- 1Boehringer Ingelheim, Duluth, GA, United States
- 2Boehringer Ingelheim, Racine, WI, United States
- 3Boehringer Ingelheim, Huntersville, NC, United States
- 4Boehringer Ingelheim, Grafton, MA, United States
- 5Boehringer Ingelheim, Los Angeles, CA, United States
- 6Boehringer Ingelheim, League City, TX, United States
Leptospirosis vaccine for dogs in the United States is considered a lifestyle or non-core vaccine, making individual veterinary practitioners responsible for determining if vaccination is necessary for their patients. Veterinary professionals often base their vaccination decisions on local rates of clinical cases. However, even subclinical leptospirosis infections have zoonotic potential. The microscopic agglutination test (MAT) is effective for screening unvaccinated animals, but previous vaccination can lead to inconsistent results and variable MAT titers over time. This prospective research survey evaluated if local experience was sufficient to justify selective vaccination for leptospirosis. MAT analyses were performed on sera collected from well-cared-for, unvaccinated dogs residing in five different geographies across the United States: South-Central (East Texas), New England, the Mid-Atlantic (North Carolina and Virginia), Midwest (Wisconsin/northern Illinois), and Southwest (southern California). Thirty-eight clinics participated, submitting a total of 1345 qualified samples from unvaccinated dogs over 1 year of age. 11.6% of these unvaccinated dogs had MAT titers for one or more serogroups of Leptospira. While seropositivity does not necessarily indicate that disease will result or that a specific serovar is involved, these MAT-positive cases do indicate that the potential for exposure exists and clinical signs or a carrier-state could result from infection. These survey results would indicate that a more aggressive vaccination protocol for leptospirosis should be considered.
Introduction
Leptospirosis is a worldwide bacterial spirochetal disease that affects humans (Weil’s Disease, Rat Catcher’s Fever, etc.), animals and is regarded as re-emerging in dogs in North America (1). Infection is spread to dogs either directly or indirectly via contact with the urine of wildlife, farm animals, or other dogs. Leptospirosis can be subclinical or cause a wide range of clinical signs including fever, myalgia, shivering, weakness, lack of appetite, increased thirst and urination, anuria/oliguria, dehydration, vomiting, cough, difficulty breathing, arrhythmias, and/or lymphadenopathy. In some cases, leptospirosis can be associated with multi-organ failure and death (2).
The microscopic agglutination test (MAT) is considered an economically viable reference test for serologic screening, diagnosis of leptospirosis. Sera are screened at 1:100 dilution and, typically, those showing agglutination are serially diluted to determine a titer endpoint. The highest MAT titers were historically considered indicative of the infective serovar, but this can be erroneous because of cross-reactions between serogroups (3). To further complicate the issue, MAT results can be negative in acute infections and do not differentiate vaccinated from infected dogs, making interpretation of results challenging (2).
Previous studies have utilized laboratory animals that are specific pathogen free (4–7) to minimize exposure complications observed with MAT titers. MAT titers were examined in a controlled prospective study using client owned dogs following vaccination (on day 0) and booster (at week 3, ±3 days) with variable MAT titers collected over the span of a year (8), which would be expected to complicate clinical diagnosis of leptospirosis using MAT titers.
The purpose of the current study was to determine Leptospira exposure rates in apparently healthy dogs 1 year of age and older, with no history of Leptospira vaccination, nor travel history outside their respective geographical regions: South-Central, New England, the Mid-Atlantic, the Midwest, and Southwest. To alleviate complications commonly associated with MAT testing; this prospective research survey assessed the level of exposure to Leptospira in well-cared-for, unvaccinated dogs, considered by practitioners to not be at risk of exposure.
Materials and methods
Forty veterinary clinics agreed to participate in this survey and enroll qualifying patients from dogs brought into the clinic for a wellness examination, targeting 50 dogs for participation per clinic. Eligible dogs were at least 1 year of age, had no current health concerns, had never been vaccinated against leptospirosis, and had not traveled outside of the area of residence.
Prior to inclusion, each pet owner signed a Pet Owner Consent Form (Appendix 1) which gave permission for the clinic staff to draw blood from the dog and send it to the laboratory for testing and acknowledging that the pet owner would not expect to receive results from the survey.
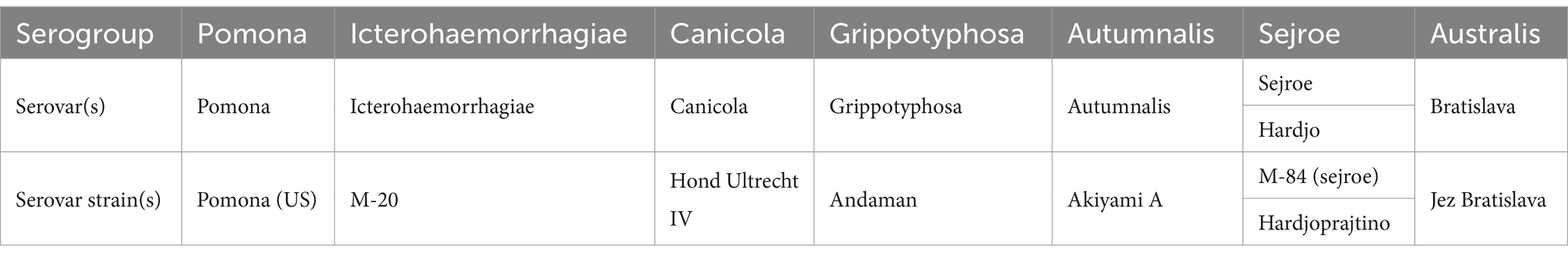
In addition, each clinic filled out the Patient Information Form, providing information including owner name, dog name, breed, age, weight, sex, and sample date on all the enrolled dogs. A sufficient volume of whole blood to secure a 2–3 mL serum sample was collected from each of the qualifying dogs. The serum vials were frozen and retained as they were received and, once all sampling was completed, all samples were submitted to the veterinary diagnostic laboratory at Texas A&M University for serologic testing using the microscopic agglutination test. A total of eight Leptospira serovars belonging to the P1 sub-clade of the pathogenic clade were reacted with sera at a 1:100 dilution; no further titrations were performed, as ascertaining potential exposure was the only intent. The seven serogroups used for testing were Pomona, Icterohaemorrhagiae, Canicola, Grippotyphosa, Bratislava, Sejroe (sejroe, hardjo), and Autumnalis, with the respective MAT diagnostic serovar strains used by the laboratory noted in Table 1.
Statistical methods
Data were analyzed using Fisher’s Exact Test on (nx2) frequency tables. When the overall sample, or one of the regional subsamples, indicated significant (p < 0.05) differences between levels of the classification variable, all pairwise combinations of levels were compared using Fisher’s Exact Test.
Results
A total of 1,363 samples were collected from 38 clinics. Eighteen of those samples were excluded for one of three reasons: poor specimen quality, lack of adequate owner consent, or dog age < 1 year. Of the 1,345 qualifying samples, 156 (11.6%) dogs were seropositive for one or more Leptospira serovars.
The Wisconsin/N. Illinois-area (Midwest US) had 55 (14.9%) of 370 dogs testing positive. The New England geography (Northeast US) had 38 (14.6%) of 261 dogs testing positive. The Mid-Atlantic US had 17 (10.8%) of 158 dogs testing positive. The east Texas geography (South-Central US) had 35 (9.1%) of 384 dogs testing positive. The southern California (Southwest US) had 11 (6.4%) of 172 dogs testing seropositive (Table 2).
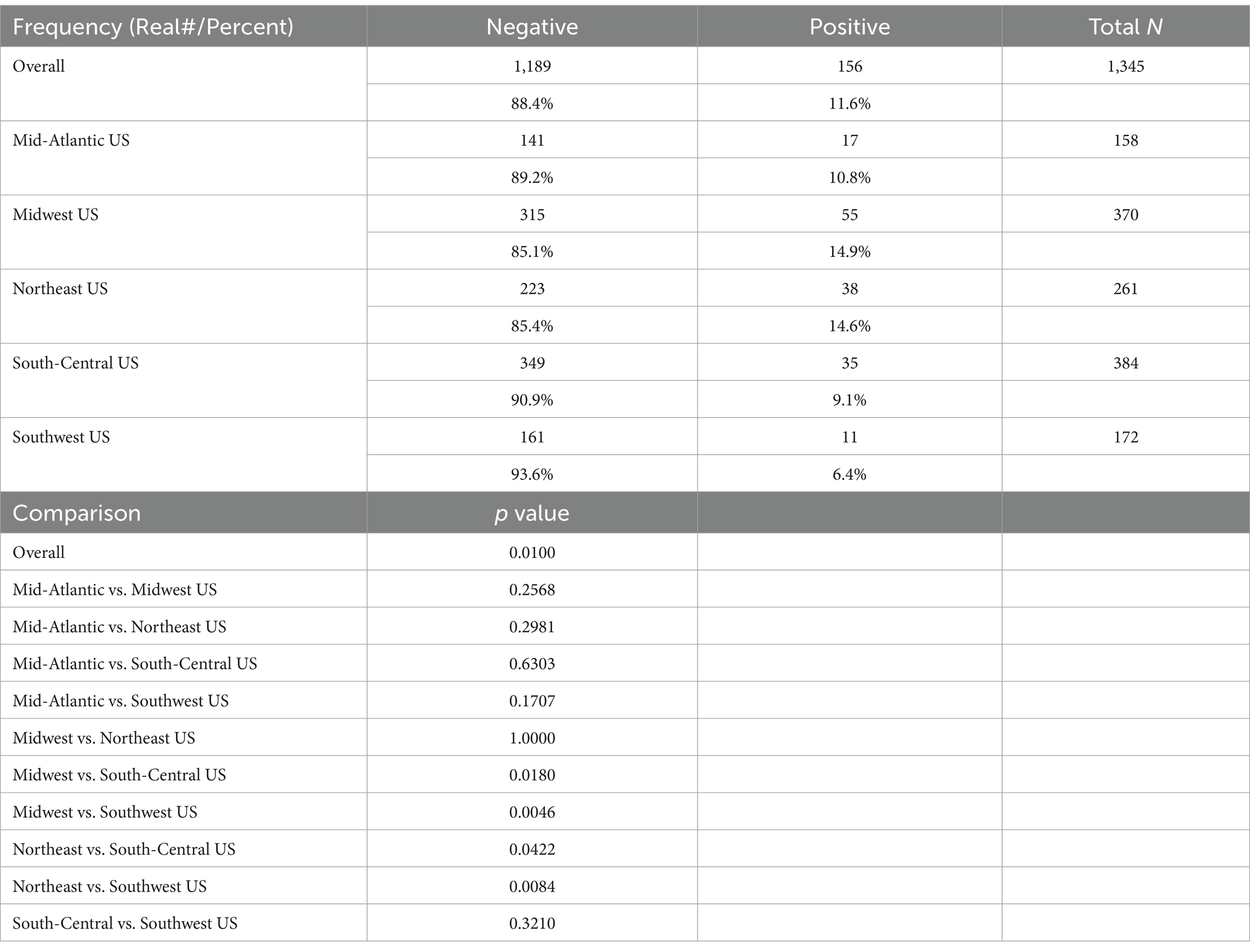
Table 2. Overall results: MAT results, %, total and stat comparisons (1,345 samples from 38 clinics in five geographic regions).
In the overall sample of 1,345 dogs, there were significant differences seen among regions. Pairwise comparisons among the regions showed that the South-Central US had a significantly lower rate of positive samples than the Midwest US (p = 0.0180) and the Northeast US (p = 0.0422); and the Southwest US had a significantly lower rate of positive samples than the Midwest US (p = 0.0046) and the Northeast US (p = 0.0084) (Table 2).
There was no significant (p > 0.10) difference in the rate of positive samples between males and females, either overall or within any of the regions (Table 3).
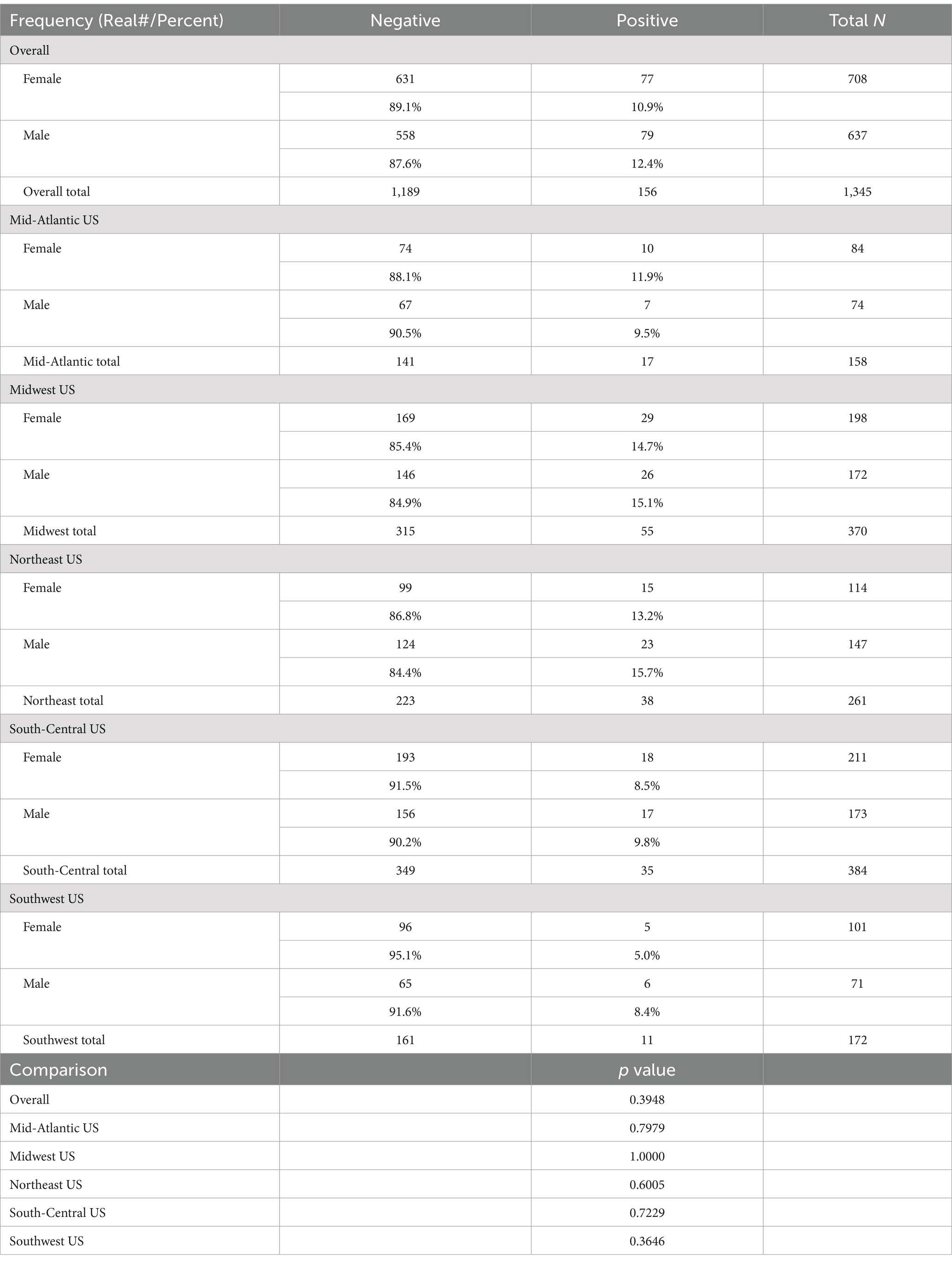
Table 3. Overall results by sex, and results by region, w/stats (females, intact or not vs. males, intact or not).
There was no significant (p > 0.10) difference in the rate of positive samples among young (1–5 years), middle (>5 to 10 years), or older (>10 years) age groups, either overall or within any of the regions (Table 4).
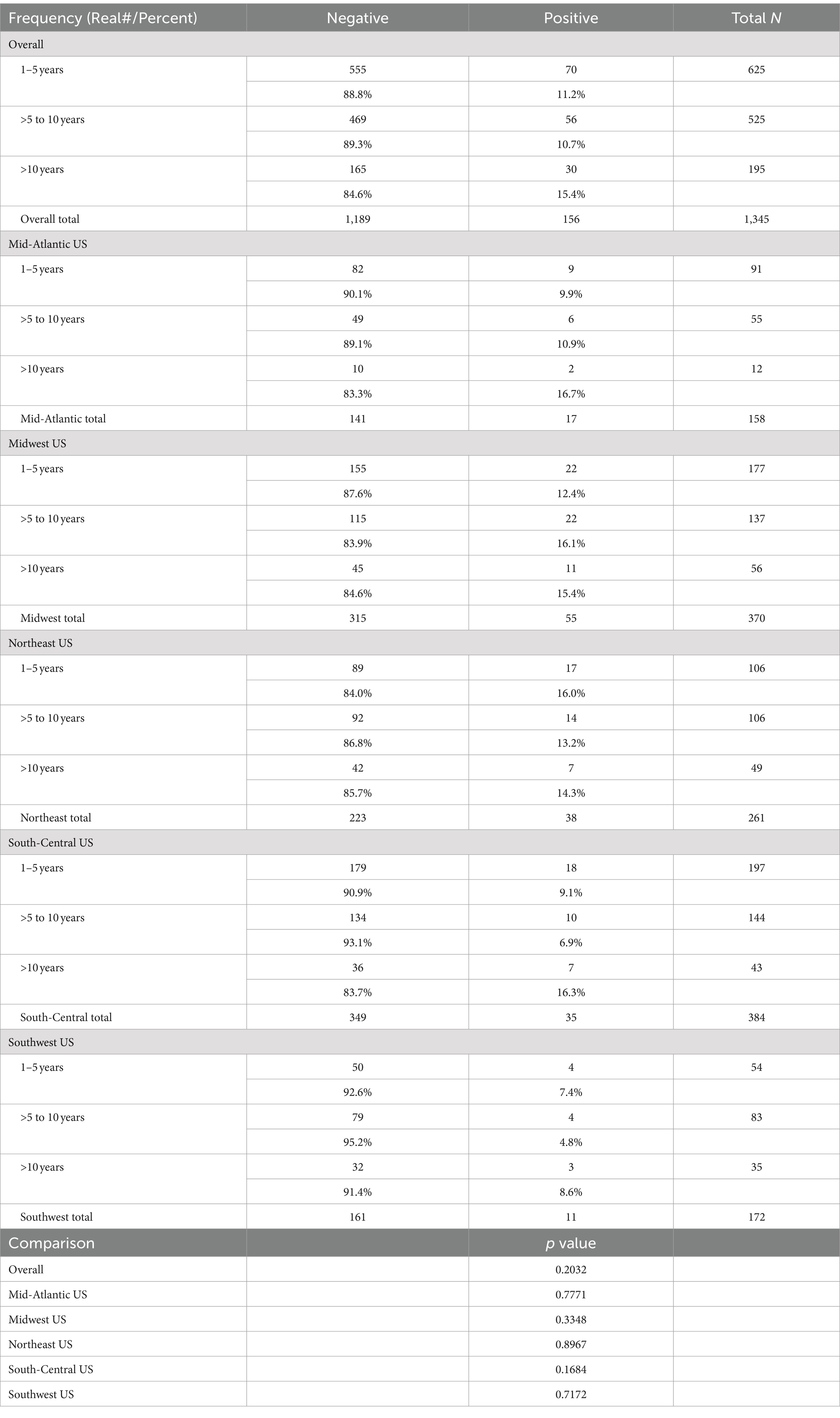
Table 4. Overall results by age group, and results by region (1–5 years, >5 to 10 years, and >10 years).
The average weight of the seropositive dogs was 50.5 pounds (range, 7–148 pounds). There were 51 seropositive dogs under 35 pounds, 45 dogs between 35.1 and 60 pounds, and 60 dogs from 60 to 108.6 pounds (Table 5).
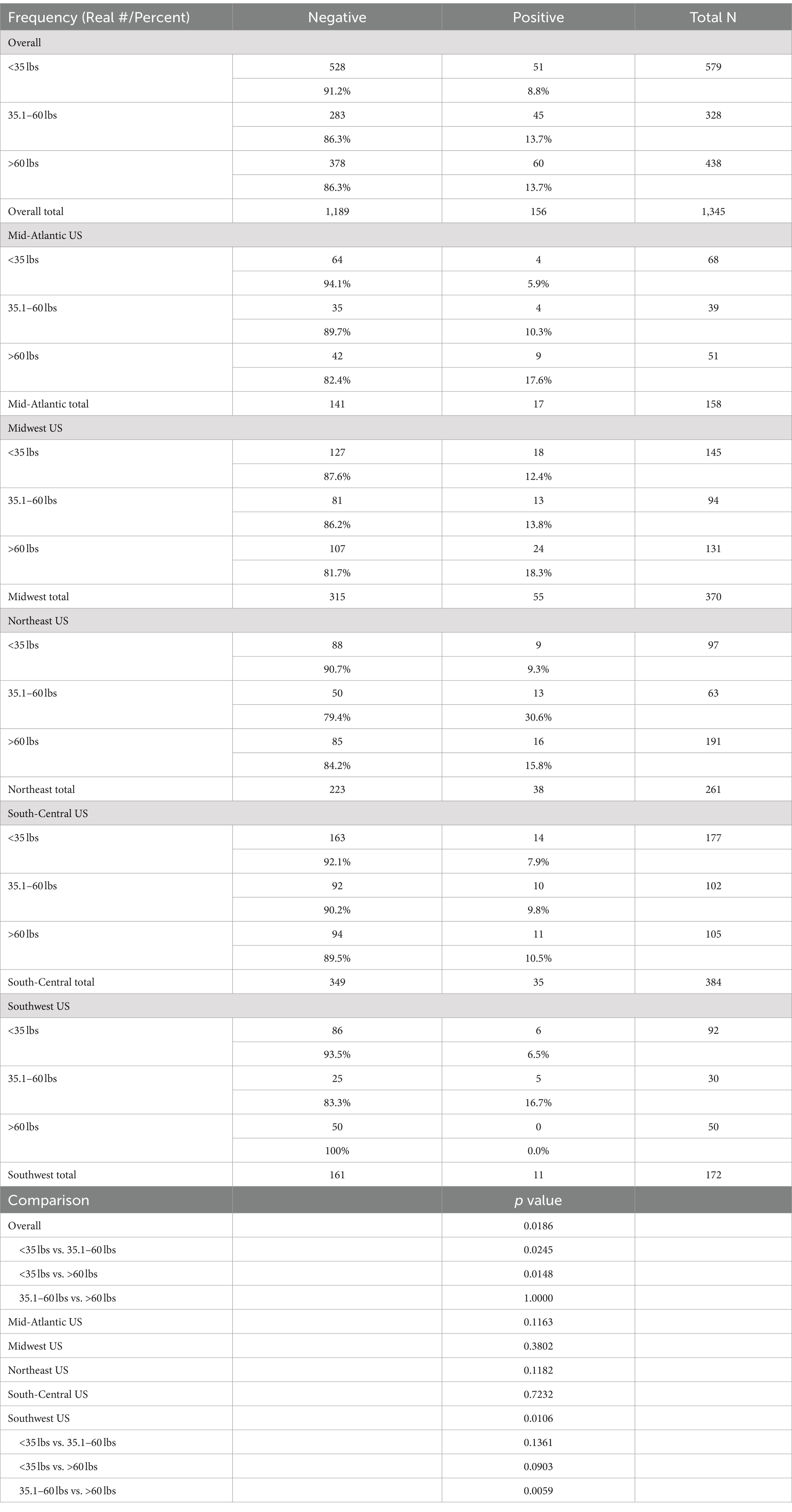
Table 5. Overall results by weight group, and results by region (<35 lbs., 35.1–60 lbs., and >60 lbs.).
In the overall sample, there were significant differences seen among weight groups. Pairwise comparisons of weight groups showed that the lightest dogs (<35 lbs.) had a significantly lower rate of positive samples than either the medium weight (35.1–60 lbs.; p = 0.0245) or the heaviest (>60 lbs.; p = 0.0148) dogs. There were no significant differences among weight groups for the Mid-Atlantic, Midwest, Northeast, and South-Central. For the Southwest, large dogs (>60 lbs.) had a significantly (p = 0.0059) lower rate of positive samples than the medium weight group (35.1–60 lbs.) (Table 5).
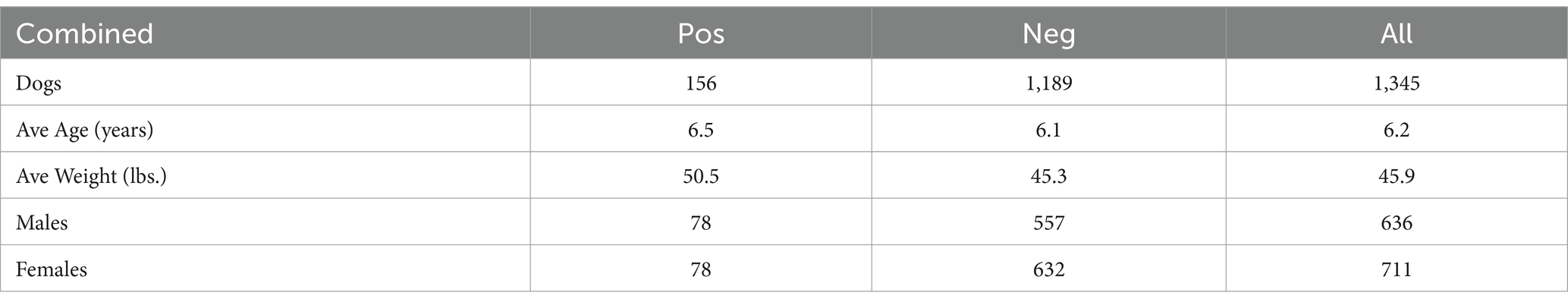
The demographic summary is in Table 6.
Of the 156 seropositive dogs, 92 were MAT positive for one serogroup, 28 were positive for two serogroups, 15 were positive for three serogroups, 15 were positive for four serogroups and six dogs were MAT positive for five serogroups (283 total serovars identified). MAT positive samples were observed for all seven serogroups. 70 dogs positive for Australis serogroup (represented by serovar bratislava), 58 for Grippotyphosa (serovar Grippotyphosa), 56 for Autumnalis (serovar autumnalis), 43 for Pomona (serovar pomona), 40 for Icterohaemorrhagiae (serovar Icterohaemorrhagiae), 14 for Canicola (serovar Canicola), and two for Sejroe (two serovar sejroe, and none for serovar hardjo). Individual sample information for Leptospira-positive samples including locations, sample numbers, dog age, weight, sex, and serovars identified are included in Appendix 2.
Discussion
These data provide an interesting insight into exposure of dogs to Leptospira in five separate geographic areas across the United States. In this survey, the dogs were all over 1 year of age and the gender and age incidence was essentially equal. The average age of the dogs included in the study was 6.18 years (range, 1–18 years). The average age of seropositive dogs was 6.54 years of age (range, 1–15 years). Of the 156 positive dogs, 78 females and 78 male dogs seroreacted to one or more of the serovars tested. Weight ranged from 7 lbs. (3.2 kg) to 148 lbs. (67.3 kg). Overall, seropositive dogs were slightly heavier than the average seronegative dog, with a 5.24 lbs. (2.4 kg) weight difference observed in this study.
These data indicated an overall 11.6% rate of exposure of qualifying dogs (healthy dogs with no history of leptospirosis vaccination, over 1 year of age and no history of traveling out of their home area) enrolled in this study from five different regions spanning the United States. The intriguing factor is that veterinarians and/or pet owners chose to not vaccinate the dogs included in this study against leptospirosis. Possible reasons for not vaccinating could be that they felt it was not necessary to vaccinate for leptospirosis, likely expecting little to no opportunity for the dogs to become infected based on their geography or the dog’s lifestyles, or that vaccination posed an unnecessary risk to the dog.
In the South-Central United States, 9.1% of 384 dogs tested positive, in New England 14.6% of 261 dogs tested positive, in the Mid-Atlantic 10.8% of 158 dogs tested positive, in the Midwest 14.9% of 370 dogs tested positive, and in the Southwest 6.4% of 172 dogs tested positive for exposure. Since they were unvaccinated, many would be at risk of developing clinical signs due to pathology consistent with leptospirosis.
In the 156 antibody seropositive dogs, there were 283 hits in all seven of the selected serogroups screened by the diagnostic laboratory. It is important to note that in the United States, available four-way leptospirosis vaccines include Canicola, Grippotyphosa, Icterohaemorrhagiae, and Pomona. Current vaccines can prevent disease resulting from experimental challenge and can decrease shedding of vaccinal serovars (2). These same four vaccine serogroups accounted for 155 of the antibody positive serogroup hits, with three of the four non-canine vaccine serogroups (Australis, Autumnalis, and Sejroe) accounting for 128 antibody positives hits. None of the dogs tested were positive for hardjo antibodies. Leptospira hardjo is well-known as a sexually transmitted infection of cattle, often causing abortions, especially in heifers. The L. Hardjo MAT was performed simply because it was part of the standard screening panel provided by the diagnostic laboratory, and the authors chose not to add any complications to the normal laboratory procedures.
One must consider that cross-reactivity between serovars within the same serogroup and to serovars in other serogroups has been reported (9, 10). However, for the purposes of this survey, the primary goal was not to identify specific serovars, but to examine the potential Lepto-exposure rate of dogs previously not considered to be at risk.
Seventy dogs harbored antibodies to Australis serogroup. Bratislava is known to cause disease in swine, but there is only limited evidence that bratislava may be a pathogen of dogs in the United States (11). Fifty-six of the antibody hits were for Autumnalis, and two were for Sejroe, both of which have been identified as causing clinical signs of leptospirosis in dogs in the United States, with Sejroe also causing issues in ruminants, and Autumnalis affecting humans and raccoons in the southern US (12). The observations in this serologic survey illustrate dogs are becoming infected, potentially becoming carriers and/or suffering clinical signs, and being involved in the spread of multiple serogroups of Leptospira infection. In this survey, it was demonstrated that dogs that were considered no-or low-risk, were seropositive at a rate approximately 10 times higher than nationwide seropositivity for heartworm disease in the United States (13).
These data support the conclusion that perceptions of Leptospira exposure risk are likely underestimating the actual risk for dogs across the United States. Further these data support the most recent ACVIM Consensus Statement, where it was stated that “All dogs are at risk of leptospirosis, regardless of signalment, geographic location, lifestyle and the time of year” (2). Thus, an annual vaccination protocol including leptospirosis should be considered for all dogs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by Boehringer Ingelheim United States studies for the studies involving animals because samples collected, with pet owner permission, as a service and part of normal veterinary wellness assessments. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
DC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EL: Investigation, Writing – review & editing. TW: Investigation, Writing – review & editing. JS: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. DP: Conceptualization, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Boehringer Ingelheim, Duluth, GA, USA. Boehringer Ingelheim was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors would like to thank all of those involved with this study, the veterinary clinics and staffs, and the pet owners. We would also like to thank Sheila Gross for her statistical analyses of these data.
Conflict of interest
DC, EL, TW, JS, AS, and DP were employees of Boehringer Ingelheim at the time of this study.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1435630/full#supplementary-material
References
1. Smith, AM, Arruda, AG, Evason, MD, Weese, JS, Wittum, TE, Szlosek, D, et al. A cross-sectional study of environmental, dog, and human-related risk factors for positive canine leptospirosis PCR test results in the United States, 2009 to 2016. BMC Vet Res. (2019) 15:412. doi: 10.1186/s12917-019-2148-6
2. Sykes, JE, Francey, T, Schuller, S, Stoddard, RA, Cowgill, LD, and Moore, GE. Updated ACVIM consensus statement on leptospirosis in dogs. J Vet Intern Med. (2023) 37:1966–82. doi: 10.1111/jvim.16903
3. Lee, HS, Guptill, L, Johnson, AJ, and Moore, GE. Signalment changes in canine leptospirosis between 1970 and 2009. J Vet Intern Med. (2014) 28:294–9. doi: 10.1111/jvim.12273
4. Minke, JM, Bey, R, Tronel, JP, Latour, S, Colombet, G, Yvorel, J, et al. Onset and duration of protective immunity against clinical disease and renal carriage in dogs provided by a bi-valent inactivated leptospirosis vaccine. Vet Microbiol. (2009) 137:137–45. doi: 10.1016/j.vetmic.2008.12.021
5. Schreiber, P, Martin, V, Grousson, D, Sanquer, A, Gueguen, S, and Lebreux, B. One-year duration of immunity in dogs for Leptospira interrogans serovar icterohaemorrhagiae after vaccination. Intern J Appl Res Vet Med. (2012) 10:305–10.
6. Bouvet, J, Cariou, C, Valfort, W, Villard, S, Hilaire, F, Oberli, F, et al. Efficacy of a multivalent DAPPi-L multi canine vaccine against mortality, clinical signs, infection, bacterial excretion, renal carriage and renal lesions caused by Leptospira experimental challenges. Vaccine Rep. (2016) 6:23–8. doi: 10.1016/j.vacrep.2016.07.003
7. Cariou, C, Herbet, G, Ripart, P, Martin-Cagnon, N, Bouvet, J, Schneider, M, et al. Development of antibody ELISA specific of Leptospira interrogans serovar grippotyphosa, canicola, and icterohaemorrhagiae to monitor vaccine immunogenicity. Vet Immunol Immunopathol. (2020) 219:109960. doi: 10.1016/j.vetimm.2019.109960
8. Martin, LER, Wiggans, KT, Jablonski Wennogle, SA, Curtis, K, Chandrashekar, R, and Lappin, MR. Vaccine-associated Leptospira antibodies in client-owned dogs. J Vet Intern Med. (2014) 28:789–92. doi: 10.1111/jvim.12337
9. Levett, PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. (2003) 36:447–52. doi: 10.1086/346208
10. Lizer, J, Velineni, S, Weber, A, Krecic, M, and Meeus, P. Evaluation of 3 serological tests for early detection of Leptospira-specific antibodies in experimentally infected dogs. J Vet Intern Med. (2018) 32:201–7. doi: 10.1111/jvim.14865
11. Hill, R.E. (2005). USDA Center for veterinary biologics notice no. 05-06: requirements for addition of Leptospira Bratislava in canine bacterins. Available at: https://www.aphis.usda.gov/sites/default/files/notice_05_06.pdf
12. Moore, GE, Guptill, LF, Glickman, NW, Caldanaro, RJ, AuCoin, D, and Glickman, LT. Canine leptospirosis, United States, 2002–2004. Emerg Infect Dis. (2006) 12:501–3. doi: 10.3201/eid1203.050809
13. Capcvet (2024). Companion Animal Parasite Council | Parasite Prevalence Maps. Available at: capcvet.org (Accessed May 25, 2024).
Keywords: leptospirosis, serovars, MAT titers, unvaccinated, exposure
Citation: Carithers D, Loebach E, Williams T, Sponseller J, Schreibman A and Platts D (2024) Field assessment of potential exposure of dogs to leptospirosis by measuring antibody titers in dogs: a multisite study in five geographic regions of the United States. Front. Vet. Sci. 11:1435630. doi: 10.3389/fvets.2024.1435630
Edited by:
Alda Natale, Experimental Zooprophylactic Institute of the Venezie (IZSVe), ItalyReviewed by:
Giovanni Sgroi, Experimental Zooprophylactic Institute of Southern Italy (IZSM), ItalySara Savic, Scientific Veterinary Institute Novi Sad, Serbia
Mario D’Incau, Experimental Zooprophylactic Institute of Lombardy and Emilia Romagna (IZSLER), Italy
Copyright © 2024 Carithers, Loebach, Williams, Sponseller, Schreibman and Platts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doug Carithers, ZG91Zy5jYXJpdGhlcnNAYm9laHJpbmdlci1pbmdlbGhlaW0uY29t
 Doug Carithers
Doug Carithers Ed Loebach2
Ed Loebach2 Troy Williams
Troy Williams