
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 25 June 2024
Sec. Zoological Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1379980
This article is part of the Research Topic Advances in Methods of Biochemical Assessment and Diagnosis of Animal Welfare in Wildlife View all 8 articles
 Lisa K. F. Lee1
Lisa K. F. Lee1 J. Mark Hipfner2
J. Mark Hipfner2 Greg Frankfurter3
Greg Frankfurter3 Carolyn Cray4
Carolyn Cray4 Scott F. Pearson5
Scott F. Pearson5 Christine Fiorello6
Christine Fiorello6 Nikolas M. T. Clyde2
Nikolas M. T. Clyde2 Sarah A. Hudson2
Sarah A. Hudson2 Sarah E. Parker7
Sarah E. Parker7 David E. Stallknecht8
David E. Stallknecht8 Emmanuelle Furst9
Emmanuelle Furst9 Katherine H. Haman5*
Katherine H. Haman5*Clinical metrics of baseline health in sentinel seabird species can offer insight into marine ecosystem dynamics, individual and population health, and assist in wildlife rehabilitation and conservation efforts. Protein electrophoresis is useful for detecting changes in acute phase proteins and immunoglobulin levels that may indicate subtle inflammatory responses and/or infectious disease. Serum biochemistry can highlight nutritional status, metabolic derangements, and organ injury and function. However, baseline values for such health parameters are largely unknown for many seabird species. Therefore, the objective of this study is to establish baseline clinical health reference intervals for serum protein electrophoresis, acute phase proteins including serum amyloid A and haptoglobin, and biochemistry parameters in the rhinoceros auklet (Cerorhinca monocerata), a key sentinel species in the North Pacific. From 2013 to 2019, 178 wild, apparently healthy breeding adult rhinoceros auklets were captured across four breeding colonies in British Columbia, Canada (Lucy Island, Pine Island, Triangle Islands, and SGang Gwaay) and from one colony in Washington, United States (Protection Island). Reference intervals were calculated for protein electrophoresis fractions and acute phase proteins (n = 163), and serum biochemistry (n = 35) following established guidelines by the American Society of Veterinary Clinical Pathology. Animals were also assessed for the presence of antibodies to the influenza A virus. Approximately 48% (70/147) of sampled birds were seropositive for influenza A virus, with a prevalence of 50% (6/12) in 2013, 75% (47/63) in 2014, and 24% (17/72) in 2019. This work provides clinical baseline health metrics of a key North Pacific sentinel species to help inform marine ecosystem monitoring, recovery, and rehabilitation efforts in the Pacific Northwest.
Serum baseline values can illustrate key features about wild seabird health at the individual and population levels. Serum biochemistry provides information about electrolyte balance, metabolism, and internal organ status (1). Acute phase proteins (APP) and protein electrophoresis (EPH) can be used to gauge inflammatory response and prognosis in avian species, and are becoming more widely used (2–4). Protein electrophoresis and APP allow researchers and clinicians to detect inflammatory changes earlier when compared to traditional hematology and serum biochemistry approaches (5). This is due to the rapid changes that occur in APP during acute disease and inflammation, such as the upregulation of serum amyloid A (SAA), haptoglobin (HP) (PIT54 is the homologous protein in birds) (6), and α- and β-globulins; and downregulation of albumin (7). Particularly in marine environments, APP such as HP provide valuable information in population health assessments of marine mammal species exposed to oil and other pollutants (8).
Maintaining established clinical baseline health parameters may serve as a pre-defined recovery goal for seabirds in the event of a marine environmental disruption (9–11). For example, oil contamination from both acute and chronic releases has long-term physiological and metabolic impacts on the health, reproduction, and survival of migratory seabirds (12–17). Baseline clinical health parameters of free-ranging seabirds can help distinguish between local or large-scale environmental impacts to marine systems, such as point-source anthropogenic stressors including oil spills and other marine pollutants (18–20). Furthermore, this information may facilitate wildlife rehabilitation and recovery efforts (21, 22). This becomes especially critical during environmental disasters such as oil spills, as it provides baseline metrics for recovery.
Climate change and severe weather are other significant contributors to global seabird species declines (23) due to decreased habitat suitability, prey abundance, and shifts in pathogen-host dynamics associated with ocean warming (24, 25). Seabirds may act as ecosystem sentinels for their pelagic and coastal habitats due to their responsiveness to environmental changes and their role as top predators in marine food webs (26). This is reflected through population health and ultimately, changes in breeding success and survival (27–29).
Seabirds can also potentially aid in disease surveillance across all flyways. Within the Pacific Flyway, influenza A virus (IAV) is of particular interest. While wild aquatic birds can act as disease reservoirs in the Pacific Flyway (30, 31), knowledge about the role of different seabird species in IAV ecology remains limited due to difficulties in sampling free-ranging seabirds (32, 33). Seabirds such as gulls are known to act as reservoirs for low pathogenic avian influenza H13 and H16 subtypes, and were able to spread highly pathogenic avian influenza (HPAI) H5 subtype rapidly due to large distances traveled (34). The ongoing H5N1 2.3.4.4b HPAI outbreak across North America further highlights the importance of IAV in seabirds (35), especially since there is a potential for catastrophic loss of species infected with H5N1 HPAI (36–38) (e.g., brown pelicans, Caspian terns). However, few studies have investigated baseline exposure levels to IAV in general.
In the North Pacific, the rhinoceros auklet (Cerorhinca monocerata) from the Alcidae family is an important indicator species for ecosystem health (18, 39). Ocean warming can lead to long-term decreases in rhinoceros auklet abundance (40), as they are sensitive to oceanographic changes that shift trophic level interactions and diet composition. This can also affect reproductive success, as reproductive performance is correlated with diet quality and availability (41–43). Interestingly, adult survival rates remained relatively stable during extreme environmental variation (44). Alcids have been particularly vulnerable to large mortality events in recent years (45) with disease playing a major role in a rhinoceros auklet mortality event in the Salish Sea (39). Assessment of clinical parameters in rhinoceros auklets can potentially provide a more comprehensive view of marine ecosystem health, especially when integrated with other attributes such as demography, reproduction, and morphometrics.
Few studies provide baseline serum biochemistry, EPH, and APP parameters for seabirds due to challenges in obtaining samples and limited APP reagent validation in avian species (46). There is also a scarcity of clinical baseline seabird population health data based on the American Society of Veterinary Clinical Pathology guidelines in reference interval (RI) generation (47), which are founded on the use of significant sample sizes and recommended statistical methods. The objectives of this study were therefore to: (1) establish baseline clinical health serum biochemistry, EPH, and APP RIs for the rhinoceros auklet on multiple breeding colonies in the core of its breeding range; and (2) assess IAV antibody prevalence among these breeding colonies.
Research protocols employed in this study were approved by Simon Fraser University Animal Care Services (#974B-94), the Western and Northern Animal Care Committee of Environment and Climate Change Canada’s Canadian Wildlife Service (14MH01, and 19MH01), ECCC Migratory Birds banding permit (10667F), and US Fish and Wildlife Federal Bird Banding Permit (22913).
Adult rhinoceros auklets were caught on land at breeding colonies at night, either by hand, with landing nets, or mist nets in July (2013, 2014, and 2019) across four colonies in British Columbia, Canada (Lucy, Pine, and Triangle Islands, plus SGang Gwaay) and one colony in Washington, United States (2019, Protection Island) in the North Pacific (Figure 1). Birds were weighed with a 1 kg (± 5 g) analog spring scale (Pesola AG, Switzerland), and the following morphometric measurements were collected: maximum flattened wing chord, tarsus, culmen length, bill depth at gonys, and horn height. Blood was taken from the brachial vein, using 27 g needles and 3 cc syringes. Whole blood was centrifuged at 10,000 rotations per minute for 5–10 min to separate erythrocytes from serum, within 4–6 h of collection. Serum was collected into tubes after the blood samples clotted, then subsequently frozen in liquid nitrogen dry shippers. Samples were stored at −20°C until shipping to the University of Miami Avian and Medicine Laboratory (Miami, FL, United States), California Animal Health and Food Safety Laboratory (Davis, CA, United States), University of Georgia (Athens, GA, United States), and/or the University of Lethbridge (Lethbridge, AB, United States). Total elapsed time between sample collection and analysis from wild birds ranged from 160 to 598 days (median: 173 days).
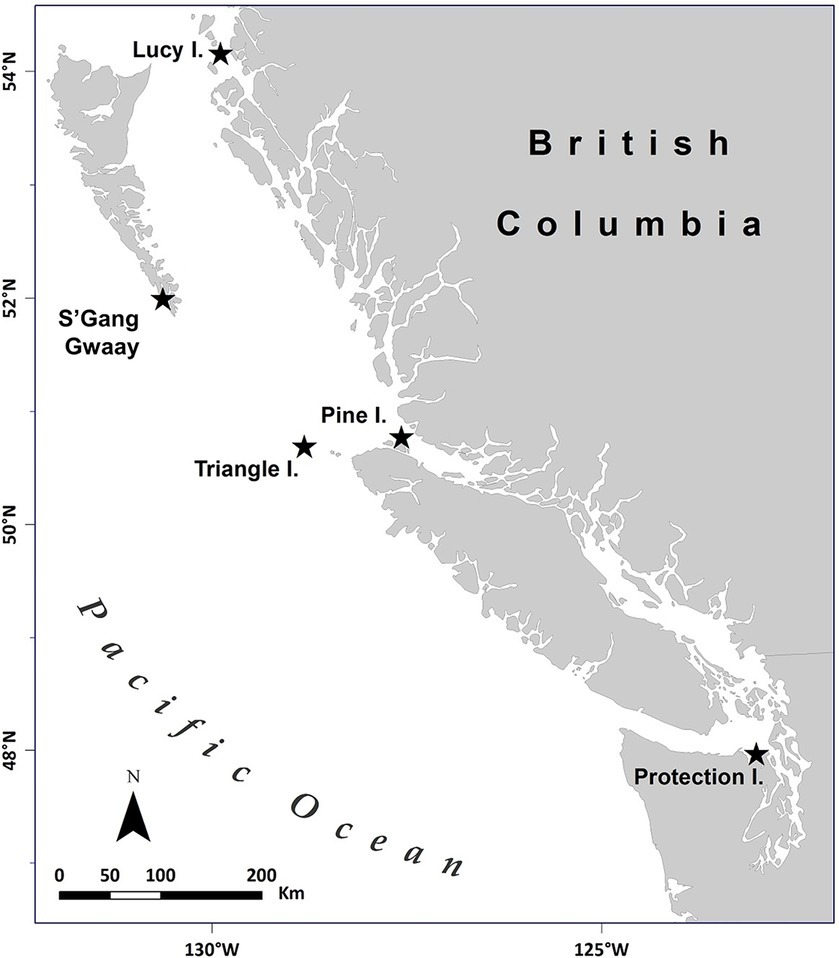
Figure 1. Map of rhinoceros auklet (Cerorhinca monocerata) breeding colonies studied in British Columbia, Canada, and Washington, United States within the North Pacific from 2013 to 2019. The seabird breeding colonies were located on Lucy Island (54.294418°N, −130.621907°W), Pine Island (50.976062°N, −127.729909°W), Triangle Island (50.851023°N, −129.066292°W), SGang Gwaay (52.092634°N, −131.225633°W) in British Columbia, and Protection Island (48.126341°N, −122.930289°W) in Washington, United States. Adapted from Environmental Pollution, Volume 239, Hipfner et al., “Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs”, Pages 215-222, Copyright Elsevier (2018).
Five captive rhinoceros auklets at the Alaska SeaLife Center were selected for measurement of SAA and HP levels from May 2007 to September 2013, due to the presence of various clinical abnormalities associated with inflammation, in most cases involving their feet (Supplementary Tables S2–S4). Four of the five birds were originally collected as eggs from Middleton Island, AK in June of 2006 and participated in a nutritional research project prior to being placed at the Alaska SeaLife Center for long-term care in early 2007. The fifth bird selected was hatched at the Alaska SeaLife Center from a mated pair of the previously described individuals. One plasma or serum sample was collected from each bird during clinical disease and when clinically normal. Serum or plasma was collected from peripheral veins (brachial, metatarsal, or jugular vein) and processed as described previously.
Serum biochemistry, protein electrophoresis, and acute phase protein analysis was conducted at the University of Miami—Avian and Medicine Laboratory (Miami, FL). Routine biochemistry testing was performed using a Vitros 250 analyzer (Ortho, Rochester, NY). Protein electrophoresis was conducted using the SPIFE 3000 system and split beta gels (Helena Laboratories, Beaumont, TX, United States). A representative electrophoretogram from a rhinoceros auklet is presented in Figure 2. Fraction delimits were placed according to conventions established for other avian species (2) and included prealbumin, albumin, and α-1, α-2, β-, and γ-globulins. The albumin to globulin ratio was calculated as the sum of prealbumin and albumin divided by the sum of the globulins. The absolute values for the fractions were calculated by multiplying the fraction percent values by the total protein. Haptoglobin levels were determined using the phase colorimetric assay (Tridelta, Morris Plains, NJ), and SAA levels were determined using the SAA-LZ immunoturbidimetric assay (Eiken Chemical Co, Tokyo, Japan). Both assays were performed on a Daytona Rx analyzer (Kearneysville, WV). Assay reactivity, as determined by stepwise dilution of high abnormal samples (100, 90, 80, …) was found to be linear under dilution. For SAA, the slope included 1 (0.89–1.21) and the y-intercept included 0 (−9.81–43.99). The runs test did indicate a significant deviation from linearity (p = 0.02). For HP, the slope included 1 (0.48–1.11) and the y-intercept included 0 (−0.11–0.62). The runs test did not indicate a significant deviation from linearity (p = 0.90). Coefficient of variation and diagnostic limits were consistent with that observed with other species (3); analysis was performed with GraphPad Prism 8.0 (GraphPad Software, San Diego, California, United States).
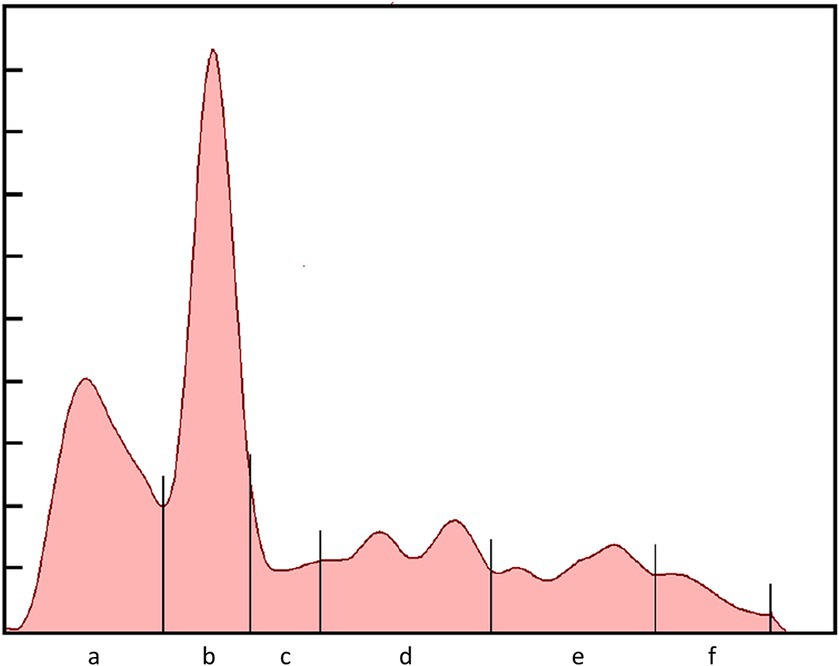
Figure 2. Electrophoretogram of a free-ranging adult rhinoceros auklet (Cerorhinca monocerata) that was presumed healthy. Protein fractions are: (A) prealbumin; (B) albumin; (C) α-1; (D) α-2; (E) β-; and (F) γ-globulins.
Sera were tested for antibodies to the IAV nucleoprotein using a commercial bELISA (IDEXX AI MultiS-Screen Ab test, IDEXX Laboratories, Westbrook, Maine, United States) according to the manufacturer’s instructions either at the California Animal Health and Food Safety Laboratory (Davis, CA) or the University of Georgia (Athens, GA). Sera were considered positive for antibodies to IAV if the serum-sample-to-negative-control (S/N) absorbance value was <0.7, based on recommendations for evaluating wildlife species (48).
DNA was extracted from blood stored in Queen’s lysis buffer using a modified Chelex protocol (49, 50) at the University of Lethbridge (Lethbridge, AB). Individuals were sexed using the Z43BF/Z43BR Primer Pair (51); the forward primer modified with M13 to allow incorporation of fluorescent marker to run on Licor gel. All PCR reactions were conducted in 10 μL reactions with 1 μL of genomic DNA. PCR cocktails contained 2.0 μL ClearFlexi Buffer 5x (Promega), 2.5 mM MgCl₂, 200 μM dNTP, 1 μM each primer, 0.05 μM M13 primer, and 0.5 units GoTaq (Promega). The following Thermocycler Conditions were used: 1 cycle of 30 s at 94°C; 35 cycles of 30 s at 94°C, and 45 s at 55°C, and 45 s at 72°C, with a final extension for 5 min at 72°C, and 5 s at 4°C. All PCR products were run on a 6% acrylamide gel. Two positive controls (one male and one female) and a negative control were included on each run.
Statistical analyses were conducted using RStudio (R Development Core Team, Vienna, Austria) and Stata (17.0, StataCorp LLC, College Station, TX, United States), with results deemed significant at p ≤ 0.05, unless otherwise stated. Sex was predicted based on the following morphometric measurements (52): A designation of female was assigned if bill depth was <16.5 mm, while males had a bill depth of >17.0 mm. Birds with ambiguous bill depths (i.e., 16.5–17.0 mm) were assigned female if they weighed <500 g, and assigned male if ≥500 g. The McNemar’s test was conducted to determine if there was a difference between genetic sex of rhinoceros auklets and when predicted by morphometric measurements.
Normality was first assessed for serum EPH, APP, and biochemistry measurands using the Shapiro–Wilk test. Measurands were considered to have a non-Gaussian distribution if p ≤ 0.3, as per guidelines from the American Society of Veterinary Clinical Pathology (53). As the majority of the parameters had a non-Gaussian distribution, the Kruskal-Wallis test was used to determine differences between colonies, years sampled, and morphometrically predicted sex. Pairwise comparisons were evaluated with Dunn’s test, with p-values adjusted with the Benjamini-Hochberg method to control the type I error rate. The distribution of IAV seropositivity status was analyzed using a multilevel mixed-effects logistic regression with colony, year, and morphometrically predicted sex, when clustering by colony. Variables were considered for multivariable model building if association with IAV seropositivity was at a threshold of p ≤ 0.20. Model fit was evaluated with Aikake’s information criteria (AIC). Inclusion of variables was tested with a likelihood ratio for successive models with forward stepwise selection.
Data collected from individuals of all colonies were pooled together to generate RIs for serum biochemistry, EPH, and APP. Reference intervals containing the central 95% of the population were calculated using Reference Value Advisor v. 2.1 Microsoft Excel add-on (54), according to guidelines established by the American Society of Veterinary Clinical Pathology (47). A nonparametric method was used as most distributions were non-normal. Lower and upper bounds represent the 2.5 and 97.5th percentiles, respectively. Outliers were identified using Tukey’s interquartile fences and excluded from RI calculations following histogram and boxplot examination. For variables with n ≥ 120, a nonparametric method was used for generating the 95% RI, with 90% confidence intervals (CI) of the upper and lower limits of the RI. For non-normally distributed variables with n ≥ 20 and < 40, the robust method was used to calculate RI and 90% CI upper and lower limits of the RI (47).
From 2013 to 2019, 178 adult rhinoceros auklets were captured from breeding colonies at the sampling sites (Figures 1, 3). On brief physical exam, no gross external abnormalities were noted and all individuals were presumed healthy. All animals had evidence of breeding present (i.e., presence of bill load for chick feeding) at time of capture. Descriptive statistics for mass and morphometric values are summarized in Table 1.
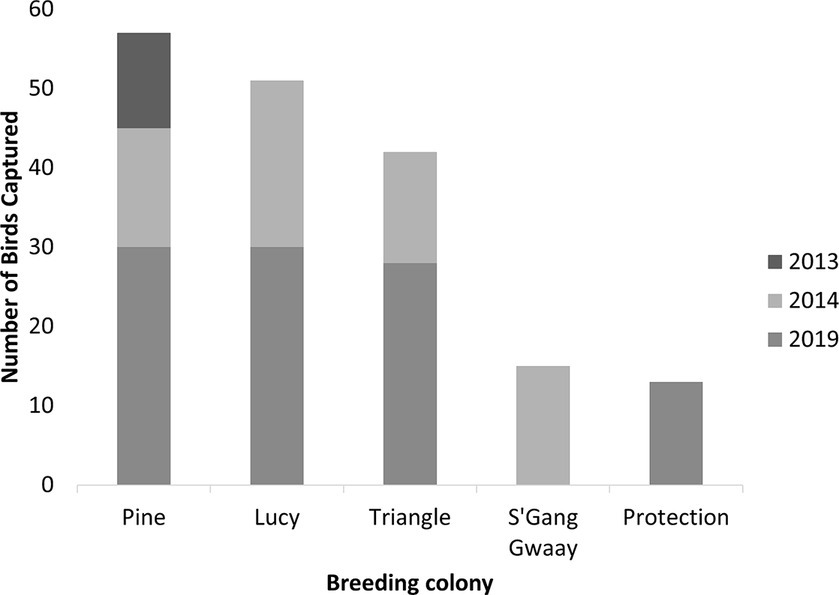
Figure 3. Number of wild breeding rhinoceros auklets (Cerorhinca monocerata) captured by year and colony from Lucy, Pine, Triangle Islands, and SGang Gwaay, British Columbia, Canada and from Protection Island, Washington, United States, 2013–2019.
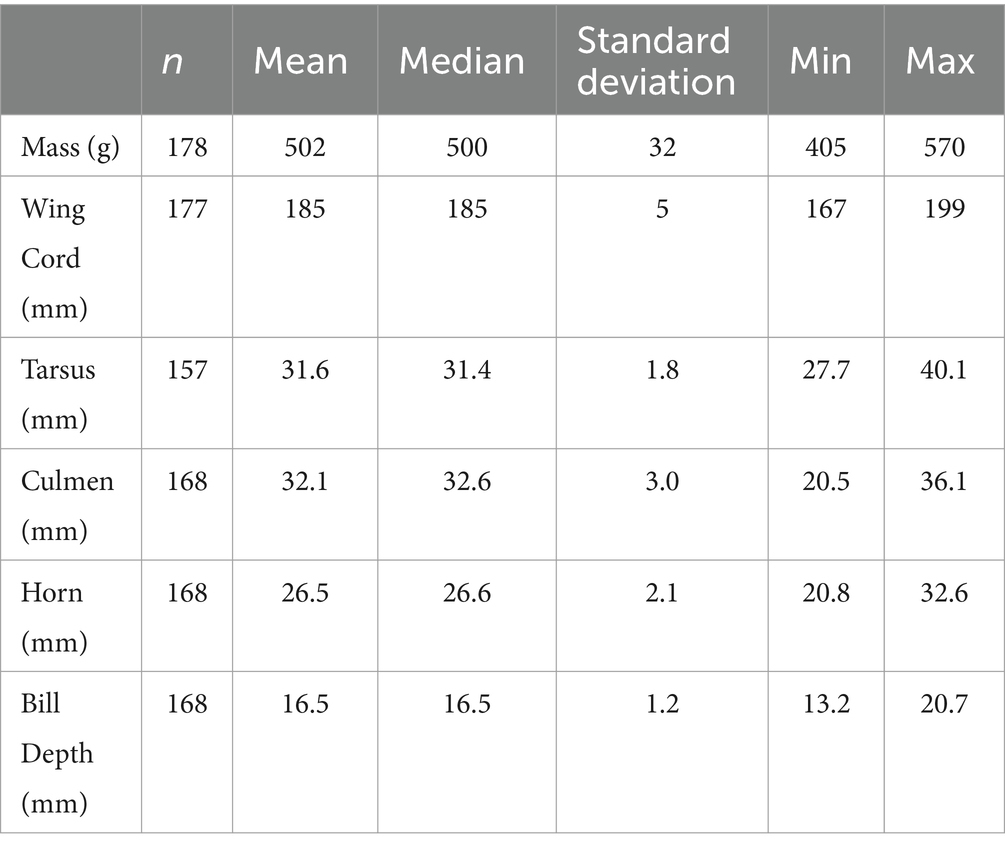
Table 1. Descriptive statistics for morphometric data of wild breeding adult rhinoceros auklets (Cerorhinca monocerata) from Lucy, Pine, Triangle, and SGang Gwaay Islands in British Columbia and Protection Island, Washington, 2013–2019.
Of the captured birds, the sex of 38 animals was determined genetically, and was composed of 19 males (50%) and 19 females (50%). Bill depth was not recorded for 2/38 of the genetically sexed birds. Based on bill depth and weight measurements as described above, there were 93 females (52%), and 75 males (42%). Ten animals (6%) were of unknown sex, as morphometric data for these birds were not collected. Supplementary Table S1 shows the cross classification of genetic sex compared to morphometric predictions in 36 animals. The McNemar’s test indicated that the proportion determined as male and female by the two tests was not significantly different (p = 1). When comparing genetic versus morphometrically predicted sex, there was a weak level of agreement (kappa = 0.44).
Blood samples were collected from 163 individuals. Lipemia occurred in two samples with a lipemic index of 1 and 2, respectively (0–4 scale, with 4 graded as severe); these animals had total protein levels comparable to other animals in the study. Most serum samples had no evidence of hemolysis; however, four and five birds had hemolysis indexes of 1+ (mild) and 2+ (moderate), respectively. Serum protein electrophoresis and biochemistry values were assessed in 163 and 35 animals, respectively. Distributions of values were non-normal, so non-parametric techniques were used. Reference intervals containing the central 95% of the population, with corresponding 90% CI for the lower and upper limit of the RI, are presented for protein electrophoresis fractions and APP (Table 2) and biochemistry data (Table 3). For individual analyte values below the limit of detection, the RI was calculated using the limit of detection (e.g., <0.1 mg/L SAA was entered as 0.1 mg/L for RI calculation). Histograms showing the distribution of serum biochemistry values are provided in Supplementary Figures S1, S2 due to smaller sample sizes (n < 40) assessed. Gamma-glutamyl transferase was assayed but RI were not calculated as 80% (28/35) of samples were below the limit of detection (< 5 U/L). The remaining gamma-glutamyl values ranged from 5 to 9 U/L. For creatinine, 13/35 (37%) samples were below the limit of detection (< 0.2 mg/dL), so RI for the blood urea nitrogen to creatinine ratio was not calculated. Serum concentrations for SAA and HP for all birds assessed are shown in Supplementary Figure S3.
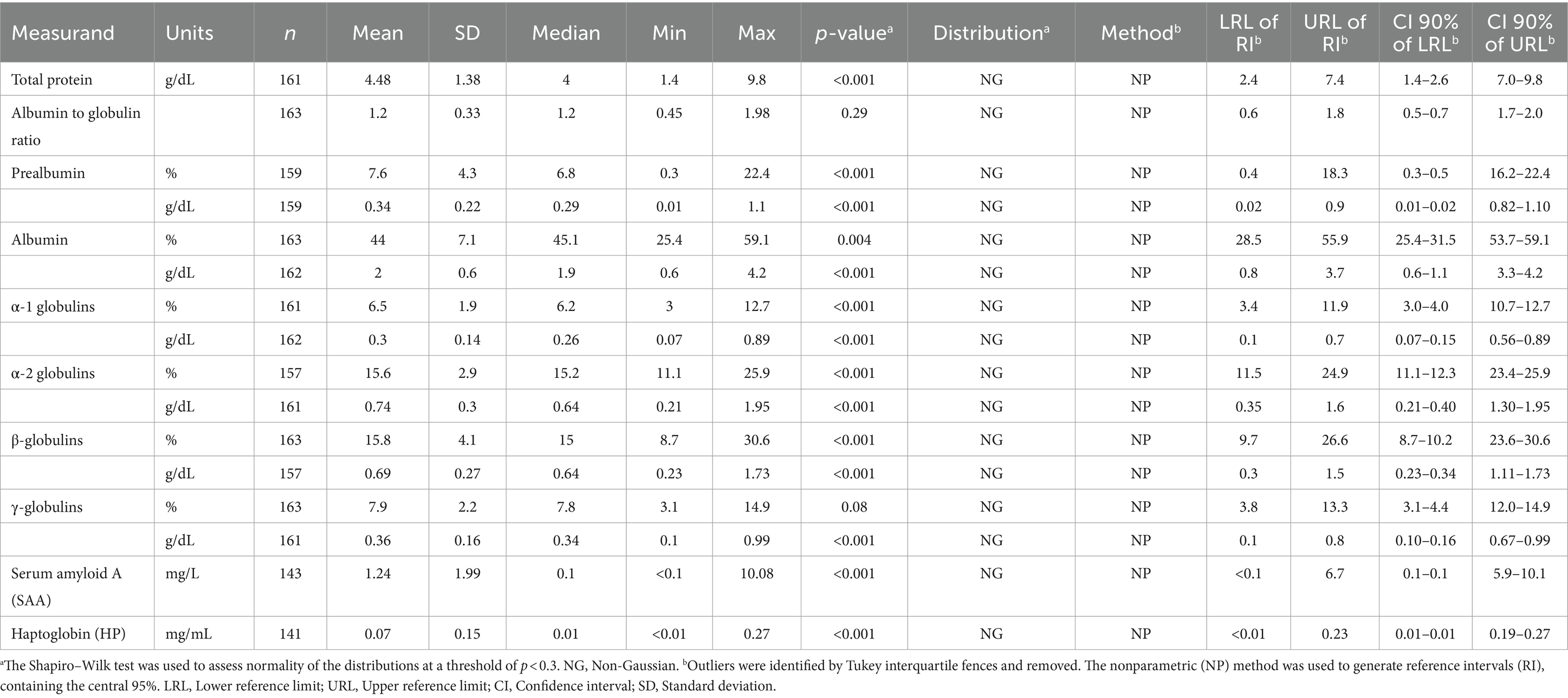
Table 2. Serum protein electrophoresis fractions and acute phase protein reference intervals (RI) for wild breeding adult rhinoceros auklets (Cerorhinca monocerata) from Lucy, Pine, Triangle, and SGang Gwaay Islands in British Columbia and Protection Island, Washington, 2013–2019.
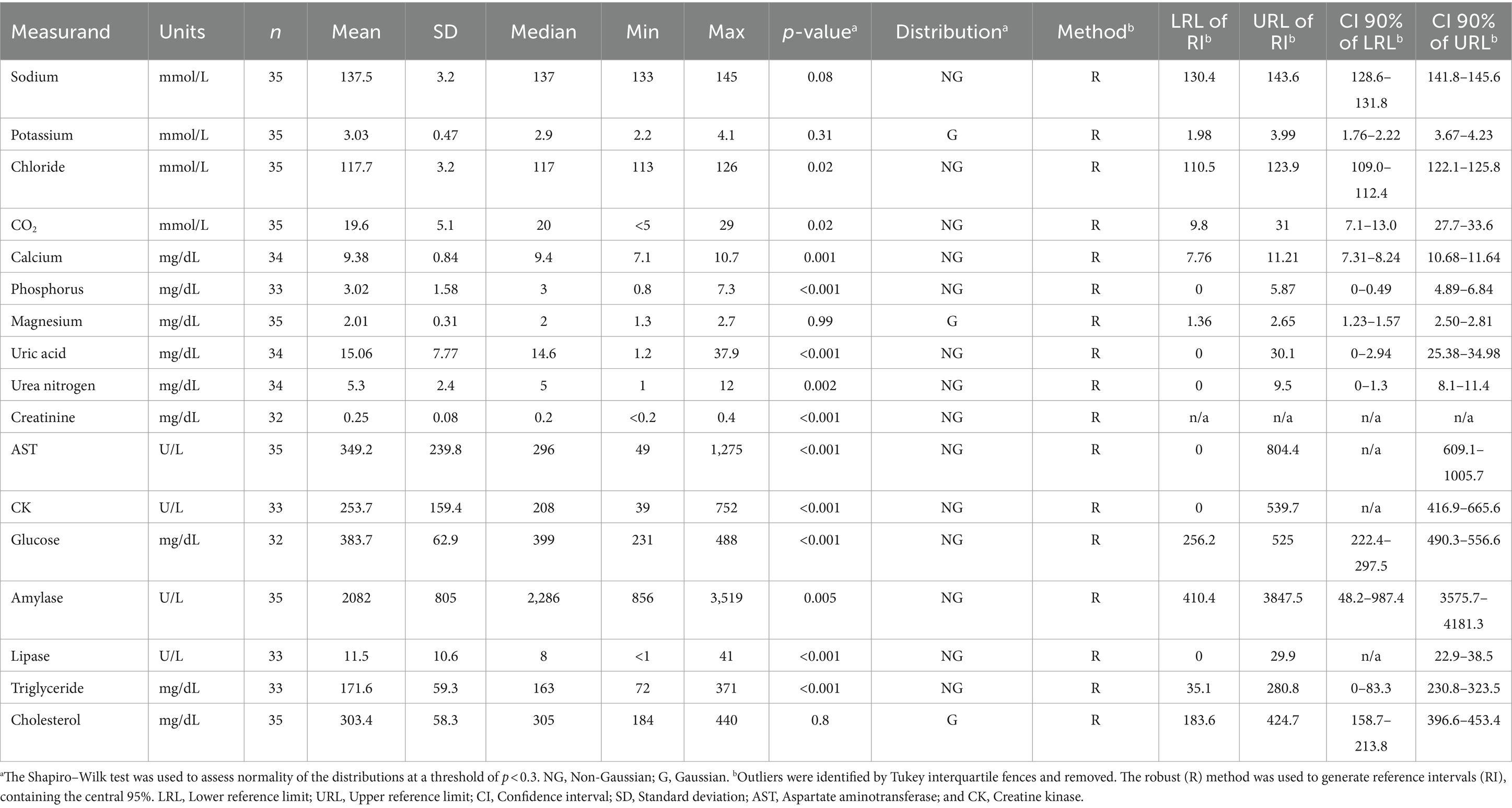
Table 3. Serum biochemistry reference intervals (RI) of wild breeding adult rhinoceros auklets (Cerorhinca monocerata) from Lucy, Pine, Triangle Islands, and SGang Gwaay in British Columbia, 2013–2019.
For comparison to birds with known illnesses, SAA and HP levels from five captive rhinoceros auklets at the Alaska SeaLife Center were assessed during clinical disease and when clinically healthy (Supplementary Tables S2–S4). Four of the five clinically ill rhinoceros auklets were diagnosed with pododermatitis with active abscessation, necrosis, cellulitis, or an active joint infection; one clinically ill rhinoceros auklet had an open diagnosis with nonspecific signs of illness that responded to supportive therapy. SAA ranged from 0.8 to 385.1 mg/L (median 43.9 mg/L) and HP ranged from 0.35 to 2.45 mg/mL (median 1.39 mg/mL). When considered clinically normal, SAA ranged from <0.1 to 15.52 mg/L (median 1.65 mg/L) and HP ranged from 0.35 to 0.87 mg/mL (median 0.5 mg/mL). EPH was also performed in three of the clinically ill birds (Supplementary Table S3).
Seropositivity for IAV was determined by enzyme-linked immunosorbent assay in 147 individuals. Year, colony, and morphometrically predicted sex were assessed for association with IAV seropositivity. Sex was not associated (p = 0.74). Year and colony were considered for further multivariate model building as p values were below a threshold of p ≤ 0.2. After adjusting for colony clustering, the prevalence of IAV in 2014 (75%; 47/63) was significantly higher than in 2019 (24%; 17/72; p < 0.001), but not different compared to in 2013 (50%; 6/12; p = 0.1). A subset analysis including only colonies with data for multiple years and only the last 2 years of sampling did not change estimates of effect (<5% difference; data not shown). The analysis of the full dataset is presented here. The model including year of sampling yielded a better fit than the base model (AIC = 173 vs. AIC = 205). Overall, including the year of sampling yielded a significantly better model (Likelihood ratio test, p < 0.0001).
This study provides protein electrophoresis, APP, and serum biochemistry RIs and IAV seroprevalence among wild adult rhinoceros auklets from large breeding colonies in the North Pacific. While various studies have described protein electrophoresis fractions in avian species previously, sample sizes have generally been limited and reference ranges only compiled for a handful of species including Xantus’s murrelets (Synthliboramphus hypoleucus), common loons (Gavia immer), and captive American flamingos (Phoenicopterus ruber) (3, 4, 55). This study, to our knowledge, provides one of the largest samplings of a free-ranging seabird to assess baseline health by protein electrophoresis to date. It is important to note though, that reference intervals are both species and laboratory-specific, and that RI calculation method (e.g., whether outliers were removed) likely affects calculated intervals. We therefore followed current guidelines from the American Society of Veterinary Clinical Pathology for RI generation.
Analysis of acute phase response is a more recent advancement in avian medicine to complement disease diagnosis, such as in the diagnosis of aspergillosis or chlamydiosis (2, 5). Pododermatitis is negatively associated with albumin levels in captive American flamingos (3), which has potential implications for wildlife rehabilitation as this disease is a common negative consequence of captivity in waterbirds (56). Serum EPH and APP profiles of the rhinoceros auklets in this study were unremarkable when compared to other species, with some exceptions. Albumin concentrations and the associated albumin to globulin ratio range were slightly elevated in rhinoceros auklets compared to wild adult Xantus’s murrelets, common loons, brown pelicans (Pelecanus occidentalis), juvenile herring gulls (Larus argentatus), and Caspian terns (Sterna caspia) (4, 55, 57, 58). By contrast, captive American flamingos exhibited a higher albumin to globulin ratio, in addition to prealbumin and HP concentrations (3).
Serum amyloid A (SAA) and HP are other biomarkers for monitoring inflammation in birds (59). Inflammation has been linked with elevations of SAA levels in peregrine falcons (Falco peregrinus) with fungal pneumonia and pododermatitis (46). Rhinoceros auklets in this study had a comparable SAA RI to healthy peregrine falcon individuals, although some rhinoceros auklets had highly elevated SAA concentrations (> 20 mg/L) subsequently identified as statistical outliers. This could represent inflammatory disease, and potentially subclinical illness. As birds were presumed healthy based on brief external examination and breeding status, this represents a limitation within the baseline health parameters generated in this study.
Haptoglobin values in this study were often below the limit of detection of the analyzer. In general, HP is considered a minor acute phase protein; HP binds free hemoglobin, minimizing oxidative damage caused during inflammation (6, 60). Plasma HP concentration has been previously associated with herpesvirus infection in frigatebird nestlings and was predictive of short-term survival (61). Haptoglobin concentrations also increased in adult mallard ducks (Anas platyrhyncos) experimentally injected with bacterial lipopolysaccharide (62). By contrast, these mallard ducks exhibited no change in HP when experimentally exposed to fuel oil (62). Wild common guillemots (Uria aalge) had plasma HP concentrations negatively correlated with exposure to polycyclic aromatic hydrocarbons in crude oil, thought to be related to Heinz body hemolytic anemia (63). Further investigation is required to understand the contextual implications of HP concentration alterations in seabirds, particularly related to oiling and infection by different pathogens.
Since there is limited information about SAA and HP values in clinically normal compared to abnormal seabirds, serum or plasma concentrations were assessed for five captive rhinoceros auklets. For SAA, all individuals with a definitive diagnosis of an acute infection had much greater SAA levels compared to the RI calculated in this study. One clinically abnormal bird with nonspecific clinical signs of illness and no definitive diagnosis had a corresponding minimal increase in SAA, potentially corresponding to a minor illness. For HP, even when all captive birds were considered clinically normal, all surprisingly had values above the RI calculated. Full medical histories are provided in Supplementary Tables S2–S4, as minor elevations in HP may correlate to subclinical low-grade pododermatitis common in managed captive flocks or to other subclinical disease not diagnosed at the time of sampling. For SAA and HP levels from the same individual, both were higher in clinically abnormal compared to normal states. This suggests possible value in comparing SAA and HP levels over time in individual animals to monitor disease state in captivity or during rehabilitation efforts. An important limitation, however, is that a very small number of rehabilitated animals were assessed here; future studies should aim to investigate the utility of monitoring serum amyloid A and HP among captive flocks and rehabilitated animals, using a larger sample size and variety of disease states.
Serum biochemistry parameters are previously described in seabirds, including Xantus’s murrelets, waved albatrosses (Phoebastria irrorata), and a few tropical seabirds (4, 55, 64, 65). Reference intervals in this study were comparable to previous waterbird studies, although amylase was notably higher in the sampled rhinoceros auklets compared to common loons and brown pelicans (4, 58). Scarce information exists regarding amylase concentrations in seabirds, and differences may be attributed to genetic variability within the species and are not necessarily diet-specific (66). Uric acid RI were also higher in rhinoceros auklets than previously reported in common loons, waved albatrosses, dark-rumped petrels (Pterodroma phaeopygia), and wedge-tailed shearwaters (Ardenna pacifica) (4, 64, 65). Rhinoceros auklets in this study were sampled at night, as they flew back from their burrows after foraging all day. Elevated uric acid could therefore be attributed to postprandial blood sampling as has been observed in captive black-footed penguins (Spheniscus demersus) and peregrine falcons (67, 68) or potentially due to dietary differences between those species. Uric acid differences could also reflect variation due to sampling at different times of days. Higher serum triglyceride concentrations in rhinoceros auklets compared to common loons may be reflective of the high lipid content during egg laying and brooding season, as demonstrated in the blue-footed booby (Sula nebouxii); alternatively, this may instead, reflect dietary differences between rhinoceros auklets and common loons (4, 69). Subclinical plastic ingestion has also been linked with increased uric acid, amylase, and cholesterol levels in flesh-footed shearwaters (Ardenna carneipes) (70). It is unknown whether plastic pollution similarly affects these parameters in rhinoceros auklets, though plastic fibers have been found in the rhinoceros auklet diet (71).
No sex differences in serum biochemical parameters were noted in this study, similar to black-browed albatrosses (Thalassarche melanophris) (72). By contrast, sex-related differences have been previously found in waterbirds including higher calcium and triglycerides in adult female Alaskan seabirds and brown pelicans (22, 58), which may potentially indicate species differences or reproductive status differences from rhinoceros auklets.
As IAV is an important pathogen for surveillance in wild birds within the Pacific Flyway, rhinoceros auklets were surveyed for antibodies. We detected seropositivity to IAV antibodies across rhinoceros auklets in all sampling years, indicating viral exposure. This highlights the utility of continued surveillance, especially in light of the H5N1 HPAI outbreak in wild birds across North America and Europe. IAV serology has previously been assessed in free-ranging adult waved albatrosses and southern giant petrels, with no animals testing positive (64, 73). Among different seabird species on the Canadian East coast, Atlantic puffins (Fratercula arctica) and common murres (Uria aalge) exhibited 22 and 44% antibody prevalence, respectively, while several other species were seronegative (33). It is unclear what the source of IAV exposure is to these rhinoceros auklets. One possibility is that sympatric species such as gulls, known to act as reservoirs for low pathogenic H13 and H16 subtypes of IAV (34), could be a potential source. Additional work is required to understand cross-species transmission and other modes of exposure to IAV.
It is important to note that while many sampled birds had antibodies against IAV, they were all clinically healthy on external examination. Therefore, while it is possible that previous IAV infection could have affected the serum EPH, APP, and biochemical analytes assessed (e.g., increased SAA with acute infection), it is probably less likely unless birds were infected at the time of sampling. Understanding the viral subtypes that rhinoceros auklets are exposed to is particularly important, as it has been experimentally shown in wood ducks that previous exposure to IAV could infer some degree of homosubtypic (homologous hemagglutinin) and heterosubtypic (heterologous hemagglutinin) protective immunity if birds are exposed to H5N1 HPAI (74). Continued serological surveillance is required to understand the role of rhinoceros auklets in IAV epidemiology, and their potential as a reservoir species.
There were several limitations to this study. This study was conducted over multiple years and colonies, and a variety of small inter-colony and inter-annual differences for parameters were observed. Notably, inter-annual differences have also previously been observed in clinical metrics for other seabird species (75, 76). Given the minor variations in these parameters that were not suggestive of biological significance, RI were compiled with inclusion of all individuals to provide a broader representation of rhinoceros auklet populations across multiple years and colonies. There was prolonged storage of some samples prior to testing, which could have affected protein presence and metabolism. However, the impact of sample storage duration is not well described in the literature and therefore was not a variable investigated in this study. Finally, only brief external exams were conducted upon animal capture and so subclinical disease may have been missed. As such, since the EPH RI generated here are broad, there may have been some ill birds within the group sampled. To counteract this limitation in potentially missing animals with subclinical disease, we utilized Tukey’s interquartile fences to identify statistical outliers as per the American Society of Veterinary Clinical Pathology guidelines. Further research is required to compare health parameters between diseased and healthy rhinoceros auklets. For example, the serum EPH, APP, and biochemistry profiles of birds from rehabilitation centers with known injuries should be further characterized to help correlate parameters with clinical disease or injuries. Future research can assess how these health parameters may vary with increased stress in birds, as a proxy for subclinical illness; this can be done by conducting hormone analysis.
In conclusion, this study provides serum EPH, APP, and biochemistry RI for rhinoceros auklets, an important alcid sentinel species of the North Pacific, following recommended guidelines by the American Society of Veterinary Clinical Pathology. Overall, rhinoceros auklet RIs are comparable to other aquatic bird species, although rhinoceros auklets appeared to have higher upper RIs for serum amylase, uric acid, and triglyceride concentrations. This work is critical for wildlife conservation and management in the North Pacific, as it facilitates the monitoring of marine ecosystem health in the face of stressors such as pandemic illness, infectious disease, climate change, plastic ingestion, pollution, increased marine vessel traffic, and large-scale environmental catastrophes such as oil spills. Furthermore, the clinical nature of these data will provide a useful basis for assessment during the rehabilitation of seabirds, especially during climatic or anthropogenic events.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Research protocols employed in this study were approved by Simon Fraser University Animal Care Services (#974B-94), the Western and Northern Animal Care Committee of Environment and Climate Change Canada’s Canadian Wildlife Service (14MH01, and 19MH01), ECCC Migratory Birds banding permit (10667F), and US Fish and Wildlife Federal Bird Banding Permit (22913).
LL: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. GF: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. CC: Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. SPe: Data curation, Formal analysis, Supervision, Writing – review & editing. CF: Writing – review & editing. NC: Writing – review & editing. SH: Writing – review & editing. SPa: Formal analysis, Writing – review & editing. DS: Methodology, Writing – review & editing. EF: Writing – review & editing. KH: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported through the Oiled Wildlife Care Network’s Research & Technology Development Program. This project was also financially supported in part by the SeaDoc Society, a program of the Karen C. Drayer Wildlife Health Center, School of Veterinary Medicine, University of California Davis, which provided a grant for improvement of the health of the Salish Sea. The Oceans Protection Plan provided funding to Environment and Climate Change Canada for colony-based work.
We acknowledge that this work was done on the traditional territories of the Coast Tsimshian, which includes both Metlakatla and Lax Kwa’alaams First Nations (Lucy Islands); Gwa’sala-‘Nakwaxda’xw Nations (Pine Island); Quatsino and Tlatlasikwala First Nations (Triangle Island); and Haida Nation (SGang Gwaay). We are grateful to Peter Hodum, Andrew Huang, Catherine Jardine, Glen Keddie, Agathe Lebeau, Megan Ross, Kate Shapiro, and Katie Studholme for their field assistance on colony-based work, and to the Canadian Coast Guard and West Coast Helicopters for safe transport to field sites. We also thank Sue Thomas and the US Fish and Wildlife Washington Maritime Complex for access to Protection Island. Finally, we thank Theresa Burg and Brendan Graham at the University of Lethbridge for genetic sexing of the auklets.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1379980/full#supplementary-material
1. Harr, KE. “Diagnostic value of biochemistry,” in Clinical Avian Medicine. Vol 2 Harrison GJ and Lightfoot TL (Spix Publishing, Inc.), (2012) 611–29.
2. Cray, C. Protein electrophoresis of non-traditional species: a review. Vet Clin Pathol. (2021) 50:478–94. doi: 10.1111/vcp.13067
3. Delk, KW, Wack, RF, Burgdorf-Moisuk, A, Kass, PH, and Cray, C. Acute phase protein and electrophoresis protein fraction values for captive American flamingos (Phoenicopterus ruber). J Zoo Wildl Med. (2015) 46:929–33. doi: 10.1638/2014-0191.1
4. Kneeland, M, Berman, E, Grade, T, Cooley, J, Vogel, H, Schoch, N, et al. Plasma biochemistry and protein electrophoresis reference intervals of the common loon (Gavia immer). J Zoo Wildl Med. (2020) 51:561–70. doi: 10.1638/2019-0168
5. Cray, C, and Tatum, LM. Applications of protein electrophoresis in avian diagnostics. J Avian Med Surg. (1998) 12:4–10. doi: 10.2307/30133137
6. Wicher, KB, and Fries, E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci USA. (2006) 103:4168–73. doi: 10.1073/pnas.0508723103
7. Eckersall, PD. Proteins, proteomics, and the dysproteinemias In: Clinical Biochemistry of Domestic Animals. 6th Edn. San Diego: Academic Press. (2008). 117–55.
8. Hooijberg, EH, and Cray, C. Acute phase reactants in nondomesticated mammals—a veterinary clinical pathology perspective. Vet Clin Pathol. (2022) 52:19–36. doi: 10.1111/vcp.13189
9. Harr, KE, Cunningham, FL, Pritsos, CA, Pritsos, KL, Muthumalage, T, Dorr, BS, et al. Weathered MC252 crude oil-induced anemia and abnormal erythroid morphology in double-crested cormorants (Phalacrocorax auritus) with light microscopic and ultrastructural description of Heinz bodies. Ecotoxicol Environ Saf. (2017a) 146:29–39. doi: 10.1016/j.ecoenv.2017.07.030
10. Khan, RA, and Ryan, P. Long term effects of crude oil on common murres (Uria aalge) following rehabilitation. Bull Environ Contam Toxicol. (1991) 46:216–22. doi: 10.1007/BF01691940
11. Newman, SH, Mazet, JK, Ziccardi, MH, Lieske, CL, Fauquier, DA, Gardner, IA, et al. Haematological changes and anaemia associated with captivity and petroleum exposure in seabirds. Comp Haematol Int. (1999) 9:60–7. doi: 10.1007/BF02585537
12. Barron, MG, Vivian, DN, Heintz, RA, and Yim, UH. Long-term ecological impacts from oil spills: comparison of Exxon Valdez, Hebei Spirit, and Deepwater horizon. Environ Sci Technol. (2020) 54:6456–67. doi: 10.1021/acs.est.9b05020
13. Champoux, L, Rail, JF, Houde, M, Giraudo, M, Lacaze, É, Franci, CD, et al. An investigation of physiological effects of the Deepwater horizon oil spill on a long-distance migratory seabird, the northern gannet. Mar Pollut Bull. (2020) 153:110953. doi: 10.1016/j.marpolbul.2020.110953
14. Dorr, BS, Hanson-Dorr, KC, Assadi-Porter, FM, Selen, ES, Healy, KA, and Horak, KE. Effects of repeated sublethal external exposure to deep water horizon oil on the avian metabolome. Sci Rep. (2019) 9:1–12. doi: 10.1038/s41598-018-36688-3
15. Fox, CH, O’Hara, PD, Bertazzon, S, Morgan, K, Underwood, FE, and Paquet, PC. A preliminary spatial assessment of risk: marine birds and chronic oil pollution on Canada’s Pacific coast. Sci Total Environ. (2016) 573:799–809. doi: 10.1016/j.scitotenv.2016.08.145
16. Harr, KE, Reavill, DR, Bursian, SJ, Cacela, D, Cunningham, FL, Dean, KM, et al. Organ weights and histopathology of double-crested cormorants (Phalacrocorax auritus) dosed orally or dermally with artificially weathered Mississippi canyon 252 crude oil. Ecotoxicol Environ Saf. (2017b) 146:52–61. doi: 10.1016/j.ecoenv.2017.07.011
17. King, MD, Elliott, JE, and Williams, TD. Effects of petroleum exposure on birds: a review. Sci Total Environ. (2021) 755:142834. doi: 10.1016/j.scitotenv.2020.142834
18. Elliott, JE, Drever, MC, Studholme, KR, Silverthorn, V, Miller, AA, Elliott, KH, et al. Exposure to persistent organic pollutants is linked to over-wintering latitude in a Pacific seabird, the rhinoceros auklet, Cerorhinca monocerata. Environ Pollut. (2021) 279:116928. doi: 10.1016/j.envpol.2021.116928
19. Elliott, JE, and Elliott, KH. Tracking marine pollution. Science. (2013) 340:556–8. doi: 10.1126/science.1235197
20. Good, TP, Pearson, SF, Hodum, P, Boyd, D, Anulacion, BF, and Ylitalo, GM. Persistent organic pollutants in forage fish prey of rhinoceros auklets breeding in Puget Sound and the northern California current. Mar Pollut Bull. (2014) 86:367–78. doi: 10.1016/j.marpolbul.2014.06.042
21. Edwards, DB, Mallory, ML, and Forbes, MR. Variation in baseline haematology of northern fulmars (Fulmarus glacialis) in the Canadian high Arctic. Comp Clin Pathol. (2006) 14:206–9. doi: 10.1007/s00580-005-0589-8
22. Newman, SH, Piatt, JF, and White, J. Hematological and plasma biochemical reference ranges of Alaskan seabirds: their ecological significance and clinical importance. Colon Waterbirds. (1997) 20:492–504. doi: 10.2307/1521600
23. Dias, MP, Martin, R, Pearmain, EJ, Burfield, IJ, Small, C, Phillips, RA, et al. Threats to seabirds: a global assessment. Biol Conserv. (2019) 237:525–37. doi: 10.1016/j.biocon.2019.06.033
24. Baker-Austin, C, Trinanes, JA, Taylor, NGH, Hartnell, R, Siitonen, A, and Martinez-Urtaza, J. Emerging vibrio risk at high latitudes in response to ocean warming. Nat Clim Chang. (2013) 3:73–7. doi: 10.1038/nclimate1628
25. Barbraud, C, Bertrand, A, Bouchón, M, Chaigneau, A, Delord, K, Demarcq, H, et al. Density dependence, prey accessibility and prey depletion by fisheries drive Peruvian seabird population dynamics. Ecography. (2018) 41:1092–102. doi: 10.1111/ecog.02485
26. Hazen, EL, Abrahms, B, Brodie, S, Carroll, G, Jacox, MG, Savoca, MS, et al. Marine top predators as climate and ecosystem sentinels. Front Ecol Environ. (2019) 17:565–74. doi: 10.1002/fee.2125
27. Gaston, AJ, Bertram, DF, Boyne, AW, Chardine, JW, Davoren, G, Diamond, AW, et al. Changes in Canadian seabird populations and ecology since 1970 in relation to changes in oceanography and food webs. Environ Rev. (2009) 17:267–86. doi: 10.1139/A09-013
28. Mallory, ML, Robinson, SA, Hebert, CE, and Forbes, MR. Seabirds as indicators of aquatic ecosystem conditions: a case for gathering multiple proxies of seabird health. Mar Pollut Bull. (2010) 60:7–12. doi: 10.1016/j.marpolbul.2009.08.024
29. Parsons, M, Mitchell, I, Butler, A, Ratcliffe, N, Frederiksen, M, Foster, S, et al. Seabirds as indicators of the marine environment. ICES J Mar Sci. (2008) 65:1520–6. doi: 10.1093/icesjms/fsn155
30. Fourment, M, Darling, AE, and Holmes, EC. The impact of migratory flyways on the spread of avian influenza virus in North America. BMC Evol Biol. (2017) 17:118–2. doi: 10.1186/s12862-017-0965-4
31. Siembieda, JL, Johnson, CK, Cardona, C, and Anchell, N. Influenza a viruses in wild birds. Vect Borne Zoo Dis. (2010) 10:793–800. doi: 10.1089/vbz.2009.0095
32. Huang, Y, Robertson, GJ, Ojkic, D, Whitney, H, and Lang, AS. Diverse inter-continental and host lineage reassortant avian influenza a viruses in pelagic seabirds. Infect Genet Evol. (2014) 22:103–11. doi: 10.1016/j.meegid.2014.01.014
33. Wille, M, Huang, Y, Robertson, GJ, Ryan, P, Wilhelm, SI, Fifield, D, et al. Evaluation of seabirds in Newfoundland and Labrador, Canada, as hosts of influenza A viruses. J Wildl Dis. (2014) 50:98–103. doi: 10.7589/2012-10-247
34. Hill, NJ, Bishop, MA, Trovão, NS, Ineson, KM, Schaefer, AL, Puryear, WB, et al. Ecological divergence of wild birds drives avian influenza spillover and global spread. PLoS Pathog. (2022) 18:e1010062. doi: 10.1371/journal.ppat.1010062
35. Caliendo, V, Lewis, NS, Pohlmann, A, Baillie, SR, Banyard, AC, Beer, M, et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci Rep. (2022) 12:1–18. doi: 10.1038/s41598-022-13447-z
36. Adlhoch, C, Fusaro, A, Gonzales, JL, Kuiken, T, Marangon, S, Niqueux, É, et al. Avian influenza overview September–December 2022. EFSA J. (2023) 21:e07786. doi: 10.2903/j.efsa.2023.7786
37. Rijks, JM, Leopold, MF, Kühn, S, In’t Veld, R, Schenk, F, Brenninkmeijer, A, et al. Mass mortality caused by highly pathogenic influenza a(H5N1) virus in Sandwich terns, the Netherlands, 2022. Emerg Infect Dis. (2022) 28:2538–42. doi: 10.3201/eid2812.221292
38. U.S. Department of Agriculture—Animal and Plant Health Inspection Service (2023). 2022–2023 Detections of Highly Pathogenic Avian Influenza in Wild Birds [WWW Document]. U.S. Department of Agriculture. Available at: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza/hpai-2022/2022-hpai-wild-birds (Accessed February 21, 2023).
39. Knowles, S, Bodenstein, BL, Berlowski-Zier, BM, Thomas, SM, Pearson, SF, and Lorch, JM. Detection of bisgaard taxon 40 in rhinoceros auklets (Cerorhinca monocerata) with pneumonia and septicemia from a mortality event in Washington, USA. J Wildl Dis. (2019) 55:246–9. doi: 10.7589/2017-12-309
40. Hyrenbach, KD, and Veit, RR. Ocean warming and seabird communities of the southern California current system (1987-98): response at multiple temporal scales. Deep Sea Res 2 Top Stud Oceanogr. (2003) 50:2537–65. doi: 10.1016/S0967-0645(03)00123-1
41. Borstad, G, Crawford, W, Hipfner, JM, Thomson, R, and Hyatt, K. Environmental control of the breeding success of rhinoceros auklets at Triangle Island, British Columbia. Mar Ecol Prog Ser. (2011) 424:285–302. doi: 10.3354/meps08950
42. Hedd, A, Bertram, DF, Ryder, JL, and Jones, IL. Effects of interdecadal climate variability on marine trophic interactions: rhinoceros auklets and their fish prey. Mar Ecol Prog Ser. (2006) 309:263–78. doi: 10.3354/meps309263
43. Wagner, E, Pearson, S, Good, T, Hodum, P, Buhle, E, and Schrimpf, M. Resilience to a severe marine heatwave at two Pacific seabird colonies. Mar Ecol Prog Ser. (2024) 737:101–20. doi: 10.3354/meps14222
44. Morrison, KW, Hipfner, JM, Blackburn, GS, and Green, DJ. Effects of extreme climate events on adult survival of three pacific auks. Auk. (2011) 128:707–15. doi: 10.1525/auk.2011.10198
45. Parrish, JK, Burgess, H, Lindsey, J, Divine, L, Kaler, R, Pearson, S, et al. Partnering with the Public: The Coastal Observation and Seabird Survey Team, Partnerships in Marine Research: Case Studies, Lessons Learned, and Policy Implications. Cambridge, MA, USA: Elsevier Inc. (2022).
46. Caliendo, V, McKinney, P, Bailey, T, Kinne, J, and Wernery, U. Serum amyloid a as an indicator of health status in falcons. J Avian Med Surg. (2013) 27:83–9. doi: 10.1647/2011-026
47. Friedrichs, KR, Harr, KE, Freeman, KP, Szladovits, B, Walton, RM, Barnhart, KF, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol. (2012) 41:441–53. doi: 10.1111/vcp.12006
48. Shriner, SA, VanDalen, KK, Root, JJ, and Sullivan, HJ. Evaluation and optimization of a commercial blocking ELISA for detecting antibodies to influenza a virus for research and surveillance of mallards. J Virol Methods. (2016) 228:130–4. doi: 10.1016/j.jviromet.2015.11.021
49. Burg, TM, and Croxall, JP. Global relationships amongst black-browed and grey-headed albatrosses: analysis of population structure using mitochondrial DNA and microsatellites. Mol Ecol. (2001) 10:2647–60. doi: 10.1046/j.0962-1083.2001.01392.x
50. Walsh, PS, Metzger, DA, and Higuchi, R. Biotechniques 30th anniversary gem Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques. (2013) 54:134–9. doi: 10.2144/000114018
51. Dawson, DA, dos Remedios, N, and Horsburgh, GJ. A new marker based on the avian spindlin gene that is able to sex most birds, including species problematic to sex with CHD markers. Zoo Biol. (2016) 35:533–45. doi: 10.1002/zoo.21326
52. Addison, B, Kitaysky, AS, and Hipfner, JM. Sex allocation in a monomorphic seabird with a single-egg clutch: test of the environment, mate quality, and female condition hypotheses. Behav Ecol Sociobiol. (2008) 63:135–41. doi: 10.1007/s00265-008-0643-z
53. ASVCP Quality Assurance and Laboratory Standards Committee (2020). Guideline Checklist for Determining Reference Intervals [WWW Document]. Available at: https://cdn.ymaws.com/www.asvcp.org/resource/resmgr/qals/reference-interval-checklist.pdf (Accessed December 17, 2023).
54. Geffré, A, Concordet, D, Braun, JP, and Trumel, C. Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft excel. Vet Clin Pathol. (2011) 40:107–12. doi: 10.1111/j.1939-165X.2011.00287.x
55. Newman, SH, Carter, HR, Whitworth, DL, and Zinkl, JG. Health assessments and stress response of Xantus’s Murrelets to capture, handling and radio-marking. Mar Ornithol. (2005) 33:147–54.
56. Fiorello, CV. Intravenous regional antibiotic perfusion therapy as an adjunctive treatment for digital lesions in seabirds. J Zoo Wildl Med. (2017) 48:189–95. doi: 10.1638/2016-0045.1
57. Grasman, KA, Armstrong, M, Hammersley, DL, Scanlon, PF, and Fox, GA. Geographic variation in blood plasma protein concentrations of young herring gulls (Larus argentatus) and Caspian terns (Sterna caspia) from the Great Lakes and Lake Winnipeg. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. (2000) 125:365–75. doi: 10.1016/S0742-8413(99)00118-8
58. Zaias, J, Fox, WP, Cray, C, and Altman, NH. Hematologic, plasma protein, and biochemical profiles of brown pelicans (Pelecanus occidentalis). Am J Vet Res. (2000) 61:771–4. doi: 10.2460/ajvr.2000.61.771
59. O’Reilly, EL, and Eckersall, PD. Acute phase proteins: a review of their function, behaviour and measurement in chickens. Worlds Poult Sci J. (2014) 70:27–43. doi: 10.1017/S0043933914000038
60. Kromann, S, Olsen, RH, Bojesen, AM, Jensen, HE, and Thøfner, I. Assessment of automated assays for serum amyloid a, haptoglobin (PIT54) and basic biochemistry in broiler breeders experimentally infected with Escherichia coli. Vet Res. (2022) 53:25. doi: 10.1186/s13567-022-01040-1
61. Sebastiano, M, Eens, M, Angelier, F, Pineau, K, Chastel, O, and Costantini, D. Corticosterone, inflammation, immune status and telomere length in frigatebird nestlings facing a severe herpesvirus infection. Conserv Physiol. (2017) 5:1–13. doi: 10.1093/conphys/cow073
62. Lee, KA, Tell, LA, and Mohr, FC. Inflammatory markers following acute fuel oil exposure or bacterial lipopolysaccharide in mallard ducks (Anas platyrhynchos). Avian Dis. (2012) 56:704–10. doi: 10.1637/10075-020712-Reg.1
63. Troisi, G, Borjesson, L, Bexton, S, and Robinson, I. Biomarkers of polycyclic aromatic hydrocarbon (PAH)-associated hemolytic anemia in oiled wildlife. Environ Res. (2007) 105:324–9. doi: 10.1016/j.envres.2007.06.007
64. Padilla, LR, Huyvaert, KP, Merkel, J, Miller, RE, and Parker, PG. Hematology, plasma chemistry, serology, and Chlamydophila status of the waved albatross (Phoebastria irrorata) on the Galapagos Islands. J Zoo Wildl Med. (2003) 34:278–83. doi: 10.1638/02-076
65. Work, TM. Weights, hematology, and serum chemistry of seven species of free-ranging tropical pelagic seabirds. J Wildl Dis. (1996) 32:643–57. doi: 10.7589/0090-3558-32.4.643
66. Brzęk, P, Ciminari, ME, Kohl, KD, Lessner, K, Karasov, WH, and Caviedes-Vidal, E. Effect of age and diet composition on activity of pancreatic enzymes in birds. J Comp Physiol B. (2013) 183:685–97. doi: 10.1007/s00360-012-0731-2
67. Kolmstetter, CM, and Ramsay, EC. Effects of feeding on plasma uric acid and urea concentrations in blackfooted penguins (Spheniscus demersus). J Avian Med Surg. (2000) 14:177–9. doi: 10.1647/1082-6742(2000)014[0177:EOFOPU]2.0.CO;2
68. Lumeij, JT, and Remple, JD. Plasma urea, creatinine and uric acid concentrations in relation to feeding in Peregrine falcons (Falco Peregrinus). Avian Pathol. (1991) 20:79–83. doi: 10.1080/03079459108418743
69. González-Medina, E, Castillo-Guerrero, JA, Herzka, SZ, and Fernández, G. High quality diet improves lipid metabolic profile and breeding performance in the blue-footed booby, a long-lived seabird. PLoS One. (2018) 13:e0193136. doi: 10.1371/journal.pone.0193136
70. Lavers, JL, Hutton, I, and Bond, AL. Clinical pathology of plastic ingestion in marine birds and relationships with blood chemistry. Environ Sci Technol. (2019) 53:9224–31. doi: 10.1021/acs.est.9b02098
71. Hipfner, JM, Galbraith, M, Tucker, S, Studholme, KR, Domalik, AD, Pearson, SF, et al. Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs. Environ Pollut. (2018) 239:215–22. doi: 10.1016/j.envpol.2018.04.009
72. Ferrer, M, Morandini, V, Perry, L, and Bechard, M. Factors affecting plasma chemistry values of the black-browed albatross Thalassarche melanophrys. Polar Biol. (2017) 40:1537–44. doi: 10.1007/s00300-017-2075-6
73. Uhart, MM, Quintana, F, Karesh, WB, and Braselton, WE. Hematology, plasma biochemistry, and serosurvey for selected infectious agents in southern giant petrels from Patagonia, Argentina. J Wildl Dis. (2003) 39:359–65. doi: 10.7589/0090-3558-39.2.359
74. Costa, TP, Brown, JD, Howerth, EW, Stallknecht, DE, and Swayne, DE. Homo- and heterosubtypic low pathogenic avian influenza exposure on H5N1 highly pathogenic avian influenza virus infection in wood ducks (Aix sponsa). PLoS One. (2011) 6:e15987. doi: 10.1371/journal.pone.0015987
75. Gallo, L, Quintana, F, Svagelj, WS, and Uhart, M. Hematology and blood chemistry values in free-living Imperial cormorants (Phalacrocorax atriceps). Avian Dis. (2013) 57:737–43. doi: 10.1637/10521-022713-Reg.1
Keywords: acute phase proteins, baseline, Cerorhinca monocerata , protein electrophoresis, rhinoceros auklets, serum biochemistry
Citation: Lee LKF, Hipfner JM, Frankfurter G, Cray C, Pearson SF, Fiorello C, Clyde NMT, Hudson SA, Parker SE, Stallknecht DE, Furst E and Haman KH (2024) Baseline health parameters of rhinoceros auklets (Cerorhinca monocerata) using serum protein electrophoresis, acute phase proteins, and biochemistry. Front. Vet. Sci. 11:1379980. doi: 10.3389/fvets.2024.1379980
Received: 31 January 2024; Accepted: 20 May 2024;
Published: 25 June 2024.
Edited by:
Ana M. Molina-López, University of Cordoba, SpainReviewed by:
Todd Schmitt, SeaWorld Entertainment, United StatesCopyright © 2024 Lee, Hipfner, Frankfurter, Cray, Pearson, Fiorello, Clyde, Hudson, Parker, Stallknecht, Furst and Haman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine H. Haman, S2F0aGVyaW5lLkhhbWFuQGRmdy53YS5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.