
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 11 September 2024
Sec. Parasitology
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1446743
A new species of nematode, Molinostrongylus longmenensis n.sp., parasite of the genus Molinostrongylus, is described based on specimens recovered from the small intestine of Scotophilus kuhlii Leach, 1822 (Chiroptera: Vespertilionidae) in Longmen County, Guangdong Province, China. To date, 135 species of bat-parasitic nematodes have been reported worldwide. Overall, 13 species belonging to seven genera in three families have been described in China. The new species is characterized by the presence of three ventral and three dorsal longitudinal cuticular ridges perpendicular to the body surface, which appear posterior to the cephalic vesicle and extend to the caudal bursa in males and the posterior end in females. The female tail has two medium-sized subventral conical processes of equal length, as well as one large dorsal conical process, and one thin spine, lateral alae that extend to the position of the vulva, with a fin-like ending. In addition, the new species was also characterized using molecular approaches, such as sequencing and analyzing the internal transcribed spacer 1 (ITS-1) of the ribosomal DNA.
The lesser yellow house bat, Scotophilus kuhlii Leach, 1822 (Chiroptera: Vespertilionidae), is a nocturnal insectivorous bat species that is mainly distributed in southern China and Southeast Asia. It inhabits buildings, dead fronds of palm plants, or the undersides of leaves. Nests under bridge piers can also be used as habitats. S. kuhlii is a dominant species in Guangdong Province, China (1–4). From January 2008 to April 2010, Professor Li Haiyan and her team (College of Life Sciences, Guangzhou University) conducted a comprehensive biodiversity survey of bats across Guangdong Province, China. This study was supported by the Natural Science Foundation of Guangdong Province (8151009101000005) (5). For our research, Professor Li's team provided the nematode specimens collected from the small intestine of S. kuhlii.
The specimens were identified as a new member of the genus Molinostrongylus (Skarbilovitch, 1934). Durette-Desset and Chabaud (6) classified the genus Molinostrongylus into five groups. The group alatus is characterized by two lateral alae, three small ventral ridges, and three small dorsal ridges, with the distal end of the spicules divided into two branches. The morphology of the specimen described enables us to categorize it within group alatus, but the new member has significant differences from other members of this group. Furthermore, the new species was characterized via molecular approaches by sequencing and analyzing the ITS-1 of the ribosomal DNA.
To date, 135 species of bat-parasitic nematodes have been reported worldwide, belonging to 4 orders, 13 families, and 26 genera. Only 13 species from seven genera in three families have been described in China, which are distributed in Henan, Yunnan, Guangdong, Guangxi, and Taiwan provinces (7, 8). Four species of parasitic nematodes have been reported in bats of the genus Scotophilus: Molinostrongylus scotophili (Ferenc, 1973) and Capillaria vietnamensis (Meszaros, 1973) parasites in Scotophilus temminckii (9) and Rictularia rhinolophi (Kagei and Sawada, 1977) (10) and Seuratum indicum (Dey, 2003) parasites in Scotophilus heathi (11).
Here, we report a new species of nematodes from S. kuhlii, thus contributing to the inventory of the helminth biodiversity of this host.
From January 2008 to April 2010, Professor Li Haiyan and her research team collected a variety of bat species. Their findings included two Rhinolophus pearsonii in Yingde City, 36 Rhinolophus sinicus, five Scotophilus heathi in Huizhou City, seven Taphozous melanopogon, five Hipposideros armiger, and 35 S. kuhlii in Longmen County. Those 35 individuals of S. kuhlii were captured using harp traps. Euthanasia of the collected bats was performed in accordance with the Laboratory Animal—Guidelines for Euthanasia (GB/T 39760-2021). Nematode specimens isolated from the intestine of this host were washed in physiological saline, fixed, and then stored in 75% ethanol. The specimens were provided to our laboratory for study.
For light microscopic studies, the nematodes were cleaned with lactophenol. Photomicrographs were captured using a Nikon® digital camera coupled with a Nikon® optical microscope. Drawings were performed using a Nikon microscope drawing attachment. For scanning electron microscopy (SEM), specimens were fixed in 4% formaldehyde solution, then post-fixed in 1% OsO4, dehydrated with ethanol and acetone, and critical point dried. The samples were coated with gold and examined using a Hitachi S-4800 scanning electron microscope at an accelerating voltage of 20 kV. Unless noted otherwise, measurements are given in micrometers (μm), with the range followed by the mean in parentheses. The nomenclature for the caudal bursa is based on Durette-Desset et al. (7) and Durette-Desset and Digiani (12). The synlophe was studied according to the method provided by Durette-Desset (13). The voucher specimens were deposited in the College of Life Sciences, Hebei Normal University, Hebei Province, China.
Two female specimens were randomly chosen for molecular analysis. According to the manufacturer's instructions, genomic DNA from each sample was extracted using a Column Genomic DNA Isolation Kit (Shanghai Sangon, China). The ITS-1 region was amplified by polymerase chain reaction (PCR) using the forward primer NC1 (forward: 5′-AACAACCCTGAACCAGACGT-3′) and the reverse primer NC5R (reverse: 5′-AATGATCCTTCCGCAGGTTCACCTAC-3′) (14). The cycling conditions were as described previously (15). The PCR products were examined on GoldView-stained 1.5% agarose gels and purified using the Column PCR Product Purification Kit (Shanghai Sangon, China). Sequencing was carried out using a Dye Deoxy Terminator Cycle Sequencing Kit (v.2, Applied Biosystems, California, USA) and an automated sequencer (ABI-PRISM 377). Sequences were aligned using ClustalW2. The DNA sequences obtained herein were compared (using the algorithm BLASTn) with those available in the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov) (16, 17).
Based on the ITS-1 sequence data, phylogenetic trees were constructed using maximum likelihood (ML) inference with MEGA 6.0 software. Amidostomoides monodon and Amidostomoides acutum (Strongylida, Trichostrongyloidea, and Amidostomatidae) were treated as the outgroup. The ingroup included 12 species of six genera in one family: Molineidae. We used a built-in function in the software MEGA 6.0 to select a best-fitting substitution model for the present sequences according to the Bayesian information criterion. The K2 (Kimura 2-parameter) + G model for the ITS-1 sequence data was identified as the optimal nucleotide substitution model. Reliabilities for the ML tree were tested using 1,000 bootstrap replications, and the BI tree was tested using 50 million generations, and bootstrap values exceeding 70% were shown in the phylogenetic tree (18, 19).
A total of 36 specimens of the genus Molinostrongylus (16 male and 20 female, sex ratios 4/5), were obtained from the small intestines of 15 host individuals among the 35 Scotophilus kuhlii examined. The overall prevalence of Molinostrongylus spp. in these bats was 43% (15 out of 35). No nematodes of the genus Molinostrongylus were detected in the other five species of bats (R. pearsonii, R. sinicus, S. heathi, T. melanopogon, and H. armiger) in this survey. A new species of nematode, Molinostrongylus longmenensis n.sp. is described based on 23 specimens (11 males and 12 females).
General: Small-sized, whitish nematodes. Body cylindrical, maximum width at about region of middle. Cuticle with fine transverse striations. Cephalic vesicles distinct in both sexes (Figures 1B, C, 2B, 3B, 4A, B). Anterior extremity with a triradiate oral aperture, surrounded by a pair of amphids and four cephalic papillae (Figure 1C). Esophagus clavate, slightly broader posteriorly than anteriorly (Figure 3A). Lateral alae are present in both sexes. Lateral alae extending to caudal bursa in males (Figures 2E, 3H), and to the vulva, ending fin-like in females (Figures 1E, F, 3C, 4E, F). Nerve ring at approximately one-third of the esophageal length. Excretory pores situated at approximately two-third of esophageal length. Deirids slightly posterior to excretory pore (Figure 3A). Cuticular excretory furrow absent.
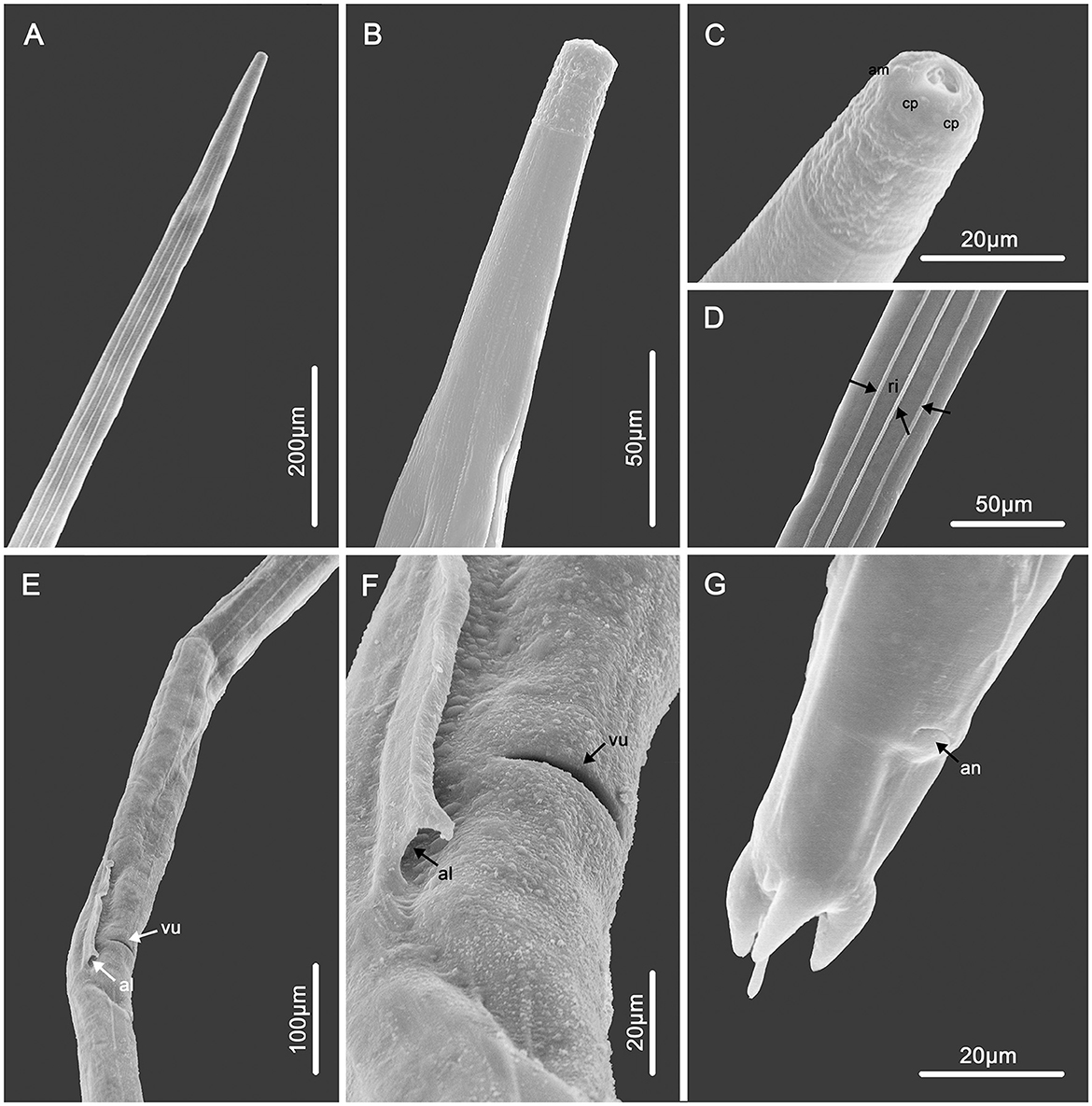
Figure 1. Scanning electron micrographs of female Molinostrongylus longmenensis n. sp. from Scotophilus kuhlii. (A) Anterior part, dorsal view. (B) Anterior part, ventral view. (C) Cephalic extremity, sub-apical view, amphid (am), and cephalic papillae (cp). (D) Mid-body, ventral view, showing the longitudinal ridges (arrows, ri). (E) Vulval region, lateral ventral view, vulva (arrow, vu), and the end of alae (arrow, al). (F) Magnified vulval region, vulva (arrow, vu), the end of alae (arrow, al). (G) Posterior end, lateral ventral view.
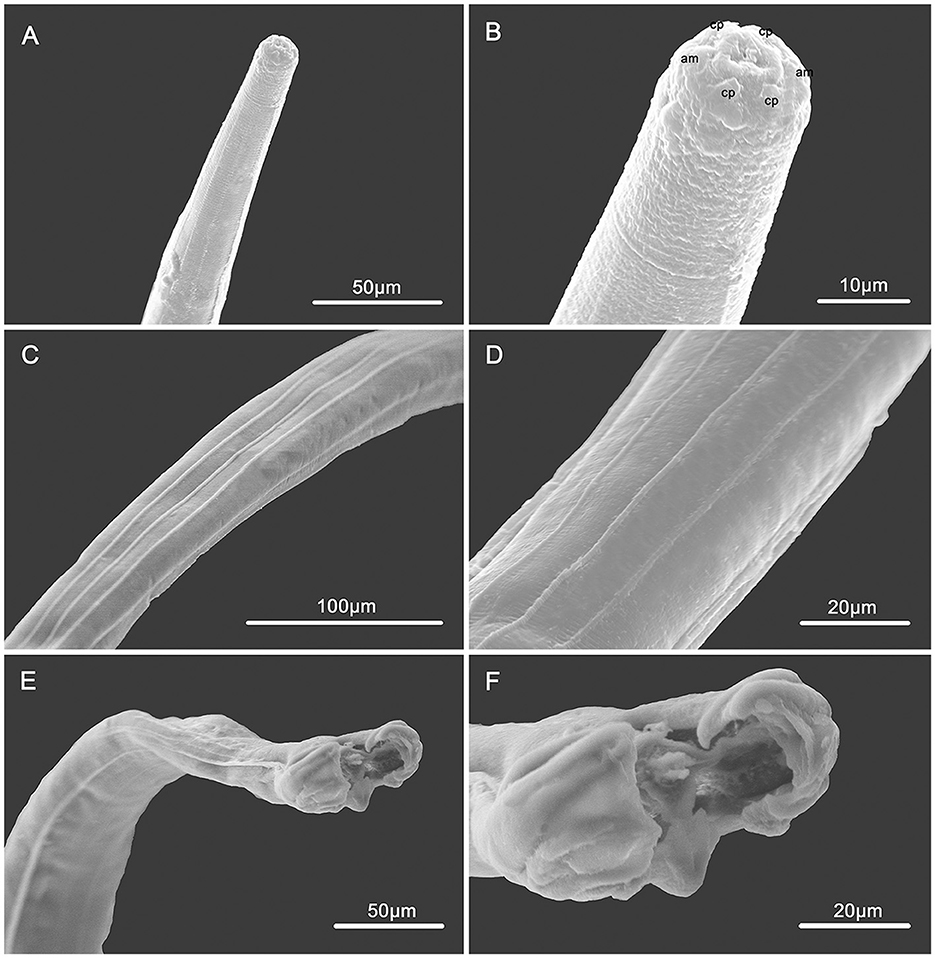
Figure 2. Scanning electron micrographs of male Molinostrongylus longmenensis n. sp. from Scotophilus kuhlii (A) Anterior part, lateral view. (B) Cephalic extremity, sub-apical view, amphid (am), and cephalic papillae (cp). (C) Mid-body, dorsal view, showing the longitudinal ridges. (D) Magnified mid-body, ventral view, showing the longitudinal ridges. (E) Posterior end, bottom view. (F) Caudal bursa, bottom view.
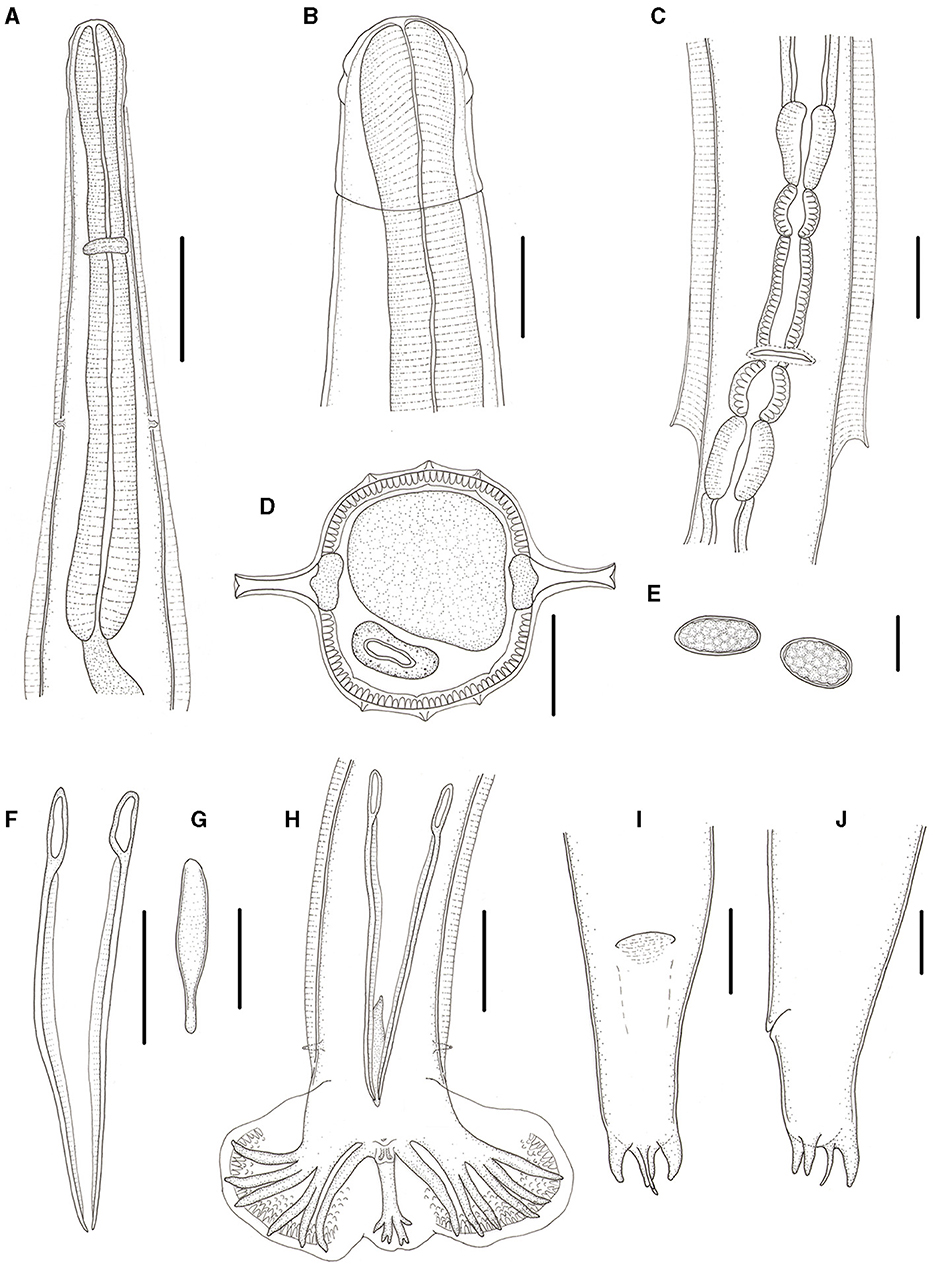
Figure 3. Molinostrongylus longmenensis n. sp. is collected from Scotophilus kuhlii (Leach; Chiroptera: Vespertilionidae) in China. (A) Anterior extremity, ventral view, male. (B) Anterior end, lateral view, female. (C) Region of vulva, ventral view. (D) Transverse section at mid-body, male. (E) Egg. (F) Spicules, ventral view. (G) Gubernaculums, ventral view. (H) Posterior end, ventral view, male. (I) Posterior end, ventral view, female. (J) Posterior end, lateral view, female. Scale bars (A, C, E–J) = 50 μm, (B) = 20 μm, and (D) = 30 μm.

Figure 4. Photomicrographs of Molinostrongylus longmenensis n. sp. collected from Scotophilus kuhlii (Leach; Chiroptera: Vespertilionidae) in China. (A) Anterior extremity, ventral view, male. (B) Anterior end, lateral view, female. (C) Posterior end, dorsal view, female. (D) Posterior end, lateral view, female. (E) Vulval region, ventral view. (F) Magnified vulval region, ventral view. (G) Magnified posterior end of male, showing the dorsal ray. (H) Posterior end, ventral view, male. Scale bars (A, E) = 100 μm; (B–D, F–H) = 20 μm, and (G) = 50 μm.
Synlophe (studied on one male and one female): In both sexes, the cuticle bears three ventral and three dorsal uninterrupted ridges, which appear posterior to the cephalic vesicle, extend to the caudal bursa in males (Figures 2A, C–F), and have a posterior end in females (Figures 1A, D, E). Longitudinal ridges are oriented perpendicularly to the body surface (Figure 3D). Three ventral and three dorsal ridges are nearly the same size in both males and females and are nearly parallel along the body.
Male (based on one holotype and nine paratypes): body 2.5–3.1 (2.8) mm long, maximum width 70–82 (75); cephalic vesicle 32–44 (35) long and 22–35 (28) wide; nerve ring, deirids, and excretory pore situated at 95–110 (103), 170–185 (177), and 165–176 (173), respectively, from anterior extremity; esophagus 240–285 (263) long, 30–50 (39) in maximum width; lateral alae extending to caudal bursa; three uninterrupted longitudinal cuticular ridges appear posterior to cephalic vesicle extending to caudal bursa. Caudal bursa symmetrical, lateral lobes with dotted ornamentation larger than dorsal lobe (Figures 2F, 3H, 4G, H). Pattern of caudal bursa being of type 3–2. Rays 3 and 4, rays 5 and 6 grouped with divergent extremities. Rays 2, 3, and 4 emerge together at the base of the trunk. Rays 3 and 4 bifurcated at the middle of the trunk. Rays 5 and 6 bifurcated at one-third of the trunk. Rays 2, 3, 4, 5, and 6 are almost equal in length with distal extremities almost reaching the bursal margin and directed ventrally. Ray 8 arises from the basis of dorsal ray, and is slightly longer than the latter. Dorsal ray bifurcates at the distal extremity into two branches, ray 9 arising first, and ray 10 divided into two branches (Figure 3H). Spicules relatively long and slender, length 169–181 (175) almost equal. Spicules with handles thicker and shorter than blade, blade with a striated ala extending from handle to tip of the blade (Figure 3F). Gubernaculum 39–50 (45) long, bowling pin shape, slightly curved in lateral view (Figure 3G). Genital cone well-developed with two presenting papillae 7 in each extremity, papillae zero presence (Figure 3H).
Female (based on 1 allotype and 10 paratypes): Body 3.0–4.2 (3.5) mm long, maximum width 80–95 (87). Cephalic vesicle 39–55 (45) long, 31–43 (36) wide. Nerve ring, deirids, and excretory pores situated at 132–147 (140), 200–221 (211), and 192–210 (202), respectively, from the anterior extremity. Esophagus 350–380 (358) mm in total length, 30–53 (40) in maximum width. Lateral alae 21–25 (23) in maximum width, extending to the vulva, and ending fin-like. Didelphic. Vulva transverse slit, 1.25–1.5 (1.35) mm from anterior extremity (Figures 1E, F). Vagina vera short and oriented perpendicular to the body surface, 15–22 (17) long, divided vestibule into two unequal parts, anterior part 50–92 (75) long, posterior part 10–22 (15) long. Anterior sphincter and infundibulum 30–45 (38) and 40–80 (62) long, respectively; posterior sphincter and infundibulum 39–46 (42) and 43–63(53) long, respectively (Figure 3C). Anterior uterine branch 0.8–1.2 (1.1) mm long, posterior uterine branch 0.8–1.3 (1.1) mm long. Each uterine branch with 8–15 eggs, the eggs are in the morula stage, irregularly oval, 50–98 (77) long and 29–65 (44) wide (Figure 3E). Anus 60–73 (68) from posterior extremity. Tail thick conical, with two medium-sized subventral conical processes equal in length, one large dorsal conical process, and one thin spine. Two of the conical processes equal in length, 13–18 (15) long; the other shorter one 6–9 (7) long. The thin spine 13–18 (15) in length (Figures 1G, 3I, J, 4C, D).
Genetic characterization: The three ITS-1 sequences of M. longmenensis n. sp., obtained in this study, are all 633 base pairs (bp) in length and correspond to a single genotype. Two ITS-1 sequences of M. spasskii obtained herein are all (632) bp in length and represent one genotype. A pairwise comparison of ITS-1 sequences between M. longmenensis n. sp. and M. spasskii displayed 1.58% nucleotide divergence. The ITS-1 sequences of M. longmenensis n. sp. and M. spasskii have been deposited in the GenBank database under the accession numbers PP853664–PP853666 (the sequences of M. longmenensis n. sp. are identical; the serial number is PP853664. The two ITS-1 sequences of M. spasskii are identical, depending on the collection location, respectively, PP853665 and PP853666).
Phylogenetic trees inferred from ML and Bayesian inference (BI) revealed that representatives of Molineidae were divided into three major clades (Figure 5). Clade I included the species of two genera, Nematodirus and Nematodirella; Nematodirus included four species, and Nematodirella included only one species (Nematodirella cameli). Nematodirella cameli displayed a closer relationship with Nematodirus. Clade II included the species of four genera, Nematodiroides, Oswaldocruzia, Durettenema, and Molinostrongylus. Among the four genera, Durettenema exhibited a closer phylogenetic relationship to Molinostrongylus than to Nematodiroides and Oswaldocruzia. Clade III included only Nematodirus battus.
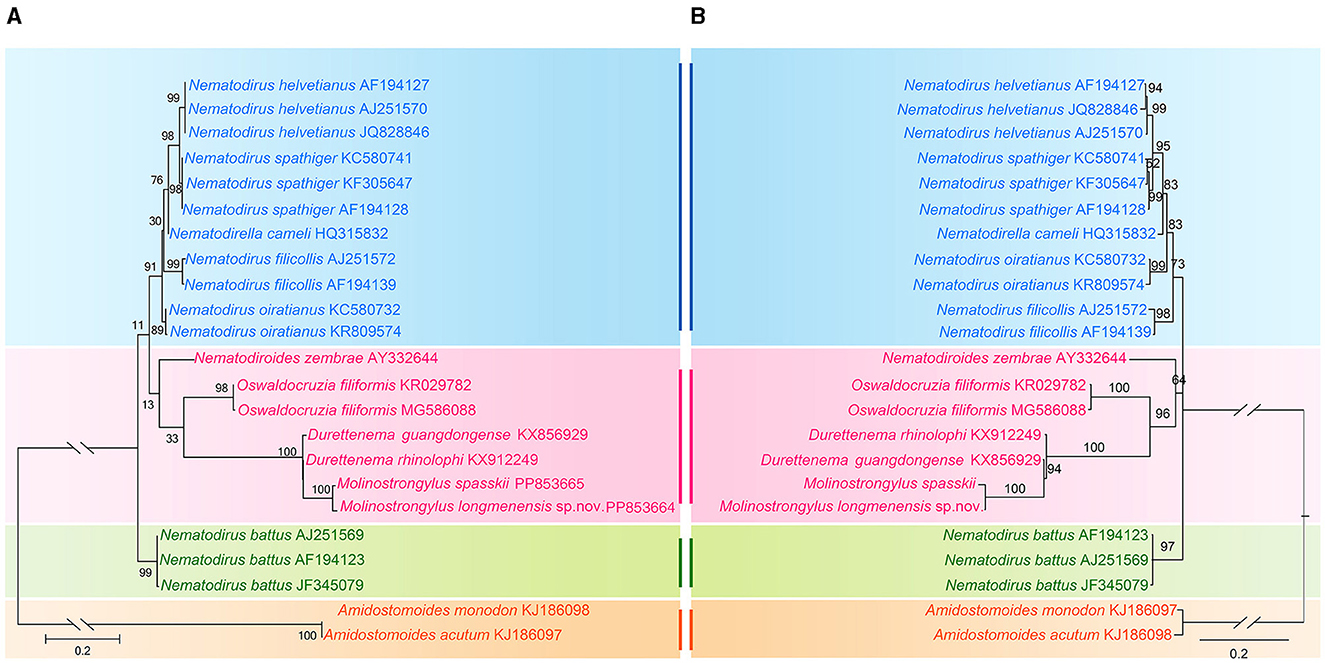
Figure 5. Phylogenetic relationships of representatives of the family Molineidae using maximum likelihood (A) and Bayesian inference (B) analyses based on the ITS-1 sequences. Amidostomoides monodon and Amidostomoides acutum (Strongylida, Trichostrongyloidea, and Amidostomatidae) was chosen as the outgroup. Bootstrap values exceeding 70% are shown in the phylogenetic trees.
Molinostrongylus longmenensis n. sp.
Type host: Scotophilus kuhlii Leach, 1822 (Chiroptera: Vespertilionidae).
Site of infection: Small intestine.
Prevalence: 43% (15 out of 35 dissected specimens were infected).
Infection intensity: 2–6 (2.4).
Type locality: Longmen County (23.65°N, 114.12°E), Guangdong Province, China.
Type specimen deposition: Holotype HBNU-M1007; Allotype HBNU-M1008; Paratypes (nine males, 10 females) HBNU-M1009. Deposited at the College of Life Sciences, Hebei Normal University, Hebei Province, China.
Etymology: The specific epithet refers to the type location, Longmen County.
The genus Molinostrongylus was established by Skarbilovitch in 1934 and M. skrjabini was described as the type species. To date, 20 species of the genus have been reported, including M. ornatus (Mönnig, 1927) (20), M. pseudornatus (Yeh, 1957) (21), M. panousei (Dollfus, 1954) (22), M. dollfusi (Thomas, 1958) (23), M. heydoni (Baylis, 1930) (24), M. rhinolophi (Yamaguti, 1941) (25), M. richardae (Durette-Desset and Chabaud, 1975), M. bauchoti (Durette-Desset and Chabaud, 1975), M. benexae Durette-Desset and Chabaud, 1975 (6), M. alatus (Ortlepp, 1932) (26), M. skrjabini (Skarbilovitch, 1934) (27, 28), M. aelleni (Durette-Desset and Chabaud, 1975) (6), M. longispicula (Yamashita and Mori, 1953) (29), M. owyangi (Durette-Desset and Chabaud, 1975) (6), M. vespertilionis (Morosov and Spassky, 1961) (27), M. colleyi (Durette-Desset and Chabaud, 1975) (6), M. morosovi (Andrejko, Pinchuk, and Skvorzov, 1968) (26), M. scotophili (Ferenc, 1973) (9), M. tsuchiyai Ohbayashi and Kamiya 1979 (30), and M. spasskii (Andrejko, Pinchuk, and Skvorzov, 1968) (23, 31).
Nematodes of the genus Molinostrongylus parasitic in bats, the general features include small sized; cephalic vesicle present; longitudinal ridges on ventral and dorsal sides extending from anterior to tail; wide lateral alae; amphids and cephalic papillae present; female tail with three conical processes and one thin spine; male caudal bursa consisting of two larger lateral lobes and one smaller dorsal lobe; rays 3 and 4 emerging together at the base of the trunk (6, 32). Characters of the present species are consistent with the features above.
Durette-Desset and Chabaud (6) classified the genus into five groups. Group orantus: two lateral alae, seven ventral ridges, and seven dorsal ridges; the distal end of spicules divided into two or three branches, parasitic in Miniopterina. Group richardae: two lateral alae, three large ventral ridges, and three large dorsal ridges, distal end of spicules divided into two branches, parasitic in Molossidae. Group alatus: two lateral alae, three small ventral ridges, and three small dorsal ridges, distal end of spicules divided into two branches, parasitic in the genus Myotis from Africa and Europe. Group skrjabini: two lateral alae, absence of dorsal and ventral ridges, distal end of spicules does not bifurcate or divide into two branches, parasitic in genre Nyctalus. Group vespertilionis: two wide and flat lateral alae, absence of dorsal and ventral ridges, distal end of spicules divided into two branches, parasitic in Pipistrellus in Europe and Tylonycteris in Malaisie (6, 33).
The present species is distinguished from the groups orantus, richardae, and vespertilionis in having three ventral ridges and three dorsal ridges instead of seven ventral ridges and seven dorsal ridges or no ridges. Moreover, group vespertilionis has two wide and flat lateral alae. Group richardae have the same pattern of ridges as our specimens, but they differ in the location of the excretory pore (anterior to the nerve ring vs. posterior to the nerve ring). Therefore, we assigned our specimens to group alatus. There are three species in group alatus, M. alatus, M. spasskii, and M. scotophili (Tables 1, 2). The present species differs from M. alatus by the significantly longer ray 9 and slightly longer ray 10. Moreover, the present species can be distinguished from M. alatus by having wide lateral alae extending to the vulva with a fin-like ending instead of extending to the posterior end.
The present species is distinguished from M. spasskii and M. scotophili in having two medium-sized subventral conical processes equal in length and one large dorsal conical process at the tip of the female tail instead of three almost equally stout conical processes (M. spasskii) or one dorsal, two subventral, and two small lateral conical processes (M. scotophili). M. scotophili is different from our specimens in the patterns of the caudal bursa being 2–3 instead of 3–2. Moreover, the synlophe of the present species is markedly distinguished from that of M. scotophili in having three ridges appearing posterior to the cephalic vesicle and extending to the caudal bursa in males and to the posterior end in females, and in having three longitudinal cuticular ridges appearing posterior to the cephalic vesicle and extending to the region of the vulva in the female, and extending to two-thirds of the body length in the male, then the number of these lines is doubled, that is, six in the last third of the body length.
Currently, the specific diagnosis of Molinostrongylus spp. is based mainly on morphology, and the genetic data of these parasites are severely limited. Based on the genetic analysis of M. longmenensis n. sp., no intraspecific nucleotide differences in the ITS-1 regions among different individuals were noted, but a high level of interspecific genetic variation in these regions among species of the other genera in the family Molineidae was clear.
Both morphological and genetic evidence supported the conclusion that our nematode specimens collected from S. kuhlii represent a new species of nematode parasite in bats. Bats are considered important reservoirs of pathogens of veterinary and medical relevance worldwide (34). Indeed, there is still an important lack of knowledge about parasites infecting bats. In this sense, further investigations into the parasitological fauna of bats are necessary, which could result in the description of numerous new species of helminths.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, PP853664–PP853666.
The manuscript presents research on animals that do not require ethical approval for their study.
H-DJ: Investigation, Writing – original draft. RJ: Data curation, Investigation, Software, Writing – original draft. S-YW: Writing – review & editing. L-YQ: Investigation, Writing – original draft. W-LG: Investigation, Writing – original draft. ZX: Investigation, Writing – review & editing, Validation, Visualization. HZ: Investigation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Hebei Province (C2022106002), the Science and Technology Program of Shijiazhuang (241460085A), and the Doctoral research Foundation of Shijiazhuang university (21BS006).
The authors are grateful to Luping Zhang (Hebei Normal University) for his guidance on the taxonomic identification of specimens and the revision of the manuscript and also express their thanks to Haiyan Li (College of Life Sciences, Guangzhou University) for collecting the specimens.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wu Y, Yu WH, Cai CY, Li XQ, Shu H, Yi ZS, et al. Preliminary study on growth and development of Scotophilus kuhlii pups in the laboratory. J Guangzhou Univ. (2013) 12:30–5.
2. Lin LK, Li LL, Zheng XQ. Bats of Taiwan. 2nd ed. Taichung: National Museum of Natural Science (2004). p. 141–3.
3. Wang Y. A Complete Checklist of Mammal Species and Subspecies in China: A Taxonomic and Geographic Reference. Beijing: China Forestry Publishing House (2003). p. 41–6.
4. Li YC, Chen Z, Long YR, Zhou F, Zhong YR. Species diversity of chiroptera in Ma'anling Volcano Area, Hainan Island. Chin J Zool. (2006) 41:106. doi: 10.13859/j.cjz.2006.03.024
5. Li HY, Wu Y, Xu T, Huang XJ. Study of bat parasitic fluke (fluke: Mesogonaceae and Proporaceae) in Guangdong Province. Guangdong Agric Sci. (2011) 38:118–20. doi: 10.3969/j.issn.1004-874X.2011.16.045
6. Durette-Desset MC, Chabaud AG. Nématodes Trichostrongyloidea Parasites De Microchiroptères. Annales de Parasitologie Humaine et Comparée. (1975) 50:303–37. doi: 10.1051/parasite/1975503303
7. Durette-Desset MC, Chabaud A. Nouvel Essai De Classification Des Nématodes Trichostrongyloidea. Annales de Parasitologie Humaine et Comparée. (1981) 56:297–312. doi: 10.1051/parasite/1981563297
8. Ju HD, Jian R, Ma N, Xu Z. Research progress on systematic taxonomy of bat parasitic nematodes. Chin J Vet Med. (2023) 59:95–102.
11. Dey Sarkar S R. Report on a collection of parasitic nematodes from the Kaimur wildlife sanctuary, Rohtas and Bhabua districts, Bihar, India, along with the descriptions of five new species. Record Zool Surv India. (2003) 101:267–86. doi: 10.26515/rzsi/v101/i3-4/2003/159563
12. Durette-Desset MC, Digiani MC. The Caudal Bursa in the Heligmonellidae (Nematoda: Trichostrongylina). Journal de la Société Française de Parasitologie. (2012) 19:3–18. doi: 10.1051/parasite/2012191003
13. Durette-Desset MC. Trichostrongyloid nematodes and their vertebrate hosts: reconstruction of the phylogeny of a parasitic group. Adv Parasitol. (1985) 24:239–306. doi: 10.1016/S0065-308X(08)60564-3
14. Ju HD, Li L, Zhang LP. Durettenema guangdongense gen. et sp. nov. (Nematoda: Molineoidea) from Hipposideros larvatus (Horsfield)(Chiroptera: Rhinolophidae) with discussion of the taxonomic status of Macielia Rhinolophi Yin, 1980. Acta Parasitol. (2017) 62:575–81. doi: 10.1515/ap-2017-0069
15. Jian R, Wang SW, Zhang WX, Zhang LP. Morphological and molecular identification of Habronema spp. (Nematoda: Habronematidae) from Donkeys in Xinjiang, China, and notes on the taxonomical status of Habronema majus (Creplin, 1849) and H. microstoma (Schneider, 1866). Systemat Parasitol. (2017) 94:511–25. doi: 10.1007/s11230-017-9714-8
16. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. Mrbayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systemat Biol. (2012) 61:539–42. doi: 10.1093/sysbio/sys029
17. Ronquist F, Teslenko M, Mark PVD, Ayres DL, Darling A, Höhna S, et al. Mega6: molecular evolutionary genetics analysis version 6.0. Mol Piol Evol. (2013) 30:2725–9. doi: 10.1093/molbev/mst197
18. Hung GC, Chilton NB, Beveridge I, Gasser RB. A molecular systematic framework for equine strongyles based on ribosomal DNA sequence data. Int J Parasitol. (2000) 30:95–103. doi: 10.1016/s0020-7519(99)00166-6
19. Bu Y, Niu H, Zhang L. Phylogenetic analysis of the genus cylicocyclus (Nematoda: Strongylidae) based on nuclear ribosomal sequence data. Acta parasitologica. (2013) 58:167–73. doi: 10.2478/s11686-013-0124-z
20. Lauro T. Revisão Da Família Trichostrongylidae Leiper, 1912. Rio de Janeiro: Monografias do Instituto Oswaldo Cruz (1937).
21. Yeh LS. Studies on a Trematode and a New Nematode from a Bat from Northern Rhodesia. J Helminthol. (1957) 31:121–5. doi: 10.1017/S0022149X0000434X
22. Tkach V, Sharpilo LD. Nematodes of the Genus Molinostrongylus (Nematoda, Molineidae) from Ukrainian Bats. Vestn Zool. (1988) 4:3–8.
23. Andreiko OF, Pinchuk LM, Skvortsov VG. New species of Nematodes from Microchiroptera. Izvestiya akademii Nauk Moldavskoi SSR., Seriya Biologicheskikh i Khimicheskikh Nauki. (1968) 1:3–8.
24. Thomas PM. Some nematode parasites from Australian hosts. Trans Royal Soc South Austr. (1959) 82:151–62.
25. Yin WZ, Zhang ZX. New records of parasitic nematodes in chiroptera from China. Acta Zootaxonomica Sinica. (1980) 4:357.
26. Genov T, Stoykova-Hajinikolova R, Meszaros F. Molinostrongylus spp. (Nematoda: Molineidae) from bats in Bulgaria, with a review of European species. Parasitol Hungar. (1992) 25:53–68. doi: 10.1016/S0016-6995(06)80403-1
27. Morozov Y, Spasski A. Molinostrongylus vespertilionis n. sp. and some morphological characteristics of M. alatus and M. skrjabini. Helminthologia. (1961) 3:244–50.
28. Barus V, Tenora F. Further discoveries of nematodes in the bats of Afghanistan. Acta Universitatis Agriculturae, Facultas Agronomica, Brno. (1970) 18:133–41.
30. Ohbayashi M, Kamiya H. Nematode parasites from Vespertilio orientalis Wallin. Jpn J Vet Res. (1979) 27:11–5.
31. Ju HD, Xie LB, Zhao W. Description of a new Chinese recorded species of nematworm. Adv Anim Med. (2022) 43:122–5.
32. Barus V, Rysavy B. An analysis of the biogeography of nematodes of the family trichostrongylidae parasitizing bats of the suborder microchiroptera. Folia Parasitologica. (1971) 18:1–14.
33. Barus V, Tenora F. First record of Molinostrongylus vespertilionis Morozov et Spassky, 1961 (Nematoda) in Norway. Folia Parasitol. (1977) 24:122.
Keywords: bats, parasite, nematode, morphology, genetic data, phylogeny
Citation: Ju H-D, Jian R, Wang S-Y, Qin L-Y, Gao W-L, Xu Z and Zhang H (2024) Molinostrongylus longmenensis n. sp. (Strongylida: Molineidae) in the bat Scotophilus kuhlii (Leach; Chiroptera: Vespertilionidae) from China. Front. Vet. Sci. 11:1446743. doi: 10.3389/fvets.2024.1446743
Received: 10 June 2024; Accepted: 20 August 2024;
Published: 11 September 2024.
Edited by:
Calin Mircea Gherman, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Osama Mohammed, King Saud University, Saudi ArabiaCopyright © 2024 Ju, Jian, Wang, Qin, Gao, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Xu, eHV6aGVuaG1AMTI2LmNvbQ==; Hong Zhang, emhzanpjZGNAMTI2LmNvbQ==
†Present address: Zhen Xu, Research and Education Division, Affiliated Hospital of Hebei University of Engineering, Handan, China
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.