- 1College of Animal Science and Technology, Southwest University, Rongchang, Chongqing, China
- 2Beef Cattle Engineering and Technology Research Center of Chongqing, Southwest University, Rongchang, Chongqing, China
High temperature induces heat stress, adversely affecting the growth and lactation performance of cows. Research has shown the protective effect of taurine against hepatotoxicity both in vivo and in vitro. This study aimed to investigate the effect of taurine on the metabolomics of mammary epithelial cells of dairy cows under high-temperature conditions. Mammary epithelial cells were exposed to 0 mmol/L (HS, control), 8 mmol/L (HT-8), and 32 mmol/L (HT-32) of taurine, then incubated at 42°C for 6 h. Metabolomics analysis was conducted using Liquid Chromatograph Mass Spectrometer (LC–MS). Compared with the HS group, 2,873 and 3,243 metabolites were detected in the HT-8 group in positive and negative ion modes. Among these, 108 and 97 metabolites were significantly upregulated in positive and negative ion modes, while 60 and 166 metabolites were downregulated. Notably, 15 different metabolites such as palmitic acid, adenine and hypoxanthine were screened out in the HT-8 group. Compared with the HS group, 2,873 and 3,243 metabolites were, respectively, detected in the HT-32 group in the positive and negative ion modes. Among those metabolites, 206 metabolites were significantly up-regulated, while 206 metabolites were significantly downregulated in the positive mode. On the other hand, 497 metabolites were significantly upregulated in the negative mode, while 517 metabolites were reported to be downregulated. Noteworthy, 30 distinct metabolites, such as palmitic acid, phytosphingosine, hypoxanthine, nonanoic acid, and octanoic acid, were screened out in the HT-32 group. KEGG enrichment analysis showed that these metabolites were mainly involved in lipid metabolism, purine metabolism and other biological processes. Overall, our study indicates that taurine supplementation alters the metabolites primarily associated with purine metabolism, lipid metabolism and other pathways to alleviate heat stress in bovine mammary epithelial cells.
1 Introduction
In livestock production, stress can be induced by factors such as environment, nutrition, management, pathogens and disease (1). Climate change-induced increases in diurnal temperatures and global warming have attracted increasing attention. Heat stress (HS) happens when an animal is not able to dissipate the heat load generated by the body’s metabolism and the environment, thereby losing the body’s thermal homeostasis. The cows under HS conditions reduce the body heat load by reducing dry matter intake (DMI), resulting in insufficient energy and nutrients being available to maintain normal production (2–4). HS reduces daily rumination time and milk production in lactating high-producing dairy cows (5, 6). Furthermore, HS poses a significant threat to dairy farming, disrupting cows’ productivity, reproductive performance, and overall health (7, 8). In addition, animal management has become a huge challenge due to the increase in the number of production animals and the increased metabolic activity due to high temperatures (9). Therefore, HS caused to huge economic loss on dairy production in the world.
The structural and functional integrity of the mammary tissue is considered a crucial factor in the performance of lactation in cows (10). Research has shown that environmental and management factors can affect mammary gland function at both molecular and cellular levels (11). For example, high temperatures can inhibit the proliferation of mammary epithelial cells, causing the occurrence of various diseases such as mastitis, and affecting the yield and milk quality of cows (12–14). HS led to a variety of protein chaperone genes up-regulated and interfere with cytoskeletal and cell transport function in bovine mammary epithelial cells in vitro (15).
Taurine, as a sulfur-containing non-protein amino acid, is one of the most abundant free amino acids in mammalian tissues (16). It is involved in many biological processes, including anti-inflammatory, antioxidant activities, bile acid binding, membrane stabilization, osmoregulation, regulation of cellular calcium flux, and immunomodulation (17, 18). It has been shown to be protective in stress models and toxic situations such as high temperatures, endotoxin excitation, and high stocking densities (19). Previous studies have shown that taurine supplementation promotes milk fat and protein synthesis and alleviates oxidative stress and inflammation in bovine mammary epithelial cells (20, 21). Moreover, taurine can reverse the decreases in the activity of superoxide dismutase and glutathione peroxidase induced by HS, then alleviate cellular oxidative stress, and thus protect bovine mammary epithelial cells against HS (22). Furthermore, the protective effect of taurine on mammary glands has been achieved by attenuating mammary gland epithelial integrity damage and inflammatory response under HS conditions (23).
The metabolites of the organism, especially the macromolecules, can directly reflect the changes in life activities in the organism (24). Metabolomics is an important method that allows for a comprehensive analysis of metabolites in the organism (25). It can be used to explore the dynamic response of living system metabolites to changes in endogenous or exogenous factors in a quantitative way (26). Previous research has demonstrated that HS results in an elevated concentration of amino acids in the mammary epithelial cells of dairy cows, thereby enhancing the transportation of amino acids and stimulating the activity of the mTOR signaling pathway (27). Additionally, 16 metabolites have been identified in the plasma of dairy cows treated with N-Carbamylglutamate (NCG), and found that NCG could relieve HS, resulting in an improvement in milk production under HS (28). Furthermore, another study using analyzing metabolomics proved the protective effect in the yeast chromium by reducing rectal temperature, decreasing serum insulin concentration, and increasing serum glucose and plasma nicotinamide concentration to improve the lactation performance of dairy cows under heat stress (29).
It has been found that heat stress in dairy cows is associated with many metabolic pathways, such as glucose metabolism, amino acid metabolism, and nucleotide metabolism (30, 31). However, the direct impact of taurine on the metabolism of bovine mammary epithelial cells under HS remains underexplored. This study aims to address this gap by employing liquid chromatography-mass spectrometry (LC-MS) to investigate the metabolite profile of bovine mammary epithelial cells supplemented with taurine under high-temperature conditions. By shedding light on the molecular mechanisms underlying taurine’s protective effects, this research seeks to provide a theoretical basis for developing targeted interventions to mitigate the adverse effects of HS in dairy cows.
2 Materials and methods
2.1 Cells and reagents
The bovine mammary epithelial cells were preserved by our laboratory (Rongchang, Chongqing); fetal bovine serum and DMEM/F-12 medium were purchased from Gibco; taurine (Taurine, 99% biotech grade) was purchased from Macklin, and methanol (chromatographically pure, HPLC-grade) were purchased from Sigma.
2.2 Cell culture and treatment
The mammary epithelial cells were cultured in DMEM/F-12 medium containing 10% fetal bovine serum and 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C, 5% CO2 in an incubator, and then cultured in 10 cm dishes at a density of 6 × 106 cells/mL until cells reached 80–90% confluence, and then replaced with culture medium containing 0 mmol/L (HS, control group), 8 mmol/L (HT-8) and 32 mmol/L (HT-32) of taurine, respectively. After cells were cultured at 37°C for 6 h, they were then transferred to 42°C for 6 h-incubation based on similar work by Salama et al. (32–34). All of the treatments were conducted in six biological replicates (n = 6).
2.3 Samples research topic
After the hyperthermia treatment, the medium was discarded and washed twice with pre-cooled (4°C) PBS. Afterwards, the dish containing the cells was exposed to liquid nitrogen for 10 s and then placed on ice. 750 μL of methanol–water (Methanol/Water = 8:2, V/V) was added to each dish, and the cells were scraped off with a cell scraper and transferred into Eppendorf tubes, while the remaining cells were scraped off with another 750 μL of methanol–water and put into the same tube. The collected samples were stored at −80°C for 30 min (mins) and then pulverized by ultrasound. The samples were centrifuged at 14,000 g for 15 min at 4°C. The supernatant was transferred in a 1.5 mL Eppendorf tube and stored at −80°C for measurement.
2.4 Detection of metabolites
LC-MS/MS analysis was performed using UHPLC equipment (UHPLC3000, Dionex, Sunnyvale, CA, Germany). Detection conditions were presented as follows.
2.4.1 Chromatographic conditions
Column: UPLC Hypersil Gold C18 column (2.1 Hypers, particle size 1.91, Thermo Fisher Science, United States) and Q-Exactive Orbitrapp (Thermo Fisher Science, United States), flow rate 0.2 mL/min, column temperature 35°C, injection volume 2 μL; mobile phases: solvent A (0.1%formic acid), solvent B (methanol containing 0.1% formic acid), solvent C (0.1%NH3), and D (methanol containing 0.1%NH3); gradient elution program: positive ions were 0–10 min, 5%B and 95%A; 10–12 min, 5%A and 95% B; 12–13 min, 5%A and 95%B; 13.1–14 min 95%A and 5%B; negative ions were 0–2.5 min, 95%C and 5%D; 2.5–16.5 min, 95%D and 5%C; 16.5–19 min, 95%D and 5%C; 19–20 min, 95%C and 5%D.
2.4.2 Mass spectrometry conditions
MS/MS spectra were acquired in an information-dependent acquisition (IDA) mode using Q-ExactiveOrbitRAP under the control of the acquisition software (X Cup, Thermo Fisher Science, United States); HASI source operating parameters: sheath and auxiliary gas flow rates of 40 and 10 arb, respectively, capillary temperature of 320°C, full mass scan range m/z 70–1,050 with a resolution of 70,000; the MS/MS scan mode was set to a data-dependent MS2 (dd-MS2) scan with a resolution of 35,000, high collisional dissociation, and a spray voltage of 3.5 kV (positive-ion mode)/−2.5 kV (negative-ion mode) in NCE mode.
2.5 Data analysis
2.5.1 Data processing
The raw data from the mass spectrometry downgauge was analyzed with Compound Discoverer 3.2 (Thermo Fisher, United States) for peak processing and peak integration. The measured mass spectral primary and mass spectral secondary information were matched with the mzCloud, Chemspider, and mzVault databases to analyze the metabolites to which they could be matched.
2.5.2 Analysis of differential metabolites
Multivariate statistical analysis was performed using SIMCA 14.1 (Umetrics, Sweden) software. The unsupervised principal component analysis (PCA) was used to categorize the ions detected in the positive and negative modes, and then the data were analyzed using Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA). The model was verified by the permutation test to find out the projected importance values (VIPs) of the variables. The metabolites were screened for differences between treatment and the control. The metabolite analysis was performed using the online software MetaboAnalyst 5.0. Kyoto Encyclopedia of Genes and Genomes (KEGG) database is a comprehensive database that integrates genomic, chemical, and systemic functional information to reveal the genetic material and chemical blueprint of life phenomena. The samples that met the criteria were compared with the KEGG database to identify the metabolites and explain their biological functions and physicochemical properties. For unidentified compounds, KEGG annotations can be used to determine their identity. Using the MBROLE 2.0 online website, identified differential metabolites were mapped to relevant metabolic pathways to better understand their functions and roles in biological systems.
p values were obtained by univariate analysis of the multiplicity of differences (Fold-Change) and T-statistical test, combined with VIP values (Variable Important for the Projection) obtained by multivariate statistical analysis OPLS-DA to screen for differential metabolites. Differential metabolites were required to fulfill the following conditions simultaneously: (1) |Log2 Fold Change| ≥ 1; (2) P-value ≤ 0.05; (3) VIP ≥ 1.
3 Results
3.1 Multivariate statistical analysis
In order to find the differential metabolites between the treatment and control groups, multivariate statistical analysis of cell lysates was performed. The stability of the model was indicated by QC samples. Figure 1 showed the plots of PCA scores for the degree of dispersion between the groups of samples with different concentrations of taurine and the control group in the positive and negative ion mode, respectively. Almost all of the samples were within the ellipse representing the 95% confidence interval, and all treatment groups were separated from the blank group in the horizontal coordinate t [1] (first principal component), and the QC samples were clustered together, which indicated that the established system was stable and reliable. Further, taurine had a significant effect on the metabolism patterns of mammary epithelial cells of cows under high-temperature conditions.
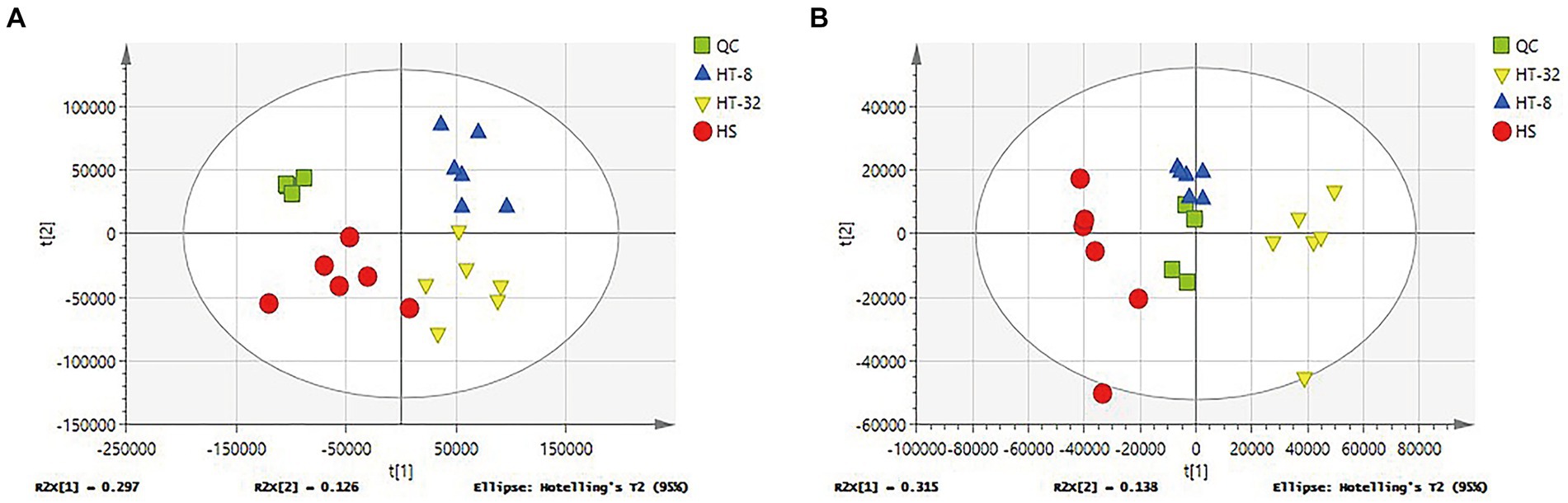
Figure 1. PCA score plots of different samples. (A) PCA score plots of three groups in cationic mode (positive ion). (B) PCA score plots of three groups in anionic mode (negative ion). Green represents the quality control group (QC), Red represents control group (HS), blue represents 8 mmol/L taurine group (HT-8), yellow represents 32 mmol/L taurine group (HT-32), (n = 6) the same as below.
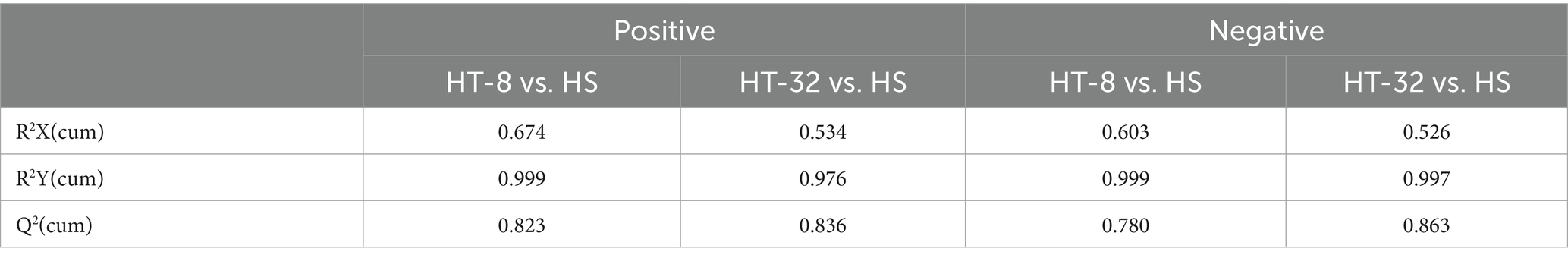
To verify the reliability of the model and to identify the potential biological markers affecting cellular metabolic patterns, orthogonal partial least squares discriminant analysis (OPLS-DA) was carried out between the HS, HT-8 and HT-32 groups in positive and negative ion modes, respectively. The parameters of the model, R2X and R2Y, represented the explanatory rate of the constructed model for the X and Y matrices, respectively. The value of Q2 indicated the predictive ability of the model, and their sizes directly reflected the reliability of the model. The OPLS-DA score plots between different groups in positive and negative ion mode were shown in Figures 2A–D, and their related model parameters, R2 and Q2 values, were shown in Table 1. The R2 values of each group were greater than 0.5, which showed that the models of the different concentrations of taurine treatment groups were stable; the Q2 values of HT-8 and HT-32 groups in positive ion mode were 0.823, 0.836, respectively, and the Q2 values of HT-8 and HT-32 group in negative ion mode were 0.780 and 0.863, respectively, indicating that the model predictability was good.
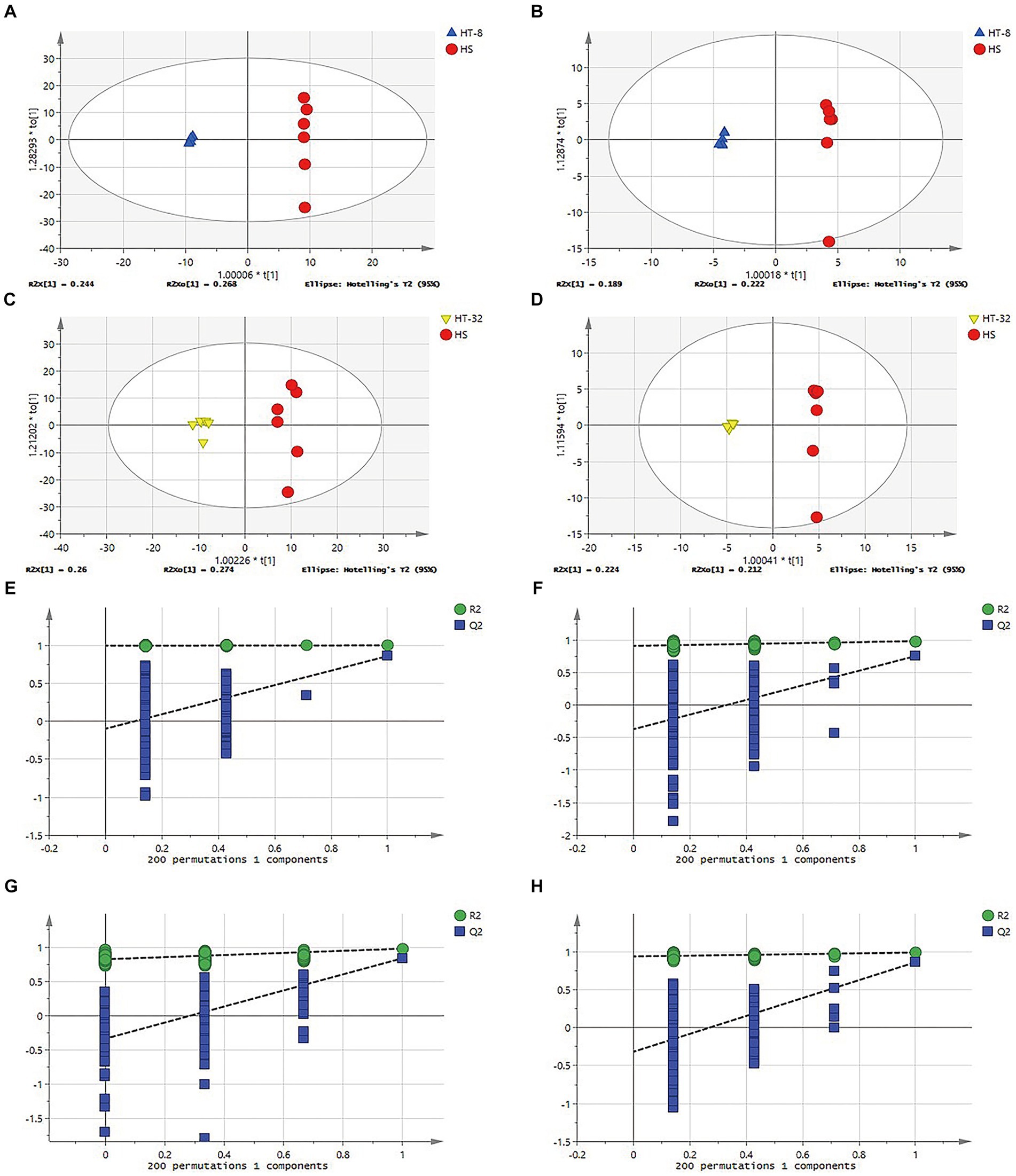
Figure 2. OPLS-DA model and verification diagram of different samples. (A)OPLS-DA scores plot of HT-8 vs. HS in cationic mode. (B) OPLS-DA scores plot of HT-8 vs. HS in anionic mode. (C) OPLS-DA scores plot of HT-32 vs. HS in cationic mode. (D) OPLS-DA scores plot of HT-32 vs. HS in anionic mode. (E–H) OPLS-DA model verification diagram of panel (A–D).
In order to decide whether the data derived from the OPLS-DA analysis were analytically significant, the data were subjected to a permutation test (Permutation test). As shown in Figures 2E–H, the slope of the fitted line of the various treatment groups was greater than 0, and the next step in the analysis could be carried out.
3.2 Effects of taurine on the metabolite profile of the mammary epithelial cells
Metabolomic analysis was conducted to reveal the changes in metabolic profiles of the mammary epithelial cells following taurine treatment under high temperatures. Compared to the control group, a total of 2,873 metabolites were detected in the positive ion mode when treated with 8 mmol/L taurine (Figure 3A), of which 108 metabolites were significantly up-regulated, 60 metabolites were significantly down-regulated, and the remaining 2,705 metabolites did not show significantly different. On the other hand, a total of 3,243 metabolites were detected in the negative ion mode, among which 97 metabolites were significantly up-regulated, 166 metabolites were significantly down-regulated, and the remaining 2,980 metabolites were not significantly different (Figure 3B). In 32 mmol/L taurine group, 2,873 metabolites were found in the positive ion mode, of which 206 metabolites were significantly up-regulated, 206 metabolites were significantly down-regulated and the other 2,461 metabolites did not show significant changes (Figure 3C). In addition, a total of 3,243 metabolites were detected in negative ion mode, of which 497 metabolites were significantly up-regulated, 517 metabolites were significantly down-regulated, and the remaining 2,229 metabolites showed no significant difference (Figure 3D).
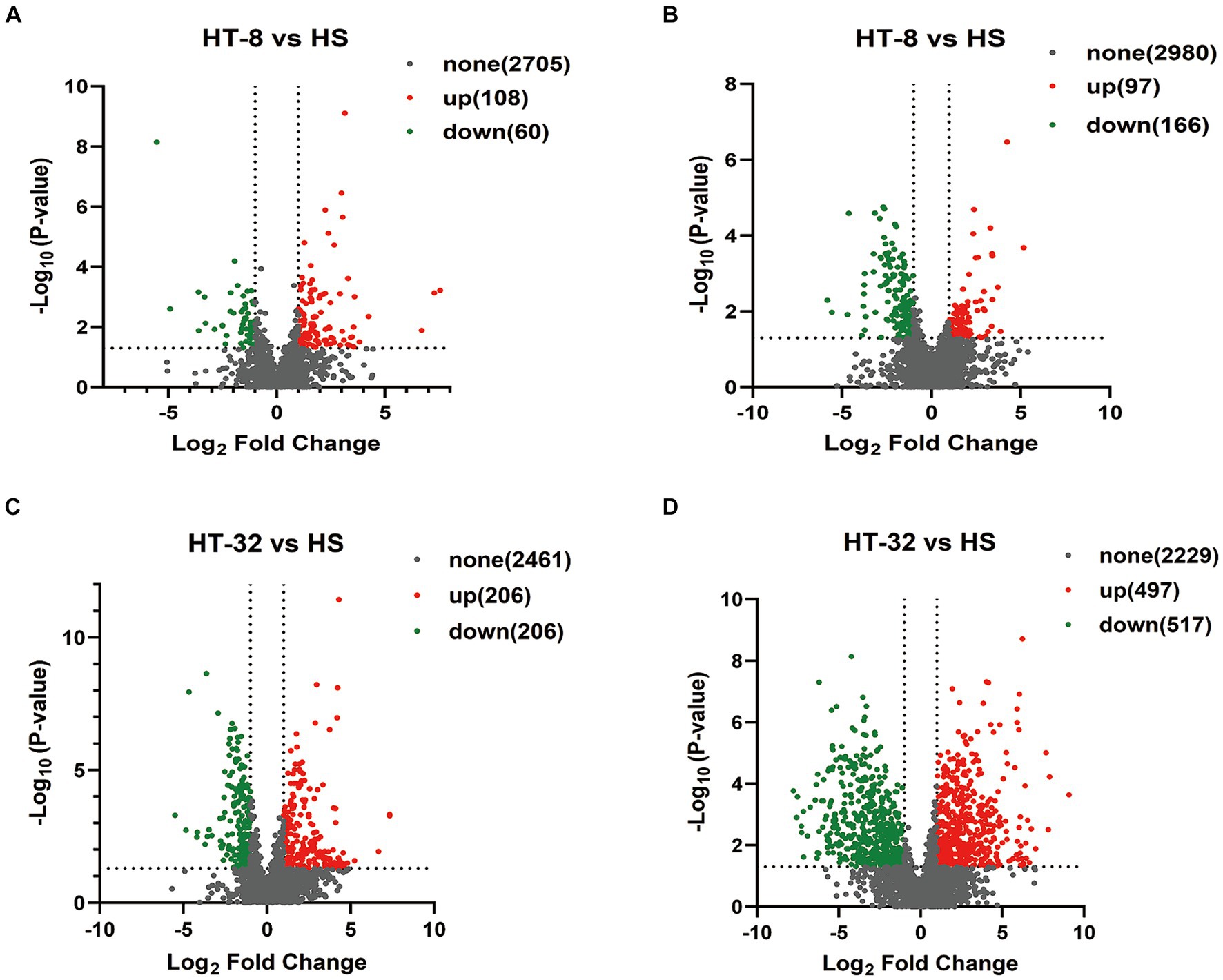
Figure 3. Volcano diagram of differential expressed metabolites between different samples. (A) Volcano diagram of HT-8 vs. HS in cationic mode. (B) Volcano diagram of HT-8 vs. HS in anionic mode. (C) Volcano diagram of HT-32 vs. HS in cationic mode. (D) Volcano diagram of HT-32 vs. HS in anionic mode. Red dots represent metabolites with significant and upregulated differences, green dots represent metabolites with significant and downregulated differences, and black represents metabolites with non-significant differences.
3.3 Metabolite clustering analysis
Heatmaps constructed from the peak areas of the differential metabolites showed the combined differentiation between treatments. The clustering of metabolite contents among groups could be clearly observed by horizontal comparison. As shown in Figure 4, the HT-8 and HT-32 groups had similar metabolite expression patterns in the positive ion mode, whereas the HT-8 and HS groups had similar metabolite expression patterns in the negative ion mode.
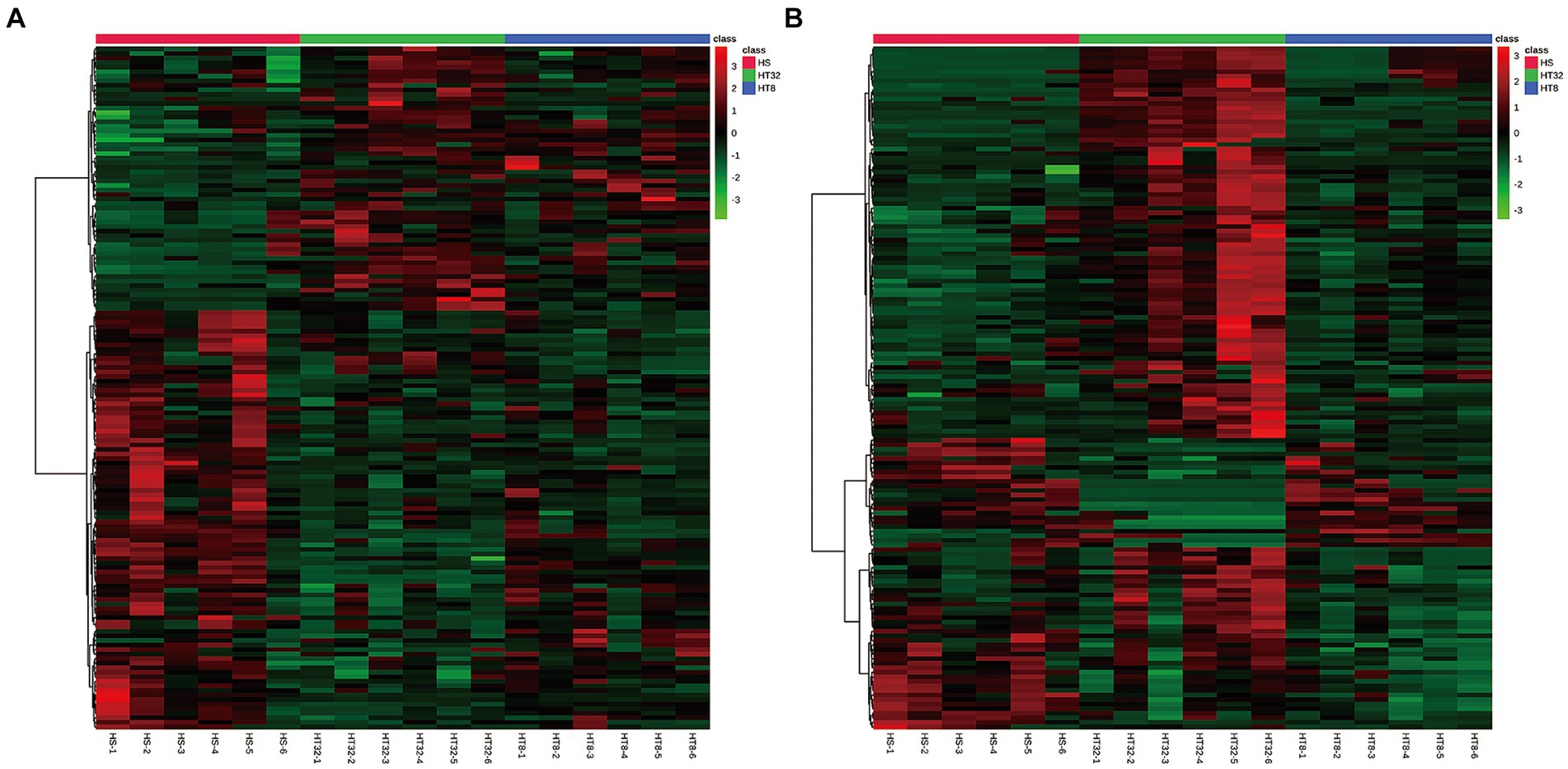
Figure 4. Cluster diagram of differentially expressed metabolites. (A) Cluster diagram of three groups in cationic mode (positive ion). (B) Cluster diagram of three groups in anionic mode (negative ion). Red dots represent up-regulated metabolites and green dots represent down-regulated metabolites. The vertical axis indicates clustering of all groups and the horizontal axis indicates clustering of all metabolites, with shorter cluster branches indicating higher similarity.
3.4 Analysis of differential metabolites
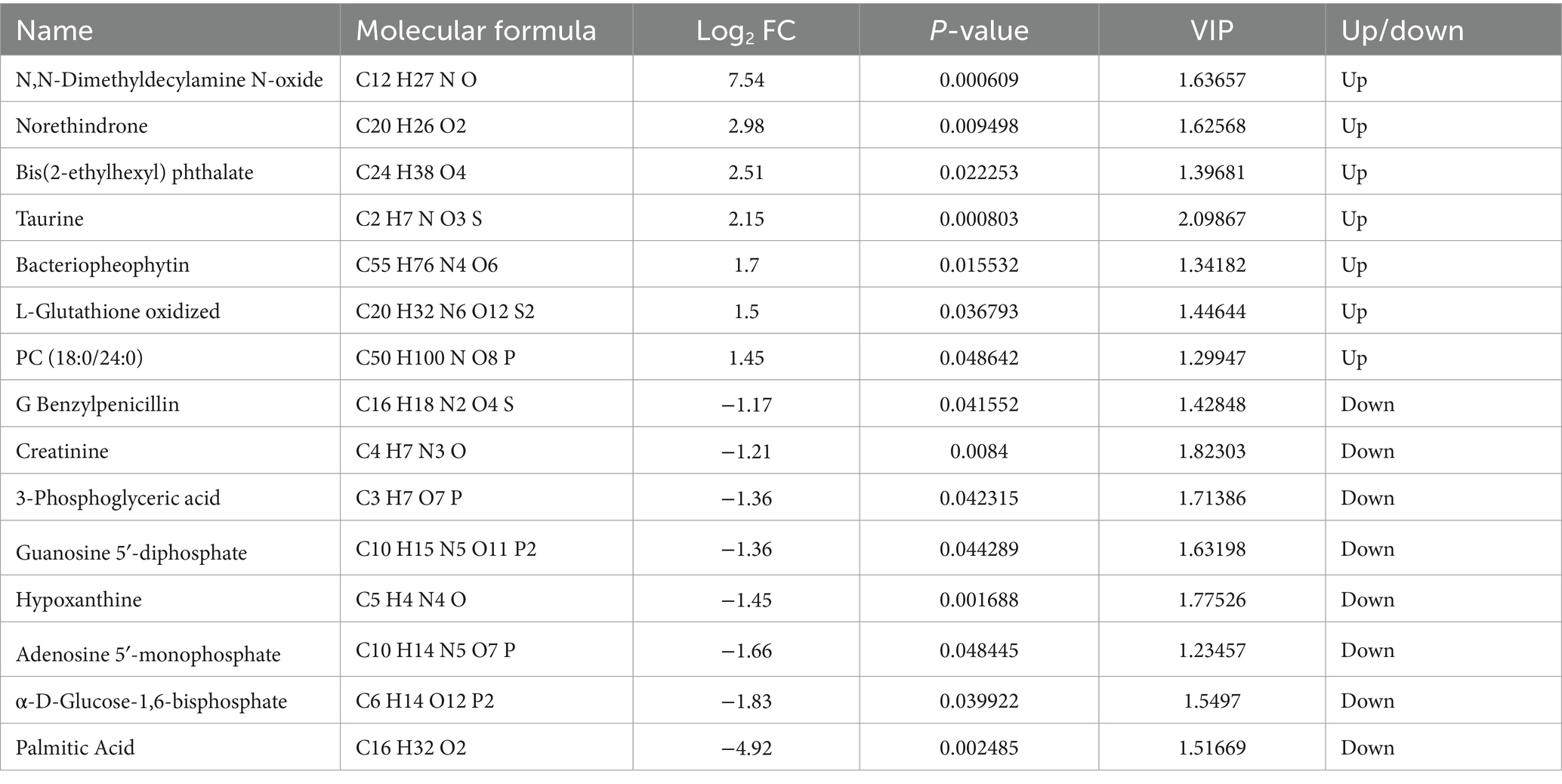
Based on the OPLS-DA assay, potential metabolic markers were screened using a “multi-criteria” technique. Differential metabolites between treatment and the control group were screened under the conditions of |log2 Fold Change| ≥ 1, VIP value > 1, and P-value ≤ 0.05. As shown in Table 2, a total of 15 differential metabolites were screened in the HT-8 group compared with the control group, of which N,N-Dimethyldecylamine N-oxide, Norethindrone, Bis(2-ethylhexyl) phthalate, Taurine, Bacteriopheophytin, L-Glutathione oxidized and PC(18:0/24:0) for seven upregulated metabolites, G Benzylpenicillin, Creatinine, 3-Phosphoglyceric acid, Guanosine 5′-diphosphate, Hypoxanthine, Adenosine 5′-monophosphate, α-D-Glucose-1,6-bisphosphate, Palmitic Acid as 8 downregulated metabolites.
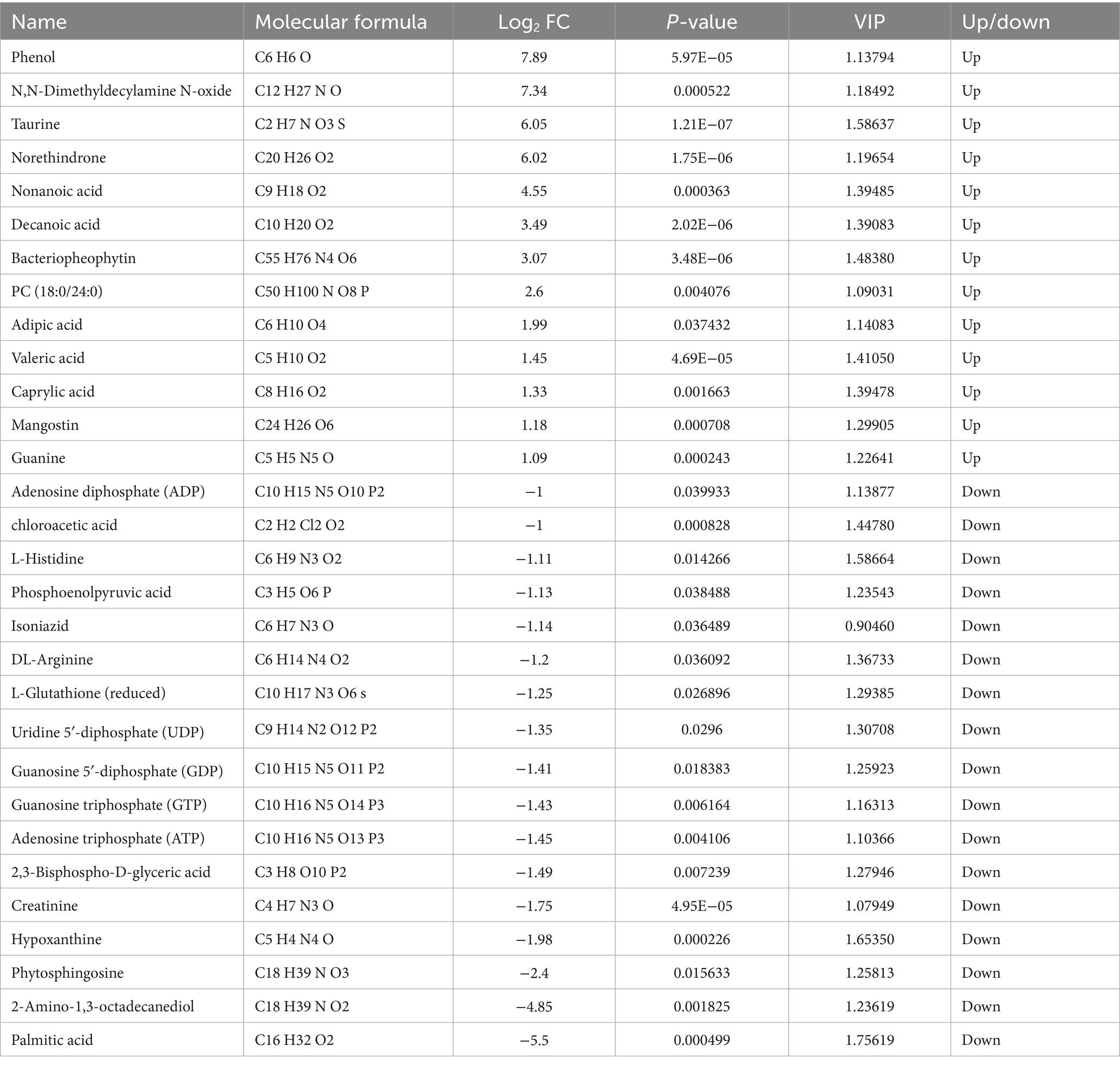
As shown in Table 3, a total of 30 differential metabolites were screened in the HT-32 group compared with the control group, including 13 upregulated metabolites such as Phenol, Norethindrone, Nonanoic acid, Decanoic acid, and Guanine, and ADP, L-Glutathione (reduced), UDP, GDP, GTP, ATP, Hypoxanthine, Phytosphingosine, 2-Amino-1,3-octadecanediol, Palmitic Acid and 17 other downregulated metabolites.
Further analysis of these differential metabolites, as shown in Figure 5, revealed that 8 mmol/L and 32 mmol/L taurine resulted in the upregulation of decanoic acid, valeric acid, octanoic acid, and oxidized glutathione, and the downregulation of ADP, ATP, hypoxanthine, phytosphingosine, 2-amino-1,3-octadecanediol (sphingolipid analogs), reduced glutathione, and palmitic acid, compared to the control.
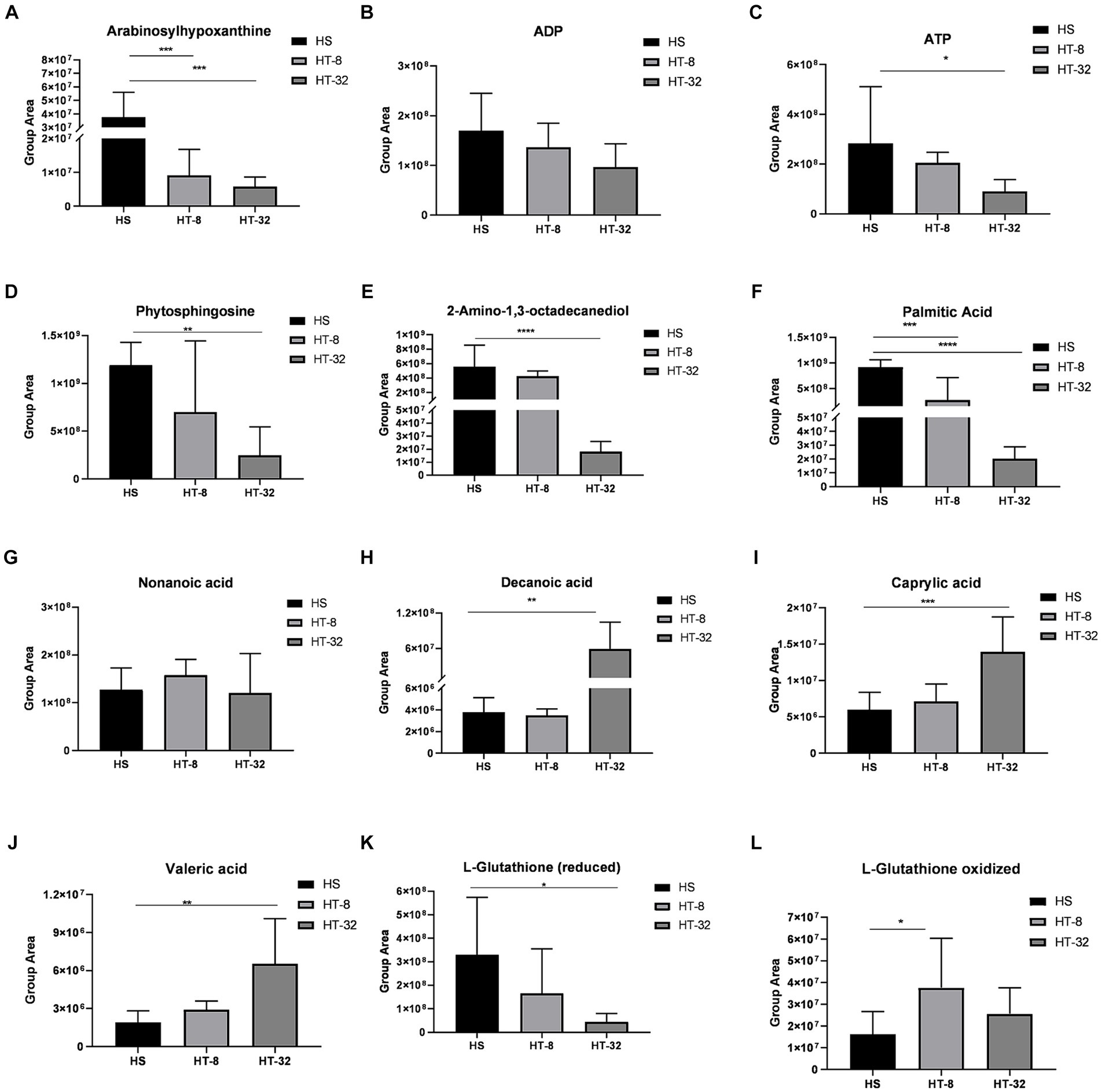
Figure 5. A plot of the peak values of several major metabolites between treatments. (A-L) indicate peak plots of Arabinosylhypoxanthine, ADP, ATP, Phytosphingosine, 2-Amino-1,3-octadecanediol, Palmitic acid, Nonanoic acid, Decanoic acid, Caprylic acid, Valeric acid, L-Glutathione (reduced) and L-Glutathione (oxidized) between treatments, respectively. Values are means ± SEM (n = 3). Asterisks (*, ** and ***) represent significant differences with p < 0.05, p < 0.01 and p < 0.001 respectively.
3.5 Analysis of differential metabolite metabolic pathways
In order to identify potential biomarkers of KEGG identification, the online software of MetaboAnalyst 5.0 was utilized to analyze KEGG. KEGG pathway enrichment analysis was performed by the MBROLE.2.0·online website. Figure 6 showed differentially expressed metabolites through the KEGG pathway analysis. Figures 6A,B showed the top 20 KEGG metabolic pathways enriched by differential metabolites between the HT-8 vs. HS and HT-32 vs. HS groups, respectively. After being treated with 8 mmol/L taurine, purine metabolism, glutathione metabolism, fatty acid biosynthesis and metabolism, linoleic acid metabolism, and mTOR signaling pathway were significantly changed (Figure 6A). The changes in purine metabolism, fatty acid metabolism, taurine and hypo-taurine metabolism, mTOR signaling pathway, and metabolic pathway were significant after 32 mmol/L taurine (HT-32) treatment (Figure 6B). In conclusion, taurine affected the metabolic pathways: purine metabolism, amino acid metabolism, glucose metabolism, fatty acid metabolism and biosynthesis in bovine mammary epithelial cells under high temperature condition.
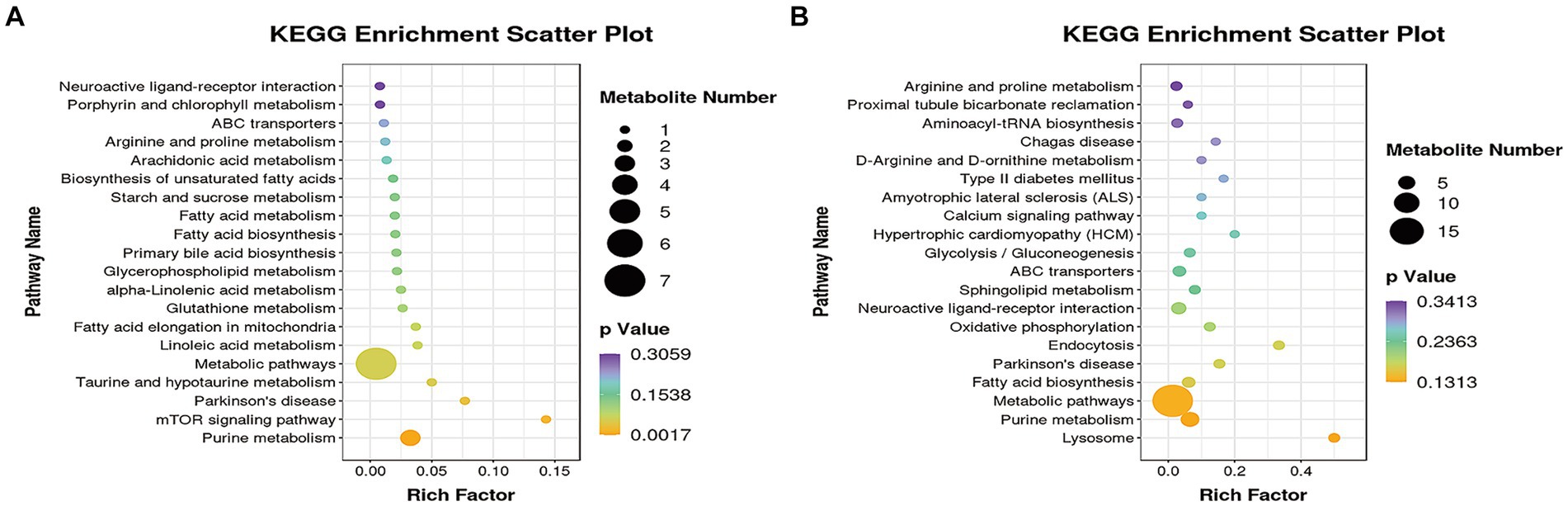
Figure 6. The KEGG pathway analysis of differentially expressed metabolites. (A) The KEGG pathway of HT-8 vs. HS. (B) The KEGG pathway of HT-32 vs. HS. The size of the solid circle in the figure indicated the number of differential metabolites enriched by the pathway, and the darkness of the color of the circle represents the size of the p-value.
4 Discussion
The aim of this study was to assess the impact of taurine supplementation on the metabolite composition of bovine mammary epithelial cells subjected to high-temperature stress. In this experiment, employing liquid chromatography-mass spectrometry (LC-MS), a total of 15 differential metabolites were identified in the mammary epithelial cells of dairy cows by supplementation of 8 mmol/L taurine under high temperature, and 30 differential metabolites were identified with the addition of 32 mmol/L taurine, compared with the control group. The KEGG enrichment analysis revealed that these metabolites mainly regulated the pathways of purine metabolism, fatty acid metabolism, fatty acid biosynthesis and sphingolipid metabolism.
Fatty acids and their derivatives are widespread in all living organisms, both as energy providers and as part of the composition of biological membranes (35). They can regulate gene expression via activating transcription factors and cell membrane receptor signaling (36). They also affect their own metabolism as well as the metabolism of other compounds by influencing gene transcription (37). Under high temperature condition, cells need to regulate membrane fluidity to maintain normal physiological function, as the amount of saturated fatty acids in the membrane affects the fluid properties of the membrane. Therefore, cells increase the synthesis of unsaturated fatty acids to regulate membrane properties in response to HS (38). The synthesis of short- and medium-chain unsaturated fatty acids are fatty acid groups consisting of a carbon chain of 3–12 carbon atoms, which have a small molecular mass and a strong ability to penetrate cell membranes. Short-chain fatty acids include formic acid, acetic acid, and valeric acid, while medium-chain fatty acids include caproic acid, octanoic acid, nonanoic acid, decanoic acid, etc. (39, 40). The medium chain fatty acids include hexanoic acid, octanoic acid, nonanoic acid and decanoic acid. Studies have shown that pathways related to lipid metabolisms, such as fatty acid biosynthesis and amino acid metabolism, are inhibited under high temperature conditions (41). In the present study, 32 mmol/L taurine significantly upregulated the expression of short-chain fatty acids, such as caproic acid, valeric acid, nonanoic acid, and decanoic acid in the mammary epithelial cells of dairy cows under high temperature conditions. Therefore, it can be hypothesized that taurine could protect the cells by regulating the expression of short and medium chain fatty acids in the fatty acid synthesis pathway, thereby increasing the expression of heat shock proteins and enhancing the tolerance to heat stress.
Palmitic acid (PA) is the most abundant saturated free fatty acid in the blood and one of the main substrates for fat synthesis (42). PA can regulate cellular metabolism via the activation of phosphatidylinositol 3-kinase (PI3K) (43). Excessive PA leads to endoplasmic reticulum stress, mitochondrial damage, and apoptosis (44–46). Excessive PA also induces programmed necrosis (necrotic apoptosis) in endothelial cells by initiating enhanced autophagy (47). Besides, PA has been reported to stimulate hepatocyte apoptosis by inducing oxidative stress through intracellular generation of reactive oxygen species (48) and apoptosis of bovine mammary epithelial cells by inducing extreme endoplasmic reticulum stress (49). In this study, we found that 8 mmol/L and 32 mmol/L of taurine downregulated the expression of palmitic acid in bovine mammary epithelial cells under high temperature conditions. Therefore, taurine may activate cellular autophagy pathways, and alleviate endoplasmic reticulum stress, mitochondrial damage, and apoptosis induced by palmitic acid. Moreover, taurine can reduce cellular autophagy by inhibiting the PI3K pathway and inhibiting the mTOR pathway through down-regulation of adenosine monophosphate (AMP) expression. It also reduces the production of intracellular reactive oxygen species under high temperature conditions, thereby alleviating oxidative stress and to some extent relieving the damage of mammary epithelial cells in dairy cows under high temperature conditions.
Sphingolipids are one of the major lipid classes in eukaryotes and play a crucial role in cells as structural components of membrane lipid bilayers and signaling molecules (50, 51). They are related to important physiological and pathological processes, including cell adhesion, recognition of viral and bacterial toxins, skin barrier formation, neural function, apoptosis, and glucose metabolism (52, 53). Phytosphingosine (PHS) is a sphingolipid found in plants and animals and is unique since it has an extra hydroxyl group compared to other long-chain bases (54). 2-Amino-1,3-octadecanediol (2-Amino-1,3-octadecanediol), a sphingosine analog that inhibits protein kinase C, is used in the treatment of skin diseases and cancer (55). Studies have shown that sphingolipid metabolites associated with PHS, as well as sphingosine 1-phosphate, can cause programmed cell death (56). They can also directly or indirectly interfere with mitochondria and induce apoptosis (57) and promote apoptosis by disrupting mitochondrial autophagy (54, 58). Furthermore, PHS causes abnormal nuclear morphology, micronuclei and DNA damage, inhibits cell proliferation by damaging DNA, and activates the ATM/P53/p21 pathway, resulting in cell cycle arrest in S phase (59). In this experiment, we found that the metabolites of PHS and its analog (2-Amino-1,3-octadecanediol) in the mammary epithelial cells of dairy cows under high temperature conditions were significantly downregulated by 32 mmol/L taurine. It means that taurine might alleviate apoptosis under high temperature conditions by down-regulating sphingomyelin metabolism.
Purine nucleotides, essential components for cell proliferation, provide cells with energy, and help cells against the adverse effects of the external environment (60, 61). When an animal is subjected to HS, it will release some endogenous substances, such as catalase and cytokines, which can affect the purine metabolic pathway and lead to changes in the activity of the relevant enzymes (62). Heat stress may also lead to a change in purine metabolism to produce excessive free radicals and oxidative stress, which may adversely affect the cells (63). Hypoxanthine is an important product of the nucleotide degradation pathway and can be used as a substrate for ATP synthesis (64, 65). It can induce apoptosis by regulating the expression of proteins associated with apoptosis (66), which induces cell death and ROS production. Studies have shown that HS first affects the animal’s feed intake and nutrient absorption, and then affects the body’s metabolism. During heat production, a large number of free radicals are generated, causing oxidative stress in the body (67, 68). In this experiment, 8 and 32 mmol/L taurine inhibited purine metabolism by significantly down-regulating the production of ATP, ADP, UTP, UDP and hypoxanthine in mammary epithelial cells of cows at high temperature. By reducing the levels of these metabolites, taurine attenuates high temperature-induced oxidative stress and free radical release. Thus, it attenuates the damage and apoptosis of mammary epithelial cells in dairy cows under high temperature conditions.
The results of this study emphasize the vital role of taurine in alleviating HS in dairy cows through various metabolic pathways. These effects not only help maintain the health and productivity of dairy herds but also have substantial implications for improving the economic sustainability of dairy farming operations. Therefore, ensuring sufficient taurine intake through careful nutritional management and feeding practices is essential for managing HS and optimizing overall herd health and performance.
In summary, the effect of taurine on the metabolome of mammary epithelial cells of dairy cows under high temperature conditions is a complicated biological process, and the potential biomarkers are mainly involved in the regulation of purine metabolism, lipid metabolism, sphingolipid metabolism, amino acid metabolism, which constitutes a complicated regulatory network. However, this study primarily utilized metabolomics analysis to pinpoint potential biomarkers indicating taurine’s capacity to mitigate HS, it did not proceed to validate the precise mechanisms underlying the action of the selected metabolites. To strengthen the findings, future research could incorporate cellular function experiments, such as assessing cell proliferation and apoptosis, to validate the metabolomics results and delve further into taurine’s specific effects on mammary epithelial cells.
5 Conclusion
The LC-MS technique and multivariate statistical analysis were utilized to screen the significantly different metabolites of the mammary epithelial cells of dairy cows under high-temperature conditions after taurine treatment with different concentrations of taurine. These metabolites are mainly involved in the pathways of purine metabolism, lipid metabolism, and sphingomyelin metabolism, which accumulates data for the in-depth study of the mitigation of heat stress in dairy cows by taurine.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Ethics Committee/Institutional Review Board. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FL: Data curation, Writing – original draft, Formal analysis, Methodology. LL: Methodology, Writing – review & editing, Visualization. ZL: Writing – review & editing, Data curation. GZ: Writing – review & editing. FZ: Methodology, Writing – review & editing. LW: Funding acquisition, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The project was supported by the Chongqing Natural Fund project (cstc2020jcyj-msxmX0436), Special Key Project of Chongqing Technology Innovation and Application Development (cstc2021jscx-gksbX0012), and Guizhou Provincial Department of Agriculture and Rural Affairs (Qian Cai Nong 2024).
Acknowledgments
Thanks to all participants for their advice and support of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Surai, PF, and Fisinin, VI. Vitagenes in poultry production: part 1. Technological and environmental stresses. Worlds Poult Sci J. (2016) 72:721–34. doi: 10.1017/S0043933916000714
2. Baumgard, LH, Wheelock, JB, Sanders, SR, Moore, CE, Green, HB, Waldron, MR, et al. Postabsorptive carbohydrate adaptations to heat stress and Monensin supplementation in lactating Holstein Cows1. J Dairy Sci. (2011) 94:5620–33. doi: 10.3168/jds.2011-4462
3. Cartwright, SL, Schmied, J, Karrow, N, and Mallard, BA. Impact of heat stress on dairy cattle and selection strategies for Thermotolerance: a review. Front Vet Sci. (2023) 10:1198697. doi: 10.3389/fvets.2023.1198697
4. Liu, J, Li, L, Chen, X, Lu, Y, and Wang, D. Effects of heat stress on body temperature, Milk production, and reproduction in dairy cows: a novel idea for monitoring and evaluation of heat stress—a review. Asian Australas J Anim Sci. (2019) 32:1332–9. doi: 10.5713/ajas.18.0743
5. Müschner-Siemens, T, Hoffmann, G, Ammon, C, and Amon, T. Daily rumination time of lactating dairy cows under heat stress conditions. J Therm Biol. (2020) 88:102484. doi: 10.1016/j.jtherbio.2019.102484
6. Heinrichs, AJ, Heinrichs, BS, Cavallini, D, Fustini, M, and Formigoni, A. Limiting Total mixed ration availability alters eating and rumination patterns of lactating dairy cows. JDS Commun. (2021) 2:186–90. doi: 10.3168/jdsc.2020-0074
7. Collier, RJ, Renquist, BJ, and Xiao, Y. A 100-year review: stress physiology including heat stress. J Dairy Sci. (2017) 100:10367–80. doi: 10.3168/jds.2017-13676
8. Tao, S, Orellana Rivas, RM, Marins, TN, Chen, Y-C, Gao, J, and Bernard, JK. Impact of heat stress on Lactational performance of dairy cows. Theriogenology. (2020) 150:437–44. doi: 10.1016/j.theriogenology.2020.02.048
9. Becker, CA, Collier, RJ, and Stone, AE. Invited review: physiological and behavioral effects of heat stress in dairy cows. J Dairy Sci. (2020) 103:6751–70. doi: 10.3168/jds.2019-17929
10. Rakib, MRH, Zhou, M, Xu, S, Liu, Y, Asfandyar Khan, M, Han, B, et al. Effect of heat stress on udder health of dairy cows. J Dairy Res. (2020) 87:315–21. doi: 10.1017/S0022029920000886
11. Tao, S, Orellana, RM, Weng, X, Marins, TN, Dahl, GE, and Bernard, JK. Symposium review: the influences of heat stress on bovine mammary gland Function1. J Dairy Sci. (2018) 101:5642–54. doi: 10.3168/jds.2017-13727
12. Molinari, PCC, and Bromfield, JJ. Inflammatory responses of bovine endometrial epithelial cells are increased under in vitro heat stress conditions. J Therm Biol. (2023) 114:103564. doi: 10.1016/j.jtherbio.2023.103564
13. Collier, RJ, Collier, JL, Rhoads, RP, and Baumgard, LH. Invited review: genes involved in the bovine heat stress Response1. J Dairy Sci. (2008) 91:445–54. doi: 10.3168/jds.2007-0540
14. Tao, S, Bubolz, JW, do Amaral, BC, Thompson, IM, Hayen, MJ, Johnson, SE, et al. Effect of heat stress during the dry period on mammary gland development. J Dairy Sci. (2011) 94:5976–86. doi: 10.3168/jds.2011-4329
15. Rhoads, ML, Rhoads, RP, VanBaale, MJ, Collier, RJ, Sanders, SR, Weber, WJ, et al. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating Somatotropin1. J Dairy Sci. (2009) 92:1986–97. doi: 10.3168/jds.2008-1641
16. Wen, C, Li, F, Zhang, L, Duan, Y, Guo, Q, Wang, W, et al. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res. (2019) 63:e1800536. doi: 10.1002/mnfr.201800536
17. Salze, GP, and Davis, DA. Taurine: a critical nutrient for future fish feeds. Aquaculture. (2015) 437:215–29. doi: 10.1016/j.aquaculture.2014.12.006
18. Marcinkiewicz, J, and Kontny, E. Taurine and inflammatory diseases. Amino Acids. (2014) 46:7–20. doi: 10.1007/s00726-012-1361-4
19. Surai, PF, Kochish, II, and Kidd, MT. Taurine in poultry nutrition. Anim Feed Sci Technol. (2020) 260:114339. doi: 10.1016/j.anifeedsci.2019.114339
20. Yu, M, Wang, Y, Wang, Z, Liu, Y, Yu, Y, and Gao, X. Taurine promotes Milk synthesis via the Gpr87-Pi3k-Setd1a signaling in Bmecs. J Agric Food Chem. (2019) 67:1927–36. doi: 10.1021/acs.jafc.8b06532
21. Li, M, Xi, P, Xu, Y, Wang, Z, Han, X, Ren, W, et al. Taurine attenuates Streptococcus Uberis-induced bovine mammary epithelial cells inflammation via Phosphoinositides/Ca2+ signaling. Front Immunol. (2019) 10:1825. doi: 10.3389/fimmu.2019.01825
22. Bai, H, Li, T, Yu, Y, Zhou, N, Kou, H, Guo, Y, et al. Cytoprotective effects of taurine on heat-induced bovine mammary epithelial cells in vitro. Cells. (2021) 10:258. doi: 10.3390/cells10020258
23. Li, H, Chen, H, Zhang, S, Wang, S, Zhang, L, Li, J, et al. Taurine alleviates heat stress-induced mammary inflammation and impairment of mammary epithelial integrity via the Erk1/2-Mlck signaling pathway. J Therm Biol. (2023) 116:103587. doi: 10.1016/j.jtherbio.2023.103587
24. Qiu, S, Cai, Y, Yao, H, Lin, C, Xie, Y, Tang, S, et al. Small molecule metabolites: discovery of biomarkers and therapeutic targets. Signal Transduct Target Ther. (2023) 8:132. doi: 10.1038/s41392-023-01399-3
25. Chen, Y, Li, E-M, and Xu, L-Y. Guide to metabolomics analysis: a bioinformatics workflow. Meta. (2022) 12:357. doi: 10.3390/metabo12040357
26. Nicholson, JK, and Wilson, ID. Opinion: understanding 'Global' Systems biology: Metabonomics and the continuum of metabolism. Nat Rev Drug Discov. (2003) 2:668–76. doi: 10.1038/nrd1157
27. Fu, L, Zhang, L, Liu, L, Yang, H, Zhou, P, Song, F, et al. Effect of heat stress on bovine mammary cellular metabolites and gene transcription related to amino acid metabolism, amino acid transportation and mammalian target of rapamycin (Mtor) signaling. Animals. (2021) 11:3153. doi: 10.3390/ani11113153
28. Ma, N, Li, Y, Ren, L, Hu, L, Xu, R, Shen, Y, et al. Effects of dietary N-Carbamylglutamate supplementation on Milk production performance, nutrient digestibility and blood metabolomics of lactating Holstein cows under heat stress. Anim Feed Sci Technol. (2021) 273:114797. doi: 10.1016/j.anifeedsci.2020.114797
29. Abdel-Moneim, AM, Al-Kahtani, MA, El-Kersh, MA, and Al-Omair, MA. Free radical-scavenging, anti-inflammatory/anti-fibrotic and Hepatoprotective actions of taurine and Silymarin against Ccl4 induced rat liver damage. PLoS One. (2015) 10:e0144509. doi: 10.1371/journal.pone.0144509
30. Chen, X, Dong, JN, Rong, JY, Xiao, J, Zhao, W, Aschalew, ND, et al. Impact of heat stress on Milk yield, Antioxidative levels, and serum metabolites in Primiparous and multiparous Holstein cows. Trop Anim Health Prod. (2022) 54:159. doi: 10.1007/s11250-022-03159-x
31. Sammad, A, Wang, YJ, Umer, S, Lirong, H, Khan, I, Khan, A, et al. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: consequences and opportunities. Animals. (2020) 10:793. doi: 10.3390/ani10050793
32. Salama, AAK, Duque, M, Wang, L, Shahzad, K, Olivera, M, and Loor, JJ. Enhanced supply of methionine or arginine alters mechanistic target of rapamycin signaling proteins, messenger Rna, and Microrna abundance in heat-stressed bovine mammary epithelial cells in vitro. J Dairy Sci. (2019) 102:2469–80. doi: 10.3168/jds.2018-15219
33. Xu, J, Wang, X-L, Zeng, H-F, and Han, Z-Y. Methionine alleviates heat stress-induced Ferroptosis in bovine mammary epithelial cells through the Nrf2 pathway. Ecotoxicol Environ Saf. (2023) 256:114889. doi: 10.1016/j.ecoenv.2023.114889
34. Wang, H, Hao, W, Yang, L, Li, T, Zhao, C, Yan, P, et al. Procyanidin B2 alleviates heat-induced oxidative stress through the Nrf2 pathway in bovine mammary epithelial cells. Int J Mol Sci. (2022) 23:7769. doi: 10.3390/ijms23147769
35. Günenc, AN, Graf, B, Stark, H, and Chari, A. Fatty acid synthase: structure, function, and regulation In: JR Harris and J Marles-Wright, editors. Macromolecular protein complexes iv: Structure and function. Cham: Springer International Publishing (2022). 1–33.
36. De Carvalho, CCCR, and Caramujo, MJ. The various roles of fatty acids. Molecules. (2018) 23:2583. doi: 10.3390/molecules23102583
37. Nakamura, MT, Yudell, BE, and Loor, JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. (2014) 53:124–44. doi: 10.1016/j.plipres.2013.12.001
38. Kishimoto, I, Ariga, I, Itabashi, Y, and Mikami, K. Heat-stress memory is responsible for acquired Thermotolerance in Bangia Fuscopurpurea. J Phycol. (2019) 55:971–5. doi: 10.1111/jpy.12895
39. Dalile, B, Van Oudenhove, L, Vervliet, B, and Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
40. Takeuchi, H, Sekine, S, Kojima, K, and Aoyama, T. The application of medium-chain fatty acids: edible oil with a suppressing effect on body fat accumulation. Asia Pac J Clin Nutr. (2008) 17:320–3.
41. Min, L, Cheng, JB, Bl, S, Yang, HJ, Zheng, N, and Wang, JQ. Effects of heat stress on serum insulin, Adipokines, amp-activated protein kinase, and heat shock signal molecules in dairy cows. J Zhejiang Univ Sci B. (2015) 16:541–8. doi: 10.1631/jzus.B1400341
42. Carta, G, Murru, E, Banni, S, and Manca, C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol. (2017) 8:902. doi: 10.3389/fphys.2017.00902
43. Calvo-Ochoa, E, Sánchez-Alegría, K, Gómez-Inclán, C, Ferrera, P, and Arias, C. Palmitic acid stimulates energy metabolism and inhibits insulin/Pi3k/Akt signaling in differentiated human neuroblastoma cells: the role of Mtor activation and mitochondrial Ros production. Neurochem Int. (2017) 110:75–83. doi: 10.1016/j.neuint.2017.09.008
44. Lolicato, F, Brouwers, JF, Chav, DL, Wubbolts, R, Aardema, H, Priore, P, et al. The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced Lipotoxicity1. Biol Reprod. (2015) 92:16. doi: 10.1095/biolreprod.114.120634
45. Sutton-McDowall, ML, Wu, LLY, Purdey, M, Abell, AD, Goldys, EM, MacMillan, KL, et al. Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental Competence1. Biol Reprod. (2016) 94:23. doi: 10.1095/biolreprod.115.131862
46. Li, H, Xiao, Y, Tang, L, Zhong, F, Huang, G, Xu, JM, et al. Adipocyte fatty acid-binding protein promotes palmitate-induced mitochondrial dysfunction and apoptosis in macrophages. Front Immunol. (2018) 9:81. doi: 10.3389/fimmu.2018.00081
47. Khan, MJ, Rizwan Alam, M, Waldeck-Weiermair, M, Karsten, F, Groschner, L, Riederer, M, et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells*. J Biol Chem. (2012) 287:21110–20. doi: 10.1074/jbc.M111.319129
48. Lambertucci, RH, Hirabara, SM, Silveira, LR, Levada-Pires, AC, Curi, R, and Pithon-Curi, TC. Palmitate increases superoxide production through mitochondrial Electron transport chain and Nadph oxidase activity in skeletal muscle cells. J Cell Physiol. (2008) 216:796–804. doi: 10.1002/jcp.21463
49. Sharmin, MM, Mizusawa, M, Hayashi, S, Arai, W, Sakata, S, and Yonekura, S. Effects of fatty acids on inducing endoplasmic reticulum stress in bovine mammary epithelial cells. J Dairy Sci. (2020) 103:8643–54. doi: 10.3168/jds.2019-18080
50. Aguilera-Romero, A, Gehin, C, and Riezman, H. Sphingolipid homeostasis in the web of metabolic routes. Biochim Biophys Acta. (2014) 1841:647–56. doi: 10.1016/j.bbalip.2013.10.014
51. Don, AS, Lim, XY, and Couttas, TA. Re-configuration of sphingolipid metabolism by oncogenic transformation. Biomol Ther. (2014) 4:315–53. doi: 10.3390/biom4010315
52. Kondo, N, Ohno, Y, Yamagata, M, Obara, T, Seki, N, Kitamura, T, et al. Identification of the Phytosphingosine metabolic pathway leading to odd-numbered fatty acids. Nat Commun. (2014) 5:5338. doi: 10.1038/ncomms6338
53. Sonnino, S, Prinetti, A, Mauri, L, Chigorno, V, and Tettamanti, G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem Rev. (2006) 106:2111–25. doi: 10.1021/cr0100446
54. Li, J, Wen, J, Sun, C, Zhou, Y, Xu, J, MacIsaac, HJ, et al. Phytosphingosine-induced cell apoptosis via a Mitochondrially mediated pathway. Toxicology. (2022) 482:153370. doi: 10.1016/j.tox.2022.153370
55. Carfagna, MA, Young, KM, and Susick, RL. Sex differences in rat hepatic Cytolethality of the protein kinase C inhibitor Safingol: role of biotransformation. Toxicol Appl Pharmacol. (1996) 137:173–81. doi: 10.1006/taap.1996.0070
56. Taha, TA, Mullen, TD, and Obeid, LM. A house divided: ceramide, sphingosine, and Sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta Biomembr. (2006) 1758:2027–36. doi: 10.1016/j.bbamem.2006.10.018
57. Nagahara, Y, Shinomiya, T, Kuroda, S, Kaneko, N, Nishio, R, and Ikekita, M. Phytosphingosine induced mitochondria-involved apoptosis. Cancer Sci. (2005) 96:83–92. doi: 10.1111/j.1349-7006.2005.00012.x
58. Schofield, JH, and Schafer, ZT. Mitochondrial reactive oxygen species and Mitophagy: a complex and nuanced relationship. Antioxid Redox Signal. (2020) 34:517–30. doi: 10.1089/ars.2020.8058
59. Sun, C, Chang, X, MacIsaac, HJ, Wen, J, Zhao, L, Dai, Z, et al. Phytosphingosine inhibits cell proliferation by damaging DNA in human cell lines. Ecotoxicol Environ Saf. (2023) 256:114840. doi: 10.1016/j.ecoenv.2023.114840
60. Soflaee, MH, Kesavan, R, Sahu, U, Tasdogan, A, Villa, E, Djabari, Z, et al. Purine nucleotide depletion prompts cell migration by stimulating the serine synthesis pathway. Nat Commun. (2022) 13:2698. doi: 10.1038/s41467-022-30362-z
61. Wu, F, and Minteer, S. Krebs cycle metabolon: structural evidence of substrate channeling revealed by cross-linking and mass spectrometry. Angew Chem Int Ed. (2015) 54:1851–4. doi: 10.1002/anie.201409336
62. Gonzalez-Rivas, PA, Chauhan, SS, Ha, M, Fegan, N, Dunshea, FR, and Warner, RD. Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci. (2020) 162:108025. doi: 10.1016/j.meatsci.2019.108025
63. Sejian, V, Bhatta, R, Gaughan, JB, Dunshea, FR, and Lacetera, N. Review: adaptation of animals to heat stress. Animal. (2018) 12:s431–44. doi: 10.1017/S1751731118001945
64. Megumi, F, Nana, S, and Ken, O. Hypoxanthine reduces radiation damage in vascular endothelial cells and mouse skin by enhancing Atp production via the salvage pathway. Radiat Res. (2022) 197:583–93. doi: 10.1667/RADE-21-00223.1
65. Garg, D, Singh, M, Verma, N, and Monika, R. Review on recent advances in fabrication of enzymatic and chemical sensors for hypoxanthine. Food Chem. (2022) 375:131839. doi: 10.1016/j.foodchem.2021.131839
66. Kim, Y-J, Ryu, H-M, Choi, J-Y, Cho, J-H, Kim, C-D, Park, S-H, et al. Hypoxanthine causes endothelial dysfunction through oxidative stress-induced apoptosis. Biochem Biophys Res Commun. (2017) 482:821–7. doi: 10.1016/j.bbrc.2016.11.119
67. Murata, H, Kunii, H, Kusama, K, Sakurai, T, Bai, H, Kawahara, M, et al. Heat stress induces oxidative stress and activates the Keap1-Nfe2l2-are pathway in bovine endometrial epithelial cells†. Biol Reprod. (2021) 105:1114–25. doi: 10.1093/biolre/ioab143
68. Quinteiro-Filho, WM, Rodrigues, MV, Ribeiro, A, Ferraz-de-Paula, V, Pinheiro, ML, Sá, LRM, et al. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal Axis Activation1. J Anim Sci. (2012) 90:1986–94. doi: 10.2527/jas.2011-3949
Keywords: metabolites, cattle, heat stress, metabolic pathways, taurine
Citation: Liu F, Liang L, Luo Z, Zhang G, Zuo F and Wang L (2024) Effects of taurine on metabolomics of bovine mammary epithelial cells under high temperature conditions. Front. Vet. Sci. 11:1393276. doi: 10.3389/fvets.2024.1393276
Edited by:
Yangchun Cao, Northwest A&F University, ChinaReviewed by:
Tatiana Ruiz-Cortés, University of Antioquia, ColombiaDa-Wei Wei, Ningxia University, China
Copyright © 2024 Liu, Liang, Luo, Zhang, Zuo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Wang, d2xpbmc4MTBAMTYzLmNvbQ==
 Feifei Liu1
Feifei Liu1 Ling Wang
Ling Wang