
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 19 July 2024
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1414767
This article is part of the Research TopicFunctional Nutritional Strategies as Alternatives to AntimicrobialsView all 19 articles
Introduction: The objective of this study was to evaluate the effects of dietary supplementation of postbiotics on growth performance, mortality rate, immunity, small intestinal health, tibia characteristics, and hematological parameters of broiler chicks. he postbiotics were derived from Bacillus subtilis ACCC 11025.
Methods: A total of 480 day-old Arbor acre broiler chicks (52.83 ± 1.38 g) were used in a 42-day study and were randomly allocated into four groups. Each group comprised 6 replicate cages, each containing 20 birds. Dietary treatments were based on a basal diet, supplemented with postbiotics at concentrations of 0.000%, 0.015%, 0.030%, or 0.045%.
Results and discussion: The results demonstrated an improvement in growth performance, antibody titers against avian influenza virus and Newcastle disease virus, serum albumin levels, and serum total protein levels, as well as a reduction in mortality rate among broiler chicks with increasing levels of postbiotic supplementation. The most significant effect were observed in the group receiving 0.015% postbiotics. Furthermore, a dose-dependent enhancement in tibia weight and tibia weight to length ratio, coupled with a reduction in the robusticity index, was noted. The most favorable outcomes for tibia health were observed in the group receiving 0.030% postbiotics. This improvement in tibia health corresponded to a linear increase in serum calcium and inorganic phosphorus contents. In summary, supplementing broiler chicks with 0.015% postbiotics effectively enhances immunity, leading to improved growth performance and reduced mortality rates. Additionally, a postbiotic dose of 0.030% is suitable for optimizing tibia health.
For an extended period, the poultry industry has benefited from antibiotic supplementation, which has been instrumental in promoting growth, enhancing intestinal health, and bolstering immunity (1). However, the detrimental effects associated with antibiotic use have gained widespread recognition (2). Consequently, in the context of antibiotic-free poultry husbandry, the industry has faced significant challenges characterized by elevated mortality rates and suboptimal growth (3). High mortality rates and poor growth performance in broiler chicks are closely linked to their immune status, intestinal health, and tibia health (4–6).
Broiler chicks are highly susceptible to various diseases due to their underdeveloped immune system. Notably, the immune system of broiler chicks does not reach maturity until around day 30 to 34 post-hatch (7). Establishing a robust and healthy gut plays a pivotal role in optimizing the growth performance and immunity of poultry. The small intestine is central to nutrient absorption and maintaining overall gut health in chicks (8, 9). Furthermore, tibia health is crucial for their overall growth and well-being, directly impacting their leg health and locomotion (10). To secure favorable body weight and survival rates among broiler chicks, it is imperative to implement measures that enhance their immune function, small intestine health, and tibia health.
In the quest for antibiotic alternatives, probiotic supplementation has emerged as an effective strategy to enhance immunity (11), support tibia health (12), maintain intestinal health (13), reduce mortality rates (14), and improve growth performance (15). It is crucial to highlight that the beneficial effects of probiotics are not solely contingent upon their viability (16). Research has shown that non-viable microorganisms, including inactive bacteria, can also confer benefits in terms of poultry growth and production (17, 18). These non-viable microorganisms are commonly referred to as “postbiotics.”
According to the International Scientific Association of Probiotics and Prebiotics in 2021, a postbiotic is defined as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” (19). A postbiotic should derive from microorganisms, does not have to be derived from probiotics, and should undergo a process to terminate cell viability. The final postbiotic must contain inactivated microbial cells or cell components, with viable cells absent or negligible in the final product (19, 20).
Bacillus subtilis, a well-established probiotic, has been extensively used in poultry diets, yielding benefits such as improved growth performance through modulation of intestinal microbiota (21), immune system regulation (22), and heightened disease resistance (23). However, the exploration of postbiotics derived from Bacillus subtilis remains relatively unexplored. While research on the effects of Bacillus subtilis-derived postbiotics in poultry is still limited, promising insights have emerged from studies such as those published by Zhu et al. (24). Their findings demonstrated that dietary supplementation with heat-inactivated Bacillus subtilis and Lactobacillus acidophilus BFI at a 1:1 ratio effectively enhanced the growth performance of native broiler chicks by influencing the composition of intestinal microbiota.
Building upon these promising preliminary findings, our research endeavors aim to explore the influence of dietary supplementation with postbiotics derived from Bacillus subtilis on growth performance, mortality rate, hematological parameters, small intestine health, tibia health, and immunity of broiler chicks. Our working hypothesized that this intervention will improve growth performance and reduce mortality rates through enhancing immunity, small intestine health, and tibia health.
A total of 480 day-old (as hatched) Arbor Acre broiler chicks (52.83 ± 1.38 g) were used in a 42-day study and were randomly allocated into four groups. Each group comprised 6 replicate cages, each containing 20 birds. Dietary treatments were based on a basal diet, supplemented with postbiotics at concentrations of 0.000, 0.015, 0.030%, or 0.045%. The postbiotics were derived from Bacillus subtilis ACCC 11025, obtained from Jiangsu Yinong Biological Co., Ltd. (Jiangsu, China). According to the manufacture, Bacillus subtilis ACCC 11025 with 1 × 109 colony-forming units per gram were inactivated by high temperature and then spray-dried to prepare the final product. The experimental protocol and all associated procedures were executed with stringent ethical standards, receiving the requisite approval and oversight from the Animal Care and Use Committee of Jinzhou Medical University (Jinzhou, China).
Birds were housed in three-tier battery cages, each measuring 1.25 meters in length, 0.80 meters in width, and 0.50 meters in height. These cages were situated within a carefully controlled experimental environment, where ambient temperature conditions were systematically managed. The temperature regimen commenced at 33°C and was subsequently decreased by 3°C on a weekly basis until it stabilized at 22°C. Concurrently, the relative humidity within the facility was consistently maintained at 65%.
Throughout the experiment, birds had uninterrupted access to both feed and water. The formulation of diets was predicated on the optimization of nutritional requirements derived from the recommendations put forth by the National Research Council. This formulation has been effectively employed in commercial practices (Boin Feed Company, Shenyang, China). The specific details of the diets are outlined in Table 1.
An electronic scale was used to measure the body weight of broiler chicks on days 1, 21 and 42 of the study. Fresh feed was provided to the broiler chicks every morning, with the amount provided weighed in advance. The feed was changed the next morning, and the remaining feed was weighed. Feed intake (FI) was calculated as the difference between the feed given and the remaining amount. Data on body weight were used to calculate the average daily gain (ADG) using the following formula:
Data on ADG and FI were used to calculate the feed conversion ratio (FCR) using the following formula:
The count of dead birds was recorded daily to calculate the mortality rate for days 1–21, 22–42, and 1–42 using the following formula:
A total of eighteen birds were randomly selected from each group (3 birds per replicate cage) for blood sampling on days 21 and 42. Blood samples were collected from the wing vein and subsequently subjected to centrifugation at 3,000 × g at 4°C for 15 min to isolate the serum. Hemagglutination inhibition (HI) tests were used to evaluate the antibodies against avian influenza (AI; subtype H9) and Newcastle disease virus (NDV) (25). Colorimetric methods were employed to measure the concentrations of total protein and albumin in the serum (26). Globulin levels were calculated as the difference between the total protein and albumin. A fully automated biochemical analyzer (SMT-120VP, Seamaty, Chengdu, China) was used to assess the concentration of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatinine, blood urea nitrogen, calcium, and inorganic phosphorus.
Following the blood sampling procedure, the birds were euthanized by administering 1 mL of Euthasol® intravenously. Subsequently, immune organs such as the spleen, bursa of Fabricius, and thymus were excised to determine the relative organ weights using the following formula:
In a similar manner, the duodenum, jejunum, and ileum were excised using the aforementioned methods, and the relative weight for each segment was calculated using the same formula. Subsequently, the length of the duodenum, jejunum, and ileum was measured using a ruler to assess their physical dimensions. Furthermore, the assessment of reddish lesions was conducted using a 4-point scoring system ranging from 0 to 3. In this scoring system, a lesion scored as 1 indicated the presence of one or two clearly visible reddish lesions on the intestinal segment. A lesion score of 3 was assigned when large reddish lesions were observed at multiple sites within the intestinal segments. Lesions scored as 2 exhibited an intermediate appearance between those scored as 1 or 3 (27).
The tibia bones of euthanized bird were extracted with drumsticks intact, boiled for 10 min, and subsequently defleshed and air-dried for 24 h. Measurements included tibia length, weight, thickness of medial and lateral walls, and medullary canal diameter at the diaphysis midpoint. The tibia weight to length ratio was calculated to assess bone density. Additionally, the tibiotarsal (28) and robusticity (29) indexes were calculated to assess structural strength and robustness of the bones using the following formulas, respectively:
In this study, the replicate cage served as the experimental unit. All collected data underwent the Kolmogorov–Smirnov test to assess normal distribution. Data showing normal distribution were analyze for linear and quadratic effects using the MIXED Model procedure of SAS Institute Inc. (Cary, NC, United States). Tukey’s test was employed to analyze the differences in data showing normal distribution. Data that did not exhibit normal distribution (mortality rate and intestinal reddish lesions) were analyzed using the nonparametric Kruskal-Wallis test. Variability within the dataset was expressed as the standard error of means, and statistical significance was defined as p < 0.05.
Supplementing the diet of broiler chicks with postbiotics resulted in dose-dependent improvements in ADG and decreased FCR. Specifically, postbiotic supplementation quadratically increased ADG on days 1–21 (p < 0.05), days 22–42 (p < 0.05), and days 1–42 (p < 0.05), and linearly on days 22–42 (p < 0.05) and 1–42 (p < 0.05). Comparative analysis among groups indicated positive effects on ADG across all postbiotic doses, with the most significant improvement observed at 0.015% supplementation (p < 0.05) (Figure 1A). Additionally, FCR linearly decreased on days 1–21 (p < 0.05), days 22–42 (p < 0.05), and days 1–42 (p < 0.05), and quadratically on days 22–42 (p < 0.05) and 1–42 (p < 0.05). Notably, the 0.015% postbiotic group showed the lowest FCR (p < 0.05) (Figure 1C). However, postbiotics supplementation did not significantly affect FI (Figure 1B).
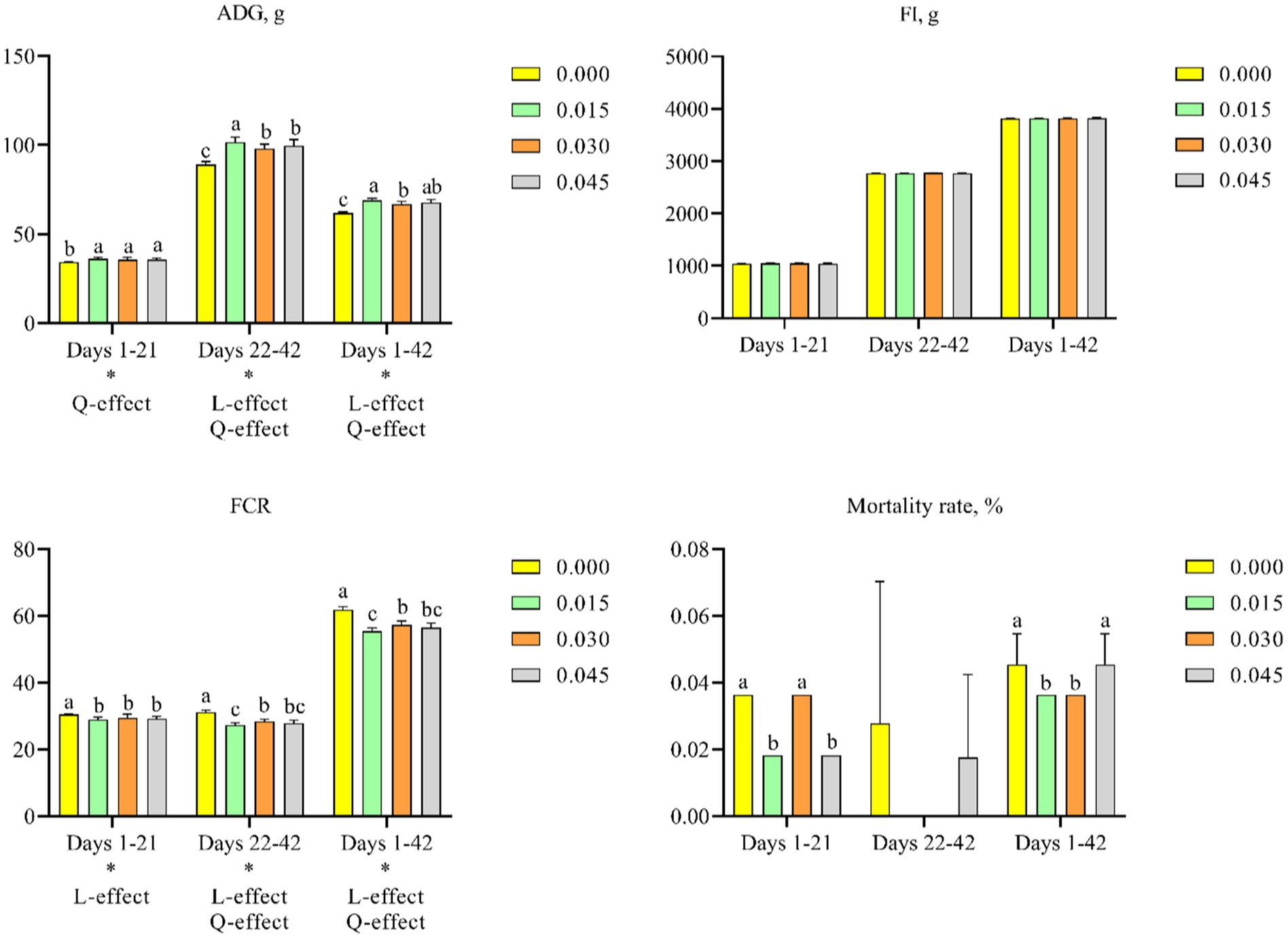
Figure 1. Effects of dietary supplementation with postbiotics on growth performance and mortality rates of broiler chicks. Values represent the means of 6 replicates per group (n = 6). a–cColumns in the same parameter with different superscript differ significantly (p < 0.05). L-effect means the parameter has a linear effect as the concentration increases. Q-effect means the parameter has a quadratic effect as the concentration increases. ADG, average daily gain; FI, feed intake; FCR, feed conversion ratio.
A significant time effect was observed for ADG (p < 0.05), FI (p < 0.05), and FCR (p < 0.05). Moreover, ADG (p < 0.05) and FCR (p < 0.05) were significantly affected by postbiotic supplementation (Figure 1). An interaction between treatment and time was also observed for ADG (p < 0.05) and FCR (p < 0.05) (Table 2).
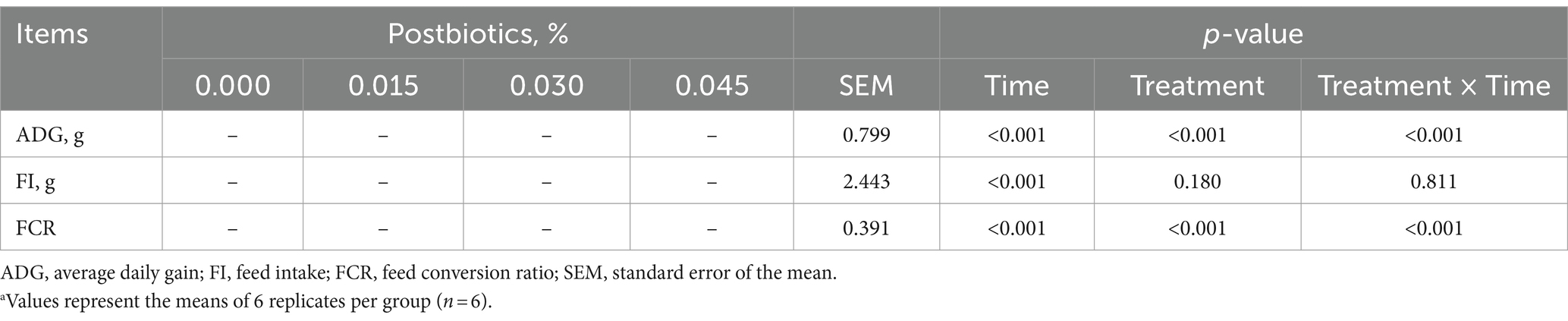
Table 2. Effects of dietary supplementation with postbiotics on growth performance of broiler chicks at different timepoints.a
Feeding broiler chicks with postbiotic-contained diet significantly decreased mortality rate. Specifically, supplementation with 0.015% postbiotics significantly decreased mortality rate on days 1–21 (p < 0.05) and days 1–42 (p < 0.05). Additionally, reductions in mortality rate were observed on days 1–21 with 0.045% postbiotics supplementation (p < 0.05), and on days 1–42 with 0.030% postbiotics supplementation (p < 0.05) (Figure 1D).
Supplementation with postbiotics linearly increased antibody levels against H9 AIV on day 42 (p < 0.05), and quadratically increased antibody levels against H9 AIV on day 21 (p < 0.05) and NDV on day 42 (p < 0.05). Post-hoc analysis revealed that only the 0.015% postbiotic dose significantly increased antibody levels against NDV on day 42 (p < 0.05). For H9 AIV antibodies, both the 0.015 and 0.030% doses were effective in increasing antibody levels on days 21 (p < 0.05) and 42 (p < 0.05) (Figures 2A,B).
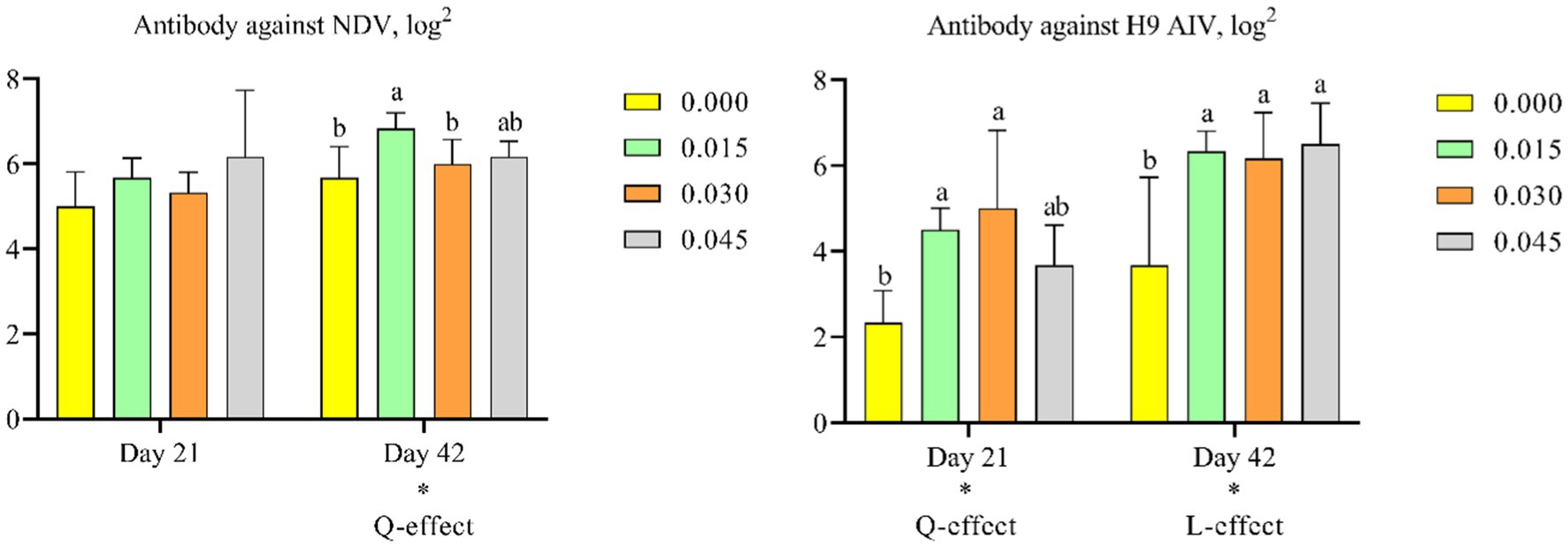
Figure 2. Effect of dietary supplementation with postbiotics on antibody titers of broiler chicks. Values represent the means of 6 replicates per group (n = 6). a,bColumns in the same parameter with different superscript differ significantly (p < 0.05). L-effect means the parameter has a linear effect as the concentration increases. Q-effect means the parameter has a quadratic effect as the concentration increases. AIV, avian influenza virus; NDV, newcastle disease virus.
No significant differences were observed in the immune organ index, including spleen, bursa of Fabricius, and thymus, among the various groups (Figure 3).
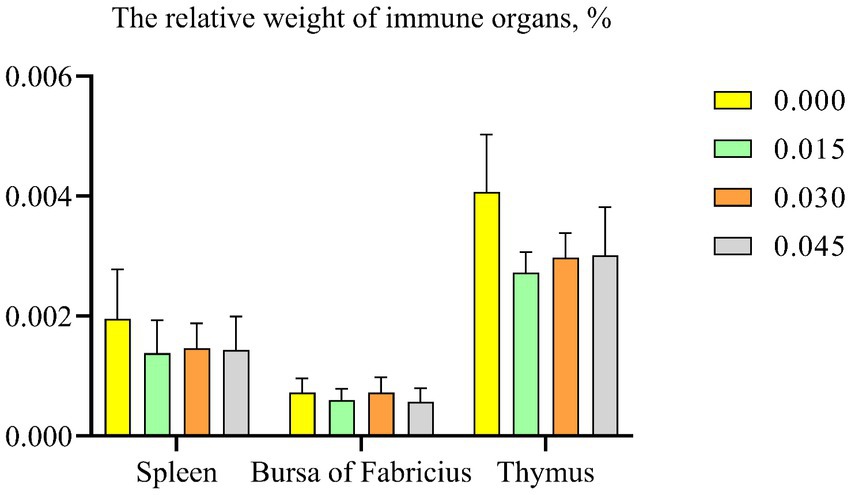
Figure 3. Effect of dietary supplementation with postbiotics on the relative weight of immune organs of broiler chicks. Values represent the means of 6 replicates per group (n = 6).
Postbiotic supplementation did not significantly affect the relative weight or length of small intestinal segments. Additionally, scores for reddish lesions in the intestines did not differ notably among the groups (Figures 4A–C).
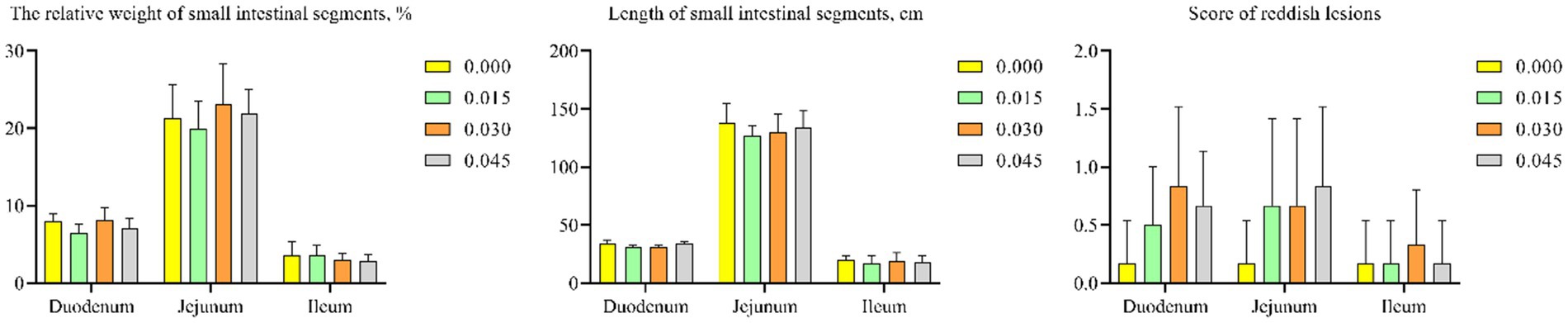
Figure 4. Effect of dietary supplementation with postbiotics on small intestinal health parameters of broiler chicks. Values represent the means of 6 replicates per group (n = 6).
Parameters of tibia health, including tibia weight (p < 0.05) and tibia weight to length ratio (p < 0.05), increased quadratically, while the robusticity index (p < 0.05) decreased linearly with postbiotic supplementation in the diet (Figure 5). Notably, significant effects on these parameters were observed only with the 0.030% dose.
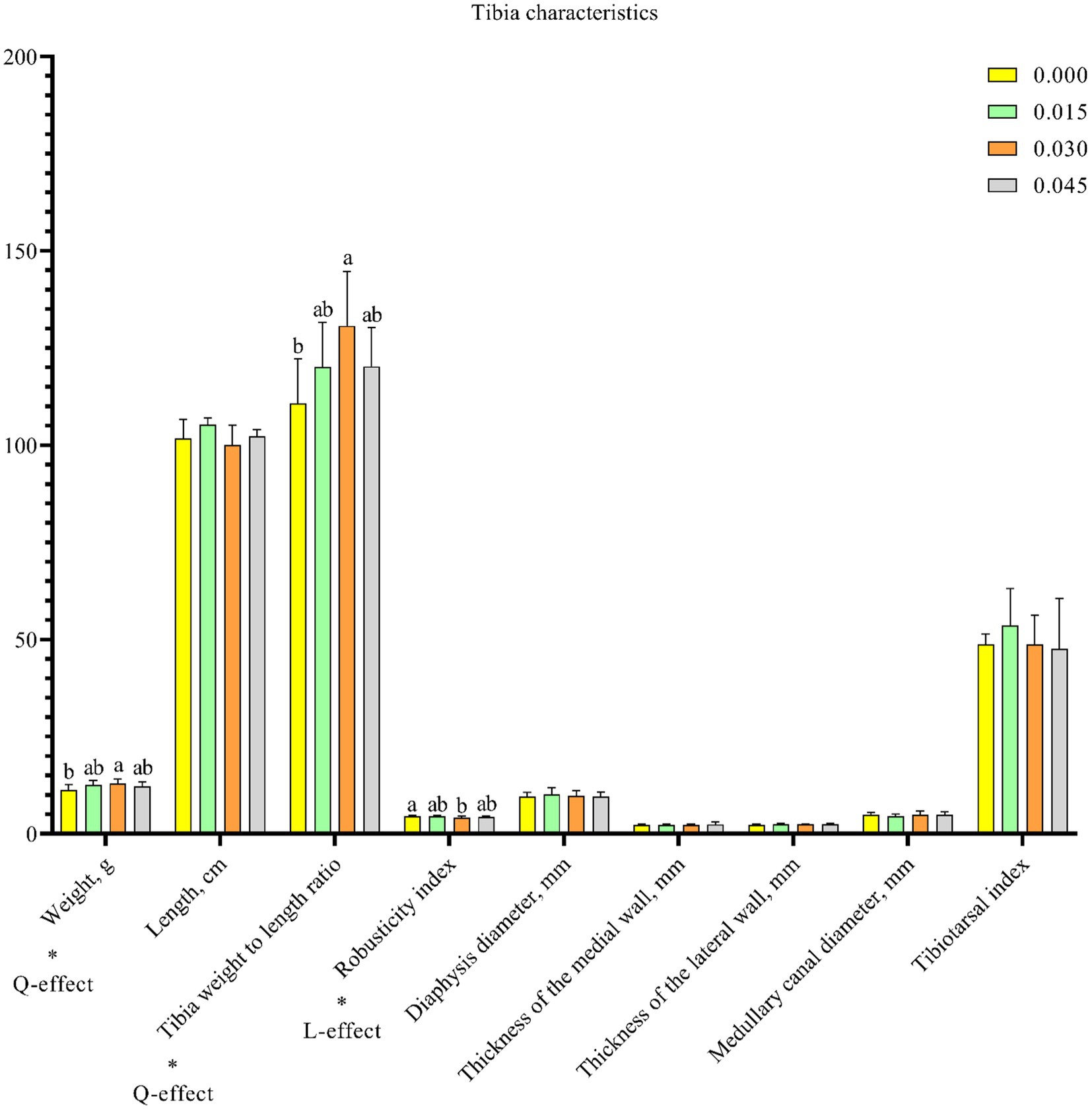
Figure 5. Effect of dietary supplementation with postbiotics on tibia characteristics of broiler chicks. Values represent the means of 6 replicates per group (n = 6). a,bColumns in the same parameter with different superscript differ significantly (p < 0.05). L-effect means the parameter has a linear effect as the concentration increases. Q-effect means the parameter has a quadratic effect as the concentration increases.
Consumption of postbiotics did not significantly affect parameters such as aspartate aminotransferase (Figure 6A), alanine aminotransferase (Figure 6B), alkaline phosphatase (Figure 6C), creatinine (Figure 6D), blood urea nitrogen (Figure 6E), and globulin (Figure 6I). However, postbiotics supplementation linearly increased the concentration of calcium on day 42 (p < 0.05; Figure 6F), inorganic phosphorus on days 21 (p < 0.05) and 42 (p < 0.05; Figure 6G), albumin on day 42 (p < 0.05; Figure 6H), and total protein on days 21 (p < 0.05) and 42 (p < 0.05; Figure 6J).
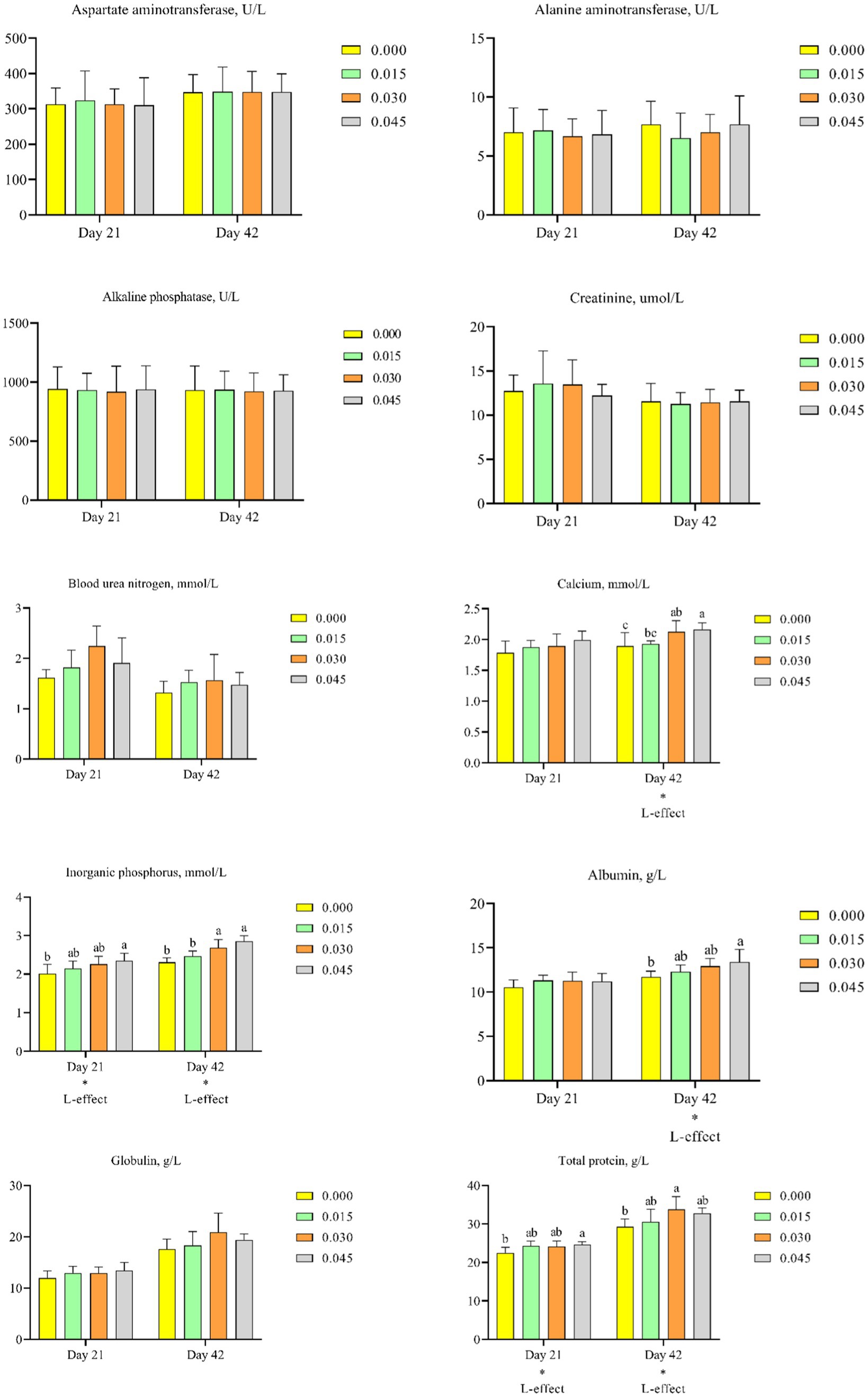
Figure 6. Effect of dietary supplementation with postbiotics on hematological parameters of broiler chicks. Values represent the means of 6 replicates per group (n = 6). a,bColumns in the same parameter with different superscript differ significantly (p < 0.05). L-effect means the parameter has a linear effect as the concentration increases.
Comparative analysis revealed that the highest calcium concentration on day 42 was observed in the 0.030 and 0.045% postbiotics groups (p < 0.05). The highest inorganic phosphorus concentration on day 21 was observed in the 0.045% group, and on day 42 in the 0.030 and 0.045% groups (p < 0.05). The highest albumin concentration on day 42 was observed in the 0.045% group (p < 0.05). The highest total protein concentration on day 21 was observed in the 0.045% group, and on day 42 in the 0.030% group (p < 0.05).
This study highlights the enhanced growth performance of broiler chicks through graded supplementation with postbiotic. These fundings are consistent with previous research, emphasizing the positive impact of such supplementation on broiler performance. For instance, Incharoen et al. (30) demonstrated that dietary supplementation with heat-killed Lactobacillus plantarum L-137 effectively improved growth performance in broiler chicks. Similarly, Khonyoung and Yamauchi (31) found that supplementation with heat-killed Lactobacillus sakei HS-1 increased ADG and decreased FCR in broiler chicks. Growth performance serves as an indirect indicator of broiler health, healthy growth and achieving target weights typically indicate good health. Conversely, slow growth patterns may indicate underlying health issues (32). Thus, the observed benefits of dietary postbiotic supplementation on growth performance suggest an improvement of overall health and well-being in broiler chicks.
Assessment of serum biochemical parameters, such as albumin, globulin, and total protein, provides valuable insights into the in vivo health status of animals (33). Circulating total protein levels are typically lower in diseased broiler chicks compared to healthy birds (34). In the current study, a linear increase in serum albumin and total protein content was observed in animals treated with postbiotics. Therefore, dietary supplementation with postbiotics was beneficially impact the overall health of birds, as indicated by increased levels of albumin and total protein in their serum. These findings align with the concept that healthier birds tend to exhibit higher levels of these serum proteins, reflecting improved health and well-being.
Indeed, there is a correlation between serum total protein, albumin, globulin, and the antibody response indicative of immunity in broiler chicks (35, 36). Antibody titers serve as indicators of antibody levels in the bloodstream. These antibodies are proteins produced by the immune system in response to exposure to pathogens such as viruses or bacteria. Elevated antibody titers indicate that the chick has encountered a specific pathogen and mounted an immune response against it. This immune response provides protection against potential future infections caused by the same pathogen. In the present study, dietary supplementation with postbiotics resulted in increased antibody titers in broiler chicks. This observation is consistent with Nofouzi et al. (18), who reported increased antibody titers against NDV and AIV in broiler chicks following administration of heat-killed Tsukamurella inchonensis. Therefore, it is reasonable to conclude that postbiotic supplementation exerts positive effects on the immunity of broiler chicks, as evidenced by enhanced antibody titers.
Immune organs such as the spleen, bursa of Fabricius, and thymus are crucial for the immunological function of broiler chicks. A reduction in the weight of these organs may indicate immunosuppression, while an increase suggests enhanced immune function (37). In the present study, dietary supplementation with postbiotics did not significant affect the weight of these immune organs. This finding is consistent with Incharoen et al. (30), who found that dietary supplementation with heat-killed Lactobacillus plantarum L-137 did not affect the spleen index. Similarly, Zhu et al. (24) observed no changes in the relative weight of the bursa of Fabricius and spleen following dietary supplementation with a compound probiotic of heat-inactivated Bacillus subtilis and Lactobacillus acidophilus BFI. Thus, it can be hypothesized that dietary supplementation with postbiotics may not significantly enhance the immunity of broiler chicks through alterations in immune organ indices.
The small intestine plays a vital role in nutrient absorption and immune function, making its health crucial for optimal growth and immunity. The relative weight and length of small intestinal segments can indicate the development of specific segments within the small intestine (38). However, in the current study, dietary supplementation with postbiotics did not significant affect the development of small intestinal segments. This finding contrasts with Khonyoung and Yamauchi (31), who reported decreased weights of the ileum, total small intestine, and ceca with increasing supplementation of heat-killed Lactobacillus sakei HS-1 in broiler chicks. Therefore, different postbiotic types may generate different effects on intestinal health.
Additionally, scoring reddish lesions in the small intestine is typically used to assess the presence and severity of lesions associated with conditions like coccidiosis or Clostridium perfringens infection, which can cause small red petechiae in the mid-intestine (39, 40). These lesions are closely related to high intestinal fragility (41). In the present study, supplementation with postbiotics did not significantly impact the score of reddish lesions, possibly due to the absence of coccidiosis or Clostridium perfringens infection within the experimental block.
Tibia health is crucial for poultry, directly impacting their overall well-being. The structural support provided by the tibia allows poultry to engage in various activities such as standing, walking, and perching. Poultry with strong tibia are better equipped to resist diseases and infections, while weakened or fragile tibia can render birds more susceptible to injuries and infections (42). In the current study, dietary supplementation with postbiotics positively affected tibia health parameters, including tibia weight and tibia weight to length ratio, while it negatively affected the robusticity index. The tibia weight to length ratio is a straightforward indicator of bone density, with higher values indicating denser bone structures (43). Similarly, higher tibia weight values indicate greater bone mineralization (44). A lower robusticity index suggests a robust bone structure (45). Calcium and phosphorus are essential minerals for bone mineralization in broilers, and their deficiency can impair bone development and related metabolic utilization parameters (46, 47). In the present study, dietary postbiotic supplementation led to increased serum calcium and phosphorus contents. As reported by Li et al. (47), broiler chicks with higher calcium and phosphorus levels in their serum tend to exhibit better bone quality. Therefore, it is reasonable to conclude that dietary supplementation with postbiotics can enhance tibia health.
Alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase are parameters that provide insight into liver health (48). An increase in their serum levels may indicate liver damage (48). Additionally, creatinine and blood urea nitrogen are indicators of renal function (49). Elevated levels in serum are typically associated with kidney injury (50). In the present study, dietary supplementation with postbiotics did not significantly affect alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, creatinine, and blood urea nitrogen levels. These observations suggest that postbiotic supplementation did not adversely affect liver or renal function in broiler chicks.
Enhancing the health condition of broiler chicks can positively impact their survival rate. In the present study, dietary supplementation with postbiotics improved immunity and tibia health in broiler chicks. This is consistent with findings from previous research. Wu et al. (51) reported that enhancing immunity effectively reduces mortality caused by Escherichia coli infection in broiler chicks. Furthermore, Liu et al. (52) found that supplementation with Lactobacillus rhamnosus improved normal growth and development of the tibia growth plate, thereby protecting broiler survival rates. Based on these findings, it can be speculated that dietary supplementation with postbiotics enhances immunity and tibia quality in broiler chicks, contributing to a reduced mortality rate.
In conclusion, this research highlights the potential benefits of dietary supplementation with postbiotics derived from Bacillus subtilis ACCC 11025 in broiler chick nutrition. This postbiotic have demonstrated the capacity to enhance growth performance, reduce mortality rates, boost immunity, and improve tibia health without compromising liver or renal function. These findings contribute to the development of effective and sustainable poultry farming practices essential for the growth and well-being of the poultry industry.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by the experimental protocol and all associated procedures were executed with stringent ethical standards. Additionally, it received the requisite approval and oversight from the Animal Care and Use Committee of Jinzhou Medical University (Jinzhou, China). The study was conducted in accordance with the local legislation and institutional requirements.
DL: Investigation, Writing – original draft, Writing – review & editing. SF: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. FH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. XF: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. TW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. ZC: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. MW: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We appreciate the support from the Collaborative Innovation Center for Prevention and Control of Zoonoses and the Innovation and entrepreneurship team of green and healthy breeding technology and product development.
TW was employed by Liaoning Kaiwei Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mehdi, Y, Létourneau-Montminy, MP, Gaucher, ML, Chorfi, Y, Suresh, G, Rouissi, T, et al. Use of antibiotics in broiler production: global impacts and alternatives. Anim Nutr. (2018) 4:170–8. doi: 10.1016/j.aninu.2018.03.002
2. Muhammad, J, Khan, S, Su, JQ, Hesham, AEL, Ditta, A, Nawab, J, et al. Antibiotics in poultry manure and their associated health issues: a systematic review. J Soils Sediments. (2020) 20:486–97. doi: 10.1007/s11368-019-02360-0
3. Cervantes, HM . Antibiotic-free poultry production: is it sustainable? J Appl Poult Res. (2015) 24:91–7. doi: 10.3382/japr/pfv006
4. Avicola, ES, Porcino, ES, and America, S. Leg health in large broilers leg health is one of the most prevalent causes of culling and late mortality during grow-out of heavy broilers according to Edgar O. Oviedo-Rondón, DVM, PhD., Dip. ACPV Department of Poultry Science North Carolina State University. Health. (2022) 17:1.
5. Ducatelle, R, Goossens, E, De Meyer, F, Eeckhaut, V, Antonissen, G, Haesebrouck, F, et al. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet Res. (2018) 49:43–9. doi: 10.1186/s13567-018-0538-6
6. Stefanetti, V, Mancinelli, AC, Pascucci, L, Menchetti, L, Castellini, C, Mugnai, C, et al. Effect of rearing systems on immune status, stress parameters, intestinal morphology, and mortality in conventional and local chicken breeds. Poult Sci. (2023) 102:103110. doi: 10.1016/j.psj.2023.103110
7. Song, B, Tang, D, Yan, S, Fan, H, Li, G, Shahid, MS, et al. Effects of age on immune function in broiler chickens. J Anim Sci Biotechnol. (2021) 12:42–12. doi: 10.1186/s40104-021-00559-1
8. Ducatelle, R, Goossens, E, Eeckhaut, V, and Van Immerseel, F. Poultry gut health and beyond. Anim Nutr. (2023) 13:240–8. doi: 10.1016/j.aninu.2023.03.005
9. Svihus, B . Function of the digestive system. J Appl Poult Res. (2014) 23:306–14. doi: 10.3382/japr.2014-00937
10. Santos, MN, Widowski, TM, Kiarie, EG, Guerin, MT, Edwards, AM, and Torrey, S. In pursuit of a better broiler: tibial morphology, breaking strength, and ash content in conventional and slower-growing strains of broiler chickens. Poult Sci. (2022) 101:101755. doi: 10.1016/j.psj.2022.101755
11. Jiang, S, Yan, FF, Hu, JY, Mohammed, A, and Cheng, HW. Bacillus subtilis-based probiotic improves skeletal health and immunity in broiler chickens exposed to heat stress. Animals. (2021) 11:1494. doi: 10.3390/ani11061494
12. McCabe, L, Britton, RA, and Parameswaran, N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. (2015) 13:363–71. doi: 10.1007/s11914-015-0292-x
13. Khan, S, Moore, RJ, Stanley, D, and Chousalkar, KK. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol. (2020) 86:e00600–20. doi: 10.1128/AEM.00600-20
14. Huff, GR, Huff, WE, Rath, NC, El-Gohary, FA, Zhou, ZY, and Shini, S. Efficacy of a novel prebiotic and a commercial probiotic in reducing mortality and production losses due to cold stress and Escherichia coli challenge of broiler chicks. Poult Sci. (2015) 94:918–26. doi: 10.3382/ps/pev068
15. Dang, DX, Zou, Q, Xu, Y, Cui, Y, Li, X, Xiao, Y, et al. Feeding broiler chicks with bacillus subtilis, clostridium butyricum, and enterococcus faecalis mixture improves growth performance and regulates cecal microbiota. Probiotics Antimicrob Proteins. (2022) 16:113–24. doi: 10.1007/s12602-022-10029-3
16. Kataria, J, Li, N, Wynn, JL, and Neu, J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. (2009) 67:546–50. doi: 10.1111/j.1753-4887.2009.00226.x
17. Khonyoung, D, and Yamauchi, KE. Effects of heat-killed Lactobacillus plantarum L-137 on morphology of intestinal villi and epithelial cells in broiler chickens. J Appl Anim Res. (2012) 40:140–7. doi: 10.1080/09712119.2011.640208
18. Nofouzi, K, Mirzazadeh, S, Khordadmehr, M, Madadi, MS, Amininia, S, Firouzamandi, M, et al. The effects of heat-killed Tsukamurella inchonensis on intestinal morphology and humoral immune responses of broiler chickens. Iran J Microbiol. (2021) 13:81. doi: 10.18502/ijm.v13i1.5496
19. Salminen, S, Collado, MC, Endo, A, Hill, C, Lebeer, S, Quigley, EM, et al. The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6
20. Vinderola, G, Sanders, ME, and Salminen, S. The concept of postbiotics. Food Secur. (2022) 11:1077. doi: 10.3390/foods11081077
21. Zhang, S, Zhong, G, Shao, D, Wang, Q, Hu, Y, Wu, T, et al. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult Sci. (2021) 100:100935. doi: 10.1016/j.psj.2020.12.032
22. Qiu, K, Li, CL, Wang, J, Qi, GH, Gao, J, Zhang, HJ, et al. Effects of dietary supplementation with Bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front Nutr. (2021) 8:786878. doi: 10.3389/fnut.2021.786878
23. Dong, Y, Li, R, Liu, Y, Ma, L, Zha, J, Qiao, X, et al. Benefit of dietary supplementation with Bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiotics Antimicrob Proteins. (2020) 12:1385–97. doi: 10.1007/s12602-020-09643-w
24. Zhu, C, Gong, L, Huang, K, Li, F, Tong, D, and Zhang, H. Effect of heat-inactivated compound probiotics on growth performance, plasma biochemical indices, and cecal microbiome in yellow-feathered broilers. Front Microbiol. (2020) 11:585623. doi: 10.3389/fmicb.2020.585623
25. Chaka, H, Thompson, PN, Goutard, F, and Grosbois, V. Evaluation of enzyme-linked immunosorbent assays and a haemagglutination inhibition tests for the detection of antibodies to Newcastle disease virus in village chickens using a Bayesian approach. Prev Vet Med. (2015) 119:21–30. doi: 10.1016/j.prevetmed.2015.01.016
26. Palladino, P, Brittoli, A, Pascale, E, Minunni, M, and Scarano, S. Colorimetric determination of total protein content in serum based on the polydopamine/protein adsorption competition on microplates. Talanta. (2019) 198:15–22. doi: 10.1016/j.talanta.2019.01.095
27. Huff, WE, Harvey, RB, Kubena, LF, and Rottinghaus, GE. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult Sci. (1988) 67:1418–23. doi: 10.3382/ps.0671418
28. Barnett, E, and Nordin, BEC. The radiological diagnosis of osteoporosis: a new approach. Clin Radiol. (1960) 11:166–74. doi: 10.1016/S0009-9260(60)80012-8
29. Riesenfeld, A . Metatarsal robusticity in bipedal rats. Am J Phys Anthropol. (1972) 36:229–33. doi: 10.1002/ajpa.1330360211
30. Incharoen, T, Charoensook, R, Onoda, S, Tatrakoon, W, Numthuam, S, and Pechkong, T. The effects of heat-killed Lactobacillus plantarum L-137 supplementation on growth performance, intestinal morphology, and immune-related gene expression in broiler chickens. Anim Feed Sci Technol. (2019) 257:114272. doi: 10.1016/j.anifeedsci.2019.114272
31. Khonyoung, D, and Yamauchi, KE. Improved growth performance due to hypertrophied intestinal absorptive epithelial cells by heat-killed Lactobacillus sakei HS-1 in broiler chickens. J Anim Sci. (2019) 97:2066–75. doi: 10.1093/jas/skz075
32. Huang, J, Liang, L, Cui, K, Li, P, Hao, G, and Sun, S. Salmonella phage CKT1 significantly relieves the body weight loss of chicks by normalizing the abnormal intestinal microbiome caused by hypervirulent Salmonella Pullorum. Poult Sci. (2022) 101:101668. doi: 10.1016/j.psj.2021.101668
33. Park, JH, and Kim, IH. The effects of betaine supplementation in diets containing different levels of crude protein and methionine on the growth performance, blood components, total tract nutrient digestibility, excreta noxious gas emission, and meat quality of the broiler chickens. Poult Sci. (2019) 98:6808–15. doi: 10.3382/ps/pez412
34. Matthew, O, Danladi, JI, Joseph, NA, Dahiru, S, Danlami, AA, Stephen, K, et al. Effects of synbiotic probiotic and prebiotic supplementation on haematology and serum total proteins of broiler chickens challenged with Eimeria tenella. Comp Clin Path. (2022) 31:53–66. doi: 10.1007/s00580-021-03305-1
35. Attia, YA, Al-Khalaifah, H, Ibrahim, MS, Abd Al-Hamid, AE, Al-Harthi, MA, and El-Naggar, A. Blood hematological and biochemical constituents, antioxidant enzymes, immunity and lymphoid organs of broiler chicks supplemented with propolis, bee pollen and mannan oligosaccharides continuously or intermittently. Poult Sci. (2017) 96:4182–92. doi: 10.3382/ps/pex173
36. Guo, S, He, L, Zhang, Y, Niu, J, Li, C, Zhang, Z, et al. Effects of vitamin a on immune responses and vitamin a metabolism in broiler chickens challenged with necrotic enteritis. Life. (2023) 13:1122. doi: 10.3390/life13051122
37. Iftikhar, H, Mahmood, MS, Arshad, MI, Akhtar, M, Mahmood, F, and Rafique, A. Immune system dysfunction in broiler chickens experimentally inoculated with fowl adenovirus serotype-4 associated with inclusion body hepatitis hydropericardium syndrome. Turk J Vet Anim. (2012) 36:223–30. doi: 10.3906/vet-0807-21
38. Alshamy, Z, Richardson, KC, Hünigen, H, Hafez, HM, Plendl, J, and Al Masri, S. Comparison of the gastrointestinal tract of a dual-purpose to a broiler chicken line: a qualitative and quantitative macroscopic and microscopic study. PLoS One. (2018) 13:e0204921. doi: 10.1371/journal.pone.0204921
39. Dahiya, JP, Hoehler, D, Van Kessel, AG, and Drew, MD. Dietary encapsulated glycine influences Clostridium perfringens and lactobacilli growth in the gastrointestinal tract of broiler chickens. J Nutr. (2007) 137:1408–14. doi: 10.1093/jn/137.6.1408
40. Peek, HW, Van der Klis, JD, Vermeulen, B, and Landman, WJM. Dietary protease can alleviate negative effects of a coccidiosis infection on production performance in broiler chickens. Anim Feed Sci Technol. (2009) 150:151–9. doi: 10.1016/j.anifeedsci.2008.08.006
41. Hokama, A, Kishimoto, K, Nakamoto, M, Kobashigawa, C, Hirata, T, Kinjo, N, et al. Endoscopic and histopathological features of gastrointestinal amyloidosis. World J Gastrointest Endosc. (2011) 3:157–61. doi: 10.4253/wjge.v3.i8.157
42. Takase, K, Haraguchi, Y, Suzuki, A, and Obi, T. Fracture status of wild cranes (Grus monacha and G. vipio) found dead or in a weak condition at Izumi plain in Japan. J Vet Med Sci. (2020) 82:823–6. doi: 10.1292/jvms.19-0273
43. Seedor, JG, Quartuccio, HA, and Thompson, DD. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res. (1991) 6:339–46. doi: 10.1002/jbmr.5650060405
44. Sanchez-Rodriguez, E, Benavides-Reyes, C, Torres, C, Dominguez-Gasca, N, Garcia-Ruiz, AI, Gonzalez-Lopez, S, et al. Changes with age (from 0 to 37 D) in tibiae bone mineralization, chemical composition and structural organization in broiler chickens. Poult Sci. (2019) 98:5215–25. doi: 10.3382/ps/pez363
45. Stock, JT, and Shaw, CN. Which measures of diaphyseal robusticity are robust? A comparison of external methods of quantifying the strength of long bone diaphyses to cross-sectional geometric properties. Am J Phys Anthropol. (2007) 134:412–23. doi: 10.1002/ajpa.20686
46. Imari, ZK, Hassanabadi, A, and Nassiri Moghaddam, H. Response of broiler chickens to calcium and phosphorus restriction: effects on growth performance, carcase traits, tibia characteristics and total tract retention of nutrients. Ital J Anim Sci. (2020) 19:929–39. doi: 10.1080/1828051X.2020.1808101
47. Li, T, Xing, G, Shao, Y, Zhang, L, Li, S, Lu, L, et al. Dietary calcium or phosphorus deficiency impairs the bone development by regulating related calcium or phosphorus metabolic utilization parameters of broilers. Poult Sci. (2020) 99:3207–14. doi: 10.1016/j.psj.2020.01.028
48. Baradaran, A, Samadi, F, Ramezanpour, SS, and Yousefdoust, S. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicol Rep. (2019) 6:788–94. doi: 10.1016/j.toxrep.2019.07.011
49. Long, CM, Yang, JR, Yang, H, Li, XC, and Wang, GX. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti inflammatory, and anti apoptotic activities. Mol Med Rep. (2016) 13:4697–704. doi: 10.3892/mmr.2016.5128
50. Wang, X, Han, C, Cui, Y, Li, S, Jin, G, Shi, W, et al. Florfenicol causes excessive lipid peroxidation and apoptosis induced renal injury in broilers. Ecotoxicol Environ Saf. (2021) 207:111282. doi: 10.1016/j.ecoenv.2020.111282
51. Wu, Z, Yang, K, Zhang, A, Chang, W, Zheng, A, Chen, Z, et al. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult Sci. (2021) 100:101323. doi: 10.1016/j.psj.2021.101323
Keywords: small intestine health, immunity, tibia characteristic, survival rate, killed probiotic
Citation: Li D, Fang S, He F, Fan X, Wang T, Chen Z and Wang M (2024) Postbiotic derived from Bacillus subtilis ACCC 11025 improves growth performance, mortality rate, immunity, and tibia health in broiler chicks. Front. Vet. Sci. 11:1414767. doi: 10.3389/fvets.2024.1414767
Received: 09 April 2024; Accepted: 10 July 2024;
Published: 19 July 2024.
Edited by:
Francesca Riva, University of the West of Scotland, United KingdomReviewed by:
Monika Proszkowiec-Weglarz, United States Department of Agriculture, United StatesCopyright © 2024 Li, Fang, He, Fan, Wang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mi Wang, d2FuZ21pX2p6QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.