- Department of Biological Sciences, Andong National University, Andong, Republic of Korea
Background: Prion diseases are irreversible infectious neurodegenerative diseases caused by a contagious form of prion protein (PrPSc). Since chronic wasting disease (CWD)-infected white-tailed deer are strong carriers of the prion seed through corpses via scavenger animals, preemptive control based on genetic information for a culling system is necessary. However, the risk of CWD-related genetic variants has not been fully evaluated. In the present study, we carried out a quantitative estimation of the risk of a G96S single nucleotide polymorphism (SNP) of the PRNP gene to CWD infection in white-tailed deer.
Methods: We carried out a literature search for genetic data of the G96S (c.286G>A) SNP of the PRNP gene from CWD-infected white-tailed deer and matched controls. We performed a meta-analysis using incorporated eligible studies to evaluate the association of the G96S SNP of the PRNP gene with susceptibility to CWD in white-tailed deer.
Results: We identified a strong association between the G96S (c.286G>A) SNP of the PRNP gene and susceptibility to CWD infection in white-tailed deer using meta-analysis. We observed the most significant association in the recessive model (odds ratio = 3.0050, 95% confidence interval: 2.0593; 4.3851, p < 0.0001), followed by the additive model (odds ratio = 2.7222, 95% confidence interval: 1.9028; 3.8945, p < 0.0001) and the heterozygote (AA vs. AG) comparison (odds ratio = 2.7405, 95% confidence interval: 1.9215; 3.9085, p < 0.0001).
Conclusion: To the best of our knowledge, this was the first meta-analysis of the association between the G96S (c.286G>A) SNP of the PRNP gene and susceptibility to CWD infection.
1 Introduction
Prion diseases are malignant contagious neurodegenerative diseases caused by an infectious form of prion protein (PrPSc) converted from a benign form of prion protein (PrPC) (1–3). Several types of prion diseases have been reported worldwide including scrapie in sheep and goats; bovine spongiform encephalopathy (BSE) in cattle; Creutzfeldt-Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann–Straussler–Scheinker syndrome (GSS), and kuru in humans; and chronic wasting disease (CWD) in the Cervidae family (2, 4, 5). PrPSc acts as a conversion seed and changes PrPC to a ß-sheet-rich aggregate. The aggregate accumulates in brain lesions and is accompanied by several neurotoxic symptoms including neuron loss, vacuolation, and astrogliosis (1, 6, 7). During the conversion process, genetic variants of the amino acid sequences of prion protein (PrP), which are encoded by the prion protein gene (PRNP), play a pivotal role in susceptibility to prion diseases (8, 9). In humans, the M129V single nucleotide polymorphism (SNP) provides resistance to CJD (10). In addition, the G127V natural genetic variant blocks kuru (11). In sheep, scrapie susceptibility is modulated by haplotypes located on codons 136, 154, and 171 (12). In goats, codons at 142, 146, 154, 211, and 222 of the PRNP gene contribute to prion disease resistance (12–14). Like other prion diseases, CWD hosts have unique susceptibility-related genetic variants (5, 15). In elk, M132L SNP is associated with CWD propagation (16). In sika deer, a study indicated that S19N is related to CWD vulnerability (17). In white-tailed deer, several studies have suggested that G96S is the most potent candidate for prion-related genetic factors (18–27). However, quantitative studies have not been performed.
Among the several types of prion disease, CWD is the most infectious because it can be vertically transmitted, as through CWD prion-contaminated soil (28). Therefore, preemptive control with a culling system using genetic information is a crucial step to prevent the extensive spread of CWD. Since some CWD-infected white-tailed deer are free-ranging, there is a greater possibility of prion contamination through corpses via scavenger animals and of the emergence of novel prion hosts and strains (29). In addition, older free-ranging deer of indeterminate slaughter age carrying germline and somatic mutations in the PRNP gene are more likely to develop prion disease. Since CWD seed inducible genetic variants have not been fully elucidated, a quantitative evaluation of the connection between G96S of the PRNP gene and CWD susceptibility in white-tailed deer is necessary.
In the present study, we searched the literature for study data on the genotype and allele distributions of the G96S SNP of the PRNP gene from CWD-infected white-tailed deer and matched controls to evaluate the association between the G96S SNP of the PRNP gene and susceptibility to CWD in white-tailed deer. Then, we carried out a meta-analysis using incorporated eligible studies.
2 Materials and methods
2.1 Search strategy
We carried out the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A literature search was conducted in the PubMed, Google Scholar, and Web of Science databases to find studies associated with the G96S (c.286G>A) SNP of the PRNP gene from CWD-infected white-tailed deer. The following terms were employed for the search: “PRNP,” “white-tailed deer,” and “CWD” or “Chronic wasting disease” combined with “polymorphism” or “SNP” (last search update: March 12, 2024).
Eligible studies adhered to the subsequent inclusion criteria: (1) relating to and containing the association between the G96S (c.286G>A) SNP and CWD-infected white-tailed deer; (2) a case-control study; (3) full text available; and (4) written in English. The exclusion criteria were as follows: (1) case reports and (2) insufficient genetic information.
2.2 Meta-analysis
The meta-analysis was performed using the meta package of the R program [https://www.r-project.org/ (accessed on March 12, 2024)] and involved calculation of the pooled odds ratios with 95% confidence intervals between the G96S (c.286G>A) SNP of the PRNP gene and susceptibility to CWD. We utilized a total of 7 genetic models: additive (A vs. G), recessive (AA vs. AG + GG), dominant (AA + AG vs. GG), and over-dominant (AG vs. AA + GG) models and homozygote (AA vs. GG) and heterozygote (AA vs. AG and AG vs. GG) comparisons. Heterogeneity was calculated using the p-value and I2 value. The fixed and random effect models were selected according to the value of the I2 test. Publication bias was estimated using Egger’s weighted regression methods.
3 Results
3.1 Study selection
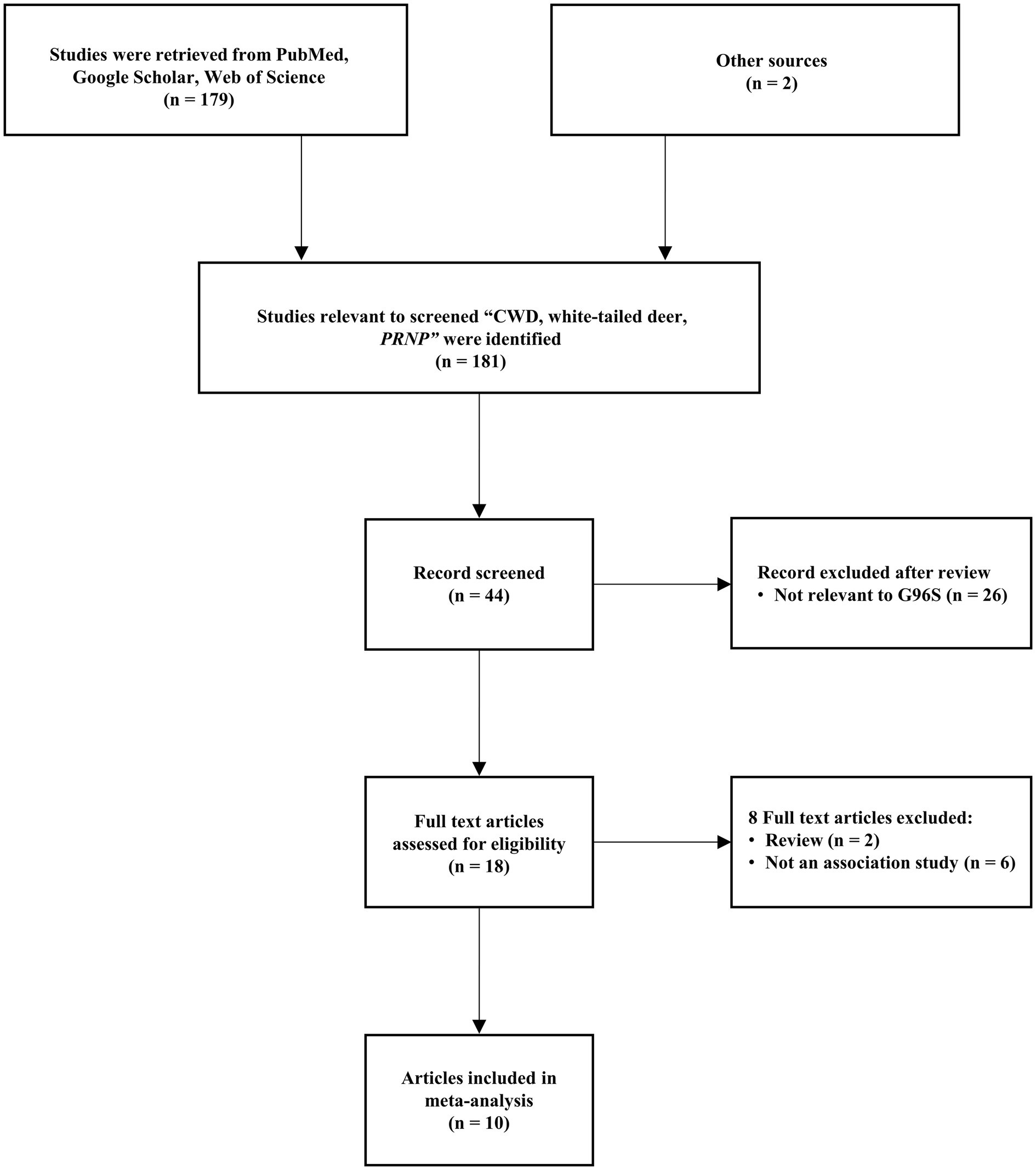
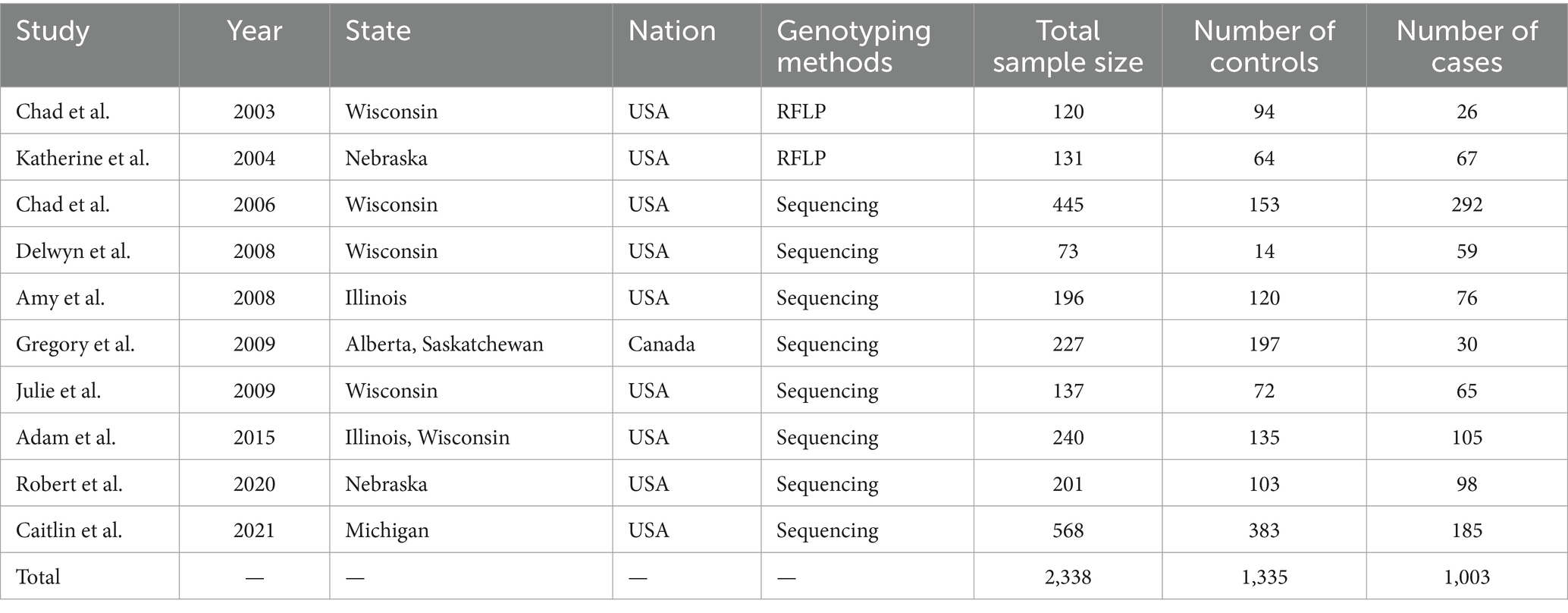
A flow chart of the study selection process is shown in Figure 1. After excluding ineligible articles based on the inclusion and exclusion criteria, 10 relevant studies were extracted from the databases (PubMed, Google Scholar, Web of Science). The meta-analysis comprised 1,003 cases and 1,335 controls (Table 1). All eligible studies utilized a case–control comparison using two genotyping methods. Among the 10 studies, 2 studies were genotyped using polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) methods. The remaining 8 studies were genotyped using PCR-amplicon sequencing methods (Table 1).
3.2 Evaluation of the association between the G96S (c.286G>A) SNP of the PRNP gene and susceptibility to CWD by meta-analysis in white-tailed deer
The pooled, weighted genotyping results were combined according to sample size to evaluate the association between the G96S SNP of the PRNP gene and susceptibility to CWD using the meta package of the R program. The pooled odds ratios with 95% confidence intervals were evaluated based on 7 genetic models: additive (A vs. G), recessive (AA vs. AG + GG), dominant (AA + AG vs. GG), and over-dominant (AG vs. AA + GG) models and homozygote (AA vs. GG) and heterozygote (AA vs. AG and AG vs. GG) comparisons. Heterogeneity was estimated using the p-value and I2 value (Table 2). The dominant model (AA + AG vs. GG) and homozygote (AA vs. GG) and heterozygote (AG vs. GG) comparisons used fixed models according to the I2 value (>0.5). The remaining 4 genetic models used random models according to the I2 value (<0.5).
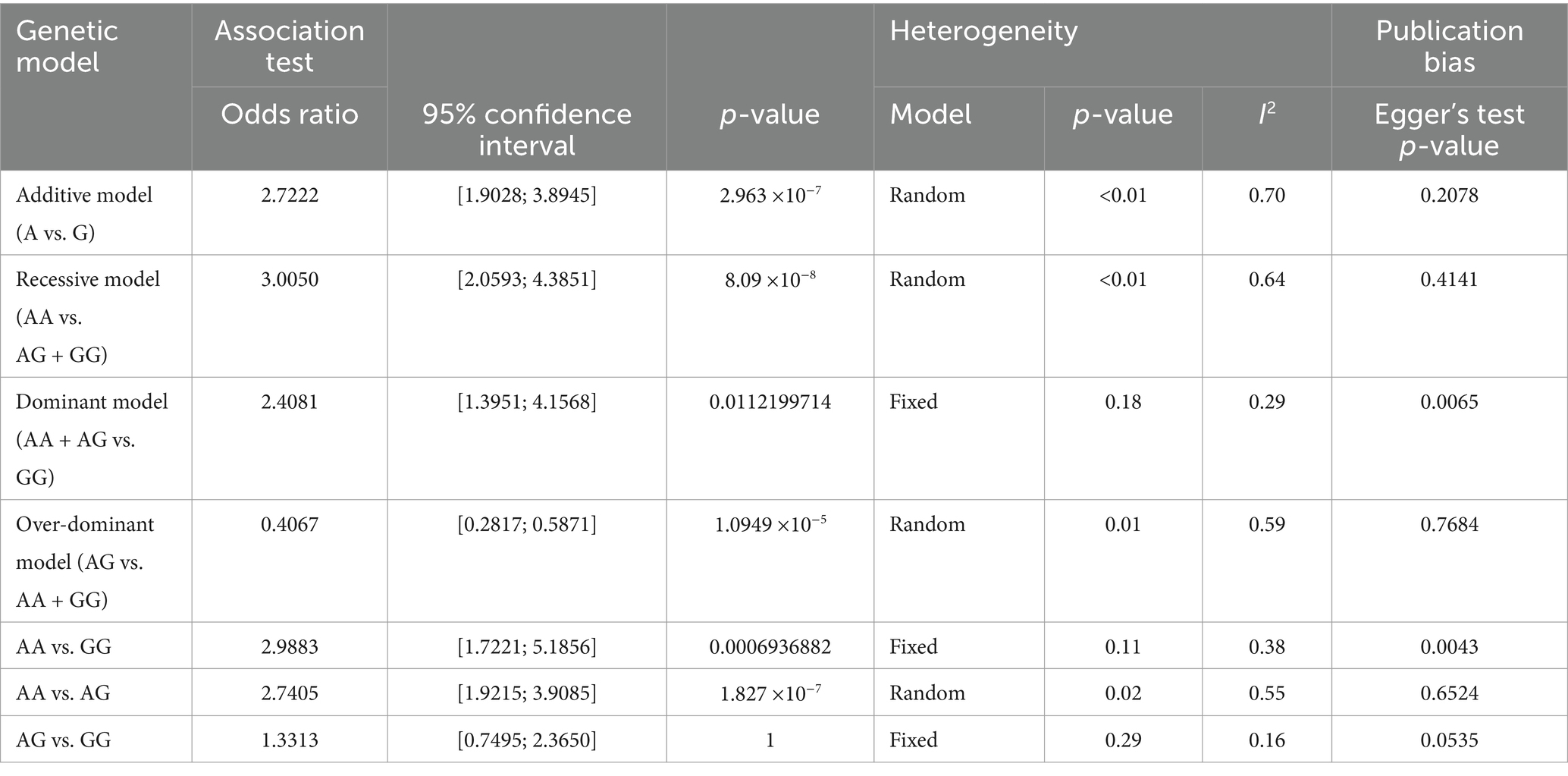
Table 2. Meta-analysis of the association between G96S (c.286G>A) of the prion protein gene (PRNP) gene and susceptibility to chronic wasting disease (CWD).
Except for the heterozygote (AG vs. GG) comparison, the data from the 9 remaining genetic models showed a strong association between the risk of CWD and the G96S (c.286G>A) SNP of the PRNP gene in the additive model (odds ratio = 2.7222, 95% confidence interval: 1.9028; 3.8945, p < 0.0001), recessive model (odds ratio = 3.0050, 95% confidence interval: 2.0593; 4.3851, p < 0.0001), dominant model (odds ratio = 2.4081, 95% confidence interval: 1.3951; 4.1568, p < 0.05), over-dominant model (odds ratio = 0.4067, 95% confidence interval: 0.2817; 0.5871, p < 0.001), homozygote comparison (odds ratio = 2.9883, 95% confidence interval: 1.7221; 5.1856, p < 0.001), and heterozygote (AA vs. AG) comparison (odds ratio = 2.7405, 95% confidence interval: 1.9215; 3.9085, p < 0.0001).
To estimate potential publication bias, Egger’s tests were carried out, and publication bias was observed in the dominant model and homozygote (AA vs. GG) and heterozygote comparisons (AG vs. GG). The details of the values are described in Table 2. Except for publication bias that violated genetic models, the most significant association was observed in the recessive model, followed by the additive model and heterozygote (AA vs. AG) comparison. Forest plots are shown in Figure 2.
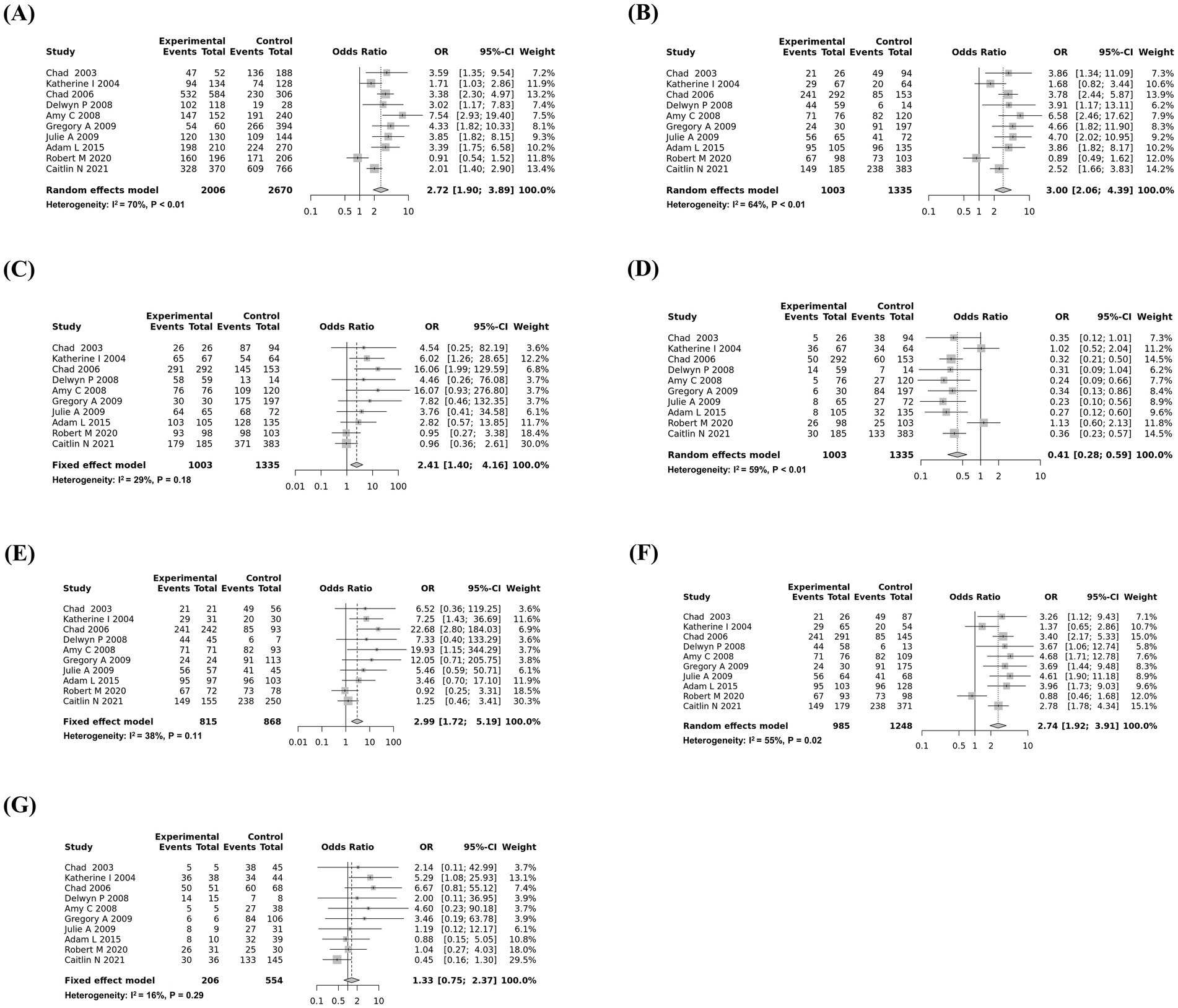
Figure 2. Forest plots of the association between the G96S (c.286G>A) single-nucleotide polymorphism (SNP) of the prion protein gene (PRNP) gene and susceptibility to chronic wasting disease (CWD). (A) Additive model (A vs. G). (B) Recessive model (AA vs. AG + GG). (C) Dominant model (AA + AG vs. GG). (D) Over-dominant model (AG vs. AA + GG). (E) Homozygote comparison (AA vs. GG). (F) Heterozygote comparison (AA vs. AG). (G) Heterozygote comparison (AG vs. GG).
4 Discussion
White-tailed deer are raised on farms or live in the wild, and CWD-infected white-tailed deer have been reported in the USA and Canada in North America (23, 30). In previous surveillance for prion diseases in Wisconsin, field cases were reported in several scavenger animals including mink, raccoon, and opossums (29). The results of that study indicated that free-ranging CWD-infected white-tailed deer are strong prion carriers and possible CWD pandemic inducers in the ecosystem. In addition, recent studies have indicated that a sporadic type of animal prion disease, atypical BSE, was identified in old animals (8 years old or older) (31). Since sporadic human prion diseases account for over 85% of the total number (32), and the slaughter age is not determined for free-ranging white-tailed deer, the possibility of sporadic occurrence may be much higher in white-tailed deer than in other farmed animals. Therefore, it is important to investigate CWD-related genetic factors for use in a culling system for preemptive prevention of CWD in white-tailed deer.
Previous genome-wide association analysis (GWAA) suggested that G96S is the large-effect risk locus; however, that study did not provide the quantitative value of the CWD risk (33). In the present study, we quantitatively evaluated the risk of the G96S SNP of the PRNP gene for CWD in white-tailed deer and observed a strong association in the recessive (AA vs. AG + GG) and additive (A vs. G) models and the heterozygote (AA vs. AG) comparison (Table 2). In summary, the 96G allele confers a risk of CWD that is more than 2.5 times higher than that of the 96S allele in these genetic models (Table 2).
Codon 96 is located on the unstructured coil region between the octapeptide repeat region and ß-sheet of cervid PrP (15). Several pathogenic mutations including P102L, P105L, and A117V of human PrP were identified in a similar region (34). Thus, this region seems to play a pivotal role in the conversion of PrPSc. However, although M129V heterozygotes provided potent resistance to CJD, human PrP with the 129M allele showed a similar structure and characteristics to human PrP with the 129V allele. The prion-resistant effect of the 129V allele is related to the homotypic amino acid sequence between the template protein and prion seed (PrPSc) (35). In addition, the results of a recent real-time quaking-induced conversion (RT-QuIC) study indicated that conversion efficiency (PrPC to PrPSc) is significantly related to the correspondence of the amino acid sequence at codon 132 of the elk PrP between the seed (PrPSc) and template protein (36). Thus, further investigation of the effects of codon 96 on seed-template interaction is required to elucidate the pathomechanism of codon 96. In white-tailed deer, several reports have indicated that codon 95 is also associated with susceptibility to CWD (15, 18). In addition, a recent study suggested that codon 116 is related to the stability of CWD prion, sensitivity to proteinase K, and lower prion seeding activity (37). Since the present study did not reflect other codons or the haplotypes of the PRNP gene, further investigation of genetic polymorphisms at other codons and the haplotypes of the PRNP gene are highly desirable in the future. Given the complexity of the pathological mechanisms of CWD, fully understanding them is challenging; therefore, we focused on a single factor (G96S SNP) in this study. However, a comprehensive analysis involving other codons will be necessary in the future to fully capture the phenomenon in real-world settings.
In conclusion, this study is the first meta-analysis to quantitatively assess the G96S SNP’s association with CWD susceptibility in white-tailed deer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
S-YW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Y-CK: Conceptualization, Funding acquisition, Investigation, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a Research Grant of Andong National University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Imran, M, and Mahmood, S. An overview of human prion diseases. Virol J. (2011) 8:1–9. doi: 10.1186/1743-422X-8-559
3. Scheckel, C, and Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat Rev Genet. (2018) 19:405–18. doi: 10.1038/s41576-018-0011-4
4. Jackson, GS, and Clarke, AR. Mammalian prion proteins. Curr Opin Struct Biol. (2000) 10:69–74. doi: 10.1016/s0959-440x(99)00051-2
5. Sigurdson, C. A prion disease of cervids: chronic wasting disease. Vet Res. (2008) 39:41. doi: 10.1051/vetres:2008018
6. Ironside, JW, Ritchie, DL, and Head, MW. Prion diseases. Handb Clin Neurol. (2018) 145:393–403. doi: 10.1016/B978-0-12-802395-2.00028-6
7. Cobb, NJ, and Surewicz, WK. Prion diseases and their biochemical mechanisms. Biochemistry. (2009) 48:2574–85. doi: 10.1021/bi900108v
8. Mead, S, Lloyd, S, and Collinge, J. Genetic factors in mammalian prion diseases. Annu Rev Genet. (2019) 53:117–47. doi: 10.1146/annurev-genet-120213-092352
9. Takada, LT, Kim, MO, Cleveland, RW, Wong, K, Forner, SA, Gala, II, et al. Genetic prion disease: experience of a rapidly progressive dementia center in the United States and a review of the literature. Am J Med Genet B Neuropsychiatr Genet. (2017) 174:36–69. doi: 10.1002/ajmg.b.32505
10. Kim, YC, and Jeong, BH. The first Meta-analysis of the M129V single-nucleotide polymorphism (SNP) of the prion protein gene (PRNP) with sporadic Creutzfeldt–Jakob disease. Cells. (2021) 10:3132. doi: 10.3390/cells10113132
11. Asante, EA, Smidak, M, Grimshaw, A, Houghton, R, Tomlinson, A, Jeelani, A, et al. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature. (2015) 522:478–81. doi: 10.1038/nature14510
12. Greenlee, JJ. Update on classical and atypical scrapie in sheep and goats. Vet Pathol. (2019) 56:6–16. doi: 10.1177/0300985818794247
13. Torricelli, M, Sebastiani, C, Ciullo, M, Ceccobelli, S, Chiappini, B, Vaccari, G, et al. PRNP polymorphisms in eight local goat populations/breeds from central and southern Italy. Animals. (2021) 11:333. doi: 10.3390/ani11020333
14. Kim, SK, Kim, YC, Won, SY, and Jeong, BH. Potential scrapie-associated polymorphisms of the prion protein gene (PRNP) in Korean native black goats. Sci Rep. (2019) 9:15293. doi: 10.1038/s41598-019-51621-y
15. Arifin, MI, Hannaoui, S, Chang, SC, Thapa, S, Schatzl, HM, and Gilch, S. Cervid prion protein polymorphisms: role in chronic wasting disease pathogenesis. Int J Mol Sci. (2021) 22:2271. doi: 10.3390/ijms22052271
16. Roh, IS, Kim, YC, Won, SY, Park, KJ, Park, HC, Hwang, JY, et al. Association study of the M132L single nucleotide polymorphism with susceptibility to chronic wasting disease in korean elk: a meta-analysis. Front Vet Sci. (2022) 8:804325. doi: 10.3389/fvets.2021.804325
17. Roh, IS, Kim, YC, Won, SY, Jeong, MJ, Park, KJ, Park, HC, et al. First report of a strong association between genetic polymorphisms of the prion protein gene (PRNP) and susceptibility to chronic wasting disease in sika deer (Cervus nippon). Transbound Emerg Dis. (2022) 69:e2073–83. doi: 10.1111/tbed.14543
18. Johnson, C, Johnson, J, Clayton, M, McKenzie, D, and Aiken, J. Prion protein gene heterogeneity in free-ranging white-tailed deer within the chronic wasting disease affected region of Wisconsin. J Wildl Dis. (2003) 39:576–81. doi: 10.7589/0090-3558-39.3.576
19. O'Rourke, KI, Spraker, TR, Hamburg, LK, Besser, TE, Brayton, KA, and Knowles, DP. Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J Gen Virol. (2004) 85:1339–46. doi: 10.1099/vir.0.79785-0
20. Johnson, C, Johnson, J, Vanderloo, JP, Keane, D, Aiken, JM, and McKenzie, D. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J Gen Virol. (2006) 87:2109–14. doi: 10.1099/vir.0.81615-0
21. Keane, DP, Barr, DJ, Bochsler, PN, Hall, SM, Gidlewski, T, O'Rourke, KI, et al. Chronic wasting disease in a Wisconsin white-tailed deer farm. J Vet Diagn Invest. (2008) 20:698–703. doi: 10.1177/104063870802000534
22. Kelly, AC, Mateus-Pinilla, NE, Diffendorfer, J, Jewell, E, Ruiz, MO, Killefer, J, et al. Prion sequence polymorphisms and chronic wasting disease resistance in Illinois white-tailed deer (Odocoileus virginianus). Prion. (2008) 2:28–36. doi: 10.4161/pri.2.1.6321
23. Wilson, GA, Nakada, SM, Bollinger, TK, Pybus, MJ, Merrill, EH, and Coltman, DW. Polymorphisms at the PRNP gene influence susceptibility to chronic wasting disease in two species of deer (Odocoileus Spp.) in western Canada. J Toxicol Environ Health A. (2009) 72:1025–1029. doi: 10.1080/15287390903084264
24. Blanchong, JA, Heisey, DM, Scribner, KT, Libants, SV, Johnson, C, Aiken, JM, et al. Genetic susceptibility to chronic wasting disease in free-ranging white-tailed deer: complement component C1q and Prnp polymorphisms. Infect Genet Evol. (2009) 9:1329–35. doi: 10.1016/j.meegid.2009.08.010
25. Brandt, AL, Kelly, AC, Green, ML, Shelton, P, Novakofski, J, and Mateus-Pinilla, NE. Prion protein gene sequence and chronic wasting disease susceptibility in white-tailed deer (Odocoileus virginianus). Prion. (2015) 9:449–62. doi: 10.1080/19336896.2015.1115179
26. Zink, RM, Najar, N, Vázquez-Miranda, H, Buchanan, BL, Loy, D, and Brodersen, BW. Geographic variation in the PRNP gene and its promoter, and their relationship to chronic wasting disease in North American deer. Prion. (2020) 14:185–92. doi: 10.1080/19336896.2020.1796250
27. Ott-Conn, CN, Blanchong, JA, and Larson, WA. Prion protein polymorphisms in Michigan white-tailed deer (Odocoileus virginianus). Prion. (2021) 15:183–90. doi: 10.1080/19336896.2021.1990628
28. Kuznetsova, A, McKenzie, D, Banser, P, Siddique, T, and Aiken, JM. Potential role of soil properties in the spread of CWD in western Canada. Prion. (2014) 8:92–9. doi: 10.4161/pri.28467
29. Jennelle, CS, Samuel, MD, Nolden, CA, Keane, DP, Barr, DJ, Johnson, C, et al. Surveillance for transmissible spongiform encephalopathy in scavengers of white-tailed deer carcasses in the chronic wasting disease area of Wisconsin. J Toxicol Environ Health A. (2009) 72:1018–24. doi: 10.1080/15287390903084249
30. Schultze, ML, Horn-Delzer, A, Glaser, L, Hamberg, A, Zellner, D, Wolf, TM, et al. Herd-level risk factors associated with chronic wasting disease-positive herd status in Minnesota, Pennsylvania, and Wisconsin cervid herds. Prev Vet Med. (2023) 218:106000. doi: 10.1016/j.prevetmed.2023.106000
31. Dudas, S, and Czub, S. Atypical BSE: current knowledge and knowledge gaps. Food Saf. (2017) 5:10–3. doi: 10.14252/foodsafetyfscj.2016028
32. Chen, C, and Dong, X-P. Epidemiological characteristics of human prion diseases. Infect Dis Poverty. (2016) 5:47. doi: 10.1186/s40249-016-0143-8
33. Seabury, CM, Lockwood, MA, and Nichols, TA. Genotype by environment interactions for chronic wasting disease in farmed US white-tailed deer. G3. (2022) 12:jkac109. doi: 10.1093/g3journal/jkac109
34. Appleby, BS, Shetty, S, and Elkasaby, M. Genetic aspects of human prion diseases. Front Neurol. (2022) 13:1003056. doi: 10.3389/fneur.2022.1003056
35. Kobayashi, A, Teruya, K, Matsuura, Y, Shirai, T, Nakamura, Y, Yamada, M, et al. The influence of PRNP polymorphisms on human prion disease susceptibility: an update. Acta Neuropathol. (2015) 130:159–70. doi: 10.1007/s00401-015-1447-7
36. Moore, SJ, Vrentas, CE, Hwang, S, West Greenlee, MH, Nicholson, EM, and Greenlee, JJ. Pathologic and biochemical characterization of PrPSc from elk with PRNP polymorphisms at codon 132 after experimental infection with the chronic wasting disease agent. BMC Vet Res. (2018) 14:80. doi: 10.1186/s12917-018-1400-9
Keywords: prion, PRNP, PrPSc, scrapie, BSE, CWD, CJD, white-tailed deer
Citation: Won S-Y and Kim Y-C (2024) The first meta-analysis of the G96S single nucleotide polymorphism (SNP) of the prion protein gene (PRNP) with chronic wasting disease in white-tailed deer. Front. Vet. Sci. 11:1437189. doi: 10.3389/fvets.2024.1437189
Edited by:
Jeff Wilson, Novometrix Research Inc., CanadaReviewed by:
João Queirós, Centro de Investigacao em Biodiversidade e Recursos Geneticos (CIBIO-InBIO), PortugalDeepanker Tewari, Pennsylvania Veterinary Laboratory, United States
Yalçın Yaman, Siirt University, Türkiye
Copyright © 2024 Won and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Chan Kim, a3ljaEBhbnUuYWMua3J6
 Sae-Young Won
Sae-Young Won Yong-Chan Kim
Yong-Chan Kim