- 1College of Animal Science and Technology, Henan Agricultural University, Zhengzhou, China
- 2Henan Technical Institute, Zhengzhou, China
- 3Henan Anjin Biotechnology Co., Ltd., Xinxiang, China
- 4Henan Delin Biological Product Co., Ltd., Xinxiang, China
Fungal probiotics have the potential as feed additives, but less has been explored in ruminant feed up to date. This study aimed to determine the effect of compound probiotics (CPs) with Aspergillus oryzae 1, Aspergillus oryzae 2 and Candida utilis on Hu sheep’s growth performance, rumen fermentation and microbiota. A total of 120 male Hu sheep, aged 2 months and with the body weight of 16.95 ± 0.65 kg were divided into 4 groups. Each group consisted of 5 replicates, with 6 sheep per replicate. Group A was the control group fed with the basal diet. Group B, C and D was supplemented with the basal diet by adding 400, 800 and 1,200 grams per ton (g/t) CPs, respectively. The feeding trial lasted for 60 days after a 10-day adaptation period. The results showed that the average daily gain (ADG) of sheep in the CPs groups were significantly higher, the feed/gain were significantly lower than those in group A in the later stage and the overall period. The addition of CPs increased the economic benefit. The levels of CD4+ and the CD4+/CD8+ ratio in the CPs groups were higher than those in Group A. The levels of GSH, IgG, IL-2, IL-6, and IFN-γ in group C were significantly elevated compared with group A. Group B showed a significant increase in rumen NH3-N and cellulase activity. There was no difference in VFAs content between group A and group B, however, with the increasing addition of CPs, the butyric acid and isobutyric acid content tended to decrease. The rumen microbiota analysis indicated that the CPs addition increased the Firmicutes and Proteobacteria abundances, decreased the Bacteroidetes abundance. The correlation analysis showed that Prevotella was negatively correlated with ADG, and the addition of 400 CPs in group B reduced Prevotella’s relative abundance, indicating CPs increased sheep growth by decreasing Prevotella abundance. The CPs addition reduced caspase-3, NF-κB and TNF-α expression in liver, jejunum and rumen tissues. In conclusion, the addition of CPs increased the sheep production performance, reduced inflammation, improved rumen and intestinal health. Considering the above points and economic benefits, the optimal addition of CPs as an additive for Hu sheep is 800 g/t.
1 Introduction
Antibiotics, beyond controlling pathogenic microorganisms, also affect many beneficial microorganisms, causing disturbances to the balance of the gastrointestinal microbiota (1). The abused of antibiotics can result in the development of drug-resistant bacteria and the presence of antibiotic residues in livestock products, which may subsequently enter the human body through the food chain and adversely affect human health (2). Adding antibiotics to feed is already banned, therefore, it is more important to study the substitutes of antibiotics. Probiotics are a class of active microorganisms that can be beneficial to the host by colonize the host’s body and alter the balance of gut microbiota (1). In recent years, probiotics have gained increasing attention as alternatives to antibiotics due to their ability to enhance animal productivity (3), inhibit pathogenic microorganism colonization (4), and maintain the balance of microflora of the digestive tract of the host (5). Probiotic preparation on the market includes bacteria, fungus and yeast (6). In the past, the application in animal husbandry mainly focused on bacteria and yeast, while little attention has been paid on to fungal probiotics supplementation in ruminants.
The fungal probiotic field is one of the developing fields nowadays (7). Numerous studies have described the role of Aspergillus oryzae as one of the most common probiotics and has been widely used as a feed additive in ruminant production (8–10). Aspergillus oryzae enhances feed dry matter degradation, reduces inflammation, and increases the energy supply of total volatile fatty acids (VFAs) in the rumen (8). Additionally, Aspergillus oryzae plays a role in the rumen and hindgut (11). Candida utilis cell wall contains β-glucan, glucomannan and mannoprotein (12), which have been shown to improve intestinal health, inhibit cellular DNA damage, antioxidant, antimutagenic and antitoxic activities (13). Many studies have shown that Candida utilis used in feed can improve animal production performance, antioxidant capacity, immune function and increase the villus height of the jejunum and ileum (14–16).
Even though the effectiveness of individual Aspergillus oryzae and Candida utilis has been studied, most studies are limited to a single strain. Still, single strains have problems such as poor stability and insignificant application effect (12). This study aimed to investigate the effect of combined additive with Aspergillus oryzae and Candida utilis on growth performance, antioxidant capacity, immunity, ruminal fermentation and microbial diversity of Hu sheep. It will provide scientific basis for the application of compound probiotics in ruminant production.
2 Materials and methods
2.1 Animal ethics statement
All animal experimental procedures were approved by the Animal Care and Use Committee of Henan Agricultural University (Approval number: HENAU-2021-025).
2.2 Materials preparation
Aspergillus oryzae 1 (CGMCC5817) used in this study was isolated from a cow rumen, and identified by 26S rDNA, it was preserved in China General Microbiological Culture Collection Center (CGMCC). Aspergillus oryzae 2 (CGMCC3.0496) and Candida utilis (CGMCC2.0615) were obtained from CGMCC. They were incubated according to the published protocols to make the probiotics powder (17). Based on the previous results obtained with response surface regression design in vitro in our laboratory. The compound probiotics (CPs) were prepared by the optimal ratio of Aspergillus oryzae 1, Aspergillus oryzae 2 and Candida utilis with 1 × 104.45, 1 × 105 and 1 × 105 colony-forming units (CFU)/ g compound probiotics powder, respectively.
2.3 Animals, diets, and experimental design
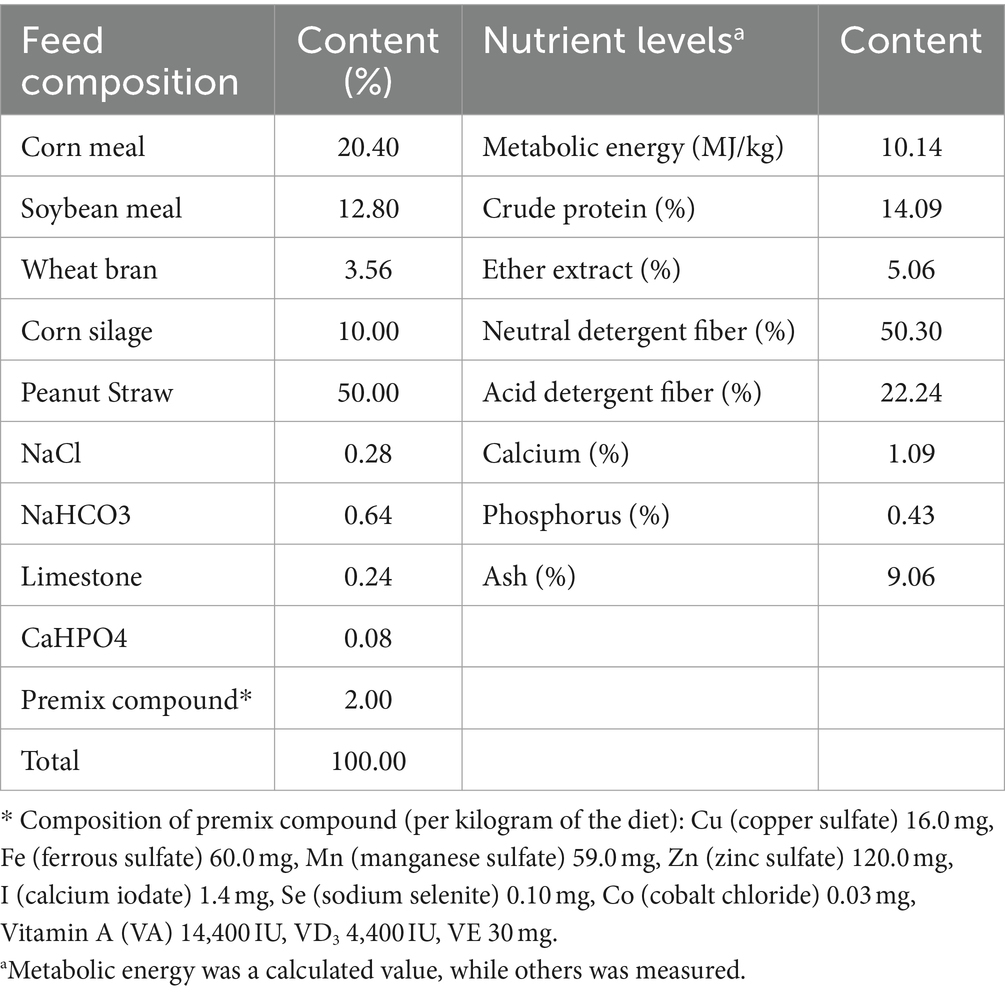
This experimental design was approved by the Sanmu Lvyuan Livestock Breeding Co., Ltd. (Dengfeng, China). A total of 120 male Hu sheep, aged 2 months and with the body weight of 16.95 ± 0.65 kg were divided into 4 groups. Each group consisted of 5 replicates, with 6 sheep per replicate. Group A was fed basal diet (control group); Group B was fed basal diet with 400 g/t CPs; Group C was fed basal diet with 800 g/t CPs; and Group D was fed basal diet with 1,200 gram per ton (g/t) CPs. The feeding experiment duration was 70 days, which included a 10-day adaptation period. The pre-trial period lasted 30 days (1–30 day), and the post-trial period for 30 days (31–60 day). The sheep were fed twice daily at 08:00 and 16:00. The amount of feed offered to each pen, as well as leftover feed, was recorded daily. They were provided access to clean, fresh water ad libitum. The diet was formulated according to Nutrition Requirement of Meat Sheep in China (NY/T 816–2021) to meet the nutritional requirements. Ingredients and chemical composition of the total mixed diet are shown in Table 1.
2.4 The determination of growth performance
Body weight for each replicate were recorded on day 1, 30, and 60, respectively, to calculate the average daily gain (ADG), average daily feed intake (ADFI) and feed/gain (F/G) for days 1 to 30, 31 to 60, and 1 to 60 (1to 60, the entire test period). ADG, ADFI, and F/G were calculated as follows:
2.5 The determination of digestibility
On days 28 and 58, fecal samples were collected from five sheep in each group for three consecutive days. Total feces from each sheep were collected, weighed, mixed, and sampled twice daily (0900 and 1700 h). Representative samples (100 g each) were mixed with 10 mL of 10% (wt/wt) hydrochloric acid and stored in a sterile plastic bag at −20°C for further analysis. Some fecal samples were dried at 65°C for 24 h and mashed to determine apparent nutrient digestibility. The apparent nutrient digestibility was calculated according to formula:
The chemical analyses of the fecal and diets samples included crude protein (CP; method 984.13; AOAC, 2006), ether extract (EE; method 920.39; AOAC, 2006), Ca (method 968.08; AOAC, 2006), and P (method 946.06; AOAC, 2006) as described by AOAC International. The NDF and ADF content was determined using the method of Van Soest et al. (18).
2.6 The determination of immune and antioxidant indices
At the end of feeding experiment, one sheep from each replicate with a similar live weight was selected for blood collection. Serum was obtained from non-anticoagulated blood through centrifugation at 3,000 rpm/min for 10 min at 4°C in order to analyze serum biochemical indicators. The concentrations of immunoglobulin M (IgM), immunoglobulin A (IgA), immunoglobulin G (IgG), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 6 (IL-6), interleukin 10 (IL-10), interferon-γ (IFN-γ), CD4+ and CD8+ were determined with immunoglobulin-based ELISA kits (Beijing solarbio Bio Tech Company, Shanghai, China). We used the corresponding kit (Shanghai Meilian Bio-Tech Company, Beijing, China) to determine the concentrations of superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione (GSH).
2.7 Rumen, hepatic, and jejunum morphology
Following the 60-day experimental period, three Hu sheep of comparable body weight were selected from both groups A and B for slaughter. The goat was weighed with empty stomach in the morning and killed by captive bolt stunning exsanguination. For each sheep, a 1 cm segment of the jejunum was cut with a scalpel, approximately 2 cm × 2 cm of liver tissue, and a 3 cm × 3 cm of the rumen wall were taken. These tissue samples were subsequently washed with saline solution, fixed in 10% formalin buffer for future morphological analysis.
Rumen, hepatic, and jejunum morphologies were detected with hematoxylin–eosin (HE) staining. The specific experimental steps of HE staining refer to the previous study (19, 20), and then observed with a microscope (Olympus Co., Ltd., Tokyo, Japan).
2.8 Immunohistochemistry
Immunohistochemical staining was done using the streptavidin-perosidase (SP) method (Thermo Fisher Scientific Company, Shanghai, China). Inflammatory factors (Caspase-3, NF-κB, and TNF-α) were detected by immunohistochemistry as described (21). Only the nuclear staining pattern was considered positive. The immunostaining was scored as “0” (negative, no or less than 5% positive cells), “1” (5–25% positive cells), “2” (26–50% positive cells) and “3” (more than 50% positive cells). The staining intensity was scored as follows: 0, colorless; 1, buff; 2, brownish yellow and 3, dark brown. The staining index was obtained by multiplying the staining intensity score by the positive tumor cell score. Based on the heterogeneity of the measure, we defined a staining index of 1–2 as weak, 3–4 as moderate, and 6–9 as strong staining (22, 23).
2.9 Rumen fermentation parameters
One sheep from each replicate with a similar live weight was selected for rumen fluid collection. The collection of ruminal fluid was performed 2 h after the morning feeding on day 60 of treatment. Rumen fluid samples were collected through the oral cavity using a rumen tube. To prevent saliva contamination, the initial 50 mL of fluid was discarded, and the next 100 mL was retained. The collected ruminal fluid was then filtered through four layers of cheesecloth. A sample of the ruminal fluid was immediately used to measure pH with a portable pH meter (PHB-4; Shanghai Leici Co., Ltd., Shanghai, China). Additionally, the remaining sample was immediately brought to the laboratory and stored in a − 80°C refrigerator to analyze ruminal bacteria, total and individual VFAs components (acetic acid, propionic acid, and butyric acid), NH3-N, protease activity, amylase activity and cellulase activity. Two milliliters of ruminal fluid were immediately stored at −80°C to assess the bacterial population. Ten milliliters of ruminal fluid were added to 2 mL of 25% metaphosphoric acid for assay of VFAs.
Thawed rumen fluid samples were centrifuged at 6000 × g for 10 min, the supernatant collected was analyzed for NH3-N based on the protocol described by Ma et al. (24). The concentration of VFAs was determined using crotonic acid as an internal standard by a gas chromatography method (Agilent 7890B, Agilent, California, United States), according to Izuddin et al. (25). The assayed cellulase and amylase activities were measured using the 3,5-dinitrosalicylic acid method (26, 27). Protease activity was determined using the Azocoll assay as previously described (28).
2.10 DNA extraction, amplification and Miseq sequencing
Rumen samples were thoroughly homogenized, followed by centrifugation of one milliliter of the rumen fluid for 10 min at 13,200 rpm. The supernatant was discarded, and the remaining pellet was resuspended in 400 mL of nuclease-free water. Microbial DNA extraction was performed utilizing a Genomic DNA Extraction Kit (Takara Biomedical Technology Co., Ltd., Beijing, China) in accordance with the manufacturer’s protocols. After assessing the integrity and purity of DNA, amplification and sequencing were performed as described by Wang et al. (29). The V4–V5 region of the bacteria 16S ribosomal RNA genes was amplified using primers of F:5′-GTGCCAGCMGCCGCGGTAA-3′ and R: 5′-CCGTCAATTCCTTTGAGTTT-3′ with the barcode attached. The concentrations of PCR products were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States), then merged according to DNA concentration. Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 250) on an Illumina MiSeq platform that was finished by Shanghai Paisennuo Biological Technology Co., Ltd. (Shanghai, China).
2.11 Data processing
QIIME2 software package was used to process the raw data. All the raw reads generated from the Illumina sequencing were trimmed using Trimmomatic to remove adapters and low-quality sequences with a phred score of 33 (30). Reads quality control and the resolution of amplicon sequence variants (ASVs) were performed using the DADA2 R package (31). Taxonomic assignment of the ASVs was performed based on the SILVA database (Release132 http://www.Arb-silva.de) (32). Differences in bacterial communities between control and treatment groups were assessed using ANOSIM with the vegan package in R.1 Redundancy analysis (RDA) was performed to analyze the correlation between environmental factors and the rumen microbial community. RDA analysis was carried out in reference to the method described by Song et al. (33).
2.12 Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) using SPSS software (version 22.0; SPSS Institute Inc., Chicago, IL, United States), and the significant differences were compared by Duncan’s multiple range test (p < 0.05). The results were expressed as mean values ± standard deviations (mean ± SD).
3 Results
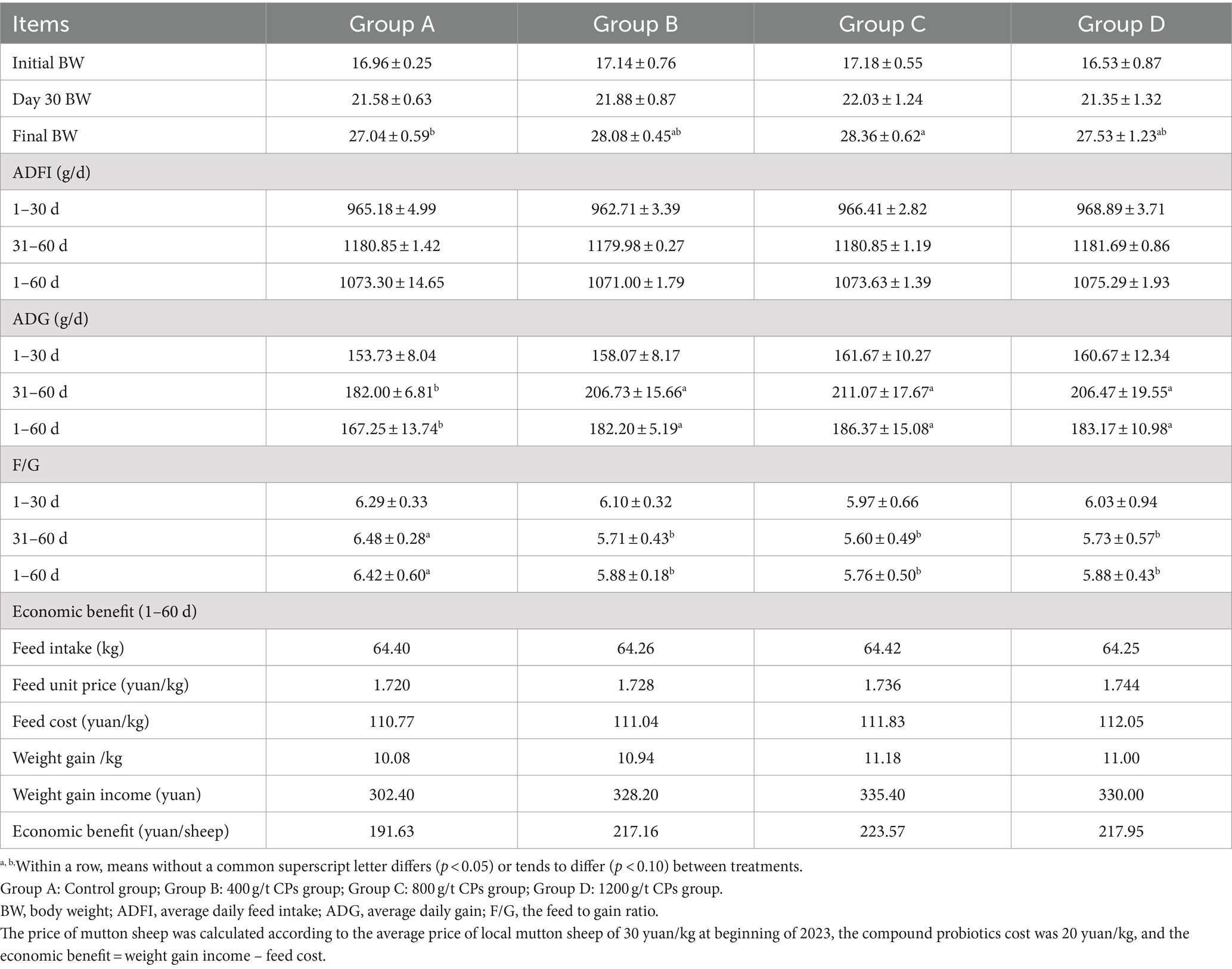
3.1 Effect of compound postbiotics on the growth performance and economic benefit of Hu sheep
The effect of CPs on Hu sheep growth performance is presented in Table 2. There was no significant difference for the 30 day body weights of the sheep in all groups. The body weight of 60 day in the groups B and C was higher than that of group A (p < 0.05).
The ADFI of Hu sheep exhibited no significant differences among the various groups during the pre-period, post-period, and the entire trial period (p > 0.05). During days 30 to 60, as well as the entire trial period, the group supplemented with CPs exhibited a higher ADG and a lower F/G compared to group A (control group) (p < 0.05). The addition of CPs increased the economic benefit of Hu sheep.
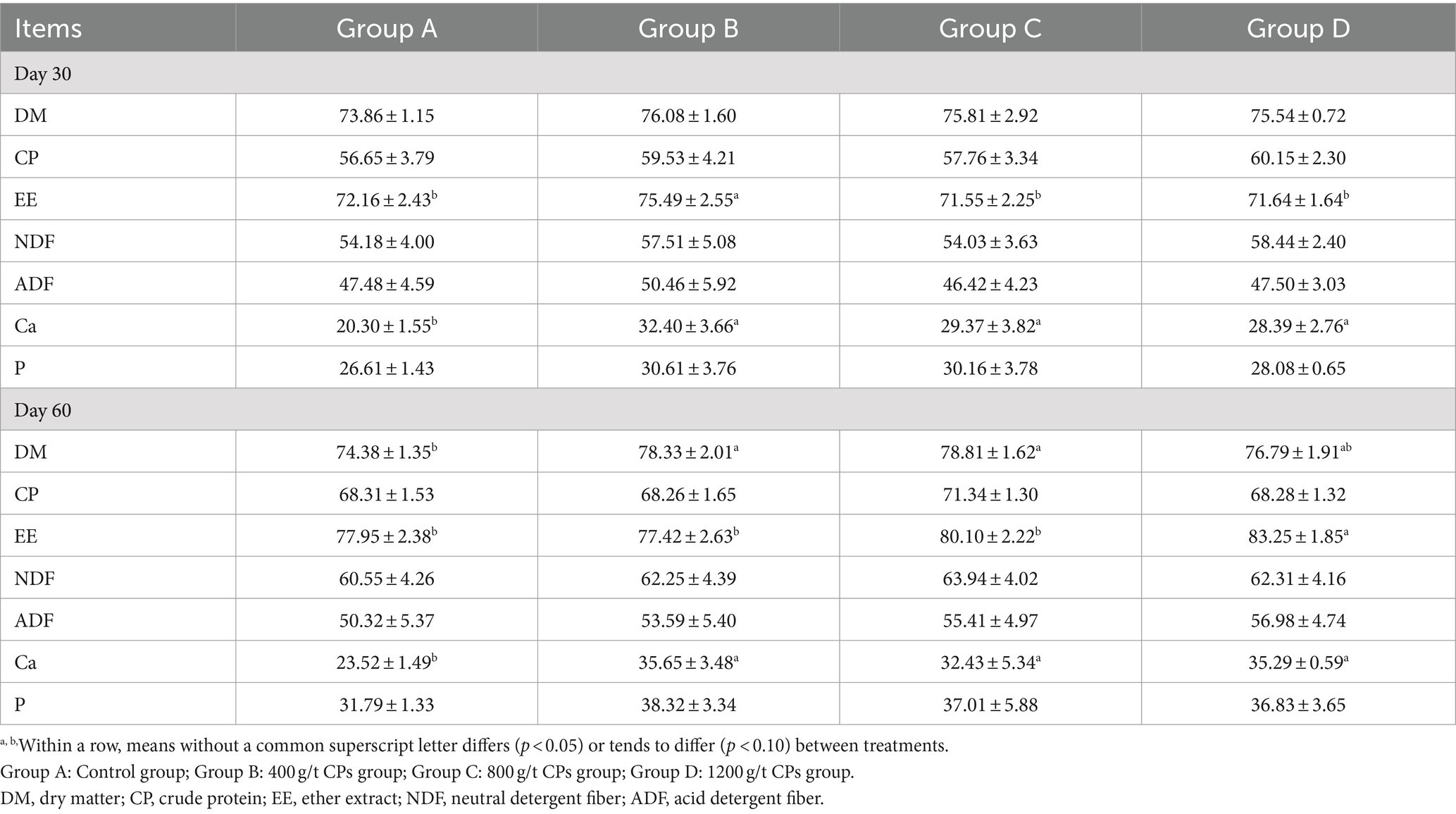
3.2 Effect of compound postbiotics on the nutrient digestibility of Hu sheep
The nutrient digestibility of Hu sheep is shown in Table 3. In the early stage, the digestibility of EE in group B was significantly higher compared with the other groups (p < 0.05), while no significant differences were observed among the remaining groups. In the post-period, EE digestibility in group D was higher than in groups A, B, and C (p < 0.05); The dry matter digestibility of groups B and C was significantly higher than that of group A. During both the pre-trial and post-trial phases, groups B, C, and D exhibited significantly higher Ca digestibility compared with group A (p < 0.05).
3.3 Effect of compound probiotics on the antioxidant and immune parameters of Hu sheep serum
Table 4 showed that the contents of GSH and IgG in group C were significantly higher than that in the other groups (p < 0.05). Additionally, the contents of IL-2 and IL-6 in group C were significantly higher than those in group A (p < 0.05). The IL-4 level in group D was significantly higher than that in group A (p < 0.05); however, no significant difference were observed among groups B, C and D. The content of IFN-γ was significantly higher in group C compared to group A (p < 0.05); nonetheless, the difference with groups B and D was nonsignificant. The content of CD4+ lymphocytes and the CD4+/CD8+ ratio were highest in group D, but not significantly different from group C.
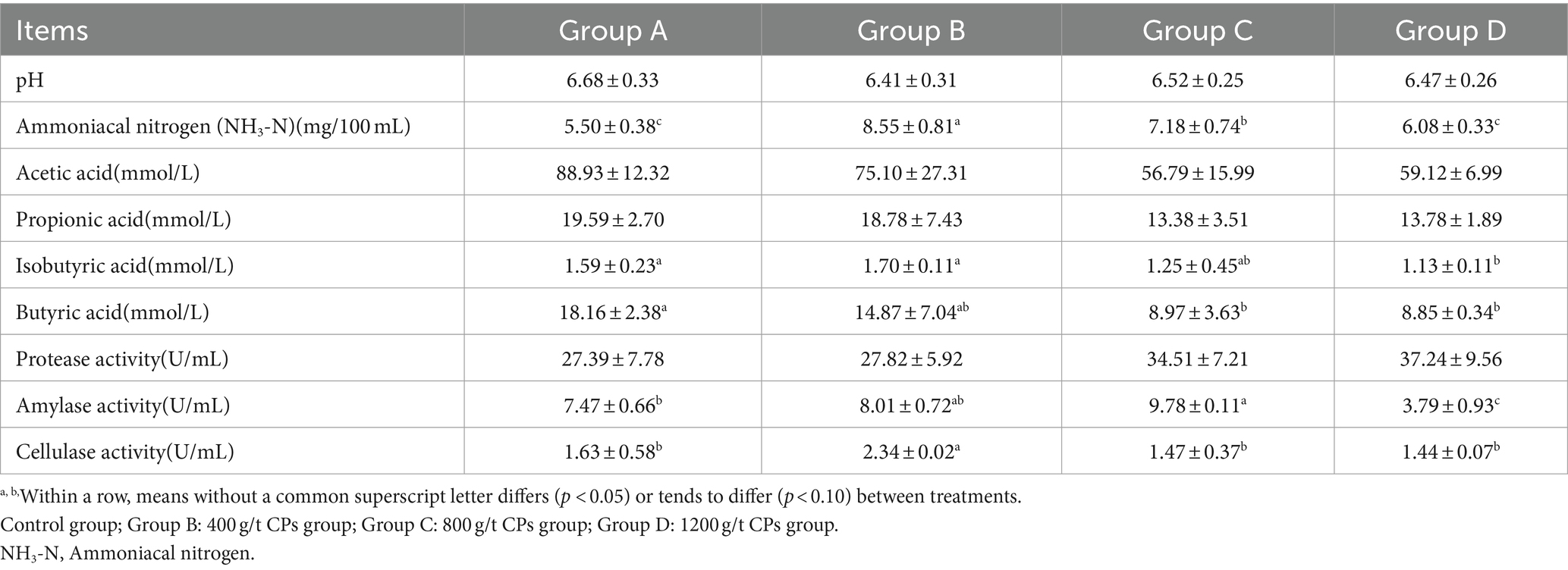
3.4 Effects of compound probiotics on rumen fermentation parameters of Hu sheep
Table 5 presents the rumen fermentation parameters, and it showed that the NH3-N content was significantly increased in groups B and C compared to group A, with group B exhibiting the highest content (p < 0.05). The rumen isobutyrate content in the group B was the highest. The butyric acid content tended to decrease as the amount of CPs added increased, however, the butyric acid content were highest in group A, with no significant differences observed between groups A and B. The amylase activity observed in group C was significantly elevated compared with group A and group D (p < 0.05), however, it did not differ significantly from that in group B (p < 0.05). Furthermore, group B showed significantly higher cellulase activity compared with all other groups.
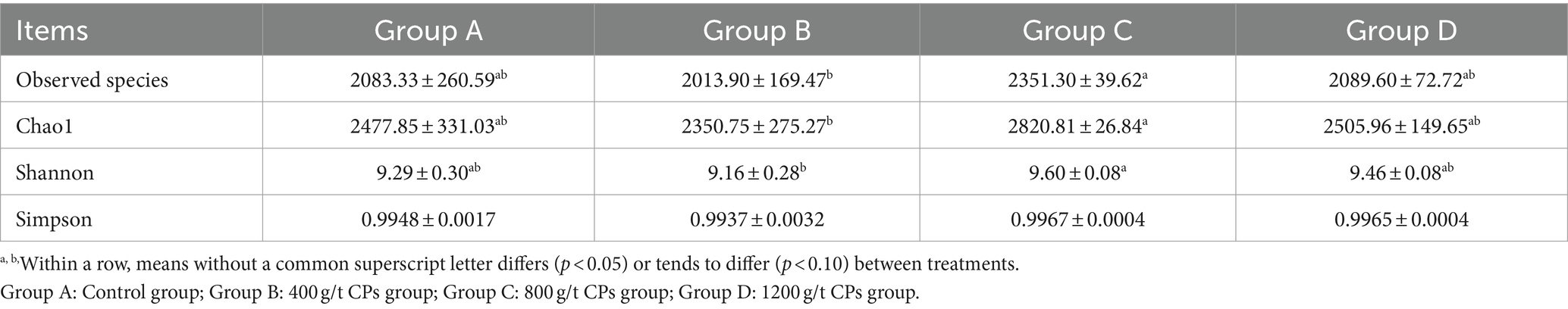
3.5 Effects of compound probiotics on rumen microbial diversity of Hu sheep
The microbial richness was estimated by observed species and Chao index, the diversity was evaluated by Shannon and Simpson index (Table 6). The Chao index, Shannon index, and the number of observed species in group C were significantly higher than those in group B (p < 0.05). Conversely, with the exception of Group C, no significant differences were observed among the other groups (p > 0.05).
3.6 Gut microbial community influenced by compound probiotics
Figure 1A showed the abundances of top 10 bacteria at phylum level. The top two major bacterial phyla were Firmicutes and Bacteroidetes. Compared with group A, the relative abundance of Firmicutes increased in the CPs addition groups, whereas the relative abundance of Bacteroidetes decreased (Table 7). At the bacterial genus level, the top 10 genera of bacteria are presented in Figure 1B and Table 7. The predominant bacterium in the rumen among the four groups was Prevotella, followed by Ruminococcus and Butyrivibrio. The relative abundance of Prevotella and Anaeroplasma in group B was lower than that in the other three groups (p > 0.05), indicating that the addition of 400 CPs decreased the relative abundance of Anaeroplasma and Prevotella (Table 7).
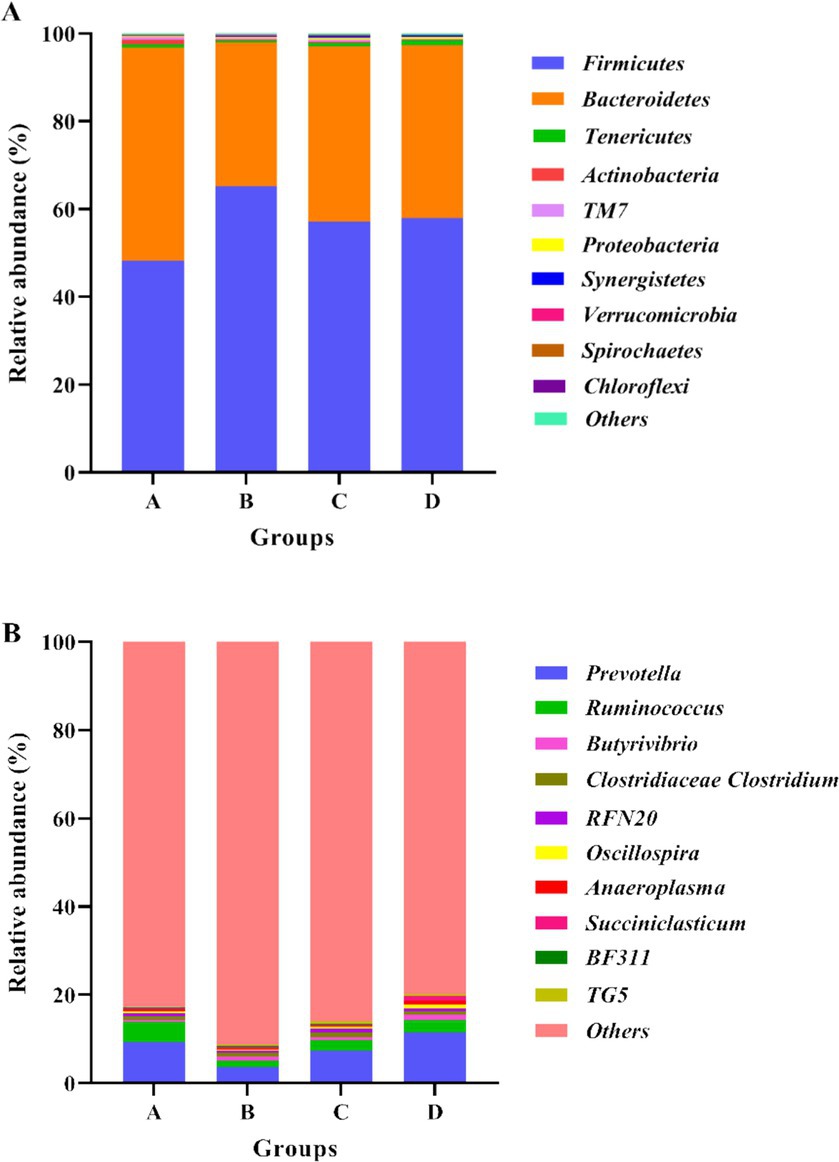
Figure 1. Bacterial compositions and abundances at phylum and genus levels. (A) Relative abundance of the top 10 phyla of rumen bacteria; (B) Relative abundance of the top 10 genus of rumen bacteria. Group A: Control group; Group B: 400 g/t CPs group; Group C: 800 g/t CPs group; Group D: 1200 g/t CPs group.
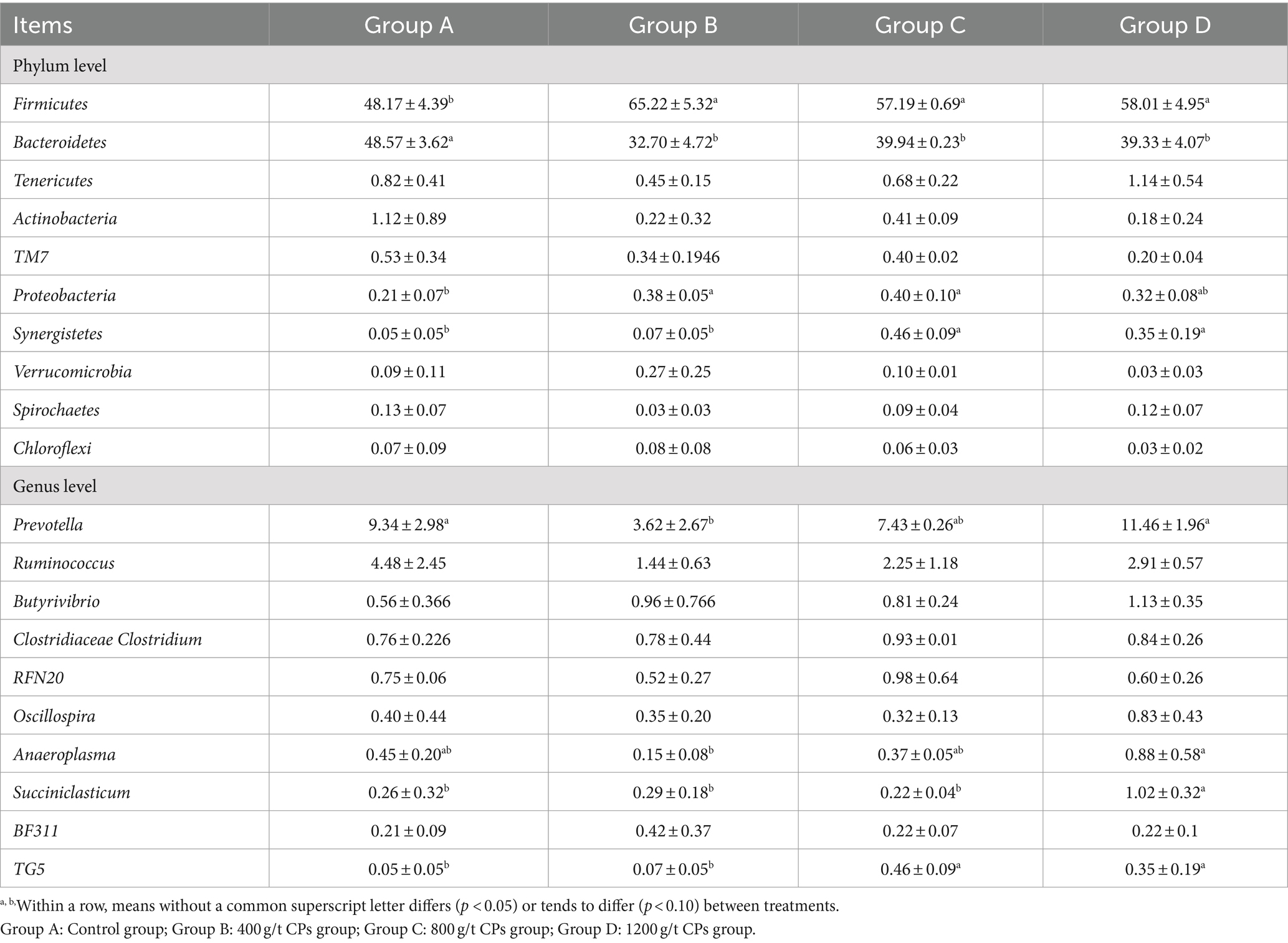
Table 7. Effects of compound probiotics on the composition and structure of rumen microflora of Hu sheep(%).
3.7 Correlations between rumen bacteria and ruminal fermentation parameters
To investigate the impact of rumen microflora on the progression of rumen fermentation, a correlation analysis was conducted using redundancy analysis (RDA). The results revealed significant correlations between influential rumen microflora at the genus level and ruminal fermentation parameters (Figure 2). Prevotella and Ruminococcus were strongly correlated with environmental factors. Specifically, Prevotella was positively correlated with ADF; Ruminococcus was positively correlated with ADF, NDF, pH, acetic acid, propanoic acid, and butyric acid. ADG and NH3-N were negatively correlated with both Prevotella and Ruminococcus.
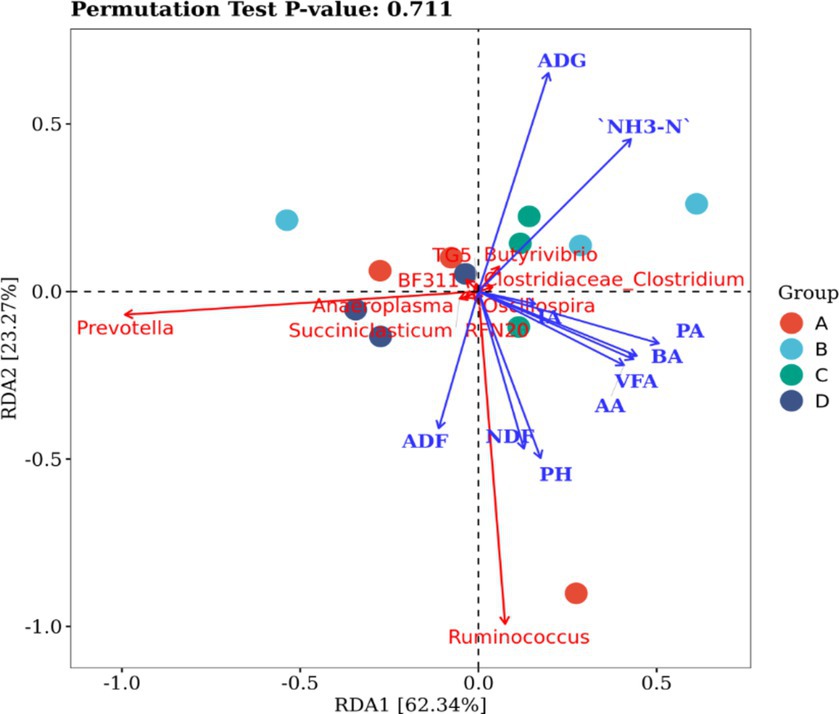
Figure 2. Correlation between bacteria and fermentation parameters in the rumen. VFA is the sum of AA, PA and BA, and NH3-N is ammonia nitrogen. AA, acetic acid; PA, propionic acid; BA, butanoic acid.
3.8 Effects of compound probiotic on the histological structure of Hu sheep
The initial analysis of the results indicated that group B and group C exhibited superior outcomes. However, the difference between the two groups was not significant. Consequently, Group B was selected for further investigation.
Oil Red O staining was performed on liver, jejunum, and rumen sections for groups A and B to verify the digestibility and immunity. The hepatocytes were visualized using HE staining, with signifies the staining of hepatocyte cytoplasm and blue denotes stained nuclei (Figure 3A). The hepatocyte morphology was normal, with hepatic cells being orderly arranged and no abnormalities observed. However, compared to the control group (Figure 3Aa), the cells in the treatment group (Figure 3Ab) exhibited a reduction in inflammatory response and bleeding point. Figure 3B shows the morphology structure of the jejunum. Compared with the control group, group B exhibited a significantly greater number of villi and a higher villus height to crypt depth ratio (Table 8). The analysis of the rumen epithelium revealed that the length and density of the ruminal papillae were significantly increased in the CPs group compared with the control group (Figure 3Cb).
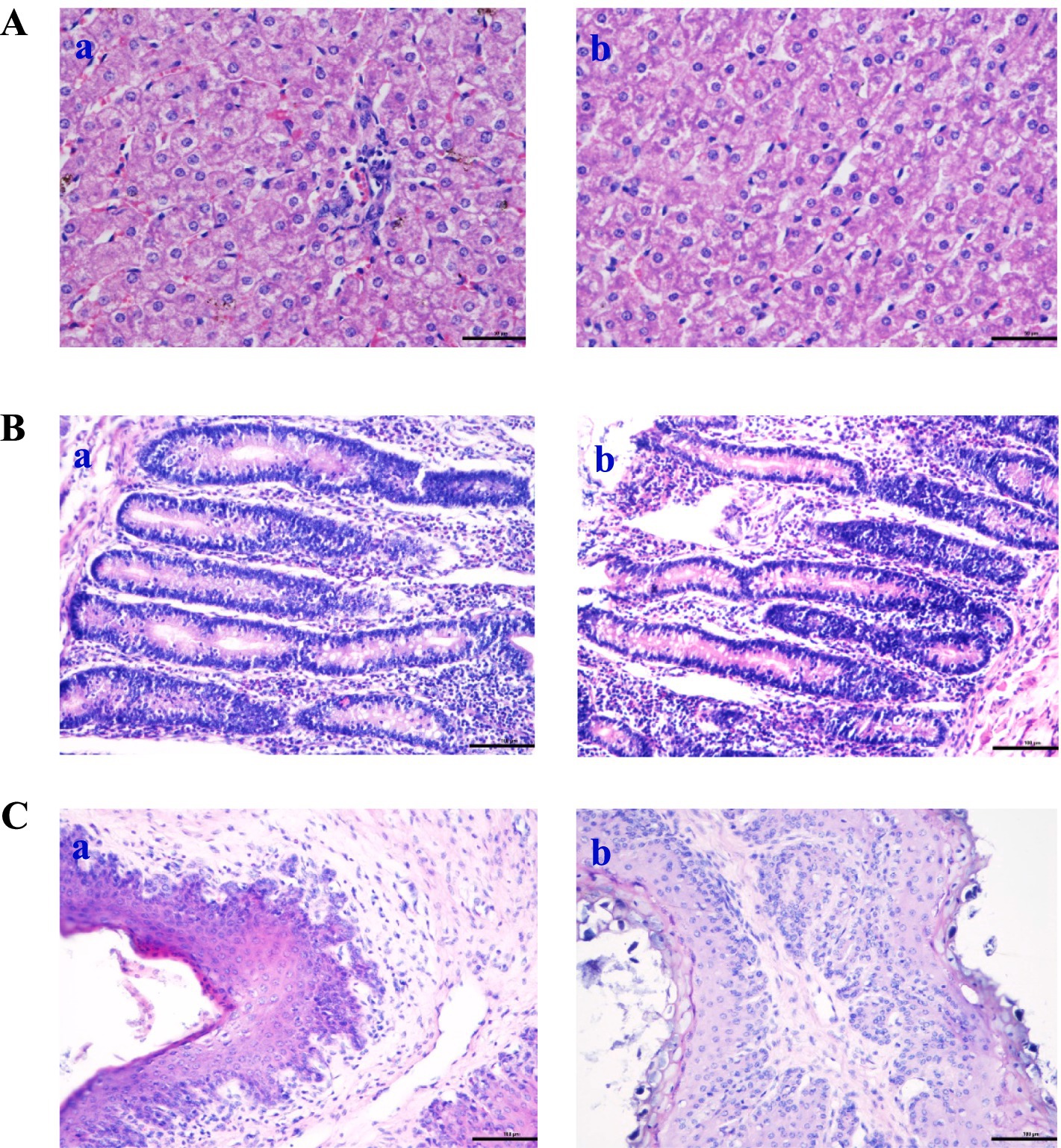
Figure 3. Effects of compound probiotics on the histological structure of Hu sheep (×50). (A) Liver tissue; (B) Jejunum structure; (C) Rumen structure. a: the control group (Group A), b: the CPs group (Group B).
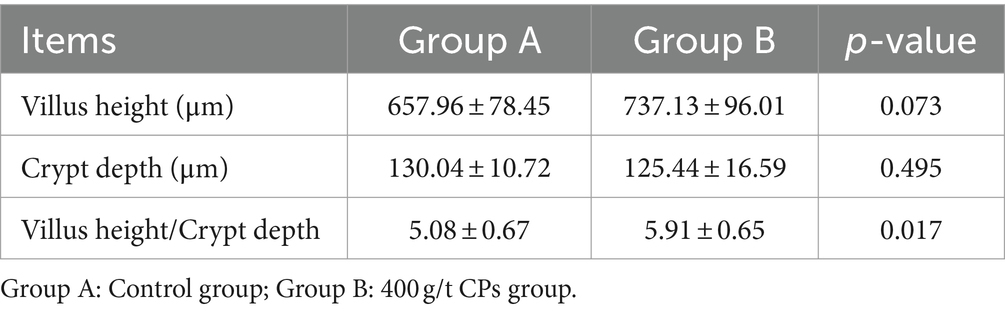
Table 8. Effects of compound probiotics on villus height, crypt depth, and villus height/crypt depth ratio of the jejunum of Hu sheep.
3.9 Effects of compound probiotics on the inflammatory cytokine expression of Hu sheep
The histological evaluation (Table 9) of liver, jejunum, and rumen specimens from groups A and B provides evidence supporting the anti-inflammatory activity of CPs. The positive cell detection algorithm measured the number of DAB-positive cells per mm2. The control group exhibited a higher number of Caspase-3, NF-κB, and TNF-α positive cells, with a darker color, compared with the CPs group. CPs significantly decreased pro-inflammatory factors, including Caspase-3 and NF-κB, in liver and jejunal tissues compared with the control group (Table 9; Figures 4, 5). The immunohistochemistry results indicated that group B had reduced Caspase-3 and TNF-α levels, fewer positive cells, and lighter staining in rumen tissue compared with group A (Table 9; Figure 6).
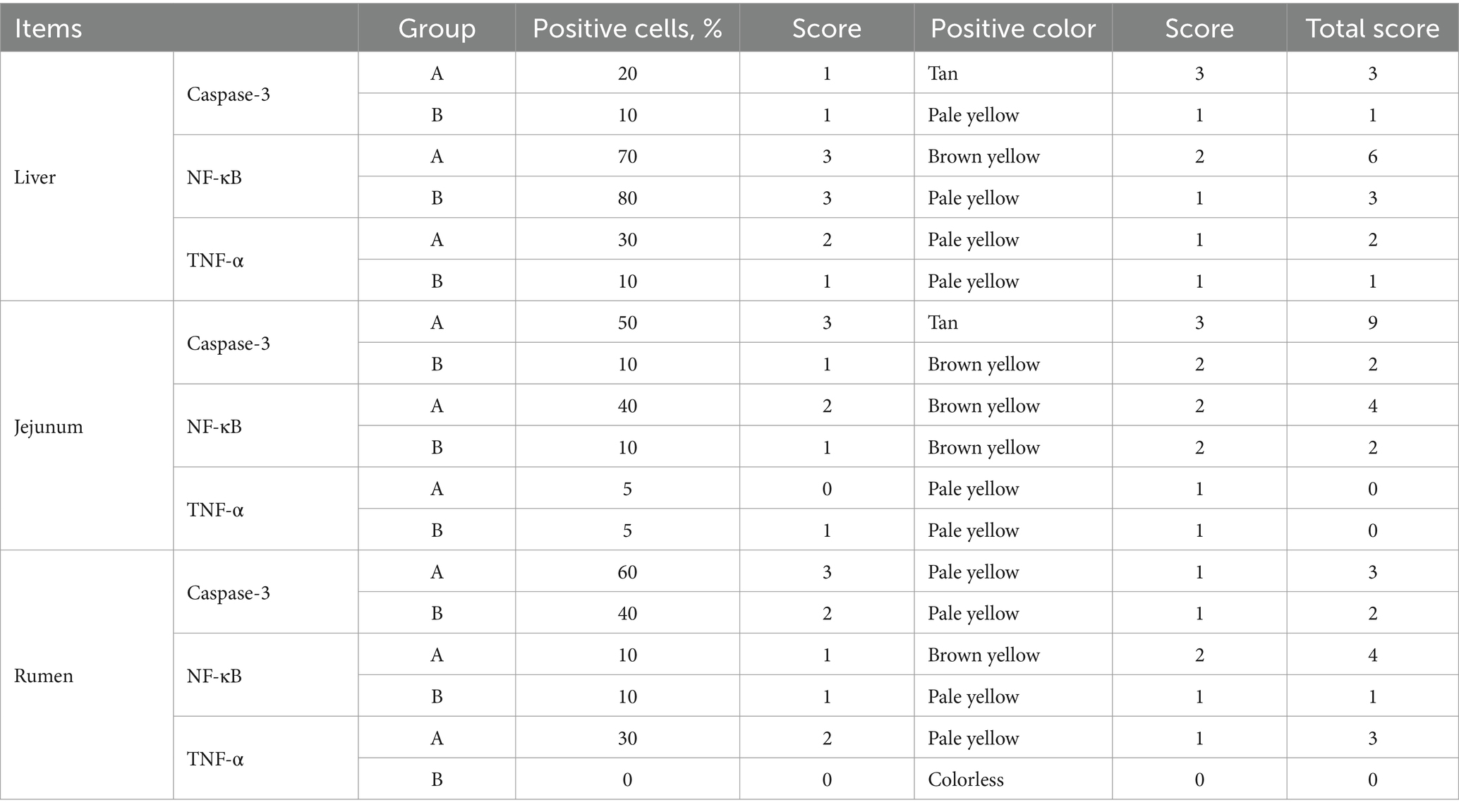
Table 9. Immunohistochemical analysis tissue and immunohistochemistry scores of live, jejunum, and rumen of Hu sheep.
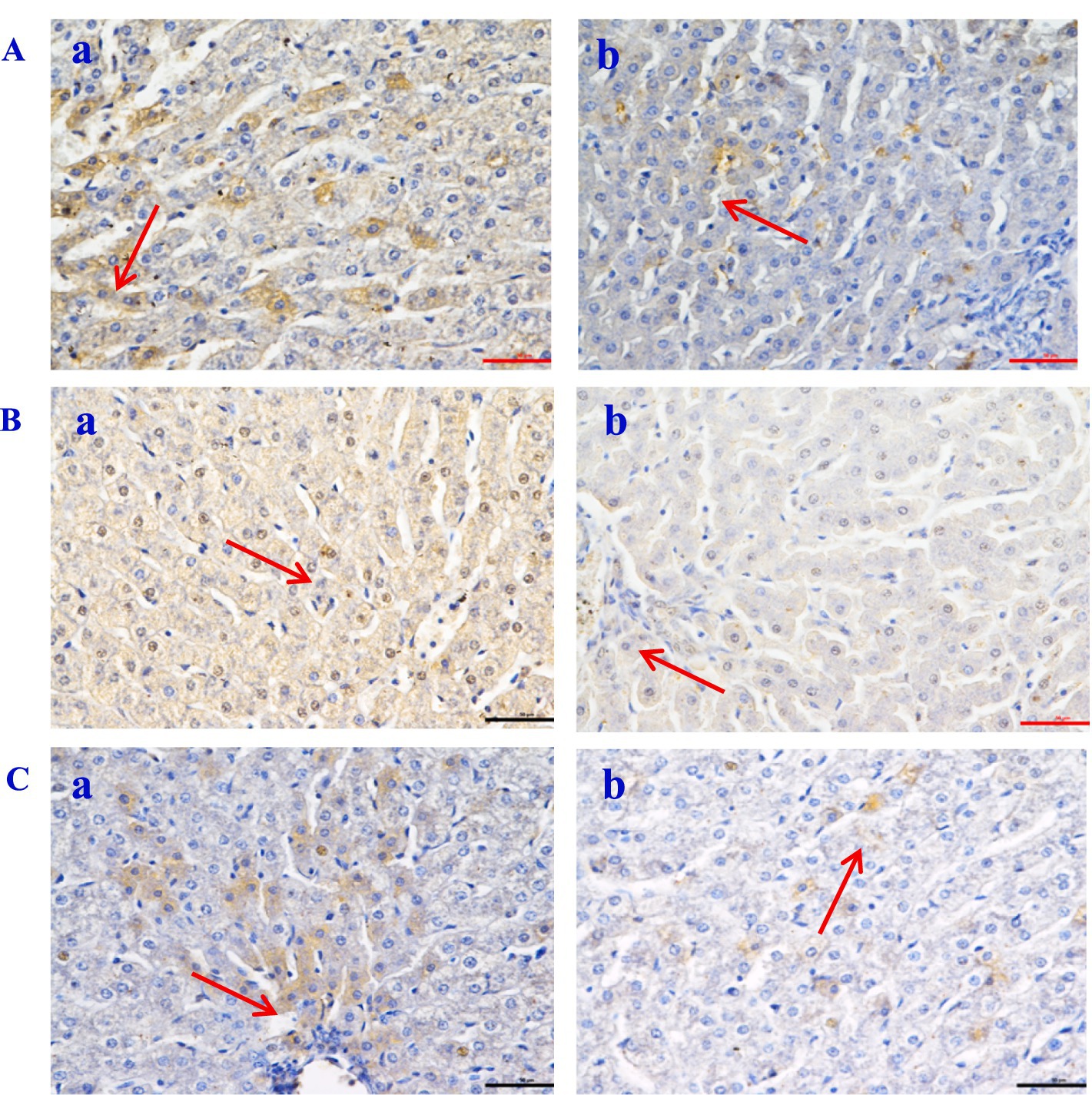
Figure 4. The expression of inflammatory factor in Hu sheep liver tissue (×50). (A) Caspase-3; (B) NF-Κb; (C) TNF-α. a: the control group (Group A), b: the CPs group (Group B).
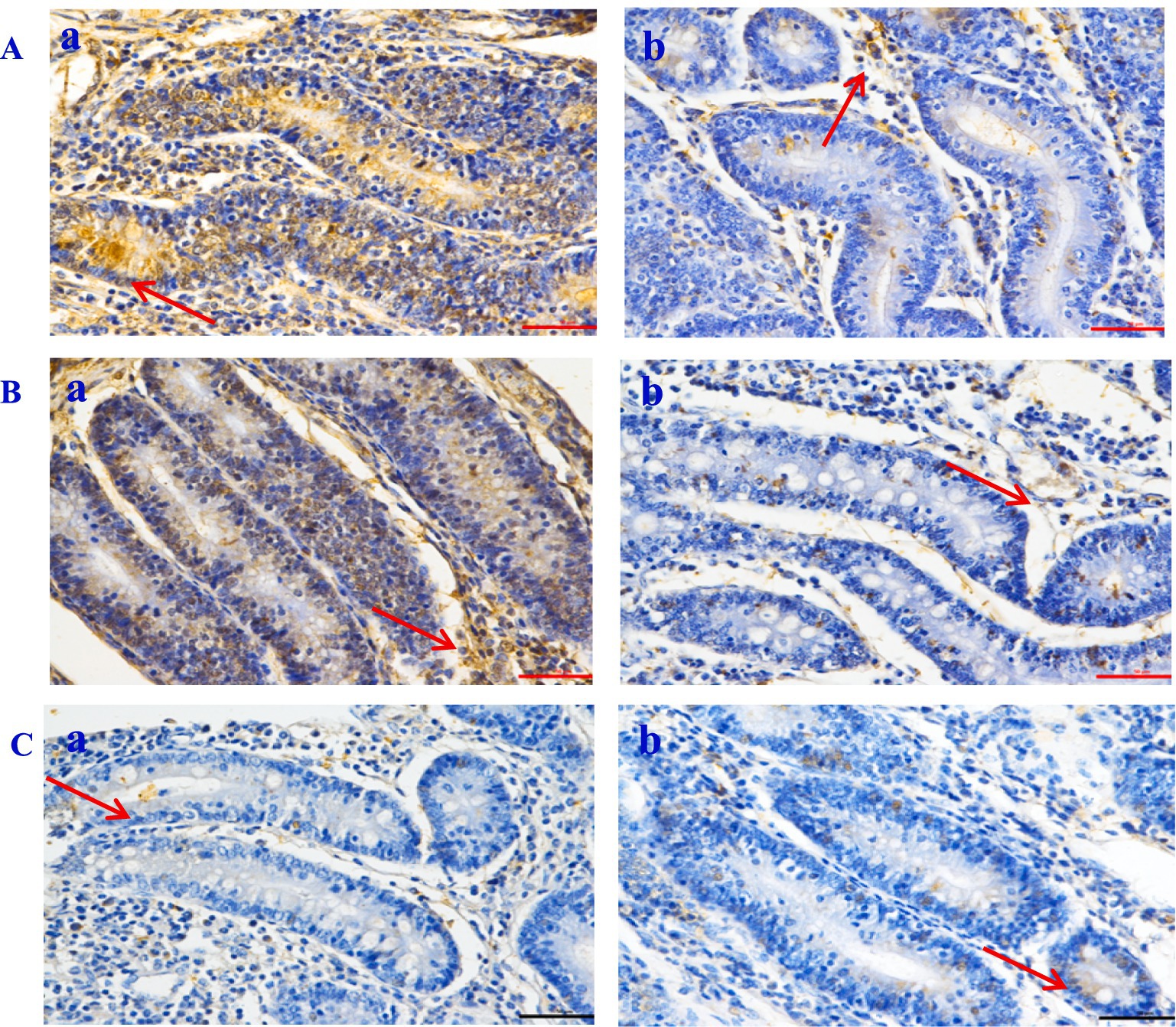
Figure 5. The expression of inflammatory factor in Hu sheep jejunal tissue (×50). (A) Caspase-3; (B) NF-Κb; (C) TNF-α. a: the control group (Group A), b: the CPs group (Group B).
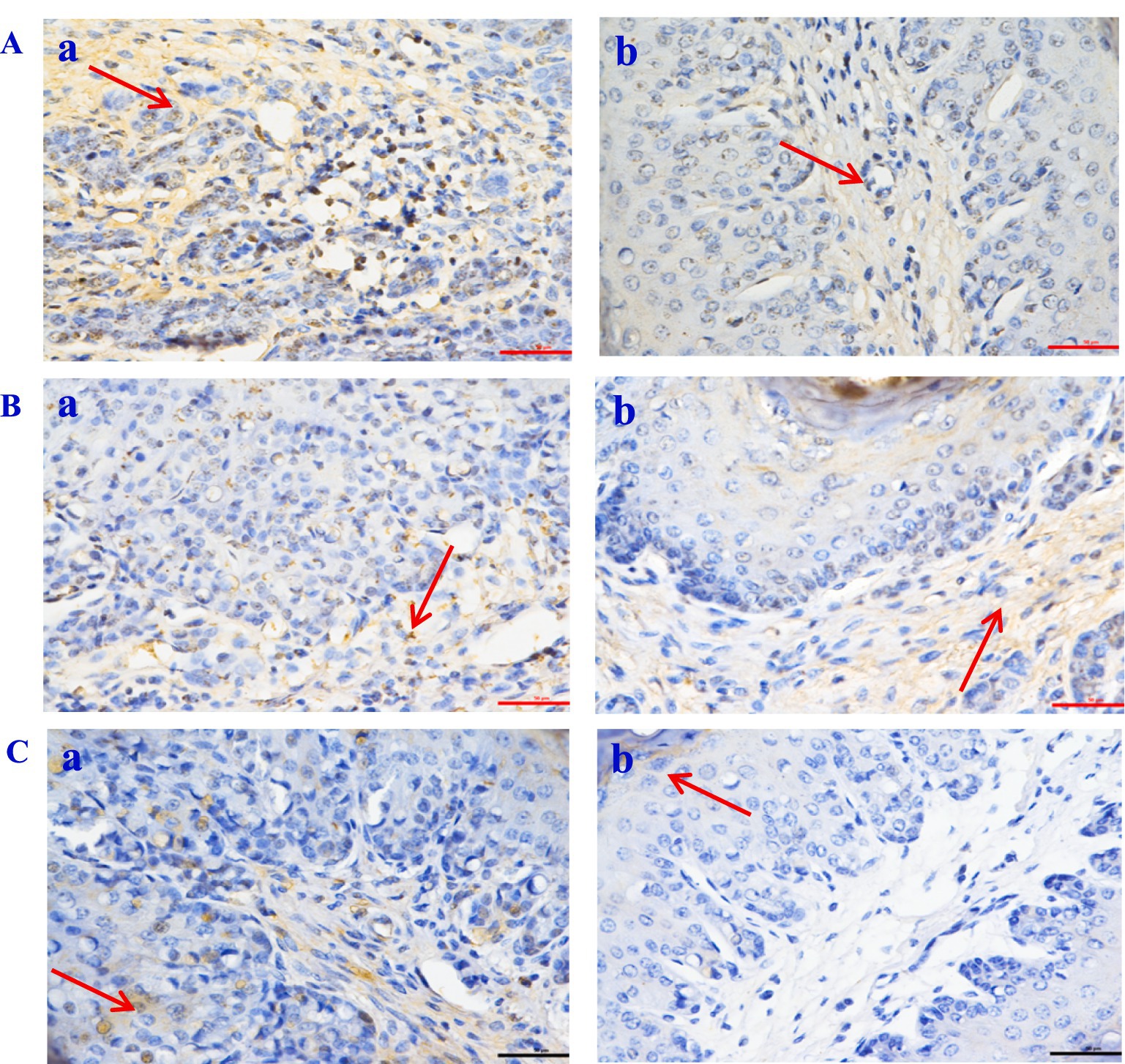
Figure 6. The expression of inflammatory factor in Hu sheep rumen tissue (×50). (A) Caspase-3; (B) NF-Κb; (C) TNF-α. a: the control group (Group A), b: the CPs group (Group B).
4 Discussion
Previously, it was shown that the compound probiotics could effectively improve the rumen environment and enhance the intestinal absorptive ability of the nutrients, and improve animal production performance (34–36). The present study showed that the compound probiotics significantly increased the ADG and decreased the F/G during days 31–60 and days 1–60. Guo et al. (8) reported that Aspergillus oryzae culture could improve feed dry matter degradation and increase the energy supply of VFAs concentration in the rumen of Hu sheep. A previous study revealed that Aspergillus oryzae and Aspergillus niger co-cultivation extract effectively improved feed digestibility and enhanced bacterial diversity (9). Candida utilis could improve feed utilization and intestinal health, consequently enhancing growth performance (37, 38). The present results were consistent with the above studies. However, no significant effects on ADG and F/G were observed during days 1–30, possibly due to the early stage being the colonization period of probiotics in the rumen. The present study indicated that CPs could increase calcium digestibility. Probiotics may influence gut morphology and gene expression to stimulate calcium absorption (39). Studies have indicated that the gut microbiome plays a role in enhancing calcium absorption, increasing calcium retention, and improving various measures of bone health (40), with serum calcium levels improving significantly after probiotic supplementation (41). Probiotics can change the rumen microbial ecosystem in ruminants and promote nutrient digestibility and feed efficiency.
IgM, IgA, and IgG are important immune factors, IgG is the sera’s major immunoglobulin, accounting for approximately 75% of immunoglobulin (42). Studies have shown that many immunoglobulins can improve the non-specific ability of the body’s immunity (43). The present study shows that the serum IgG content increased significantly in group C compared with group A. This result corroborates the findings of previous work on CPs, which can enhance animal immunity and anti-infection ability (44, 45). Strong antioxidant-destroying free radicals were formed when the soybean flour were fermented with Aspergillus oryzae (46). In addition, probiotics have been shown to increase GSH levels and reduce intestinal oxidative stress in several experimental models (47, 48). In the study by Lutgendorff et al. (49), it was shown that multispecies probiotics promoted the production of GSH and elevated its levels both in the pancreas and throughout the body. This study is consistent with previous work showing an antioxidant role of CPs. A cytokine is a group of proteins synthesized and secreted by immune and non-immune cells when stimulated (50). In the present study, the levels of cytokines IL-2, IL-4, IL-6, and IFN-γ in the serum of experimental sheep from the CPs groups were significantly upregulated compared with the control group. Studies have shown that CD4+ T cells are classified into Th1 and Th2 types, which secrete IFN-γ, IL-2 and IL-15 related to cellular immune responses, and IL-4, IL-6, and IL-10 that regulate the production of antibodies (51, 52). Thus, our study implicates that CPs effectively stimulated the Th1 and Th2 type cellular immune responses in sheep. Notably, group C showed the highest levels of both cellular and humoral immunity; however, there was no difference in serum CD4+ content among the three levels of CPs groups. The above results suggested that CPs could protect against oxidative stress, improve the body’s immunity, and enhance immune function.
Probiotics have been reported to elevate the efficiency of rumen function by stabilizing rumen pH (25, 53). Study has shown that yeast (Candida albicans) can maintain rumen pH stability (54), which was consistent with this study that CPs did not change the rumen pH. The probiotic treatment did not affect the concentrations of major ruminal VFAs, acetic acid and propionic acid in the present study. This agrees with previous reports in which ruminal main VFAs was not affected by a probiotic (55, 56). The levels of butyric acid and isobutyric acid showed a decreasing trend with higher CPs addition (800 CPs and 1,200 CPs). This outcome may be attributed to CPs (Yeast) stimulating the expression of transporters in the rumen epithelium, which could increase the rate of VFAs absorption and thereby decrease in the levels of butyric acid and isobutyric acid (57, 58). The present study showed that the 400 CPs probiotics addition increased the concentration of NH3-N, probably due to the reproduction of microorganisms, which increased protein degradation and produced more NH3-N (25). Aspergillus oryzae 1 and Aspergillus oryzae 2 have been proven by our previous research to have the ability to secrete proteases, amylase, and cellulase activity (59, 60). The incorporation of 400 CPs probiotics also improved the amylase and cellulase activity in the rumen, which contributed to improving the digestibility of nutrients.
In this study, the relative abundances of bacteria at the phylum level showed that Firmicutes in the CP groups were significantly higher, while Bacteroidetes were significantly lower than those in the control group. It was reported that the low ratio between Bacteroidetes and Firmicutes was beneficial to fat deposition (61), so it can be inferred that CPs addition contributes to sheep body weight gain by decreasing the ratio. At genus levels, the Prevotella, Ruminococcus, and Butyrivibrio were the three dominant genera. The genus Prevotella plays an important role in polysaccharide degradation and fermentation in the rumen. Carbohydrates are fermented by Prevotella to produce acetic acid (62). This is in keeping with the decrease of acetic acid content in the 400 CPs groups. Furthermore, Prevotella and Ruminococcus showed a positive correlation with environmental factors. The correlative analysis showed that Prevotella was negatively correlated with ADG, while group B with 400 CPs added decreased its relative abundance, indicating CPs increased sheep growth by decreasing Prevotella abundance. The specific mechanism needs further study. Ruminococcus is a cellulolytic bacterium, producing various cellulolytic enzymes, including cellulase (63, 64). The correlation analysis suggested that there was positive correlation between Ruminococcus and NDF. In this study, Group B exhibited the lowest relative abundance of Ruminococcus, although this difference was not statistically significant compared with the other groups. Nevertheless, Group B demonstrated the highest cellulase activity, suggesting that the CPs possess the capacity to produce cellulase for the degradation of cellulose.
Intestinal morphology is directly linked to intestinal development and health status (65). The increase in villus height and the ratio of villus height to crypt depth resulted in improved intestinal digestion and absorption efficiency, due to an increase in the absorption area (66). In this experiment, the villus height and the ratio of villus height to crypt depth in the jejunum of sheep fed on CPs were improved compared with the control group. In a study by Yang et al. (37), it was found that Candida utilis supplementation increased the villus height of the ileal mucosa and the ratio of villus height to crypt depth while decreasing the crypt depth. These findings suggest that Candida utilis has the potential to promote the health of intestinal tissues. This is one of the reasonable explanations for the CPs that can improve the performance and digestibility of the above study. Alkaline protease secreted by Aspergillus oryzae has anti-inflammatory activity, which may be useful for treating TNF-α-dependent inflammatory bowel diseases (67). TNF-α increases the phagocytic activity of neutrophils and stimulates the inflammatory response (68, 69). In the present study, the number of TNF-α and Caspase-3 positive cells in the liver and rumen in the CPs group was significantly lower than that in the control group. Previous studies have suggested that TNF-α can cause hepatocyte apoptosis via activating the caspase-3 signaling pathway (70, 71); the results of this study are consistent with those previous findings. In this study, the number of NF-κB and Caspase-3 positive cells in the jejunum in the CPs groups was significantly decreased compared with the control group. Furthermore, research has indicated that the NF-κB signaling pathway can activate caspase-3 (72), and there is a positive correlation between the expression of caspase-3 and NF-κB (73). This mechanism reasonably explains the decrease in NF-κB and Caspase-3 observed in the present study. Taken together, these findings suggest that CPs decrease the amount of inflammatory cytokines and the degree of inflammation, thereby reducing cell damage.
5 Conclusion
Based on the analysis presented above, it is concluded that adding 800 g/t of composite probiotics to the diet of Hu sheep yielded the most favorable outcomes. These included increased daily weight gain, improved feed-to-meat ratio, enhanced immune and anti-inflammatory functions, improved live, rumen and jejunum tissue structure, increased villus height and crypt depth in the jejunum, improved rumen fermentation function, and increased the relative abundance of Firmicutes and Proteobacteria at the phylum level. In conclusion, the findings of this study indicated the potential utility of compound probiotics in ruminant production.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Scientific Ethics Committee of Henan Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LW: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ZL: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. XN: Formal analysis, Methodology, Writing – original draft. ZY: Formal analysis, Methodology, Writing – review & editing. PW: Data curation, Writing – review & editing. CL: Formal analysis, Methodology, Writing – review & editing. SJ: Software, Writing – original draft. XL: Data curation, Formal analysis, Writing – review & editing. QY: Conceptualization, Funding acquisition, Writing – original draft. QZ: Methodology, Writing – review & editing. JC: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research and Development Projects of Henan Province (231111111600 and 241111113700), the Key Project of Science and Technology of Henan Province (232102110103), and the Natural Science Foundation of Henan Province (242300421573).
Conflict of interest
ZY was employed by Henan Anjin Biotechnology Co., Ltd. QZ was employed by Henan Delin Biological Product Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Melara, EG, Avellaneda, MC, Valdivié, M, García-Hernández, Y, Aroche, R, and Martínez, Y. Probiotics: symbiotic relationship with the animal host. Animals. (2022) 12:719. doi: 10.3390/ani12060719
2. Yang, M, Yin, Y, Wang, F, Bao, X, Long, L, Tan, B, et al. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J Anim Sci. (2021) 99:skab237. doi: 10.1093/jas/skab237
3. Tian, S, Wang, J, Yu, H, Wang, J, and Zhu, W. Changes in ileal microbial composition and microbial metabolism by an early-life galacto-oligosaccharides intervention in a neonatal porcine model. Nutrients. (2019) 11:1753. doi: 10.3390/nu11081753
4. Kober, A, Riaz Rajoka, MS, Mehwish, HM, Villena, J, and Kitazawa, H. Immunomodulation potential of probiotics: a novel strategy for improving livestock health, immunity, and productivity. Microorganisms. (2022) 10:388. doi: 10.3390/microorganisms10020388
5. Wei, H, Geng, W, Yang, XY, Kuipers, J, van der Mei, HC, and Busscher, HJ. Activation of a passive, mesoporous silica nanoparticle layer through attachment of bacterially-derived carbon-quantum-dots for protection and functional enhancement of probiotics. Mater Today Bio. (2022) 15:100293. doi: 10.1016/j.mtbio.2022.100293
6. Li, Y, Wang, Y, Liu, Y, Li, X, Feng, L, and Li, K. Optimization of an economical medium composition for the coculture of Clostridium butyricum and Bacillus coagulans. AMB Express. (2022) 12:19. doi: 10.1186/s13568-022-01354-5
7. Shruthi, B, Deepa, N, Somashekaraiah, R, Adithi, G, Divyashree, S, and Sreenivasa, MY. Exploring biotechnological and functional characteristics of probiotic yeasts: a review. Biotechnol Rep (Amst). (2022) 34:e00716. doi: 10.1016/j.btre.2022.e00716
8. Guo, L, Zhang, D, Du, R, Li, F, Li, F, and Ran, T. Supplementation of aspergillus oryzae culture improved the feed dry matter digestibility and the energy supply of total volatile fatty acid concentrations in the rumen of Hu sheep. Front Nutr. (2022) 9:847156. doi: 10.3389/fnut.2022.847156
9. Kong, F, Lu, N, Liu, Y, Zhang, S, Jiang, H, Wang, H, et al. Aspergillus oryzae and aspergillus Niger co-cultivation extract affects in vitro degradation, fermentation characteristics, and bacterial composition in a diet-specific manner. Animals. (2021) 11:1248. doi: 10.3390/ani11051248
10. Hymes-Fecht, UC, and Casper, DP. Adaptation and withdrawal of feeding dried aspergillus oryzae fermentation product to dairy cattle and goats on in vitro NDF digestibility of selected forage sources. Transl Anim Sci. (2021) 5:txab051. doi: 10.1093/tas/txab051
11. Zhang, J, Jin, W, Jiang, Y, Xie, F, and Mao, S. Response of milk performance, rumen and hindgut microbiome to dietary supplementation with aspergillus oryzae fermentation extracts in dairy cows. Curr Microbiol. (2022) 79:113. doi: 10.1007/s00284-022-02790-z
12. Miadoková, E, Svidová, S, Vlcková, V, Dúhová, V, Nad'ová, S, Rauko, P, et al. Diverse biomodulatory effects of glucomannan from Candida utilis. Toxicol In Vitro. (2006) 20:649–57. doi: 10.1016/j.tiv.2005.12.001
13. Vlcková, V, Dúhová, V, Svidová, S, Farkassová, A, Kamasová, S, Vlcek, D, et al. Antigenotoxic potential of glucomannan on four model test systems. Cell Biol Toxicol. (2004) 20:325–32. doi: 10.1007/s10565-004-0089-7
14. Yang, B, Wang, D, Wei, G, Liu, Z, and Ge, X. Selenium-enriched Candida utilis: efficient preparation with l-methionine and antioxidant capacity in rats. J Trace Elem Med Biol. (2013) 27:7–11. doi: 10.1016/j.jtemb.2012.06.001
15. Chaucheyras-Durand, F, Ameilbonne, A, Auffret, P, Bernard, M, Mialon, MM, Dunière, L, et al. Supplementation of live yeast based feed additive in early life promotes rumen microbial colonization and fibrolytic potential in lambs. Sci Rep. (2019) 9:19216. doi: 10.1038/s41598-019-55825-0
16. Cruz, A, Håkenåsen, IM, Skugor, A, Mydland, LT, Åkesson, CP, Hellestveit, SS, et al. Candida utilis yeast as a protein source for weaned piglets: effects on growth performance and digestive function. Livest Sci. (2019) 226:31–9. doi: 10.1016/j.livsci.2019.06.003
17. Huang, W, Chang, J, Wang, P, Liu, C, Yin, Q, Zhu, Q, et al. Effect of the combined compound probiotics with mycotoxin-degradation enzyme on detoxifying aflatoxin B(1) and zearalenone. J Toxicol Sci. (2018) 43:377–85. doi: 10.2131/jts.43.377
18. Van Soest, PJ, Robertson, JB, and Lewis, BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
19. Fei, L, Sun, G, Sun, J, and Wu, D. The effect of N6-methyladenosine (m6A) factors on the development of acute respiratory distress syndrome in the mouse model. Bioengineered. (2022) 13:7622–34. doi: 10.1080/21655979.2022.2049473
20. Song, K, Zeng, X, Xie, X, Zhu, R, Liang, J, Chen, G, et al. Dl-3-n-butylphthalide attenuates brain injury caused by cortical infarction accompanied by cranial venous drainage disturbance. Stroke Vasc Neurol. (2022) 7:222–36. doi: 10.1136/svn-2021-001308
21. Yue, D, Zhao, J, Chen, H, Guo, M, Chen, C, Zhou, Y, et al. MicroRNA-7, synergizes with RORα, negatively controls the pathology of brain tissue inflammation. J Neuroinflammation. (2020) 17:28. doi: 10.1186/s12974-020-1710-2
22. Piao, J, Shang, Y, Liu, S, Piao, Y, Cui, X, Li, Y, et al. High expression of DEK predicts poor prognosis of gastric adenocarcinoma. Diagn Pathol. (2014) 9:67. doi: 10.1186/1746-1596-9-67
23. He, W, Hou, M, Zhang, H, Zeng, C, He, S, Chen, X, et al. Clinical significance of circulating tumor cells in predicting disease progression and chemotherapy resistance in patients with gestational choriocarcinoma. Int J Cancer. (2019) 144:1421–31. doi: 10.1002/ijc.31742
24. Ma, T, Tu, Y, Zhang, NF, Deng, KD, and Diao, QY. Effect of the ratio of non-fibrous carbohydrates to neutral detergent fiber and protein structure on intake, digestibility, rumen fermentation, and nitrogen metabolism in lambs. Asian Australas J Anim Sci. (2015) 28:1419–26. doi: 10.5713/ajas.15.0025
25. Izuddin, WI, Loh, TC, Samsudin, AA, Foo, HL, Humam, AM, and Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet Res. (2019) 15:315. doi: 10.1186/s12917-019-2064-9
26. Prauchner, CA, Kozloski, GV, and Farenzena, R. Evaluation of sonication treatment and buffer composition on rumen bacteria protein extraction and carboxymethylcellulase activity. J Sci Food Agric. (2013) 93:1733–6. doi: 10.1002/jsfa.5959
27. Miller, GL, Blum, R, Glennon, WE, and Burton, AL. Measurement of carboxymethylcellulase activity. Anal Biochem. (1960) 1:127–32. doi: 10.1016/0003-2697(60)90004-X
28. Elolimy, AA, Arroyo, JM, Batistel, F, Iakiviak, MA, and Loor, JJ. Association of residual feed intake with abundance of ruminal bacteria and biopolymer hydrolyzing enzyme activities during the peripartal period and early lactation in Holstein dairy cows. J Anim Sci Biotechnol. (2018) 9:43. doi: 10.1186/s40104-018-0258-9
29. Wang, L, Zhang, G, Xu, H, Xin, H, and Zhang, Y. Metagenomic analyses of microbial and carbohydrate-active enzymes in the rumen of Holstein cows fed different forage-to-concentrate ratios. Front Microbiol. (2019) 10:649. doi: 10.3389/fmicb.2019.00649
30. Bolger, AM, Lohse, M, and Usadel, B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. (2014) 30:2114–20. doi: 10.1093/bioinformatics/btu170
31. Callahan, BJ, McMurdie, PJ, Rosen, MJ, Han, AW, Johnson, AJ, and Holmes, SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. (2016) 13:581–3. doi: 10.1038/nmeth.3869
32. Quast, C, Pruesse, E, Yilmaz, P, Gerken, J, Schweer, T, Yarza, P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
33. Song, H, Singh, D, Tomlinson, KW, Yang, X, Ogwu, MC, Slik, JWF, et al. Tropical forest conversion to rubber plantation in Southwest China results in lower fungal beta diversity and reduced network complexity. FEMS Microbiol Ecol. (2019) 95:fiz092. doi: 10.1093/femsec/fiz092
34. Lynch, HA, and Martin, SA. Effects of Saccharomyces cerevisiae culture and Saccharomyces cerevisiae live cells on in vitro mixed ruminal microorganism fermentation. J Dairy Sci. (2002) 85:2603–8. doi: 10.3168/jds.S0022-0302(02)74345-2
35. Uyeno, Y, Akiyama, K, Hasunuma, T, Yamamoto, H, Yokokawa, H, Yamaguchi, T, et al. Effects of supplementing an active dry yeast product on rumen microbial community composition and on subsequent rumen fermentation of lactating cows in the mid-to-late lactation period. Anim Sci J. (2017) 88:119–24. doi: 10.1111/asj.12612
36. Sittisak, K, Pala, C, Uthai, K, Rungson, S, and Metha, W. Manipulation of rumen ecology by malate and yeast in native cattle. Pak J Nutr. (2009) 8:1048–51. doi: 10.3923/pjn.2009.1048.1051
37. Yang, Z, Wang, Y, He, T, Ziema Bumbie, G, Wu, L, Sun, Z, et al. Effects of dietary yucca schidigera extract and oral candida utilis on growth performance and intestinal health of weaned piglets. Front Nutr. (2021) 8:685540. doi: 10.3389/fnut.2021.685540
38. Shen, YB, Piao, XS, Kim, SW, Wang, L, Liu, P, Yoon, I, et al. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci. (2009) 87:2614–24. doi: 10.2527/jas.2008-1512
39. Tiihonen, K, Ouwehand, AC, and Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res Rev. (2010) 9:107–16. doi: 10.1016/j.arr.2009.10.004
40. Wallace, TC, Marzorati, M, Spence, L, Weaver, CM, and Williamson, PS. New frontiers in fibers: innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. (2017) 36:218–22. doi: 10.1080/07315724.2016.1257961
41. Franck, A. Prebiotics stimulate calcium absorption: a review. Milchwissenschaft. (2005) 45:11–6. doi: 10.1016/j.nut.2017.06.011
42. Cheng, X, He, F, Si, M, Sun, P, and Chen, Q. Effects of antibiotic use on saliva antibody content and oral microbiota in Sprague dawley rats. Front Cell Infect Microbiol. (2022) 12:721691. doi: 10.3389/fcimb.2022.721691
43. Wang, Z, Zhang, F, Xiang, L, Yang, Y, Wang, W, Li, B, et al. Successful use of extracorporeal life support and continuous renal replacement therapy in the treatment of cardiogenic shock induced by tumor lysis syndrome in a pediatric patient with lymphoma: a case report. Front Med. (2021) 8:762788. doi: 10.3389/fmed.2021.762788
44. Xu, X, Yang, C, Chang, J, Wang, P, Yin, Q, Liu, C, et al. Dietary supplementation with compound probiotics and berberine alters piglet production performance and fecal microbiota. Animals. (2020) 10:511. doi: 10.3390/ani10030511
45. Liu, X, Zhao, W, Yu, D, Cheng, JG, Luo, Y, Wang, Y, et al. Effects of compound probiotics on the weight, immunity performance and fecal microbiota of forest musk deer. Sci Rep. (2019) 9:19146. doi: 10.1038/s41598-019-55731-5
46. Handa, CL, de Lima, FS, Guelfi, MFG, Fernandes, MDS, Georgetti, SR, and Ida, EI. Parameters of the fermentation of soybean flour by Monascus purpureus or aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. (2019) 271:274–83. doi: 10.1016/j.foodchem.2018.07.188
47. Tang, Y, Han, L, Chen, X, Xie, M, Kong, W, and Wu, Z. Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under aeromonas hydrophila challenge. Probiotics Antimicrob Proteins. (2019) 11:545–58. doi: 10.1007/s12602-018-9409-8
48. Peran, L, Camuesco, D, Comalada, M, Nieto, A, Concha, A, Adrio, JL, et al. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int J Color Dis. (2006) 21:737–46. doi: 10.1007/s00384-005-0773-y
49. Lutgendorff, F, Trulsson, LM, van Minnen, LP, Rijkers, GT, Timmerman, HM, Franzén, LE, et al. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. (2008) 295:G1111–21. doi: 10.1152/ajpgi.00603.2007
50. Zhang, J, Zhang, Y, Wang, Q, Li, C, Deng, H, Si, C, et al. Interleukin-35 in immune-related diseases: protection or destruction. Immunology. (2019) 157:13–20. doi: 10.1111/imm.13044
51. Toda, M, and Ono, SJ. Genomics and proteomics of allergic disease. Immunology. (2002) 106:1–10. doi: 10.1046/j.1365-2567.2002.01407.x
52. Kanai, K, Asano, K, Watanabe, S, Kyo, Y, and Suzaki, H. Epinastine hydrochloride antagonism against interleukin-4-mediated T cell cytokine imbalance in vitro. Int Arch Allergy Immunol. (2006) 140:43–52. doi: 10.1159/000092001
53. O'Callaghan, TF, Ross, RP, Stanton, C, and Clarke, G. The gut microbiome as a virtual endocrine organ with implications for farm and domestic animal endocrinology. Domest Anim Endocrinol. (2016) 56:S44–55. doi: 10.1016/j.domaniend.2016.05.003
54. Liu, Y, Xiao, Y, Ma, T, Diao, Q, and Tu, Y. Candida tropicalis as a novel dietary additive to reduce methane emissions and nitrogen excretion in sheep. Environ Sci Pollut Res Int. (2023) 30:82661–71. doi: 10.1007/s11356-023-28245-x
55. Chiquette, J, Allison, MJ, and Rasmussen, MA. Prevotella bryantii 25A used as a probiotic in early-lactation dairy cows: effect on ruminal fermentation characteristics, milk production, and milk composition. J Dairy Sci. (2008) 91:3536–43. doi: 10.3168/jds.2007-0849
56. Ghorbani, GR, Morgavi, DP, Beauchemin, KA, and Leedle, JA. Effects of bacterial direct-fed microbials on ruminal fermentation, blood variables, and the microbial populations of feedlot cattle. J Anim Sci. (2002) 80:1977–85. doi: 10.2527/2002.8071977x
57. Shah, AM, Cai, Y, Zou, H, Zhang, X, Wang, L, Xue, B, et al. Effects of supplementation of branches and leaves trimmed from tea plant on growth performance, rumen fermentation and meat composition of nanjiang yellow goats. Animals. (2019) 9:590. doi: 10.3390/ani9090590
58. Petri, RM, Wetzels, SU, Qumar, M, Khiaosa-Ard, R, and Zebeli, Q. Adaptive responses in short-chain fatty acid absorption, gene expression, and bacterial community of the bovine rumen epithelium recovered from a continuous or transient high-grain feeding. J Dairy Sci. (2019) 102:5361–78. doi: 10.3168/jds.2018-15691
59. Chang, J, Cheng, W, Yin, Q, Zuo, R, Song, A, Zheng, Q, et al. Effect of steam explosion and microbial fermentation on cellulose and lignin degradation of corn Stover. Bioresour Technol. (2012) 104:587–92. doi: 10.1016/j.biortech.2011.10.070
60. Chang, J, Wang, T, Wang, P, Yin, Q, Liu, C, Zhu, Q, et al. Compound probiotics alleviating aflatoxin B(1) and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol Environ Saf. (2020) 194:110420. doi: 10.1016/j.ecoenv.2020.110420
61. Turnbaugh, PJ, Ley, RE, Mahowald, MA, Magrini, V, Mardis, ER, and Gordon, JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444:1027–31. doi: 10.1038/nature05414
62. Shinkai, T, Ikeyama, N, Kumagai, M, Ohmori, H, Sakamoto, M, Ohkuma, M, et al. Prevotella lacticifex sp. nov., isolated from the rumen of cows. Int J Syst Evol Microbiol. (2022) 72:5278. doi: 10.1099/ijsem.0.005278
63. Kawahara, R, Saburi, W, Odaka, R, Taguchi, H, Ito, S, Mori, H, et al. Metabolic mechanism of mannan in a ruminal bacterium, Ruminococcus albus, involving two mannoside phosphorylases and cellobiose 2-epimerase: discovery of a new carbohydrate phosphorylase, β-1,4-mannooligosaccharide phosphorylase. J Biol Chem. (2012) 287:42389–99. doi: 10.1074/jbc.M112.390336
64. Zhang, XM, Medrano, RF, Wang, M, Beauchemin, KA, Ma, ZY, Wang, R, et al. Corn oil supplementation enhances hydrogen use for biohydrogenation, inhibits methanogenesis, and alters fermentation pathways and the microbial community in the rumen of goats. J Anim Sci. (2019) 97:4999–5008. doi: 10.1093/jas/skz352
65. Huang, D, Zhu, J, Zhang, L, Ge, X, Ren, M, and Liang, H. Dietary supplementation with eucommia ulmoides leaf extract improved the intestinal antioxidant capacity, immune response, and disease resistance against streptococcus agalactiae in genetically improved farmed tilapia (GIFT; Oreochromis niloticus). Antioxidants. (2022) 11:1800. doi: 10.3390/antiox11091800
66. Sun, H, Tang, JW, Yao, XH, Wu, YF, Wang, X, and Feng, J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop Anim Health Prod. (2013) 45:987–93. doi: 10.1007/s11250-012-0322-y
67. Hong, K, Yu, J, and Parmely, MJ. Alkaline protease purified from aspergillus oryzae prevents TNF-a-induced acute inflammation in the mouse small intestine. FASEB J. (2006) 20:A144. doi: 10.1096/fasebj.20.4.A144
68. Carmona-Rivera, C, Khaznadar, SS, Shwin, KW, Irizarry-Caro, JA, O'Neil, LJ, Liu, Y, et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood. (2019) 134:395–406. doi: 10.1182/blood.2018892752
69. Matte, A, Recchiuti, A, Federti, E, Koehl, B, Mintz, T, El Nemer, W, et al. Resolution of sickle cell disease-associated inflammation and tissue damage with 17R-resolvin D1. Blood. (2019) 133:252–65. doi: 10.1182/blood-2018-07-865378
70. Guerra, AD, Yeung, OWH, Qi, X, Kao, WJ, and Man, K. The anti-tumor effects of M1 macrophage-loaded poly (ethylene glycol) and gelatin-based hydrogels on hepatocellular carcinoma. Theranostics. (2017) 7:3732–44. doi: 10.7150/thno.20251
71. Wang, S, Li, L, and Shi, L. Identification of a key candidate gene-phenotype network mediated by glycyrrhizic acid using pharmacogenomic analysis. Mol Med Rep. (2019) 20:2657–66. doi: 10.3892/mmr.2019.10494
72. Milani, A, Basirnejad, M, and Bolhassani, A. Heat-shock proteins in diagnosis and treatment: an overview of different biochemical and immunological functions. Immunotherapy. (2019) 11:215–39. doi: 10.2217/imt-2018-0105
Keywords: Hu sheep, compound probiotics, growth performance, immune function, rumen bacteria
Citation: Wang L, Lv Z, Ning X, Yue Z, Wang P, Liu C, Jin S, Li X, Yin Q, Zhu Q and Chang J (2024) The effects of compound probiotics on production performance, rumen fermentation and microbiota of Hu sheep. Front. Vet. Sci. 11:1440432. doi: 10.3389/fvets.2024.1440432
Edited by:
Suban Foiklang, Maejo University, ThailandReviewed by:
Anusorn Cherdthong, Khon Kaen University, ThailandHangshu Xin, Northeast Agricultural University, China
Lifeng Dong, Chinese Academy of Agricultural Sciences, China
Maharach Matra, Khon Kaen University, Thailand
Copyright © 2024 Wang, Lv, Ning, Yue, Wang, Liu, Jin, Li, Yin, Zhu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Chang, Y2hhbmdqdWFuMjAwMEAxMjYuY29t
†These authors have contributed equally to this work
 Lijun Wang
Lijun Wang Zhanqi Lv1†
Zhanqi Lv1† Ping Wang
Ping Wang Sanjun Jin
Sanjun Jin Qingqiang Yin
Qingqiang Yin Juan Chang
Juan Chang