- Yunnan Provincial Key Laboratory of Animal Nutrition and Feed, Faculty of Animal Science and Technology, Yunnan Agricultural University, Kunming, China
Introduction: The ban on antibiotics in animal husbandry underscores the crucial need for safe, natural feed additives. This study investigates the effects of Ampelopsis grossedentata flavonoids (AGF) and Tween 80 on the growth performance, blood indexes, and rumen microbiota of Nubian goats, evaluating their potential as alternative feed additives in livestock management.
Methods: Thirty-two goats were randomly divided into four groups. The control group (CON group) was provided with a basal diet, while the experimental groups received diets supplemented with various dietary additives for a duration of 100 days: either a basal diet supplemented with 25 mg/kg of monensin (MN group), a basal diet containing 2.0 g/kg of Ampelopsis grossedentata flavonoids (AGF group), or a basal diet containing 7.5 mL/kg of Tween 80 (TW group). Blood and rumen fluid samples were collected for analysis at the end of the feeding period. Growth performance was monitored through regular weighing and feed intake measurements. Blood indexes were analyzed using standard biochemical techniques, while the microbial composition of the rumen fluid was determined through high throughput 16S rRNA gene sequencing to assess microbial diversity and function. The effects of the dietary treatments on growth performance, blood indexes, and rumen microbial composition were then evaluated.
Results: The AGF group exhibited significantly increased average daily gain, and decreased feed-to-gain ratio (p < 0.05). Blood indexes analysis revealed no differences between the CON and AGF groups, with both showing higher concentrations of triglyceride, low-density lipoprotein cholesterol, glutamic-pyruvic transaminase, alkaline phosphatase, and lactate dehydrogenase compared to the monensin group (p < 0.05). The TW group had significantly higher glucose, glutamic-oxaloacetic transaminase, and glutamic-pyruvic transaminase levels than the MN group (p < 0.05). Microbial diversity analysis revealed that the TW group had significantly greater alpha-diversity than other groups, while beta-diversity analysis showed closer similarity between the rumen microbiota of the AGF and CON groups. LEfSe analysis identified Proteobacteria, Deferribacteres, Ehryarchaeoia, and Elusimicrobia as biomarkers distinguishing the rumen microbiota among the groups. In conclusion, AGF supplementation increased the relative abundance of beneficial bacteria in the rumen of Nubian goats, and thus enhanced the growth performance. TW supplementation significantly increased rumen microbial diversity and abundance, suggesting benefits for rumen health despite poor palatability. These findings highlight the potential of AGF as a new green additive with important implications for the efficiency and development of animal husbandry.
1 Introduction
Antibiotic additives have previously been widely used in animal husbandry (1). However, due to issues like drug residues, their use has been banned in many countries (2). This prohibition has posed significant challenges to animal husbandry as a whole, underscoring the critical need to explore natural, green, and safe feed additives. This study aimed to assess the effects of two potential alternatives—Ampelopsis grossedentata flavone (AGF) derived from natural plant extracts and Tween 80, widely used as an emulsifier—as feed additives on growth performance, blood indexes and rumen microbiota in Nubian goats.
AGF, an acronym for Ampelopsis grossedentata flavonoids, represents a group of natural plant extracts derived from the Ampelopsis grossedentata vine, known for their significant antioxidative properties. Its main component is dihydromyricetin (3), which has various physiological functions such as anti-inflammation, anti-oxidative stress (4), antibacterial (5), anti-apoptosis (6), immune regulation (7), and plays an important role in improving animal growth performance and enhancing animal immunity (8). Recent reports indicate that AGF not only shows strong antioxidant activity, but also has excellent liver protective properties that can be developed into products aimed at anti-diabetes, antioxidants and liver protection (9–11). The study found that rattan tea prevented metabolic disorders caused by a high-fat diet by improving glucose homeostasis in rats. Wang and colleagues' research found that the antioxidant qualities of AGF play a protective role in maintaining the integrity of the intestinal barrier within the porcine epithelial cell line (12). Zhu et al. found that incorporating AGF into the diet not only enhances the microbial ecosystem but also holds significant potential for advancing the growth of juvenile livestock, thereby contributing to economic gains (13). However, the specific role of AGF as feed additives in ruminants still requires further investigation.
The chemical name of Tween 80 is polyoxyethylene sorbide monooleate. It can increase the amount of enzyme release and improve enzyme activity, promote the interaction between enzyme and substrate (14). It is widely used as an emulsifier in food processing (15). Food-grade Tween 80 is generally considered safe as a feed additive (16). Experiments have shown that Tween 80 has a certain regulatory effect on rumen microorganisms, which can enhance the activity of cellulase and promote the growth of non-cellulolytic bacteria (17, 18). Martha's study found that Tween 80 and its derivative oleic acid promoted the growth of corynebacterium accolens (19). Research has demonstrated that the addition of the surfactant Tween-80 effectively boosts the natural ability of bacteria to biodegrade naphthalene (20). However, only a few studies have verified the effect of Tween 80 in ruminants, with particularly few reports on its application in meat sheep production.
Therefore, the aim of this study was to comprehensively investigate the effects of dietary supplementation with AGF and Tween 80 on growth performance, blood indexes, and rumen microbiota in Nubian goats, as well as to evaluate the feasibility of these two additives as alternatives to monensin. Through this study, we hope to provide a theoretical basis for the promotion and application of these natural and safe feed additives in the animal husbandry industry.
2 Materials and methods
The study was conducted at the farm in Zhaotong County, Yunnan Province, China. Nestled in the mid-mountainous terrain of Houlong Mountain at coordinates N27°31′13″, E103°54′51″, the farm stands proudly at an elevation of 1,614 m. This locale experiences a climatic climate characterized by high humidity levels ranging from 20% to 60% RH, and a penchant for precipitation. Throughout the summer months, spanning June to September, the daily temperatures peak at an average temperature of 26°C. However, a conspicuous diurnal-temperature delta. The research proposal and the relevant experimental procedures were approved by the institutional Animal Care and Use Committee of Yunnan Agricultural University (Case Number: 202200301).
2.1 Animals and experiment design
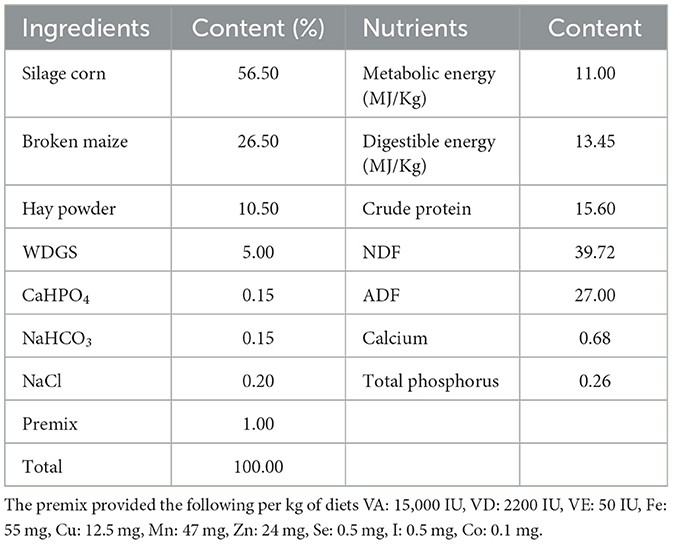
The study was carried out using a completely randomized design approach. Four groups were formed from a total of thirty-two Nubian goats (initial mean ± SE; 27 ± 1.39 kg body weight, and 5 ± 0.5 months of age) designated for fattening, with each group comprising eight goats (half male and half female). The basal diet formulations followed in this study were based on the guidelines provided by the NRC (21) and NY/T816-2004 (Table 1). The control group (CON group) was fed a standard basal diet. In contrast, the experimental groups received variations of this diet: one with an addition of 2.0 g/kg of Ampelopsis grossedentata flavonoids (AGF group), another incorporating 25 mg/kg of monensin (MN group), and the last one enhanced with 7.5 mL/kg of Tween 80 (TW group). We uniformly feed and manage all experimental goats. Spanning 100 days, the experiment was initiated with a 10-day preparatory phase dedicated to vaccination, deworming, management adjustments, and dietary transitions. The specific arrangement is shown in Figure 1A. The goats were fed twice daily, at 8:00 and 16:00 h with free access to fresh water.
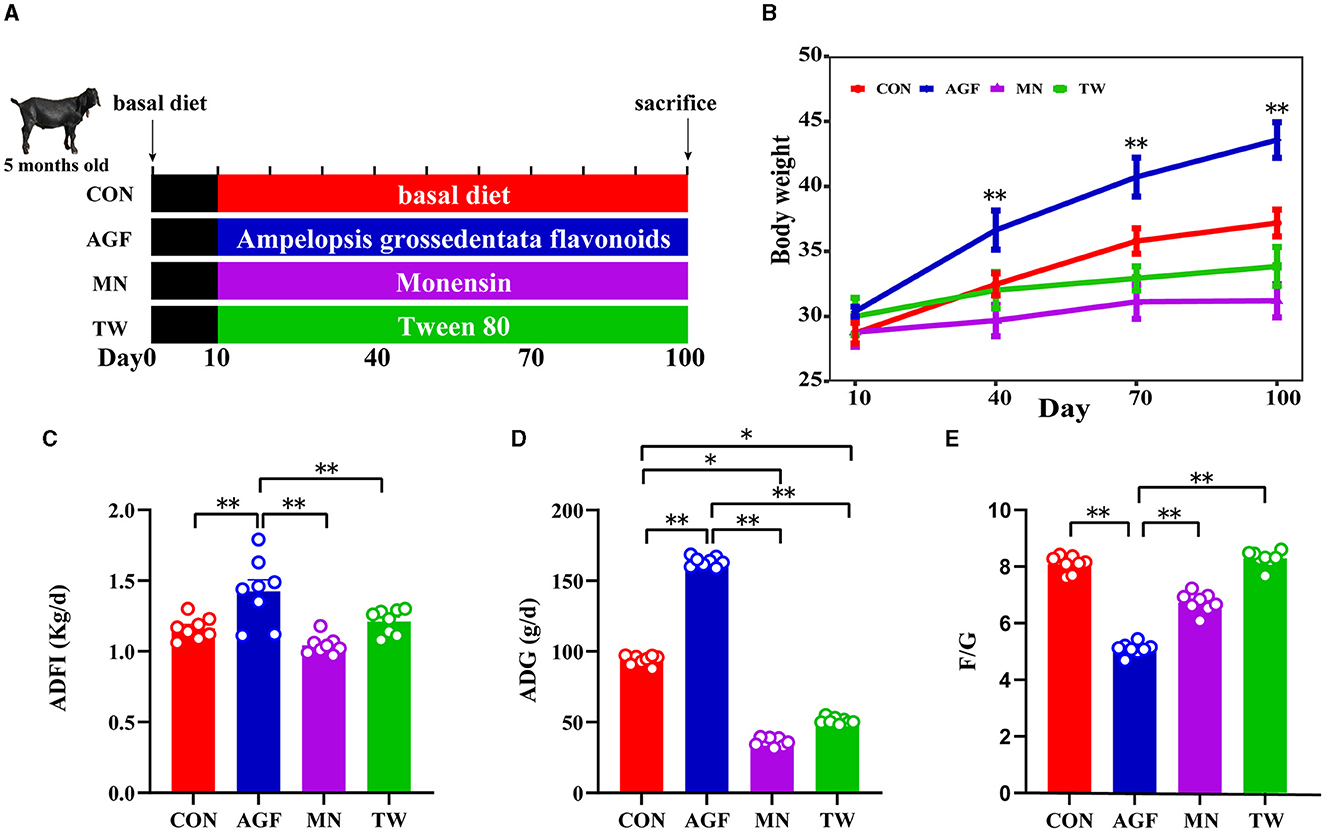
Figure 1. Influences of various additives on the growth performance in goats. (A) Schematic diagram of treatment of feed additives. (B) Changes in body weight of goats. (C) Average daily feed intake of goats. (D) The average daily gain of goats. (E) Feed to weight ratio of goats. * Indicates significant difference (p < 0.05), and ** indicates extremely significant difference (p < 0.01).
2.2 Sample collection
On the 101st day, at 07:00, we collected blood samples from the jugular veins of eight goats in each group, using vacutainer tubes. These samples were immediately spun down at 3,000 rpm and a temperature of 4°C. Following centrifugation, we separated the plasma and stored it at −20°C for future analysis. Additionally, on the same day, before feeding, we gathered rumen fluid samples from six goats in each group, selected for their weights being closest to the group's average, using a vacuum pump. For microbial analysis, we employed high-throughput sequencing to identify unique microbial sequences in the 16S region (22).
2.3 Growth performance
Daily records were kept of feed consumption. At the scheduled time points (07:00) on the 11th, 41st, 71st, and 101st days, goats undergo weight assessment after fasting. The feed conversion ratio was determined by applying the standard formula.
2.4 Plasma physiology and biochemistry
An automated biochemistry analyzer (Roche, Basel, Switzerland) was utilized to measure plasma physiology and biochemistry indices. These included triglyceride (TG), total protein (TP), glucose (GLU), UREA, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-CH), low-density lipoprotein cholesterol (LDL-CH), globulin (GLOB), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH).
2.5 16S rRNA gene sequencing
Microbial DNA was extracted using the HiPure Soil DNA Kits (Magen, Guangzhou, China) according to the manufacturer's protocols. The NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA) was employed for DNA quantification, ensuring the procurement of high-quality genomic DNA in adequate amounts (23). The V3-V4 regions of the 16S rDNA genes were then amplified by PCR, using specific forward (5′-CCTACGGGNGGCWGCAG-3′) and reverse (5′-GGACTACHVGGGTATCTAAT-3′) primers (24). PCR was used to amplify the ribosomal RNA gene, with the protocol starting at 94°C for 2 min, followed by 30 cycles of 98°C for 10 s, 62°C for 30 s, and 68°C for 30 s, culminating in a final extension at 68°C for 5 min. The resulting amplicons were then extracted from 2% agarose gels and underwent purification via the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA), as per the guidelines provided by the manufacturer. Quantification was carried out using the ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, CA). After purification, the amplicons underwent paired-end sequencing on the Illumina platform (PE250) following standard procedures.
FASTP (version 0.18.0) (25) was employed to filter raw data from the Illumina platform, resulting in clean reads for subsequent assembly analysis. The software FLASH (version 1.2.11) (26) was utilized to merge clean reads into tags based on a minimum overlap of 10bp and a maximum mismatch rate of 2%. Following the filtering criteria outlined in the literature (27), QIIME (version 1.9.1) (28) was employed to further filter low-quality tags, yielding a set of high-quality clean tags. Utilizing the UCHIME algorithm (29) with a reference database (version r20110519), chimera checking was performed on the clean tags. The resultant clean tags, post chimera filtering, were subjected to subsequent analysis. Using UPARSE software (version 7.1) (24), operational taxonomic units (OTUs) underwent reclustering at a 97% sequence similarity threshold (30). Chimeric sequences were identified and removed employing the Uchime algorithm (31, 32).
Principal Component Analysis (PCA) based on OTU abundance tables was conducted using the R language Vegan package (version 2.5.3) (33). The species abundance heatmap was generated using the R language heatmap package (version 1.0.12) (34). Pearson correlation analysis of species was computed using the psych package (version 1.8.4) (35). The species correlation network graph was constructed using the R language graph package (version 1.1.2) (36). Alpha-diversity indices, including Chao1 and Simpson, were calculated using QIIME version 1.9.1 (28). Diversity indices, including dilution curves and rank-abundance curves, were visualized through the ggplot2 package in R (version 2.2.1) (37). Non-metric Multidimensional Scaling (NMDS) analyses were conducted using the vegan package (version 2.5.3) in R, with resulting plots generated through the ggplot2 package (version 2.2.1) (38, 39). Intergroup Upset analysis, aimed at identifying unique and shared species, was performed using the UpSetR package in R (40). The LEfSe software (version 1.0) was utilized to identify biomarker features within each group (41). PICRUSt2 (version 1.0) was employed to infer KEGG pathway analysis of the OTUs (35). Between-group differences were evaluated through Welch's t-test and Wilcoxon rank-sum test for pairwise comparisons, alongside Tukey's Honestly Significant Difference (HSD) test and Kruskal-Wallis H test for multiple-group analyses, all conducted using the Vegan package (version 2.5.3) in R (33).
2.6 Data analysis
The impact of four diets on goats' growth performance, plasma metabolites, rumen pH, and rumen microbiota was analyzed using a one-way ANOVA in the SPSS 25.0 program (IBM Corp, Armonk, NY, USA), with results presented as means ± pooled SE (42). LSD and Duncan's multiple range tests facilitated multiple comparisons of means, categorizing differences as extremely significant (p < 0.01), significant (p < 0.05), or non-significant (p > 0.05) based on p values.
3 Results
3.1 Growth performance
The initial body weight comparison indicated no significant differences between the CON, AGF, MN, and TW groups (p > 0.05). However, a 90-day intake of Ampelopsis grossedentata flavonoids led to a significant increase in body weight in goats compared to the CON group (Figure 1B). The average daily feed intake (ADFI) and average daily gain (ADG) in the AGF group were significantly higher than those in the CON group, as well as those in the MN and TW groups (p < 0.01; Figures 1C, D). Additionally, the feed-to-gain ratio (F/G) in the AGF group was significantly lower than that in the CON group, and also significantly lower than that in the MN and TW groups (p < 0.01; Figure 1E).
3.2 Blood indexes
There were no significant differences in plasma TP and UREA concentrations between the four groups (p> 0.05) (Figures 2B, D). At the end of the experiment, there was no significant difference in plasma TG concentration between the AGF and CON groups (p > 0.05). However, the TG concentration in both groups was significantly higher than that in the MN and TW groups (p < 0.05; Figure 2A). There were no significant differences in plasma T-CHO and ALB concentrations between the AGF, TW, and CON groups (p > 0.05). However, the CON group had significantly higher T-CHO and ALB concentrations than the MN group (p < 0.05). The differences in plasma LDL-CH concentrations between the AGF, TW, and CON groups were not significant (p > 0.05). However, the LDL-CH concentration in the AGF group was significantly higher than that in the MN group (p < 0.05; Figures 2E, F). There were no significant differences (p > 0.05) in the plasma concentrations of GLU, AST, and ALT between the AGF, TW, and CON groups. The concentrations of GLU, AST, and ALT in the MN group were significantly lower than those in the TW group (p < 0.05), but there were no significant differences (p > 0.05) between the MN and AGF groups. In particular, the plasma concentration of ALT in the MN group was significantly lower than that in the CON and AGF groups (Figures 2C, G). The concentrations of ALP and LDH in MN group were significantly lower than those in AGF group and CON group, respectively (p < 0.05) (Figure 2H).
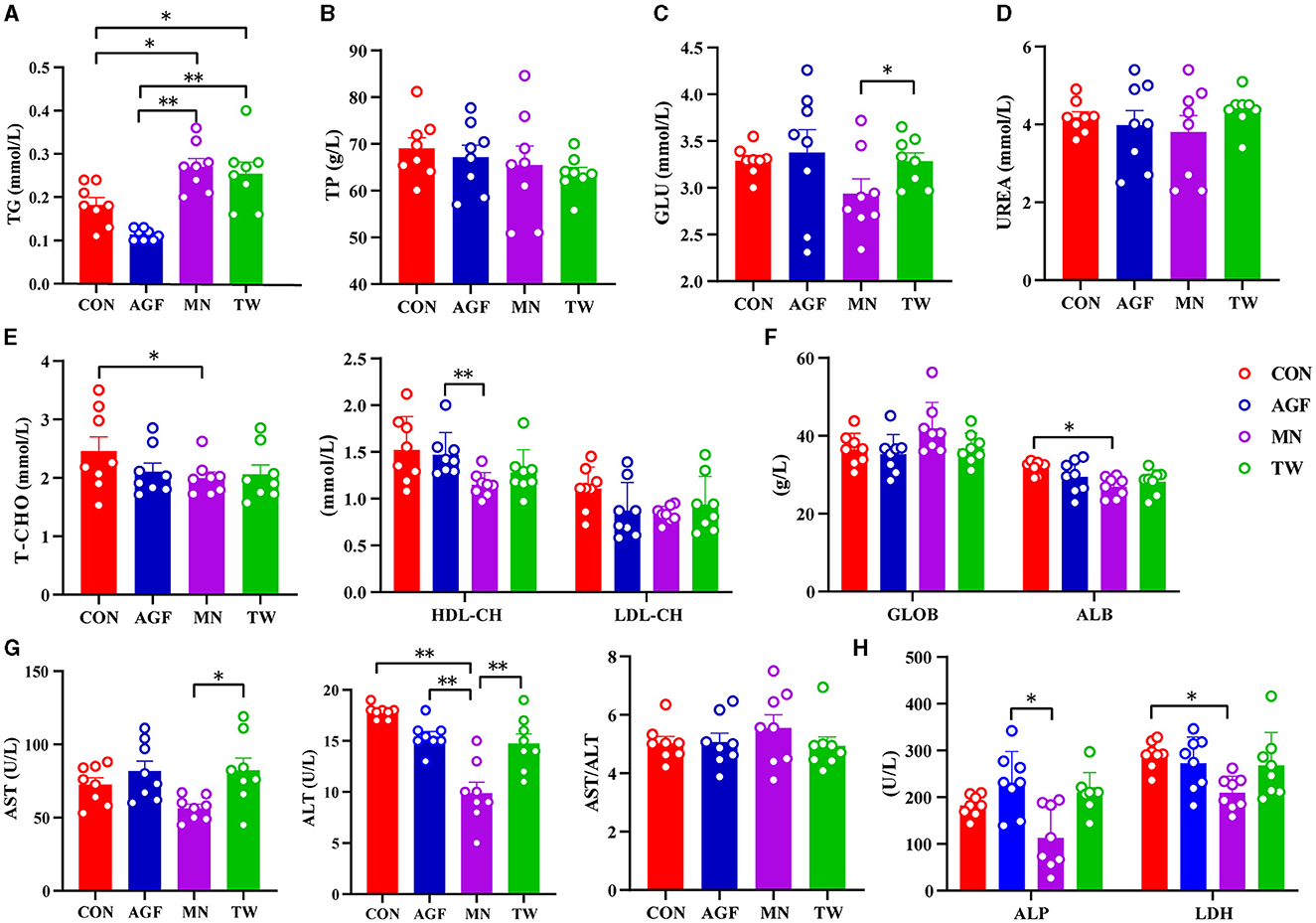
Figure 2. Effects of different additives on plasma metabolites of goats. (A) Hepatic triglyceride (TG) level. (B) Total protein (TP) level. (C) Glucose (GLU) level. (D) UREA level. (E) Cholesterol level. (F) Globulin (GLOB) and albumin (ALB) levels. (G) Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. (H) Alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels. *Indicates a significant difference (p < 0.05), **Indicates an extremely significant difference (p < 0.01).
3.3 Composition and diversity of the rumen microbiota
3.3.1 Rumen pH
Rumen pH was measured across different groups, yielding values of 6.71 ± 0.03 (CON), 6.62 ± 0.02 (AGF), 6.42 ± 0.05 (MN), and 6.61 ± 0.06 (TW), respectively. The analysis indicated that the various additives did not significantly affect rumen pH in goats (p > 0.05).
3.3.2 Analysis of microbial diversity in the rumen
The Good's coverage of this sequencing exceeded 99%, ranging from 99.70% to 99.84% (Figure 3A). A diversity analysis was performed on OTUs to further understand the richness and diversity of microbial composition among the CON, AGF, MN, and TW groups. Compared to the CON group, all five α-diversity indices in the AGF group were significantly lower (p < 0.05). In contrast, there were no significant differences in the five α-diversity indices between the TW and CON groups (p > 0.05). The α-diversity in the AGF group was significantly lower than that in the MN and TW groups (p < 0.05). The microbial species richness in the AGF group was significantly decreased (p < 0.05; Figure 3B), whereas there was no significant difference in evenness (p > 0.05; Figure 3C). Principal Coordinate Analysis (PCoA) is a method of dimensionality reduction that employs a two-dimensional plane to visualize the distances between samples. Coordinates 1 represents 14.44% of the variation and coordinates 2 represents 11.11% of the variance (Figure 3D). The rumen microbial composition exhibited greater similarity between the TW and MN groups.
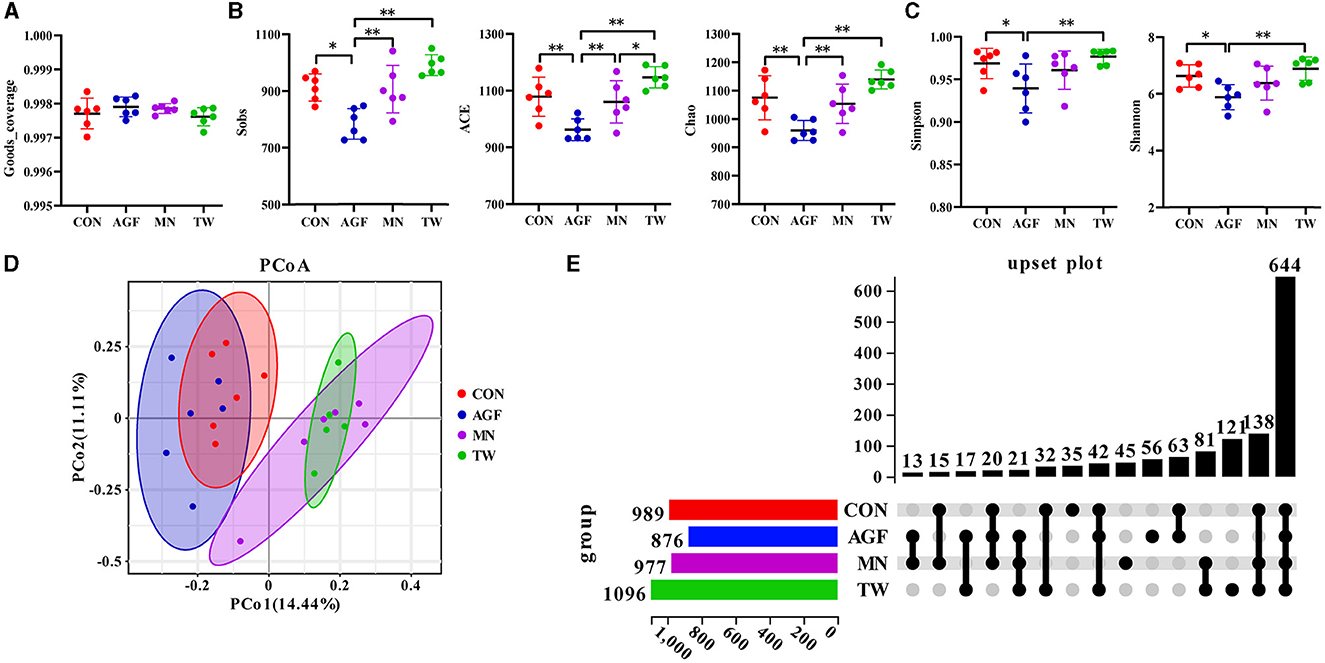
Figure 3. Diversity of rumen microbiota community as affected by additives. (A) Good's coverage index. (B) Sobs, ACE, and Chao1 indexes. (C) Shannon and Simpson indexes. (D) PCoA analysis. (E) Upset plot diagram at the OTU level of rumen microbiota in four groups. *Indicates a significant difference (p < 0.05), **Indicates an extremely significant difference (p < 0.01).
3.3.3 OTU cluster
Following the exclusion of tags deemed of low quality or lacking biological significance, analysis of the 32 samples yielded a total of 2,879,868 effective tags. Each sample generated between 110,629 and 128,204 effective tags, with an effective rate of over 92% (Supplementary Table S1). A total of 989, 876, 977, and 1,096 operational taxonomic units (OTUs) were observed in the CON, AGF, MN, and TW groups, with 35, 56, 45, and 121 OTUs being unique to the CON, AGF, MN, and TW groups, respectively (Figure 3E). There were 644 OTUs common to all four groups, and intersections of CON and AGF, MN, TW groups contained 63, 15, and 32 OTUs, respectively (Figure 3E).
3.3.4 Microbial composition
Within the phylum level, Bacteroidetes and Firmicutes constituted the predominant phyla, collectively accounting for over 90% of the microorganisms across all four groups (Figure 4A), and their relative abundances ranging from 33.38% to 76.59% and 18.07% to 56.22%, respectively (Supplementary Table S2). Compared to the CON group, there were no significant differences in the relative abundance of Bacteroides and Firmicutes in the AGF and TW groups (p > 0.05). Additionally, the relative abundances of Proteobacteria and Kiritimatiellaeota in the AGF group did not show significant differences (p > 0.05). However, in the TW group, the relative abundance of Proteobacteria decreased significantly, while the relative abundance of Kiritimatiellaeota increased significantly (p < 0.05). Compared to the MN group, the relative abundances of Bacteroides and Proteobacteria were significantly higher in the AGF group (p < 0.01), whereas the relative abundances of Firmicutes and Kiritimatiellaeota were significantly lower (p < 0.05). There were no significant differences in the relative abundances of these taxa in the TW group (p > 0.05) (Figure 4B).
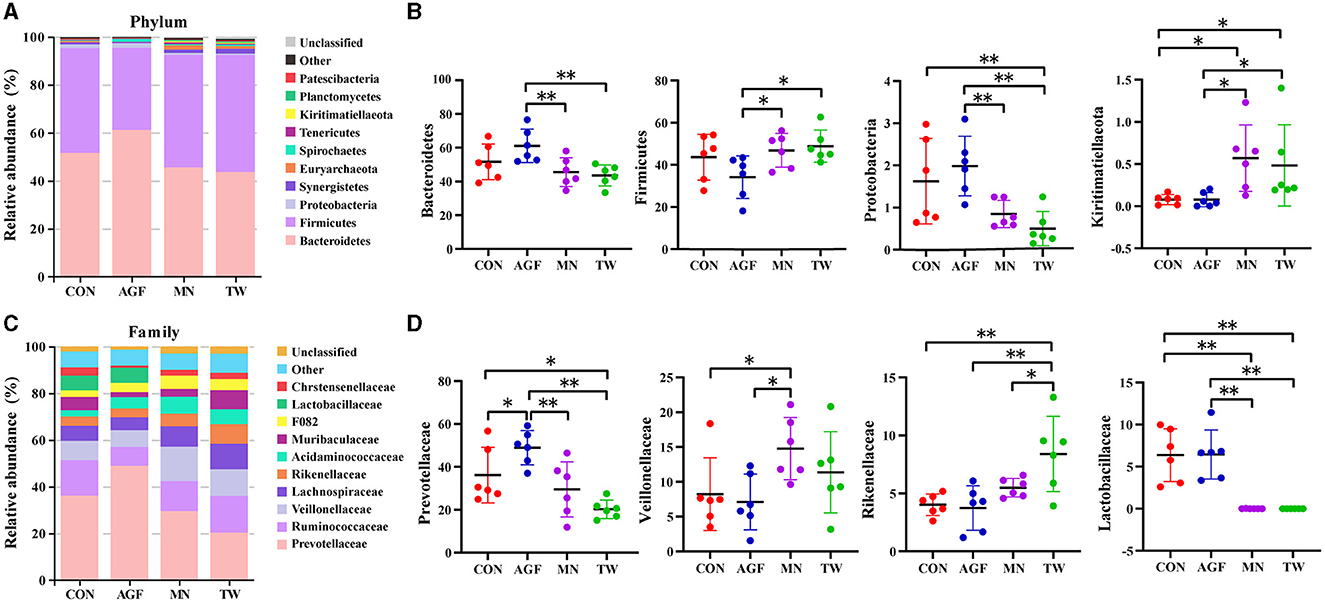
Figure 4. Species composition analysis of rumen microorganisms of goat at phylum and family level. (A) Top 10 dominant species at phylum level. (B) Dominant species of significant differences at phylum level. (C) Top 10 dominant species at family level. (D) Dominant species of significant differences at family level. *Indicates a significant difference (p < 0.05), **Indicates an extremely significant difference (p < 0.01).
Within the family level, Prevotellaceae emerged as the dominant microbiota (Figure 4C). Compared with CON group, the relative abundance of Prevotellaceae in AGF group was significantly increased, while that in TW group was significantly decreased (p < 0.05). Compared with MN group, the relative abundance of Prevotellaceae in AGF group was significantly increased (p < 0.05), but not in TW group (p > 0.05). Compared with CON group, the relative abundance of Veillonellaceae, Rikenellaceae, and Lactobacillaceae in AGF group was not significantly different (p > 0.05), while the relative abundance of Rikenellaceae in TW group was significantly increased (p < 0.05). The relative abundance of Lactobacillaceae was significantly decreased (p < 0.01). Compared with the MN group, the relative abundance of Veillonellaceae in AGF group was significantly decreased, and the relative abundance of Lactobacillaceae was significantly increased (p < 0.05). The relative abundance of Rikenellaceae in TW group was significantly increased, and the relative abundance of Lactobacillaceae was significantly decreased (p < 0.05) (Figure 4D).
Within the genus level, compared with CON group, the relative abundance of Prevotella_1 in AGF group is significantly higher (p < 0.05), the relative abundance of Rikenellaceae_RC9_gut_group and Lactobacillus in TW group is not significantly different, and the relative abundance of Prevotella_1 in TW group is not significantly different (p > 0.05) (Figures 5A, B). The relative abundance of Rikenellaceae_RC9_gut_group was significantly increased, and the relative abundance of Lactobacillus was significantly decreased (p < 0.05). Compared with MN group, the relative abundance of Prevotella_1 and Lactobacillus in AGF group was significantly increased (p < 0.05), while the relative abundance of Rikenellaceae_RC9_gut_group was not significantly different (p > 0.05) (Figure 5B).
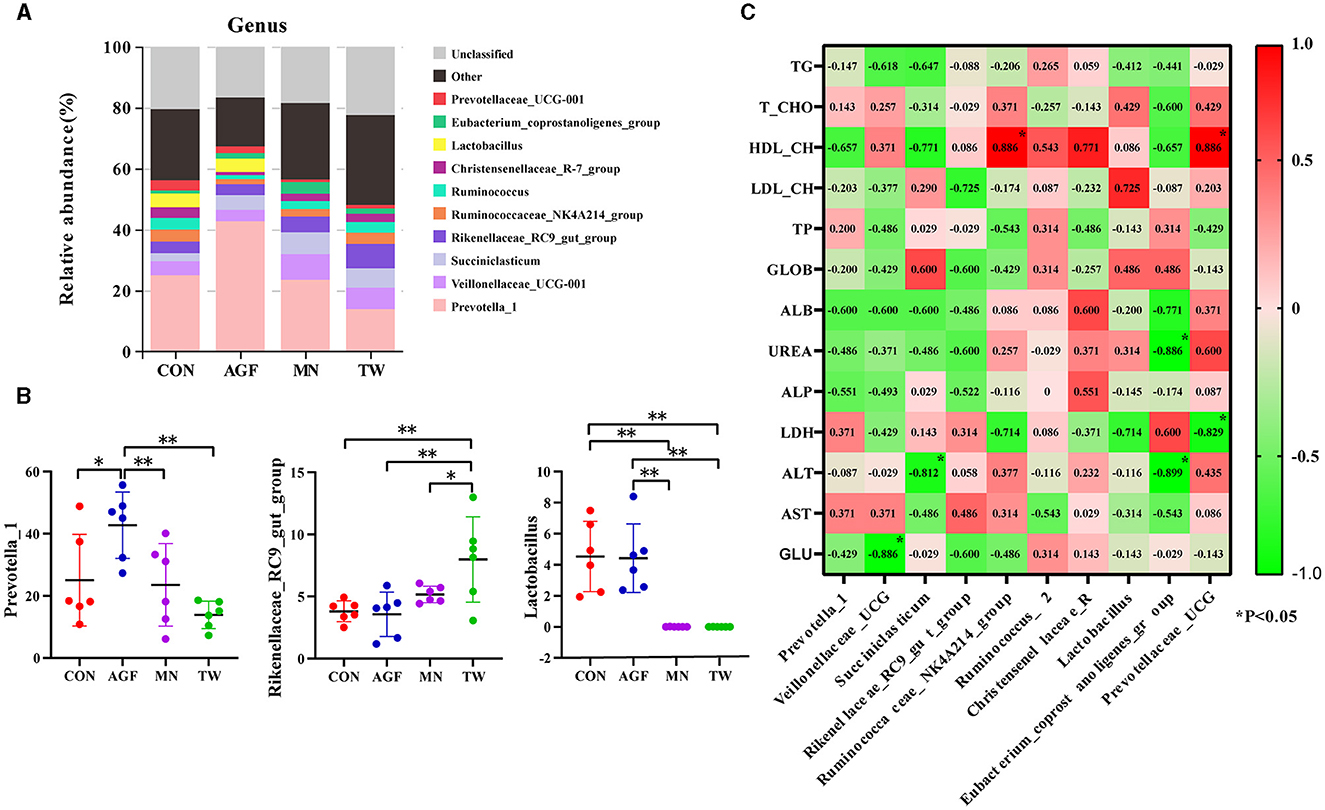
Figure 5. Species analysis at genus level. (A) Top 10 dominant species at genus level. (B) Dominant species of significant differences at genus level. (C) Analysis of the correlation between dominant genera in the rumen microbiota and plasma metabolites. *Indicates a significant difference (p < 0.05), **Indicates an extremely significant difference (p < 0.01).
Correlation analysis was conducted between dominant rumen microbial genera in AGF group and various physiological and biochemical parameters in plasma, and the results were shown in Figure 5C. Veillonellaceae_UCG-001 is significantly positively correlated with GLU (p < 0.05). Succiniclasticum is significantly negatively correlated with ALT (p < 0.05). The Ruminococcaceae_NK4A214_group and Prevotellaceae_UCG-001 exhibited a significant positive correlation with HDL-CH (p < 0.01). Conversely, the Eubacterium_coprostanoligenes_group showed a significant negative correlation with UREA and AST (p < 0.01).
3.3.5 Analysis of rumen differential microbiota
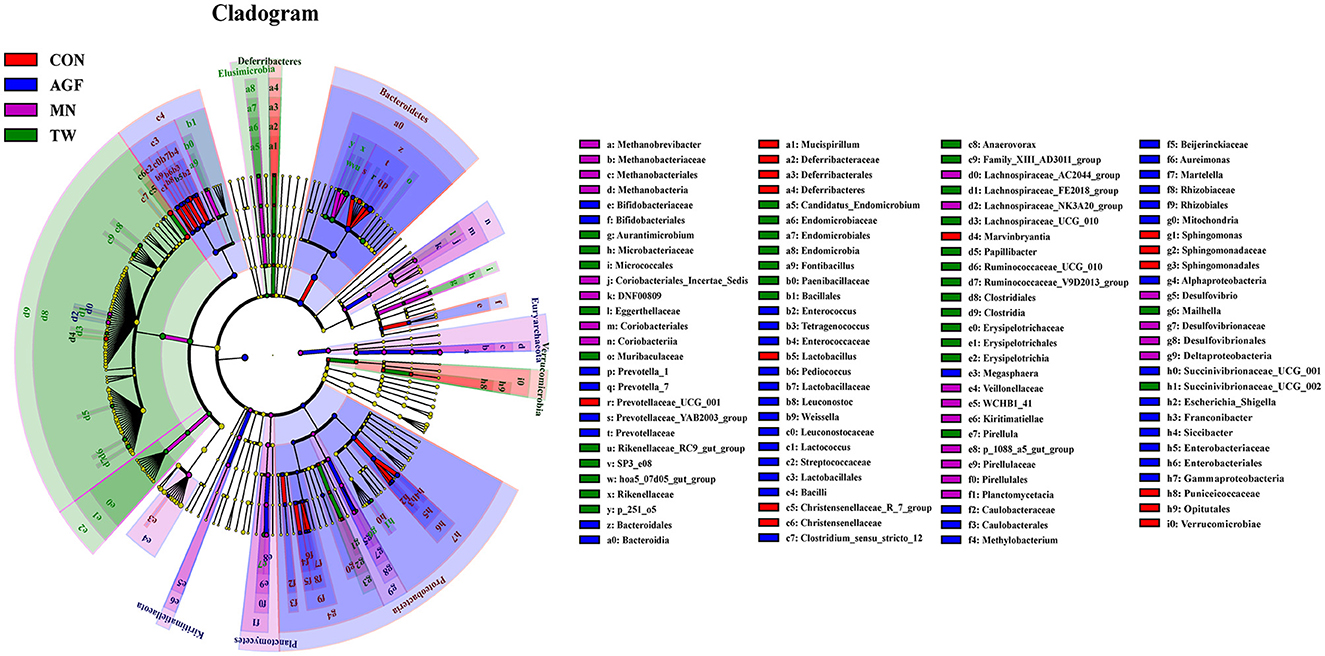
In the AGF, CON, MN, and TW groups, a total of 117 biomarkers across various taxonomic levels were identified. Notably, Proteobacteria, Deferribacteres, Euryarchaeota, and Elusimicrobia emerged as key taxa that contributed to the observed differences in rumen microbiota among these groups (Figure 6). In the AGF and MN groups, a total of 112 biomarkers across various taxonomic levels were identified. Notably, Prevotellaceae and Clostridia emerged as key taxa that contributed to the observed differences in rumen microbiota among these groups (Supplementary Table S3). In the AGF and CON groups, a total of 24 biomarkers across various taxonomic levels were identified. Notably, Clostridia and Pseudomonadales emerged as key taxa contributed to the observes differences in rumen microbiota among these groups (Supplementary Table S4). In the TW and MN groups, a total of 12 biomarkers across various taxonomic levels were identified. Notably, Erysipelotrichia and Bacterium_VCD2007 emerged as key taxa contributed to the observes differences in rumen microbiota among these groups (Supplementary Table S5). In the TW and CON groups, a total of 92 biomarkers across various taxonomic levels were identified. Notably, Rikenellaceae and Prevotellaceae emerged as key taxa contributed to the observes differences in rumen microbiota among these groups (Supplementary Table S6).
3.3.6 Microbiota functional profile prediction
Functional prediction of the rumen microbiota was performed on the PICRUSt2 platform. Welch's t-test was applied to identify significant differences in functional pathways among the four groups at level 2. Two functional categories exhibited significant differences between the AGF and CON groups (p < 0.05). In the AGF group, both glycan biosynthesis and metabolism, and the biosynthesis of other secondary metabolites, were significantly elevated compared to the CON group (p < 0.05) (Figure 7A). Significant disparities were observed across five functional categories between the MN and CON groups (p < 0.05). In the MN group, the levels of amino acid metabolism, folding, sorting and degradation, and cell motility were significantly elevated compared to the CON group (p < 0.05) (Figure 7B). Eight functional classifications in the TW and CON groups showed statistically significant differences, with the TW group being significantly lower than the CON group (p < 0.05) (Figure 7C). At level 3, a total of twenty functional categories within the rumen microbiota of goats were predicted by the study. The AGF and MN groups showed similar enrichment in eighteen functional categories (Figure 7D).
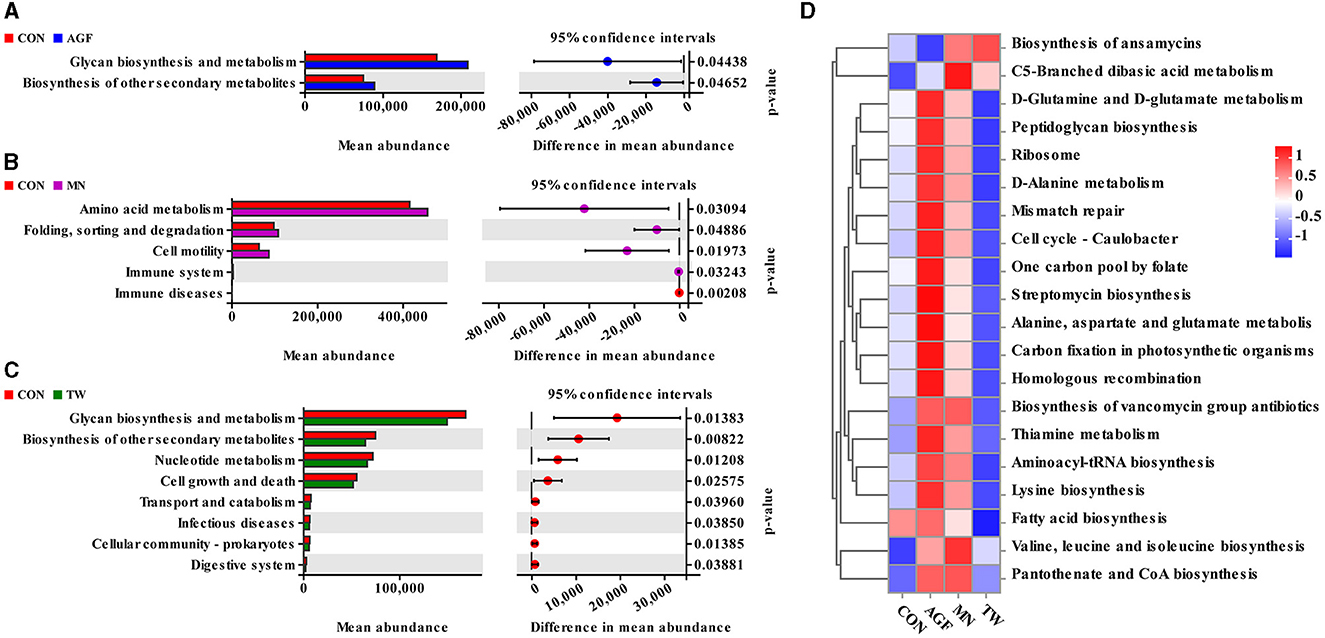
Figure 7. Forecasting the functions of microbiota using the PICRUSt2 platform. (A) Markedly distinct signaling pathways at level 2 between the AGF group and the CON group (p < 0.05). (B) Markedly distinct signaling pathways at level 2 between the MN group and the CON group (p < 0.05). (C) Markedly distinct signaling pathways at level 2 between the TW group and the CON group. (D) Level 3 heat map.
4 Discussion
The comprehensive “ban” may have a certain impact on the production capacity of animal husbandry in the short term. At present, it has been found that plant extracts (43), probiotics (44), enzyme preparations (45), chitosan (46) and antimicrobial peptides (47) have the characteristics of high efficiency and no side effects. This research group has conducted experiments on natural plant additives and food additives, so as to overcome the defects of antibiotics to different degrees. Among these, Ampelopsis grossedentata flavonoids, a plant extract (48), and Tween 80, a non-ionic surfactant (49), both of which are non-toxic and harmless, are expected to become new pollution-free green feed additives. However, information on the use of AGF in ruminants remains scarce. Information regarding AGF application in ruminant animals is still limited. Thus, the objective of this study is to determine the effectiveness of AGF and TW by analyzing their influences on goats' growth performance, plasma metabolites, and rumen microbiota, all of which are key indicators of the animals' growth and health condition.
The improvement of goats' growth performance is closely related to ADFI and feed utilization. The results showed that both ADFI and ADG were higher in AGF group than in other groups, with the lowest F/G ratio, indicating high feed utilization and an effective fattening effect. This effectiveness is attributed to Ampelopsis grossedentata flavonoids' ability to alleviate oxidative stress responses, reduce gastrointestinal damage, and promote the gastrointestinal growth and development of goats (4). Contrary to expectations, the TW group demonstrated an adverse effect, differing from previous studies. For instance, McAllister et al. (16) observed that Tween 80 treatment in goats significantly reduced DMI and daily gain compared to untreated controls. Lee et al. (17) reported an increase in DMI and milk production in cows treated with Tween 80. The differing responses may be attributed to Ampelopsis grossedentata flavonoids' appealing fragrance enhancing feed intake, whereas Tween 80′s slightly unpleasant smell and bitter taste could deter goat from eating. Considering these factors, Ampelopsis grossedentata flavonoids show promise as a monensin substitute for enhancing growth performance, yet further rigorous testing is required.
Changes in plasma composition can reflect the nutritional level and organ function of ruminants (50). ALT and AST are two types of aminotransferases, play crucial roles in evaluating liver function, amino acids and proteins, metabolism, and indirectly influence the decomposition and synthesis of fats and sugars (51). Normally, AST levels in goats ranges from 40.0 to 123.0 U/L, but can increase when the liver is damaged (52). In this experiment, the plasma AST activity in all goat groups was within the normal range. ALP, an enzyme extensively found in various tissues such as the liver, bones, intestines, kidneys, and placenta, is secreted from the liver into the bile. It is associated with bone growth in animals, as well as the transport and utilization of sugars, lipids, proteins, and phosphoric acid. ALP levels reflect the growth and development of livestock and poultry (53). The ALP activity in goat plasma of the AGF group was significantly higher than in the MN group, warranting further investigation. HDL-CH, considered good cholesterol, transports lipids from tissues to the liver. In this experiment, HDL-CH levels in the AGF group were significantly higher than in the MN group. AGF is helpful to the absorption and transport of lipids in ruminants. In summary, AGF has shown to improve growth and development and lipid levels and lipid profiles in goats.
Rumen fluid pH reflects rumen fermentation status mainly by affecting rumen microbial activity. Studies have shown that dietary composition, saliva secretion, rumen fermentation product utilization, absorption efficiency, and before and after feeding can all cause rumen fluid pH changes, and dietary composition is the key factor causing pH changes (54). In addition, when rumen pH is 6.4 to 6.8, cellulose decomposing bacteria can achieve a suitable environment (55). When the pH of the rumen was 6.2 to 7.0, the rumen microbial ecosystem was relatively stable, which could ensure the normal fermentation of rumen (56). In this experiment, supplementing the diet with various feed additives did not markedly influence the pH of rumen fluid, which remained within the range of 6.4 to 6.8, indicating that the rumen environment was relatively stable, and the measured number of rumen microorganisms accurately reflected the microflora.
Rumen microorganisms and their hosts constitute a complex microecosystem, which plays a significant role in ruminant nutrition. In-depth study of rumen microecosystem is conducive to further understanding the mechanism of ruminant nutrition (57). Alpha-diversity refers to the diversity within a particular habitat or ecosystem. Good's Coverage reflects the sequencing saturation of the sample. Sobs, Chao1, and ACE indexes are mainly concerned with the species richness of samples. Simpson and Shannon comprehensively reflect the richness and evenness of species, so the level of the index will also be affected by evenness. The more evenly the species are distributed in the sample, the higher the diversity will be. In this experiment, the sequencing saturation of each group was sufficient. The Sobs, ACE, Chao1, Shannon, and Simpson indexes of the TW group were significantly higher than those of the other three groups. In this experiment, TW group had the most OTU and AGF group had fewer OTUs, which also corresponded to the diversity of results. In a nutshell, the findings suggest that the TW group exhibited the greatest species richness and evenness in the goat rumen fluid, thereby possessing the highest level of species diversity as well.
Firmicutes and Bacteroidetes emerged as the two predominant bacterial phyla in this study, corroborating the findings of earlier research (58). Among the top 10 phyla, the relative abundance of Bacteroides and Proteobacteria in the TW group is significantly lower than that in the AGF group, while the relative abundance of Firmicutes and Kiritimatiellaeota shows the opposite trend. Bacteroides play a key role in metabolizing polysaccharides and oligosaccharides, thereby furnishing nutrients and vitamins to both the host and other microbes residing in the intestines (59). Gut Firmicutes, stimulated by dietary fiber, and their metabolites display significant functions that promote health (60). Prevotella_1 is the dominant genus, which is consistent with the results of previous studies, and its relative abundance in the AGF group is significantly higher than in other groups. Moreover, Prevotella is capable of utilizing starch, monosaccharides, and various non cellulosic polysaccharides (60). These results indicate that the synthesis and metabolism of sugars in the AGF group are stronger, which is consistent with the functional prediction in this experiment.
The Rikenellaceae_RC9_gut_group, part of the Rikenellaceae family, primarily ferments unabsorbed polysaccharides in the host's gut, leading to production of short-chain fatty acids like acetate, propionate, and butyrate (61). In this experiment, the relative abundance of Rikenellaceae_RC9_gut_group in the TW group was significantly higher than that in other groups. However, the glucose metabolism function of the TW group was significantly lower than that of the CON and AGF groups, which is speculated to be due to the lower abundance of Rikenellaceae_RC9_gut_group compared to Prevotella_1 among the total bacterial genera. Lactobacillus is regarded as a beneficial bacterium, crucial for preserving the equilibrium of intestinal microbiota and thwarting the invasion of pathogens (62). The relative abundance of Lactobacillus in the AGF and CON groups was significantly higher than that in the TW and MN groups. In conclusion, the rumen microbial balance and glucose anabolic ability of the AGF group were relatively strong, and the diversity of AGF group was closer to that of the CON group, making it a good choice for green additives.
5 Conclusions
The adjustment diagram of this study is shown in Figure 8, which is drawn according to the experimental results. In conclusion, dietary addition of Ampelopsis grossedentata flavonoids can significantly increase the relative abundance of beneficial bacteria and affect the plasma physiological and biochemical indexes of goats. Further improve the average daily feed intake and daily gain of goats and reduce the ratio of feed to gain. Dietary supplementation of Tween 80 significantly increases the diversity and abundance of rumen microorganisms, thereby optimizing the microbial community and improving rumen health. However, despite these benefits, the abundance of beneficial bacteria is lower than in the control group, which suggests that improvements in rumen microbial diversity alone may not fully compensate for the lower palatability. Consequently, the feeding effect is poorer compared to the AGF group, indicating that the feeding method needs further improvement. All in all, Ampelopsis grossedentata flavonoids show potential as a new green additive to replace monensin and reduce stress in animal husbandry.
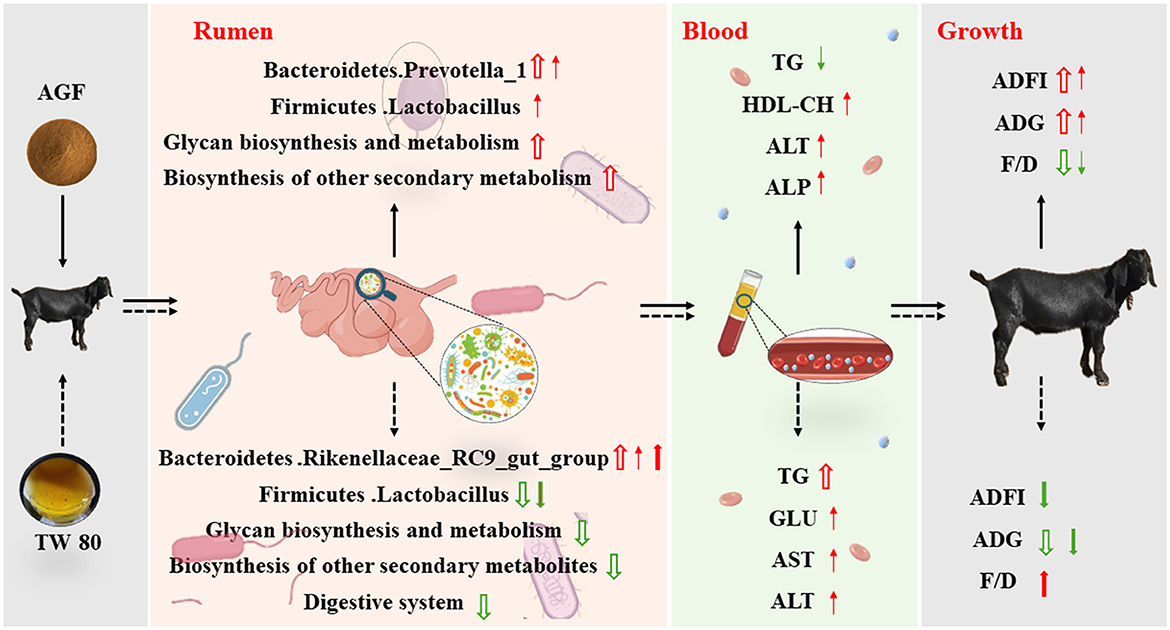
Figure 8. Regulation of Ampelopsis grossedentata flavonoids and Tween 80 on rumen microbial, plasma metabolites and growth performance of goats. Red arrows signify increases, while blue arrows indicate decreases. The hollow arrow indicates comparison with the CON group, the thin arrow indicates comparison with the MN group, and the thick arrow indicates comparison with the AGF group.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJna956984.
Ethics statement
The animal studies were approved by Animal Care and Use Committee of Yunnan Agricultural University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. ZG: Investigation, Software, Writing – review & editing. YC: Investigation, Software, Writing – review & editing. ML: Formal analysis, Investigation, Writing – review & editing. WD: Investigation, Resources, Writing – review & editing. DX: Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Research and Development Project (2022YFD1100408).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1411071/full#supplementary-material
References
1. Wen R, Li C, Zhao M, Wang H, Tang Y. Withdrawal of antibiotic growth promoters in China and its impact on the foodborne pathogen campylobacter coli of swine origin. Front Microbiol. (2022) 13:1004725. doi: 10.3389/fmicb.2022.1004725
2. Toutain PL, Ferran AA, Bousquet-Melou A, Pelligand L, Lees P. Veterinary medicine meeds new green antimicrobial drugs. Front Microbiol. (2016) 7:1196. doi: 10.3389/fmicb.2016.01196
3. Zhang S, Gao S, Chen Y, Xu S, Yu S, Zhou J. Identification of hydroxylation enzymes and the metabolic analysis of dihydromyricetin synthesis in Ampelopsis Grossedentata. Genes. (2022) 13:2318. doi: 10.3390/genes13122318
4. Hou X, Tong Q, Wang W, Xiong W, Shi C, Fang J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci. (2015) 130:38–46. doi: 10.1016/j.lfs.2015.03.007
5. Wu Y, Bai J, Zhong K, Huang Y, Gao H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2r,3r-dihydromyricetin from pine needles of cedrus deodara against staphylococcus aureus. Food Chem. (2017) 218:463–70. doi: 10.1016/j.foodchem.2016.07.090
6. Zhang Y, Yang H, Li S, Li WD, Wang Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: a cohort study in the Uk biobank. PLoS Med. (2021) 18:e1003830. doi: 10.1371/journal.pmed.1003830
7. Zhang X, Wang L, Peng L, Tian X, Qiu X, Cao H, et al. Dihydromyricetin protects huvecs of oxidative damage induced by sodium nitroprusside through activating Pi3k/Akt/Foxo3a signalling pathway. J Cell Mol Med. (2019) 23:4829–38. doi: 10.1111/jcmm.14406
8. Wu RR, Li X, Cao YH, Peng X, Liu GF, Liu ZK, et al. China medicinal plants of the Ampelopsis Grossedentata-a review of their botanical characteristics, use, phytochemistry, active pharmacological components, and toxicology. Molecules. (2023) 28:7145. doi: 10.3390/molecules28207145
9. Xiao XN, Wang F, Yuan YT, Liu J, Liu YZ Yi X. Antibacterial activity and mode of action of dihydromyricetin from Ampelopsis Grossedentata leaves against food-borne bacteria. Molecules. (2019) 24:2831. doi: 10.3390/molecules24152831
10. Liang H, He K, Li T, Cui S, Tang M, Kang S, et al. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against staphylococcus aureus. Sci Rep. (2020) 10:21416. doi: 10.1038/s41598-020-78379-y
11. Luo QJ, Zhou WC, Liu XY, Li YJ, Xie QL, Wang B, et al. Chemical constituents and a-glucosidase inhibitory, antioxidant and hepatoprotective activities of Ampelopsis Grossedentata. Molecules. (2023) 28:7956. doi: 10.3390/molecules28247956
12. Wang Z, Jiang Q, Li P, Shi P, Liu C, Wang W, et al. The water extract of Ampelopsis Grossedentata alleviates oxidative stress and intestinal inflammation. Antioxidants. (2023) 12:0547. doi: 10.3390/antiox12030547
13. Zhu J, Liu X, Lu Y, Yue D, He X, Deng W, et al. Exploring the impact of Ampelopsis Grossedentata Flavonoids on growth performance, ruminal microbiota, and plasma physiology and biochemistry of kids. Animals. (2023) 13:2454. doi: 10.3390/ani13152454
14. Akin DE. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. (1980) 39:242–52. doi: 10.1128/aem.39.1.242-252.1980
15. Lu Y, Wang YY, Yang N, Zhang D, Zhang FY, Gao HT, et al. Food emulsifier polysorbate 80 increases intestinal absorption of Di-(2-ethylhexyl) phthalate in rats. Toxicol Sci. (2014) 139:317–27. doi: 10.1093/toxsci/kfu055
16. McAllister TA, Stanford K, Bae HD, Treacher RJ, Hristov AN, Baah J, et al. Effect of a surfactant and exogenous enzymes on digestibility of feed and on growth performance. Canadian J Animal Sci. (2000) 80:35–44. doi: 10.4141/A99-053
17. Lee SS, Ahn BH, Kim HS, Kim CH, Cheng KJ, Ha JK. Effects of non-ionic surfactants on enzyme distributions of rumen contents, anaerobic growth of rumen microbes, rumen fermentation characteristics and performances of lactating cows. Asian Austral J Animal Sci. (2003) 16:104–15. doi: 10.5713/ajas.2003.104
18. Okino S, Ikeo M, Ueno Y, Taneda D. Effects of Tween 80 on cellulase stability under agitated conditions. Bioresour Technol. (2013) 142:535–9. doi: 10.1016/j.biortech.2013.05.078
19. Menberu MA, Hayes AJ, Liu S, Psaltis AJ, Wormald PJ, Vreugde S. Tween 80 and its derivative oleic acid promote the growth of corynebacterium accolens and inhibit staphylococcus aureus clinical isolates. Int Forum Allergy Rhinol. (2021) 11:810–3. doi: 10.1002/alr.22730
20. Olukanni OD, Albert AA, Farinto M, Awotula AO, Osuntoki AA. Tween-80 enhanced biodegradation of naphthalene by klebsiella quasipneumoniae. Antonie Van Leeuwenhoek. (2023) 116:697–709. doi: 10.1007/s10482-023-01839-8
21. National Research Council (NRC) (2007). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC: The National Academies Press.
22. Lodge-Ivey SL, Browne-Silva J, Horvath MB. Technical note: bacterial diversity and fermentation end products in rumen fluid samples collected via oral lavage or rumen cannula. J Anim Sci. (2009) 87:2333–7. doi: 10.2527/jas.2008-1472
23. Thermo Fisher Scientific (2009). NanoDrop 2000/2000c Spectrophotometers. [User Manual]. Available online at: https://www.thermofisher.com/nanodrop
24. Guo M, Wu F, Hao G, Qi Q, Li R, Li N, et al. Bacillus subtilis improves immunity and disease resistance in rabbits. Front Immunol. (2017) 8:354. doi: 10.3389/fimmu.2017.00354
25. Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one fastq preprocessor. Bioinformatics. (2018) 34:i884–i90. doi: 10.1093/bioinformatics/bty560
26. Magoč T, Salzberg SL. Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
27. Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat Methods. (2013) 10:57–9. doi: 10.1038/nmeth.2276
28. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6. doi: 10.1038/nmeth.f.303
29. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics. (2011) 27:2194–200. doi: 10.1093/bioinformatics/btr381
30. Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. (2004) 32:1792–7. doi: 10.1093/nar/gkh340
31. Price MN, Dehal PS, Arkin AP. Fasttree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE. (2010) 5:e9490. doi: 10.1371/journal.pone.0009490
32. Lozupone C, Knight R. Unifrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. (2005) 71:8228–35. doi: 10.1128/AEM.71.12.8228-8235.2005
33. Oksanen JB, Kindt FG. R Vegan: community ecology package. R package. (2010). Available online at: https://cran.r-project.org (accessed February 10, 2024).
34. Kolde RK. M R. Package ‘Pheatmap'. R package (2015). Available online at: https://CRAN.R-project.org/package=pheatmap (accessed February 14, 2024).
35. Revelle WR MW. Package ‘Psych'. The Comprehensive R Archive Network. (2015). Available online at: https://CRAN.R-project.org/package=psychTools (accessed February 13, 2024).
36. Csardi MG. Package‘Igraph'. (2013) 3:2013. Available online at: https://CRAN.R-project.org/package=igraph (accessed February 10, 2024).
37. Wickham HC. W Ggplot2: an implementation of the grammar of graphics. R package version 07. (2008). Available online at: http://CRANR-projectorg/package=~ggplot2 (accessed February 10, 2024).
38. Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16s rRNA marker gene Sequences. Nat Biotechnol. (2013) 31:814–21. doi: 10.1038/nbt.2676
39. Ward TL, J. Meulemans, J. Bugbase predicts organism level microbiome phenotypes. BioRxiv. (2017). p. 133462. doi: 10.1101/133462
40. Conway JR, Lex A, Gehlenborg N. Upsetr: an R package for the visualization of intersecting sets and their properties. Bioinformatics. (2017) 33:2938–40. doi: 10.1093/bioinformatics/btx364
41. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. (2011) 12:R60. doi: 10.1186/gb-2011-12-6-r60
42. Pollesel M, Tassinari M, Frabetti A, Fornasini D, Cavallini D. Effect of does parity order on litter homogeneity parameters. Ital J Anim Sci. (2020) 19:1188–94. doi: 10.1080/1828051X.2020.1827990
43. Jayanegara A, Yogianto Y, Wina E, Sudarman A, Kondo M, Obitsu T, et al. Combination effects of plant extracts rich in tannins and saponins as feed additives for mitigating in vitro ruminal methane and ammonia formation. Animals. (2020) 10:1531. doi: 10.3390/ani10091531
44. Markowiak P, Sliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. (2017) 9:1021. doi: 10.3390/nu9091021
45. Peters A, Lebzien P, Meyer U, Borchert U, Bulang M, Flachowsky G. Effect of exogenous fibrolytic enzymes on ruminal fermentation and nutrient digestion in dairy cows. Arch Anim Nutr. (2010) 64:221–37. doi: 10.1080/17450391003693142
46. Swiatkiewicz S, Swiatkiewicz M, Arczewska-Wlosek A, Jozefiak D. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J Anim Physiol Anim Nutr. (2015) 99:1–12. doi: 10.1111/jpn.12222
47. Liu Q, Yao S, Chen Y, Gao S, Yang Y, Deng J, et al. Use of antimicrobial peptides as a feed additive for juvenile goats. Sci Rep. (2017) 7:12254. doi: 10.1038/s41598-017-12394-4
48. Chen J, Wu Y, Zou J, Gao K. A-glucosidase inhibition and antihyperglycemic activity of flavonoids from ampelopsis grossedentata and the flavonoid derivatives. Bioorg Med Chem. (2016) 24:1488–94. doi: 10.1016/j.bmc.2016.02.018
49. Kaur G, Mehta SK. Developments of polysorbate (Tween) based microemulsions: preclinical drug delivery, toxicity and antimicrobial applications. Int J Pharm. (2017) 529:134–60. doi: 10.1016/j.ijpharm.2017.06.059
50. Nozad S, Ramin AG, Moghadam G, Asri-Rezaei S, Babapour A, Ramin S. Relationship between blood urea, protein, creatinine, triglycerides and macro-mineral concentrations with the quality and quantity of milk in dairy holstein cows. Veter Res Forum. (2012) 3:55–9. doi: 10.2298/AVB1101003N
51. Mohammadi V, Anassori E, Jafari S. Measure of energy related biochemical metabolites changes during peri-partum period in makouei breed sheep. Veter Res Forum. (2016) 7:35–9.
52. Russell KE, Roussel AJ. Evaluation of the ruminant serum chemistry profile. Veter Clinics North Am Food Animal Pract. (2007) 23:403–26. doi: 10.1016/j.cvfa.2007.07.003
53. Poupon R. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology. (2015) 61:2080–90. doi: 10.1002/hep.27715
54. Jiang FG, Lin XY, Yan ZG, Hu ZY, Wang Y, Wang ZH. Effects of forage source and particle size on chewing activity, ruminal Ph, and saliva secretion in lactating holstein cows. Anim Sci J. (2019) 90:382–92. doi: 10.1111/asj.13153
55. Liang J, Zhang P, Zhang R, Chang J, Chen L, Wang G, et al. Response of rumen microorganisms to Ph during anaerobic hydrolysis and acidogenesis of lignocellulose biomass. Waste Manag. (2024) 174:476–86. doi: 10.1016/j.wasman.2023.12.035
56. Mackie RI, Gilchrist FM. Changes in lactate-producing and lactate-utilizing bacteria in relation to Ph in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl Environ Microbiol. (1979) 38:422–30. doi: 10.1128/aem.38.3.422-430.1979
57. Pan X, Li Z, Li B, Zhao C, Wang Y, Chen Y, et al. Dynamics of rumen gene expression, microbiome colonization, and their interplay in goats. BMC Genomics. (2021) 22:288. doi: 10.1186/s12864-021-07595-1
58. Cui X, Wang Z, Yan T, Chang S, Wang H, Hou F. Rumen bacterial diversity of tibetan sheep (ovis aries) associated with different forage types on the qinghai-tibetan plateau. Can J Microbiol. (2019) 65:859–69. doi: 10.1139/cjm-2019-0154
59. Zafar H, Saier MH. Gut bacteroides species in health and disease. Gut Microbes. (2021) 13:1–20. doi: 10.1080/19490976.2020.1848158
60. Sun Y, Zhang S, Nie Q, He H, Tan H, Geng F, et al. Gut firmicutes: relationship with dietary fiber and role in host homeostasis. Crit Rev Food Sci Nutr. (2023) 63:12073–88. doi: 10.1080/10408398.2022.2098249
61. Su XL, Tian Q, Zhang J, Yuan XZ, Shi XS, Guo RB, et al. Acetobacteroides hydrogenigenes gen. nov, sp nov, an anaerobic hydrogen-producing bacterium in the family rikenellaceae isolated from a reed swamp. Int J System Evolut Microbiol. (2014) 64:2986–91. doi: 10.1099/ijs.0.063917-0
Keywords: Ampelopsis grossedentata flavonoids, Tween 80, Nubian goats, growth performance, blood indexes, rumen microbiota
Citation: Zhu J, Lu Y, Gao Z, Chong Y, Li M, Deng W and Xi D (2024) Exploring the additive effect of Ampelopsis grossedentata flavonoids and Tween 80 on feeding Nubian goats. Front. Vet. Sci. 11:1411071. doi: 10.3389/fvets.2024.1411071
Received: 02 April 2024; Accepted: 25 June 2024;
Published: 12 July 2024.
Edited by:
Haoyu Liu, Yangzhou University, ChinaReviewed by:
Rongfang Li, Hunan Agricultural University, ChinaHouqiang Luo, Wenzhou Vocational College of Science and Technology, China
Copyright © 2024 Zhu, Lu, Gao, Chong, Li, Deng and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongmei Xi, ZG14aXluYXVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Junhong Zhu
Junhong Zhu Ying Lu†
Ying Lu† Zhendong Gao
Zhendong Gao Weidong Deng
Weidong Deng Dongmei Xi
Dongmei Xi