (Table 1) has been linked to cattle (Table 2).
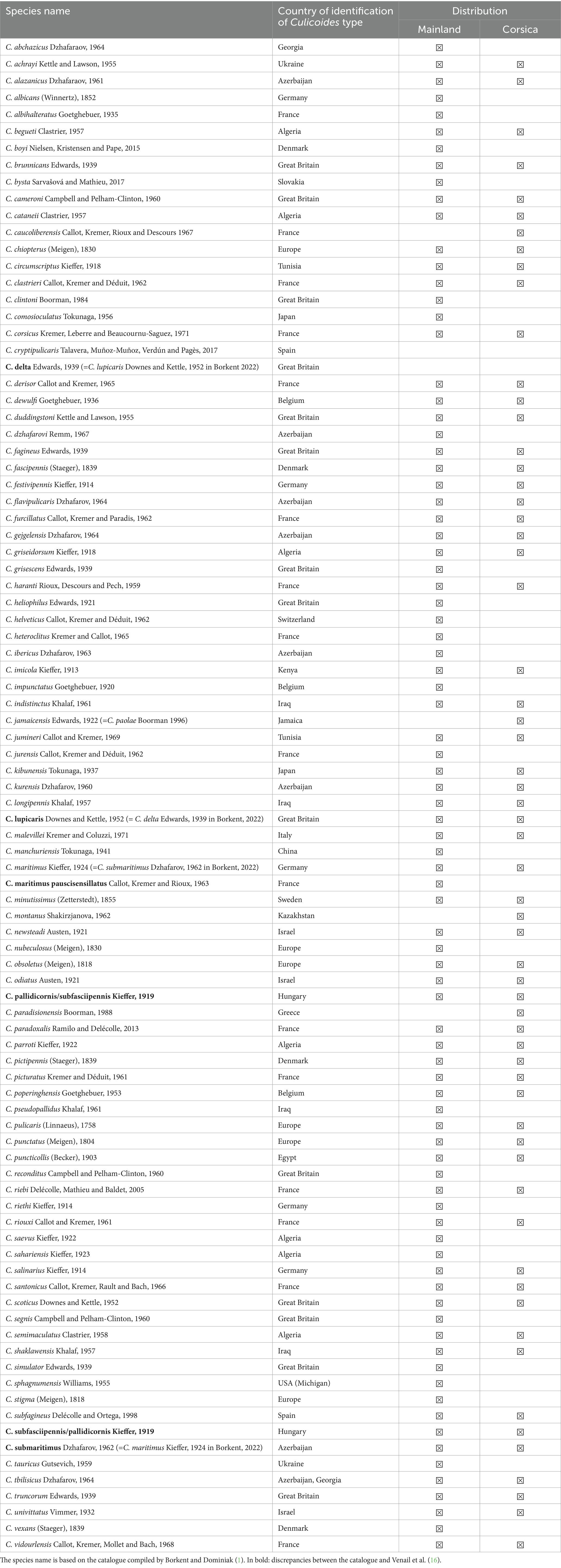
Table 1. Checklist of Culicoides species reported in mainland France and Corsica with the countries where types were first identified.
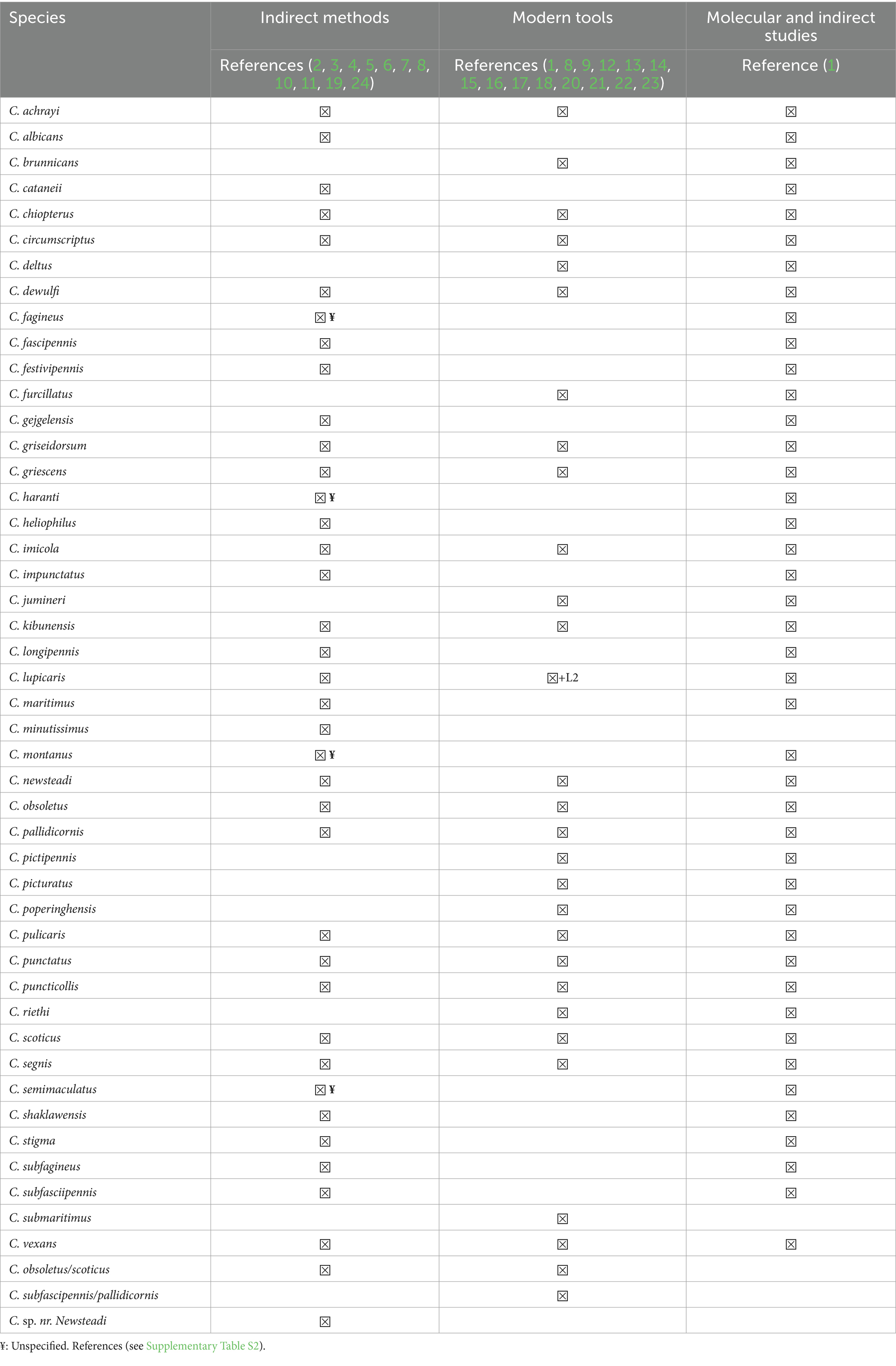
Table 2. Cattle preference of Culicoides species in France based on molecular analysis of engorged Culicoides females and other techniques (animal baits, direct aspiration, light traps, glue strips).
Secondly, documents, selected by Prudhomme et al. (19), are analysed in Supplementary Table S1. Publications selected (focus on biting midges) were reviewed in full text for inclusion of cattle (cattle OR livestock OR bovine OR cow OR beef OR calf OR calves OR heifer).
Results
In all, we found 45 species that take blood meals from cattle (Table 2). Four species (C. fagineus Edwards C. haranti Rioux, Descours and Pech, C. montanus Shakirzjanova and C. semimaculatus Clastrier) have been reported to feed on cattle without information as to the method of collection (noted ¥ in Table 2). Finally, 14 species (C. albicans (Winnertz), C. cataneii Clastrier, C. fascipennis (Staeger), C. festivipennis Kieffer, C. gejgelensis Dzhafarov, C. heliophilus Edwards, C. impunctatus C. longipennis Khalaf, C. maritimus Kieffer, C. minutissimus, C. shaklawensis Khalaf, C. stigma (Meigen). C. subfagineus Delécolle and Ortega and C. subfasciipennis Kieffer) have been reported to feed on cattle based only on indirect investigations.
Second, based on 62 number of articles reviewed, only 4 articles, were used to compile the current species distribution list among the cattle (21–24). Overall, 18 species were reported in cattle: C. achrayi Kettle and Lawson, C. brunnicans Edwards, C. chiopterus (Meigen), C. deltus Edwards, C. dewulfi Goetghebuer, C. duddingstoni Kettle and Lawson, C. festivipennis, C. furcillatus Callot, Kremer and Paradis, C. lupicaris Downes and Kettle, C. minutissimus, C. newsteadi Austen, C. obsoletus (Meigen), C. pallidicornis Kieffer, C. pictipennis (Staeger), C. pulicaris (Linnaeus), C. punctatus (Meigen), C. scoticus Downes and Kettle, C. vexans Obsoletus group and C. obsoletus/C. scoticus.
Discussion
The epidemiology of vector-borne diseases is linked to the preferred host and feeding behaviour of the arthropod vector. Our results reveal that 45 species feed on cattle (Table 2). The vertebrate hosts of 31 (9) and 37 (10) Culicoides species have previously been identified by molecular means. Indirect and molecular biology methods are in fact compatible (Table 2). There are more reports on the preferred hosts of Culicoides spp. by indirect methods than molecular tools (Table 2). Finally, research using proteomics to identify host proteins reveals a more diverse host pool than research using a traditional molecular approach (24). For C. imicola Kieffer, for example, the number of hosts varies between 1.5 and 5. Future studies on blood meal sources (i.e., hosts) could use a combination of PCR and proteomics-based methods to better characterise the Culicoides host spectrum.
Prudhomme et al. (19) proposed applying a step with three items (France OR Corsica OR French) AND (cattle OR livestock OR bovine OR cow OR beef OR calf OR calves OR heifer) AND (haematophag* OR hematophag* OR vector* OR arthropod* OR insect* OR tick* OR mite* OR acari*) to search for literature on the presence of Culicoides among cattle. They reported 94 Culicoides species recorded on different types of cattle farms in mainland France and Corsica. However, their review contains many mistakes: (i) many of the references are based on larval ecology (25–30) and these papers did not describe either the animals or any farms in the vicinity of the muds collected; (ii) references (25, 31–33) do not comply with the item “cattle OR livestock OR bovine OR cow OR beef OR calf OR calves OR heifer”; (iii) C. cameroni Campbell and Pelham-Clinton and C. clintoni Boorman were collected in natural areas and not on farms (34), so C. clintoni was not found with cattle; (iv) many Culicoides have been collected through the national surveillance programme in France (35). Traps were implemented among several types of livestock in both Corsica [sheep, cattle, horses (36); sheep (37); sheep, cattle (16)] and the French mainland [sheep (37); sheep and cattle (16, 21, 38, 39)]. Thus, the references using results of Culicoides trapped during national surveillance programme activities did not separate farms with cattle only from farms with other animals too. Consequently, the resulting list, which is said to focus only on cattle, is unreliable; (v) at the beginning of the Culicoides national surveillance programme, the composition of the Pulicaris group included three species (C. pulicaris, C. lupicaris Downes and C. flavipulicaris Dzhafarov) (16). Nowadays, in France the Pulicaris group includes six species (C. boyi, C. bysta, C. cryptipulicaris, C. pulicaris, C. lupicaris and C. flavipulicaris) (see Material and Methods and Table 1) but this new group has not been incorporated in the last publication (40); (vi) Culicoides ibericus Dzhafarov, C. manchuriensis Tokunaga, C. sahariensis, C. tauricus and C. vexans are found in mainland France but not Corsica (16); (vii) C. pseudopallidus Khalaf 1961 is present in France but C. pseudoheliophilus is not (16); (viii) finally, C. accraensis Carter, Ingram and Macfie, C. albipennis Kieffer, C. musicola (invalid species), C. pumilus (Winnertz), C. sergenti (Kieffer) and C. sigrosignatus (invalid species) have not been reported in France (14, 16). In our opinion, the Culicoides species presented in this systematic review (19) are not aligned with field reality. Our data show that 18 species are present among cattle in France (mainland without Corsica). The review of Prudhomme et al. (19) uses much results obtained by the national surveillance. But publications did not separate farms with cattle only from farms with other animals (See above). A great deal of vigilance must be exercised when we elaborate a review (based on requests in databases), in particular to choose of items.
The references used by Prudhomme et al. (19) include four techniques for characterising the presence of Culicoides biting midges among cattle: animal baits, larval ecology, light traps and the blood meals of Culicoides females. While light traps offer a good description of diversity (7), they do not accurately reflect the proportions of biting midges in an area (41). The light trap is not suited for midges feeding on an animal (42). Trapped engorged females with a blood meal in their abdomen can be used to accurately reveal host preferences by identifying the origin of the blood meal, but there is a flagrant bias because only night species are caught, and the attractiveness of light traps is limited. Animal baits can be used to identify host preferences and attack rates by counting and identifying the numbers of biting midges that can be captured from a specific host. Larval ecology offers knowledge of substrates suitable for Culicoides larval development. This method is not suitable for investigating relationships between a species and a host [in Prudhomme et al. (19) there is only one reference (23)]. A study on Belgian cattle farms has shown that 13 Culicoides species were obtained by incubation of soil samples (43) with 11 species presenting on cattle. In contrast, Uslu and Dik (44) describes the breeding sites of 18 Culicoides species in Turkey without describing the environmental sites. Future studies on larval development in the immediate surroundings of cattle farms will shed light on the microhabitats of Culicoides biting midges.
Five criteria are used to consider an arthropod as a biological vector of arboviruses (45, 46), including the presence of virus (es) and abundance of the suspected vector. Light traps underestimate the numbers of Culicoides present in an area (41), so C. chiopterus (Meigen) had not been seriously considered as a potential vector of BTV (41). The French national surveillance programme (35) has reported species in “groups or complex” to show species distribution and seasonal dynamics (16). Epidemiological studies can use wing characteristics to group together species with similar markings without needing to mount the specimens (47). But in this case, the results are not suitable for characterising the abundance of specific species. But, surveillance programme allows to model the abundance of Culicoides spp. in order to identify risk periods in France (48).
Contacts between competent vertebrate hosts and insect vectors are vital for vector-borne pathogens to successfully complete their transmission cycle (49). Cattle are very attractive to Culicoides, with 45 species feeding on them (Table 2) out of 92 species present in France. Among the species that feed on cattle, C. chiopterus, C. dewulfi, C. imicola, C. obsoletus, C. pulicaris, C. punctatus, C. scoticus are confirmed or probable vectors of BTV (50) and SBV (51). In addition, two species of the newsteadi complex and two species (C. nubeculosus C. lupicaris) are recorded or suspected of being involved in BTV and SBV transmission (50, 51), respectively. Most Culicoides species are opportunistic and may change host depending on availability (9, 10). For example, C. scoticus can switch from its preferential (predominant) mammal host to other hosts according to site and host availability (20). Many Culicoides species are known to feed on birds and to transmit the avian Haemoproteus parasite (52, 53). The ornithophilic Culicoides list includes 18 species (C. alazanicus Dzhafaraov, C. cataneii, C. chiopterus, C. circumscriptus Kieffer, C. clastrieri Callot, Kremer and Déduit, C. festivipennis, C. griseidorsum Kieffer, C. impunctatus C. kibunensis Tokunaga, C. obsoletus, C. pallidicornis, C. pictipennis, C. pulicaris, C. punctatus, C. scoticus, C. segnis, C. seminaculatus and C. univittatus Vimmer) (9, 52–54). Fourteen of these species may occasionally switch to cattle (Table 2). In contrast, several species usually take their blood meals from mammals or birds indifferently, and can switch from one to another (9, 10). The relationship between insects and their host is thus a key factor in the transmission of pathogens, including host availability (attractiveness and acceptability) (55). Culicoides species feed disproportionately on different host species (11, 42). For example, Culicoides spp. have been reported as feeding on cattle nine times more than on sheep (42), showing their attraction to cattle over sheep (11). Nevertheless, host diversity impacts Culicoides species diversity (7, 11, 22, 34, 42). Culicoides use hosts differently according to their preferential feeding locations on the body. Moreover, in one study Culicoides species switched between hosts (a cow and ewe) and between parts of the body attacked (42). More biting midges were collected from the head than the back, belly/flank and legs (11). One possible explanation could be the adaptation of Culicoides to a preferential (predominant) host through specific morphology of antennae, palpi and the number and/or distribution of sensilla (9, 10). This means that the sensory structures can be used to distinguish between ornithophilic, mammalophilic, or ornithophilic & mammalophilic species. Culicoides blood meals are not systematically included in Culicoides research literature (only 24 references in the Palaeartic region). As discussed, it is nonetheless a key factor for identifying the host spectrum of a species in order to anticipate/understand the transmission of pathogens. Hence, further studies that include both molecular approaches and indirect investigations and that focus on host preference by Culicoides species with nocturnal and diurnal activities would provide greater insights into Culicoides feeding patterns and vector-host interactions.
In conclusion, our study investigated the relationship between cattle and biting midges based on direct and indirect investigations. Data in the literature reports that all Culicoides species in France are present among cattle but our results reveal that only 18 species are present among cattle and 45 species feed on cattle out of the 92 species present. It could be misleading to state that species found near cattle interact with those cattle through blood meals, and research should focus on proving the midge vector-host relationship through both modern and traditional means in order to identify both the species involved in viral transmission and the viruses transmitted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
CM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LH-H: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1451442/full#supplementary-material
References
1. Borkent, A, and Dominiak, P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. (2020) 4787:377. doi: 10.11646/zootaxa.4787.1.1
2. Borkent, A. The biting midges, the Ceratopogonidae (Diptera) In: WC Marquardt, editor. Biology of disease vectors. Amsterdam: Elsevier Science & Technology: Elsevier Academic Press (2005). 113–26.
3. Bessell, PR, Auty, HK, Searle, KR, Handel, IG, Purse, BV, and de C Bronsvoort, BM. Impact of temperature, feeding preference and vaccination on Schmallenberg virus transmission in Scotland. Sci Rep. (2014) 4:5746. doi: 10.1038/srep05746
4. Blackwell, A, Mordue, AJ, and Mordue, W. Identification of blood meals of the Scottish biting midge, Culicoides impunctatus, by indirect enzyme-linked immunosorbent assay (ELISA). Med Vet Entomol. (1994) 8:20–4. doi: 10.1111/j.1365-2915.1994.tb00378.x
5. Bartsch, S, Bauer, B, Wiemann, A, Clausen, P-H, and Steuber, S. Feeding patterns of biting midges of the Culicoides obsoletus and Culicoides pulicaris groups on selected farms in Brandenburg, Germany. Parasitol Res. (2009) 105:373–80. doi: 10.1007/s00436-009-1408-y
6. Lassen, SB, Nielsen, S, and Kristensen, M. Identity and diversity of blood meal hosts of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasit Vectors. (2012) 5:143. doi: 10.1186/1756-3305-5-143
7. Viennet, E, Garros, C, Gardes, L, Rakotoarivony, I, Allene, X, Lancelot, R, et al. Host preferences of Palaearctic Culicoides biting midges: implications for transmission of orbiviruses. Med Vet Entomol. (2013) 27:255–66. doi: 10.1111/j.1365-2915.2012.01042.x
8. Santiago-Alarcon, D, Havelka, P, Schaefer, HM, and Segelbacher, G. Bloodmeal analysis reveals avian plasmodium infections and broad host preferences of Culicoides (Diptera: Ceratopogonidae) vectors. PLoS One. (2012) 7:e31098. doi: 10.1371/journal.pone.0031098
9. Martínez-De La Puente, J, Figuerola, J, and Soriguer, R. Fur or feather? Feeding preferences of species of Culicoides biting midges in Europe. Trends Parasitol. (2015) 31:16–22. doi: 10.1016/j.pt.2014.11.002
10. Augot, D, Hadj-Henni, L, Strutz, S, Slama, D, Millot, C, Depaquit, J, et al. Association between host species choice and morphological characters of main sensory structures of Culicoides in the Palaeartic region. Peer J. (2017) 5:e3478. doi: 10.7717/peerj.3478
11. Ayllón, T, Nijhof, AM, Weiher, W, Bauer, B, Allène, X, and Clausen, PH. Feeding behaviour of Culicoides spp. (Diptera: Ceratopogonidae) on cattle and sheep in Northeast Germany. Parasit Vectors. (2014) 7:34. doi: 10.1186/1756-3305-7-34
12. Talavera, S, Muñoz-Muñoz, F, Durán, M, Verdún, M, Soler-Membrives, A, Oleaga, Á, et al. Culicoides species communities associated with wild ruminant ecosystems in Spain: tracking the way to determine potential bridge vectors for arboviruses. PLoS One. (2015) 10:e0141667. doi: 10.1371/journal.pone.0141667
13. Ramilo, D, Garros, C, Mathieu, B, Benedet, C, Allène, X, Silva, E, et al. Description of Culicoides paradoxalis sp. nov. from France and Portugal (Diptera: Ceratopogonidae). Zootaxa. (2013) 3745:243–56. doi: 10.11646/zootaxa.3745.2.4
14. Augot, D, Hadj-Henni, L, Millot, C, Lehrter, V, Cousinat, M, and Depaquit, J. First report of Culicoides boyi (Diptera: Ceratopogonidae) in France. Annal Soc Entomol Fr. (2016) 52:171–8. doi: 10.1080/00379271.2016.1207486
15. Sarvašová, A, Kočišová, A, Candolfi, E, and Mathieu, B. Description of Culicoides (Culicoides) bysta n. sp., a new member of the Pulicaris group (Diptera: Ceratopogonidae) from Slovakia. Parasit Vectors. (2017) 10:279. doi: 10.1186/s13071-017-2195-4
16. Venail, R, Balenghien, T, Guis, H, Tran, A, Setier-Rio, ML, Delécolle, JC, et al. Assessing diversity and abundance of vector populations at a national scale: example of Culicoides surveillance in France after bluetongue virus emergence In: H Mehlhorn, editor. Arthropods as vectors of emerging diseases. Parasitology research monographs. Berlin Heidelberg: Springer-Verlag (2012). 77–102.
17. Talavera, S, Muñoz-Muñoz, F, Verdún, M, and Pagès, N. Morphology and DNA barcoding reveal three species in one: description of Culicoides cryptipulicaris sp. nov. and Culicoides quasipulicaris sp. nov. in the subgenus Culicoides. Med Vet Entomol. (2017) 31:178–91. doi: 10.1111/mve.12228
18. Dähn, O, Werner, D, Mathieu, B, and Kampen, H. Development of conventional multiplex PCR assays for the identification of 21West palaearctic biting midge taxa (Diptera: Ceratopogonidae) belonging to the Culicoides subgenus Culicoides, including recently discovered species and genetic variants. Diversity. (2023) 15:699. doi: 10.3390/d15060699
19. Prudhomme, J, Depaquit, J, Fite, J, Quillery, E, Bouhsira, E, and Liénard, E. Systematic review of hematophagous arthropods present in cattle in France. Parasite. (2023) 30:56. doi: 10.1051/parasite/2023059
20. Ninio, C, Augot, D, Delecolle, JC, Dufour, B, and Depaquit, J. Contribution to the knowledge of Culicoides (Diptera: Ceratopogonidae) host preferences in France. Parasitol Res. (2011) 108:657–63. doi: 10.1007/s00436-010-2110-9
21. Baldet, T, Delecolle, JC, Cêtre-Sossah, C, Mathieu, B, Meiswinkel, R, and Gerbier, G. Indoor activity of Culicoides associated with livestock in the bluetongue virus (BTV) affected region of northern France during autumn 2006. Prev Vet Med. (2008) 87:84–97. doi: 10.1016/j.prevetmed.2008.06.014
22. Garros, C, Gardes, L, Allene, X, Rakotoarivony, I, Viennet, E, Rossi, S, et al. Adaptation of a species-specific multiplex PCR assay for the identification of blood meal source in Culicoides (Ceratopogonidae: Diptera): applications on Palaearctic biting midge species, vectors of Orbiviruses. Infect Genet Evol. (2011) 11:1103–10. doi: 10.1016/j.meegid.2011.04.002
23. Ninio, C, Augot, D, Dufour, B, and Depaquit, J. Emergence of Culicoides obsoletus from indoor and outdoor breeding sites. Vet Parasitol. (2011) 183:125–9. doi: 10.1016/j.vetpar.2011.07.020
24. Díaz-Sánchez, S, Hernández-Jarguín, A, Torina, A, Fernández de Mera, IG, Estrada-Peña, A, Villar, M, et al. Biotic and abiotic factors shape the microbiota of wild-caught populations of the arbovirus vector Culicoides imicola. Insect Mol Biol. (2018) 27:847–61. doi: 10.1111/imb.12526
25. Kremer, M. Contribution à l'étude du genre Culicoides Latreille, particulièrement en France. Paris (France): Lechevalier P (1965).
26. Kremer, M, Leberre, G, and Beaucournu-Saguez, F. 1971. Notes sur les Culicoides (Dipt. Ceratopogonidae) de Corse. Description de C. corsicus n. sp. Ann. Parasitol. Hum. Comp. (1971) 46:653–60. doi: 10.1051/parasite/1971465653
27. Kremer, M, Rieb, J, and Rebholtz, C. Ecology of the Ceratopogonids of the Alsace plain. I. The genus Culicoides from the humid soils of the Ried. Ann Parasitol Hum Comp. (1978) 53:101–15.
28. Chacker, E. Description of larvae of six species of Culicoides. Proceedings of the Fifth International Symposium on Ceratopogonidae, Strasbourg (1982).
29. Rieb, JP, Mialhe, E, and Quiot, JM. Ceratopogonidae larvae infected by an Iridovirus. Proceedings of the Fifth International Symposium on Ceratopogonidae, Strasbourg (1982).
30. Rieb, JP. L'estivo-hibernation et le contrôle de la dynamique du cycle évolutif dans le genre Culicoides (Diptères, Cératopogonidés). Vie et Milieu. (1987) 37:23–37.
31. Rageau, J, and Mouchet, J. Les arthropodes hématophages de Camargue. Cahier ORSTOM: Sér Entomol Méd Parasitol. (1967) 5:263–81.
32. Mathieu, B, Delecolle, JC, Garros, C, Balenghien, T, Setier-Rio, ML, Candolfi, E, et al. Simultaneous quantification of the relative abundance of species complex members: application to Culicoides obsoletus and Culicoides scoticus (Diptera: Ceratopogonidae), potential vectors of bluetongue virus. Vet Parasitol. (2011) 182:297–306. doi: 10.1016/j.vetpar.2011.05.052
33. Kluiters, G, Carpenter, S, Gardes, L, Guis, H, Baylis, M, and Garros, C. Morphometric discrimination of two sympatric sibling species in the Palaearctic region, Culicoides obsoletus Meigen and C. scoticus Downes & Kettle (Diptera: Ceratopogonidae), vectors of bluetongue and Schmallenberg viruses. Parasit Vectors. (2016) 9:1–15. doi: 10.1186/s13071-016-1520-7
34. Rossi, S, Balenghien, T, Viarouge, C, Faure, E, Zanella, G, Sailleau, C, et al. Red deer (Cervus elaphus) did not play the role of maintenance host for bluetongue virus in France: the burden of proof by long-term wildlife monitoring and Culicoides snapshots. Viruses. (2019) 11:903–29. doi: 10.3390/v11100903
35. Balenghien, T, Garros, C, Mathieu, B, Setier-Rio, M-L, Allène, X, Gardes, L, et al. La surveillance des Culicoides en France. Bull Epidémiol Santé Animale et Alimentation. (2010) 35:8–9.
36. Delécolle, JC, and De La Rocque, S. Contribution à l'étude des Culicoides de Corse. Liste des espèces recensées en 2000/2001 et redescription du principal vecteur de la Fièvre catarrhale ovine: Culicoides imicola Kieffer, 1913 (Diptera, Ceratopogonidae). Bull Sociét Entomolo Fr. (2002, 2002) 107:371–9. doi: 10.3406/bsef.2002.16876
37. Baldet, T, Mathieu, B, Delécolle, JC, Gerbier, G, and Roger, F. Emergence de la fièvre catarrhale ovine dans le Bassin méditerranéen et surveillance entomologique en France. Rev Élevage Méd Vét Pays Trop. (2005) 58:125–32. doi: 10.19182/remvt.9923
38. Baldet, T, and Delécolle, JC. Studies on Culicoides found in association with livestock in the bluetongue virus (BTV) affected region of northern France. EFSA Panel on Animal Health and Welfare, 2007. Report on Epidemiological analysis of the 2006 bluetongue virus serotype 8 epidemic in north-western Europe, EFSA Journal 2007. (2007) 5:366. doi: 10.2903/j.efsa.2007.34r
39. Mignotte, A, Garros, C, Dellicour, S, Jacquot, M, Gilbert, M, Gardès, L, et al. High dispersal capacity of Culicoides obsoletus (Diptera: Ceratopogonidae), vector of bluetongue and Schmallenberg viruses, revealed by landscape genetic analyses. Parasit Vectors. (2021) 14:93. doi: 10.1186/s13071-020-04522-3
40. Garros, C. Complete data of Culicoides captures realized by the surveillance network in France in 2010. (2022)
41. Carpenter, S, Szmaragd, C, Barber, J, Labuschagne, K, Gubbins, S, and Mellor, P. An assessment of Culicoides surveillance techniques in northern Europe: have we underestimated a potential bluetongue virus vector? J Appl Ecol. (2008) 2008:1237–45. doi: 10.1111/j.1365-2664.2008.01511.x
42. Elbers, ARW, and Meiswinkel, R. Culicoides (Diptera: Ceratopogonidae) host preferences and biting rates in the Netherlands: comparing cattle, sheep and the black-light trap. Vet Parasitol. (2014) 205:330–7. doi: 10.1016/j.vetpar.2014.06.004
43. Zimmer, JY, Brostaux, Y, Haubruge, E, and Francis, F. Larval development sites of the main Culicoides species (Diptera: Ceratopogonidae) in northern Europe and distribution of coprophilic species larvae in Belgian pastures. Vet Parasitol. (2014) 205:676–86. doi: 10.1016/j.vetpar.2014.08.029
44. Uslu, U, and Dik, B. Description of breeding sites of Culicoides species (Diptera: Ceratopogonidae) in Turkey. Parasite. (2007) 14:173–7. doi: 10.1051/parasite/2007142173
45. DeFoliart, GR, Grimstad, PR, and Watts, DM. Advances in mosquito-borne arbovirus/vector research. Annu Rev Entomol. (1987) 32:479–505. doi: 10.1146/annurev.en.32.010187.002403
46. Standfast, HA, and Dyce, AL. Potential vectors of arboviruses of cattle and buffalo in Australia. Aust Vet J. (1972) 48:224–7. doi: 10.1111/j.1751-0813.1972.tb05139.x
47. Rawlings, P. A key, based on wing patterns of biting midges (genus Culicoides Latreille-Diptera: Ceratopogonidae) in the Iberian Peninsula, for use in epidemiological studies. Graellsia. (1996) 52:57–71. doi: 10.3989/graellsia.1996.v52.i0.376
48. Villard, P, Muñoz, F, Balenghien, T, Baldet, T, Lancelot, R, and Hénaux, V. Modeling Culicoides abundance in mainland France: implications for surveillance. Parasit Vectors. (2019) 12:391. doi: 10.1186/s13071-019-3642-1
49. Takken, W, and Verhulst, NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. (2013) 58:433–53. doi: 10.1146/annurev-ento-120811-153618
50. Kundlacz, C, Caignard, G, Sailleau, C, Viarouge, C, Postic, L, Vitour, D, et al. Bluetongue virus in France: an illustration of the European and Mediterranean context since the 2000s. Viruses. (2019) 11:672. doi: 10.3390/v11070672
51. Ségard, A, Gardès, L, Jacquier, E, Grillet, C, Mathieu, B, Rakotoarivony, I, et al. Schmallenberg virus in Culicoides Latreille (Diptera: Ceratopogonidae) populations in France during 2011-2012 outbreak. Transbourd Emerg Dis. (2018) 65:e94–e103. doi: 10.1111/tbed.12686
52. Žiegytė, R, Platonova, E, Kinderis, E, Mukhin, A, Palinauskas, V, and Bernotienė, R. Culicoides biting midges involved in transmission of haemoproteids. Parasit Vectors. (2021) 14:27. doi: 10.1186/s13071-020-04516-1
53. Žiegytė, R, Bernotienė, R, and Palinauskas, V. Culicoides segnis and Culicoides pictipennis biting midges (Diptera, Ceratopogonidae), new reported vectors of Haemoproteus parasites. Microorganisms. (2022) 10:898. doi: 10.3390/microorganisms10050898
54. Braverman, Y, and Linley, JR. Fecundity and proportions of gravid females in populations the bluetongue vector Culicoides imicola (Diptera: Ceratopogonidae) and several other species in Israel. J Med Entomol. (1994) 31:838–43. doi: 10.1093/jmedent/31.6.838
Keywords: France, cattle, Culicoides , vectors, pathogen
Citation: Millot C, Hadj-Henni L and Augot D (2024) Culicoides biting midges among cattle in France: be wary of data in the literature. Front. Vet. Sci. 11:1451442. doi: 10.3389/fvets.2024.1451442
Edited by:
Faham Khamesipour, Tehran University of Medical Sciences, IranReviewed by:
Chris Chase, South Dakota State University, United StatesKarien Labuschagne, Agricultural Research Council of South Africa (ARC-SA), South Africa
Copyright © 2024 Millot, Hadj-Henni and Augot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine Millot, Y2hyaXN0aW5lLm1pbGxvdEB1bml2LXJlaW1zLmZy; Denis Augot, ZGVuaXMuYXVnb3RAYW5zZXMuZnI=
†ORCID: Denis Augot, orcid.org/0000-0001-5425-5525
 Christine Millot1*
Christine Millot1* Denis Augot
Denis Augot