- 1Department of Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, VA, United States
- 2School of Animal Sciences, Virginia Tech, Blacksburg, VA, United States
- 3Department of Large Animal Clinical Sciences, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, VA, United States
- 4Department of Population Health Sciences, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, VA, United States
Introduction: Theileria orientalis Ikeda genotype is an emerging cattle disease in the US. Since 2017, when T. orientalis Ikeda was discovered in beef cattle in two counties in Virginia, cattle infections have risen to include ~67% of Virginia counties and 14 states. Consistent with New Zealand studies, many infected herds in Virginia were >90% positive upon initial testing without overt evidence of infection. Central bull tests present a unique opportunity to study the effects of T. orientalis Ikeda infections, as bulls from multiple source herds are consolidated. The objective of this study was to determine if infection with T. orientalis Ikeda affected the average daily gain (ADG), adjusted yearling weight (AYW) and breeding soundness of bulls at two test stations in Virginia over a period of years.
Materials and methods: The bulls were fed and housed similarly to compare their growth performance and breeding soundness. For T. orientalis Ikeda testing, DNA was extracted from whole blood for quantitative polymerase chain reaction.
Results: The number of bulls infected with T. orientalis Ikeda at initial delivery to the stations increased significantly over the years studied. Multivariable linear regression models, using Angus bulls from Virginia test stations, indicated no significant effect on ADG or AYW in bulls that became test positive during the test or were positive for the duration, compared to Angus bulls that were negative for the duration. At LOC A, the odds of passing a breeding soundness exam (BSE) were not significantly different for bulls that turned positive during the test or were positive for the duration, compared to bulls that were negative for the duration of the test. At LOC B, bulls that became positive during the test were 2.4 times more likely (95% CI: 1.165–4.995, p = 0.016) to pass their BSE compared to bulls that remained negative throughout the test.
Discussion: We do not suppose that an obscured infection of T. orientalis Ikeda is protective for bulls to pass a BSE. However, this study demonstrates an obscured infection of T. orientalis Ikeda does not negatively affect weight gain or achievement of a satisfactory BSE rating at the central bull test stations in Virginia.
1 Introduction
In 2017, Theileria orientalis genotype Ikeda was first detected in the United States (US) in beef cattle from multiple counties in Virginia (1). In Virginia between 2019 and 2021, surveillance testing of market cattle for T. orientalis Ikeda, increased from <2 to ~35% of samples testing positive in the Northern and Southwest markets with an overall prevalence of 8.7% in 2021 (2). This emerging cattle disease has since been discovered in 14 states as of November 2023 (3). T. orientalis is a tick-borne non-transforming hemoprotozoan (4) with 11 genotypes as determined by molecular characterizations of the major piroplasm surface protein (MPSP) (5). Of the 11 genotypes of T. orientalis, type 2 (Ikeda) is meaningfully associated with clinical disease in Australia, New Zealand, Japan (4) and the US (1).
The primary biologic vector of T. orientalis Ikeda in New Zealand, Japan (4) and the US is Haemaphysalis longicornis, the Longhorned tick (LT) (6, 7). As of March 2024, the LT has been found in 19 states (8). The biology of the LT includes parthenogenetic reproduction, aggressive biting, minimal host specificity, and swarming behavior which allows for rapid increases in both population numbers and infested territories (9, 10). This biology, and that the LT spends the majority of its lifecycle off the host in the environment, makes LT control measures problematic (11). The expanding distribution of the LT has followed predictions in the Eastern US (12, 13), while expanding farther west, north and south than expected. With the geographic distribution of T. orientalis Ikeda closely mirroring that of the LT, spread of T. orientalis Ikeda to non-endemic regions through expansion of the LT distribution must be anticipated.
Acute clinical infection from T. orientalis Ikeda can cause anemia, icterus, ill-thrift, abortions and death in naïve cattle particularly if they are stressed due to parturition, transportation or a significant change in husbandry (1, 4, 14). A sequela of T. orientalis Ikeda infection is the cattle become asymptomatic carriers, potentially for life (15). However, in other countries, positive beef herds without overt clinical signs have been described and associated with decreased live weight gain in weaned beef calves (16) and decreased mean daily gain in suckling beef calves (17). It was thought the persistently infected cattle could recrudesce into clinical disease particularly under times of stress, however it does not appear to frequently occur (18). However, theileriosis, even if apparently asymptomatic or in the persistently infected chronic phase, “decreases the fitness of the animal to tolerate other endemic diseases and deficiencies” (19). Additionally, there are no available treatments or vaccines for T. orientalis in the US, and only one effective treatment available elsewhere requiring extended meat withdrawal and cost (4). As the LT and cattle theileriosis spread in the US, creating endemicity, it will be crucial to understand the effects of T. orientalis Ikeda infections on beef production parameters such as growth performance, reproductive soundness and longevity within the herd.
The Virginia Beef Cattle Improvement Association has operated bull performance test stations for over 60 years, and currently operates two separate stations annually. Bull test stations provide bull producers an opportunity to compare their bull's growth performance to other bulls in a controlled environment, culminating in a value-added sale based upon the bull's performance. Bull test stations provide an excellent opportunity to study the effects of T. orientalis Ikeda infection on weight gain, as feed and environment are the same for all the bulls at each location. Another advantage of the test stations is that the entry requirements provide a generally homogenous population of bulls with respect to age and health status to study reproductive soundness.
The purpose of this retrospective study was to determine if weight gain or reproductive soundness of bulls at Virginia bull test stations was affected by the bulls' T. orientalis Ikeda status. Our hypothesis was that those bulls that were negative for T. orientalis Ikeda for the duration of the bull test would perform better with respect to gain and reproductive soundness compared to bulls that were positive for the whole duration or became positive during the bull test.
2 Materials and methods
2.1 Bull management and growth data
The bull test stations were operated at two locations in Virginia by the Virginia Beef Cattle Improvement Association. At LOC A (in Louisa County), fall born bulls (SR) were delivered to the test facility in Late June at 8–10 months of age. At LOC B (in Wythe County), both the fall born (SR) and spring-born (JR) bulls were delivered to the test facility in early October at 10–12 and 7–9 months of age respectively. At both stations, growth performance was evaluated over a 112-day period with qualifying bulls sold at a special sale ~6 weeks later. For this study, bulls were placed on the study at the time of delivery to their respective station until BSE's were completed after the growth performance test, which was for ~5 months. All bulls originated from cooperating farms in Virginia or bordering states.
At delivery, bulls were accompanied by a Certificate of Veterinary Inspection, tested negative to anaplasmosis and bovine viral diarrhea (BVD) persistent infection and met post-weaning management and vaccination requirements. Requirements included a 7-strain Clostridial, Pasteurella, and a modified live 5-way respiratory viral vaccination. At delivery, bulls were examined for reproductive soundness by measuring testicular circumference and unsound bulls were eliminated. Bulls received a 7-strain Clostridium booster, 5-way respiratory virus booster, and were treated for internal and external parasites with pour-on Cydectin® (Elanco Animal Health, Greenfield IN; 5 mg/ml moxidectin, dose 0.5 mg/kg topically) at delivery. Lastly, at delivery, all bulls were bled via the caudal tail vein into an EDTA anticoagulant tube for molecular diagnostics and testing for T. orientalis Ikeda as described below. Only single use needles were used for all injections. The blood sampling was reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Tech (protocol #21-248).
Each bull's attitude and appearance were examined daily, however unless they appeared ill or anorexic, they were only put the through the chute system periodically for weighing. At LOC A, bulls were housed in 12-acre grass lots with the woods on the edges mostly fenced out, in groups of ~50 according to breed, age, and weight. At LOC B, bulls were housed in 12–30-acre grass lots that contained some woods, but grouped similarly. At both locations, a corn-silage based total mixed ration was fed with a target average daily gain (ADG) of 1.6 kg/day, formulated using the National Research Council Nutrient Requirements of Beef Cattle (20). Following a 14–21-day diet acclimation period, weight gain was measured over a 112-day period. Average daily gain (ADG) and adjusted yearling weight (AYW) were calculated along with ratios for animals of the same breed and age (SR vs. JR) according to Guidelines for Uniform Beef Improvement Programs standards (21). Because the LOC B test occurred over winter, the bulls were also treated for external parasites in early January. At completion of the test every bull's scrotal circumference was measured, caudal tail vein blood was obtained for T. orientalis Ikeda testing, and SR bulls had a breeding soundness exam (BSE) performed. Approximately 10%−20% of SR bulls did not receive a BSE as they were not sale eligible based on poor test growth performance, structural unsoundness, illness or other issue.
Both bull tests consisted of a 2–3-week transition period, 112 days on feed performance testing, and then an ~6-week period until the sale of the bulls. At the end of the feed performance test, a complete breeding soundness exam (BSE) is performed on sale-eligible bulls. T. orientalis Ikeda was first detected in 2019 when the bulls arrived at the southwest Virginia bull test station, but testing was not conducted on the second location until 2021. An acute case of T. orientalis Ikeda with clinical signs severe enough to require pulling a bull from its group, for exam by bull station staff or a veterinarian, was not noted at either test station to date.
2.2 Breeding soundness exam
Components of the BSE for SR bulls included a brief physical exam, rectal palpation of the accessory sex glands, palpation of the testis, determination of scrotal circumference, and electroejaculation. Electroejaculation utilized the same program for each bull via the Pulsator IV (prior to 2022) and the Pulsator V (after 2022) (Lane Manufacturing, Denver CO). Semen motility was evaluated by wet mount chute side with 30% progressive motility required for a satisfactory rating. Morphology was determined using Eosin-stained slides examined under oil immersion at 100X with 70% normal sperm required for a satisfactory rating. If a bull was deferred at the initial BSE that occurred at the end of the growth performance test, a second BSE was performed ~3 weeks later for final determination of BSE result. For the purposes of this study, a bull was graded either “not-eligible,” satisfactory or unsatisfactory.
2.3 Molecular diagnostics
DNA was extracted from K2-EDTA anticoagulated blood (DNeasy blood and tissue kit; Qiagen) following the manufacturer's protocol using pre-warmed (56°C elution buffer. The initial blood volume for DNA extraction was 100 μl. We used a duplex TaqMan-based rtPCR assay to detect the major surface protein 1b (msp1b) gene of Anaplasma marginale and the major piroplasm surface protein (MPSP) gene of T. orientalis as described by our lab previously (22). The primers and probes for MPSP are specific for T. orientalis but are not genotype-specific. To further characterize genotype a second multiplex rtPCR assay targeting Ikeda, Chitose, and Buffeli genotypes as described by our lab previously (22).
Each year, the bulls were tested for T. orientalis Ikeda twice; at delivery and at the end of the feed performance test when BSEs were performed on eligible bulls. Based upon the T. orientalis Ikeda results from the start of the test to the end of the feed performance test, bulls were classified as either negative throughout the test (NN), positive throughout the test (PP), or negative on intake and positive at the end of the test (NP). There are two scenarios where a bull could be classified as a NP bull; they were infected shortly before delivery and did not have enough time to become test positive at delivery sampling or they became positive while at the bull test station.
2.4 Statistical analysis
Data were entered into spreadsheets (Excel; Microsoft Corp.) and subsequently analyzed using JMP® Pro (JMP®, Version 16. SAS Institute Inc., Cary, NC 1989–2013). Prevalence (95% CI) of bulls positive at intake to the test station were compared for each location by year and tested with a Fisher's exact test at LOC A or Cochran-Armitage test for trend at LOC B. There were three distinct populations of bulls from the two stations; LOC A all SR bulls (2021–2022), LOC B SR bulls (2020–2023) and LOC B JR bulls (2020–2023). Angus was the majority breed in all three populations of bulls with too few bulls of other breeds for analysis, so analyses for average daily gain (ADG) and adjusted yearling weight (AYW) were restricted to purebred Angus bull data due to well documented breed differences expected with gain (23–25). Means for ADG and AYW were determined with 95% confidence intervals (CI). A one-way ANOVA with Dunnets method for means comparison was used to compare the NP and PP bulls to the control group NN. In multivariable linear regression models for ADG and AYW, T. orientalis Ikeda status was forced into each model and other potential covariables included year of test, age and weight at delivery, group within test, and state of origin. The cut-off for potential covariates to be included in the final models was p < 0.05. Then using the Dunnets method, within the model, the T. orientalis Ikeda statuses of NP and PP were compared to the control group NN. To assess effects of infection on eligibility for a BSE, the NP and PP bulls were separately compared to the NN group to determine the ratio of success between the groups, with the 95% CIs and Fisher's exact test. In multivariable logistic regression models for BSE eligibility, T. orientalis Ikeda status was forced into each model and other potential covariables included year of test, ADG ratio, and AYW ratio. The ADG and AYW ratios were used in the BSE models because all breeds were represented in the BSE data, and the ratios normalized the breed differences in gain. To assess effects of infection on achieving a satisfactory rating in the BSE, the NP and PP bulls were separately compared to the NN group to determine the ratio of successes between groups, with the 95% CIs and Fisher's exact test. In multivariable logistic regression models for BSE success, T. orientalis Ikeda status was forced into each model. We used forward stepwise multivariable logistic regression analyses with Bayesian Information Criteria as the stopping rule and p < 0.05 as the cut-off for potential covariates: year, state of origin, pen number, breed, bull conception type, age at delivery, weight at delivery, ADG and AYW.
3 Results
3.1 Bull test station populations
A total of 584 senior and 416 junior bulls were examined in this study (Table 1). For all three populations the majority of the bulls were purebred Angus, though there was representation from many different breeds. At LOC A, ~92% of the bulls were from Virginia with <8% of bulls from West Virginia and North Carolina. At LOC B for both JR and SR groups, ~62% of the bulls were from Virginia. Approximately 23% of the LOC B bulls were from Tennessee, while <15% of the LOC B bulls were from West Virginia and North Carolina. For the two SR populations of bulls ~68% of the bulls passed a BSE with a satisfactory rating. JR bulls were too young for a BSE. The three populations of purebred Angus bulls used in the growth performance analyses are described in Table 2.
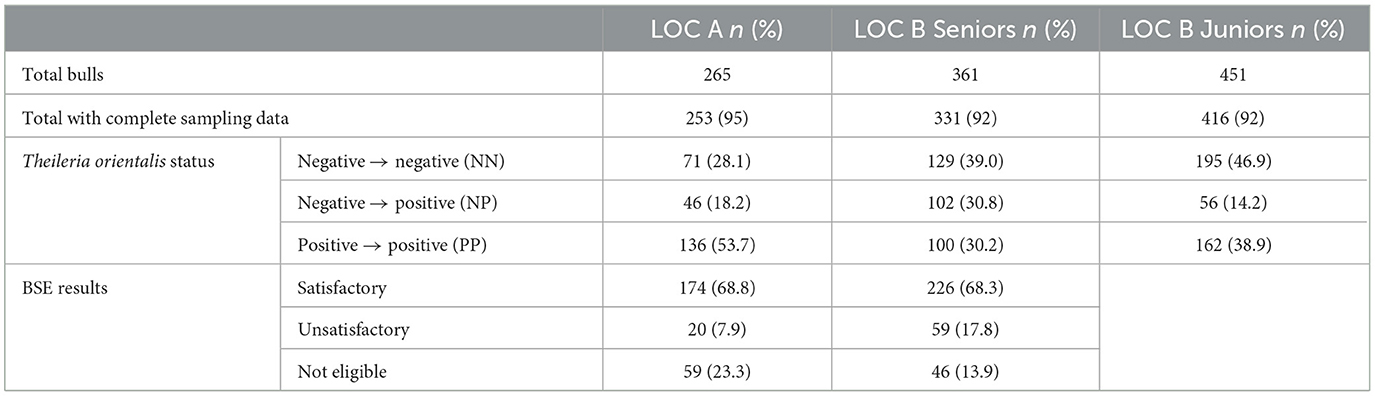
Table 1. Descriptive statistics of the three populations of bulls including all breeds; LOC A (years 2021–2022), LOC B seniors (years 2020–2023) and LOC B juniors (years 2020–2023).
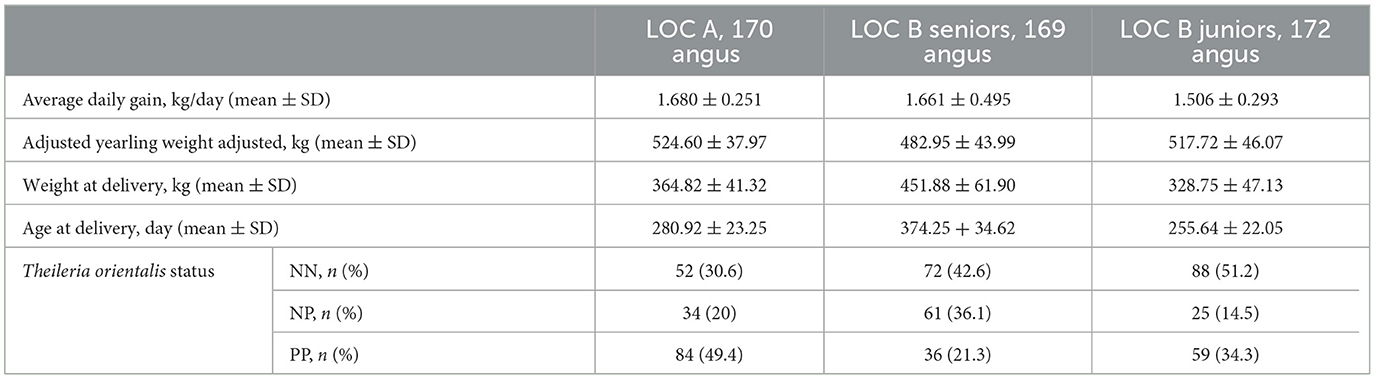
Table 2. Descriptive statistics of the Angus bulls within each subpopulation from the Virginia bull test stations, for the variables used in the multivariable models of average daily gain and adjusted yearling weight.
3.2 Theileria orientalis Ikeda testing
At both Virginia bull test stations, there has been a significant (p < 0.0001) increase in the percent of bulls that tested positive to T. orientalis Ikeda at delivery over the years tested (Figure 1).
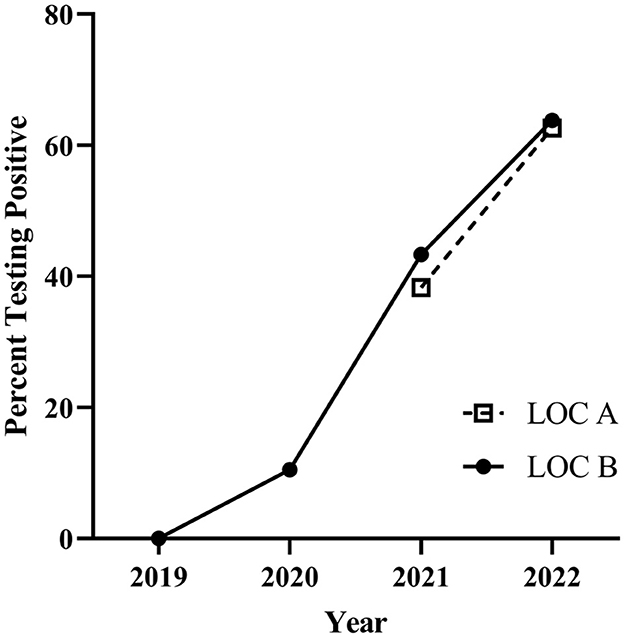
Figure 1. Theileria orientalis Ikeda percentage of positive bulls on intake to the bull test stations in Virginia for years indicated. LOC A–2021 40.3% (31.8–49.5 95% CI), 2022 64.7% (56.5–72.2 95% CI). LOC B–2019 0.8% (0.1–4.5 95% CI), 2020 10.4% (6.9–15.4 95% CI), 2021 49.1% (42.5–55.7 95% CI), 2022 64.4% (57.7–70.6 95% CI). CI: 95% confidence interval.
3.3 Average daily gain and adjusted yearling weight
With univariate analysis, ADG was significantly higher among NP bulls compared to NN bulls in both the LOC B JR and SR populations and for PP bulls compared to NN bulls among the LOC B SR population (Table 3). After adjusting for year of test, age at delivery and weight at delivery; there were no significant differences regardless of T. orientalis Ikeda status.
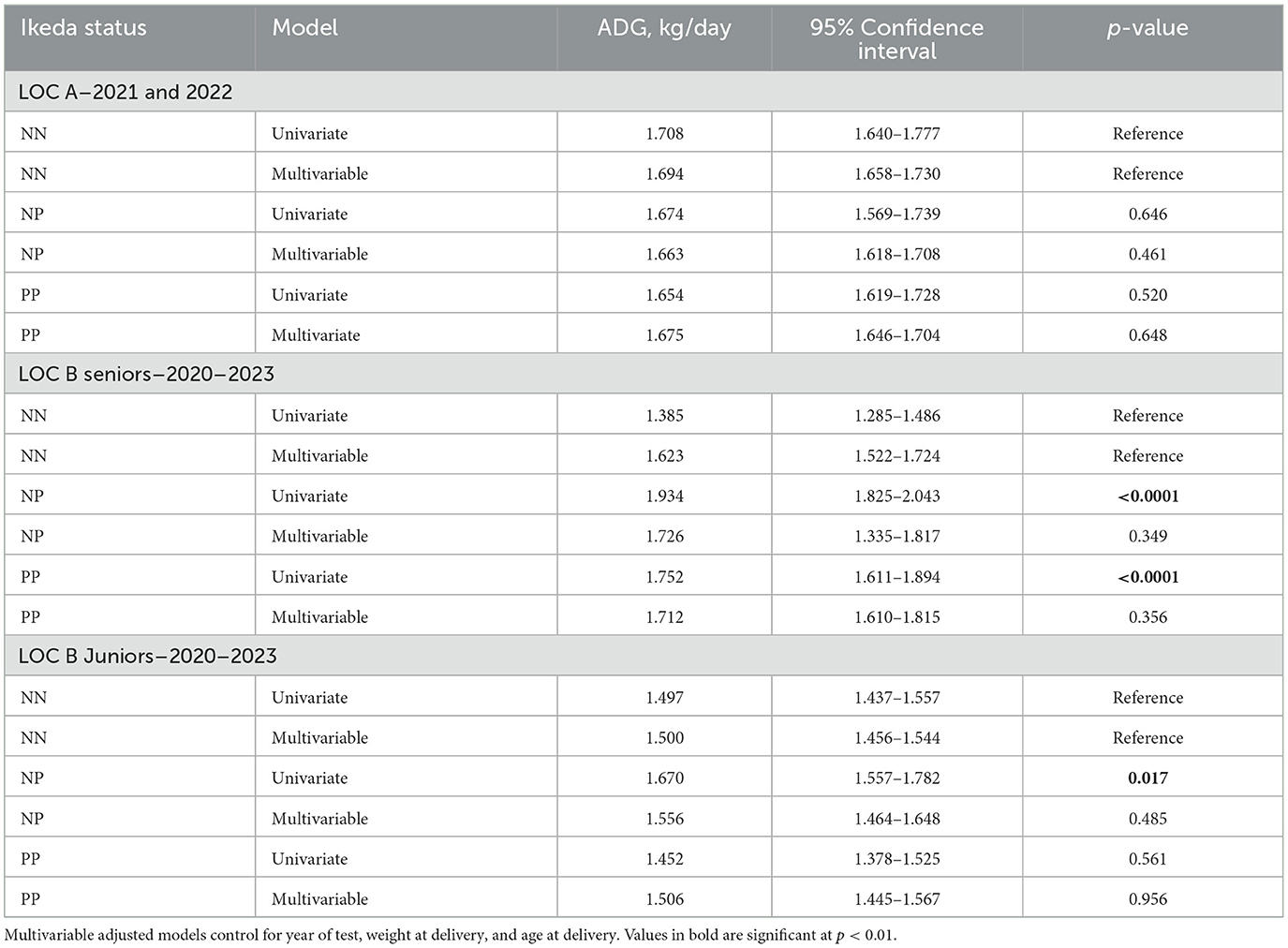
Table 3. Unadjusted and adjusted ADG (kg/day) of the NP and PP bulls, compared to NN bulls, within the three populations at the Virginia bull test stations for the years indicated.
In the univariate comparison, LOC B senior bulls with a NP T. orientalis Ikeda status had a significantly higher AYW compared to NN bulls (Table 4). However, after adjustment for year of test, age at delivery and weight at delivery, this difference vanished. There were no significant differences in AYW for any comparisons between NN and NP senior bulls in either the LOC B or LOC A (Table 4).
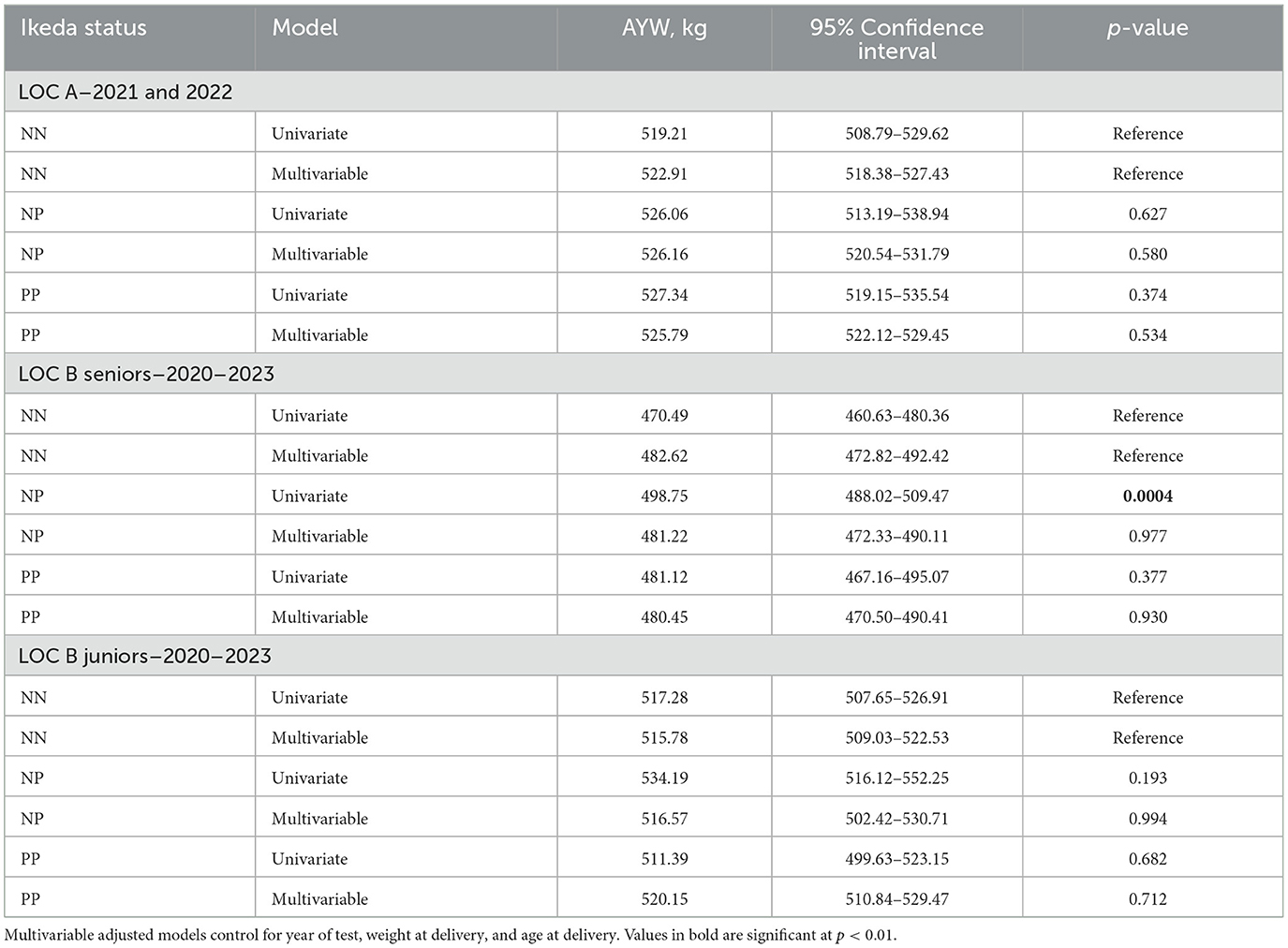
Table 4. Unadjusted and adjusted AYW (kg) of the NP and PP bulls, compared to the NN bulls, within the three populations at the Virginia bull test stations for the years indicated.
3.4 Breeding soundness exam
There was no significant difference in the likelihood of a NN bull being eligible for a BSE as compared to the NP or PP bulls, at either location (p > 0.3 for all multivariable models). BSE eligibility was determined in part by gain during the feed test period, therefore it is no surprise that at both locations the ADG ratio (p < 0.002) and AYW ratio (p < 0.002) were significantly lower if the bull was ineligible for a BSE, regardless of T. orientalis Ikeda status or year of test.
Among bulls of different T. orientalis Ikeda status, the only significant difference was a higher likelihood for NP bulls to achieve a satisfactory BSE, compared to NN bulls at LOC B (Table 5). None of the tested covariables were found to be significant at LOC A, therefore only univariate results were reported. However, at LOC B year of test was significant (p = 0.0005) when comparing NN and NP bulls regardless of T. orientalis Ikeda status. And when comparing NN and PP bulls at LOC B, ADG ratio increased significantly (p < 0.01) with the odds of achieving a satisfactory rating on the BSE regardless of T. orientalis Ikeda status.
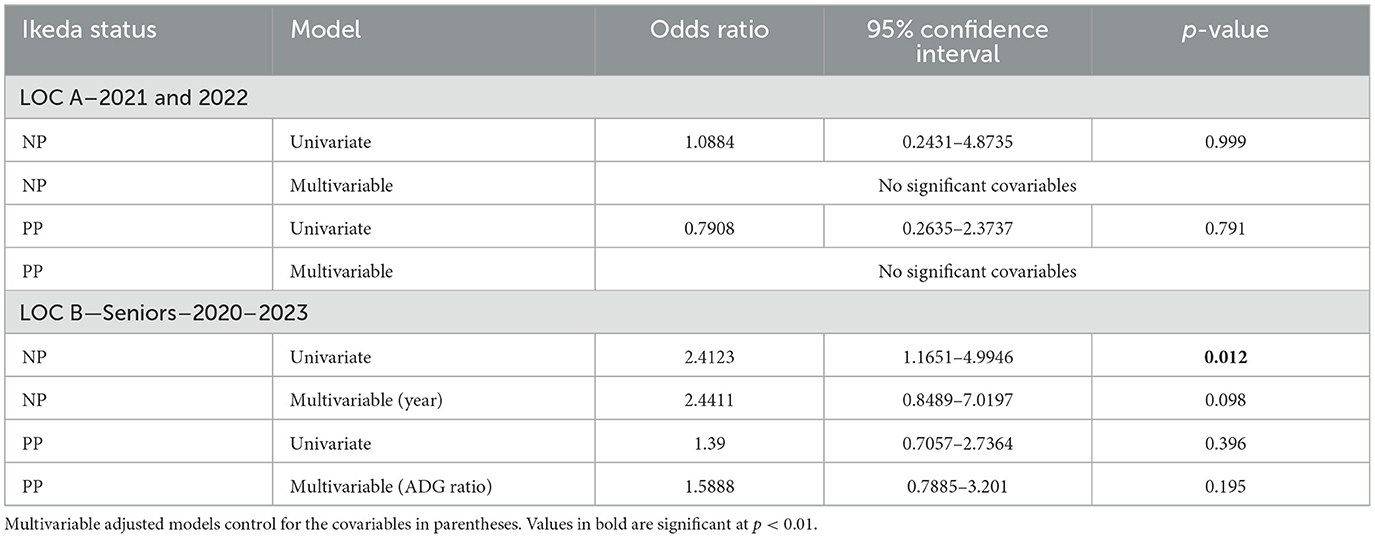
Table 5. The odds of Theileria orientalis Ikeda status NP or PP bull, using the NN bulls as reference, achieving a satisfactory rating in the BSE at the Virginia bull test stations for the years indicated.
4 Discussion
Cattle T. orientalis Ikeda infections are spreading and already endemic in some areas where the LT is present in the US. At this time, a true prevalence of Ikeda infection in Virginia cattle is difficult to ascertain as the majority of surveillance sampling has been convenience sampling at Virginia livestock markets (2) or sampling of specific populations of cattle, such as this study. The increasing number of bulls delivered to the test stations infected over the years studied and the number of bulls obtaining a NP status during our study, confirm T. orientalis Ikeda is highly infective. Other authors have found that virulence is highly variable (19) due to the presence of multifactorial risk factors, the relationship of which have yet to be elucidated. This study focused on whether infection in young beef bulls negatively impacted bull performance. These overarching effects of infection are pertinent for beef cow/calf producers, as the vast majority use live cover for breeding. We found no significant differences in test ADG or AYW between Angus bulls that were negative for T. orientalis Ikeda infection at both the start and end of the test period; those that were positive at the start and end; or those that became infected during the 5–6 months on test. The bulls at the Virginia bull test stations were all beef breed bulls, 7–14 months of age, and they all gained appropriately for the nutrition provided and according to previous literature (25). Similarly, previous work in 2-year-old dairy bulls that were experimentally infected with T. orientalis Ikeda, through intravenous injection of infected blood, found no differences in gain, live weight, hematocrit, or temperature between the infected (n = 10) and non-infected (n = 7) bulls over the 20-week study (26). At day 70 post-infection, a subset (n = 4) of the infected bulls started gaining significantly less than the other six bulls of the infected group. However, a reason for this apparent gain difference among infected animals was not identified.
Two other studies possibly indicate younger beef calves may experience reduced gain if infected with T. orientalis Ikeda. In Australia, a group of 30 weaned beef calves negative for T. orientalis were moved to an area where T. orientalis Ikeda was proven to be in both H. longicornis ticks and resident cattle (16). By 3 weeks after arrival, all 30 calves were found to be infected with T. orientalis Ikeda. The authors calculated average daily live weight gain (ADLG) every 3 weeks and there were two periods of significantly lower gain in the 24 weeks, the second of which did not appear to be related to parasitemia and was considered a period of declining nutrition as it occurred in the middle of winter (16). At 24 weeks the weight of the 30 calves was less than was expected based upon regional benchmarks of ADLG from years prior. The lack of uninfected calves for comparison and the recognized period of poor nutrition makes these findings difficult to interpret. A New Zealand study investigated gain in 123 suckling beef calves on pasture with their dams that were ~2 months old at the start of the study and 5–7 months old at the end (17). The bred dams had been purchased from an area in New Zealand that was endemically stable for T. orientalis Ikeda but were not tested prior to the study and were then moved to a new area of sparser tick distribution prior to calving. Calves that became infected during the study, had a lower mean daily gain in the latter half of the study yielding a ~4.5 kg lower live weight at the end of the study. The T. orientalis infection status, genetics and milk production of their dams was unknown. For some chronic infectious diseases, negative health and production effects occur during times of stress or less than optimal nutrition (27). Bulls at the Virginia test stations are managed on a high plane of nutrition with an extensive preventive program against other diseases. This may reduce the stresses upon their immune system which would equip the bulls to better manage T. orientalis Ikeda parasitemia.
Other studies have used experimental inoculation of infected blood or pasture-based tick-borne transmission with evidence of T. orientalis Ikeda infected ticks proven prior to putting cattle in the environment. At both locations of the Virginia bull test stations, no ticks were found on the bulls at delivery or at any processing or weighing event during the test. Additionally, all bulls were treated with an ectoparasiticide upon delivery (and a second time at LOC B), and individual needles are used for all injections throughout the test. A bull that was infected shortly before delivery may have not reached a detectable parasitemia at delivery. And to limit stress and maintain a viable test station, the bulls were not examined frequently for transient fevers or anemia, therefore a mild but acute infection could have been missed. A low environmental burden of ticks creating a minimal number of ticks on the bulls could also have been overlooked with infrequent examinations. A low number of ticks on the bulls or mechanical transmission by hematogenous flies or lice may be responsible for the transmission of the T. orientalis Ikeda. Cattle infected by mechanical transfer or smaller tick burdens take longer to turn positive, have a lower parasitemia, experience a mild anemia if any, are unlikely to experience severe symptoms of disease, and could go undetected unless tested regularly (6, 26, 28). This also may have mitigated the negative effects of infection that occurred at the test stations.
With regard to reproductive soundness, there was no significant difference in the odds of the NP or PP bulls achieving a satisfactory rating compared to the NN bulls at LOC A. This is in agreement with a New Zealand study (29) where dairy bulls were serially tested for reproductive soundness following infection and no changes were found in semen quality. At our LOC B bull test, we found that NP bulls were significantly more likely to achieve a satisfactory rating compared to NN bulls. We do not suggest that T. orientalis Ikeda infection enhances the reproductive soundness of the NP bulls. However, it is evidence that acquiring a T. orientalis Ikeda infection, without having clinical diseases, does not appear to hinder the future reproductive soundness of younger bulls.
This New Zealand study (29), also included serial measurements of libido and found a 2-week period of decreased libido following infection. This was thought to be a manifestation of the mild anemia and anorexia. In the US, the bull BSE does not typically include a measurement of libido (30). In our study the BSE was not serially performed but was only done at the end of the feeding period to ensure reproductive soundness before sale and libido was not assessed. Therefore, we could not assess transient effects on bull reproductive performance.
In a related study, bulls that became clinically ill following infection with A. marginale, another cause of clinical bovine infectious anemia, had significantly decreased scrotal circumference and reduced sperm quality for weeks beyond clinical resolution of the disease (31). There is anecdotal evidence from regional herds in Virginia, showing a gap of 5–7 weeks in pregnancies when bulls were acutely infected in the field during the breeding season (Lahmers, K.K. personal communication). Similarly, the Northland Index herd in New Zealand, reported a 47% decrease in successful pregnancies during their 6-week natural breeding season following infection with theileriosis (19). A clinical infection of T. orientalis Ikeda during the breeding season, resulting in decreased libido or decreased sperm quality, would explain these results. Acute disease is more likely with a heavy infected tick load because the LT amplifies infective sporonts by the thousands in their salivary glands, which are then deposited into the bovine host when feeding (32, 33).
There is large variability in the herd-level manifestation of clinical theileriosis in both New Zealand and US outbreaks with some herds experiencing up to a 5% mortality rate and others becoming nearly 100% T. orientalis Ikeda positive without any signs of clinical disease (1, 3, 19). Whether this variability is due to nutritional status, genetics or method of transmission is unknown at this time (34). However, the results of this study are encouraging in that bulls that are positive for T. orientalis Ikeda or acquire the infection without overt clinical signs, continue to grow appropriately and are able to achieve a satisfactory rating on a BSE exam.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Virginia Tech Institutional Animal Care and Use Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
SGu: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Investigation. SGr: Methodology, Writing – review & editing, Data curation. JC: Investigation, Writing – review & editing, Methodology. ST: Methodology, Writing – review & editing. AA: Methodology, Writing – review & editing. LH: Methodology, Writing – review & editing, Formal analysis. KL: Funding acquisition, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Development of Control Strategies for Theileria orientalis, USDA ARS Nonassistance cooperative agreement (8/1/22-7/30/25), Surveillance for Theileria orientalis, USDA APHIS Cooperative agreement (8/1/21-7/30/23; AP21VSSP0000C068) to KL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oakes VJ, Yabsley MJ, Schwartz D, LeRoith T, Bissett C, Broaddus C, et al. Theileria orientalis Ikeda genotype in cattle, Virginia, USA. Emerg Infect Dis. (2019) 25:1653–9. doi: 10.3201/eid2509.190088
2. Telionis A, Lahmers K, Todd M, Carbonello A, Broaddus CC, Bissett CJ, et al. Distribution of Theileria orientalis in Virginia Market Cattle, 2018–2020. Pathogens. (2022) 11:1353. doi: 10.3390/pathogens11111353
3. Currin JF, Lahmers K, Eastwood G, Day ER, Dellinger TA, McCormick LE. Asian longhorned tick and Theileria orientalis Ikeda: Current Thoughts and Understandings. (2023). Available online at: https://www.pubs.ext.vt.edu/content/pubs_ext_vt_edu/en/APSC/apsc-196/apsc-196.html. (accessed February 5, 2024).
4. Watts JG, Playford MC, Hickey KL. Theileria orientalis: a review. NZ Vet J. (2016) 64:3–9. doi: 10.1080/00480169.2015.1064792
5. Sivakumar T, Hayashida K, Sugimoto C, Yokoyama N. Evolution and genetic diversity of Theileria. Infect Genet E. (2014) 27:250–63. doi: 10.1016/j.meegid.2014.07.013
6. Dinkel KD, Herndon DR, Noh SM, Lahmers KK, Todd SM, Ueti MW, et al. A U.S. isolate of Theileria orientalis, Ikeda genotype, is transmitted to cattle by the invasive Asian longhorned tick, Haemaphysalis longicornis. Parasit Vectors. (2021) 14:157. doi: 10.1186/s13071-021-04659-9
7. Thompson AT, White S, Shaw D, Egizi A, Lahmers K, Ruder MG, et al. Theileria orientalis Ikeda in host-seeking Haemaphysalis longicornis in Virginia, USA. Ticks Tick-Borne Dis. (2020) 11:101450. doi: 10.1016/j.ttbdis.2020.101450
8. USDA APHIS. National Haemaphysalis longicornis (Asian longhorned tick) Situation Report. (2024). Available online at: https://www.aphis.usda.gov/sites/default/files/longhorned-tick-sitrep-2024-03-07.pdf. (accessed March 8, 2024).
9. Heath A. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. NZ Vet J. (2016) 64:10–20. doi: 10.1080/00480169.2015.1035769
10. Trout Fryxell RT, Vann DN, Butler RA, Paulsen DJ, Chandler JG, Willis MP, et al. Rapid discovery and detection of Haemaphysalis longicornis through the use of passive surveillance and collaboration: building a state tick-surveillance network. Int J Environ Res Public Health. (2021) 18:7980. doi: 10.3390/ijerph18157980
11. Schappach BL, Krell RK, Hornbostel VL, Connally NP, Gouge D. Exotic Haemaphysalis longicornis (Acari: Ixodidae) in the United States: biology, ecology, and strategies for management. J Integr Pest Manag. (2020) 11:1–11. doi: 10.1093/jipm/pmaa019
12. Raghavan RK, Barker SC, Cobos ME, Barker D, Teo EJM, Foley DH, et al. Potential spatial distribution of the newly introduced long-horned tick, Haemaphysalis longicornis in North America. Sci Rep. (2019) 9:498. doi: 10.1038/s41598-018-37205-2
13. Rochlin I. Modeling the asian longhorned tick (Acari: Ixodidae) suitable habitat in North America. J Med Entomol. (2019) 56:384–91. doi: 10.1093/jme/tjy210
14. Forshaw D, Alex SM, Palmer DG, Cotter J, Roberts WD, Jenkins C, et al. Theileria orientalis Ikeda genotype infection associated with anaemia, abortion and death in beef cattle in Western Australia. Aust Vet J. (2020) 98:290–7. doi: 10.1111/avj.12937
15. Yam J, Bogema D, Jenkins C. Oriental theilieriosis. In:Abubakar M, Perera PF, , editors. Ticks and Tick-Borne Pathogens. Rijeka: IntechOpen (2019). p. 11. doi: 10.5772/intechopen.81198
16. Emery D, Zhang S, Loo C, Shirley C. A longitudinal study of infection with genotypes of Theileria orientalis in calves and introduced cattle at Dorrigo, New South Wales, and the effect on weight gains. Vet Parasitol. (2021) 296:109487. doi: 10.1016/j.vetpar.2021.109487
17. Lawrence KE, Lawrence BL, Hickson RE, Hewitt CA, Gedye KR, Fermin LM, et al. Associations between Theileria orientalis Ikeda type infection and the growth rates and haematocrit of suckled beef calves in the North Island of New Zealand. NZ Vet J. (2019) 67:66–73. doi: 10.1080/00480169.2018.1547227
18. Lawrence KE, Gedye K, Pomroy WE. A longitudinal study of the effect of Theileria orientalis Ikeda type infection on three New Zealand dairy farms naturally infected at pasture. Vet Parasitol. (2019) 276:108977. doi: 10.1016/j.vetpar.2019.108977
19. Lawrence K, Gedye K, McFadden A, Pulford D, Heath A, Pomroy W, et al. Review of the New Zealand Theileria orientalis Ikeda type epidemic and epidemiological research since 2012. Pathogens. (2021) 10:1346. doi: 10.3390/pathogens10101346
20. National National Academies of Sciences Engineering and Medicine. Nutrient Requirements of Beef Cattle: Eighth Revised Edition. Washington, DC: The National Academies Press (2016).
21. BIF Guidelines Wiki. Guidelines for Uniform Beef Improvement Plans. (2023). Available online at: http://guidelines.beefimprovement.org/index.php?title=Guidelines_for_Uniform_Beef_Improvement_Programsandoldid=2679 (accessed February 5, 2024).
22. Oakes VJ, Todd SM, Carbonello AA, Michalak P, Lahmers KK. Coinfection of cattle in Virginia with Theileria orientalis Ikeda genotype and Anaplasma marginale. J Vet Diagn Invest. (2022) 34:36–41. doi: 10.1177/10406387211057627
23. Cain M, Wilson L. Factors influencing individual bull performance in central test stations. J Anim Sci. (1983) 57:1059–66. doi: 10.2527/jas1983.5751059x
24. Tong A. Effects of initial age and weight on test daily gains of station-tested bulls. Can J Anim Sci. (1982) 62:671–8. doi: 10.4141/cjas82-082
25. Townsend HGG, Meek AH, Lesnick TG, Janzen ED. Factors associated with average daily gain, fever and lameness in beef bulls at the saskatchewan central feed test station. Ca J Vet Res. (1989) 53:349–54.
26. Lawrence KE, Gibson M, Hickson RE, Gedye K, Hoogenboom A, Fermin L, et al. Experimental infection of Friesian bulls with Theileria orientalis (Ikeda) and effects on the haematocrit, live weight, rectal temperature and activity. Vet Parasitol Reg Stud Rep. (2018) 14:85–93. doi: 10.1016/j.vprsr.2018.09.004
27. Carroll JA, Forsberg NE. Influence of stress and nutrition on cattle immunity. Vet Clin North Am Food Anim Pract. (2007) 23:105–49. doi: 10.1016/j.cvfa.2007.01.003
28. Hammer JF, Jenkins C, Bogema D, Emery D. Mechanical transfer of Theileria orientalis: possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasit Vectors. (2016) 9:34. doi: 10.1186/s13071-016-1323-x
29. Gibson MJ, Lawrence KE, Hickson RE, How R, Gedye KR, Jones G, et al. Effects of Theileria orientalis Ikeda type infection on libido and semen quality of bulls. Anim Reprod Sci. (2020) 214:106312. doi: 10.1016/j.anireprosci.2020.106312
30. Koziol JH, Armstrong CL. Society for Theriogenology Manual for Breeding Soundess Examination of Bulls. Providence, RI: Society for Theriogenology (2018).
31. Lovett AC, Reppert EJ, Jaeger JR, Kang Q, Flowers MR, Bickmeier NP, et al. Satisfactory breeding potential is transiently eliminated in beef bulls with clinical anaplasmosis. BMC Vet Res. (2022) 18:381. doi: 10.1186/s12917-022-03470-7
32. Florin-Christensen M, Schnittger L. Piroplasmids and ticks: a long-lasting intimate relationship. FBL. (2009) 14:3064–73. doi: 10.2741/3435
33. Shaw M. Theileria development and host cell invasion. In:DAE Dobbelaere, DJ McKeever, , editors. World Class Parasites: Volume 3 Theileria. New York, NY: Kluwere Academic Publishers (2002), p. 1–23. doi: 10.1007/978-1-4615-0903-5_1
34. Hickson RE, Lawrence BL, Lawrence KE, Gedye K, Fermin LM, Coleman LW, et al. Genetic susceptibility to Theileria orientalis (Ikeda) in Angus- and Hereford-sired yearling cattle born to dairy cattle on an endemically infected farm in New Zealand. New Zeal J Agric Res. (2023) 1–9. doi: 10.1080/00288233.2023.2181360
Keywords: beef cattle, Theileria orientalis (Ikeda), average daily gain (ADG), breeding soundness evaluation (BSE), bull
Citation: Guynn SR, Greiner SP, Currin JF, Todd SM, Assenga A, Hungerford LL and Lahmers KK (2024) Theileria orientalis Ikeda infection does not negatively impact growth performance or breeding soundness exam results in young beef bulls at bull test stations. Front. Vet. Sci. 11:1432228. doi: 10.3389/fvets.2024.1432228
Received: 13 May 2024; Accepted: 03 July 2024;
Published: 18 July 2024.
Edited by:
Camila Hamond, Animal and Plant Health Inspection Service (USDA), United StatesReviewed by:
Remil Galay, University of the Philippines Los Baños, PhilippinesChengming Wang, Auburn University, United States
Copyright © 2024 Guynn, Greiner, Currin, Todd, Assenga, Hungerford and Lahmers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sierra R. Guynn, c2d1eW5uQHZ0LmVkdQ==
†These authors share last authorship
 Sierra R. Guynn
Sierra R. Guynn Scott P. Greiner2
Scott P. Greiner2 S. Michelle Todd
S. Michelle Todd Laura L. Hungerford
Laura L. Hungerford Kevin K. Lahmers
Kevin K. Lahmers