
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 20 December 2024
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1450241
This article is part of the Research TopicExploring Antinutritional Factors in Animal Feed: Implications for Health and ProductivityView all 8 articles
 Jiajin Sun
Jiajin Sun Zhonghao Wang
Zhonghao Wang Xinyu Yan
Xinyu Yan Yuqi Zhao
Yuqi Zhao Li Tan
Li Tan Xuning Miao
Xuning Miao Rong Zhao
Rong Zhao Wenjie Huo
Wenjie Huo Lei Chen
Lei Chen Qinghong Li
Qinghong Li Qiang Liu
Qiang Liu Cong Wang
Cong Wang Gang Guo*
Gang Guo*Aflatoxin B1 (AFB1) has been recognized as a serious health risk for ruminant animals. From a molecular perspective, indole-3-acid (IAA) possesses the potential to enhance the removal of AFB1 by rumen microbiota. Therefore, this study aims to investigate the impact of different concentrations of IAA on the removal of AFB1 by rumen microbiota using an in vitro technique. Experiment 1: interaction between AFB1 and rumen fermentation. Experiment 2: The study used a randomized design with five IAA levels (0, 15, 150, 1,500, and 7,500 mg/kg) to examine the effect of IAA on AFB1 removal and its impact on rumen fermentation. The results showed: (1) the content of AFB1 gradually decreased, removal rate of up to 75.73% after 24 h. AFB1 exposure altered the rumen fermentation pattern, with significantly decreased in the acetic acid/propionic acid ratio (p < 0.05). It significantly reduced the relative proportions of R. amylophilus, P. ruminicola, and F. succinogenes (p < 0.05). (2) As the content of IAA increased, AFB1 exposure decreased. A total of 15 and 150 mg/kg IAA significantly mitigated the negative impact of AFB1 on key rumen bacteria (R. amylophilus, P. ruminicola and F. succinogenes), increased acetate levels and acetate/propionate ratio (p < 0.05). However, 1,500 mg/kg IAA lowered levels of propionate and isovalerate, adversely affected enzyme activities (pectinase, xylan and Carboxymethyl-cellulase) and relative proportions of microbiota (R. flavefaciens, P. ruminicola and F. succinogenes). In conclusion, IAA significantly removed AFB1, and in the range of 150 mg/kg of IAA reduced the negative effects of AFB1 on in vitro fermentation characteristics and fermentation end-products.
Aflatoxin B1 (AFB1) are toxic secondary metabolites produced during the growth of molds and commonly found in grains and animal feed (1–3). AFB1 exposure may reduce feed intake and weight gain, disrupt rumen microbiota balance, impair liver and kidney function, and negatively affect overall health (4, 5). Additionally, AFB1 can be transferred into animal products (meat and milk), thereby posing a risk to human health (6, 7). A recent review by Eskola et al. (8) suggested that about 60 to 80% of the global food crops are contaminated with mycotoxins. In the subtropical region, the highest concentration was 3.76 mg/kg (9). Ma et al. (10) collected 742 feed ingredients samples from various regions of China. Among them, more than 83.3% of the samples was contaminated AFB1 at different concentrations. The highest concentration in China exceeded the standard of 1.6 mg/kg (9). Previous studies had shown that rumen microbiota have the ability to remove AFB1 (7, 11, 12), but the ability is limited. Therefore effective methods of enhancing the removal capacity of the original rumen microbiota for AFB1 are necessary to be developed.
Regarding the food safety, dietary interventions with plant-derived additives are a promising approach to promoting the removal of AFB1 by rumen microbiota. In mammals, Indoleacetic-3-acid (IAA) is an important indole-derivative catabolized from dietary tryptophan by the intestinal microbiota, but intestinal dysbiosis can influence IAA production (13). IAA has various special functions in microbiota metabolism (14, 15), and has a certain regulatory effect on the synthesis of tryptophan and cytochromes (16). Cytochrome CYP can oxidize AFB1, forming AFB1-8, 9-epoxide (17, 18). Moreover, IAA was able to restore the intestinal microbiota balance and maintain its stability (19). But there are few reports on the removal of rumen microbiota and AFB1 by IAA. The objective of the present study was therefore to evaluate different dose of IAA in the rumen effect of the removal rate, ruminal fermentation profile, enzyme activity, and microbiota in vitro.
Experiment 1: They were divided into 80 bottles and allocated to two groups: (1) the CK group, blank control group without AFB1; (2) the AFB1 group, with 1 mg/kg AFB1. The in vitro fermentation was independently conducted seven times for 0, 1, 2, 3, 4, 5, 24, and 48 h. Experiment 2: The removal rate of AFB1 in Experiment 1 was used to select 24 h as the fermentation time for Experiment 2. They were divided into 20 bottles and allocated to five groups: (1) the AFB1 group, with 1 mg/kg AFB1; (2) 1 mg/kg AFB1 + 15 mg/kg IAA group; (3) 1 mg/kg AFB1 + 150 mg/kg IAA group; (4) 1 mg/kg AFB1 + 1,500 mg/kg IAA group; (5) 1 mg/kg AFB1 + 7,500 mg/kg IAA group. Each treatment was performed inquintuplicate. The fermentation liquid was collected and stored in liquid nitrogen, pre-column derivatization (20) was carried out to detect AFB1 content and rumen fermentation indicators.
In vitro ruminal fermentation followed the method described by Longland et al. (21). The rumen fluid was collected from three Holstein dry cows (650 kg ± 20 kg) before the morning feeding. The ingredients and chemical composition of total mixed ration are presented in Table 1. The mixture of the rumen fluid was filtered by four layers of gauze, mixed with buffer solution (v/v = 1:1), and kept at 39°C in a water bath while continually flushed with CO2. A total of 0.2 g alfalfa silage (DM, 364.20 g·kg−1 FM; CP, 173.2 g·kg−1 DM; NDF, 389.5 g·kg−1 DM; ADF, 285.5 g·kg−1 DM) and 0.2 g starch (DM, 85.24 g·kg−1 FM; Starch, 612.1 g·kg−1 DM; CP, 55.8 g·kg−1 DM; NDF, 87 g·kg−1 DM; ADF, 21.9 g·kg−1 DM) as the fermentation substrate and placed in nylon bags. Afterwards, 60 mL of the diluted rumen fluid was divided into individual fermentation bottles, sealed, and placed in a shaker (120 rpm) at 39°C (22). Upon at 24 h, it was immediately removed, stopped in an ice bath, and samples were taken for detection of various indicators.
A total 1 mL of the collected rumen fluid was subjected to pre-column derivatization (20). The content of AFB1 was determined using an Agilent 1,260 Infinity II high-performance liquid chromatograph (HPLC) with a C18 column, 4.6 × 250 nm, 5 μm. The mobile phase was acetonitrile-water (20:80); column temperature: 40°C; mobile phase flow rate: 1.0 mL/min; injection volume: 20 μL; excitation wavelength 360 nm, emission wavelength 440 nm; detection time 20 min. The degradation rate of AFB1 = (AFB1 content in the blank control - AFB1 content in the experimental group)/AFB1 content in the blank control × 100%.
pH was measured using a pH meter (LE438, Mettler Toledo Instruments Co., Ltd. China). For the other analyses, 25% meta-phosphoric acid was added to the fermentation fluid (1/5, v/v), and then samples were centrifuged for 10 min at 10,000× g at 4°C using a high-speed freezing centrifuge (Eppendorf 5810R, Eppendorf AG, Hamburg, Germany). The supernatant was collected and stored at −80°C for NH3-N and VFA determinations. The content of ammonia nitrogen (NH3-N) was determined using the phenol-hypochlorous acid colorimetric method (23). The content of volatile fatty acids (VFA) (Acetic acid, Propionic acid, Butyric acid, Isobutyric acid, Valeric acid and Isovaleric acid) was determined using a high-performance gas chromatograph (GC-TRACE 1300, column model 30 m × 0.25 mm × 0.25 μm) (24). The determination of the four types of cellulase (Carboxymethyl cellulase, Pectinase, Xylanase, α-glicosidase) was carried out according to the method described by Agarwal et al. (25).
Ruminal microbial DNA was extracted according to the method of Edrington TS (26). qPCR (primers were listed in Table 2) was used to determine the relative abundance of 10 bacteriain the incubation fluid. Real-time PCR was carried out on an Applied Biosystems Step One Plus Fast Real-Time PCR System (Applied Biosystems Co., USA). The reaction mixture (20 μL) were mixed with SYBR Premix TaqTM (10 μL, TaKaRa Biotechnology Co., Ltd., Dalian, China), ddH2O (7.0 μL), PCR forward or reverse primer (0.2 μmol L−1, 0.8 μL), ROX Reference Dye (0.4 μL, 50×) and the template DNA (1 μL). The number of cycles required to reach a threshold adjusted for each taxon (Ct) was recorded for eachsample. The PCR programs included initial denaturation (1 cycle of 50°C for 2 min and 95°C for 2 min), primer annealing and product elongation [40 cycles of 95°C for 15 s and 60°C for 1 min (27, 28)].
Data of experimental 1 using Statistical Analysis System (paired sample T-test). Data of experimental 2 using the general linear model (GLM) procedure of Statistical Analysis System (SAS, 1999). The repeated measures model accounted for the fixed effects at different level of treatment. Tukey’s test was used for the multiple comparisons among mean values and linear and quadratic effects were calculated at p < 0.05, (*p < 0.05; **p < 0.001). p < 0.05 was accepted as statistically significant, and p-values between 0.05 and 0.10 were considered to represent a statistical trend. Figures were drawn using KingDrawPc V3.0.2.20 (Qingdao Qingyuan Precision Agriculture Technology Co., Ltd., Qingdao, China), GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA), and Adobe Illustrator 2022–26.0 (Adobe Systems, San Jose, CA, USA) for graphic illustration.
The removal efficiency of AFB1 exhibited a stable and significant increasing trend with the extension of incubation time (Figure 1). The removal rate of AFB1 increased rapidly in the first 24 h, after which the rate of removal slowed down between until 48 h. At 48 h, the removal rate of AFB1 can reach 80.09%. Considering the factors of removal efficiency, this experiment selects 24 h as the final fermentation time. Compared to the control group, AFB1 exposure significantly reduced the content of acetic acid (p < 0.05); meanwhile, the content of propionic acid, isobutyric acid valeric acid, and isovaleric acid significantly increased (p < 0.05). Further analysis shows that AFB1 exposure led to a significant decrease in the acetic acid/propionic acid ratio (p < 0.05). In addition, AFB1 exposure did not significantly affect the pH value and NH3-N content of the in vitro fermentation fluid.
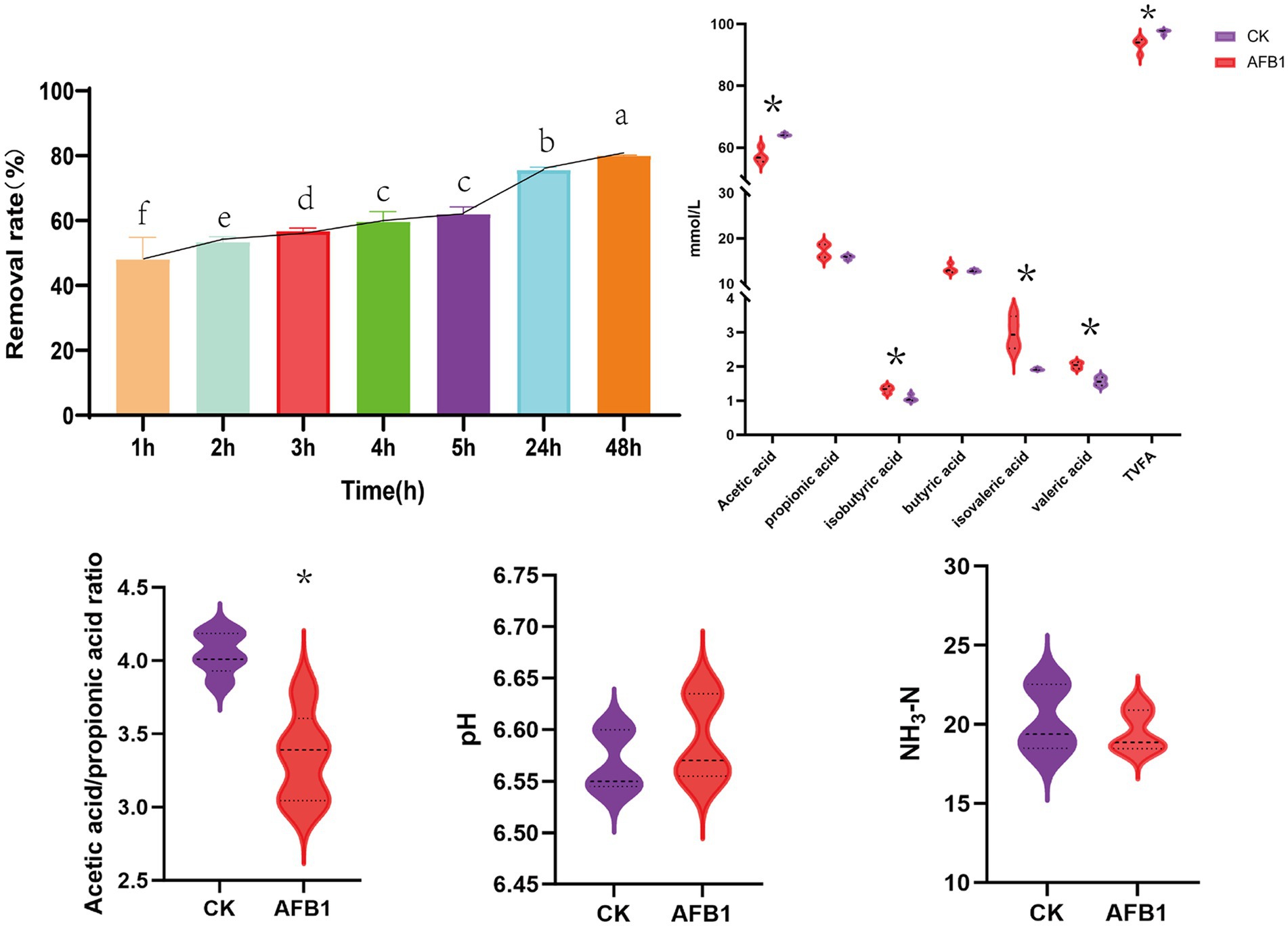
Figure 1. Interaction between AFB1 and rumen fermentation. CK: blank control group without AFB1, AFB1: with 1 mg/kg AFB1. *p < 0.05; **p < 0.001. Different letters indicate a significant difference.
Compared to the control group, AFB1 exposure has a significant (p < 0.05) effect on reducing the activity of xylanase, while it does not significantly affect the activity of pectinase, carboxymethyl cellulase and α-glucosidase (Figure 2).
As shown in Figure 3, the relative proportions of Prevotella ruminicola and Fusobacterium succinogenes significantly (p < 0.05) decreased after AFB1 exposure, indicating that the presence of AFB1 inhibits the growth of these two types of bacteria.
It can be observed that the content of IAA added showed a positive correlation with the removal rate of AFB1 (Figure 4). At concentration of 7,500 mg/kg IAA, AFB1 removal rate reached a maximum of 75.1%.
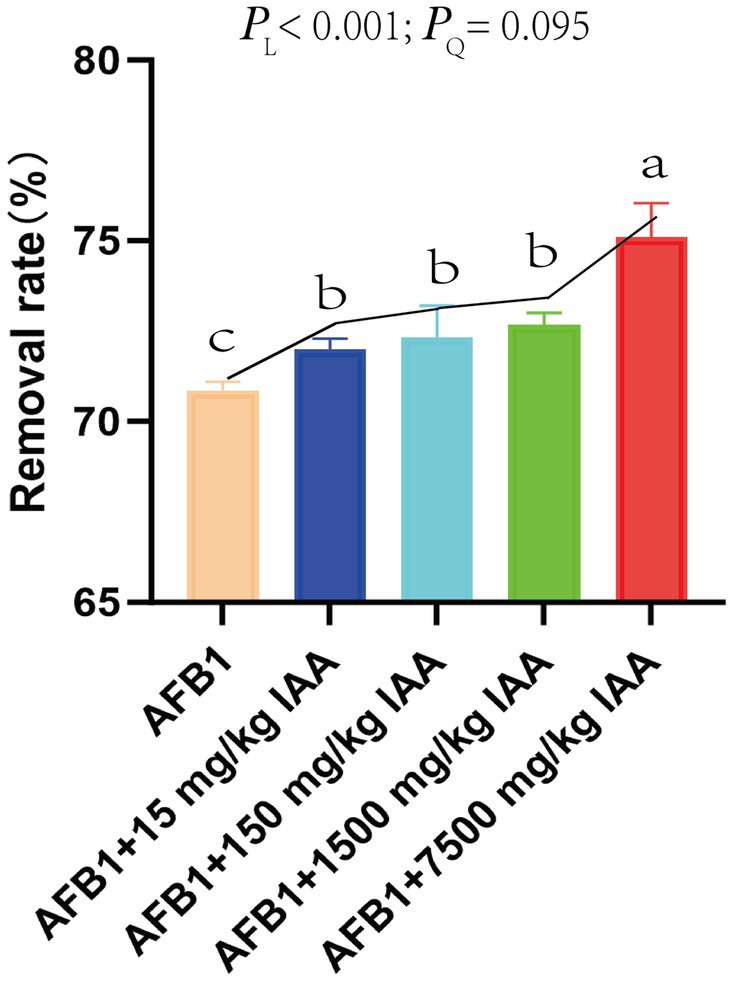
Figure 4. Effect of IAA supplementation on rumen fermentation and AFB1 removal. PL represents a linear response, PQ represents a quadratic linear response.
IAA at concentrations of 15 mg/kg and 150 mg/kg showed a positive correlation with the content of volatile fatty acids. Specifically, IAA at concentrations of 15 mg/kg and 150 mg/kg had a significant increasing trend in the content of acetic acid (PL = 0.001), propionic acid (PL = 0.012), butyric acid (PL = 0.005), isobutyric acid (PQ < 0.001), and valeric adid (PL = 0.002), isovaleric acid (PQ = 0.001) and total volatile fatty acids (TVFA) (PL = 0.031). Furthermore, at 1500 mg/kg IAA, the content of acetic acid (PL = 0.001), valeric acid (PL = 0.002), the ratio of acetic acid/propionic acid (PL = 0.001), and TVFA (PL = 0.031) in the fermentation broth significantly increased, but the content of propionic acid (PL = 0.012), butyric acid (PL = 0.005), isobutyric acid (PQ < 0.001), and isovaleric acid (PQ = 0.001) significantly decreased trend. The addition of IAA did not significantly affect the pH and NH3-N content in the fermentation fluid (Figure 5).
The impact of adding different concentrations of IAA on the activity of major fiber-degrading enzymes in vitro fermentation fluid is shown in Figure 6. A total of 15 and 150 mg/kg IAA significantly increased the activity of xylanase (p < 0.05) and showed an enhancing trend for pectinase (PL = 0.002) and carboxymethyl cellulase (PQ = 0.005). In contrast, 1,500 mg/kg concentrations of IAA significantly decreased the activity of pectinase (p < 0.05), exhibited a certain inhibitory effect on the activity of xylanase and carboxymethyl cellulase.
That 15, 150 and 1,500 mg/kg of IAA were added to the fermentation fluid, there was an increasing trend in the relative proportions of total bacteria (PL = 0.026), B. fibrisolvens (PL = 0.019), R. albus (PL = 0.014), R. amylophilsus (PL = 0.012), total methanogenic archaea (PL = 0.003), P. ruminicola (PL = 0.046) and F. succinogenes (PL = 0.003) (Figure 7). However, the relative proportions of R. flavefaciens had a downward trend (PL = 0.059).
Previous studies had found that some ruminal microbiota have the ability to removal AFB1 (26, 29). A total 1 mg/kg of AFB1 can cause a disturbance in the rumen microbiota and reduce the abundances of Prevotella and P. butyrivibrio (30). But higher concentrations (2.5 mg/kg) of AFB1 not only alter rumen fermentation pattern but also compromise the safety of liver function (26). In our study, as the fermentation time increased, AFB1 exposure gradually decreased, reaching a stable state after a period of time. Especially when the fermentation time reached 48 h, the removal efficiency of AFB1 can reach about 80%. This phenomenon can be attributed to two key factors: (1) ruminal microbiota reduce the toxicity of AFB1 through adsorption; (2) enzymes secreted by ruminal microbiota can break down the structure of AFB1, converting it into weakly toxic substances. In addition, research has shown that AFB1, as a harmful secondary metabolite widely present in feed and its raw materials. AFB1 can disrupt the balance of microbiota in the rumen when ingested by ruminants and enter the rumen, leading to change the rumen microbiota and affecting the normal fermentation process of the rumen (31, 32). In our study, the pH showed no significant changes, indicating that the in vitro fermentation test was normal. VFAs are the main source of energy for ruminants to obtain from feed (33), but the exposure of AFB1 has a significant inhibitory effect on the rumen microbiota, leading to decrease in the total volatile fatty acids (TVFA) and change in the fermentation type. This corresponds to previous studies by Cao et al. (30). In vitro fermentation experiments showed that after AFB1 exposure, the relative proportions of core microbiota in the rumen such as Prevotella and Fusobacterium decreased, these presented the similar results as acetic acid changes. In addition, AFB1 also reduced the activity of xylanase, consistent with the changes of B. fibrisolvens, while the activity of other enzymes such as pectinase did not change significantly, which may be related to the amount of AFB1 exposure. AFB1 exposure can lead to produce changes in pectase activity (34). Although the removal mechanism of AFB1 unclear, growing evidence has revealed that rumen microbiota dysbiosis is involved in the exposure of AFB1. Therefore, the restoration of rumen microbiota balance is likely to contribute to AFB1 removal.
IAA is a crucial biological regulatory substance for the growth of plants, which is considered to be an effective and environmentally friendly additive with the potential to modulate the balance of animal microbiota. Although research on IAA in rumen microbiota is still in its infancy, this experiment has confirmed that adding different concentrations of IAA to rumen fluid containing AFB1 can effectively reduce the content of AFB1. IAA can activates the expression of cytochrome genes, such as cytochrome P450 enzymes have been found in different organisms, that is a key enzyme that involve in the biotransformation of AFB1 and affects its toxicity (17). Moreover, IAA can maintain microbiota homeostasis and improve microbiota disorders by activating signal transduction pathways through its specific ligands (35). Although the mechanism by which IAA removes AFB1 is not yet clear, a growing body of evidence suggests that the rumen microbiota is involved in the removal of AFB1. Therefore, we have focused on the metabolic mechanisms of rumen microbiota with the addition of IAA. Considering that high concentrations of IAA may affect the balance of the rumen, according to the “Safety Use Specifications for Feed Additives” the recommended amount of tryptophan (the precursor of IAA) in the diet of ruminants is 0.1% (1 g/kg). The dosage of IAA is based on previous reports (50 mg/kg body weight) (36) and our preliminary studies, and with a rumen volume of 50 L, it is considered safe for cattle. In addition, some studies have shown that excessively high concentrations (50 mg/kg body weight) of IAA may change the animal microbiota (19). Therefore, this experiment further explored the specific impact of IAA additions of 15, 150, and 1,500 mg/kg on rumen fermentation parameters, aimed to verify the addition of a reasonable range of IAA concentrations to mitigate the impact of AFB1 on the rumen fermentation process, thereby enhancing animal health.
In our study, found that 150 and 15 mg/kg concentrations of IAA can significantly increase the content of TVFA, while 1,500 mg/kg concentrations of IAA tend to decrease. Indicate that the addition of IAA, enhances the rumen microbiota ability to utilize carbon sources and stimulates the degradation of nutrients within the rumen (37). In the experiment, after the addition of IAA, the content of acetic acid showed a linear upward trend, this is attributed to the metabolic fermentation products of R. amylophilus and P. ruminicola being primarily acetic acid (38, 39). With the addition of IAA, the observed changes in the microbiota, such as Prevotella and Fusobacterium, are closely related to the adjustment of VFAs composition (40, 41). These microbiota decompose the fiber in the feed, promoting the production of acetic acid and propionic acid. Propionic acid is key in the gluconeogenesis process, affecting body fat and lactose synthesis, this is primarily due to F. succinogene impact on the production of propionic acid through the propionate metabolism pathway (42–46). The concentration of IAA has a significant regulatory effect on propionic acid production, with 150 and 15 mg/kg concentrations of IAA causing a linear increase in propionic acid content, while 1,500 mg/kg concentrations of IAA reduce the level of propionic acid. F. succinogene also exhibited a trend of increasing first and then decreasing in this study. The change in butyric acid content is also related to the amount of IAA added, with 150 mg/kg and 15 mg/kg concentrations of IAA increasing butyric acid content, while 1,500 mg/kg concentrations of IAA reduce butyric acid content. B. fibrisolvens metabolize the production of butyric acid in rumen fluid, and the addition of 1,500 mg/kg IAA may make the microbiota rapidly metabolize and compete for rumen nutrients, resulting in the poor competitiveness of B. fibrisolvens and reducing the production of butyric acid (47). In addition, the regulation of the acetic acid/propionic acid ratio affects microbiota protein synthesis and the structure of the rumen microbiota, which relates to the digestion and nutritional metabolism of the whole body (48, 49). In the rumen ecosystem, bacteria can degrade and utilize starch and plant cell wall polysaccharides, such as xylan and pectin. These bacteria play an important role in the degradation of protein and the absorption and fermentation of peptides (50–53). The addition of IAA has a significant impact on the changes in the rumen microbiota and the activity of the main fiber-degrading enzymes. A total of 15 and 150 mg/kg concentrations of IAA had increased trend of the activity of pectinase, xylanase, and carboxymethyl cellulase, while 1,500 mg/kg concentrations of IAA reduced the activity of these enzymes. These results show that IAA alleviates the imbalance of the rumen fermentation caused by AFB1 by regulating the rumen microbiota and enzyme activity. R. albus, B. fibrisolvens and R. flavefaciens, as the main fiber-degrading bacteria, can produce xylanase. In this study, the significant increase in R. albus and B. fibrisolvens is consistent with the changes in xylanase. The decrease in xylanase with the addition of 1,500 mg/kg concentrations of IAA may be related to the downward trend of R. flavefaciens. The changes in carboxymethyl cellulose are mainly caused by P. ruminicola, and pectinase follows the relative changes of R. flavefaciens and B. fibrisolvens (36). In summary, the appropriate addition of IAA had a positive impact on the rumen microbiota and metabolic products, but 1,500 mg/kg concentrations of IAA may inhibit the rumen fermentation process. These findings provide important information for optimizing rumen fermentation and improving the nutritional absorption of ruminants.
This experiment preliminarily explored the impact of in vitro addition of IAA on the removal rate of AFB1 and rumen fermentation. It was found that IAA in the range of 150 mg/kg addition could improve the removal rate of AFB1 and reduce the negative effects of AFB1 on in vitro fermentation characteristics and fermentation end-products. This provides a new strategy to mitigate the potential threat of AFB1 to animal health.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The animal studies were approved by the Animal Welfare and Ethics Committee of Shanxi Agricultural University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
JS: Data curation, Writing – original draft, Writing-review & editing, Formal analysis. ZW: Investigation, Methodology, Writing – original draft. XY: Validation, Writing – review & editing. YZ: Software, Writing-review & editing. LT: Resources, Writing-review & editing. XM: Visualization, Writing-review & editing. RZ: Visualization, Writing – review & editing. WH: Funding acquisition, Writing-review & editing. LC: Visualization, Writing – review & editing. QinL: Conceptualization, Writing-review & editing. QiaL: Project administration, Writing – review & editing. CW: Supervision, Writing – review & editing. GG: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (32001405).
The authors thank the National Natural Science Foundation of China for financially supporting our experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fang, MX, Hu, W, and Liu, B. Protective and detoxifying effects conferred by selenium against mycotoxins and livestock viruses: a review. Front Vet Sci. (2022) 9:956814. doi: 10.3389/fvets.2022.956814
2. Yao, G, Yue, Y, Fu, Y, Fang, Z, Xu, Z, Ma, G, et al. Exploration of the regulatory mechanism of secondary metabolism by comparative transcriptomics in aspergillus flavus. Front Microbiol. (2018) 9:1568. doi: 10.3389/fmicb.2018.01568
3. Ismail, A, Akhtar, S, Riaz, M, Gong, YY, Routledge, MN, and Naeem, I. Prevalence and exposure assessment of aflatoxins through black tea consumption in the Multan city of Pakistan and the impact of tea making process on a flatoxins. Front Microbiol. (2020) 11:446. doi: 10.3389/fmicb.2020.00446
4. Wang, Q, Zhang, Y, Zheng, N, Guo, L, Song, X, Zhao, S, et al. Biological system responses of dairy cows to aflatoxin B1 exposure revealed with Metabolomic changes in multiple biofluids. Toxins. (2019) 11:77. doi: 10.3390/toxins11020077
5. Liew, WP, and Mohd-Redzwan, S. Mycotoxin: its impact on gut health and microbiota. Front Cell Infect Microbiol. (2018) 8:60. doi: 10.3389/fcimb.2018.00060
6. Pleadin, J, Lešić, T, Milićević, D, Markov, K, Šarkanj, B, Vahčić, N, et al. Pathways of mycotoxin occurrence in meat products: a review. PRO. (2021) 9:2122. doi: 10.3390/pr9122122
7. Rahimi, E, Bonyadian, M, Rafei, M, and Kazemeini, H. Occurrence of aflatoxin M1 in raw milk of five dairy species in Ahvaz Iran. Food Chem Toxicol. (2009) 48:129–31. doi: 10.1016/j.fct.2009.09.028
8. Eskola, M, Kos, G, Elliott, CT, Hajšlová, J, Mayar, S, and Krska, R. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25. Crit Rev Food Sci Nutr. (2020) 60:2773–89. doi: 10.1080/10408398.2019.1658570
9. Jallow, A, Xie, H, Tang, X, Qi, Z, and Li, P. Worldwide aflatoxin contamination of agricultural products and foods: from occurrence to control. Compr Rev Food Sci Food Saf. (2021) 20:2332–81. doi: 10.1111/1541-4337.12734
10. Ma, R, Zhang, L, Liu, M, Su, YT, Xie, WM, Zhang, NY, et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins. (2018) 10:113. doi: 10.3390/toxins10030113
11. Guo, C, Fan, L, Yang, Q, Ning, M, Zhang, B, and Ren, X. Characterization and mechanism of simultaneous degradation of aflatoxin B1 and zearalenone by an edible fungus of Agrocybe cylindracea GC-Ac2. Front Microbiol. (2024) 15:1292824. doi: 10.3389/fmicb.2024.1292824
12. Tang, Y, Liu, X, Dong, L, and He, S. Screening and identification of an aflatoxin B1-degrading strain from the Qinghai-Tibet plateau and biodegradation products analysis. Front Microbiol. (2024) 15:1367297. doi: 10.3389/fmicb.2024.1367297
13. Roager, HM, and Licht, TR. Microbial tryptophan catabolites in health and disease. Nat Commun. (2018) 9:3294. doi: 10.1038/s41467-018-05470-4
14. Wang, F, Niu, H, Xin, D, Long, Y, Wang, G, Liu, Z, et al. OsIAA18, an aux/IAA transcription factor gene, is involved in salt and drought tolerance in Rice. Front Plant Sci. (2021) 12:738660. doi: 10.3389/fpls.2021.738660
15. Ahmad, I, Song, X, Hussein Ibrahim, ME, Jamal, Y, Younas, MU, Zhu, G, et al. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front Plant Sci. (2023) 14:1108507. doi: 10.3389/fpls.2023.1108507
16. Chen, Q, Wang, J, Zhang, H, Shi, H, Liu, G, Che, J, et al. Microbial community and function in nitrogen transformation of ectopic fermentation bed system for pig manure composting. Bioresour Technol. (2021) 319:124155. doi: 10.1016/j.biortech.2020.124155
17. He, XY, Tang, L, Wang, SL, Cai, QS, Wang, JS, and Hong, JY. Efficient activation of aflatoxin B1 by cytochrome P 450 2A13, an enzyme predominantly expressed in human respiratory tract. Int J Cancer. (2006) 118:2665–71. doi: 10.1002/ijc.21665
18. Huang, L, Duan, C, Zhao, Y, Gao, L, and Li, S. Reduction of aflatoxin b1 toxicity by lactobacillus plantarum c88: a potential probiotic strain isolated from chinese traditional fermented food “tofu”. PLoS One. (2017) 12:e0170109. doi: 10.1371/journal.pone.0170109
19. Shen, J, Yang, L, You, K, Chen, T, Su, Z, Cui, Z, et al. Indole-3-acetic acid alters intestinal microbiota and alleviates ankylosing spondylitis in mice. Front Immunol. (2022) 13:762580. doi: 10.3389/fimmu.2022.762580
20. Haidukowski, M, Casamassima, E, Cimmarusti, MT, Branà, MT, and Longobardi, F. Aflatoxin B1-adsorbing capability of Pleurotus eryngii mycelium: efficiency and modeling of the process. Front Microbiol. (2019) 10:1386. doi: 10.3389/fmicb.2019.01386
21. Longland, AC, Theodorou, MK, Sanderson, R, Sanderson, R, Lister, SJ, Powell, CJ, et al. Non-starch polysaccharide composition and in vitro fermentability of tropical forage legumes varying in phenolic content. Anim Feed Sci Tech. (1995) 55:161–77. doi: 10.1016/0377-8401(95)00808-Z
22. Givens, DI, Owen, E, and Omed, HM. Forage evaluation in ruminant nutrition. CABI. (2000) 21:63–6. doi: 10.1079/9780851993447.0000
23. Weatherburn, MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. (1967) 39:971–4. doi: 10.1021/ac60252a045
24. Wang, C, Liu, Q, Li, HQ, Wu, XX, Guo, G, Huo, WJ, et al. Effects of rumen-protected pantothenate supplementation on lactation performance, ruminal fermentation, nutrient digestion and blood metabolites in dairy cows. J Sci Food Agric. (2018) 98:2098–104. doi: 10.1002/jsfa.8691
25. Mattei, V, Murugesan, S, Al Hashmi, M, Mathew, R, James, N, Singh, P, et al. Evaluation of methods for the extraction of microbial DNA from vaginal swabs used for microbiome studies. Front Cell Infect Microbiol. (2019) 9:197. doi: 10.3389/fcimb.2019.00197
26. Edrington, TS, Harvey, RB, and Kubena, LF. Effect of aflatoxin in growing lambs fed ruminally degradable or escape protein sources. J Anim Sci. (1994) 72:1274–81. doi: 10.2527/1994.7251274x
27. Wang, RF, Cao, WW, and Cerniglia, CE. PCR detection of Ruminococcus spp. in human and animal faecal samples. Mol Cell Probes. (1997) 11:259–65. doi: 10.1006/mcpr.1997.0111
28. Denman, SE, and McSweeney, CS. Developmentofa real-time PCR assay formonitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. (2006) 58:572–82. doi: 10.1111/j.1574-6941.2006.00190.x
29. Lin, LX, Cao, QQ, Zhang, CD, Xu, TT, Yue, K, Li, QH, et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol Environ Saf. (2022) 232:113225. doi: 10.1016/j.ecoenv.2022.113225
30. Cao, QQ, Lin, LX, Xu, TT, Lu, Y, Zhang, CD, Yue, K, et al. Aflatoxin B1 alters meat quality associated with oxidative stress, inflammation, and gut-microbiota in sheep. Ecotoxicol Environ Saf. (2021) 225:112754. doi: 10.1016/j.ecoenv.2021.112754
31. Guo, W, Fan, Z, Fan, K, Meng, J, Nie, D, Tangni, EK, et al. In vivo kinetics and biotransformation of aflatoxin B1 in dairy cows based on the establishment of a reliable UHPLC-MS/MS method. Front Chem. (2021) 9:809480. doi: 10.3389/fchem.2021.809480
32. Seeling, K, Lebzien, P, Dänicke, S, Spilke, J, Südekum, KH, and Flachowsky, G. Effects of level of feed intake and Fusarium toxin-contaminated wheat on rumen fermentation as well as on blood and milk parameters in cows. J Anim Physiol Anim Nutr (Berl). (2006) 90:103–15. doi: 10.1111/j.1439-0396.2005.00570.x
33. Zhao, YX, Bai, C, Ao, CJ, Lv, KX, Xu, SS, and Wang, XY. Effects of allium mongolicum regel essential oil on in vitro rumen fermentation and substrate dry matter degradation rate of sheep. Chin J Anim Nutr. (2021) 33:4740–7. doi: 10.3969/j.issn.1006-267x.2021.08.052
34. Krishnan, S, Ding, Y, Saedi, N, Choi, M, Sridharan, GV, Sherr, DH, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. (2018) 23:1099–111. doi: 10.1016/j.celrep.2018.03.109
35. Zhou, R, He, D, Xie, J, Zhou, Q, Zeng, H, Li, H, et al. Panax Quinquefoliusthe synergistic effects of polysaccharides and Ginsenosides from American ginseng (L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol. (2021) 12:665901. doi: 10.3389/fimmu.2021.665901
36. Ji, Y, Gao, Y, Chen, H, Yin, Y, and Zhang, W. Indole-3-acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients. (2019) 11:2062. doi: 10.3390/nu11092062
37. Huws, SA, Creevey, CJ, Oyama, LB, Mizrahi, I, Denman, SE, Popova, M, et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: past, present, and future. Front Microbiol. (2018) 9:2161. doi: 10.3389/fmicb.2018.02161
38. Liu, YR, Wang, C, Liu, Q, Guo, G, Huo, WJ, Zhang, YL, et al. Effects of branched-chain volatile fatty acids and fibrolytic enzyme on rumen development in pre-and post-weaned Holstein dairy calves. Anim Biotechnol. (2020) 31:512–9. doi: 10.1080/10495398.2019.1633340
39. Peng, K, Shirley, DC, Xu, ZJ, Huang, QQ, Tim, A, et al. Effect of purple prairie clover (Dalea purpurea vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim Feed Sci Tech. (2016) 222:100–10. doi: 10.1016/j.anifeedsci.2016.10.012
40. Ozbayram, EG, Ince, O, Ince, B, Harms, H, and Kleinsteuber, S. Comparison of rumen and manure microbiomes and implications for the inoculation of anaerobic digesters. Microorganisms. (2018) 6:15. doi: 10.3390/microorganisms6010015
41. Wu, ST, Ransom, L, Li, W, Li, C, Connor, EE, and Li, RW. The bacterial community composition of the bovine rumen detected using pyrosequencing of 16s rrna genes. Meta. (2012) 1:1–11. doi: 10.4303/mg/235571
42. Whitelaw, FG, Eadie, JM, Bruce, LA, and Shand, WJ. Methane formation in faunated and ciliate-free cattle and its relationship with rumen volatile fatty acid proportions. Br J Nutr. (1984) 52:261–75. doi: 10.1079/bjn19840094
43. Belanche, A, Palma-Hidalgo, JM, Jiménez, E, and Yáñez-Ruiz, DR. Enhancing rumen microbial diversity and its impact on energy and protein metabolism in forage-fed goats. Front Vet Sci. (2023) 10:1272835. doi: 10.3389/fvets.2023.1272835
44. Comtet-Marre, S, Parisot, N, Lepercq, P, Chaucheyras-Durand, F, Mosoni, P, Peyretaillade, E, et al. Metatranscriptomics reveals the active bacterial and eukaryotic Fibrolytic communities in the rumen of dairy cow fed a mixed diet. Front Microbiol. (2017) 8:67. doi: 10.3389/fmicb.2017.00067
45. Kido, K, Tejima, S, Haramiishi, M, Uyeno, Y, Ide, Y, Kurosu, K, et al. Provision of beta-glucan prebiotics (cellooligosaccharides and Kraft pulp) to calves from pre-to post-weaning period on pasture. Anim Sci J. (2019) 90:1537–43. doi: 10.1111/asj.13299
46. Palevich, N, Palevich, FP, Attwood, GT, and Kelly, WJ. Complete genome sequence of the rumen bacterium Butyrivibrio fibrisolvens D1T. Microbiol Resour Announc. (2024) 13:e00267–24. doi: 10.1128/mra.00267-24
47. Yin, ZH, Wang, MZ, Wang, HR, Zhang, J, and Yu, LH. Pattern and microorganisms diversities in rumen fluid in vitro. Chin J Anim Nutr. (2011) 23:2129–35. doi: 10.1046/j.1472-765X.1999.00671.x
48. Tedeschi, LO, Muir, JP, Naumann, HD, Norris, AB, Ramírez-Restrepo, CA, and Mertens-Talcott, SU. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front Vet Sci. (2021) 8:628445. doi: 10.3389/fvets.2021.628445
49. McAllister, TA, Cheng, KJ, Rode, LM, and Forsberg, CW. Digestion of barley, maize, and wheat by selected species of ruminal bacteria. Appl Environ Microbiol. (1990) 56:3146–53. doi: 10.1128/aem.56.10.3146-3153.1990
50. Cotta, MA. Interaction of ruminal bacteria in the production and utilization of maltooligosaccharides from starch. Appl Environ Microbiol. (1992) 58:48–54. doi: 10.1128/aem.58.1.48-54.1992
51. Stewart, CS, Flint, HJ, and Bryant, MP. Flint Bacteroides (Fibrobacter) succinogenes, a cellulolytic anaerobic bacterium from the gastrointestinal tract. Appl Microbiol Biotechnol. (1989) 30:433–9. doi: 10.1007/BF00263846
52. Burnet, MC, Dohnalkova, AC, Neumann, AP, Lipton, MS, Smith, RD, Suen, G, et al. Evaluating models of cellulose degradation by Fibrobacter succinogenes S85. PLoS One. (2015) 10:e0143809. doi: 10.1371/journal.pone.0143809
Keywords: indole-3-acetic acid, aflatoxin B1, rumen fermentation, rumen microbiota, removal rate
Citation: Sun J, Wang Z, Yan X, Zhao Y, Tan L, Miao X, Zhao R, Huo W, Chen L, Li Q, Liu Q, Wang C and Guo G (2024) Indole-3-acetic acid enhances ruminal microbiota for aflatoxin B1 removal in vitro fermentation. Front. Vet. Sci. 11:1450241. doi: 10.3389/fvets.2024.1450241
Received: 25 June 2024; Accepted: 25 November 2024;
Published: 20 December 2024.
Edited by:
Yun Ji, China Agricultural University, ChinaReviewed by:
Jinsong Liang, Hebei University of Technology, ChinaCopyright © 2024 Sun, Wang, Yan, Zhao, Tan, Miao, Zhao, Huo, Chen, Li, Liu, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Guo, Z2dnZzE5ODRAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.