- 1State Engineering Technology Institute for Karst Desertfication Control, School of Karst Science, Guizhou Normal University, Guiyang, China
- 2Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment, Guizhou Normal University, Guiyang, China
- 3Hunan Polytechnic of Environment and Biology, College of Biotechnology, Hengyang, China
The application of Bacillus spp. as probiotics in the swine industry, particularly for piglet production, has garnered significant attention in recent years. This review aimed to summarized the role and mechanisms of Bacillus spp. in promoting growth and maintaining gut health in piglets. Bacillus spp. can enhance intestinal barrier function by promoting the proliferation and repair of intestinal epithelial cells and increasing mucosal barrier integrity, thereby reducing the risk of pathogenic microbial invasion. Additionally, Bacillus spp. can activate the intestinal immune system of piglets, thereby enhancing the body’s resistance to diseases. Moreover, Bacillus spp. can optimize the gut microbial community structure, enhance the activity of beneficial bacteria such as Lactobacillus, and inhibit the growth of harmful bacteria such as Escherichia coli, ultimately promoting piglet growth performance and improving feed efficiency. Bacillus spp. has advantages as well as challenges as an animal probiotic, and safety evaluation should be conducted when using the newly isolated Bacillus spp. This review provides a scientific basis for the application of Bacillus spp. in modern piglet production, highlighting their potential in improving the efficiency of livestock production and animal welfare.
Introduction
Weaning is one of the key events in the life cycle of pigs. Changes in diet and environment during weaning usually result in reduced feed intake, intestinal inflammation, and imbalances in intestinal microbial composition, leading to diarrhea in weaned piglets (1–3). In practical production, the addition of antibiotics (4, 5) or high doses of zinc oxide (ZnO) (2, 6–8) to the diets has been found to effectively alleviate diarrhea in piglets after weaning and promote their growth and development. However, while acknowledging the positive effects of antibiotics and high doses of ZnO on weaned piglets, it is also necessary to recognize their negative effects. Long-term or excessive of antibiotics in swine will lead to the imbalance of pig intestinal microbiota, the increase of antibiotic-resistant pathogens, and the residue of antibiotics in pork, which will directly or indirectly harm human health (9, 10). Excessive or long-term use of high doses of ZnO may lead to dull skin and coarse hair, inhibit the growth and development of pigs, reduce the bioavailability of ZnO, and may also lead to an increase in the percentage of intestinal bacteria resistant to multiple drugs, causing waste of zinc resources and environmental pollution (11–13). The prohibition or restriction of dietary antibiotics, and the reduction of ZnO in feed have formed a broad consensus in the world. Therefore, the search for green, safe and efficient non-antibiotic additives and ZnO substitutes, such as essential oils (14), organic acids (15), prebiotics (16), and probiotics (17), and so on to improve intestinal health and reduce diarrhea of weaned piglets has become a hot field of animal nutrition.
Probiotics are live bacteria that can improve the beneficial flora of the host with single or multiple bacteria (18). Previous studies have demonstrated that probiotics can improve the immune function, enhance the ability to resist bacterial infection, regulate the balance of intestinal flora, and thus improve production performance of piglets (19–22). There are many kinds of probiotics, including Bifidobacteria, Lactobacillus, yeast, Bacillus spp., etc. Among these probiotics, Bacillus spp. has gained increasing attention as an alternative to antibiotics or ZnO due to its advantages such as heat resistance during feed granulation, resistance to low pH in the stomach, and stability at ambient temperatures (23, 24).
Bacillus spp. are Gram-positive aerobic or facultative anaerobic bacteria capable of producing resistant endospores (25). Bacillus spp. can adapt to a variety of environments and are widely distributed around the world because their spores are highly resistant to ultraviolet radiation, high temperatures, strong acids, ionizing radiation, and many toxic chemicals (26, 27), making it widely used in industrial, agricultural, medical and other fields (25, 28–30). This is also the advantage of Bacillus spp. as a probiotic for animals. In the field of animal nutrition and feed science, compared with other types of probiotics, Bacillus spp. has more advantages. First of all, in the process of feed processing, Bacillus spp. can resist high temperature because of the presence of spores, and therefore, Bacillus spp. can maintain biological activity after processing, and its spore-forming ability is beneficial for long-term storage compared to that of non- pore-forming bacteria (31, 32). For example, Amerah et al. (33) showed that Bacillus spores are resistant to pelleting temperatures of up to 90 ° C, and more than 90% of spores are still alive in feed samples. Second, because of the strong stress resistance of Bacillus spores, they can tolerate cholate and stomach acid after entering the gastrointestinal tract, and successfully colonize and play their role in the intestine (23, 24). For example, Dong et al. (34) showed that Bacillus licheniformis (B. licheniformis) WBL009 had strong acid and bile salt resistance. The survival rate of B. licheniformis WBL009 was 35.8% after 2 h treatment in artificial gastric juice with pH value of 2.5, and 51.6% after 12 h treatment with bile salt concentration of 0.3%.
Numerous studies have shown that Bacillus spp. can produce extracellular enzymes such as glycoenzyme, protease and lipase, and also has antibacterial and immune regulation effects, thereby improving the nutrient digestibility, reducing the colonization of harmful bacteria such as, Escherichia coli and Clostridium perfringens of piglets, and thus improving the growth performance of piglets (35–40). There are many types of Bacillus spp., including Bacillus subtilis (B. subtilis), B. licheniformis, Bacillus coagulans (B. coagulans), Bacillus megaliformis (B. megaliformis), Bacillus cereus (B. cereus), and so on. However, not all Bacillus spp. are effective probiotics, some strains cannot be used because of limitations such as poor colonization effect or virulence factors, and some Bacillus spp. strains have been identified as opportunistic pathogens that causing food spoilage and toxin release in the hosts (41). Therefore, although most of the Bacillus spp. are generally recognized as safe for animal consumption, it is necessary to summarize the application effect of different Bacillus spp. on piglets. In this paper, the effects of Bacillus spp. in piglet production were summarized from the perspective of growth promotion and health benefits, so as to provide a reference for the rational utilization of Bacillus spp. in swine production.
Growth promoting effect of Bacillus spp. on piglets
Early weaning technology is the production method commonly used in most intensive pig farms at present. The weaning process of piglets is accompanied by a series of changes in diet, society and environment, which can easily cause weaning stress (3). The transition from high digestibility liquid milk to non-digestible solid feed is a great challenge for the immature gastrointestinal tract of the piglets, which usually causes diarrhea due to indigestion (42). Due to the combination of sudden separation from the sow, social factors, and other influences, piglets experience anxiety, leading to a reduction in feed intake or even refusal to eat (3, 42, 43). It is generally believed that the production performance of the whole pig farm is determined by the production performance of the piglets, and diarrhea is the culprit leading to the low production performance of the piglets. Therefore, the control of post-weaning diarrhea of piglets is of great significance to improve the economic benefits of pig production.
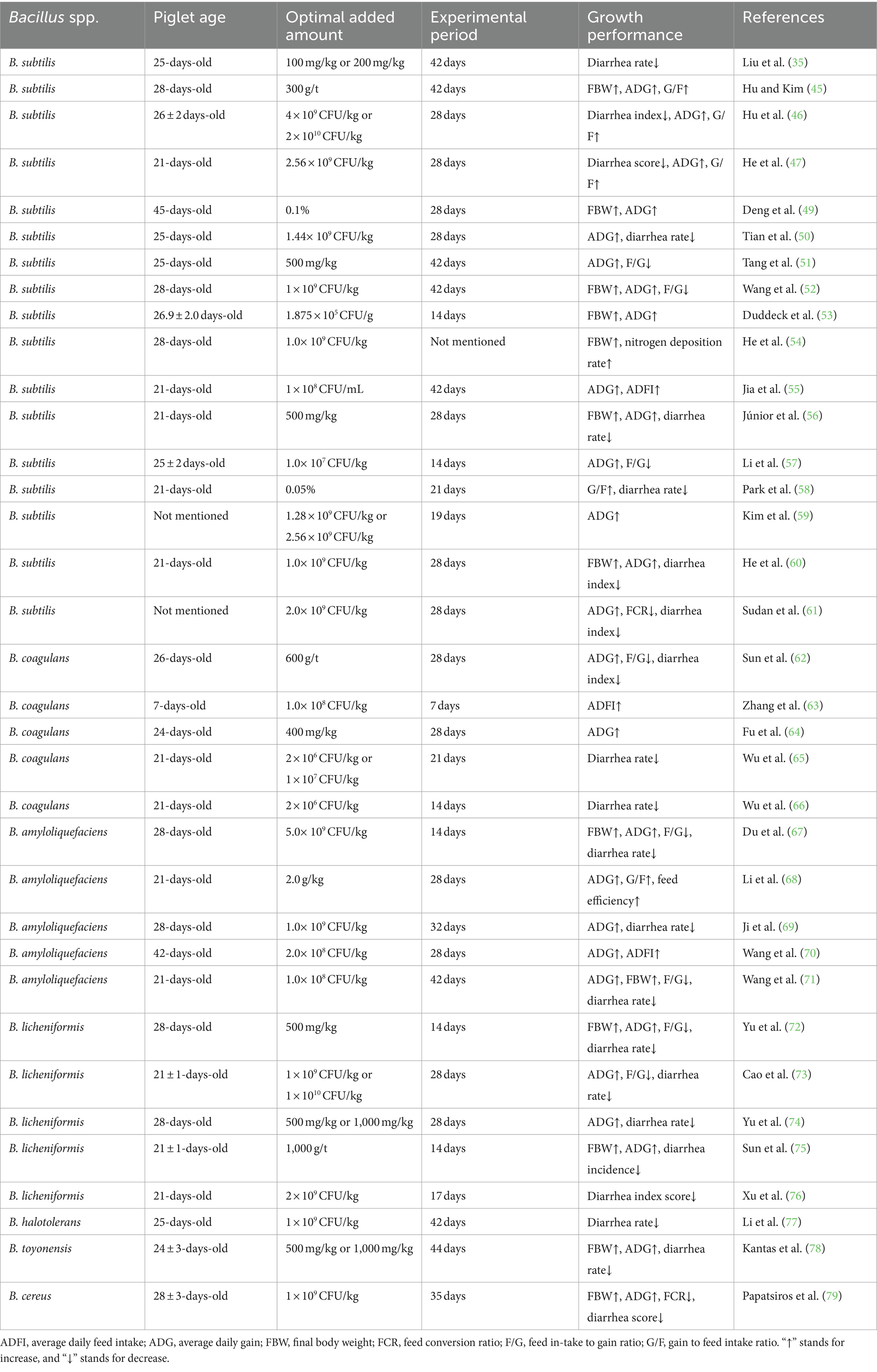
Previous studies have shown that the addition of Bacillus spp. can improve the intestinal health of pigs by altering the intestinal flora, thereby inhibiting the growth of pathogens, enhancing immune function, improving nutrient utilization and digestibility, reducing the incidence of diarrhea, and ultimately improving the growth performance of piglets (44–48). The growth promoting effect of single Bacillus spp. strains on piglets were summarized in Table 1. These studies indicated that the addition of single Bacillus spp. strains, including B. subtilis (35, 45–47, 49–61), B. coagulans (62–66), B. amyloliquefaciens (44, 67–71), B. licheniformis (72–76), B. halotolerans (77), B. toyonensis (78), B. cereuscould (79), and so on could promote the growth performance and effectively prevent the occurrence of diarrhea of piglets.
In addition to the addition of single strains of Bacillus spp., the combination of Bacillus spp. with other probiotics or functional substances has shown a synergistic effect in promoting the growth performance of piglets. For example, Liu et al. (35) showed that the combination of B. subtilis QST713 (100 mg/kg) with β-mannanase (150 mg/kg) effectively decreased the feed conversion ratio (FCR) of piglets throughout the trial period. Jiao et al. (37) demonstrated that compared to the control group, the combination of Bacillus spp. (1.3 × 109 CFU/kg; B. licheniformis and B. subtilis with the ratio of 1: 1) with medium-chain fatty acid (0.588 g/kg) significantly increased the average daily gain (ADG) and dry matter intake of piglets in phase 1 (days 1 to 9), and significantly increased the ADG of piglets in the whole period (days 1 to 36). Liu et al. (80) showed that the combination of B. subtilis with Lactobacillus plantarum (L. plantarum) jointly increased average daily feed intake (ADFI) and ADG of weaned piglets in d 14 ~ 28 and d 28 ~ 42. Pu et al. (81) showed that piglets fed a diet added with benzoic acid (3,000 g/t) and B. coagulans (400 g/t) mixture significantly increased the final body weight (FBW) and ADG, and decreased FCR compared with piglets fed a basal diet. Phaengphairee et al. (82) showed that dietary supplementation of full-fat black soldier fly larvae in combination with multi-probiotics (B. subtilis, B. licheniformis, and Saccharomyces cerevisiae) significantly increased the ADG, gain-to-feed ratio (G/F), and nutrient digestibility of weaned piglets.
Although numerous studies have confirmed the growth-promoting effect of Bacillus spp. alone or in combination with other additives on weaned piglets, some studies have shown that Bacillus spp. has no effect on the growth performance of weaned piglets. For example, Jiao et al. (37), Huting et al. (38), Kritas and Morrison (83), Luise et al. (84), and Ding et al. (85) showed that dietary supplementation with Bacillus spp. alone has no effect on the growth performance of piglets. The different effects of Bacillus spp. on alleviating diarrhea and improving growth performance of weaned piglets may be closely related to the different types of Bacillus spp., dosage, adding stage, use mode, diet composition and physiological health status of piglets. Although the effect of Bacillus spp. on the growth performance of weaned piglets is inconsistent, it is certain that Bacillus spp. has some other benefits, such as promoting intestinal health.
Intestinal health benefits of Bacillus spp. on piglets
Weaning is the transitional period when piglets shift from relying on maternal milk nutrition to solid feed. During this time, the digestive system of the piglets faces significant challenges, making them susceptible to intestinal dysfunction and diseases (86, 87). Promoting intestinal development and intestinal health of weaned piglets is the key to cope with weaning stress. In previous studies, there are many ways to promote the intestinal health of piglets, among which probiotics, especially Bacillus spp., are prominent in promoting the intestinal health of piglets.
Bacillus spp. promotes intestinal development
The gut is the main site for the digestion and absorption of nutrients in animals and serves as a selective barrier for the body to prevent exogenous harmful substances from entering the circulatory system (3, 87). The gastrointestinal digestive system of piglets is still immature, and external stress will further damage the intestinal structure of piglets, such as intestinal villi shedding, crypt hyperplasia, intestinal mucosal atrophy, etc., and then destroy the intestinal mucosal barrier function and digestive absorption capacity (3, 88, 89). Previous studies have shown that Bacillus spp. has good nutritional physiological effects on intestinal development of piglets, including the maintenance of intestinal morphology and structure (50, 71, 73, 75, 85, 90), promoting the secretion of intestinal digestive enzymes (49, 75, 91, 92), and reducing intestinal permeability (59, 90, 91).
Bacillus spp. have a positive effect on the intestinal morphological structure and function of piglets, and can improve the intestinal damage caused by various stresses, so as to maintain the integrity of intestinal mucosa (65, 66). Bacillus spp. can increase the intestinal villus height (VH), decrease the intestinal crypt depth (CD), increase the intestinal villus height to crypt depth ratio (VCR) and reduce the apoptosis of intestinal mucosal epithelial cells of piglets (50, 67, 68, 92). For instance, Du et al. (67) showed that dietary supplementation of B. amyloliquefaciens (5.0× 109 CFU/kg) significantly increased intestinal VH and VCR, while decreased intestinal CD. Hu et al. (92) reported that dietary supplementation with B. subtilis PB6 can improve intestinal damage of suckling piglets caused by intrauterine growth retardation (IUGR), as shown by higher VH and VCR, and lower CD than that of IUGR piglets without B. subtilis supplementation. Du et al. (93) showed that dietary supplementation with B. amyloliquefaciens SC06 (2.0 × 108 CFU/kg) significantly increased intestinal villus length, and intestinal villi morphology was improved. Wang et al. (36) showed that the combination of B. subtilis with B. licheniformis significantly increased the ileum VH and the jejunum and ileum VCR, and decreased the jejunum CD of piglets. The increase in VH and the decrease in CD on the intestinal mucosa effectively increase the surface area of the intestinal tract in contact with the digested food, thus improving the absorption efficiency of nutrients.
Most Bacillus spp. have a strong enzyme production capacity and can produce a variety of extracellular enzymes, such as cellulase, xylanase, amylase, protease, lipase (94–98), which can assist animals to digest feed and improve nutrient absorption. In addition to the exogenous enzymes secreted by themselves, Bacillus spp. can also stimulate the secretion of various digestive enzymes in the intestines of piglets (49, 75, 91, 92). For example, Deng et al. (49) showed that B. subtilis supplementation significantly increased ileum lipase, amylase, lactase, and maltase activities compared to control group, and significantly increased ileum lactase, and maltase activities compared to antibiotic group. Hu et al. (91) showed that replacement of aureomycin with B. amyloliquefaciens (2 × 108 cfu/Kg) significantly improved intestinal amylase, disaccharides and Na+/K+-ATPase activities of piglets, and half replacement of aureomycin with B. amyloliquefaciens (1 × 108 cfu/Kg), the activity of intestinal chymotrypsin was significantly improved. Many studies have shown that dietary addition of Bacillus spp. can significantly improve the nutrient digestibility of piglets (38, 45, 99–101), which may be related to the secretion of intestinal digestive enzymes. For instance, Huting et al. (38) showed that dietary addition of multi-strains of Bacillus spp. significantly improved the apparent total tract digestibility (ATTD) of dry matter (DM) and organic matter (OM) of piglets. Hu and Kim (45) showed that dietary B. subtilis C-3102 supplementation significantly improved the apparent the ATTD of DM, crude protein (CP), and energy of piglets. Cai et al. (99) and Lewton et al. (100) showed that piglets fed with Bacillus spp. based direct-fed microbial had a higher protein utilization, as indicated by increased the ATTD of nitrogen. Cui et al. (101) showed that the ether extract (EE) and phosphate digestibility was increased when piglets fed with B. subtilis.
When exposed to stress conditions such as weaning stress, Escherichia coli (E. coli) infection, IUGR and lipopolysaccharide (LPS) stimulation, piglets are susceptible to intestinal mucosal injury, potentially leading to increased intestinal permeability (3, 87, 102–104). In general, intestinal permeability is assessed by quantifying the passage of small molecules like horseradish peroxidase (59), monitoring plasma levels of D-lactic acid, endotoxins, and diamine oxidase (DAO) (37, 44, 105), as well as by measuring trans-epithelial electrical resistance (TEER) in intestinal tissues (106). Studies have shown that Bacillus spp. can reduce intestinal permeability of piglets. Kim et al. (59) demonstrated that a high dosage of B. subtilis (2.56 × 109 CFU/kg) supplementation lowered both intestinal intercellular and intracellular permeability in E. coli infected pigs. Hu et al. (91) showed that substituting aureomycin with B. amyloliquefaciens (2 × 108 cfu/Kg) preserved intestinal integrity, as evidenced by significantly decreased DAO activity. Xie et al. (107) showed that a combination of Lactobacillus acidophilus and B. subtilis reduced serum DAO level in piglets. In an IPEC-J2 cell model of deoxynivalenol (DON)-induced injury, Gu et al. (108) reported that TEER was higher in cells treated with B. subtilis compared to non-B. subtilis treated cells.
Bacillus spp. promotes intestinal mucosal barrier
The intestinal epithelium serves as the primary barrier, facilitating nutrient breakdown and absorption via brush border enzymes and various transport proteins at the apical and basolateral membranes, while also protecting against antigenic invasion (3). Tight junctions are vital components of the intestinal mucosal barrier against harmful pathogens, predominantly made up of membrane protein complexes, including zonula occludens proteins (ZOs), occludin, and claudins (109). Alterations in the structure and function of tight junctions may directly impair the integrity of the intestinal mucosal barrier, leading to the infiltration of pathogens and other undesirable substances through the epithelial layer (110). Previous studies have confirmed the regulatory role of Bacillus spp. on intestinal tight junction proteins in piglets (35, 75, 105, 111–113). For instance, in a study using an IPEC-J2 cells model infected with E. coli, Sudan et al. (37) demonstrated that B. subtilis significantly upregulated the expression of ZO-1, claudin-1, and occludin genes. Similarly, Li et al. (77) found that B. halotolerans markedly increased both the gene and protein expression levels of ZO-1, claudin-1, and Occludin in weaned piglets suffering from E. coli-induced diarrhea. Sun et al. (75) showed that dietary inclusion of B. licheniformis significantly promoted the expression of Occludin and ZO-1 in jejunum mucosa of piglets. Additionally, Fu et al. (105) demonstrated that piglets supplemented with B. licheniformis, either alone or in combination with Clostridium butyricum significantly up-regulated the protein expression of ZO-1 and Occludin in the jejunum, as well as ZO-1, Claudin-1 and Occludin in the ileum of piglets. In addition, Yang et al. (114) showed that dietary supplementation with a low dose (3.9 × 108 CFU/day) of a mixture of B. licheniformis and B. subtilis significantly up-regulated the protein expression of ZO-1 in the jejunum of piglets infected with E. coli, thereby preventing the loss of intestinal epithelial barrier integrity.
Another important molecule in the intestinal mucosal barrier is mucins (MUCs), which are secreted by intestinal goblet cells (87, 115). As principal constituents of the mucus layer, MUCs facilitate gut lubrication and form the initial line of defense within the mucosal barrier, which can promote the colonization of symbiotic bacteria, inhibit the attachment of pathogens, and maintain the homeostasis of the intestinal environment (116, 117). Therefore, the differentiation and proliferation of intestinal goblet cells, along with the normal secretion of MUCs, are crucial for maintaining intestinal health. Stress commonly disrupts these processes in piglets, leading to compromised cellular functions. For example, studies by Li et al. (77), Zhang et al. (118), and Xu et al. (119) showed that the number of intestinal goblet cells decreased significantly when piglets were infected with E. coli. Conversely, Bacillus spp. have been shown to enhance the maturation and differentiation of intestinal goblet cells and the secretion of MUCs in piglets (60, 68, 80, 93, 107, 118). Specifically, research by He et al. (60) revealed that dietary inclusion of B. pumilus significantly increased the number of goblet cells in duodenal villi, while supplementation of B. subtilis significantly up-regulated the expression of MUC2 gene in the jejunum mucosa of piglets. Liu et al. (80) showed that the combination of B. subtilis with L. plantarum at dosage of 1 kg/t significantly increased the number of colonic goblet cells of weaned piglets. Zhang et al. (118) demonstrated that dietary supplementation a dosage of 7.8 × 108 CFU/kg Bacillus spp. probiotics mixture significantly increased the number of ileal goblet cells, and up-regulated the expression of MUC2 gene in the ileum of piglets infected with E. coli. In conclusion, the protective effect of Bacillus spp. on intestinal mucosal barrier of piglets is achieved by promoting the expression of intestinal tight junction proteins and the secretion of MUCs.
Bacillus spp. promotes intestinal immune function and inhibits inflammatory response
The intestine is not only the most important digestive organ in animals but also the largest immune organ. The gut immune system recognizes and combats pathogens, such as bacteria, viruses, and parasites, that traverse the gut surface to prevent them from entering the bloodstream and causing systemic infections, thus playing a pivotal role in maintaining overall health (3). The intestinal development of piglets is still immature, and piglets will encounter many pathogenic and non-pathogenic challenges due to physiological and psychological factors during weaning, leading to the destruction of intestinal immune barrier function (3, 120). Numerous in vitro and in vivo studies have confirmed that Bacillus spp. can enhance the intestinal immune function of piglets by promoting the proliferation of immune cells, enhancing immune function, and inhibiting inflammatory responses (44, 47, 59, 114, 121).
Initially, Bacillus spp. enhances the intestinal immune capacity of piglets by stimulating the activity and proliferation of immune cells. The intestinal immune system of piglets is not fully matured in their early stages, and Bacillus spp. can stimulate immune cells in the intestinal submucosa, particularly T cells and B cells in the gastrointestinal-associated lymphoid tissue (GALT), to promote their proliferation and differentiation (114, 122, 123). This proliferation helps to establish a stronger and more active immune defense network, thereby enhancing the ability to resist pathogenic invasions. For instance, Xie et al. (107) showed that dietary supplementation with L. acidophilus and B. subtilis mixture increased CD4+ T cells and sIgA+ cells in intestinal mucosa of piglets, and Zhang et al. (121) showed that oral administration of B. subtilis significantly increased the number of IgA secreting cells and CD3+ T cells in intestinal tract of piglets, which suggests a direct influence on the mucosal immune response. Yang et al. (114) demonstrated that dietary supplementation with high-dose (7.8 × 108 CFU/day) of B. licheniformis and B. subtilis mixture significantly increased the percentage of CD4−CD8−T cells in the inflamed intestine of piglets challenged with E. coli. Similarly, Zhou et al. (122) showed that adding a combination of B. licheniformis and B. subtilis to the diet notably increased the percentage of CD4 + Foxp3+ T regulatory cells among the intraepithelial lymphocytes. It also increased the presence of CD4 + IL-10+ T cells in the Peyer’s patches and the lamina propria of small intestines in E. coli-infected piglets.
Secondly, Bacillus spp. enhances the intestinal immune function of piglets by producing a variety of antimicrobial substances, such as antimicrobial peptides (AMPs) and short-chain fatty acids (SCFAs), which directly inhibit the growth of pathogens, thereby protecting piglets from infections (77, 105, 107, 124). For example, Xie et al. (107) observed that dietary inclusion of a combination of L. acidophilus and B. subtilis upregulated the gene expression of AMPs, including porcine beta defensin-2 (PBD-2), PBD-3 and regenerating islet-derived IIIγ (RegIIIγ), and short-chain fatty acid receptors including GPR43, GPR41and GPR109A in the ileum mucosa of piglets. Fu et al. (105) showed that the use of B. licheniformis alone had no effect on the expression of AMPs genes, while the combination of B. licheniformis and Clostridium butyricum could significantly increase the expression of intestinal AMPs genes, including PBD-1, PBD-2, PBD-3 and PR-39 in piglets. At the same time, the use of B. licheniformis alone or in combination with Clostridium butyricum significantly increased the content of acetic, propionic acid, butyric acid, and total acid in the ileal contents of piglets.
Finally, Bacillus spp. also plays an important role in inhibiting inflammation by regulating the production of inflammatory cytokines, such as reducing the release of pro-inflammatory factors (such as TNF-α, IL-1β, and IL-6), while increasing the production of anti-inflammatory cytokines (such as IL-10, and IL-22), thereby preventing intestinal damage caused by excessive immune activity (47, 59, 72, 80, 105, 107). For example, Yu et al. (72) showed that dietary B. licheniformis supplementation significantly increased anti-inflammatory factors (IL-10), and reduced pro-inflammatory factors (TNF-α and IL-6) levels in the jejunal mucosa of piglets challenged with LPS. Li et al. (77) showed that B. halotolerans can inhibit the expression of various inflammatory factors in intestines of piglets by suppressed the activation of the toll-like receptor (TLR)2/TLR4-myeloid differentiation factor 88 (MyD88)-nuclear transcription factor-κB (NF-κB) pathway. Xie et al. (107) demonstrated that dietary inclusion of a combination of L. acidophilus and B. subtilis upregulated the expression of IL-22 in the ileum mucosa of piglets. In an E. coli-infected IPEC-1 model, Ji et al. (69) showed that B. amyloliquefaciens can inhibit the mRNA expression of IL-1α, IL-6, IL-8, and TNF-α by suppression of mitogen-activated protein kinase (MAPK) signaling pathways. In addition, Bacillus spp. can promote intestinal immune function by promoting the secretion of intestinal secretory immunoglobulin A (sIgA), which is also an important mechanism for suppressing excessive immune responses and inflammation (44, 77, 80, 107).
Bacillus spp. inhibits pathogenic bacteria and regulates intestinal flora homeostasis
The gastrointestinal tract of animals is inhabited by a large number of microorganisms, and these microorganisms and their metabolites play an important role in host health in terms of nutrition, intestinal barrier and immunity through interaction with intestinal mucosa (125, 126). Changes in diet, environment, and other factors impact (3, 87). The optimization of intestinal microbial structure of piglets by nutritional strategies is one of the current research hotspots (127–129). Among which, the use of probiotics including Bacillus spp. have a significant effect on the regulation of intestinal microbial homeostasis in piglets (76, 130–133). For example, Wang et al. (36) showed that dietary supplementation with B. subtilis and B. licheniformis mixture decreased the abundance of Blautia and Clostridium, while increased the abundances of Bacteroidetes and Ruminococcaceae. Hu et al. (46), Wang et al. (52), and Li et al. (77) showed that piglets fed Bacillus spp. significantly increased the number of intestinal Lactobacillus and decreased the number of E. coli. In which, Bacteroidetes is benefits for the degradation of proteins and carbohydrates (134), and the activation of host’s immune system (135). Ruminococcaceae are associated with energy production and can ferment cellulose and hemicellulose to produce SCFAs (136). Clostridium is associated with diarrhea, and high abundance of intestinal Clostridium increases the risk of diarrhea in piglets (137). The abnormal increase of E. coli abundance can cause intestinal oxidative damage, reduce immune function and destroy intestinal integrity, making it a primary pathogen causing diarrhea of piglets (77, 138). Lactobacillus plays an important role in intestinal health, such as preventing diarrhea and intestinal infections, so it is considered to be a beneficial bacterium to maintain the balance of intestinal flora (46). Previous studies shown that an important sign of piglet diarrhea is primarily distinguished by the increased number of E. coli and the decreased number of Lactobacillus in intestines (46, 77, 138).
Therefore, the potential mechanisms by which Bacillus spp. modulate the gut microbial composition of piglets involve the promotion of beneficial bacterial proliferation and the suppression of pathogenic bacterial growth. On one hand, Bacillus spp. competes with opportunistic pathogens for adhesion sites and nutrients, thereby inhibiting the attachment and colonization of harmful microbes in the intestinal tract (139). On the other hand, Bacillus spp. ferments carbohydrates to produce a large amount of L-lactic acid, which reduces intestinal pH value, forms an anaerobic acidic environment conducive to the growth of beneficial bacteria such as Lactobacillus and bifidobacterium, and prevents the invasion of aerobic and eosinophilic pathogens (140, 141). In addition, Bacillus spp. secretes a variety of antibacterial substances, such as MUCs, AMPs, sIgA, and SCFAs, which exhibit marked antagonistic activity against various pathogens, thereby regulating the balance of intestinal microbiota (80, 107, 118).
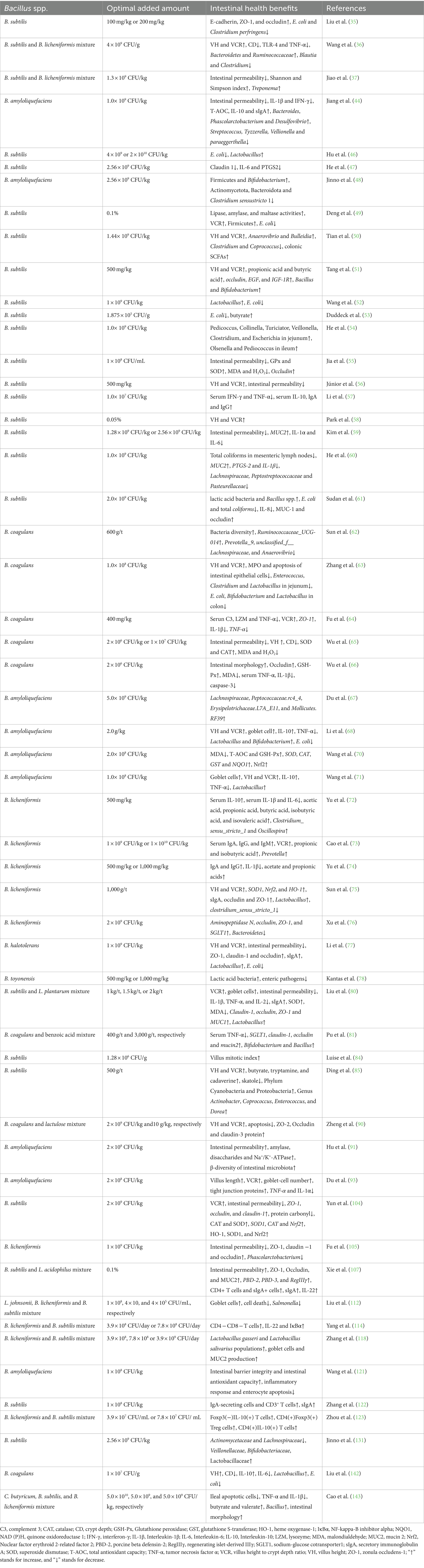
The beneficial effects of Bacillus spp. on the intestinal health of piglets are summarized in Table 2. A review of these literatures revealed that Bacillus spp. exert a positive influence on intestinal health of piglets. Firstly, Bacillus spp. facilitates intestinal development and reduces gut permeability in piglets. Secondly, Bacillus spp. enhances the function of the intestinal mucosal barrier by upregulating tight junction proteins and stimulating MUCs secretion. Thirdly, Bacillus spp. enhances intestinal immunity by activating immune cells within the gut, modulating the secretion of both pro-inflammatory and anti-inflammatory cytokines, and increasing the production of secretory immunoglobulins. Lastly, Bacillus spp. helps maintain a dynamic balance of the intestinal microbiota by encouraging the growth of beneficial microorganisms and suppressing the proliferation of pathogenic bacteria.
Numerous studies have shown that a healthy gut can promote the digestion and absorption of nutrients, thus promoting animal growth (3). Analysis of the existing literatures found that Bacillus spp. had good regulatory effects on the intestinal tract of piglets which can promote intestinal development, promote intestinal mucosal barrier, promote intestinal immune function, inhibit inflammatory response, inhibit pathogenic bacteria and regulate intestinal flora homeostasis. Moreover, most of the Bacillus can promote the growth of piglets. Given the close connection between intestinal health and animal growth, we can speculate that Bacillus spp. promotes growth by promoting intestinal health of piglets.
Potential risks and concerns of Bacillus spp. as probiotics
Over the past decade, concerns about the overuse of antibiotics have shifted attention to probiotics in the animal feed industry as they can improve growth performance and reduce disease risk (32, 144). The spore-forming Bacillus spp. has received extensive scientific and commercial attention, and their beneficial effects have been widely reported and acknowledged (32, 41). However, with a better understanding of their positive role, many questions have been raised about their safety and the relevance of spore formation in the practical application of this class of microorganisms. The first is the safety concerns of the Bacillus spp. Some strains of Bacillus can cause infections such as bacteremia and endocarditis when they enter the bloodstream (27, 41). For example, A study by Deng et al. (145) who had collected 50 commercial probiotic products and isolated bacillus from the products, which showed that 34 probiotic products (68%) exhibited hemolysis, including 19 human probiotics, 9 animal probiotics, and 6 plant probiotics. 19 of 28 B. cereus isolates maintained to exhibit hemolysis after heat treatment. Secondly, pathogenic potential. Some species within the Bacillus genus are known to be pathogenic. For example, Bacillus cereus is notorious for causing food poisoning, which causes great harm to food safety and animal health by producing enterotoxin and vomitoxin (146, 147). For example, Li et al. (146) showed that piglets received Bacillus cereus caused diarrhea, weight loss, and reduced IgG titers of swine fever virus (CSFV) and porcine epidemic diarrhea (PED). The potential for probiotic strains to switch from a beneficial to a pathogenic state is a concern. The third risk factor is antimicrobial and antibiotic resistance issues. There is a worry that the use of Bacillus spp. as probiotics could contribute to the spread of antibiotic resistance genes, particularly in the context of their extensive use in the food industry and as a biological control agent in agriculture (41, 148). For example, Deng et al. (145) showed that all 48 Bacillus spp. isolates exhibited resistance to lincomycin, and 5 were resistant to tetracycline. Zhu et al. (149) evaluated the safety of 15 strains of Bacillus cereus and found that nearly half of the strains carried the antimicrobial resistance gene tet (45). In one strain, tet (45) is located on a mobile genetic element that encodes site-specific recombination mechanisms and is transferred to Staphylococcus aureus and B. subtilis by electrical transformation. Zhai et al. (150) tested the antimicrobial resistance and antibiotic resistance of 114 isolates of Bacillus spp., and the antibiotic susceptibility tests showed that the intrinsic resistance rates of Bacillus to ampicillin and penicillin were 80 and 86%, respectively. Bacillus strains with acquired antibiotic resistance may spread antibiotic resistance between Bacillus and other clinical pathogens through horizontal gene transfer. Fourth, strain-specificity concerns. As probiotics, the action of Bacillus spp. appears to be strain-specific, which means that not all strains will have the same benefits or risks. This adds a layer of complexity to their use and regulation (41). Fifth, lack of standardization. There is a lack of standardization in the production and use of Bacillus spp. probiotics, which can lead to variability in efficacy and safety (27). Lastly, regulatory challenges. The regulatory framework for probiotics, including Bacillus spp., varies by country and is not always clear, leading to challenges in ensuring the safety and quality of probiotic products (27). In conclusion, Bacillus spp. has advantages as well as challenges as an animal probiotic, and safety evaluation should be conducted when using the newly isolated Bacillus spp.
Conclusion
The use of Bacillus spp. as probiotics in piglets offers a promising approach to promote growth and enhance intestinal health. The mechanisms by which Bacillus spp. exert their beneficial effects include improving growth performance, enhancing intestinal mucosal barrier function, improving intestinal immune function and producing antimicrobial compounds, as well as modulating gut microbiota. Further research is needed to identify the most effective strains and optimal application strategies to maximize the benefits of Bacillus spp. as probiotics in piglets.
Author contributions
XT: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. YZ: Writing – original draft, Formal analysis. KX: Writing – review & editing, Funding acquisition. JZ: Writing – review & editing, Software, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by grants from the Major Special Project of Provincial Science and Technology Program of Guizhou (No. 5411 2017 QKHPTRC); Guizhou Provincial Science and Technology Foundation (No. 267 2023 Qiankehe Jichu-ZK Yiban); China Overseas Expertise Introduction Program for Discipline Innovation (D17016); and Guizhou Normal University Academic New Seedling Fund project (No. B16 2021Qianshi Xinmiao).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Upadhaya, SD, and Kim, IH. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets-a review. Animals. (2021) 11:2418. doi: 10.3390/ani11082418
2. Su, W, Li, Z, Gong, T, Wang, F, Jin, M, Wang, Y, et al. An alternative ZnO with large specific surface area: preparation, physicochemical characterization and effects on growth performance; diarrhea, zinc metabolism and gut barrier function of weaning piglets. Sci Total Environ. (2023) 882:163558. doi: 10.1016/j.scitotenv.2023.163558
3. Tang, X, Xiong, K, Fang, R, and Li, M. Weaning stress and intestinal health of piglets: a review. Front Immunol. (2022) 13:1042778. doi: 10.3389/fimmu.2022.1042778
4. Barton, MD . Impact of antibiotic use in the swine industry. Curr Opin Microbiol. (2014) 19:9–15. doi: 10.1016/j.mib.2014.05.017
5. Hu, Q, Liu, C, Zhang, D, Wang, R, Qin, L, Xu, Q, et al. Effects of low-dose antibiotics on gut immunity and antibiotic Resistomes in weaned piglets. Front Immunol. (2022) 11:903. doi: 10.3389/fimmu.2020.00903
6. Ming, D, Wang, J, Yin, C, Chen, Y, Li, Y, Sun, W, et al. Porous zinc oxide and plant polyphenols as a replacement for high-dose zinc oxide on growth performance, diarrhea incidence, intestinal morphology and microbial diversity of weaned piglets. Animals. (2024) 14:523. doi: 10.3390/ani14030523
7. Schokker, D, Kar, SK, Willems, E, Bossers, A, Dekker, RA, and Jansman, AJM. Dietary supplementation of zinc oxide modulates intestinal functionality during the post-weaning period in clinically healthy piglets. J Anim Sci Biotechnol. (2023) 14:122. doi: 10.1186/s40104-023-00925-1
8. Peng, P, Deng, D, Chen, S, Li, C, Luo, J, Romeo, A, et al. The effects of dietary porous zinc oxide supplementation on growth performance, inflammatory cytokines and tight Junction's gene expression in early-weaned piglets. J Nutr Sci Vitaminol. (2020) 66:311–8. doi: 10.3177/jnsv.66.311
9. Wang, L, Wang, C, Peng, Y, Zhang, Y, Liu, Y, Liu, Y, et al. Research progress on anti-stress nutrition strategies in swine. Anim Nutr. (2023) 13:342–60. doi: 10.1016/j.aninu.2023.03.006
10. Zhang, M, Yang, Z, Wu, G, Xu, F, Zhang, J, Luo, X, et al. Effects of probiotic-fermented feed on the growth profile, immune functions, and intestinal microbiota of Bamei piglets. Animals. (2024) 14:647. doi: 10.3390/ani14040647
11. Lei, XJ, Liu, ZZ, Park, JH, and Kim, IH. Novel zinc sources as antimicrobial growth promoters for monogastric animals: a review. J Anim Sci Technol. (2022) 64:187–96. doi: 10.5187/jast.2022.e1
12. Ekhlas, D, Sanjuán, JMO, Manzanilla, EG, Leonard, FC, Argüello, H, and Burgess, CM. Comparison of antimicrobial resistant Escherichia coli isolated from Irish commercial pig farms with and without zinc oxide and antimicrobial usage. Gut Pathog. (2023) 15:8. doi: 10.1186/s13099-023-00534-3
13. Bonetti, A, Tugnoli, B, Piva, A, and Grilli, E. Towards zero zinc oxide: feeding strategies to manage post-weaning diarrhea in piglets. Animals. (2021) 11:642. doi: 10.3390/ani11030642
14. Liang, C, Fu, R, Chen, D, Tian, G, He, J, Zheng, P, et al. Effects of mixed fibres and essential oils blend on growth performance and intestinal barrier function of piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Physiol Anim Nutr. (2023) 107:1356–67. doi: 10.1111/jpn.13866
15. Ma, J, Piao, X, Shang, Q, Long, S, Liu, S, and Mahfuz, S. Mixed organic acids as an alternative to antibiotics improve serum biochemical parameters and intestinal health of weaned piglets. Anim Nutr. (2021) 7:737–49. doi: 10.1016/j.aninu.2020.11.018
16. Watthanaphansak, S, and Kim, HB. Gut microbial shifts by synbiotic combination of Pediococcus acidilactici and lactulose in weaned piglets challenged with Shiga toxin-producing Escherichia coli. Front Vet Sci. (2023) 9:1101869. doi: 10.3389/fvets.2022.1101869
17. Xu, X, Chang, J, Wang, P, Liu, C, Liu, M, Zhou, T, et al. Combination of glycyrrhizic acid and compound probiotics alleviates deoxynivalenol-induced damage to weaned piglets. Ecotoxicol Environ Saf. (2023) 256:114901. doi: 10.1016/j.ecoenv.2023.114901
18. Ali, MS, Lee, EB, Hsu, WH, Suk, K, Sayem, SAJ, Ullah, HMA, et al. Probiotics and Postbiotics as an alternative to antibiotics: an emphasis on pigs. Pathogens. (2023) 12:874. doi: 10.3390/pathogens12070874
19. Vieira, AM, Sessin, AP, Soratto, TAT, Pires, PGDS, Cardinal, KM, Wagner, G, et al. Effect of functional oils or probiotics on performance and microbiota profile of newly weaned piglets. Sci Rep. (2021) 11:19457. doi: 10.1038/s41598-021-98549-w
20. Yang, J, Qian, K, Wang, C, and Wu, Y. Roles of probiotic lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob Proteins. (2018) 10:243–50. doi: 10.1007/s12602-017-9273-y
21. Azizi, AFN, Uemura, R, Omori, M, Sueyoshi, M, and Yasuda, M. Effects of probiotics on growth and immunity of piglets. Animals. (2022) 12:1786. doi: 10.3390/ani12141786
22. Grandmont, A, Rhouma, M, Létourneau-Montminy, MP, Thériault, W, Mainville, I, Arcand, Y, et al. Characterization of the effects of a novel probiotic on Salmonella colonization of a piglet-derived intestinal microbiota using improved bioreactor. Animals. (2024) 14:787. doi: 10.3390/ani14050787
23. Tang, X, Liu, X, and Liu, H. Effects of dietary probiotic (Bacillus subtilis) supplementation on carcass traits, meat quality, amino acid, and fatty acid profile of broiler chickens. Front Vet Sci. (2021) 8:767802. doi: 10.3389/fvets.2021.767802
24. Li, CL, Wang, J, Zhang, HJ, Wu, SG, Hui, QR, Yang, CB, et al. Qi GH. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front Physiol. (2019) 9:1968. doi: 10.3389/fphys.2018.01968
25. Poveda, J, and González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl Microbiol Biotechnol. (2021) 105:8629–45. doi: 10.1007/s00253-021-11492-8
26. Polonca, S . Environment shapes the intra-species diversity of Bacillus subtilis isolates. Microb Ecol. (2020) 79:853–64. doi: 10.1007/s00248-019-01455-y
27. Todorov, SD, Ivanova, IV, Popov, I, Weeks, R, and Chikindas, ML. Bacillus spore-forming probiotics: benefits with concerns? Crit Rev Microbiol. (2022) 48:513–30. doi: 10.1080/1040841X.2021.1983517
28. Contesini, FJ, Melo, RR, and Sato, HH. An overview of Bacillus proteases: from production to application. Crit Rev Biotechnol. (2018) 38:321–34. doi: 10.1080/07388551.2017.1354354
29. Adeniji, AA, Loots, DT, and Babalola, OO. Bacillus velezensis: phylogeny, useful applications, and avenues for exploitation. Appl Microbiol Biotechnol. (2019) 103:3669–82. doi: 10.1007/s00253-019-09710-5
30. Gupta, AK, and Maity, C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: a prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT compliant]. Medicine. (2021) 100:e23641. doi: 10.1097/MD.0000000000023641
31. Cutting, SM . Bacillus probiotics. Food Microbiol. (2011) 28:214–20. doi: 10.1016/j.fm.2010.03.007
32. Khalid, F, Khalid, A, Fu, Y, Hu, Q, Zheng, Y, Khan, S, et al. Potential of Bacillus velezensis as a probiotic in animal feed: a review. J Microbiol. (2021) 59:627–33. doi: 10.1007/s12275-021-1161-1
33. Amerah, AM, Quiles, A, Medel, P, Sánchez, J, Lehtinen, MJ, and Gracia, MI. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim Feed Sci Technol. (2013) 180:55–63. doi: 10.1016/j.anifeedsci.2013.01.002
34. Dong, W, Gong, X, Zheng, F, Liu, Y, Zhang, L, Zhang, X, et al. Isolation, screening and biological characteristics of Bacillus licheniformis from pigs. J Gansu Agric Univ. (2021) 56:8–17. doi: 10.13432/j.cnki.jgsau.2021.05.002
35. Liu, J, Ma, X, Zhuo, Y, Xu, S, Hua, L, Li, J, et al. The effects of Bacillus subtilis QST713 and β-mannanase on growth performance, intestinal barrier function, and the gut microbiota in weaned piglets. J Anim Sci. (2023) 101:skad 257. doi: 10.1093/jas/skad257
36. Wang, X, Tian, Z, Azad, MAK, Zhang, W, Blachier, F, Wang, Z, et al. Dietary supplementation with Bacillus mixture modifies the intestinal ecosystem of weaned piglets in an overall beneficial way. J Appl Microbiol. (2021) 130:233–46. doi: 10.1111/jam.14782
37. Jiao, S, Zheng, Z, Zhuang, Y, Tang, C, and Zhang, N. Dietary medium-chain fatty acid and Bacillus in combination alleviate weaning stress of piglets by regulating intestinal microbiota and barrier function. J Anim Sci. (2023) 101:skac 414. doi: 10.1093/jas/skac414
38. Huting, AMS, Lagos, LV, Hansen, LHB, and Molist, F. Effect of supplementing a Bacillus multi-strain probiotic to a post-weaning diet on nutrient utilisation and nitrogen retention of piglets. Animals. (2023) 13:3597. doi: 10.3390/ani13233597
39. Sudan, S, Zhan, X, and Li, J. A novel probiotic Bacillus subtilis strain confers Cytoprotection to host pig intestinal epithelial cells during Enterotoxic Escherichia coli infection. Microbiol Spectr. (2022) 10:e0125721. doi: 10.1128/spectrum.01257-21
40. Liu, Y, Yue, Z, Sun, Z, and Li, C. Harnessing native Bacillus spp. for sustainable wheat production. Appl Environ Microbiol. (2023) 89:e0124722. doi: 10.1128/aem.01247-22
41. Elshaghabee, FMF, Rokana, N, Gulhane, RD, Sharma, C, and Panwar, H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol. (2017) 8:1490. doi: 10.3389/fmicb.2017.01490
42. Campbell, JM, Crenshaw, JD, and Polo, J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. (2013) 4:19. doi: 10.1186/2049-1891-4-19
43. Heo, JM, Opapeju, FO, Pluske, JR, Kim, JC, Hampson, DJ, and Nyachoti, CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. (2013) 97:207–37. doi: 10.1111/j.1439-0396.2012.01284.x
44. Jiang, Z, Su, W, Li, W, Wen, C, Du, S, He, H, et al. Bacillus amyloliquefaciens 40 regulates piglet performance, antioxidant capacity, immune status and gut microbiota. Anim Nutr. (2022) 12:116–27. doi: 10.1016/j.aninu.2022.09.006
45. Hu, J, and Kim, IH. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, nutrient digestibility, diarrhea score, intestinal microbiota, and excreta odor contents in weanling piglets. Animals. (2022) 12:316. doi: 10.3390/ani12030316
46. Hu, Y, Dun, Y, Li, S, Zhao, S, Peng, N, and Liang, Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and Faecal bacterial Flora of weaned piglets. Asian Australas J Anim Sci. (2014) 27:1131–40. doi: 10.5713/ajas.2013.13737
47. He, Y, Kim, K, Kovanda, L, Jinno, C, Song, M, Chase, J, et al. Bacillus subtilis: a potential growth promoter in weaned pigs in comparison to carbadox. J Anim Sci. (2020) 98:skaa 290. doi: 10.1093/jas/skaa290
48. Jinno, C, Wong, B, Klünemann, M, Htoo, J, Li, X, and Liu, Y. Effects of supplementation of Bacillus amyloliquefaciens on performance, systemic immunity, and intestinal microbiota of weaned pigs experimentally infected with a pathogenic enterotoxigenic E. coli F18. Front Microbiol. (2023) 14:1101457. doi: 10.3389/fmicb.2023.1101457
49. Deng, B, Wu, J, Li, X, Zhang, C, Men, X, and Xu, Z. Effects of Bacillus subtilis on growth performance, serum parameters, digestive enzyme, intestinal morphology, and colonic microbiota in piglets. AMB Express. (2020) 10:212. doi: 10.1186/s13568-020-01150-z
50. Tian, Z, Wang, X, Duan, Y, Zhao, Y, Zhang, W, Azad, MAK, et al. Dietary supplementation with Bacillus subtilis promotes growth and gut health of weaned piglets. Front Vet Sci. (2021) 7:600772. doi: 10.3389/fvets.2020.600772
51. Tang, W, Qian, Y, Yu, B, Zhang, T, Gao, J, He, J, et al. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets 1. J Anim Sci. (2019) 97:2125–38. doi: 10.1093/jas/skz090
52. Wang, H, Kim, KP, and Kim, IH. Influence of Bacillus subtilis GCB-13-001 on growth performance, nutrient digestibility, blood characteristics, faecal microbiota and faecal score in weanling pigs. J Anim Physiol Anim Nutr. (2019) 103:1919–25. doi: 10.1111/jpn.13199
53. Duddeck, KA, Petersen, TE, Adkins, HJ, Smith, AH, Hernandez, S, Wenner, SJ, et al. Dose-dependent effects of supplementing a two-strain Bacillus subtilis probiotic on growth performance, blood parameters, fecal metabolites, and microbiome in nursery pigs. Animals. (2023) 14:109. doi: 10.3390/ani14010109
54. He, F, Jin, X, Sun, K, Zhao, L, Yang, W, Zhang, X, et al. Bacillus subtilis JATP-3 improves nitrogen metabolism by regulating intestinal Flora and AKG in weaned piglets. Probiotics Antimicrob Proteins. (2023). doi: 10.1007/s12602-023-10196-x [Epub ahead of print].
55. Jia, R, Sadiq, FA, Liu, W, Cao, L, and Shen, Z. Protective effects of Bacillus subtilis ASAG 216 on growth performance, antioxidant capacity, gut microbiota and tissues residues of weaned piglets fed deoxynivalenol contaminated diets. Food Chem Toxicol. (2021) 148:111962. doi: 10.1016/j.fct.2020.111962
56. Júnior, DTV, de Amorim, RG, Soares, MH, Silva, CB, Frank, EO, Gonzalez-Vega, JC, et al. Supplementation of Bacillus subtilis DSM 32540 improves performance and intestinal health of weaned pigs fed diets containing different fiber sources. Livest Sci. (2023) 270:105202. doi: 10.1016/j.livsci.2023.105202
57. Li, H, Jiang, X, and Qiao, J. Effect of dietary Bacillus subtilis on growth performance and serum biochemical and immune indexes in weaned piglets. J Appl Anim Res. (2021) 49:83–8. doi: 10.1080/09712119.2021.1877717
58. Park, S, Lee, JW, Jerez Bogota, K, Francis, D, González-Vega, JC, Htoo, JK, et al. Growth performance and gut health of Escherichia coli-challenged weaned pigs fed diets supplemented with a Bacillus subtilis direct-fed microbial. Transl. Anim Sci. (2020) 4:txaa 172. doi: 10.1093/tas/txaa172
59. Kim, K, He, Y, Xiong, X, Ehrlich, A, Li, X, Raybould, H, et al. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J Anim Sci Biotechnol. (2019) 10:52. doi: 10.1186/s40104-019-0364-3
60. He, Y, Jinno, C, Kim, K, Wu, Z, Tan, B, Li, X, et al. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J Anim Sci Biotechnol. (2020) 11:101. doi: 10.1186/s40104-020-00498-3
61. Sudan, S, Fletcher, L, Zhan, X, Dingle, S, Patterson, R, Huber, LA, et al. Comparative efficacy of a novel Bacillus subtilis-based probiotic and pharmacological zinc oxide on growth performance and gut responses in nursery pigs. Sci Rep. (2023) 13:4659. doi: 10.1038/s41598-023-31913-0
62. Sun, T, Miao, H, Zhang, C, Wang, Y, Liu, S, Jiao, P, et al. Effect of dietary Bacillus coagulans on the performance and intestinal microbiota of weaned piglets. Animal. (2022) 16:100561. doi: 10.1016/j.animal.2022.100561
63. Zhang, Y, Tian, X, Dong, Y, Li, R, Shen, M, Yi, D, et al. Bacillus coagulans prevents the decline in average daily feed intake in young piglets infected with enterotoxigenic Escherichia coli K88 by reducing intestinal injury and regulating the gut microbiota. Front Cell Infect Microbiol. (2023) 13:1284166. doi: 10.3389/fcimb.2023.1284166
64. Fu, R, Liang, C, Chen, D, Yan, H, Tian, G, Zheng, P, et al. Effects of dietary Bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets. J Anim Physiol Anim Nutr. (2021) 105:898–907. doi: 10.1111/jpn.13529
65. Wu, T, Zhang, Y, Lv, Y, Li, P, Yi, D, Wang, L, et al. Beneficial impact and molecular mechanism of Bacillus coagulans on Piglets' intestine. Int J Mol Sci. (2018) 19:2084. doi: 10.3390/ijms19072084
66. Wu, T, Zhang, Q, Xu, H, Li, P, Zhao, D, Wang, L, et al. Protective effects of α-terpineol and Bacillus coagulans on intestinal function in weaned piglets infected with a recombinant Escherichia coli expressing heat-stable enterotoxin STa. Front Vet Sci. (2023) 10:1118957. doi: 10.3389/fvets.2023.1118957
67. Du, H, Yao, W, Kulyar, MF, Ding, Y, Zhu, H, Pan, H, et al. Effects of Bacillus amyloliquefaciens TL106 isolated from Tibetan pigs on probiotic potential and intestinal microbes in weaned piglets. Microbiol Spectr. (2022) 10:e0120521. doi: 10.1128/spectrum.01205-21
68. Li, Y, Zhang, H, Su, W, Ying, Z, Chen, Y, Zhang, L, et al. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J Anim Sci Biotechnol. (2018) 9:22. doi: 10.1186/s40104-018-0236-2
69. Ji, J, Hu, S, Zheng, M, Du, W, Shang, Q, and Li, W. Bacillus amyloliquefaciens SC06 inhibits ETEC-induced pro-inflammatory responses by suppression of MAPK signaling pathways in IPEC-1 cells and diarrhea in weaned piglets. Livest Sci. (2013) 158:206–14. doi: 10.1016/j.livsci.2013.09.017
70. Wang, Y, Wu, Y, Wang, B, Cao, X, Fu, A, Li, Y, et al. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. AMB Express. (2017) 7:52. doi: 10.1186/s13568-017-0353-x
71. Wang, Q, Wang, F, Tang, L, Wang, Y, Zhou, Y, Li, X, et al. Bacillus amyloliquefaciens SC06 alleviated intestinal damage induced by inflammatory via modulating intestinal microbiota and intestinal stem cell proliferation and differentiation. Int Immunopharmacol. (2024) 130:111675. doi: 10.1016/j.intimp.2024.111675
72. Yu, X, Cui, Z, Qin, S, Zhang, R, Wu, Y, Liu, J, et al. Effects of Bacillus licheniformis on growth performance, diarrhea incidence, antioxidant capacity, immune function, and fecal microflora in weaned piglets. Animals. (2022) 12:1609. doi: 10.3390/ani12131609
73. Cao, G, Yang, S, Wang, H, Zhang, R, Wu, Y, Liu, J, et al. Effects of Bacillus licheniformis on the growth performance, antioxidant capacity, Ileal morphology, intestinal short chain fatty acids, and colonic microflora in piglets challenged with lipopolysaccharide. Animals. (2023) 13:2172. doi: 10.3390/ani13132172
74. Yu, X, Dai, Z, Cao, G, Cui, Z, Zhang, R, Xu, Y, et al. Protective effects of Bacillus licheniformis on growth performance, gut barrier functions, immunity and serum metabolome in lipopolysaccharide-challenged weaned piglets. Front Immunol. (2023) 14:1140564. doi: 10.3389/fimmu.2023.1140564
75. Sun, W, Chen, W, Meng, K, Cai, L, Li, G, Li, X, et al. Dietary supplementation with probiotic Bacillus licheniformis S6 improves intestinal integrity via modulating intestinal barrier function and microbial diversity in weaned piglets. Biology. (2023) 12:238. doi: 10.3390/biology12020238
76. Xu, H, Gong, J, Lu, P, Azevedo, P, Li, L, Yu, H, et al. Functional evaluation of Bacillus licheniformis PF9 for its potential in controlling enterotoxigenic Escherichia coli in weaned piglets. Transl Anim Sci. (2024) 8:txae 050. doi: 10.1093/tas/txae050
77. Li, M, Zhao, D, Guo, J, Pan, T, Niu, T, Jiang, Y, et al. Bacillus halotolerans SW207 alleviates enterotoxigenic Escherichia coli-induced inflammatory responses in weaned piglets by modulating the intestinal epithelial barrier, the TLR4/MyD88/NF-κB pathway, and intestinal microbiota. Microbiol Spectr. (2024) 12:e0398823. doi: 10.1128/spectrum.03988-23
78. Kantas, D, Papatsiros, VG, Tassis, PD, Giavasis, I, Bouki, P, and Tzika, ED. A feed additive containing Bacillus toyonensis (Toyocerin (®)) protects against enteric pathogens in postweaning piglets. J Appl Microbiol. (2015) 118:727–38. doi: 10.1111/jam.12729
79. Papatsiros, VG, Tassis, PD, Tzika, ED, Papaioannou, DS, Petridou, E, Alexopoulos, C, et al. Effect of benzoic acid and combination of benzoic acid with a probiotic containing Bacillus cereus var. Toyoi in weaned pig nutrition. Pol J Vet Sci. (2011) 14:117–25. doi: 10.2478/v10181-011-0017-8
80. Liu, Y, Gu, W, Liu, X, Zou, Y, Wu, Y, Xu, Y, et al. Joint application of Lactobacillus plantarum and Bacillus subtilis improves growth performance, immune function and intestinal integrity in weaned piglets. Vet Sci. (2022) 9:668. doi: 10.3390/vetsci9120668
81. Pu, J, Chen, D, Tian, G, He, J, Zheng, P, Mao, X, et al. Effects of benzoic acid, Bacillus coagulans and oregano oil combined supplementation on growth performance, immune status and intestinal barrier integrity of weaned piglets. Anim Nutr. (2020) 6:152–9. doi: 10.1016/j.aninu.2020.02.004
82. Phaengphairee, P, Boontiam, W, Wealleans, A, Hong, J, and Kim, YY. Dietary supplementation with full-fat Hermetia illucens larvae and multi-probiotics, as a substitute for antibiotics, improves the growth performance, gut health, and antioxidative capacity of weaned pigs. BMC Vet Res. (2023) 19:7. doi: 10.1186/s12917-022-03550-8
83. Kritas, SK, and Morrison, RB. Evaluation of probiotics as a substitute for antibiotics in a large pig nursery. Vet Rec. (2005) 156:447–8. doi: 10.1136/vr.156.14.447
84. Luise, D, Bertocchi, M, Motta, V, Salvarani, C, Bosi, P, Luppi, A, et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J Anim Sci Biotechnol. (2019) 10:74. doi: 10.1186/s40104-019-0380-3
85. Ding, H, Zhao, X, Azad, MAK, Ma, C, Gao, Q, He, J, et al. Dietary supplementation with Bacillus subtilis and xylo-oligosaccharides improves growth performance and intestinal morphology and alters intestinal microbiota and metabolites in weaned piglets. Food Funct. (2021) 12:5837–49. doi: 10.1039/d1fo00208b
86. Shin, D, Chang, SY, Bogere, P, Won, K, Choi, JY, Choi, YJ, et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS One. (2019) 14:e0220843. doi: 10.1371/journal.pone.0220843
87. Tang, X . Probiotic roles of Clostridium butyricum in piglets: considering aspects of intestinal barrier function. Animals. (2024) 14:1069. doi: 10.3390/ani14071069
88. Tang, W, Liu, J, Ma, Y, Wei, Y, Liu, J, and Wang, H. Impairment of intestinal barrier function induced by early weaning via autophagy and apoptosis associated with gut microbiome and metabolites. Front Immunol. (2021) 12:804870. doi: 10.3389/fimmu.2021.804870
89. Wu, Z, Yan, Y, Li, W, Li, Y, and Yang, H. Expression profile of mi R-199a and its role in the regulation of intestinal inflammation. Animals. (2023) 13:1979. doi: 10.3390/ani13121979
90. Zheng, W, Zhao, Z, Yang, Y, Ding, L, and Yao, W. The synbiotic mixture of lactulose and Bacillus coagulans protects intestinal barrier dysfunction and apoptosis in weaned piglets challenged with lipopolysaccharide. J Anim Sci Biotechnol. (2023) 14:80. doi: 10.1186/s40104-023-00882-9
91. Hu, S, Cao, X, Wu, Y, Mei, X, Xu, H, Wang, Y, et al. Effects of probiotic Bacillus as an alternative of antibiotics on digestive enzymes activity and intestinal integrity of piglets. Front Microbiol. (2018) 9:2427. doi: 10.3389/fmicb.2018.02427
92. Hu, L, Peng, X, Chen, H, Yan, C, Liu, Y, Xu, Q, et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr. (2017) 56:1753–65. doi: 10.1007/s00394-016-1223-z
93. Du, W, Xu, H, Mei, X, Cao, X, Gong, L, Wu, Y, et al. Probiotic Bacillus enhance the intestinal epithelial cell barrier and immune function of piglets. Benef Microbes. (2018) 9:743–54. doi: 10.3920/BM2017.0142
94. Latorre, JD, Hernandez-Velasco, X, Wolfenden, RE, Vicente, JL, Wolfenden, AD, Menconi, A, et al. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front Vet Sci. (2016) 3:95. doi: 10.3389/fvets.2016.00095
95. Parrado, J, Rodriguez-Morgado, B, Tejada, M, Hernandez, T, and Garcia, C. Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzym Microb Technol. (2014) 57:1–7. doi: 10.1016/j.enzmictec.2014.01.001
96. Olmos, SJ . Bacillus probiotic enzymes: external auxiliary apparatus to avoid digestive deficiencies, water pollution, diseases, and economic problems in marine cultivated animals. Adv Food Nutr Res. (2017) 80:15–35. doi: 10.1016/bs.afnr.2016.11.001
97. Akhtar, N, Cai, HY, Kiarie, EG, and Li, J. A novel Bacillus sp. with rapid growth property and high enzyme activity that allows efficient fermentation of soybean meal for improving digestibility in growing pigs. J Appl Microbiol. (2022) 133:3–17. doi: 10.1111/jam.15268
98. Vojnovic, S, Aleksic, I, Ilic-Tomic, T, Stevanovic, M, and Nikodinovic-Runic, J. Bacillus and Streptomyces spp. as hosts for production of industrially relevant enzymes. Appl Microbiol Biotechnol. (2024) 108:185. doi: 10.1007/s00253-023-12900-x
99. Cai, L, Indrakumar, S, Kiarie, E, and Kim, IH. Effects of a multi-strain Bacillus species-based direct-fed microbial on growth performance, nutrient digestibility, blood profile, and gut health in nursery pigs fed corn-soybean meal-based diets. J Anim Sci. (2015) 93:4336–42. doi: 10.2527/jas.2015-9056
100. Lewton, JR, Woodward, AD, Moser, RL, Thelen, KM, Moeser, AJ, Trottier, NL, et al. Effects of a multi-strain Bacillus subtilis-based direct-fed microbial on weanling pig growth performance and nutrient digestibility. Transl Anim Sci. (2021) 5:txab 058. doi: 10.1093/tas/txab058
101. Cui, K, Lv, X, Diao, Q, and Zhang, N. Effects of dietary supplementation with Bacillus subtilis and yeast culture on growth performance, nutrient digestibility, serum indices and faeces microbiota of weaned piglet. J Anim Feed Sci. (2019) 28:328–36. doi: 10.22358/jafs/114238/2019
102. Cao, S, Zhang, Q, Wang, C, Wu, H, Jiao, L, Hong, Q, et al. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets. Innate Immun. (2018) 24:221–30. doi: 10.1177/1753425918769372
103. Che, L, Xu, Q, Wu, C, Luo, Y, Huang, X, Zhang, B, et al. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br J Nutr. (2017) 118:949–58. doi: 10.1017/S0007114517003051
104. Yun, Y, Ji, S, Yu, G, Jia, P, Niu, Y, Zhang, H, et al. Effects of Bacillus subtilis on jejunal integrity, redox status, and microbial composition of intrauterine growth restriction suckling piglets. J Anim Sci. (2021) 99:skab 255. doi: 10.1093/jas/skab255
105. Fu, J, Wang, T, Xiao, X, Cheng, Y, Wang, F, Jin, M, et al. Clostridium Butyricum ZJU-F1 benefits the intestinal barrier function and immune response associated with its modulation of gut microbiota in weaned piglets. Cells. (2021) 10:527. doi: 10.3390/cells10030527
106. Tang, X, and Xiong, K. Effects of epidermal growth factor on glutamine and glucose absorption by IPEC-J2 cells challenged by lipopolysaccharide using the ussing chamber system. Pak J Zool. (2021) 53:417–22. doi: 10.17582/journal.pjz/20200117080156
107. Xie, Z, Li, M, Qian, M, Yang, Z, and Han, X. Co-cultures of Lactobacillus acidophilus and Bacillus subtilis enhance mucosal barrier by modulating gut microbiota-derived short-chain fatty acids. Nutrients. (2022) 14:4475. doi: 10.3390/nu14214475
108. Gu, MJ, Song, SK, Park, SM, Lee, IK, and Yun, CH. Bacillus subtilis protects porcine intestinal barrier from Deoxynivalenol via improved zonula Occludens-1 expression. Asian Australas J Anim Sci. (2014) 27:580–6. doi: 10.5713/ajas.2013.13744
109. Suzuki, T . Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim Sci J. (2020) 91:e13357. doi: 10.1111/asj.13357
110. Zhao, Z, Sun, M, Cui, X, Chen, J, Liu, C, and Zhang, X. Bacillus coagulans MZY531 alleviates intestinal mucosal injury in immunosuppressive mice via modulating intestinal barrier, inflammatory response, and gut microbiota. Sci Rep. (2023) 13:11181. doi: 10.1038/s41598-023-38379-0
111. Zong, X, Wang, TH, Lu, ZQ, Song, DG, Zhao, J, and Wang, YZ. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest Sci. (2019) 220:137–42. doi: 10.1016/j.livsci.2018.12.024
112. Liu, X, Xia, B, He, T, Li, D, Su, JH, Guo, L, et al. Oral Administration of a Select Mixture of Lactobacillus and Bacillus alleviates inflammation and maintains mucosal barrier integrity in the ileum of pigs challenged with Salmonella Infantis. Microorganisms. (2019) 7:135. doi: 10.3390/microorganisms7050135
113. Li, Q, Li, L, Chen, Y, Yu, C, Azevedo, P, Gong, J, et al. Bacillus licheniformis PF9 improves barrier function and alleviates inflammatory responses against enterotoxigenic Escherichia coli F4 infection in the porcine intestinal epithelial cells. J Anim Sci Biotechnol. (2022) 13:86. doi: 10.1186/s40104-022-00746-8
114. Yang, GY, Zhu, YH, Zhang, W, Zhou, D, Zhai, CC, and Wang, JF. Influence of orally fed a select mixture of Bacillus probiotics on intestinal T-cell migration in weaned MUC4 resistant pigs following Escherichia coli challenge. Vet Res. (2016) 47:71. doi: 10.1186/s13567-016-0355-8
115. Yang, S, and Yu, M. Role of goblet cells in intestinal barrier and mucosal immunity. J Inflamm Res. (2021) 14:3171–83. doi: 10.2147/JIR.S318327
116. Gustafsson, JK, and Johansson, MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. (2022) 19:785–803. doi: 10.1038/s41575-022-00675-x
117. Li, G, Gao, M, Zhang, S, Dai, T, Wang, F, Geng, J, et al. Sleep deprivation impairs intestinal mucosal barrier by activating endoplasmic reticulum stress in goblet cells. Am J Pathol. (2024) 194:85–100. doi: 10.1016/j.ajpath.2023.10.004
118. Zhang, W, Zhu, YH, Zhou, D, Wu, Q, Song, D, Dicksved, J, et al. Oral Administration of a Select Mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be Enterotoxigenic E. coli F4ab/ac receptor negative. Appl Environ Microbiol. (2017) 83:e02747–16. doi: 10.1128/AEM.02747-16
119. Xu, J, Jia, Z, Xiao, S, Long, C, and Wang, L. Effects of Enterotoxigenic Escherichia coli challenge on Jejunal morphology and microbial community profiles in weaned crossbred piglets. Microorganisms. (2023) 11:2646. doi: 10.3390/microorganisms11112646
120. Bailey, M, Haverson, K, Inman, C, Harris, C, Jones, P, Corfield, G, et al. The development of the mucosal immune system pre-and post-weaning: balancing regulatory and effector function. Proc Nutr Soc. (2005) 64:451–7. doi: 10.1079/pns2005452
121. Wang, Q, Wang, F, Zhou, Y, Li, X, Xu, S, Jin, Q, et al. Bacillus amyloliquefaciens SC06 relieving intestinal inflammation by modulating intestinal stem cells proliferation and differentiation via AhR/STAT3 pathway in LPS-challenged piglets. J Agric Food Chem. (2024) 72:6096–109. doi: 10.1021/acs.jafc.3c05956
122. Zhang, P, Huang, L, Zhang, E, Yuan, C, and Yang, Q. Oral administration of Bacillus subtilis promotes homing of CD3+ T cells and IgA-secreting cells to the respiratory tract in piglets. Res Vet Sci. (2021) 136:310–7. doi: 10.1016/j.rvsc.2021.03.006
123. Zhou, D, Zhu, YH, Zhang, W, Wang, ML, Fan, WY, Song, D, et al. Oral administration of a select mixture of Bacillus probiotics generates Tr1 cells in weaned F4ab/ac R-pigs challenged with an F4+ ETEC/VTEC/EPEC strain. Vet Res. (2015) 46:95. doi: 10.1186/s13567-015-0223-y
124. Xu, J, Zhong, F, Zhang, Y, Zhang, J, Huo, S, Lin, H, et al. Construction of Bacillus subtilis strain engineered for expression of porcine β-defensin-2/cecropin P 1 fusion antimicrobial peptides and its growth-promoting effect and antimicrobial activity. Asian Australas J Anim Sci. (2017) 30:576–84. doi: 10.5713/ajas.16.0207
125. Park, W . Gut microbiomes and their metabolites shape human and animal health. J Microbiol. (2018) 56:151–3. doi: 10.1007/s12275-018-0577-8
126. Barathan, M, Ng, SL, Lokanathan, Y, Ng, MH, and Law, JX. The profound influence of gut microbiome and extracellular vesicles on animal health and disease. Int J Mol Sci. (2024) 25:4024. doi: 10.3390/ijms25074024
127. Fan, MZ, Cheng, L, Wang, M, Chen, J, Fan, W, Jashari, F, et al. Monomodular and multifunctional processive endocellulases: implications for swine nutrition and gut microbiome. Anim Microbiome. (2024) 6:4. doi: 10.1186/s42523-024-00292-w
128. Galli, GM, Andretta, I, Levesque, C, Stefanello, T, Carvalho, CL, Perez Pelencia, JY, et al. Using probiotics to improve nutrient digestibility and gut-health of weaned pigs: a comparison of maternal and nursery supplementation strategies. Front Vet Sci. (2024) 11:1356455. doi: 10.3389/fvets.2024.1356455
129. Aderibigbe, AS, Park, CS, Johnson, T, Velayudhan, DE, Vinyeta, E, and Adeola, O. Efficacy of a novel multi-enzyme feed additive on growth performance, nutrient digestibility, and gut microbiome of weanling pigs fed corn–wheat or wheat–barley-based diet. J Anim Sci. (2024) 102:skae 064. doi: 10.1093/jas/skae064
130. Sampath, V, Cho, S, Jeong, J, Mun, S, Lee, CH, Hermes, RG, et al. Dietary Bacillus spp. supplementation to both sow and progenies improved post-weaning growth rate, gut function, and reduce the pro-inflammatory cytokine production in weaners challenged with Escherichia coli K88. Anim Microbiome. (2024) 6:3. doi: 10.1186/s42523-024-00290-y
131. Jinno, C, Li, X, and Liu, Y. Dietary supplementation of Bacillus subtilis or antibiotics modified intestinal microbiome of weaned pigs under enterotoxigenic Escherichia coli infection. Front Microbiol. (2022) 13:1064328. doi: 10.3389/fmicb.2022.1064328
132. Cuevas-Gómez, I, de Andrés, J, Cardenas, N, Espinosa-Martos, I, and Jiménez, E. Feed supplementation with Ligilactobacillus salivarius PS21603 optimises intestinal morphology and gut microbiota composition in weaned piglets. Benef Microbes. (2024) 15:195–210. doi: 10.1163/18762891-bja00001
133. Poulsen, AR, Jonge, N, Nielsen, JL, Højberg, O, Lauridsen, C, Cutting, SM, et al. Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS One. (2018) 13:e0207382. doi: 10.1371/journal.pone.0207382
134. Thomas, F, Hehemann, JH, Rebuffet, E, Czjzek, M, and Michel, G. Environmental and gut bacteroidetes: the food connection. Front Microbiol. (2011) 2:93. doi: 10.3389/fmicb.2011.00093
135. Bousbaine, D, Fisch, LI, London, M, Bhagchandani, P, Rezende de Castro, TB, Mimee, M, et al. A conserved Bacteroidetes antigen induces anti-inflammatory intestinal T lymphocytes. Science. (2022) 377:660–6. doi: 10.1126/science.abg5645
136. Liang, J, Kou, S, Chen, C, Raza, SHA, Wang, S, Ma, X, et al. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. (2021) 21:85. doi: 10.1186/s12866-021-02143-z
137. Rist, VT, Weiss, E, Sauer, N, Mosenthin, R, and Eklund, M. Effect of dietary protein supply originating from soybean meal or casein on the intestinal microbiota of piglets. Anaerobe. (2014) 25:72–9. doi: 10.1016/j.anaerobe.2013.10.003
138. Li, J, Feng, S, Wang, Z, He, J, Zhang, Z, Zou, H, et al. Limosilactobacillus mucosae-derived extracellular vesicles modulates macrophage phenotype and orchestrates gut homeostasis in a diarrheal piglet model. NPJ Biofilms Microbiomes. (2023) 9:33. doi: 10.1038/s41522-023-00403-6
139. Nakano, MM, and Zuber, P. Anaerobic growth of a "strict aerobe" (Bacillus subtilis). Ann Rev Microbiol. (1998) 52:165–90. doi: 10.1146/annurev.micro.52.1.165
140. Cao, J, Yu, Z, Liu, W, Zhao, J, Zhang, H, Zhai, Q, et al. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J Funct Foods. (2020) 64:103643. doi: 10.1016/j.jff.2019.103643
141. Suzuki, H, Fujiwara, Y, Thongbhubate, K, Maeda, M, and Kanaori, K. Spore-forming lactic acid-producing bacterium Bacillus coagulans synthesizes and excretes spermidine into the extracellular space. J Agric Food Chem. (2023) 71:9868–76. doi: 10.1021/acs.jafc.3c02184
142. Liu, C, Xiao, M, Zhao, P, Li, Q, Bai, H, and Cheng, Z. Effects of dietary Bacillus coagulans on growth performance and intestinal health of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Chinese J Anim Nutr. (2021) 33:4373–83. doi: 10.3969/j.issn.1006-267x.2021.08.018
143. Cao, G, Tao, F, Hu, Y, Li, Z, Zhang, Y, Deng, B, et al. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. (2019) 10:2926–34. doi: 10.1039/c8fo02370k
144. Mingmongkolchai, S, and Panbangred, W. Bacillus probiotics: an alternative to antibiotics for livestock production. J Appl Microbiol. (2018) 124:1334–46. doi: 10.1111/jam.13690
145. Deng, F, Chen, Y, Sun, T, Wu, Y, Su, Y, Liu, C, et al. Antimicrobial resistance, virulence characteristics and genotypes of Bacillus spp. from probiotic products of diverse origins. Food Res Int. (2021) 139:109949. doi: 10.1016/j.foodres.2020.109949
146. Li, X, Li, Q, Wang, Y, Han, Z, Qu, G, Shen, Z, et al. Gastric ulceration and immune suppression in weaned piglets associated with feed-borne Bacillus cereus and Aspergillus fumigatus. Toxins. (2020) 12:703. doi: 10.3390/toxins12110703
147. Zuo, Z, Li, Q, Guo, Y, Li, X, Huang, S, Hegemann, JH, et al. Feed-borne Bacillus cereus exacerbates respiratory distress in chickens infected with Chlamydia psittaci by inducing haemorrhagic pneumonia. Avian Pathol. (2020) 49:251–60. doi: 10.1080/03079457.2020.1716940
148. Zhang, Y, Overbeck, TJ, Skebba, VLP, and Gandhi, NN. Genomic and phenotypic safety assessment of probiotic Bacillus coagulans strain JBI-YZ6.3. Probiotics Antimicrob Proteins. (2024). doi: 10.1007/s12602-024-10305-4 [Epub ahead of print].
149. Zhu, K, Hölzel, CS, Cui, Y, Mayer, R, Wang, Y, Dietrich, R, et al. Probiotic Bacillus cereus strains, a potential risk for public health in China. Front Microbiol. (2016) 7:718. doi: 10.3389/fmicb.2016.00718
Keywords: Bacillus spp., growth performance, gut microbial, intestinal health, piglets
Citation: Tang X, Zeng Y, Xiong K and Zhong J (2024) Bacillus spp. as potential probiotics: promoting piglet growth by improving intestinal health. Front. Vet. Sci. 11:1429233. doi: 10.3389/fvets.2024.1429233
Edited by:
Elena De Felice, University of Camerino, ItalyReviewed by:
Zhenguo Yang, Southwest University, ChinaNuvee Prapasarakul, Chulalongkorn University, Thailand
Copyright © 2024 Tang, Zeng, Xiong and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaopeng Tang, dGFuZ3hpYW9wZW5nMTEwQDEyNi5jb20=; Kangning Xiong, eGlvbmdrYW5nbmluZzIwMjFAMTI2LmNvbQ==
 Xiaopeng Tang
Xiaopeng Tang Yan Zeng2
Yan Zeng2 Kangning Xiong
Kangning Xiong