- 1Institute of Animal Husbandry and Veterinary Medicine of Hebei Province, Baoding, China
- 2College of Traditional Chinese Veterinary Medicine, Hebei Agricultural University, Baoding, China
- 3Hebei General Station of Animal Husbandry, Shijiazhuang, China
- 4Animal Husbandry Technology Promotion Institution of Zhangjiakou, Zhangjiakou, China
The present study was designed to evaluate the effect of a mixture of Chinese medicinal residues (CMRs) consisting of Salvia miltiorrhiza residues (SMR) and Isatidis Radix residues (IRR) on productive performance, egg quality, serum lipid and hormone levels, liver and blood antioxidant capacity, oviduct inflammation levels, and gut microbiota in the late-laying stage. A total of 288 fifty-four-week-old BaShang long-tailed hens were divided into four groups. The feed trial period was 8 weeks. The control group was fed the basic diet as a CCMR group, supplemented with 3, 4, and 6% for the experimental groups LCMR, MCMR, and HCMR. The egg production rate of the MCMR group was 8.1% higher than that of the CCMR group (p < 0.05). Serum triglyceride (TG) levels of hens of the CMR-supplemented group were significantly decreased than those of the CCMR group (p < 0.05). The group supplemented with different levels of CMR had significantly higher serum HDL-C levels compared with the control group (p < 0.05). Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels were remarkably increased for the LCMR and MCMR groups and significantly decreased for the HCMR group compared to CCMR (p < 0.05). Serum and liver glutathione peroxidase (GSH-PX) activities were significantly increased, and malondialdehyde (MDA) levels were significantly decreased in the MCMR group compared to the CCMR group (p < 0.05). The expression levels of tubal inflammatory factor markers (IL-4, IL-1β, TNF-α) in the MCMR and HCMR groups were consistent with the pathological findings of the sections. As for cecal microbiota, supplementation with CMR affected the alpha diversity of the cecum microbiome at the genus level. The Shannon index was significantly higher in the MCMR group than in the CCMR and HCMR groups (p < 0.05). Supplementation with different levels of CMR mainly regulated the ratio of intestinal Firmicutes to Bacteroidetes and the abundance of phyla such as Proteobacteria. In addition, CMR supplementation at different levels in the diet enriched lipid-metabolizing bacteria, such as Bacteroides and Ruminococcus_gnavus_group. Furthermore, according to linear discriminant analysis (LDA) effect size (LEfSe) analysis, the MCMR group showed an increase in the number of short-chain fatty acid-producing bacteria Romboutsia and fiber-degrading specialized bacteria Monoglobus. Therefore, supplementation of appropriate amounts of CMR to the diet of laying hens enhanced reproductive hormone levels, hepatic antioxidant capacity, and lipid metabolism, alleviated the levels of oviductal inflammatory factors, and modulated the abundance structure of bacterial flora to improve the late-laying performance and egg quality. The results of the current study showed that CMR is a beneficial feed supplement for chickens when added in moderation.
1 Introduction
The policy of reducing and banning the use of antibiotics is closely linked to hen welfare. The problems of drug residues and bacterial resistance caused by the misuse of antibiotics are a serious threat to animal health and food safety. In 2006, the European Union completely banned the use of growth-promoting antimicrobials in poultry feeds (1). According to the Ministry of Rural Affairs Announcement No. 194, in China, all growth-enhancing drug feed additives, except for traditional Chinese medicines, have been banned since 2020. Therefore, research and development of alternatives to antibiotics should be accelerated to meet the needs of the poultry industry and the demand for healthy green egg and poultry products. Chinese medicine resources plant-feeding functional products both to prevent disease and promote growth. The dietary addition of hawthorn leaf flavonoids can alleviate decreased ovarian function and improve hepatic lipid metabolism in older breeders (2). Marigold fortificant is a good colorant for egg yolks (3).
Salvia miltiorrhiza (SM) was first documented in “Shen Nong herbal classic,” which can relieve pain and blood stasis. Its main components include polysaccharides, water-soluble phenolic acids, and fat-soluble diterpenoids (4). Modern pharmacophore studies have shown that phenolic acid ingredient salvianolic acid A binds to disease targets sphingomyelin synthase 2 to exert therapeutic effects on hyperlipidemia (5). The liver is protected in vivo by the anti-inflammatory and antioxidant effects of SM polysaccharides (6). Isatidis Radix (IR) is a representative Chinese drug that is frequently used for heat-clearing and detoxification. Pharmacological studies have confirmed that IR mainly contains alkaloids, sulfur-containing compounds, phenylpropanoids, and other 19 categories of ingredients (7). IR has hypolipidemic pharmacological activity and can effectively inhibit adipocyte differentiation and lipid synthesis (8). IR polysaccharides have anti-inflammatory (9), lipid regulating (10), and immune cell proliferation effects (11). The use of SM and IR combination can function to tonify the liver and nourish blood. SMR and IRR are by-products of the deep processing of medicinal herbs. The deep processing industries such as traditional Chinese medicine and pharmaceuticals, which consume medicinal herbs as raw materials, produce over 70 million tons of by-products each year (12). The water content of CMRs after the extraction of active ingredients can be as high as 70%, and large piles of CMRs will decay rapidly, leading to the pollution of water sources and the emission of irritating gases (13). The herbal residue contains cellulose, hemicellulose, and lignin, as well as many medicinal components such as flavonoids, alkaloids, and organic acids. There is an urgent need to explore ways to utilize CMR resources in a high value-added way, while at the same time alleviating the pressure of environmental pollution. Previous studies have shown that the polysaccharide SMWP-U&E, isolated from SMR, was confirmed in in vivo experiments to improve gut morphology and flora, immunity, and antioxidant capacity (14). Residual glucan composition in IRR has been reported to be as high as 39% (15).
The liver is the primary organ for lipid synthesis and catabolism during egg production. Excessive reactive oxygen species (ROS) accumulation in the liver or bloodstream can lead to disorders of lipid metabolism due to elevated levels of fatty acid synthase and impaired synthesis of very low-density lipoprotein (VLDL) (16, 17). The liver interacts directly with the gut microbiota through the portal circulation to influence gut health (18). Intestinal flora composition and its metabolites, such as 5-methoxyindoleacetic acid and indole, remotely regulate hepatic function via the liver-gut axis (19). However, the poultry age and nutrients drive intestinal flora change. Due to the special reproductive structure and performance of laying hens, pathogenic bacteria in the intestine can be transferred to the oviducts through the cloaca to cause inflammation and immune reactions (20). Feedback regulation of hormones will directly affect ovarian follicle development and hen performance.
The BaShang long-tailed hen is the only local poultry variety in Hebei Province that has been listed in the “Poultry Genetic Resources in China.” It is physically resistant to rough feeding. However, extremely low egg production rates in the late-phase laying limit the useful cycle of the species. Therefore, this experiment investigated the effects of CMRs, specifically the mixture of SMR and IRR at a ratio of 1:1, supplementation on production performance, blood lipid levels, antioxidant status, reproductive hormone levels, oviductal inflammation levels, and cecum microbiota in the late-laying stage. This study aimed to provide a preliminary assessment of the potential value of CMR as a dietary supplement for layers.
2 Materials and methods
2.1 Drugs and reagents
The trial materials SMR and IRR were derived from Shineway Pharmaceutical Group Ltd., Shijiazhuang, China. Drying and crushing of single-extracted residue through 100 mesh sieve 1:1 mixing. The assay kits of total superoxide dismutase (T-SOD), GSH-PX, catalase (CAT), MDA, total cholesterol (TC), TG, low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) were purchased from Nanjing Jiancheng Biotechnology Research Institute, Nanjing, China. The assay kits of estrogen (E2), FSH, LH, and progesterone (Prog) were obtained from Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China. The M5 Universal Plus RNA Mini Kit (MF167-01) and M5 HiPer SYBR Premix Es Taq (MF787-01) were obtained from Mei5 Biotechnology Co., Ltd., Beijing, China. The SweScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (G3337-50) was purchased from Wuhan Service Bio Technology Co., Ltd., Wuhan, China.
2.2 Animal groups and handling
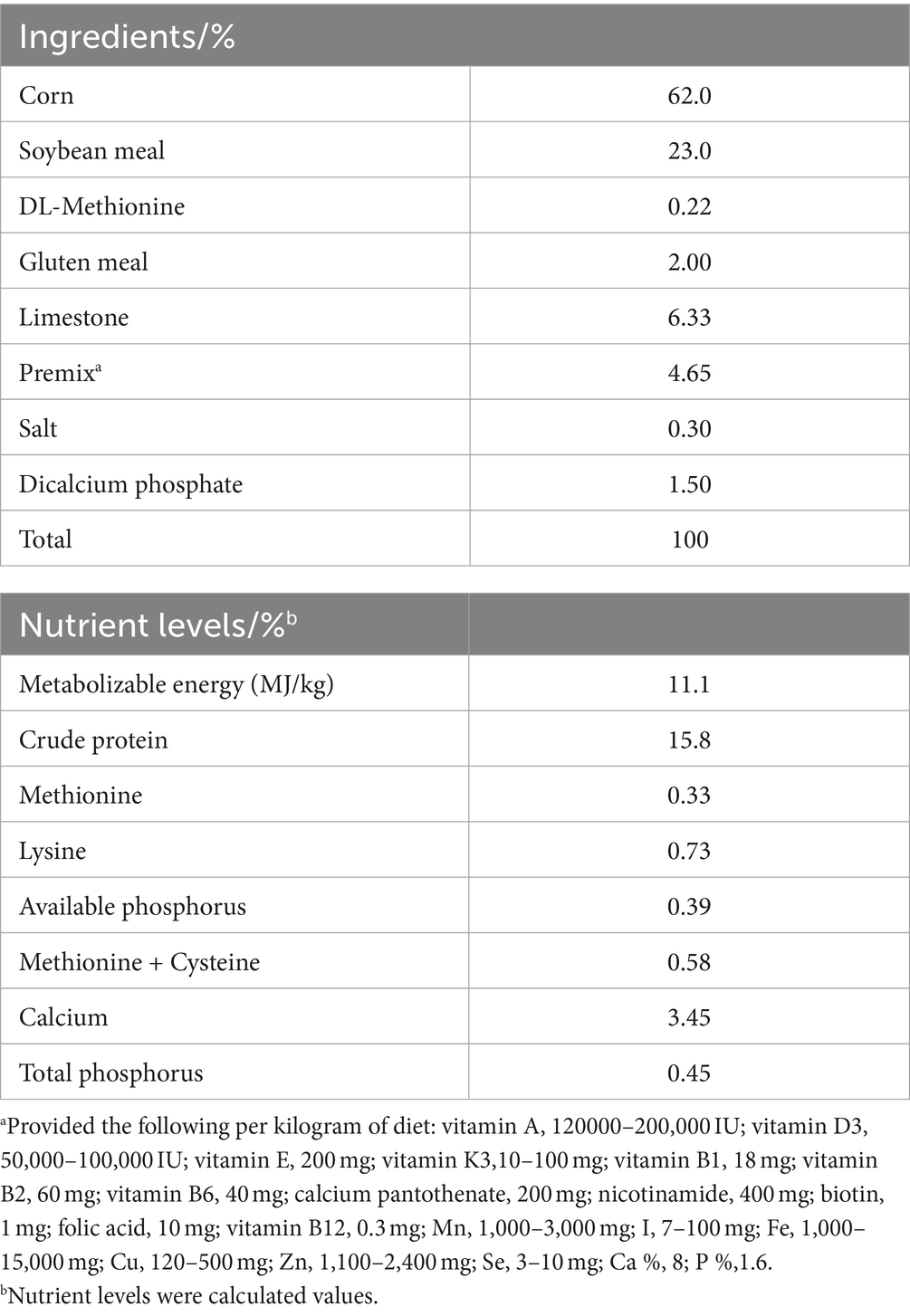
This experiment was sanctioned by the Animal Conservation and Utilization Committee of Hebei Agricultural University (grant No.2022161). A total of 288 healthy 54-week-old BaShang long-tailed hens with similar body weights were obtained from Hebei Green Field Poultry Technology Co., Ltd. (Zhangjiakou, China). Laying hens were kept in indoor cages (3 hens per cage). Each cage contained water bottles and feed buckets. The indoor temperature and humidity were 25°C and 65%, respectively. Furthermore, 16 h of light in the room were maintained, and the room was kept clean and tidy. A total of 72 hens were in each group, with six replicates in each group. Randomly divided into a control group (CCMR), 3% CMR group (LCMR), 4% CMR group (MCMR), and 6% CMR group (HCMR), basal diet supplemented with 3, 4, and 6% CMR, respectively. CMR was added to the trial for 8 weeks. The basal diets were formulated to meet nutrient requirements (Table 1).
2.3 Sample collection
Eggs are collected and recorded at 3 p.m. daily. At the end of the 8th week, two chickens were taken from each replicate group. Blood samples (5 mL) were collected from each chicken under the wings after 8 h of fasting. These two chickens were then euthanized by jugular bloodletting. Serum samples were separated using a centrifuge at 1,500 × g for 10 min at 4°C and stored at −20°C for measurement. Magnum and shell gland of oviducts and cecum contents of (three hens’ ceca contents were mixed into one sample) laying hens were stored in a refrigerator at −80°C for measurement. Oviduct magnum and shell gland tissues were fixed with 4% paraformaldehyde.
2.4 Analysis of nutrient and medicinal components in CMR
In the experiment, the SMR and IRR mixture at a ratio of 1:1 was weighed at 3 g. Three parallel replicate samples were set up. Crude protein was assayed using the Kjeldahl method. Crude ash was assayed using the cauterization method. Crude fat was assayed using the Soxhlet extraction method. The analysis of crude fiber, acid detergent fiber, and neutral detergent fiber was done using automatic fiber apparatus (A2000i, ANKOM, America). The content of total flavonoids in CMR was determined using the NaNO2-Al (NO3)3 color developing method (21). For crude polysaccharide, alkaloids, and total saponin test, refer to the total flavonoid test method. Glucose was used as the standard to establish the standard curve. The crude polysaccharide was determined using the phenol-sulfuric acid method at 490 nm with a UV spectrophotometer (UV-6850, JENWAY, United States). Reisner salt can bind to alkaloids in acidic media to form a complex precipitate that forms a purplish-red solution detected at UV 525 nm. Oleanolic acid was used as the standard to establish the standard curve. The samples were added with vanillin-glacial acetic acid solution and perchloric acid, respectively, to determine the absorbance at UV 540 nm and calculate the content of total saponins in CMR.
2.5 Production performance and egg quality
During the trial, the number of eggs laid, egg weight, average daily feed intake, dead elimination quantity, and daily feed consumption were recorded for each replicate, and the total egg production rate and feed-to-egg ratio were calculated. At the end of weeks 4 and 8, 12 eggs were taken from each group. Egg weight, Eggshell weight, and yolk weight were weighed using an electronic balance. An egg quality analyzer (EA-01, Orka Food Ltd., Israel) was used to determine albumen height, yolk color, and Haugh unit. Eggshell thickness at the large, middle, and small ends of the egg and the long and short diameter of the egg were determined using electronic digital calipers. The egg shape index and shell index were calculated. Eggshell index = Eggshell weight / 4.68 × (Egg weight)2/3 (22). F / E = feed consumption to egg weight ratio.
2.6 Serum biochemical and hormone level tests
The serum levels of TC (A111-1-1), TG (A110-1-1), LDL-C (A113-1-1), and HDL-C (A112-1-1) were determined by an enzyme label (DG5033A, East China Electronics Group Medical Equipment Co., Ltd., Nanjing, China) with commercial diagnostic kits. The serum levels of E2 (YJ023194), FSH (YJ042764), LH (YJ611689), and Prog (YJ059935) were determined by an enzyme label (RT-6100, Rayto Life and Analytical Sciences Co., Ltd., China) with commercial diagnostic kits.
2.7 Antioxidant indicator tests
The liver tissue was ground with saline in a ratio of 1:10, centrifuged, and the supernatant was taken for testing. Total protein in the supernatant was detected using the Total Protein Assay Kit (Caulmers Brilliant Blue). Measurement of T-SOD (A001-1-2), GSH-PX (A005-1-2), CAT (A007-1-1) activities, and MDA (A003-1-1) contents of serum and liver was determined by enzyme labeling apparatus (DG5033A, East China Electronics Group Medical Equipment Co., Ltd., Nanjing, China) with commercial diagnostic kits.
2.8 Histomorphometric analysis
The 4% paraformaldehyde-soaked oviduct magnum and shell gland were taken, fixed, dehydrated using graded alcohol, and soaked in wax. They were paraffin-embedded and sectioned using Leica RM2016; slides were fished and dried, followed by hematoxylin–eosin (HE) staining. Histopathological changes were viewed under a photomicroscope (NIKON ECLIPSE E100, NIKON, Japan).
2.9 Real-time polymerase chain reaction analysis
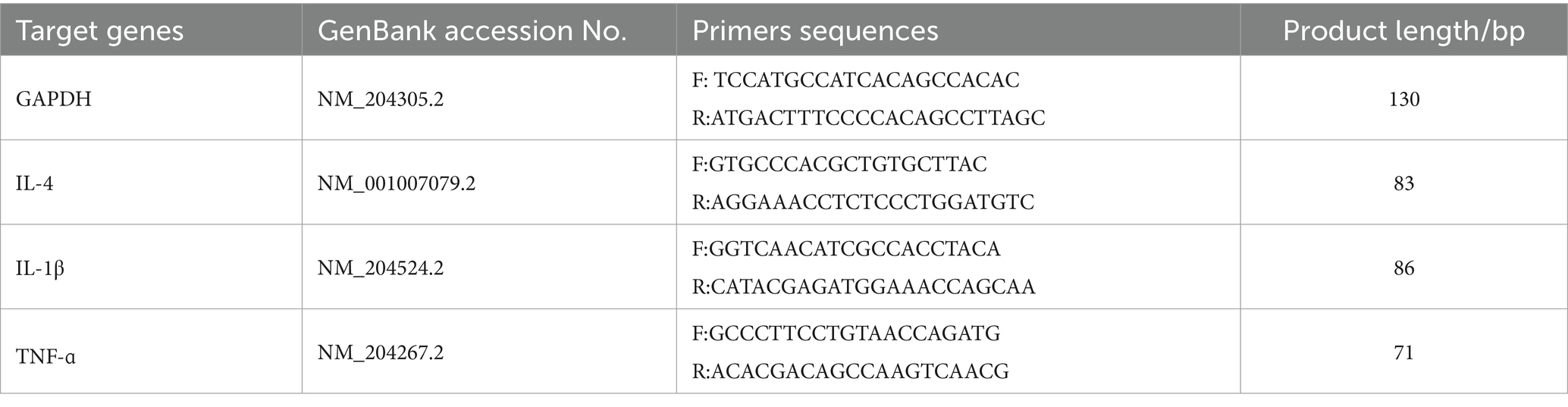
Gene expressions in the oviduct magnum and shell gland (IL-4, IL-1β, TNF-α) were detected using real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) (BIO-RAD, Hercules, CA). Total RNA was extracted from the magnum and shell gland using M5 Universal Plus RNA Mini Kit, and reverse transcription using the SweScript All-in-One First-strand cDNA Synthesis SuperMix for qPCR Kit. Quantitative real-time PCR was conducted using the M5 HiPer SYBR Premix Es Taq kit. Primer sequences are shown in Table 2. The relative expression of mRNA was determined by the 2−ΔΔCt method.
2.10 Bacterial DNA extraction and 16S rRNA gene sequencing
Genomic DNA was extracted using the CTAB method. The extracted DNA concentration was measured using the NanoDrop 2000 spectrophotometer (TFS, Waltham, MA, EUA). The extracted DNA is amplified by PCR in the reaction system. Primers 341F and 806R were used for PCR amplification of the V3-V4 region. The PCR products were purified by Qiagen using the gel recovery kit, and the libraries were constructed using the NEBNext® Ultra™ IIDNA Kit, and libraries were tested and quantified by Q-PCR using the Agilent 5,400. The sequencing and bioinformatics analysis were performed by Novo Genome Bioinformatics Technology Co., Ltd. (Beijing, China).
Each de-duplicated sequence generated after noise reduction using DADA2 was called ASVs (amplicon sequence variants). Subsequently, the QIIME2 classify-sklearn algorithm was used. Species annotation was performed for each ASV using a pre-trained Naive Bayes classifier. Subjected to diversity analysis (Shannon), a metric-free multidimensional calibration method (NMDS) analysis was performed using the Bray-Curtis method on ASVs to show diversity. LEfSe analyzed inter-group differences (LDA score > 3). The Spearman rank correlation was used to investigate the correlation between serum biochemical and microbial populations.
2.11 Statistical analysis
The data were analyzed using IBM SPSS Statistics software (version 25.0, SPSS, Chicago, IL). One-way analysis of variance (ANOVA) and Duncan’s multiple comparison tests were performed between groups. A p-value of less than 0.05 (p ≤ 0.05) indicates a significant difference among groups.
3 Results
3.1 Main medicinal components and nutrition in CMRs
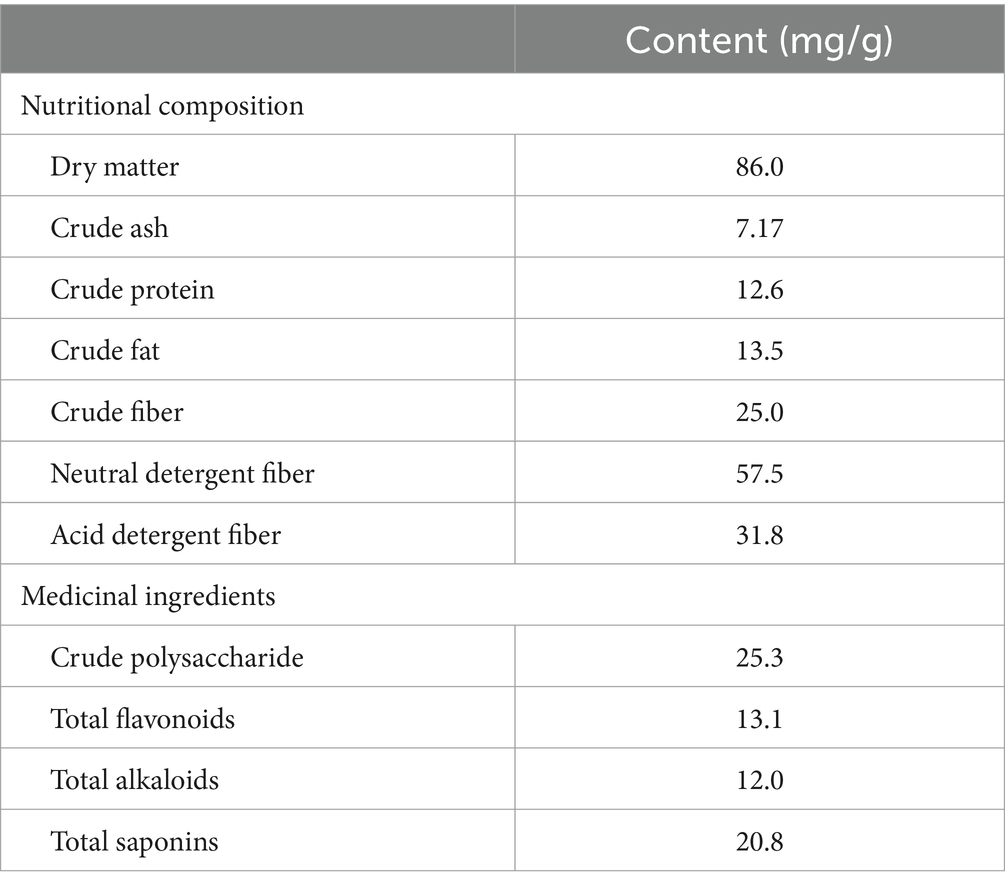
The main medicinal and nutritional ingredients in CMRs are shown in Table 3. The content of crude polysaccharide in CMRs was 25.3 mg/kg, followed by a saponin content of 20.8 mg/g, containing small amounts of flavonoids and alkaloids of 13.1 mg/g and 12.0 mg/g, respectively. The nutritional analysis of CMRs revealed a crude fiber content of 25 mg/g and lower crude protein and crude fat content. The disadvantages of using it as animal feed are poor palatability, low digestibility, and low nutritional value.
3.2 Effect of CMR supplementation in the diet on production performance and egg quality
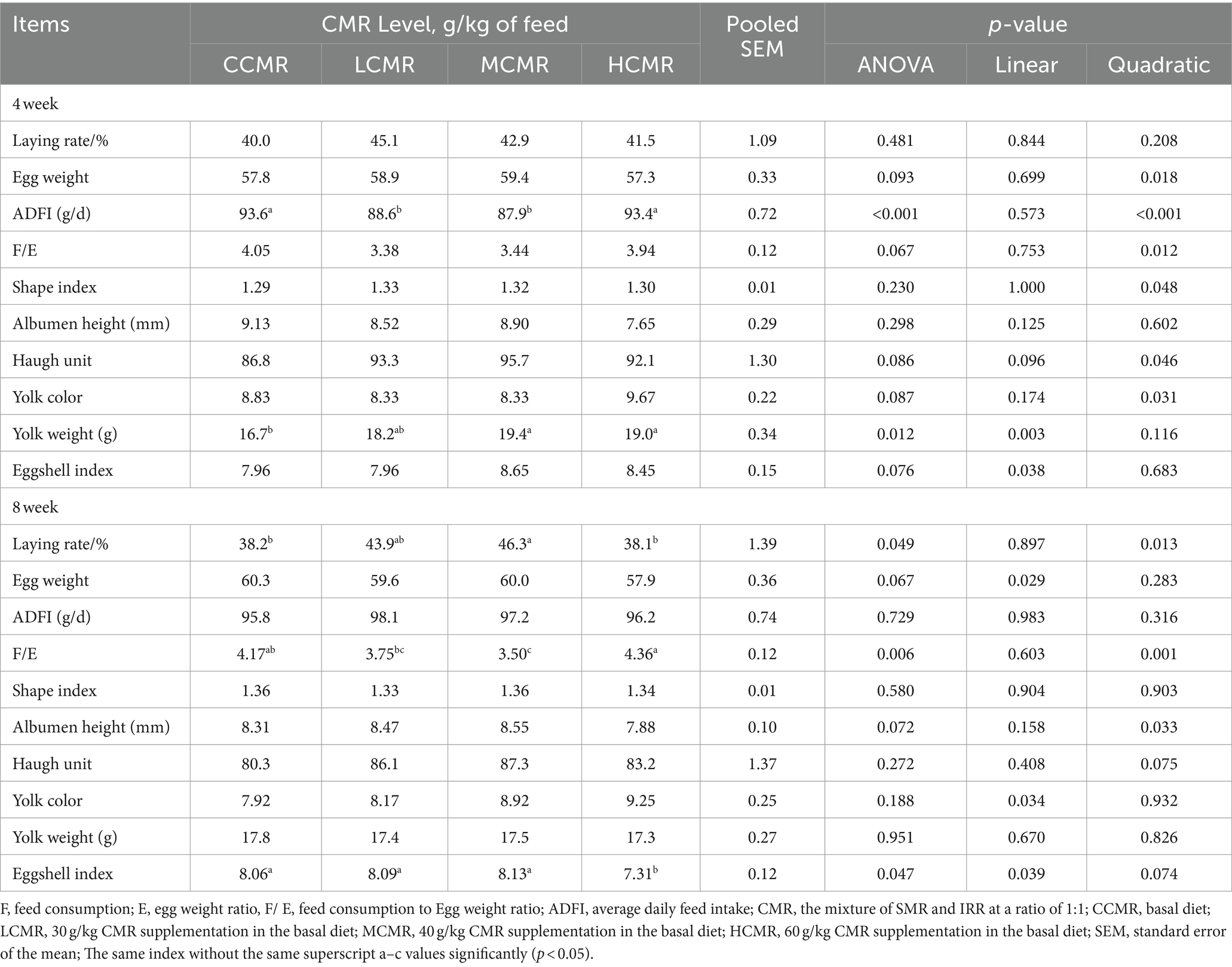
In the fourth week, yolk weight was significantly increased (p < 0.05) in the MCMR and HCMR groups compared with the CCMR group, and there was no significant difference between the CMR groups (p > 0.05; Table 4). Differences in ADFI levels between groups were significant and were significantly quadratic with changes in CMR supplementation levels (p < 0.05). In the eighth week, the MCMR group had a significantly lower (p < 0.05) feed-to-egg ratio, but the egg production rate of the MCMR group was 8.1% higher than that of the CCMR group and 8.2% higher than that of the HCMR group (p < 0.05). The eggshell index of the HCMR group was significantly lower than that of the other groups (p < 0.05).
3.3 Effects of CMR supplementation in the diet on serum biochemical and hormonal parameters
The serum levels of TG were significantly lower, and HDL-C levels were significantly higher in laying hens after CMR supplementation in the diets compared with the CCMR group (p < 0.05; Figure 1). The serum LDL-C levels were significantly lower in the MCMR and HCMR groups than in the CCMR group (p < 0.05), but there was an insignificant difference among the three supplemental CMR groups (p > 0.05).
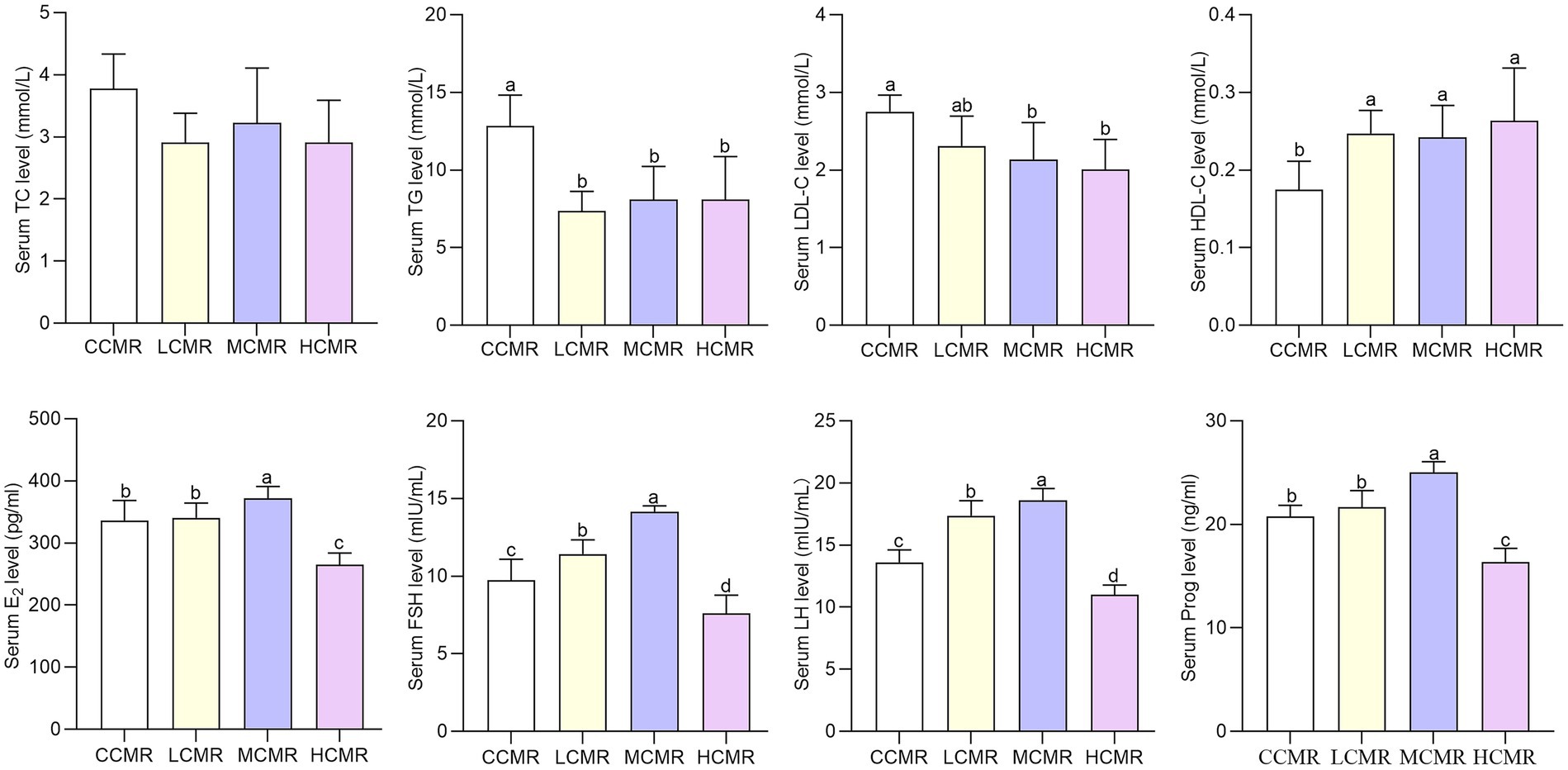
Figure 1. Effect of CMR supplementation in diets on serum biochemical and hormone parameters. CCMR (basal diet), LCMR (30 g/kg CMR supplementation in the basal diet), MCMR (40 g/kg CMR supplementation in the basal diet), and HCMR (60 g/kg CMR supplementation in the basal diet). The same index without the same superscript a-d value significantly (p < 0.05).
Serum levels of FSH and LH were significantly higher in the LCMR and MCMR groups than in the CCMR group (p < 0.05, Figure 1). The serum levels of Prog and E2 in laying hens in the MCMR group were significantly higher, whereas the serum levels of E2 and Prog in laying hens in the HCMR group were significantly lower than those in the CCMR group (p < 0.05). Serum FSH and LH levels of laying hens in the MCMR group were significantly higher than those in the LCMR group (p < 0.05).
3.4 Effect of dietary CMR supplementation on histomorphology
The results of the impact of CMR addition to the diet on the oviductal histomorphology of hens are shown in Figure 2. A small amount of focal infiltration of lymphocytes can be seen in the oviduct magnum submucosa of the CCMR group. In the HCMR group, there were many secondary folds of cilia in the magnum mucosa layer, and there were severe wrinkles. The columnar ciliated cell layer in the CMR-supplemented group was thickened to varying degrees. There is an occasional increase in white blood cell count in the oviduct shell gland of the MCMR group. In the other three groups, the cytoplasm of the gland cells in the shell gland is vacuolated.
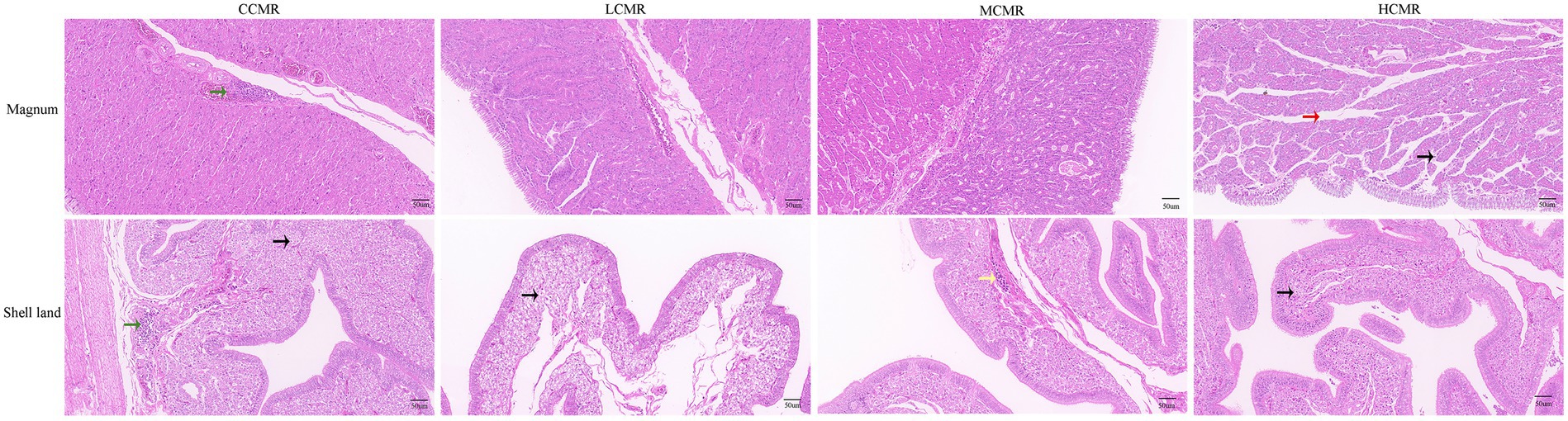
Figure 2. Histomorphometric analysis of the oviduct magnum and shell gland. The black arrows in the figure indicate cytoplasm showing vacuolation; the red arrows indicate secondary folds; the green arrows indicate foci of lymphocytic inflammatory infiltrate; and the yellow arrows indicate leukocytes.
3.5 Effect of dietary CMR supplementation on antioxidation indices
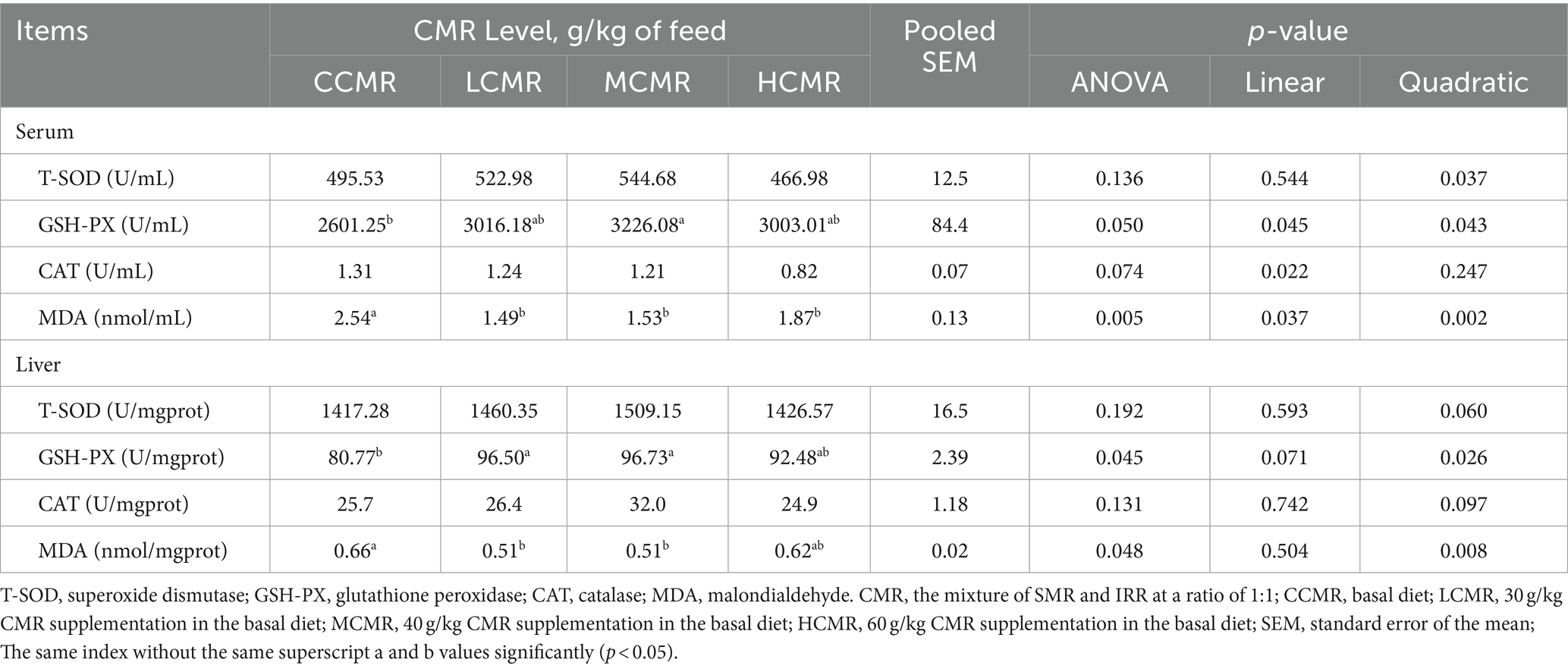
The effect of CMR on serum and liver antioxidant capacity is shown in Table 5. There were insignificant differences in serum and liver T-SOD activity and CAT activity between the groups (p > 0.05). The activity of GSH-PX in the MCMR group was significantly higher, and MDA content was significantly lower compared with the CCMR group (p < 0.05). There was an insignificant difference in GSH-PX activity and MDA content among the CMR-supplemented groups (p > 0.05). Significant secondary changes in serum and hepatic GSH-PX activity, as well as MDA content, were observed with increasing levels of the CMR additive (p < 0.05).
3.6 Effect of dietary CMR supplementation on oviduct-related mRNA gene expression
Inflammatory factor IL-4 expression levels in the oviduct magnum were significantly decreased after supplementation of MCMR in the diets compared with those in the CCMR group, whereas IL-1β expression levels were significantly increased in the HCMR group (p < 0.05; Figure 3A). The level of TNF-ɑ expression was lowest in the MCMR group compared with the CMR-supplemented group (p < 0.05). The difference in the expression level of inflammatory factors IL-4 and TNF-α in the oviduct shell gland was significant among groups (p < 0.05; Figure 3B).

Figure 3. Inflammatory factor gene expression in the oviduct. (A) Oviduct magnum. (B) Oviduct shell gland. The same index without the same superscript a–d values significantly (p < 0.05).
3.7 Effect of dietary CMR supplementation on cecal microbiota composition
In the microbial analysis, an average of 74,410 filtered high-quality sequences were obtained. The alpha diversity analysis using the Shannon index showed that the MCMR group had a significantly higher diversity than the control and HCMR groups (p < 0.05, Figure 4A). The stress values were less than 0.2 (p = 0.069; Figure 4C) for each group indicating the feasibility of using NMDS to accurately respond to different levels in different samples. The dominant flora were Firmicutes and Bacteroidetes followed by Verrucomicrobiota and Proteobacteria at the phylum level (Figure 4B). Enrichment abundance varied considerably between groups at the genus level, with the MCMR group dominated by Bacteroidetes, Rikenellaceae_RC9_gut_group, and Lactobacillus (Figure 4D). LEfSe analysis of bacteria as biomarkers differentiated the microbiota of the control and MCMR groups. The MCMR group was enriched with the organic matter-degrading bacteria Romboutsia, Veillonellaceae, Barnesiella, and Monoglobus (Figure 4E). The correlation between cecum microbiota genus levels and serum biochemical indices was analyzed using the Spearman analysis, and the results are shown in Figure 4F. Blautia, Sellimonas, and Clostridium_sensu_stricto_1 were negatively correlated with serum lipid metabolism (TC, TG, and LDL-C). Muribaculaceae, Parabacteroides, Helicobacter, and streptococcus had a positive correlation with serum lipid metabolism.
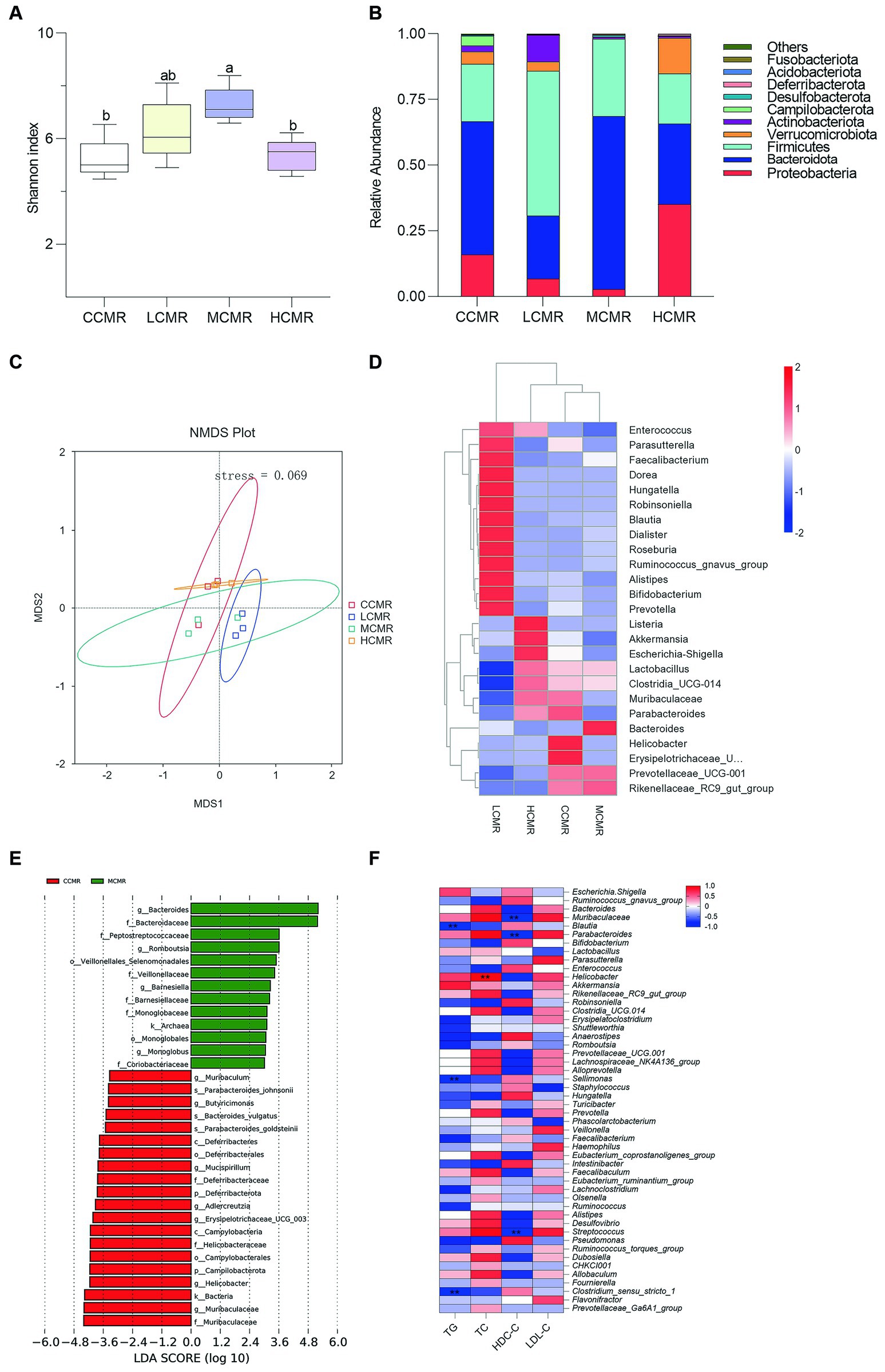
Figure 4. Analysis of cecum gut microbiota. (A) The Shannon index; (B) phylum level relative abundance; (C) NMDS analysis; (D) genus-level species abundance clustering heat map; (E). LEfSe analysis (LDA score > 3); and (F) Spearman analysis at the genus level between serum biochemical indices and cecal microbiota (Three hens’ ceca contents were mixed into one sample).
4 Discussion
The active ingredients are still present in the residue of Chinese medicines after decoction or extraction. For example, the HPLC analysis of the residue after Sophora flavescens water extraction by ethyl acetate ultrasonic re-extraction revealed that the main component contained various flavonoids (kurarinone and sophoraflavanone G) (23). Our study revealed that polysaccharides, flavonoids, alkaloids, and saponins were detected in CMR. Modern pharmacology has proved that its constituents have anti-inflammatory, antioxidant, hepatoprotective, and other biological activities (24, 25). Furthermore, CMRs contain some basic nutrients. Therefore, CMRs have the conditions to be developed into feed supplements.
Production performance is normally used to assess the condition of the laying hens’ bodies. Our study showed that supplementation of MCMR in the diet increased egg production and reduced the feed-to-egg ratio. The increased egg production and reduced feed-to-egg ratio may be due to improved hormone levels and an increased abundance of beneficial bacteria that degrade carbohydrates. The hypothalamic–pituitary (FSH and LH)-gonadal (E2) axis regulates follicular development and productive performance. The reproductive hormone FSH functions to promote follicular growth, development, and maturation, and LH induces follicular ovulation. Levels of serum FSH and LH were significantly higher in the LCMR and MCMR groups compared to the group without added CMR. Serum E2 and Prog levels were also significantly increased in the MCMR group compared to the other groups. Therefore, supplementation with CMR enhances the synergistic effect of secreted hormones, thereby promoting production performance in laying hens. The high content of crude fiber and lignin in the diet reduces palatability, and anti-nutritional factors such as tannins and phytic acid reduce the apparent digestibility of nutrients. Small mammals that consume high-fiber foods, in addition to increasing food intake to compensate for decreased digestibility, additionally reduce basal metabolic rate and non-shivering thermogenesis levels to compensate for reduced energy acquisition efficiency by reducing energy expenditure (26, 27). There was evidence that reduced egg production was directly correlated with reproductive hormones and liver metabolism (28, 29). The mean daily feed intake at 1–4 weeks and egg production rate at 4–8 weeks in the HCMR group were insignificantly different from those of the control group. Meanwhile, the disturbed intestinal flora of the HCMR group may affect the brain-intestinal-hepatic-ovarian axis, leading to a significant decrease in reproductive hormone levels and affecting egg production, and the specific mechanism is subject to further study.
The expression of anti-inflammatory and pro-inflammatory factors produced by the mucosal microwell environment of the oviduct of the laying hen is in dynamic balance under healthy conditions. The process of Gram-negative bacterial death or multiplication releases lipopolysaccharides (LPS), leading to elevated levels of pro-inflammatory factors. IL-4 cytokines are mainly secreted by a subpopulation of Th2 T cells and promote immune responses by binding specifically to interleukin-4 receptors on the surface of target cells (30). Other studies have shown that IL-4 and TNF-α have a dual role in resisting Gram-negative bacterial infections in the organism (31). The expression of anti-inflammatory factor IL-4 and pro-inflammatory factors TNF-ɑ and IL-1β genes was reduced in the MCMR group, probably because the levels of anti-inflammatory factors were correspondingly lowered by the organism to maintain relative homeostasis. The pro-inflammatory cytokine IL-1β affects eggshell ultrastructure through inhibition of calcium-binding protein expression and ca2+ transport in the endometrium (32). The HE staining results corroborate the molecular results that CMR can reduce inflammation in the fallopian tube tissue. The eggshell index refers to the eggshell weight per unit area. Escherichia-Shigella has been reported to be associated with eggshell quality in eggs (33). Due to the special reproductive structure of laying hens, the oviduct increases exposure to harmful bacteria, so abnormal calcium synthesis in the oviduct shell gland and impaired immune function caused a decrease in the eggshell index in the HCMR group.
After the high-intensity metabolic phase of the peak laying period in laying hens, many diseases of the body are caused by the imbalance of the redox system disrupted by reactive oxygen species. Enzymatic antioxidant defense systems include T-SOD, GSH-PX, and CAT. MDA is a peroxidation product of lipids during oxidative stress. It has been shown that the addition of quercetin and daidzein, a natural antioxidant, significantly increased antioxidant enzyme levels and decreased the content of MDA in laying hens (34). The same results were found in our study, where the serum and liver of CMR-supplemented laying hens showed different increases in GSH-PX activity and decreases in MDA content compared to the normal diet group. The metabolic disorders of hepatic lipids in the late egg-laying period directly affect the production of yolk precursor substances (28). Our study showed a decrease in the serum LDL-C/HDL-C ratio and a significant decline in serum TG levels in the CMR-supplemented group, suggesting an improvement in hepatic lipid metabolism levels in laying hens. Bifidobacterium and Lactobacillus are beneficial bacteria for lipid metabolism (35). The LCMR group was enriched with Bifidobacterium, while Lactobacillus was enriched in both the HCMR and MCMR groups with a higher abundance than the control group. Prior studies have confirmed the role of Parabacteroides in regulating lipid metabolism through transforming bile acid and succinic acid (36). The abundance of Parabacteroides in our study was low in the LCMR and MCMR groups but high in the HCMR and CCMR groups. However, lipid levels remained low for the HCMR group, which may be related to Akkermansia. Oral Akkermansia has been reported to reverse metabolic abnormalities caused by a high-fat diet (37).
Gut microflora are important in the maintenance of intestinal physiological functions and related activities. Insoluble fiber plant feed expands when it encounters water. This expansion increases the contact area between surimi and the epithelial cells of intestinal villi, prolongs the retention time and retention rate of surimi in the gastrointestinal tract, and accelerates the reproduction of harmful bacteria. This can potentially lead to intestinal diseases and reduce the feed conversion rate (38, 39). The relative abundance ratio of Bacteroidetes to Firmicutes increased during the late egg-laying period, while the abundance of Proteobacteria decreased (40). The results of this trial are consistent with previous ones, and we found that the LCMR and MCMR groups increased the diversity of the cecum microflora, dominated by Firmicutes and Bacteroidota. However, over-supplementation decreased the diversity of the flora, dominated by Proteobacteria and Bacteroidota. Proteobacteria include some conditionally pathogenic bacteria, such as Salmonella and E. coli. Similarly, the HCMR group enriched for Listeria and Escherichia-Shigella may be a causative agent unfavorable to the organism. Previous studies suggest Escherichia-Shigella may contribute to hepatic inflammation with fibrosis in NAFLD pathogenesis (41).
Cluster analysis of the intestinal genus level. The LCMR group was enriched with the Ruminococcus_gnavus_group, which has been shown to correlate with serum TC levels (42). The MCMR group was enriched with Bacteroides and Rikenellaceae_RC9_gut_group. Changes in the relative abundance of Bacteroides may alter body glycolipid metabolism (43). However, in our study, Rikenellaceae_RC9_gut_group was a mixed-review bacterial genus that was positively correlated with serum TC and TG levels, or possibly with host species. LEfSe analysis for the control and MCMR groups revealed that the MCMR group was enriched with the short-chain fatty acid-producing bacterium Romboutsia (44). It has been shown that Barnesiella is associated with the regulation of bile acids (45). Alterations in gut microbiology mediated by abnormal bile acid secretion are influenced by a high-fat diet (46). Monoglobus is a specialized bacterium that breaks down pectin, the main non-cellulosic component of plant cell wall polysaccharides (47, 48). Monoglobus promotes the production of short-chain fatty acids and improves the intestinal microenvironment (49). Gut microbes and their metabolites are hubs connecting the liver-brain-reproductive signaling axis to nutritional strategies that regulate laying hen performance, health, and welfare.
5 Conclusion
In summary, the supplementation of 40 g/kg CMR in the hen diet improved antioxidant capacity, reduced the blood lipid level, alleviated the inflammation of oviducts, and regulated the flora structure. Thus, CMRs alleviated the state of health in late-phase laying hens to improve performance. Note that over-addition of CMRs can have adverse effects on laying hens, and the exact mechanism needs to be studied in depth. Moderate amounts of SMR and IRR have the potential to be developed as feed supplements for roughage-tolerant poultry.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found at: https://www.ncbi.nlm.nih.gov/bioproject/, PRJNA1060451.
Ethics statement
The animal studies were following approval of study protocols by the Animal Protection and Utiliza-tion Committee of the Hebei Agricultural University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
ZL: Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. NM: Data curation, Software, Writing – original draft, Writing – review & editing. XG: Methodology, Supervision, Writing – review & editing. WS: Project administration, Supervision, Writing – review & editing. XM: Conceptualization, Writing – review & editing. JY: Data curation, Writing – review & editing. ZZ: Conceptualization, Writing – review & editing. JL: Funding acquisition, Supervision, Writing – review & editing.
Funding
This study was supported by the Key Research and Development Program of Hebei Province (No. 21326603D), the Chinese medicinal materials industry technology system funding project of Hebei Province (No. HBCT2023080202), the National Key R&D Program of China (No. 2022YFD1801104), and the Hebei Agriculture Research System (No.HBCT2024280402).
Conflict of interest
JY was employed by Animal Husbandry Technology Promotion Institution of Zhangjiakou.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Castanon, JI. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. (2007) 86:2466–71. doi: 10.3382/ps.2007-00249
2. Dai, H, Lv, Z, Huang, Z, Ye, N, Li, S, Jiang, J, et al. Dietary hawthorn-leaves flavonoids improves ovarian function and liver lipid metabolism in aged breeder hens. Poult Sci. (2021) 100:101499. doi: 10.1016/j.psj.2021.101499
3. Titcomb, TJ, Kaeppler, MS, Cook, ME, Simon, PW, and Tanumihardjo, SA. Carrot leaves improve color and xanthophyll content of egg yolk in laying hens but are not as effective as commercially available Marigold Fortificant 1. Poult Sci. (2019) 98:5208–13. doi: 10.3382/ps/pez257
4. Jiang, JS, Gu, QC, Feng, ZM, Yuan, X, Zhang, X, Zhang, PC, et al. The Tanshinones from the Plant of Salvia Miltiorrhiza. Phytochemistry. (2023) 210:113673. doi: 10.1016/j.phytochem.2023.113673
5. Sun, G, Li, X, Wei, J, Zhang, T, Li, B, Chen, M, et al. Pharmacodynamic substances in Salvia Miltiorrhiza for prevention and treatment of hyperlipidemia and coronary heart disease based on Lipidomics technology and network pharmacology analysis. Biomed Pharmacother. (2021) 141:111846. doi: 10.1016/j.biopha.2021.111846
6. Geng, Y, Lu, C, Jin, G, Li, S, Cui, Y, Han, C, et al. Study on the mechanism of Salvia Miltiorrhiza polysaccharides in relieving liver injury of broilers induced by Florfenicol. Environ Sci Pollut Res Int. (2022) 29:3372–85. doi: 10.1007/s11356-021-15687-4
7. Chen, J, Zhu, Z, Gao, T, Chen, Y, Yang, Q, Fu, C, et al. Isatidis Radix and Isatidis folium: a systematic review on Ethnopharmacology, Phytochemistry and Pharmacology. J Ethnopharmacol. (2022) 283:114648. doi: 10.1016/j.jep.2021.114648
8. Yuan, Y, Wang, DN, Zhang, WY, Sun, TT, Feng, YT, Liu, MX, et al. Protective effects of Radix Isatidis polysaccharide ameliorates obesity via promotion Ampk pathway in high-fat-diet-induced obese rats and 3t3-L1 adipocyte cells. J Pharm Pharmacol. (2022) 74:1170–9. doi: 10.1093/jpp/rgac027
9. Fan, Z, Cai, L, Wang, Y, Zhu, Q, Wang, S, and Chen, B. The acidic fraction of Isatidis Radix regulates inflammatory response in Lps-stimulated raw 264.7 macrophages through Mapks and Nf-Κb pathway. Evid Based Complement Alternat Med. (2021) 2021:8879862. doi: 10.1155/2021/8879862
10. Li, JP, Yuan, Y, Zhang, WY, Jiang, Z, Hu, TJ, Feng, YT, et al. Effect of Radix Isatidis polysaccharide on alleviating insulin resistance in type 2 diabetes mellitus cells and rats. J Pharm Pharmacol. (2019) 71:220–9. doi: 10.1111/jphp.13023
11. Tao, W, Fu, T, He, ZJ, Zhou, HP, and Hong, Y. Immunomodulatory effects of Radix Isatidis polysaccharides in vitro and in vivo. Exp Ther Med. (2021) 22:1405. doi: 10.3892/etm.2021.10841
12. Meng, F, Yang, S, Wang, X, Chen, T, Wang, X, Tang, X, et al. Reclamation of Chinese herb residues using probiotics and evaluation of their beneficial effect on pathogen infection. J Infect Public Health. (2017) 10:749–54. doi: 10.1016/j.jiph.2016.11.013
13. Zhou, Y, Selvam, A, and Wong, JW. Effect of Chinese medicinal herbal residues on microbial community succession and anti-pathogenic properties during co-composting with food waste. Bioresour Technol. (2016) 217:190–9. doi: 10.1016/j.biortech.2016.03.080
14. Jiang, YY, Li, YB, Yu, J, Chen, H, Zhou, J, Wang, L, et al. Preliminary structure and bioactivities of Polysaccharide Smwp-U & E Isolated from Salvia Miltiorrhiza Bunge residue. Int J Biol Macromol. (2020) 157:434–43. doi: 10.1016/j.ijbiomac.2020.04.092
15. Zhu, Z, Wu, S, Qi, B, Wang, C, Luo, J, and Wan, Y. High-solids enzymatic Saccharification of starch-rich raw herbal biomass residues for producing high titers of glucose. Environ Sci Pollut Res Int. (2023) 30:86232–43. doi: 10.1007/s11356-023-28501-0
16. Emami, NK, Jung, U, Voy, B, and Dridi, S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants (Basel). (2020) 10:35. doi: 10.3390/antiox10010035
17. Lee, J, Homma, T, Kurahashi, T, Kang, ES, and Fujii, J. Oxidative stress triggers lipid droplet accumulation in primary cultured hepatocytes by activating fatty acid synthesis. Biochem Biophys Res Commun. (2015) 464:229–35. doi: 10.1016/j.bbrc.2015.06.121
18. Bao, J, Zhang, Y, Zhang, L, Gong, X, Shi, W, Liu, L, et al. Therapeutic effect of Schisandrin a on avian Colibacillosis through gut-liver Axis. Poult Sci. (2021) 100:101371. doi: 10.1016/j.psj.2021.101371
19. Saeedi, BJ, Liu, KH, Owens, JA, Hunter-Chang, S, Camacho, MC, Eboka, RU, et al. Gut-resident lactobacilli activate hepatic Nrf 2 and protect against oxidative liver injury. Cell Metab. (2020) 31:956–68.e5. doi: 10.1016/j.cmet.2020.03.006
20. Nii, T, Shinkoda, T, Isobe, N, and Yoshimura, Y. Intravaginal injection of Lactobacillus Johnsonii may modulates Oviductal microbiota and mucosal barrier function of laying hens. Poult Sci. (2023) 102:102699. doi: 10.1016/j.psj.2023.102699
21. Wang, D, Lv, J, Fu, Y, Shang, Y, Liu, J, Lyu, Y, et al. Optimization of microwave-assisted extraction process of Total flavonoids from Salicornia Bigelovii Torr. And its Hepatoprotective effect on alcoholic liver injury mice. Food Secur. (2024) 13:647. doi: 10.3390/foods13050647
22. Ma, LL, Feng, J, Wang, J, Qi, GH, Ma, YB, Wu, SG, et al. The changes of eggshell quality in the laying cycle of Hy-line Brown layers. Sci Agric Sin. (2021) 54:3766–79. doi: 10.3864/j.issn.0578-1752.2021.17.017
23. Ma, H, Huang, Q, Qu, W, Li, L, Wang, M, Li, S, et al. In vivo and in vitro anti-inflammatory effects of Sophora Flavescens residues. J Ethnopharmacol. (2018) 224:497–503. doi: 10.1016/j.jep.2018.06.019
24. Wang, X, Liu, W, Zhang, D, Jiao, Y, Zhao, Q, Liu, Y, et al. Salvia Miltiorrhiza polysaccharides alleviate Florfenicol-induced inflammation and oxidative stress in Chick livers by regulating phagosome signaling pathway. Ecotoxicol Environ Saf. (2023) 249:114428. doi: 10.1016/j.ecoenv.2022.114428
25. Deng, JL, Tao, YL, He, YQ, and Chen, WS. Research Progress on active components and mechanism of Isatidis Radix for influenza virus. Zhongguo Zhong Yao Za Zhi. (2021) 46:2029–36. doi: 10.19540/j.cnki.cjcmm.20201230.601
26. Zhao, ZJ, and Wang, DH. Plasticity in the physiological energetics of Mongolian gerbils is associated with diet quality. Physiol Biochem Zool. (2009) 82:504–15. doi: 10.1086/603630
27. Zhang, W, Jia, T, Zhang, H, and Zhu, W. Effects of high-Fiber food on gut microbiology and energy metabolism in Eothenomys Miletus at different altitudes. Front Microbiol. (2023) 14:1264109. doi: 10.3389/fmicb.2023.1264109
28. Wu, H, Yuan, J, Yin, H, Jing, B, Sun, C, Nguepi Tsopmejio, IS, et al. Flammulina Velutipes stem regulates oxidative damage and synthesis of yolk precursors in aging laying hens by regulating the liver-blood-ovary Axis. Poult Sci. (2023) 102:102261. doi: 10.1016/j.psj.2022.102261
29. Gu, YF, Chen, YP, Jin, R, Wang, C, Wen, C, and Zhou, YM. Age-related changes in liver metabolism and antioxidant capacity of laying hens. Poult Sci. (2021) 100:101478. doi: 10.1016/j.psj.2021.101478
30. Ranasinghe, C, Trivedi, S, Wijesundara, DK, and Jackson, RJ. Il-4 and Il-13 receptors: roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. (2014) 25:437–42. doi: 10.1016/j.cytogfr.2014.07.010
31. Giampietri, A, Grohmann, U, Vacca, C, Fioretti, MC, Puccetti, P, and Campanile, F. Dual effect of Il-4 on resistance to systemic gram-negative infection and production of Tnf-alpha. Cytokine. (2000) 12:417–21. doi: 10.1006/cyto.1999.0576
32. Nii, T, Isobe, N, and Yoshimura, Y. Effects of interleukin-1β and −6 on the expression of ion transporters involved in eggshell mineralization in cultured hen uterine mucosal tissue. J Poult Sci. (2018) 55:142–9. doi: 10.2141/jpsa.0170138
33. Jia, F, Lu, M, Wang, J, Zhang, H, Qiu, K, Qi, G, et al. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J Anim Sci Biotechnol. (2021) 12:72. doi: 10.1186/s40104-021-00600-3
34. Liu, J, Fu, Y, Zhou, S, Zhao, P, Zhao, J, Yang, Q, et al. Comparison of the effect of quercetin and Daidzein on production performance, anti-oxidation, hormones, and Cecal microflora in laying hens during the late laying period. Poult Sci. (2023) 102:102674. doi: 10.1016/j.psj.2023.102674
35. Bernini, LJ, Simão, AN, Alfieri, DF, Lozovoy, MA, Mari, NL, de Souza, CH, et al. Beneficial effects of Bifidobacterium Lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition. (2016) 32:716–9. doi: 10.1016/j.nut.2015.11.001
36. Wang, K, Liao, M, Zhou, N, Bao, L, Ma, K, Zheng, Z, et al. Parabacteroides Distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. (2019) 26:222–35.e5. doi: 10.1016/j.celrep.2018.12.028
37. Anhê, FF, Pilon, G, Roy, D, Desjardins, Y, Levy, E, and Marette, A. Triggering Akkermansia with dietary polyphenols: a new weapon to combat the metabolic syndrome? Gut Microbes. (2016) 7:146–53. doi: 10.1080/19490976.2016.1142036
38. Farías-Kovac, C, Nicodemus, N, Delgado, R, Ocasio-Vega, C, Noboa, T, Abdelrasoul, RA, et al. Effect of dietary insoluble and soluble fibre on growth performance, digestibility, and nitrogen, energy, and mineral retention efficiency in growing rabbits. Animals (Basel). (2020) 10:1346. doi: 10.3390/ani10081346
39. Jiménez-Moreno, E, González-Alvarado, JM, de Coca-Sinova, A, Lázaro, RP, Cámara, L, and Mateos, GG. Insoluble Fiber sources in mash or pellets diets for young broilers. 2. Effects on gastrointestinal tract development and nutrient digestibility 1. Poult Sci. (2019) 98:2531–47. doi: 10.3382/ps/pey599
40. Joat, N, Van, TTH, Stanley, D, Moore, RJ, and Chousalkar, K. Temporal dynamics of gut microbiota in caged laying hens: a field observation from hatching to end of lay. Appl Microbiol Biotechnol. (2021) 105:4719–30. doi: 10.1007/s00253-021-11333-8
41. Xin, FZ, Zhao, ZH, Liu, XL, Pan, Q, Wang, ZX, Zeng, L, et al. Escherichia Fergusonii promotes nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own Msrna 23487. Cell Mol Gastroenterol Hepatol. (2022) 13:827–41. doi: 10.1016/j.jcmgh.2021.12.003
42. Vojinovic, D, Radjabzadeh, D, Kurilshikov, A, Amin, N, Wijmenga, C, Franke, L, et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat Commun. (2019) 10:5813. doi: 10.1038/s41467-019-13721-1
43. Sung, MM, Kim, TT, Denou, E, Soltys, CM, Hamza, SM, Byrne, NJ, et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes. (2017) 66:418–25. doi: 10.2337/db16-0680
44. Song, B, Li, P, Yan, S, Liu, Y, Gao, M, Lv, H, et al. Effects of dietary Astragalus polysaccharide supplementation on the Th17/Treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis. Front Immunol. (2022) 13:781934. doi: 10.3389/fimmu.2022.781934
45. Zhuang, P, Li, H, Jia, W, Shou, Q, Zhu, Y, Mao, L, et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs Axis in Db/Db mice. Microbiome. (2021) 9:185. doi: 10.1186/s40168-021-01126-6
46. Song, Z, Chen, J, Ji, Y, Yang, Q, Chen, Y, Wang, F, et al. Amuc attenuates high-fat diet-induced metabolic disorders linked to the regulation of fatty acid metabolism, bile acid metabolism, and the gut microbiota in mice. Int J Biol Macromol. (2023) 242:124650. doi: 10.1016/j.ijbiomac.2023.124650
47. Kim, CC, Kelly, WJ, Patchett, ML, Tannock, GW, Jordens, Z, Stoklosinski, HM, et al. Nov., a Pectinolytic bacterium isolated from human Faeces. Int J Syst Evol Microbiol. (2017) 67:4992–8. doi: 10.1099/ijsem.0.002395
48. Wu, J, Shen, S, Gao, Q, Yu, C, Cheng, H, Pan, H, et al. Rg-I domain matters to the in vitro fermentation characteristics of Pectic polysaccharides recycled from Citrus canning processing water. Food Secur. (2023) 12:943. doi: 10.3390/foods12050943
Keywords: Chinese medicine residue, hens, liver, oviduct, gut microbiota
Citation: Li Z, Ma N, Gong X, Shi W, Meng X, Yan J, Zhao Z and Li J (2024) Effects of herbal dregs supplementation of Salvia miltiorrhiza and Isatidis Radix residues improved production performance and gut microbiota abundance in late-phase laying hens. Front. Vet. Sci. 11:1381226. doi: 10.3389/fvets.2024.1381226
Edited by:
Yangchun Cao, Northwest A&F University, ChinaReviewed by:
Yun Peng Fan, Northwest A&F University, ChinaHouqiang Luo, Wenzhou Vocational College of Science and Technology, China
Copyright © 2024 Li, Ma, Gong, Shi, Meng, Yan, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiefeng Li, bGlqaWVmZW5nNDE3QDEyNi5jb20=
 Zhaonian Li1,2
Zhaonian Li1,2 Ning Ma
Ning Ma Jiefeng Li
Jiefeng Li