- Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
In human medicine, hemostatic disorders such as thrombocytopenia, hyperfibrinolysis, and disseminated intravascular coagulopathy (DIC) have been associated with many cancers. Acute hemorrhage secondary to hyperfibrinolysis has been predominantly reported with prostatic adenocarcinoma in human patients. To the author’s knowledge, severe bleeding due to paraneoplastic hyperfibrinolysis has not yet been reported in veterinary medicine. The case involves an 8-year-old neutered male Border Collie who was evaluated for progressive and recurrent epistaxis, having a history of 1 year of treatment for metastatic nasal adenocarcinoma. A progressive and severe coagulopathy thought to be related to the known cancer was diagnosed. Advanced coagulation testing was consistent with a chronic DIC and secondary hyperfibrinolysis. Throughout 1 week of hospitalization, the dog was treated with multiple blood products, vitamin K1, and anti-fibrinolytic medications. While the dog was initially discharged home, the dog re-presented the following day and was humanely euthanized due to a perceived poor quality of life. Post-mortem analysis revealed a histopathologic diagnosis of disseminated adenocarcinoma. In dogs with disseminated nasal adenocarcinoma that are experiencing severe bleeding, paraneoplastic secondary hyperfibrinolysis should be considered as a differential. Knowing this association could help guide treatment recommendations for optimal patient management.
Introduction
Though not completely understood, the interplay between neoplastic processes and the coagulation system is characterized by an imbalance between procoagulant and anticoagulant factors (1). Currently, it is theorized that the hemostatic system gets activated by procoagulant factors such as tissue factor that is produced by cancer cells. This activation results in local production of thrombin leading to fibrin deposition and platelet recruitment and ultimately resulting in thrombosis and consumption of coagulation factors (2). Paraneoplastic hyperfibrinolysis is thought to occur in two ways; the tumor cells either (A) produce profibrinolytic proteins, such as urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) or (B) tumor cell membranes carry the specific uPA receptor which can trigger the assembly of fibrinolytic components and subsequently result in excessive activation of the fibrinolytic cascade (3). In people, this phenomenon has been documented with prostatic adenocarcinoma (4–6). Among individuals with prostatic adenocarcinoma, the prevailing hemostatic disorder is disseminated intravascular coagulopathy (DIC). While prostatic adenocarcinoma-associated hyperfibrinolysis has been documented, it has only been reported to affect up to 1.65% of patients and typically manifests after surgical interventions (3). In veterinary medicine, severe bleeding caused by paraneoplastic disorders with secondary hyperfibrinolysis has not yet been reported. This report demonstrates the development of paraneoplastic secondary hyperfibrinolysis with severe bleeding in a dog with metastatic nasal adenocarcinoma.
Case description
An 8-year-old male neutered Border Collie (25.7 kg) was serially evaluated at a Veterinary Teaching Hospital (VTH) in January 2023 for recurrent epistaxis.
Significant medical history
The dog was diagnosed with nasal adenocarcinoma via nasal biopsy, without evidence of metastasis on thoracic or abdominal CT. In June 2022, as part of an ongoing clinical trial, the dog was treated with losartan (10 mg/kg PO q12h) and propranolol (1 mg/kg PO q12h) for 7 days followed by 3 days of stereotactic body radiation therapy (SBRT) using three fractions of 10 Gy photon therapy. Following completion of SRBT, the dog’s treatment regimen was maintained with losartan (10 mg/kg PO q12h), propranolol (1 mg/kg PO q12h), and carprofen (2.2 mg/kg PO q12h). In September 2022 (5 months post-SBRT), repeat CT revealed that the nasal tumor was reduced in size. However, fine-needle aspirates and cytology of the left mandibular lymph node were consistent with metastatic adenocarcinoma. On physical exam, the dog had mild bilateral serosanguinous nasal discharge, which later became more hemorrhagic but self-resolved and was subsequently discharged. Approximately 2 weeks later, the dog was represented to the VTH for re-evaluation, as part of the clinical trial, at which time there was no evidence of nasal discharge. The dog underwent general anesthesia for tumor biopsy after which significant hemorrhage from the bilateral nares was appreciated. Nasal flushing was performed to clear the hemorrhage, and oxymetazoline hydrochloride solution and epinephrine were administered. The dog was subsequently hospitalized with orders for cold compress application and sedation (gabapentin 10 mg/kg PO q12h, trazodone 7 mg/kg PO q8-12h, and acepromazine 0.02 mg/kg PO PRN). The next day, no further epistaxis was appreciated, and the dog was discharged home. No coagulation testing was performed at this visit. In November 2022 (7 months post-SBRT), the dog underwent extirpation of the left mandibular lymph node. Surgery was routine with no adverse events noted in the perioperative or post-operative period. In December 2022 (8 months post-SBRT), a repeat CT scan was performed, and the nasal tumor was decreased further in size. However, enlargement of the retropharyngeal and parotid lymph nodes was noted, and FNA with cytology confirmed further metastatic spread.
Serial clinical presentations and investigations
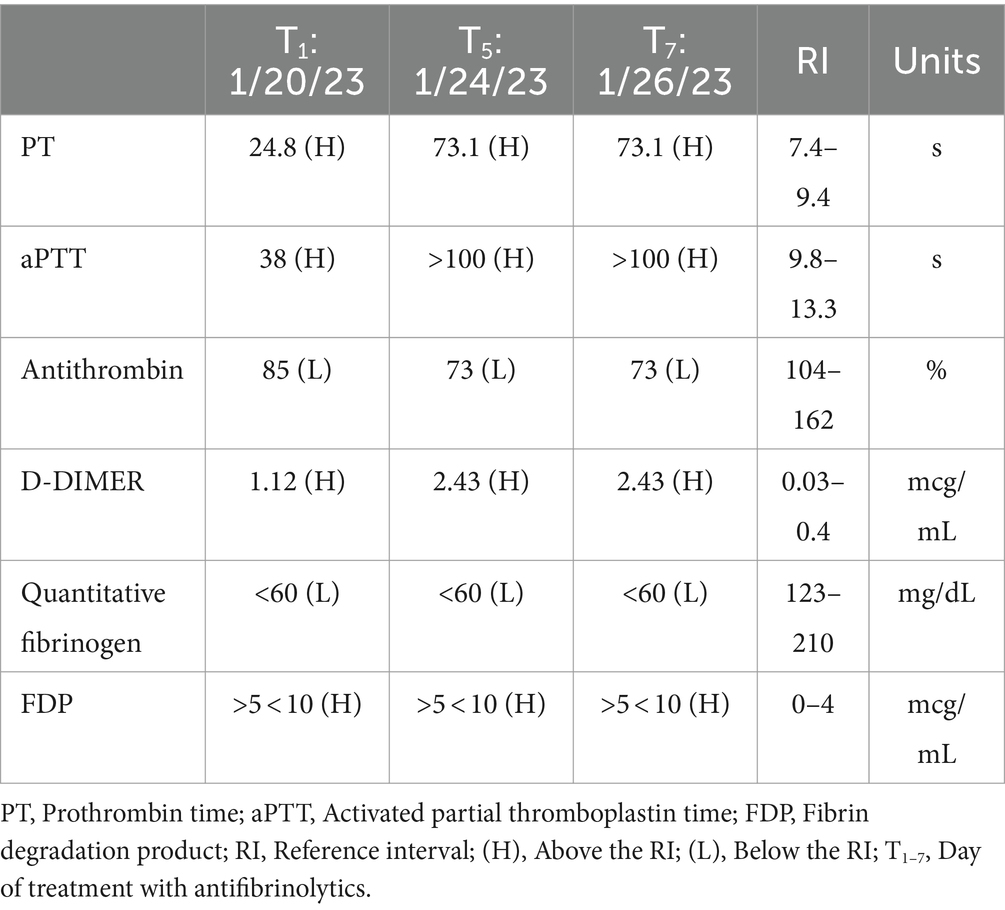
In January 2023 (9 months post-SBRT), the dog was presented to the CSU VTH for a reported prolonged unilateral epistaxis for approximately 18 h. Physical examination only confirmed unilateral epistaxis and was otherwise unremarkable. Coagulation testing revealed a markedly prolonged prothrombin time (PT), partial thrombin time (PTT), increased D-dimers, hypofibrinogenemia, mildly increased fibrin degradation product (FDP), and mildly decreased antithrombin (Table 1). On complete blood count (CBC), only a mild leukocytosis [26.9 × 103/μL, reference interval (RI): 4.5–15 × 103/μL] was noted. The biochemical profile showed mild hypoalbuminemia (ALB: 2.9 g/dL, RI: 3–4.3 g/dL) and mild hypomagnesemia (Mg2+: 1.7 mg/dL, RI: 1.8–2.4 mg/dL) and was otherwise within normal limits. The dog was hospitalized overnight on aminocaproic acid (ACA) (50 mg/kg IV q6h for four doses), maropitant (1 mg/kg IV q24h), and trazodone (4 mg/kg PO q8h/PRN) and was discharged the next day due to resolution of the epistaxis. The dog was discharged home with ACA (50 mg/kg PO q8h), gabapentin (12 mg/kg PO q8h), and trazodone (4 mg/kg PO q8h/PRN), with instructions to continue administration until the next scheduled recheck visit.
The dog presented 4 days later for continued bilateral epistaxis. At this visit, laboratory findings revealed that the dog has progressive and strongly regenerative anemia (hematocrit: 26%, RI: 40–55%, hemoglobin: 8 g/dL, RI: 13–20 g/dL, and absolute reticulocyte count: 335.1 × 103/μL, RI: 0–100 × 103/μL), mild thrombocytopenia with platelet clumping noted on blood film review (platelet count: 90 × 103/μL, RI: 200–500 × 103/μL), static leukocytosis (WBC: 27.7 × 103/μL, RI: 4.5–15 × 103/μL), mild hypoglycemia (glucose: 64 mg/dL, RI: 70–115 mg/dL), and mild hypoalbuminemia (albumin: 2.8 g/dL, RI: 3–4.3 g/dL). Thoracic radiographs revealed a newly identified pulmonary nodule. A coagulation panel revealed a progressively worsened coagulopathy with continued hypofibrinogenemia (Table 1). Native thromboelastography (TEG) (using no activators) demonstrated a prolonged initiation to clot formation, in addition to delayed clot kinetics and an extremely weak clot (Figure 1). A clot was not able to be formed in the tissue-factor + tPA TEG tracing (Figure 1). Platelet aggregometry was performed with a multiple electrode aggregometer (Multiplate 5.0 Analyzer, DiaPharma Group Inc., West Chester, Ohio) using whole blood impedance aggregometry and showed decreased aggregation compared with institution reference ranges in the presence of two platelet agonists [adenosine diphosphate (ADP) and arachidonic acid (ASPI)] (Figure 2). The dog was hospitalized on fluid therapy, maropitant (1 mg/kg IV q24h), and ACA (50 mg/kg IV q6h) as initial treatments. However, given aforementioned findings, the dog received a fresh frozen plasma (FFP) transfusion (250 mL IV over 4 h, 10 mL/kg), and vitamin K1 (5 mg/kg SC q12h) was added to the treatments. On the second day of hospitalization, repeat testing found progressive anemia [packed cell volume (PCV): 19%, RI: 29–51%], and the dog developed hyperkinetic femoral pulses, decreased mentation, and progressive lethargy. An abdominal ultrasound was performed which showcased a scant amount of anechoic peritoneal effusion, not a sufficient quantity for sampling. Although the gallbladder exhibited a small volume of echogenic debris in its lumen, the rest of the abdomen, including the spleen and liver, appeared ultrasonographically normal. Given the clinical anemia progression and evidence of hypovolemia, packed red blood cell transfusion (10 mL/kg IV for 4 h), additional FFP transfusion (10 mL/kg IV for 4 h), and two units of cryoprecipitate (1.2 mL/kg IV for 1 h) were administered. After those treatments, the dog demonstrated marked improvement in circulatory parameters, non-appreciable epistaxis, and improved anemia (PCV: 29%, RI: 29–51%). On the third day of hospitalization, the dog’s recheck coagulation profile remained unchanged (Table 1). Given the clinical improvements, the owner elected to take the dog home at that time, and the dog was discharged from the VTH with instructions to transition to oral vitamin K1 and ACA at the aforementioned doses.
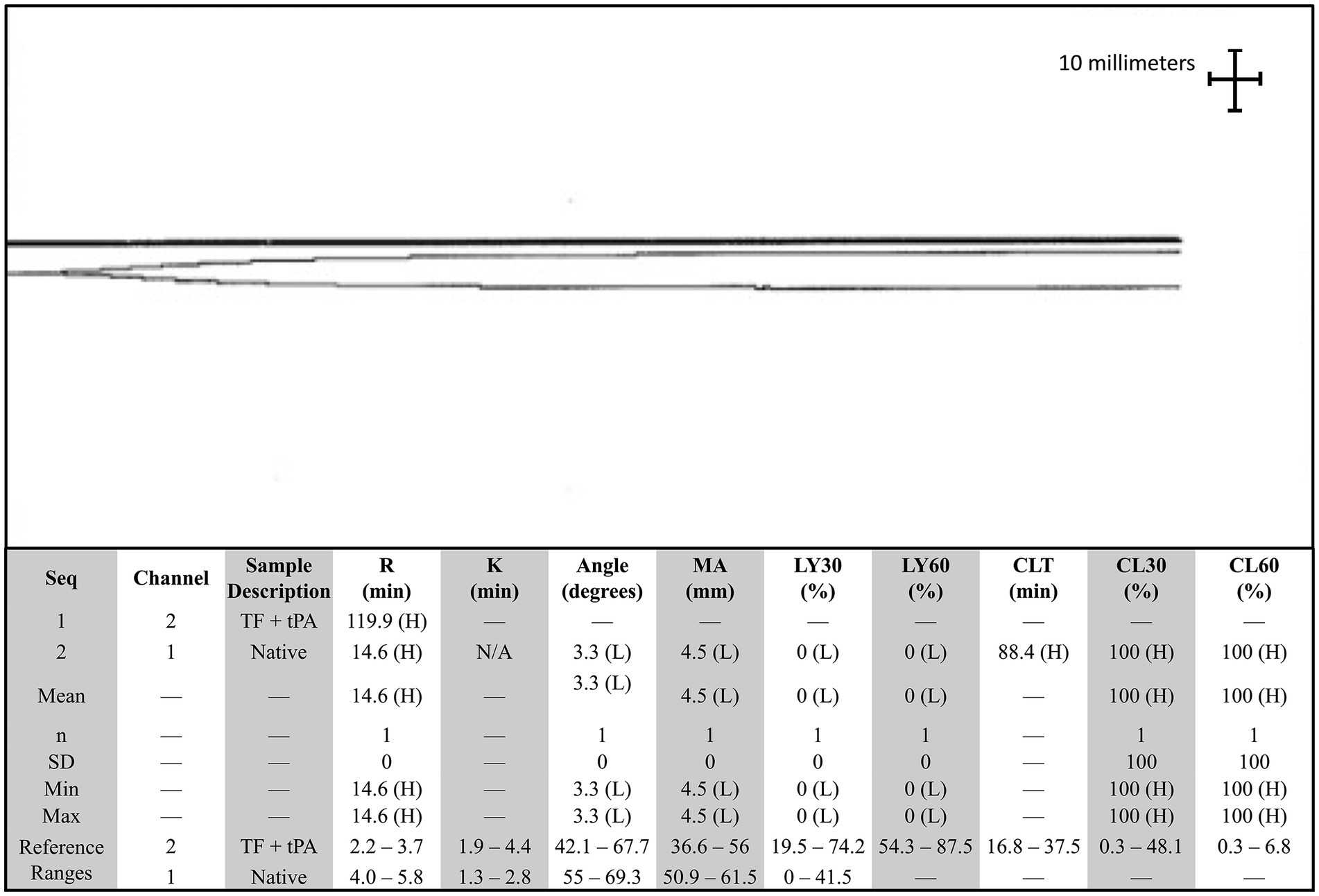
Figure 1. Native and tissue-factor + tissue plasminogen activator thromboelastography (TEG) tracing in a dog with persistent epistaxis. The top tracing is the tissue factor and tissue plasminogen activator TEG tracing, and the bottom tracing is the native tracing. A clot was not able to be formed in the presence of tissue plasminogen activator. Angle, Alpha angle; CLT, Clot lysis time; G, Shear elastic modulus; K, Clot formation time; LY30, Clot Lysis at 30 min; LY60, Clot lysis at 60 min; MA, Maximum amplitude; R, Reaction time; SD, Standard deviation; TEG, Thromboelastography; TF, Tissue factor; tPA, Tissue plasminogen activator.
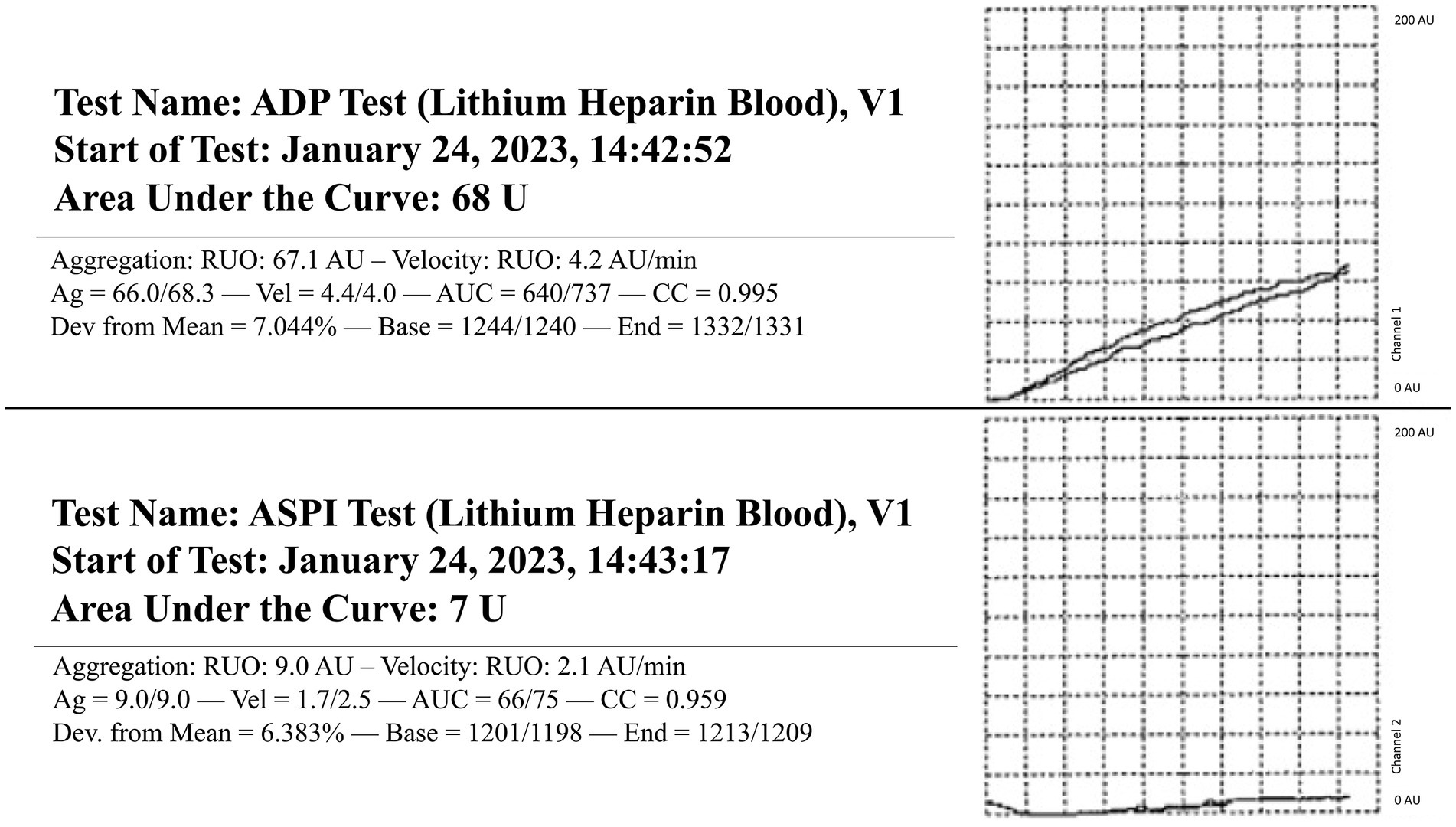
Figure 2. Whole blood impedance platelet aggregometry in a dog with persistent epistaxis. Two platelet agonists were evaluated: adenosine diphosphate (ADP) and arachidonic acid (ASPI). RI, Reference interval. ADP RI (175–294), ASPI RI (131–325).
The next day following discharge, the dog was represented for severe lethargy, bilateral epistaxis, and new serosanguinous oral discharge. Given the poor prognosis and perceived decline in quality of life, the owner elected for humane euthanasia. Before euthanasia, additional blood was sampled for specific coagulation factor, fibrinogen, and von Willebrand factor analysis which revealed normal factor II and von Willebrand factor, decreased factors VII, VIII, and X, and a severely decreased fibrinogen (Table 2). Necropsy was performed which found metastatic nasal adenocarcinoma in the nasal sinuses, regional lymph nodes, and lungs. The nasal mass, located within the nasal sinus, was decalcified and found to contain neoplastic epithelial cells, accompanied by significant osteonecrosis characterized by irregular bony trabeculae lacking osteocytes within lacunae, as well as scalloped trabeculae displaying deeply basophilic reversal lines. Additionally, the nasal turbinates showed an invasive, densely cellular mass of neoplastic epithelial cells, featuring solid and occasionally tubular formations, moderate anisocytosis and anisokaryosis, high mitotic activity (>100 per 10 high-power fields), multifocal necrosis with associated inflammatory infiltrate, and scattered regions of well-differentiated respiratory epithelium and submucosa. Examination of the right retropharyngeal lymph node revealed that nodal architecture is expanded by a multilobulated mass comprised of neoplastic epithelial cells, which are similar to those of the nasal mass and turbinates. In the lung, focally, the pulmonary parenchyma is expanded by a nodule comprised of neoplastic epithelial cells. There are multifocal tumor emboli within the lymphovascular structures and pulmonary vessels. In the liver, chronic moderate midzonal to periportal hepatocellular degeneration with a focal intravascular tumor embolus was identified.
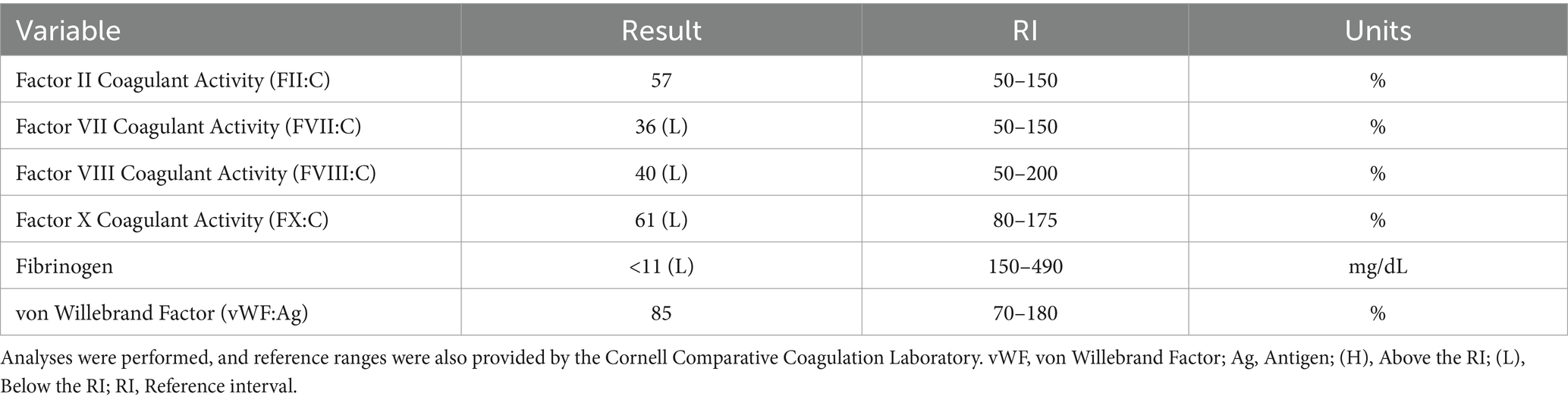
Table 2. Specific factor activity levels, fibrinogen, and vWF analysis in a dog with persistent epistaxis.
Discussion
Disseminated intravascular coagulopathy is a complex disorder characterized by abnormal hemostatic activation, with clinical manifestation depending on the cause. Laboratory diagnosis of DIC is characterized by decreased platelet count, prolonged PT, prolonged aPTT, decreased plasma fibrinogen concentration, decreased plasma antithrombin (AT) activity, increased D-dimers, and increased plasma FDP (7, 8). While these criteria help identify DIC in veterinary medicine, this description does not encompass the various DIC phenotypes observed in human medicine. The International Society on Thrombosis and Hemostasis (ISTH) characterizes two distinct phenotypes of DIC: thrombotic and fibrinolytic. The thrombotic form often leads to organ dysfunction due to clotting, while the fibrinolytic form causes severe bleeding due to excessive clot breakdown (9, 10). In many cases, hemostatic abnormalities can precede clinical and diagnostic evidence of DIC and are reportedly triggered by a multitude of causes, including cancer (11–13). In veterinary medicine, such severe bleeding secondary to hyperfibrinolysis has not been reported in association with disseminated malignancy.
Primary sinonasal neoplasms are relatively uncommon, accounting for less than 2% of all known canine cancers (14, 15). The typical clinical signs include epistaxis, facial deformities, regional lymphadenopathy, and nasal airflow obstruction (16). Fortunately, these clinical signs often respond well with appropriate therapy. Interestingly, Maruyama et al. (7) have observed an association between DIC and dogs with solid malignant tumors, occurring in 9.6% of cases (20/208). Among these cases, 18 dogs were diagnosed with nasal adenocarcinoma, and the majority (16 of 18) showed no clinical or clinicopathologic evidence of DIC (7). This data suggest that delayed, spontaneous severe bleeding in dogs with nasal adenocarcinoma is uncommon.
When considering the present case report, approximately 5 months after radiotherapy, the first bleeding event was noted after tumor biopsy. At that time, no coagulation or platelet function testing was performed, and the excessive bleeding ceased overnight without any additional interventions. When the dog was represented 9 months after radiotherapy for persistent epistaxis, coagulation testing was suggestive of DIC and hyperfibrinolysis (Table 1). In human medicine, malignancy-associated DIC is categorized as subacute, acute, and chronic and has been most often associated with prostatic adenocarcinomas (4–6, 17–19). While the mechanisms underlying the association between cancer and DIC are multifactorial and partially understood, the most prominent theory centers on uPA acting as a primary agent in malignancy-associated DIC (19). uPA has been associated with cancer cell migration, invasion, and modulation of cell adhesion, thereby promoting the formation and progression of various cancers (19, 20). uPA is also known to catalyze the conversion of plasminogen into plasmin without negative feedback, resulting in the breakdown of fibrin clots and the degradation of the extracellular matrix (18, 21, 22). This pathogenesis results in the development of secondary hyperfibrinolysis as the resultant hypofibrinogenemia is the result of imbalanced excessive activation of structurally normal fibrinolytic enzymes or enhanced susceptibility of fibrin to proteolysis (23). In this case, the dog also had deficiencies in factors VII, VIII, and X, along with severe hypofibrinogenemia, all of which are suggestive of an impaired ability to form a clot and hemostatic dysfunction (Table 2). Moreover, the reduction in factor VIII specifically has been associated with upregulation of the fibrinolytic system, resulting in secondary hyperfibrinolysis (23). Thus, when taken in tandem with the dog’s history of confirmed metastatic adenocarcinoma, thrombocytopenia, regenerative anemia, hypocoagulable profile on TEG (Figure 1), and decreased platelet aggregation on impedance aggregometry (Figure 2), the most likely cause for the dog’s progressive clinical decline is chronic DIC with a concurrent paraneoplastic secondary hyperfibrinolysis, a rare diagnosis in veterinary medicine (21).
When exploring other reasons for severe bleeding, severe hypofibrinogenemia is unusual and rarely linked to neoplasia in veterinary medicine or more specifically with dogs with nasal adenocarcinoma. Differentials such as primary hyperfibrinolysis, acquired hypofibrinogenemia, and late-radiation toxicity were considered as potential causes for the dog’s excessive bleeding. In this case, the dog had severe hypofibrinogenemia, which could represent primary hyperfibrinolysis, or an absolute quantitative or qualitative abnormality of proteins was directly involved in the fibrinolytic process (23). However, primary hyperfibrinolysis typically occurs with increased FDP values and normal D-dimers, which would not fit with coagulation abnormalities of the dog. Moreover, TEG analysis which is indicative of primary hyperfibrinolysis would showcase rapid clot formation and subsequent rapid clot lysis (24). In this case, this dog’s TEG profile revealed a prolonged initiation to clot formation, delayed clot kinetics, and a weak clot, consistent with fibrin degradation and DIC or secondary hyperfibrinolysis, further supporting the notion of a secondary hyperfibrinolysis, as observed in the context of underlying malignancy-induced coagulopathy. Next, due to cytotoxic agents (chemotherapy), hypofibrinogenemia is considered as it has been linked to acute myeloid leukemia in people (25). Notably, this phenomenon has never been reported in veterinary medicine. Moreover, in this case, the dog had not received any cytotoxic chemotherapeutic agents. Instead, immunotherapy and radiation therapy had been pursued for approximately 9 months prior to the bleeding events, and thus, it was also excluded as a differential. Other causes for this type of severe hypofibrinogenemia and other coagulation abnormalities could have included synthetic liver failure, but clinically, diagnostically, or histologically there was no evidence to support this diagnosis. Finally, we also considered late radiation toxicity after radiotherapy, but it was deemed unlikely due to the delayed onset of bleeding, unusual tissue response, and the presence of other clotting abnormalities. Irrespective of the etiology, the treatment of choice for hypofibrinogenemia is cryoprecipitate, which contains factor VIII, von Willebrand factor (vWF), fibrinogen, factor XIII, and fibronectin (26). In this case, treatments such as cryoprecipitate infusions, multiple FFP transfusions, and several days of ACA administration were short lived, further reinforcing the diagnosis of severe secondary paraneoplastic hyperfibrinolysis (27–29).
Finally, regenerative anemia and mild thrombocytopenia of the dog could have represented a response to ongoing hemorrhage experienced for multiple days. The decreased platelet aggregability that was noted in this patient is suspected to be related to several factors such as a consumptive process from the severe epistaxis, an in vitro change due to the thrombocytopenia, and platelet dysfunction secondary to metastatic neoplasia (30–33).
Conclusion
This case report presents the first documented instance of paraneoplastic secondary hyperfibrinolysis in a dog with metastatic nasal adenocarcinoma, necessitating multiple blood product transfusions and a prolonged course of ACA due to severe and recurrent bleeding. Although coagulopathies account for less than 5% of cases in dogs with epistaxis (34), the literature suggests that coagulopathies occur in approximately 56% of dogs with malignant neoplasia (7). As such, clinicians should consider systematic coagulation testing in patients with malignant neoplasia presenting with acute bleeding episodes. Such coagulation profiles can guide the administration of anti-fibrinolytic medications and blood products.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because this animal study is a case report. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
KG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. TP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. M-KB: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – review & editing. LG: Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the small animal diagnostic imaging service at the James L. Voss Veterinary Teaching Hospital for their initial interpretation of radiographs, ultrasound, and computed tomography, as well as the anatomic pathology service for their post-mortem analysis and interpretation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iba, T, Levy, JH, Thachil, J, Wada, H, and Levi, M. The progression from coagulopathy to disseminated intravascular coagulation in representative underlying diseases. Thromb Res. (2019) 179:11–4. doi: 10.1016/j.thromres.2019.04.030
2. Camerer, E, Qazi, AA, Duong, DN, Cornelissen, I, Advincula, R, and Coughlin, SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. (2004) 104:397–401. doi: 10.1182/blood-2004-02-0434
3. Okajima, K, Kohno, I, Soe, G, Okabe, H, Takatsuki, K, and Binder, BR. Direct evidence for systemic fibrinogenolysis in patients with acquired α2-plasmin inhibitor deficiency. Am J Hematol. (1994) 45:16–24. doi: 10.1002/ajh.2830450104
4. Kulić, A, Cvetković, Z, and Libek, V. Primary hyperfibrinolysis as the presenting sign of prostate cancer: a case report. Vojnosanit Pregl. (2016) 73:877–80. doi: 10.2298/VSP150525076K
5. Jafri, MA, Cohen, JV, Much, MA, Petrylak, DP, and Podoltsev, NA. A patient with pancytopenia, intractable epistaxis, and metastatic prostate cancer: how correct diagnosis of primary hyperfibrinolysis helps to stop the bleeding. Clin Genitourin Cancer. (2016) 14:e545–8. doi: 10.1016/j.clgc.2016.05.002
6. Hyman, DM, Soff, GA, and Kampel, LJ. Disseminated intravascular coagulation with excessive fibrinolysis in prostate cancer: a case series and review of the literature. Oncology. (2011) 81:119–25. doi: 10.1159/000331705
7. Maruyama, H, Miura, T, Sakai, M, Koie, H, Yamaya, Y, Shibuya, H, et al. The incidence of disseminated intravascular coagulation in dogs with malignant tumor. J Vet Med Sci. (2004) 66:573–5. doi: 10.1292/jvms.66.573
8. Stokol, T . Plasma D-dimer for the diagnosis of thromboembolic disorders in dogs. Vet Clin Small Anim Pract. (2003) 33:1419–35. doi: 10.1016/S0195-5616(03)00096-2
9. Wada, T, and Gando, S. Phenotypes of disseminated intravascular coagulation. Thromb Haemost. (2023) 100:958–9.
10. Levi, M . Disseminated intravascular coagulation in cancer: an update In: Seminars in Thrombosis and Hemostasis. New York, NY, USA: Thieme Medical Publishers (2019).
11. Falanga, A, Panova-Noeva, M, and Russo, L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. (2009) 22:49–60. doi: 10.1016/j.beha.2008.12.009
12. Lu, G, Jia, L, Yang, R, Lv, Z, and Cui, J. Acquired hyperfibrinolysis as the presenting sign of metastatic breast cancer: a case report. Oncol Lett. (2023) 25:1–6. doi: 10.3892/ol.2023.13692
13. Winther-Larsen, A, Sandfeld-Paulsen, B, and Hvas, A-M. Hyperfibrinolysis in patients with solid malignant neoplasms: a systematic review In: Seminars in Thrombosis and Hemostasis, New York, NY 10001, USA: Thieme Medical Publishers, Inc. (2021). vol. 47:581–8.
14. Madewell, B, Priester, W, Gillette, E, and Snyder, S. Neoplasms of the nasal passages and paranasal sinuses in domesticated animals as reported by 13 veterinary colleges. Am J Vet Res. (1976) 37:851–6.
15. Mortier, J, and Blackwood, L. Treatment of nasal tumours in dogs: a review. J Small Anim Pract. (2020) 61:404–15. doi: 10.1111/jsap.13173
16. Strasser, JL, and Hawkins, EC. Clinical features of epistaxis in dogs: a retrospective study of 35 cases (1999–2002). J Am Anim Hosp Assoc. (2005) 41:179–84. doi: 10.5326/0410179
17. Smith, JA Jr, Soloway, MS, and Young, MJ. Complications of advanced prostate cancer. Urology. (1999) 54:8–14. doi: 10.1016/S0090-4295(99)00448-3
18. Prokopchuk-Gauk, O, and Brose, K. Tranexamic acid to treat life-threatening hemorrhage in prostate cancer associated disseminated intravascular coagulation with excessive fibrinolysis. Cureus. (2015) 7:e428. doi: 10.7759/cureus.428
19. Ong, SY, Taverna, J, Jokerst, C, Enzler, T, Hammode, E, Rogowitz, E, et al. Prostate cancer-associated disseminated intravascular coagulation with excessive fibrinolysis treated with degarelix. Case Rep Oncol Med. (2015) 2015:1–6. doi: 10.1155/2015/212543
20. Mahmood, N, Mihalcioiu, C, and Rabbani, SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. (2018) 8:24. doi: 10.3389/fonc.2018.00024
21. Zoia, A, Drigo, M, Caldin, M, Simioni, P, and Piek, CJ. Fibrinolysis in dogs with Intracavitary effusion: a review. Animals. (2022) 12:2487. doi: 10.3390/ani12192487
22. Chapin, JC, and Hajjar, KA. Fibrinolysis and the control of blood coagulation. Blood Rev. (2015) 29:17–24. doi: 10.1016/j.blre.2014.09.003
23. Kolev, K, and Longstaff, C. Bleeding related to disturbed fibrinolysis. Br J Haematol. (2016) 175:12–23. doi: 10.1111/bjh.14255
24. Carey, M, and Cressey, DM. Hyperfibrinolysis–is it common? Measurement and treatment including the role of thromboelastography. Clin Risk. (2009) 15:188–91. doi: 10.1258/cr.2009.090028
25. Elamil, ZG, Tuncer, HH, Tara, R, and Homsi, SAA. Consistent Hypofibrinogenemia associated with induction anti-tumor chemotherapy for acute myeloid leukemia. Am Soc Hematol. (2010) 116:1400. doi: 10.1182/blood.V116.21.1400.1400
26. Pantanowitz, L, Kruskall, MS, and Uhl, L. Cryoprecipitate: patterns of use. Am J Clin Pathol. (2003) 119:874–81. doi: 10.1309/56MQVQAQG8YU90X9
27. Plaut, D . The laboratory's role in disseminated intravascular coagulation. J Contin Educ Top Issues. (2011) 13:24–9.
28. Fisher, VR, Scott, MK, Tremblay, CA, Beaulieu, GP, Ward, DC, and Byrne, KM. Disseminated intravascular coagulation: laboratory support for management and treatment. Lab Med. (2013) 44:e10–4. doi: 10.1309/LMTCAVNHBUGWTP7K
29. Toh, C-H, Alhamdi, Y, and Abrams, ST. Current pathological and laboratory considerations in the diagnosis of disseminated intravascular coagulation. Ann Lab Med. (2016) 36:505–12. doi: 10.3343/alm.2016.36.6.505
30. Crispin, P, and Gardiner, EE. Platelets and cancer… the plot doesn’t always thicken. J Thromb Haemost. (2020) 18:2482–5. doi: 10.1111/jth.14944
31. Braun, A, Anders, H-J, Gudermann, T, and Mammadova-Bach, E. Platelet-cancer interplay: molecular mechanisms and new therapeutic avenues. Front Oncol. (2021) 11:665534. doi: 10.3389/fonc.2021.665534
32. Sweeney, JD, Labuzetta, JW, and Fitzpatrick, JE. The effect of the platelet count on the aggregation response and adenosine triphosphate release in an impedance lumi-aggregometer. Am J Clin Pathol. (1988) 89:655–9. doi: 10.1093/ajcp/89.5.655
33. Müller, MR, Salat, A, Pulaki, S, Stangl, P, Ergun, E, Schreiner, W, et al. Influence of hematocrit and platelet count on impedance and reactivity of whole blood for electrical aggregometry. J Pharmacol Toxicol Methods. (1995) 34:17–22. doi: 10.1016/1056-8719(94)00075-F
34. Bissett, SA, Drobatz, KJ, McKnight, A, and Degernes, LA. Prevalence, clinical features, and causes of epistaxis in dogs: 176 cases (1996–2001). J Am Vet Med Assoc. (2007) 231:1843–50. doi: 10.2460/javma.231.12.1843
Glossary
Keywords: paraneoplastic, DIC, secondary hyperfibrinolysis, adenocarcinoma, dog
Citation: Granger KL Jr, Paulos T, Boss M-K, Guieu L and Shropshire S (2024) Case report: Chronic disseminated intravascular coagulopathy with concurrent paraneoplastic secondary hyperfibrinolysis in a dog with metastatic nasal adenocarcinoma. Front. Vet. Sci. 11:1375507. doi: 10.3389/fvets.2024.1375507
Edited by:
Carlos Eduardo Fonseca-Alves, Paulista University, BrazilReviewed by:
Alex Lynch, North Carolina State University, United StatesJulie Michele Walker, University of Wisconsin-Madison, United States
Copyright © 2024 Granger, Paulos, Boss, Guieu and Shropshire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle L. Granger Jr., a3lsZS5ncmFuZ2VyQGNvbG9zdGF0ZS5lZHU=
†These authors have contributed equally to this work
 Kyle L. Granger Jr.
Kyle L. Granger Jr. Trish Paulos†
Trish Paulos† Mary-Keara Boss
Mary-Keara Boss