- 1Key Laboratory of Livestock Disease Prevention of Guangdong Province, Key Laboratory of Avian Influenza and Other Major Poultry Diseases Prevention and Control, Ministry of Agriculture and Rural Affairs, Institute of Animal Health, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 2Wen’s Group Academy, Wen’s Foodstuffs Group Co., Ltd., Xinxing, Guangdong, China
Coccidiosis is a costly intestinal disease of chickens caused by Eimeria species. This infection is associated with high mortality, reduced feed efficiency, and slowed body weight gain. The diagnosis and control of coccidiosis becomes challenging due to the fact that chickens can be infected by seven different Eimeria species and often occur mixed-species co-infections. Grasping the epidemiology of Eimeria species is crucial to estimate the efficiency of poultry management. This study aimed to explore the distribution of Eimeria species in broiler chickens in China after administering live anticoccidial vaccines. A total of 634 samples were obtained, and the survey results showed that the prevalence of Eimeria was 86.12% (546/634), and the most common species were E. acervulina (65.62%), E. necatrix (50.95%), E. mitis (50.79%), E. tenella (48.42%), and E. praecox (41.80%). Most samples indicated mixed-species infections (an average of 3.29 species per positive sample). Notably, 63.98% of samples contain 3 to 5 Eimeria species within a single fecal sample. The most prevalent combinations were E. acervulina–E. tenella (38.96%) and E. acervulina–E. necatrix (37.22%). Statistical analysis showed that flocks vaccinated with trivalent vaccines were significantly positive for E. necatrix in grower chickens (OR = 3.30, p < 0.05) compared with starter chickens, and tetravalent vaccinated flocks showed that starter chickens demonstrated a higher susceptibility to E. tenella–E. brunetti (OR = 2.03, p < 0.05) and E. acervulina–E. maxima (OR = 2.05, p < 0.05) compared with adult chickens. Geographically, in the case of tetravalent vaccine-immunized flocks, a substantial positive association was observed between E. necatrix infection rates and flocks from eastern (OR = 3.88, p < 0.001), central (OR = 2.65, p = 0.001), and southern China (OR = 3.17, p < 0.001) compared with southwestern China. This study also found a positive association between E. necatrix (OR = 1.64, p < 0.05), E. acervulina (OR = 1.59, p < 0.05), and E. praecox (OR = 1.81, p < 0.05) infection and coccidiosis occurrence compared with non-infected flocks in tetravalent vaccinated flocks. This molecular epidemiological investigation showed a high prevalence of Eimeria species in the field. The emergent species, E. brunetti and E. praecox, might be incorporated into the widely-used live vaccines in the future. These insights could be useful in refining coccidiosis control strategies in the poultry industry.
1 Introduction
Coccidiosis is a worldwide intestinal disease of chickens caused by protozoan parasites belonging to the genus Eimeria (1). Seven Eimeria species have been well-known to be responsible for avian coccidiosis (2, 3), and recently, three cryptic species designated as Operational Taxonomic Units (OTUs) X, Y, and Z, have been suggested and assigned by Blake et al. (4). These species of Eimeria exhibit varying degree of pathogenicity, with E. necatrix standing out as the most virulent species, while E. tenella is more prevalent, both inflicting bloody lesions, high morbidity, and high mortality in naïve chickens (5); E. brunetti is highly pathogenic and associated with hemorrhagic coccidiosis (6); E. acervulina, E. maxima, E. mitis, and E. praecox are usually less pathogenic, incurring malabsorption and enteritis (6). Avian coccidiosis can cause substantial financial losses, with an estimated global cost to the poultry industry approximately £10.36 billion annually (1).
Currently, the primary methods of controlling coccidiosis encompass preventative chemotherapy using in-feed anticoccidial drugs and live vaccines. However, the emergence of drug resistance in various regions worldwide has led to a reduction in the efficacy of anticoccidial agents (7). Additionally, concerns regarding the efficacy of live vaccine have been on the rise (8). In China, three types of live anticoccidial vaccines are available for use (National Veterinary Drug Basic Database Online),1 in which two of them are attenuated (namely a trivalent vaccine containing E. tenella, E. acervulina, and E. maxima and a tetravalent vaccine containing E. tenella, E. necatrix, E. acervulina, and E. maxima). Another one is non-attenuated, known as Coccivac™ containing E. maxima, E. mivati, E. acervulina, and E. tenella. To evaluate the efficiency of poultry management, including the selection of Eimeria type in vaccines, a comprehensive understanding of the epidemiology of Eimeria species is essential.
Previous reports have monitored the patterns of oocyst accumulation in litter following vaccination (8, 9). The results have revealed a notable increase, marked by a small peak in the reproduction of vaccine strains between 2 and 4 weeks after vaccination at 1 week, followed by another slightly higher peak occurred at 4–7 weeks, which represented a challenge from the local virulent population. Subsequently, oocyst production reduced due to flock immunity after approximately 7 or 8 weeks, with no detectable oocysts remaining in the litter using conventional techniques. Similarly, changes in Eimeria oocyst concentration and species composition in litter of broiler farms subjected to various cycles of anticoccidial drug or live Eimeria oocyst vaccine control have been reported by Jenkins et al. (9). The influence of anticoccidial methods on the presence and composition of Eimeria species in the litter has also been underscored (10). These studies suggest that understanding the species composition in litter during live vaccine control may serve as a means for assessing the efficacy of a particular control program. However, comprehensive data regarding the prevalence and risk factors associated with Eimeria infection in broiler chickens administrated with live anticoccidial vaccines in China have been lacking.
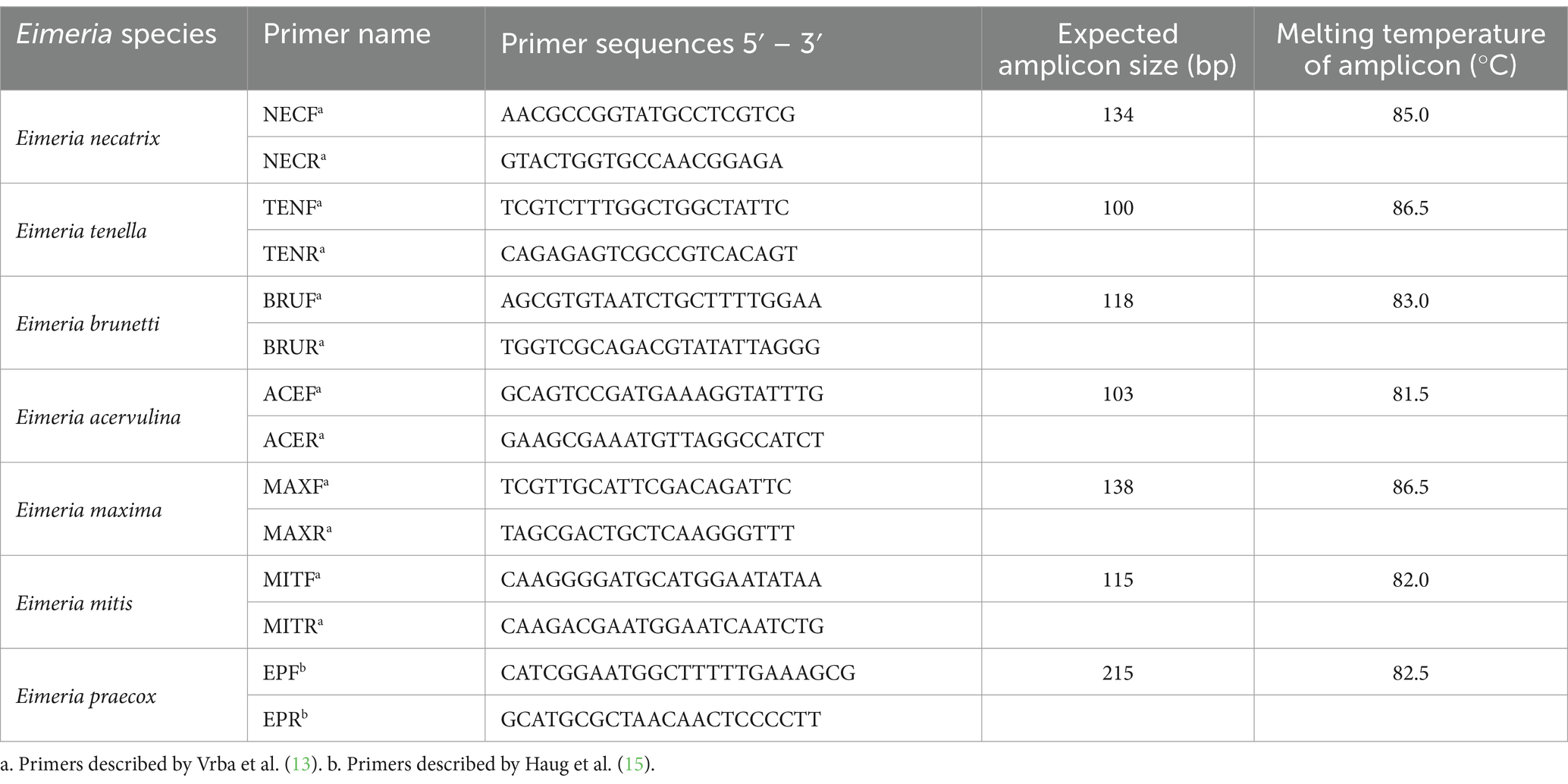
Traditional classification of Eimeria species depends on morphological characteristics, the affected region of the intestinal tract, and the pre-patent period of Eimeria after passage through experimentally infected chickens. However, these methods may not achieve a species-specific diagnosis due to overlap in these characteristics among certain species (11). Recently, polymerase chain reaction (PCR) was used to identify all seven avian Eimeria species. These techniques employ genetic markers in the internal transcribed spacer-1 (ITS-1) (12, 13), ITS-2 (14), and sequence characterized amplified region (SCAR) (12). In the present study, we applied quantitative real-time PCR (qPCR) to conduct a comprehensive survey aimed at assessing the distribution of Eimeria species in broiler flocks vaccinated with live vaccine across China. This survey was designed to determine the prevalence of Eimeria infection and the risk factors in flocks.
2 Materials and methods
2.1 Sample area and farms
The study was conducted from September to November 2022, encompassing a research area that spanned 15 provinces. The region extends between 20°09′–38°24′ north latitude and 97°21′–123°10′ east longitude, located in southern part of China, covering an area of 2,920,300 km2. The provinces in the eastern China experienced temperate and subtropical monsoon climates and subtropical humid climates. Central China had temperate and subtropical monsoon climates, while southern China had subtropical monsoon climates characterized by long summer. In southwestern China, the climates varied, encompassing subtropical humid climates, temperate and subtropical monsoon climates, and mountain climates. During the autumn of 2022, the monthly average temperature ranged from 14 to 25°C. The relative humidity levels fluctuated between 62 and 79%, while average rainfall varied approximately from 30.03 mm to 62.50 mm, as reported by World Weather Online.2 This study involved 49 broiler farms, with 14 located in eastern China, 6 in central China, 18 in southern China, and 11 in southwestern China (Figure 1). Each farm was equipped with 2–20 houses, housing between 2000 and 11,000 birds at a density ranging from 10 to 14 birds/m2. The litter material used was either wood shavings or rice husk. The most common broiler breeds were yellow-feathered chickens, followed by hybrid broilers. All these farms administrated bio-shuttle program to prevent and control coccidiosis. Live anticoccidial vaccines were administrated in the drinking water between 3 and 5 days of age. The trivalent vaccine comprising E. tenella, E. acervulina, and E. maxima or tetravalent vaccine containing E. tenella, E. necatrix, E. acervulina, and E. maxima were used in this study. An anticoccidial drug (e.g., nicarbazin, maduramicin, diclazuril, monensin, or narasin) was added to the grower feed to decrease the adverse effects on performance during peak lesion and oocyst shedding.
Figure 1. Geographic location of the sampling sites in China. Samples from Eastern, Central, Southern, and Southwestern China are shaded as indicated. This image was obtained from the Ministry of Natural Resources of the People’s Republic of China, with drawing review number [GS(2022)4301]. Reproduced with permission.
2.2 Study flocks and samples
We collected fecal samples from 9 to 18 flocks per farm. Flocks from each farm were subsequently categorized into three groups according to the age: starter (1–4 weeks), grower (5–8 weeks), and adult (over 8 weeks). Approximately one-third of the samples were selected from each group on each farm. Each individual sample weighed approximately 250 g and was consisted of 30 fresh fecal droppings collected randomly from various locations with each poultry house. A total of 634 fecal samples were collected from 49 farms and placed in labeled zipped plastic bags. All the samples were sent immediately to the laboratory and stored at 4°C for further use.
2.3 Genomic DNA extraction from samples
Before extracting DNA, each sample was added to an equal volume of sterile ddH2O and was homogenized using a blender. Aliquots of 200 μL were transferred to 1.5 mL Eppendorf tubes for DNA extraction. Genomic DNA extraction was performed using the E.Z.N.A.® Stool DNA Kit, according to the manufacturer’s protocol (Omega, D4015). The resulting DNA was stored at −20°C until further analysis.
2.4 Molecular characterization of Eimeria species using qPCR
Identification of Eimeria species was accomplished via qPCR using species-specific primers (Table 1), as previously described by Vrba et al. (13) and Haug et al. (15), with some modifications. For each sample, the total volume of 20 μL was mixed containing 10 μL of TB Green Premix Ex Taq II (Takara, RR820B), 1 μL (100 nm) of species-specific forward and reverse primers, 2 μL of DNA sample, and 6 μL of ultra-pure H2O. The amplification process was performed using CFX Connect™ Real-Time PCR System (Bio-Rad, United States), employing a cycling condition that commenced with an initial hold at 95°C for 30 s, followed by 40 repeat cycles of hold at 95°C for 5 s and annealing at 60°C for 30 s. Upon the completion of the cyclic amplification, a melting curve analysis ranging from 65°C to 95°C was determined.
2.5 Statistical analysis
All statistical analyses were performed using software IBM SPSS Statistics 27.0 (SPSS Inc.).3 Descriptive statistics including bird age, coccidiosis vaccination status, and the presence of clinical signs (e.g., bloody feces, gross lesions of Eimeria species) of coccidiosis were obtained from the questionnaires. Initially, the prevalence of Eimeria spp. infections with a 95% confidence interval (CI) was calculated. Subsequently, predictor variables associated with the presence of Eimeria spp. were assessed using univariable logistic regression models. Chi-square test or Fisher’s exact test was used to compare the prevalence of one or more Eimeria infection in variables according to age, vaccination, clinical sign, and region. Odds ratio (OR, with 95% CI) was calculated to assess the associations between participants’ characteristics and Eimeria spp. infection. Data with p values ≤0.05 were considered as statistical significance.
3 Results
3.1 Eimeria species occurrence
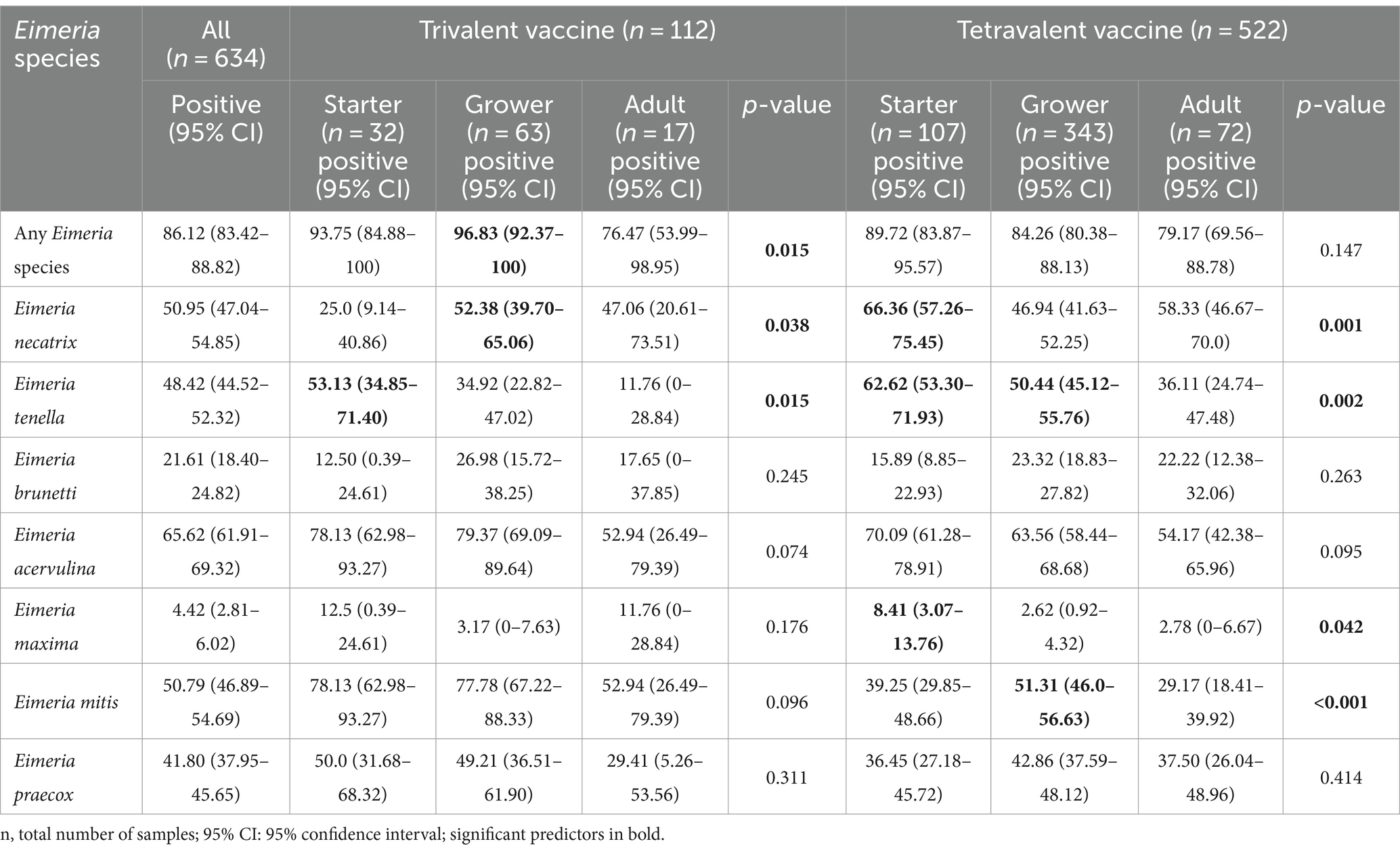
A total of 634 samples were collected from broiler farms across four regions in China (Figure 1). Out of these, 546 (86.12%) from 634 flocks were identified to be positive for one or more Eimeria species using species-specific qPCR (Table 2). All seven Eimeria species were detected in chickens vaccinated with either trivalent or tetravalent live vaccines with different detection rates, among which E. acervulina (65.62%), E. necatrix (50.95%), E. mitis (50.79%), E. tenella (48.42%), and E. praecox (41.80%) were the most common species in China. However, the prevalence of E. maxima (4.42%) was much lower than the other six Eimeria species, which ranged from 21.61 to 65.62%. In flocks that used trivalent vaccines, the distribution of E. necatrix in grower flocks (52.38%, p < 0.05) was more widespread than those in starter flocks, and E. tenella in starter flocks (53.13%, p < 0.05) was more widespread than those in adult flocks. In flocks that used tetravalent vaccines, the prevalence of E. necatrix was significantly higher in starter flocks (66.36%, p = 0.001) compared with grower flocks, as well as the prevalence of E. tenella in both starter flocks (62.62%, p < 0.05) and grower flocks (50.44%, p < 0.05) was significantly higher compared with adult flocks.
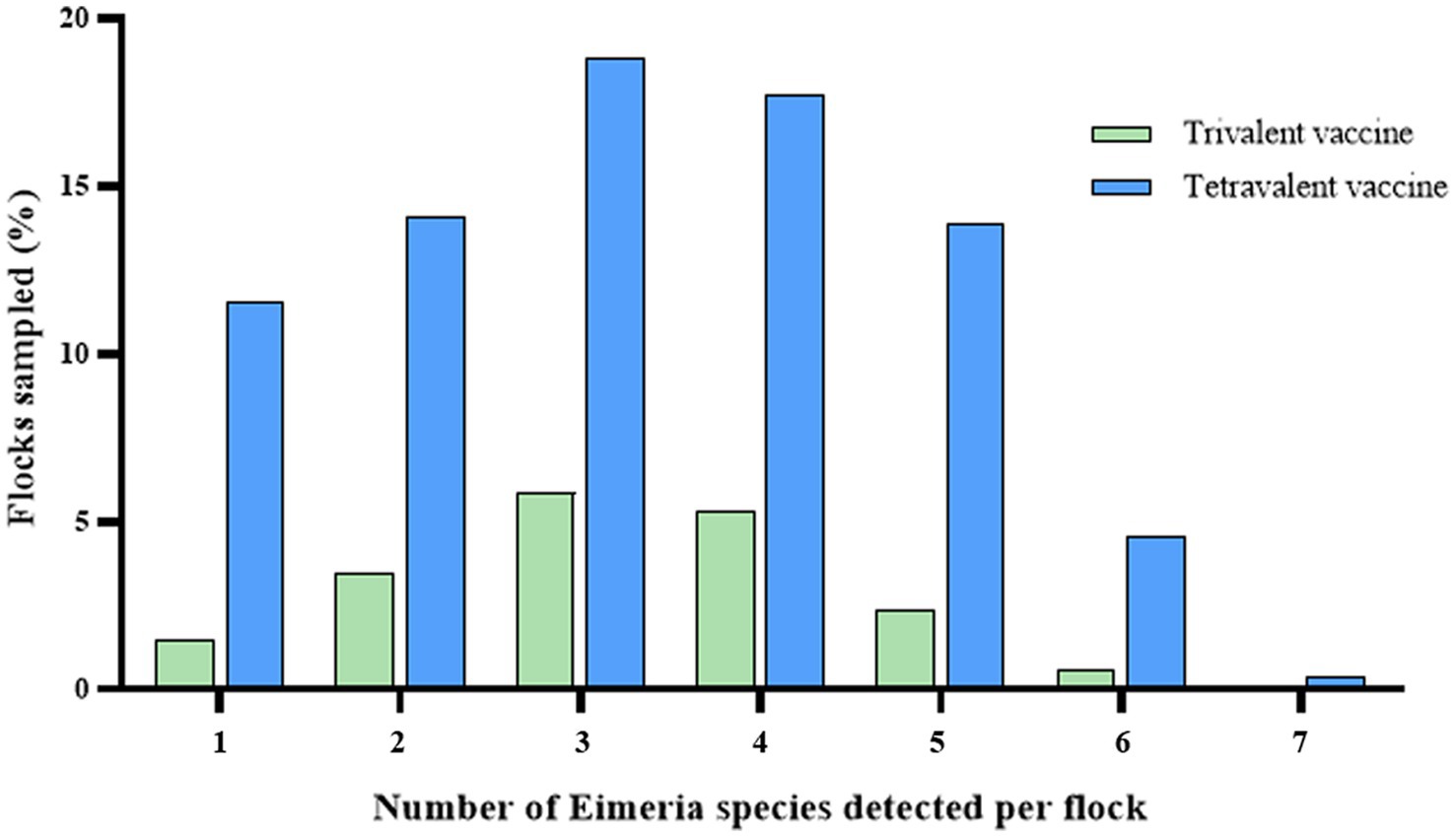
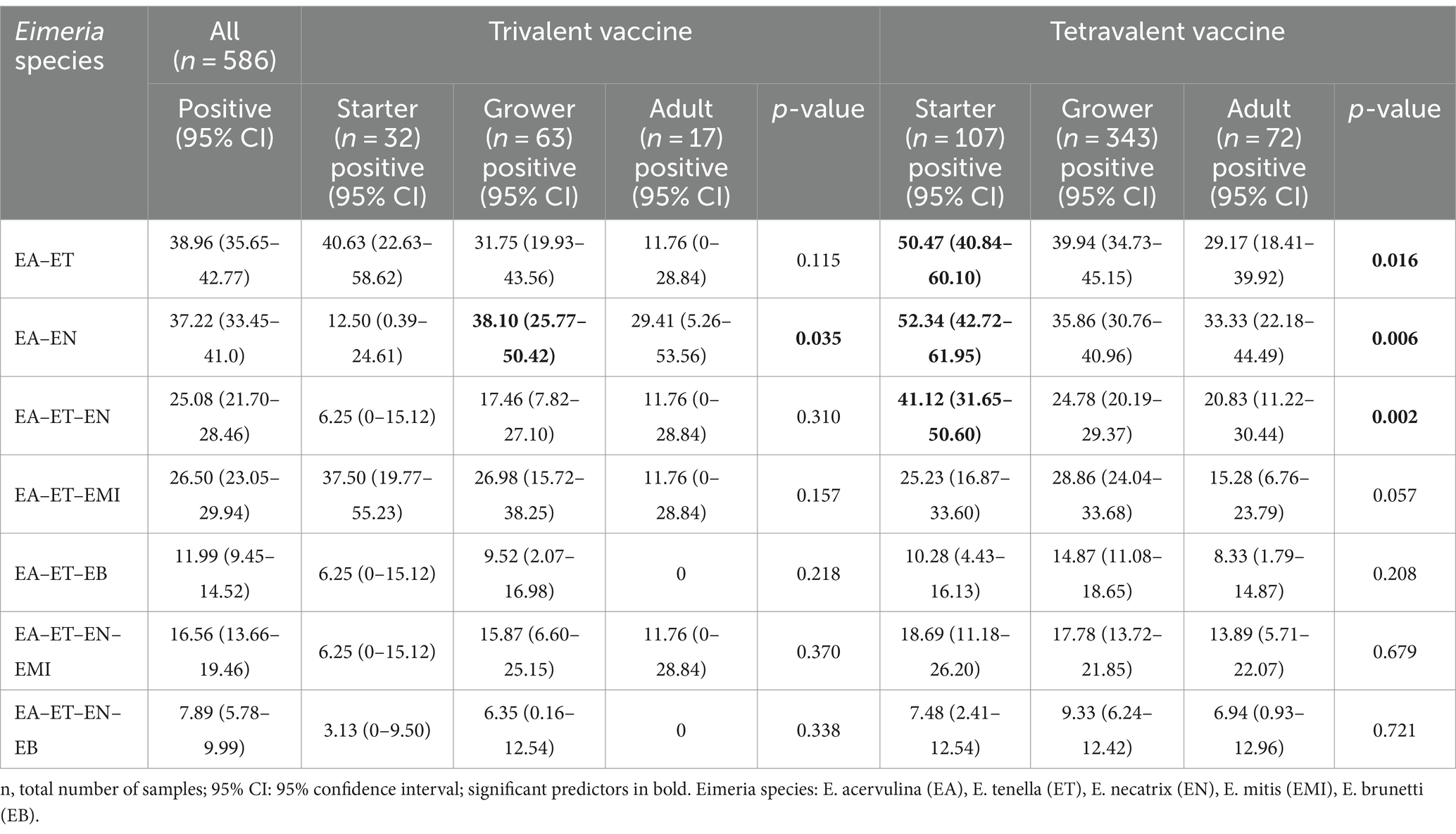
Mixed-species infections were common in China, with an average of 3.29 species per positive sample. Notably, 63.98% of samples contained 3 to 5 Eimeria species within a single fecal sample (Figure 2). The prevalence of mixed infections with three species was higher in both trivalent vaccine-immunized flocks (5.85%) and tetravalent vaccine-immunized flocks (18.83%; Table 3). The most prevalent combinations Eimeria species included E. acervulina–E. tenella (38.96%), E. acervulina–E. necatrix (37.22%), and E. acervulina–E. tenella–E. necatrix (25.08%). Specifically, the prevalence of E. acervulina–E. necatrix was significantly higher in grower flocks (38.10%, p < 0.05) compared with starter flocks within trivalent vaccine-immunized flocks. Furthermore, the prevalence of E. acervulina–E. necatrix (52.34%, p < 0.05), E. acervulina–E. tenella (50.47%, p < 0.05), and E. acervulina–E. tenella–E. necatrix (41.12%, p < 0.05) was significantly higher in starter flocks compared with adult flocks in tetravalent vaccine-immunized flocks.
3.2 Risk factors associated with Eimeria species occurrence
Odds ratio associated with the likelihood of occurrence of Eimeria was calculated based on bird age, presence of clinical sign, and sample region (Table 4). The results of the statistical analysis reveal significant associations between the type of vaccines used and the prevalence of specific Eimeria species. In flocks vaccinated with trivalent vaccines, a significant positive association was observed for E. necatrix infection in grower chickens (OR = 3.30, 95% CI: 1.29–8.45; p < 0.05) compared with starter chickens, and these flocks exhibited a significant positive association with E. mitis–E. praecox infection in both starter chickens (OR = 3.85, 95% CI: 1.05–14.16; p < 0.05) and grower chickens (OR = 4.71, 95% CI: 1.47–15.15; p < 0.05) compared with adult chickens. In the case of tetravalent vaccinated flocks, starter chickens were more likely to be positive for E. tenella–E. brunetti (OR = 2.03, 95% CI: 1.10–3.73; p < 0.05) and E. acervulina–E. maxima (OR = 2.05, 95% CI: 1.10–3.85; p < 0.05) compared with adult chickens, followed by a higher likelihood of positive results for E. mitis–E. praecox infection in grower chickens (OR = 2.0, 95% CI: 1.20–3.34; p < 0.05) compared with adult chickens.
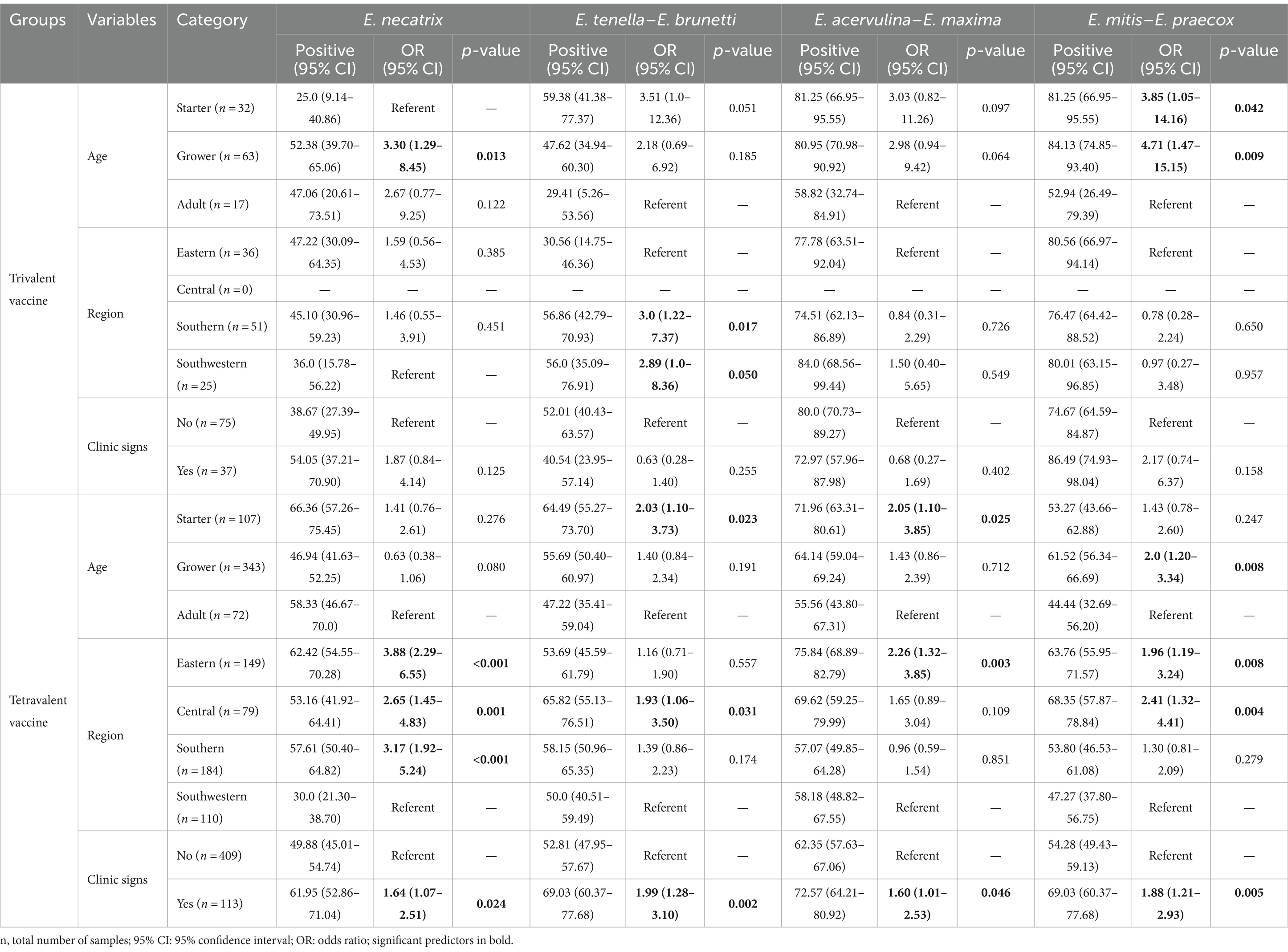
Table 4. Univariable logistic regression analysis of risk factors associated with prevalence of Eimeria species in broiler chickens in China.
Geographically, a significant positive association was identified between E. tenella–E. brunetti infection rate and flocks vaccinated with trivalent vaccine in southern China (OR = 3.0, 95% CI: 1.22–7.37; p < 0.05) and southwestern China (OR = 2.89, 95% CI: 1.0–8.36; p = 0.05) compared with eastern China (Table 4). In the case of tetravalent vaccine-vaccinated flocks, there was a significant positive association between E. necatrix infection and flocks from eastern China (OR = 3.88, 95% CI: 2.29–6.55; p < 0.001), central China (OR = 2.65, 95% CI: 1.45–4.83; p = 0.001), and southern China (OR = 3.17, 95% CI: 1.92–5.24; p < 0.001) compared with southwestern China. Similarly, the tetravalent vaccinated flocks in central China were more likely to be positive for E. tenella–E. brunetti (OR = 1.93, 95% CI: 1.06–3.50; p < 0.05) compared with southwestern China. Interestingly, in flocks vaccinated with tetravalent vaccines, there was a significant positive association between the occurrence of clinical coccidiosis and the prevalence of E. necatrix (OR = 1.64, 95% CI: 1.07–2.51; p < 0.05), E. tenella–E. brunetti (OR = 1.99, 95% CI: 1.28–3.10; p < 0.05), E. acervulina–E. maxima (OR = 1.60, 95% CI: 1.01–2.53; p < 0.05), and E. mitis–E. praecox (OR = 1.88, 95% CI: 1.21–2.93; p < 0.05) compared with flocks without clinical signs.
3.3 Eimeria species profiles associated with coccidiosis occurrence
Univariable logistic regression analysis was performed to identify Eimeria profiles associated with trivalent vaccines or tetravalent vaccines used strategies in broiler flocks. In flocks that used trivalent vaccines, those infected with E. maxima were more likely to develop clinical coccidiosis compared with non-infected flocks (OR = 7.07, 95% CI: 1.35–36.95; p < 0.05; Table 5). Furthermore, in flocks that used tetravalent vaccines, those infected with E. necatrix (OR = 1.64, 95% CI: 1.07–2.51; p < 0.05), E. acervulina (OR = 1.59, 95% CI: 1.01–2.51; p < 0.05), and E. praecox (OR = 1.81, 95% CI: 1.19–2.75; p < 0.05) were more likely to occur clinical coccidiosis compared with non-infected flocks. However, no significant associations were observed between the infection of E. tenella, E. brunetti, and E. mitis and the occurrence of clinical coccidiosis in both trivalent vaccines and tetravalent vaccines used in flocks.
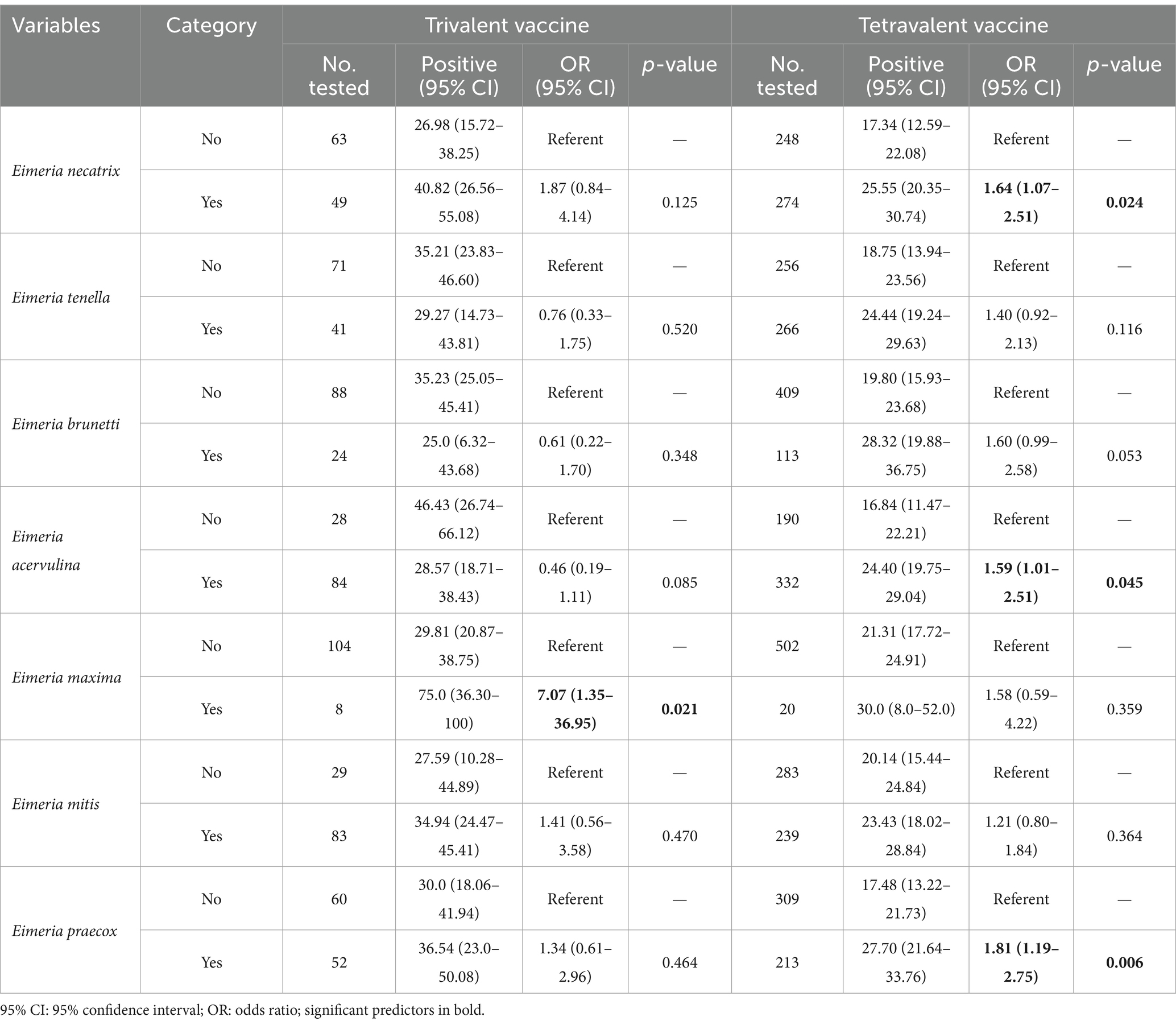
Table 5. Univariable logistic regression analysis of risk factors associated with Eimeria species presence and coccidiosis occurrence in broiler chickens in China.
4 Discussion
Coccidiosis is a disease of significant economic concern worldwide in the poultry industry. The epidemiological monitoring of Eimeria species present in broiler chickens which are vaccinated with live vaccines is vital for the selection of prevention and controlling strategies for coccidiosis. The investigation in our study included seven well-known Eimeria species, excluding the three cryptic species OTUX, OTUY, and OTUZ. This survey was conducted based on the fact that the main prevalence species in China are the seven known species (16–19). Additionally, there have been few studies reporting the presence of unidentifiable OTUs in chickens immunized with transgenic Eimeria in China (20). The overall prevalence of coccidiosis in China is 86.12% (546 of 634 flocks). A comparably high prevalence has been reported in provinces in China such as Anhui (87.75%) (18), Hubei (97.79%), and Henan (96.70%) (16) and other countries including Greece (85.7%) (21), northeastern Algeria (99.5%) (22), Colombia (96.3%) (23), and Australia (98%) (24). Conversely, the prevalence was notably lower in Serbia, north India, Korean, northeastern Brazil, and southwestern Nigeria with rates of 59, 28.5, 75, 59, and 41.3% (25–29), respectively. The variance in Eimeria species occurrence can be attributed to differences in control methods, sampling times, animal management practices, and climatic conditions (30). Previous monitoring trials found that flocks fed with anticoccidial agent in diet produced a considerably higher count of the oocysts in vaccinated flocks, especially between 4 and 8 weeks (8). Consistently, our study also found that the prevalence in vaccinated flocks (86.12%) was lower than in flocks administered with anticoccidial agent (97.17%), as previously reported (16). Southern China’s warm and humid autumn climates, with an average temperature of 25°C and a relative humidity of 79%, may contribute to the elevated prevalence of Eimeria in broiler flocks. However, it is noteworthy that we did not find an increased number of Eimeria-positive flocks in southern China compared with the other three regions in China, suggesting more effective control measures being implemented in those areas.
Seven species of Eimeria were populated in broiler chicken farms across China. The most prevalent species included E. acervulina (65.62%), E. necatrix (50.95%), E. mitis (50.79%), and E. tenella (48.42%). Interactions among Eimeria species, coupled with crowding effect, are known as the most important factors affecting oocyst production (31). Notably, E. acervulina and E. tenella exhibit a higher reproductive potential, and in co-infections, E. acervulina reduces the oocyst production of E. necatrix, E. maxima, and E. brunetti (31). The mixed infection of E. acervulina–E. tenella (38.96%) and E. acervulina–E. necatrix (37.22%) was the dominant co-infection in our study. Interestingly, younger flocks vaccinated with tetravalent vaccines and aged younger than 4 weeks showed a higher likelihood of detecting positive for E. acervulina–E. tenella (50.47%, p < 0.05), E. acervulina–E. necatrix (52.34%, p < 0.05), and E. acervulina–E. tenella–E. necatrix (41.12%, p < 0.05) compared with older ones. This is in agreement with previous studies indicating that found younger chickens are more susceptible to Eimeria species (8, 30, 32). An exception was observed with E. maxima, in which the positive rate was extremely low (4.42%). This might be attributed to all the studied flocks being vaccinated with E. tenella, E. acervulina, and E. maxima or E. tenella, E. necatrix, E. acervulina, and E. maxima. One possible explanation for this could be that the primers for E. maxima failed to detect all strains present in the field samples, as demonstrated by Lew et al. (33) and Sun et al. (34). Alternatively, another reason may be some associated factors affecting the reproductive potential of the live E. maxima vaccine strains, since the positive rate of E. maxima was not higher in starter chickens compared with grower and adult chickens, especially in trivalent vaccines used flocks. This is inconsistent with the finding reported by Snyder et al. (35) that vaccinated flocks had peak oocyst shedding during weeks 2 to 4. Since the intestinal lesions caused by coccidiosis (especially E. maxima) were a well-known predisposing factor for necrotic enteritis (NE). The prevention of coccidiosis and NE was intimately connected due to the risk of NE was greater if the number of oocysts is excessive for E. maxima. The low frequency of E. maxima in this study might reduce the occurrence of NE in live vaccines used flocks. Nevertheless, it is worth noting that the relatively high prevalence of E. brunetti peaked at 21.61% in broiler chickens compared with the previous finding (6.6%) in China (16). Given its increased prevalence, E. brunetti may need to be included in the vaccine in China, echoing similar recommendation made in Australia as previous report (36).
Different Eimeria species distribution, control strategies, and geographical features could affect the occurrence of avian coccidiosis. E. necatrix is known to have lower fecundity and to be a ‘poor competitor’ compared with other species (8). However, In the case of tetravalent vaccinated flocks, the prevalence of E. necatrix was the highest in starter flocks (66.36%) while decreased in grower flocks (46.94%), followed by a slight increase in adult flocks (58.33%). Furthermore, there was a significant positive association between E. necatrix infection rate and coccidiosis occurrence (OR = 1.64, 95% CI: 1.07–2.51; p < 0.05), hinting that flocks might challenge by wild-type strains. This association was consistent with the finding identifying E. necatrix as a contributing factor to coccidiosis occurrence (8, 35). This finding suggested that factors related to the host (e.g., underlying subclinical non-parasitic infections) and environmental factors (e.g., crowding, air quality, and stress) may negatively affect the health of vaccinated chickens, which increased the susceptibility of chickens to coccidiosis, similar to previous reports (21, 29, 36). Moreover, our study found that flocks infected with E. acervulina were also at a significantly higher risk of coccidiosis (OR = 1.59, 95% CI: 1.01–2.51; p < 0.05) when vaccinated with tetravalent vaccines. Known for its rapid reproduction and short life cycle, E. acervulina’s preponderance in broiler farms and its link to coccidiosis outbreaks resonates with previous study (7). However, flocks vaccinated with trivalent vaccines and infected with E. maxima showed a significantly elevated risk of coccidiosis (OR = 7.07, 95% CI: 1.35–36.95; p < 0.05). This may be due to the vaccine strain of E. maxima that induced incomplete cross-strain immune protection against other wild-type strains (37). Surprisingly, flocks positive for E. praecox in tetravalent vaccine-immunized farms were also at an increased risk of coccidiosis (OR = 1.59, 95% CI: 1.02–2.47; p < 0.05). Despite often being overlooked, in our study, the high prevalence of E. praecox (41.80%) was consistent with previous survey in Henan and Hubei provinces in China (33.33%), Brazil (25.1%), and Australia (34.4%) (16, 24, 28). The pathogenicity of E. praecox was observed by Williams et al. for the first time to cause decrease in weight gain and increase in feed conversion ratio (38). Therefore, the high frequencies and risk associated with E. necatrix, E. acervulina, and E. praecox in broilers emphasize the importance of incorporating them into a comprehensive broiler vaccine.
5 Conclusion
In summary, suboptimal post-immunization poultry practices, following the administration of live attenuated vaccines, precipitate either isolated or combined infections of E. acervulina, E. necatrix, E. tenella, E. mitis, and E. praecox, whether as isolated or combined infections, leading to a pronounced susceptibility to morbidity. Furthermore, trivalent or tetravalent attenuated live vaccines are available in China. It is worth noting that the presence of new dominated E. brunetti and E. praecox could be included in the widely used live vaccines. Further investigations are needed to evaluate the epidemiology of virulent species in clinical contexts and discern their associated morbidity implications. By formulating an evidence-based immunization strategy and enhancing post-immunization poultry practices, we can regulate the prevalence of clinical coccidian taxa and assiduously curtail their affiliated pathological ramifications. Notably, our study is the first to report the prevalence of Eimeria species in flocks vaccinated with live vaccines in China, based on molecular analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by the Animal Care and Use Committee of the Institute of Animal Health, Guangdong Academy of Agricultural Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SL: Conceptualization, Data curation, Investigation, Software, Writing – original draft. XL: Conceptualization, Data curation, Investigation, Writing – original draft. QZ: Conceptualization, Investigation, Methodology, Writing – original draft. ZW: Investigation, Methodology, Writing – original draft. ZY: Investigation, Methodology, Writing – original draft. DW: Investigation, Methodology, Writing – original draft. GS: Investigation, Methodology, Writing – original draft. JL: Data curation, Methodology, Writing – original draft. ML: Data curation, Methodology, Writing – original draft. JH: Data curation, Methodology, Writing – original draft. HC: Data curation, Methodology, Writing – original draft. YS: Data curation, Methodology, Writing – original draft. XC: Data curation, Methodology, Writing – original draft. YZ: Data curation, Methodology, Writing – original draft. LY: Data curation, Methodology, Writing – original draft. JZ: Methodology, Resources, Supervision, Writing – original draft. NQ: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. MS: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2023YFD1801205), The open competition program of top ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2023SDZG02), Key Realm R&D Program of Guangdong Province (2023B0202150001), Opening Project of State Key Laboratory of Swine and Poultry Breeding Industry (2023QZ-NK05, 2022GZ07), Science and technology project of Yunfu (2022020202), Science and technology project of Guangzhou (2023B04J0137, 2023A04J0789), Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (202110TD, 202122TD, R2020PY-JC001, R2019YJ-YB3010, R2020PY-JG013, R2020QD-048, R2021PY-QY007, R2023PY-JG018), The Project of Collaborative Innovation Center of GDAAS (XTXM202202).
Conflict of interest
QZ, ZW, ZY, DW, and GS are employed by Wen’s Foodstuffs Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1375026/full#supplementary-material
Footnotes
References
1. Blake, DP, Knox, J, Dehaeck, B, Huntington, B, Rathinam, T, Ravipati, V, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res. (2020) 51:115. doi: 10.1186/s13567-020-00837-2
2. Blake, DP, and Tomley, FM. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. (2014) 30:12–9. doi: 10.1016/j.pt.2013.10.003
3. Clark, EL, Tomley, FM, and Blake, DP. Are Eimeria genetically diverse, and does it matter? Trends Parasitol. (2017) 33:231–41. doi: 10.1016/j.pt.2016.08.007
4. Blake, DP, Vrba, V, Xia, D, Jatau, ID, Spiro, S, Nolan, MJ, et al. Genetic and biological characterisation of three cryptic Eimeria operational taxonomic units that infect chickens (Gallus gallus domesticus). Int J Parasitol. (2021) 51:621–34. doi: 10.1016/j.ijpara.2020.12.004
5. Blake, DP, Clark, EL, Macdonald, SE, Thenmozhi, V, Kundu, K, Garg, R, et al. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci USA. (2015) 112:E5343–50. doi: 10.1073/pnas.1506468112
6. Long, PL, Millard, BJ, Joyner, LP, and Norton, CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. (1976) 6:201–17.
7. Györke, A, Pop, L, and Cozma, V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. (2013) 20:50. doi: 10.1051/parasite/2013052
8. Williams, RB. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol. (1998) 28:1089–98. doi: 10.1016/s0020-7519(98)00066-6
9. Jenkins, MC, Parker, C, and Ritter, D. Eimeria oocyst concentrations and species composition in litter from commercial broiler farms during anticoccidial drug or live Eimeria oocyst vaccine control programs. Avian Dis. (2017) 61:214–20. doi: 10.1637/11578-010317-Reg.1
10. Lee, KW, Lillehoj, HS, Jang, SI, Pagès, M, Bautista, DA, Pope, CR, et al. Effects of in ovo vaccination and anticoccidials on the distribution of Eimeria spp. in poultry litter and serum antibody titers against coccidia in broiler chickens raised on the used litters. Res Vet Sci. (2012) 93:177–82. doi: 10.1016/j.rvsc.2011.05.005
11. Long, PL, and Joyner, LP. Problems in the identification of species of Eimeria. J Protozool. (1984) 31:535–41. doi: 10.1111/j.1550-7408.1984.tb05498.x
12. Olufemi, AS, Olatoye, IO, Oladele, DO, Adejimi, JO, and Ogundipe, GAT. Morphometric and molecular identification of Eimeria species from commercial chickens in Nigeria. J Dairy Vet Anim Res. (2020) 9:104–8. doi: 10.15406/jdvar.2020.09.00288
13. Vrba, V, Blake, DP, and Poplstein, M. Quantitative real-time PCR assays for detection and quantification of all seven Eimeria species that infect the chicken. Vet Parasitol. (2010) 174:183–90. doi: 10.1016/j.vetpar.2010.09.006
14. Gasser, RB, Woods, WG, Wood, JM, Ashdown, L, Richards, G, and Whithear, KG. Automated, fluorescence-based approach for the specific diagnosis of chicken coccidiosis. Electrophoresis. (2001) 22:3546–50. doi: 10.1002/1522-2683(200109)22:16<3546::AID-ELPS3546>3.0.CO;2-8
15. Haug, A, Thebo, P, and Mattsson, JG. A simplified protocol for molecular identification of Eimeria species in field samples. Vet Parasitol. (2007) 146:35–45. doi: 10.1016/j.vetpar.2006.12.015
16. Geng, T, Ye, C, Lei, Z, Shen, B, Fang, R, Hu, M, et al. Prevalence of Eimeria parasites in the Hubei and Henan provinces of China. Parasitol Res. (2021) 120:655–63. doi: 10.1007/s00436-020-07010-w
17. Lan, LH, Sun, BB, Zuo, BX, Chen, XQ, and Du, AF. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang province, China. Poult Sci. (2017) 96:2104–9. doi: 10.3382/ps/pew499
18. Huang, Y, Ruan, X, Li, L, and Zeng, M. Prevalence of Eimeria species in domestic chickens in Anhui province, China. J Parasit Dis. (2017) 41:1014–9. doi: 10.1007/s12639-017-0927-1
19. Zhang, JJ, Wang, LX, Ruan, WK, and An, J. Investigation into the prevalence of coccidiosis and maduramycin drug resistance in chickens in China. Vet Parasitol. (2013) 191:29–34. doi: 10.1016/j.vetpar.2012.07.027
20. Tang, X, Suo, J, Li, C, Du, M, Wang, C, Hu, D, et al. Transgenic Eimeria tenella expressing profilin of Eimeria maxima elicits enhanced protective immunity and alters gut microbiome of chickens. Infect Immun. (2018) 86:e00888–17. doi: 10.1128/IAI.00888-17
21. Andreopoulou, M, Schares, G, Koethe, M, Chaligiannis, I, Maksimov, P, Joeres, M, et al. Prevalence and molecular characterization of toxoplasma gondii in different types of poultry in Greece, associated risk factors and co-existence with Eimeria spp. Parasitol Res. (2023) 122:97–111. doi: 10.1007/s00436-022-07701-6
22. Djemai, S, Ayadi, O, Khelifi, D, Bellil, I, and Hide, G. Prevalence of Eimeria species, detected by ITS1-PCR, in broiler poultry farms located in seven provinces of northeastern Algeria. Trop Anim Health Prod. (2022) 54:250. doi: 10.1007/s11250-022-03252-1
23. Mesa, C, Gómez-Osorio, LM, López-Osorio, S, Williams, SM, and Chaparro-Gutiérrez, JJ. Survey of coccidia on commercial broiler farms in Colombia: frequency of Eimeria species, anticoccidial sensitivity, and histopathology. Poult Sci. (2021) 100:101239. doi: 10.1016/j.psj.2021.101239
24. Godwin, RM, and Morgan, JA. A molecular survey of Eimeria in chickens across Australia. Vet Parasitol. (2015) 214:16–21. doi: 10.1016/j.vetpar.2015.09.030
25. Pajić, M, Todorović, D, Knežević, S, Prunić, B, Velhner, M, Andrić, DO, et al. Molecular investigation of Eimeria species in broiler farms in the province of Vojvodina, Serbia. Life (Basel). (2023) 13:1039. doi: 10.3390/life13041039
26. Khursheed, A, Yadav, A, Sofi, OM, Kushwaha, A, Yadav, V, Rafiqi, SI, et al. Prevalence and molecular characterization of Eimeria species affecting backyard poultry of Jammu region, North India. Trop Anim Health Prod. (2022) 54:296. doi: 10.1007/s11250-022-03290-9
27. Flores, RA, Nguyen, BT, Cammayo, PLT, Võ, TC, Naw, H, Kim, S, et al. Epidemiological investigation and drug resistance of Eimeria species in Korean chicken farms. BMC Vet Res. (2022) 18:277. doi: 10.1186/s12917-022-03369-3
28. da Silva, JT, Alvares, FBV, de Lima, EF, da Silva Filho, GM, da Silva, ALP, Lima, BA, et al. Prevalence and diversity of Eimeria spp. in free-range chickens in northeastern Brazil. Front vet sci. (2022) 9:1031330. doi: 10.3389/fvets.2022.1031330
29. Ola-Fadunsin, SD. Investigations on the occurrence and associated risk factors of avian coccidiosis in Osun state, southwestern Nigeria. J Parasitol Res. (2017) 2017:9264191. doi: 10.1155/2017/9264191
30. Chengat Prakashbabu, B, Thenmozhi, V, Limon, G, Kundu, K, Kumar, S, Garg, R, et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol. (2017) 233:62–72. doi: 10.1016/j.vetpar.2016.12.003
31. Williams, RB. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol. (2001) 31:1056–69. doi: 10.1016/s0020-7519(01)00235-1
32. Long, PL, Tompkins, RV, and Millard, BJ. Coccidiosis in broilers: evaluation of infection by the examination of broiler house litter for oocysts. Avian Pathol. (1975) 4:287–94. doi: 10.1080/03079457509353877
33. Lew, AE, Anderson, GR, Minchin, CM, Jeston, PJ, and Jorgensen, WK. Inter- and intra-strain variation and PCR detection of the internal transcribed spacer 1 (ITS-1) sequences of Australian isolates of Eimeria species from chickens. Vet Parasitol. (2003) 112:33–50. doi: 10.1016/s0304-4017(02)00393-x
34. Sun, XM, Pang, W, Jia, T, Yan, WC, He, G, Hao, LL, et al. Prevalence of Eimeria species in broilers with subclinical signs from fifty farms. Avian Dis. (2009) 53:301–5. doi: 10.1637/8379-061708-Resnote.1
35. Snyder, RP, Guerin, MT, Hargis, BM, Page, G, and Barta, JR. Monitoring coccidia in commercial broiler chicken flocks in Ontario: comparing oocyst cycling patterns in flocks using anticoccidial medications or live vaccination. Poult Sci. (2021) 100:110–8. doi: 10.1016/j.psj.2020.09.072
36. Morris, GM, Woods, WG, Richards, DG, and Gasser, RB. Investigating a persistent coccidiosis problem on a commercial broiler-breeder farm utilising PCR-coupled capillary electrophoresis. Parasitol Res. (2007) 101:583–9. doi: 10.1007/s00436-007-0516-9
37. Smith, AL, Hesketh, P, Archer, A, and Shirley, MW. Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity. Infect Immun. (2002) 70:2472–9. doi: 10.1128/IAI.70.5.2472-2479.2002
Keywords: broiler chicken, coccidiosis, Eimeria spp., prevalence, risk factors
Citation: Liao S, Lin X, Zhou Q, Wang Z, Yan Z, Wang D, Su G, Li J, Lv M, Hu J, Cai H, Song Y, Chen X, Zhu Y, Yin L, Zhang J, Qi N and Sun M (2024) Epidemiological investigation of coccidiosis and associated risk factors in broiler chickens immunized with live anticoccidial vaccines in China. Front. Vet. Sci. 11:1375026. doi: 10.3389/fvets.2024.1375026
Edited by:
Ruediger Hauck, Auburn University, United StatesReviewed by:
Luis-Miguel Gómez-Osorio, Independent Researcher, Medellin, ColombiaKatarzyna B. Miska, Agricultural Research Service (USDA), United States
Copyright © 2024 Liao, Lin, Zhou, Wang, Yan, Wang, Su, Li, Lv, Hu, Cai, Song, Chen, Zhu, Yin, Zhang, Qi and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanshan Qi, cWluYW5zaGFuQGdkYWFzLmNu; Mingfei Sun, c3VubWluZ2ZlaUBnZGFhcy5jbg==
†These authors have contributed equally to this work
 Shenquan Liao
Shenquan Liao Xuhui Lin1†
Xuhui Lin1†