- 1Southeast Veterinary Referral Center, Miami, FL, United States
- 2Ozark Veterinary Specialty Care, Springdale, AR, United States
- 3Veterinary Diagnostic Laboratory, Michigan State University, East Lansing, MI, United States
- 4CriticalCareDVM.com, Omaha, NE, United States
A 10-year-old spayed female Dachshund presented with abdominal pain and generalized severe ileus. An exploratory laparotomy was performed, confirming a severe ileus of undetermined origin. Multiple intestinal biopsy results confirmed acute intestinal leiomyositis. Immunohistochemistry (IHC) stains confirmed a T-cell predominant inflammatory infiltrate. Intravenous immunoglobulin (hIVIG) was administered prior to immunosuppressive therapy. Within 10 days of hIVIG treatment, functional peristaltic activity returned, and symptoms resolved. Long-term management, including the use of mycophenolate, resulted in sustained functional peristaltic recovery. Further studies are needed to explore the potential benefits of hIVIG treatment in the stabilization phase of this commonly fatal, treatment-refractory disease.
Introduction
This case report describes the successful treatment of pseudo-obstructive intestinal leiomyositis in a dog utilizing conventional therapy with adjunctive intravenous immunoglobulin. Immunopathology performed documents a T-cell predominant myo-inflammatory infiltrate. To our knowledge, functional peristaltic long-term recovery has not been previously documented in a dog.
Case description
A 10-year-old spayed female Dachshund weighing 5.5 kg was presented to her primary care veterinarian for evaluation of weight loss, anorexia, vomiting, and lethargy for several days’ duration. Physical examination abnormalities included mild sinus tachycardia (heart rate 160 bpm), weight loss (body weight 5.7 kg; 1.8 kg loss over 1 month), depression, and mild dehydration. Point of care complete blood count (CBC), biochemical profile (CHEM), and urinalysis (UA) were performed (IDEXX1). Biochemical abnormalities included hyperglycemia 240 mg/dL (70–143 mg/dL), BUN elevation 44 mg/dL (7–27 mg/dL), normal creatinine 0.5 mg/dL (0.5–1.8 mg/dL), hyponatremia 110 mmol/L (144–160 mmoL/L), hypochloridemia 68 mmol/L (109–122 mmoL/L), and hypokalemia 2.7 mmol/L (3.5–5.8 mmoL/L). Urinalysis collected by cystocentesis revealed isosthenuria 1.014 (1.015–1.045) without proteinuria or glucosuria. Supportive care was initiated, including intravenous (IV) fluids (Normosol-R); an initial 10 mL/kg IV bolus followed by 10 mL/kg/h IV; potassium chloride (20 mEq/L); and maropitant (1 mg/kg IV). The patient was subsequently referred for continued care and further diagnostic investigation.
Upon arrival at the Referral Center Emergency Service (AESC2), the vital signs were normal, and Doppler blood pressure was measured at 156 mmHg (reference range: 90–140 mmHg). Point-of-care testing showed a packed cell volume (PCV) of 57% and total solids (TS) of 7.0 g/dL (reference ranges: 42–54% and 5.9–7.8 g/mL, respectively). C-reactive protein was elevated at 8.6 mg/dL (references: < 1.0 mg/dL), baseline cortisol was >10.0 (reference: 2.0–9.0 ug/dL); and symmetric dimethylarginine (SDMA) was at the upper limit of normal at 14 (reference: 0–14 ug/dL). Venous blood gas analysis showed the following: pH 7.59 (reference: 7.35–7.43), HCO3 46.4 mmol/L (reference: 22.2–22.4 mmol/L), PCO2 52.0 mmHg (reference: 29–42 mmHg), tCO2 48.0 mmol/L, sodium 128 mmol/L (reference: 146–154 mmol/L), potassium 2.3 mmol/L (reference: 3.8–5.3 mmol/L), and chloride 85 mmol/L (reference: 105–115 mmol/L), cumulatively consistent with hypochloremic metabolic alkalosis.
Abdominal radiography revealed generalized dilation of the gastrointestinal tract with no radiopaque foreign body (Figure 1). Thoracic radiography was unremarkable. Supportive care was continued, including intravenous lactated ringers solution (30 mL/h) with KCl supplementation (20 mEq/L), maropitant (1 mg/kg IV q24h), and ampicillin/sulbactam (20–22 mg/kg IV q8h).
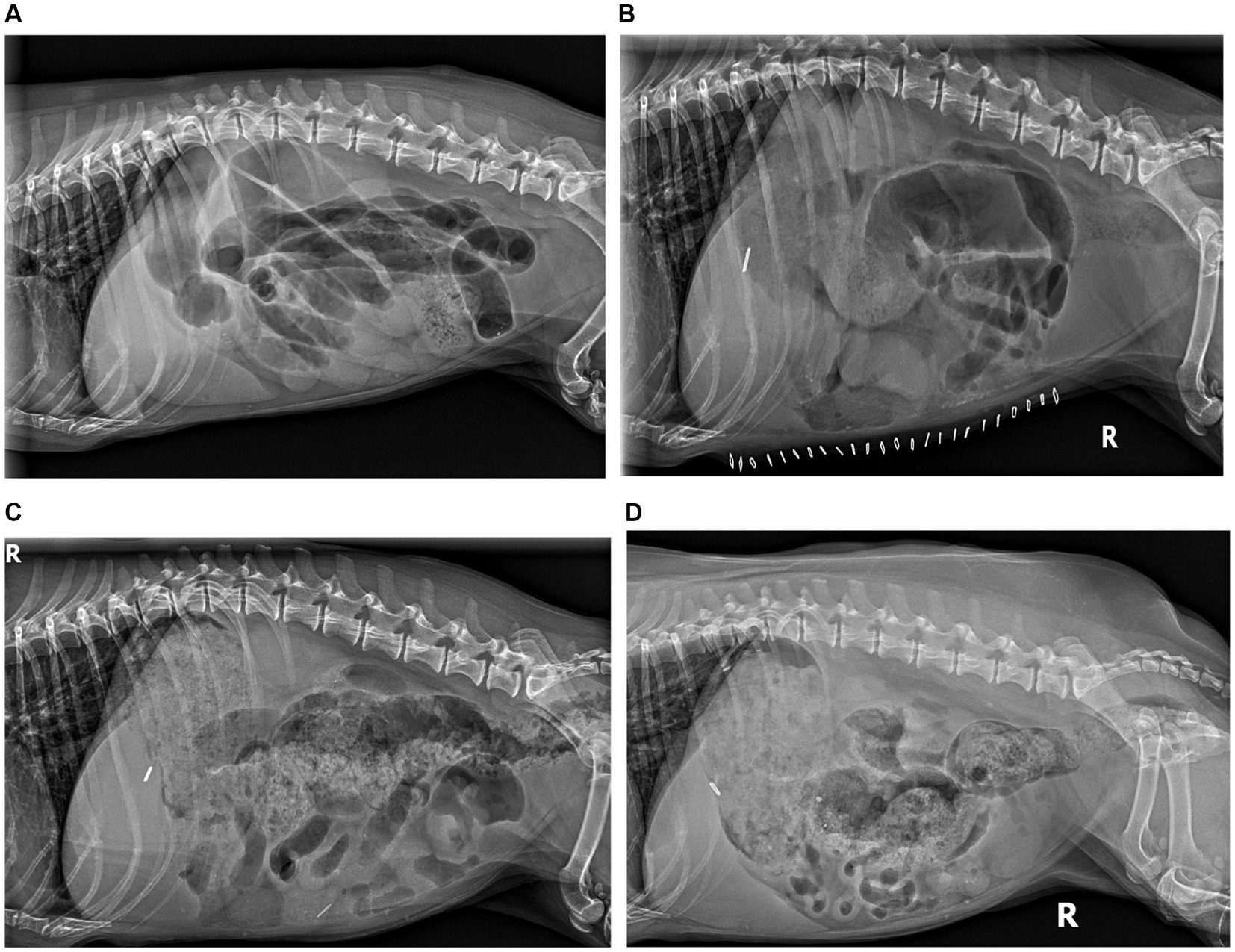
Figure 1. Radiographic peristaltic recovery. (A) Day 0 RLAT: severe generalized gaseous ileus, segmental luminal fluid, and luminal density consistent with functional and physical obstruction. (B) Day 7 RLAT: severe ileus persists with variable segmental fluid distension; no visible ingesta, consistent with ongoing hyporexia. (C) Day 15 RLAT: significant ingesta is present, colonic gaseous distension with formed fecal matter, significantly diminished small bowel distension, and peristaltic contractions are evident. (D) Day 111 RLAT: normal gastric ingesta, formed stool present in the colon, resolved small bowel distension with frequent segmental peristaltic contractions evident.
Comprehensive abdominal ultrasonography was performed by a board-certified veterinary small animal internal medicine specialist (OVSC3) and revealed severe, generalized ileus of the small intestine and stomach. The bowel luminal content was reported as admixed fluid and gas without coordinated propulsion. Additional findings included mild mesenteric lymphadenomegaly, moderate-to-marked gall bladder distension with heterogeneous dynamic inspissated bile, mild heterogenous hepatic parenchyma, and bilateral prominent adrenal glands. Due to the severe ileus, a positive contrast gastrointestinal (GI) study was not performed, and an exploratory laparotomy was recommended. Premedication included lidocaine 2% (2 mg/kg IV), midazolam (0.25 mg/kg IV), and hydromorphone (0.1 mg/kg IV). General anesthesia was induced with propofol (4 mg/kg IV) and maintained with isoflurane while providing mechanical ventilation (ADS20004).
A ventral midline celiotomy was performed. The stomach and small intestine were found to be severely distended and atonic. A plug of compressible ingesta was palpable in the distal ileum and successfully digitally advanced antegrade through the ileocecocolic valve; this plug was considered insufficient to primarily obstruct the GI tract. Incisional biopsies were obtained from the stomach, duodenum, jejunum, and ileum using a standard incisional technique. The liver appeared normal in color and size; a wedge biopsy was collected from the right lateral lobe. The gall bladder was moderately distended with palpably granular material, necessitating a cholecystectomy. Additionally, colonic distension and atony were observed, and the adrenal glands were prominent and nodular. Recovery from anesthesia and surgery was uneventful.5,6
Postoperative care included dexamethasone sodium phosphate (0.1 mg/kg IV once), cobalamin (500 mcg SC once), vitamin B complex (0.5 mL SC once), ampicillin/sulbactam (22 mg/kg IV 8 h), enrofloxacin (10 mg/kg IV q24h), lidocaine (25 mcg/kg/min IV CRI), hydromorphone (.013 mg/kg/h IV CRI), metoclopramide (1 mg/kg/day IV CRI), 0.9% NaCl (120 mL/kg/day IV CRI x 18 h), and KCl (30 mEq/L). As the patient tolerated alimentation, IV fluid support was gradually weaned, and the patient was transitioned to oral medication equivalents, including prednisolone (0.46 mg/kg PO Q24h), enrofloxacin (11.7 mg/kg PO q24h), metronidazole (22 mg/kg PO q12h), ursodiol (13 mg/kg PO q24h), metoclopramide (0.43 mg/kg PO q12h), tramadol (4.3 mg/kg PO q6h), and gabapentin (17 mg/kg PO 12 h). Tramadol was chosen over a pure mu agonist to avoid opioid-exacerbated ileus. Daily laboratory monitoring revealed normalization of electrolyte deficiencies by 72 h postoperatively. A commercial prescription low-fat diet (Hills I/D LF7) was prescribed postoperatively and continued during the recovery period.
Histopathology of the duodenum, jejunum, and ileum revealed severe, diffuse, chronic lymphoplasmacytic-to-neutrophilic leiomyositis of the tunica muscularis, as well as moderate lymphoplasmacytic enteritis with luminal bacteria and protozoa (suspected ciliates). In all sections of the small intestine, the tunica muscularis was infiltrated by moderate numbers of lymphocytes, plasma cells, and neutrophils. The smooth muscle within the affected areas was severely disrupted, with smooth myocytes variably vacuolated, fragmented, or replaced by plump fibroblasts amid fine collagen fibers, resulting in the loss of the muscular architecture of the circular longitudinal muscle layers. Neurons within the myenteric plexus within the tunica muscularis remained intact. The villous lamina propria contained moderate numbers of lymphocytes and plasma cells, as well as fewer eosinophils and neutrophils (Supplementary Figure S1). Immunohistochemical staining of the small intestine for lymphocyte markers CD3 and CD21 showed mixed lymphocytic and neutrophilic inflammation with T-cell predominance (Supplementary Figure S2). The stomach was within normal limits. The gall bladder lamina propria was diffusely expanded by moderate numbers of neutrophils, lymphocytes, and plasma cells, consistent with moderate suppurative and lymphoplasmacytic cholecystitis; segmental cystic mucinous hyperplasia was also present. Liver histopathology revealed mild centrilobular hepatocellular vacuolation. Bile aerobic bacterial culture grew Escherichia coli and Enterococcus hirae, susceptible to enrofloxacin and amoxicillin/clavulanate, respectively. Serum cobalamin was normal at 697 ng/L (251–908 ng/L), and mild hypofolatemia at 6 (7.7–24.4 ug/L) was documented. The vitamin D profile revealed an elevated parathyroid hormone at 24.8 (1.1–10.6 pmol/L), mild ionized hypocalcemia at 1.15 (1.25–1.45 mmoL/L), and normal 25-hydroxyvitamin D at 239 (109–423 nmol/L), most consistent with secondary hyperparathyroidism.
The patient was evaluated 1 week postoperatively. The primary symptoms reported were inappetence without vomiting, regurgitation, or diarrhea. Point-of-care CBC showed mild neutrophilia (13,410/uL) and mild eosinopenia (10/uL), while CHEM and electrolyte profiles were normal [IDEXX (see text footnote 1)]. A single lateral abdominal radiograph revealed a persistently severe ileus with no evidence of peritoneal effusion. The patient was treated with human intravenous immunoglobulin G [hIVIg; 5 grams IV (1.1 g/kg)] over 6 h (Gamunex9). Dexamethasone sodium phosphate (0.1 mg/kg IV) and vitamin B complex (0.5 mL SC) were administered pre-infusion. Dexamethasone was administered to help mitigate potential hIVIG reactions. Amoxicillin/clavulanate (11.5 mg/kg PO q12h) was prescribed for 3 weeks based on bile culture. Multimodal immunomodulatory therapy was also prescribed (prednisolone at 0.46 mg/kg PO q24h for 7 days, then every 48 h; mycophenolate at 9.25 mg/kg PO q12h). Treatment with tramadol, gabapentin, metoclopramide, enrofloxacin, ursodiol, metronidazole, and a low-fat diet were continued as previously prescribed.
The patient was reevaluated 8 days following the hIVIg infusion. The patient’s appetite and thirst had improved, and bowel movements were reportedly normal. Abdominal radiography revealed food in the stomach, consistent with recent meals, and feces in the colon. Small bowel luminal gas was present but markedly improved, with visible peristaltic contractions in the small bowel. Reference laboratory testing (Antech10) revealed a mildly elevated ALP (152 U/L), mild thrombocytosis (508,000/uL), and mild neutrophilia (11,760/uL). Continued therapy included Vitamin B complex (0.5 mL SC q7 days x 4 weeks, then q14 days), prednisolone (0.46 mg/kg PO q24h), metoclopramide (0.43 mg/kg PO q12h), and ursodiol (13 mg/kg PO q24h). Mycophenolate had not been started as instructed but was eventually started approximately 4 weeks postoperatively.
At 111 days following hIVIg administration, the patient exhibited normal digestive cycles and weight gain, reaching a body weight of 8.5 kg. Abdominal radiography revealed continued subjective normalization of peristalsis (Figure 1). Metoclopramide was discontinued, and instructions were provided for the gradual weaning of immunomodulatory therapy, starting first with a reduction in prednisolone from 0.29 mg/kg/day to every other day. At the time of writing, more than 2 years postoperatively, the patient remains in functional remission and taking mycophenolate every other day (approximately 5.8 mg/kg, weight gain adjusted) and ursodiol daily (9.8 mg/kg once daily, adjusted for weight gain) The treatment and progress timeline is provided in Table 1.
Discussion
To the authors’ knowledge, this is the first case reporting the use of hIVIg for functional stabilization of intestinal pseudo-obstruction (CIPO) secondary to small intestinal leiomyositis in a dog. The term “intestinal pseudo-obstruction” was introduced in the late 1950s by Dudley et al., who reported 13 human cases of intestinal obstruction unexplained by mechanical origin; this was referred to as “spastic ileus” (1–3). In human medicine, intestinal pseudo-obstruction may be acute or chronic. The acute form has been associated with abdominal surgery, peritonitis, hypokalemia, spinal or pelvic trauma, viral enteritis, myocardial infarction, retroperitoneal hemorrhage, and anticholinergic or opioid treatment (1–5). The chronic form is primarily characterized as congenital or acquired and can be further classified as primary (idiopathic) or secondary disorders (2–5) Acquired forms may occur secondary to neurologic, metabolic, endocrine, paraneoplastic, autoimmune, or infectious etiologies (1–5). CIPO is a rare and highly morbid syndrome that may be considered an insufficiency of the intestinal pump, impairing gastrointestinal propulsion and causing symptoms of functional obstruction without mechanical origin (1, 2, 4–8). Chronic intestinal pseudo-obstruction has been reported in various animal species, including dogs, horses, cats, and birds (4, 6, 9–19).
Affected dogs are presented with non-specific signs, including abdominal pain, nausea, vomiting, regurgitation, bloating, diarrhea, anorexia, abdominal distension, and weight loss of variable onset. Diagnostic imaging reveals marked gastric and small intestinal dilatation with severe hypomotility (4, 7–11, 13–16, 20). In veterinary medicine, clinical and radiologic/ultrasonographic evidence of intestinal obstruction is an indication for exploratory surgery (9). If no evidence of mechanical obstruction is found, full-thickness biopsies should be obtained from each small intestinal segment (9).
Intestinal leiomyositis, characterized by infiltration of the smooth muscle fibers of the tunica muscularis by lymphocytes, is the most frequent CIPO lesion in dogs (6, 9–11, 15, 16). Infiltration of lymphocytes between functional myocytes affects the contractility of the enteric smooth muscle cells, causing subsequent ileus (4, 21). Pathologic features of visceral myopathies reflect degenerative changes, including varying degrees of myofiber atrophy and vacuolar degeneration (22). In the reports of both human and canine patients with visceral myopathies, the predominant inflammatory cell infiltrating atrophic muscle layers are T cells, suggesting a cell-mediated inflammatory reaction directed at smooth muscle cells that can lead to the destruction of the muscularis mucosa (4, 13, 22, 23). This T-cell myopathic-predominant inflammation was identified in the dog presented in this case. With disease progression, smooth muscle is replaced by fibrosis (23). In cases of intestinal leiomyositis, the mucosa, submucosa, and neural plexuses are relatively spared (4, 11, 12, 22). The lack of mucosal lesions makes endoscopic biopsies inadequate to establish a diagnosis (4, 9). The diagnostic yield of conventional, endoscopic superficial mucosal biopsies is low, as submucosal neuromuscular structures are usually missed (4, 24, 25). It is suspected that CIPO is underdiagnosed in dogs, given the lack of full-thickness intestinal biopsies in many dogs with chronic enteropathies and the awareness of this condition among veterinarians (4).
Management of CIPO in people is largely directed at maintaining adequate caloric intake, providing parenteral nutrition, promoting gastrointestinal motility, and treating complications (e.g., bacterial overgrowth, intractable pain) (1, 3, 4, 7). Prokinetic treatment is a mainstay of treatment. Cisapride has been shown to increase the antroduodenal motility index and improve enteral feeding in people; it has also been shown to increase lower esophageal sphincter pressure and decrease gastric reflux in dogs (4, 25–28). In dogs, cisapride is a more potent and effective prokinetic agent compared to metoclopramide (1, 26). Other drugs, such as erythromycin, azithromycin, or mitemcinal (an erythromycin-derived motilin agonist), have been shown to stimulate antral motility in humans and dogs with functional or experimentally-induced gastric obstructions (4, 29, 30). However, to date, none have been shown to reliably improve gastrointestinal function in dogs with leiomyositis (4, 19).
Immunomodulatory agents, including corticosteroids, cyclosporine, cyclophosphamide, and azathioprine, have been used in humans and canine patients with documented intestinal leiomyositis (3, 4, 12, 20, 23, 31, 32). Such therapy is thought to be most successful when initiated early in the course of the disease, before mural muscular atrophy and fibrosis develop (3, 4, 12, 31, 32). Despite treatment, the overall prognosis for both human and canine patients remains poor (4).
Statistically justified pathology and treatment-specific guidelines for intestinal leiomyositis are not available in canine species. Considering the cumulative case knowledge and outcomes reported in veterinary databases, commonly used antimicrobial, antiemetic, antisecretory, and analgesic therapies may have reasonable application but do not appear to affect outcomes significantly, with average survival periods often reported in single-digit weeks (4, 6, 19, 33, 34). In cases that have employed immunosuppressive therapy, outcomes remain poor overall when the pathophysiologic component of mural myofiber atrophy or fibrosis is observed (4, 6, 9, 13, 15). However, treatment success with a functional outcome was reported in a single dog using combined immunosuppressive therapy with prednisolone and azathioprine before the development of mural muscular atrophy and fibrosis (32).
A comprehensive review of cumulative pathologic findings relative to treatment responses may be helpful but is beyond the scope of this report. A brief overview is provided (Table 2).
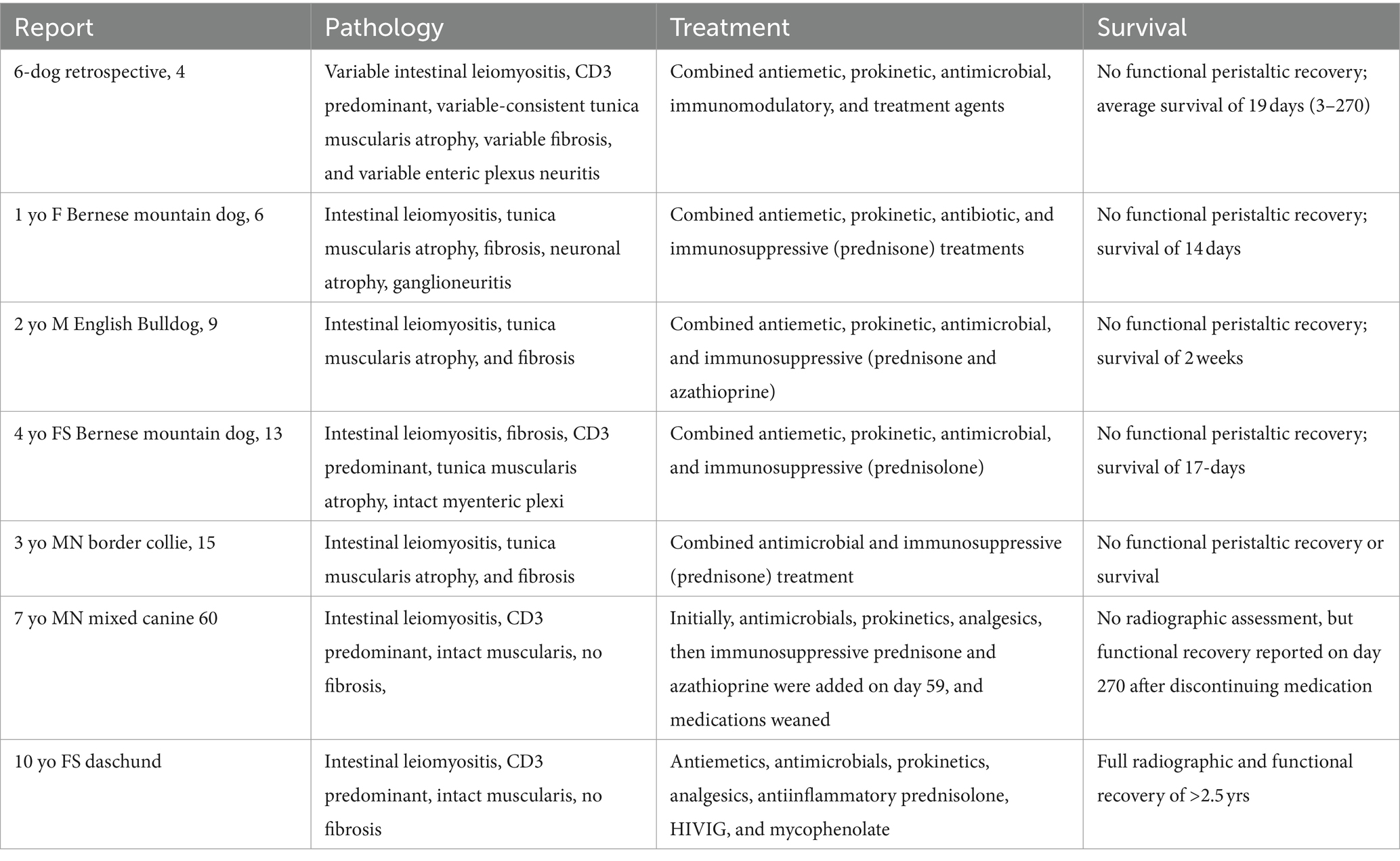
Table 2. Summary of reported histopathology, treatment(s), and survival of canine intestinal leiomyositis/chronic intestinal pseudo-obstruction.
As the predominant inflammatory cell in intestinal leiomyositis is the T cell, hIVIg represents a reasonable therapeutic option. Treatment with hIVIg was chosen due to the historically poor prognosis associated with standard multimodal immunomodulatory and prokinetic therapy for the treatment of intestinal leiomyositis. Given the phase of surgical healing, conventional immunosuppression could introduce significant recovery risk without predictable benefits. Human IVIg is not suspected to negatively affect tissue healing and is used perioperatively in humans with specific conditions (35). Specific effects of hIVIg on tissue healing are not well described. hIVIg does not appear to provide long-term immune-suppressive benefits (36).
Human intravenous immunoglobulin (hIVIg) is composed of highly purified immunoglobulin G, obtained from large pools of donated human plasma, and has been used for more than 45 years to treat a variety of diseases in both humans and dogs (36–42) (Table 3). Its mechanism of action is complex and includes modulation of expression and function of Fc receptors, interference with the activation of B and T cells and complement, and a decrease in immunoglobulin production (37, 38). Disorders that have reportedly responded to hIVIG include a wide spectrum of diseases mediated by autoantibodies or believed to depend primarily on autoaggressive T cells; hIVIg is a component of therapy used for categorical autoimmune gastrointestinal motility disorders in humans, under which leiomyositis broadly falls (38, 54).
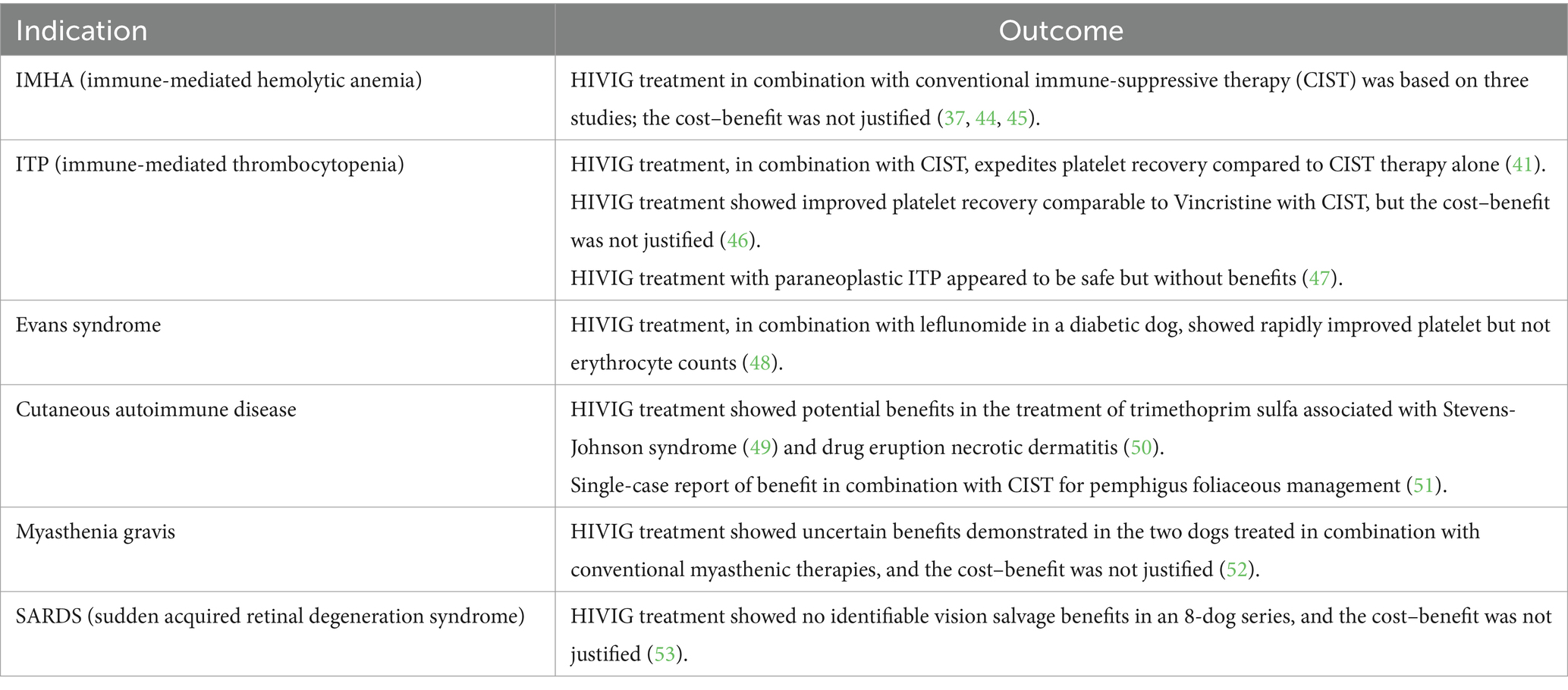
Table 3. Indication and efficacy of HIVIG therapy in dogs (43).
Treatment with hIVIg has not been previously reported in a veterinary patient with intestinal leiomyositis. This patient’s median survival time far exceeded those reported in previous publications (4, 11, 15). Serial radiography well documents functional peristaltic recovery that was not previously reported. This patient was treated with hIVIg 1 week postoperatively, and we propose that early intervention positively contributed to our patient’s response.
Dexamethasone sodium phosphate, administered immediately and 1 week postoperatively, would not have a predictable long-term immunomodulatory effect. Prednisolone therapy was initiated at an antiinflammatory dose after treatment with hIVIg. Mycophenolate therapy was prescribed to control T-cell-initiated immunopathology but was not started until after 1 month postoperatively. Clinical and radiographic improvements were temporally correlated with hIVIG therapy but cannot be verified by this single case.
It is unclear if cholecystectomy and the resolution of bacterial cholecystitis provided a long-term impact on this patient’s response to the prescribed therapies. A recent report by Viljoen et al. documented a correlation between inflammatory enteropathies and cholecystitis (55). In human patients, infectious molecular mimicry such as prodromal enteritis has been reported (31, 56). To our knowledge, there is no known association between cholecystitis and leiomyositis in dogs.
The functional long-term response to therapy, including hIVIg in this patient, warrants further investigation as a treatment option during the stabilization phase of intestinal leiomyositis in dogs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal studies were approved by OVSC Internal Medicine Clinical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MO: Writing – original draft, Writing – review & editing. JW: Writing – review & editing. NU: Writing – review & editing. SC: Writing – review & editing. CB: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1373882/full#supplementary-material
Footnotes
1. ^IDEXX Laboratories, Inc., Westbrook, Maine 04092.
2. ^Animal Emergency and Specialty Center, Springdale, AR 72762.
3. ^Ozark Veterinary Specialty Care, Springdale, AR 72762.
4. ^A.D.S. 2000, Engler Engineering Corporation, Hialeah, FLA 33013.
5. ^Michigan State University, Veterinary Diagnostic Lab, Lansing, MI 48910.
6. ^Texas A&M University, Gastrointestinal Lab, College Station, TX 77843.
7. ^Hills Pet Nutrition, Inc., Topeka, KS 66601.
9. ^Gamunex-C, Grifols Inc., Research Triangle Park, NC 27709.
10. ^Antech Diagnostics, Inc., Fountain Valley, CA 92728.
References
1. de Giorgio, R, Cogliandro, RF, Barbara, G, Corinaldesi, R, and Stanghellini, V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin N Am. (2011) 40:787–807. doi: 10.1016/j.gtc.2011.09.005
2. Dudley, HA, Sinclair, IS, McLaren, IF, McNair, TJ, and Newsam, JE. Intestinal pseudo-obstruction. J R Coll Surg Edin. (1958) 3:206–17. doi: 10.1136/bmj.292.6529.1157
3. Antonucci, A, Fronzoni, L, Cogliandro, L, Cogliandro, RF, Caputo, C, Giorgio, RD, et al. Chronic intestinal pseudo-obstruction. World J Gastroenterol. (2008) 14:2953–61. doi: 10.3748/wjg.14.2953
4. Zacuto, AC, Pesavento, PA, Hill, S, McAlister, A, Rosenthal, K, Cherbinsky, O, et al. Intestinal leiomyositis: a cause of chronic intestinal pseudo-obstruction in 6 dogs. J Vet Intern Med. (2016) 30:132–40. doi: 10.1111/jvim.13652
5. de Giorgio, R, Sarnelli, G, Corinaldesi, V, and Stanghellini, V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. (2004) 53:1549–52. doi: 10.1136/gut.2004.043968
6. Gianella, P, Tecilla, M, Bellino, C, Buracco, P, Martano, M, Zanatta, R, et al. An unusual case of intestinal leiomyositis in a Bernese Mountain dog. Schweizer Archiv fur Tierheilkunde. (2015) 157:563–7. doi: 10.17236/sat00038
7. Connor, FL, and Di Lorenzo, C. Chronic intestinal pseudo-obstruction: assessment and management. Gastroenterology. (2006) 130:S29–36. doi: 10.1053/j.gastro.2005.06.081
8. Gabbard, SL, and Lacy, BE. Chronic intestinal pseudo-obstruction. Nutr Clin Pract. (2013) 28:307–16. doi: 10.1177/0884533613485904
9. Dvir, E, Leiswitz, AL, and Van Der Lugt, JJ. Chronic idiopathic intestinal pseudo-obstruction in an English bulldog. J Small Anim Pract. (2001) 42:243–7. doi: 10.1111/j.1748-5827.2001.tb02029.x
10. Eastwood, JM, McInnes, EF, White, RN, Elwood, CM, and Stock, G. Caecal impaction and chronic intestinal pseudo-obstruction in a dog. J Vet Med. (2005) 52:43–4. doi: 10.1111/j.1439-0442.2004.00681.x
11. Johnson, CS, Fales-Williams, AJ, Reimer, SB, Lotsikas, PJ, and Haynes, JS. Fibrosing gastrointestinal leiomyositis as a cause of chronic intestinal pseudo-obstruction in an 8-month-old dog. Vet Pathol. (2007) 44:106–9. doi: 10.1354/vp.44-1-106
12. Harvey, AM, Hall, EJ, Day, MJ, Moore, AH, Battersby, IA, and Tasker, S. Chronic intestinal pseudo-obstruction in a cat caused by visceral myopathy. J Vet Intern Med. (2005) 19:111–4. doi: 10.1892/0891-6640(2005)19<111:cipiac>2.0.co;2.
13. Couraud, L, Jermyn, K, Yam, PS, Ramsey, IK, and Philbey, AW. Intestinal pseudo-obstruction, lymphocytic leiomyositis and atrophy of the muscularis externa in a dog. Vet Rec. (2006) 159:86–7. doi: 10.1136/vr.159.3.86
14. Arrick, RH, and Kleine, LJ. Intestinal pseudoobstruction in a dog. J Am Vet Med Assoc. (1978) 172:1201–5.
15. Lamb, WA, and France, MP. Chronic intestinal pseudo-obstruction in a dog. Aust Vet J. (1994) 71:84–6. doi: 10.1111/j.1751-0813.1994.tb03334.x
16. Moore, R, and Carpenter, J. Intestinal sclerosis with pseudo-obstruction in three dogs. J Am Vet Med Assoc. (1984) 184:830–3.
17. Chénier, S, Macieira, SM, Sylvestre, D, and Jean, D. Chronic intestinal pseudo-obstruction in a horse: a case of myenteric ganglionitis. Can Vet J. (2011) 42:242–7.
18. Weissenböck, H, Bakonyi, T, Sekulin, K, Ehrensperger, F, Doneley, RJT, Dürrwald, R, et al. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis. (2009) 15:1453–9. doi: 10.3201/eid1509.090353
19. Jung, J, and Choi, M. Primary Myopathic chronic intestinal Pseudo-obstruction in a Maltese dog. J Vet Clin. (2016) 33:179–82. doi: 10.17555/jvc.2016.06.33.3.179
20. Ruuska, TH, Karikoski, R, Smith, VV, and Milla, PJ. Acquired myopathic intestinal pseudo-obstruction may be due to autoimmune enteric leiomyositis. Gastroenterology. (2002) 122:1133–9. doi: 10.1053/gast.2002.92396
21. McDonald, GB, Schuffler, MD, Kadin, ME, and Tytgat, GNJ. Intestinal pseudo-obstruction is caused by diffuse lymphoid infiltration of the small intestine. Gastroenterology. (1985) 89:882–9. doi: 10.1016/0016-5085(85)90587-6
22. Domizio, P, and Martin, JE. Muscular and mechanical disorders of the small intestine In: NA Shepherd and BF Warren, editors. Williams GT, et al. Morson and Dawson’s gastrointestinal pathology. 5th ed. Hoboken, NJ: Blackwell (2013). 305–14.
23. Kapur, RP. Intestinal motor disorders In: P Russo and ED Ruchelli, editors. Poccoli DA: Pathology of pediatric gastrointestinal and liver disease. 2nd ed. Cham, Switzerland: Springer (2014). 249–316.
24. Valli, PV, Pohl, D, Fried, M, Caduff, R, and Bauerfeind, P. Diagnostic use of endoscopic full-thickness wall resection (eFTR) – a novel minimally invasive technique for colonic tissue sampling in patients with severe gastrointestinal motility disorders. Neurogastroenterol Motil. (2018) 30:1–6. doi: 10.1111/nmo.13153
25. Knowles, CH, de Giorgio, R, Kapur, RP, Bruder, E, Farrugia, G, Geboes, K, et al. The London classification of gastrointestinal neuromuscular pathology: report on behalf of the gastro 2009 international working group. Gut. (2010) 59:882–7. doi: 10.1136/gut.2009.200444
26. Kempf, J, Lewis, F, Reusch, CE, and Kook, PH. High-resolution manometric evaluation of the effects of cisapride and metoclopramide hydrochloride administered orally on lower esophageal sphincter pressure in awake dogs. Am J Vet Res. (2014) 75:361–6. doi: 10.2460/ajvr.75.4.361
27. Zacuto, AC, Marks, SL, Osborn, J, Douthitt, KL, Hollingshead, KL, Hayashi, K, et al. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J Vet Intern Med. (2012) 26:518–25. doi: 10.1111/j.1939-1676.2012.00929.x
28. Abell, TL, Camilleri, M, DiMagno, EP, Hench, VS, Zinsmeister, AR, and Malagelada, JR. Long-term efficacy of oral cisapride in symptomatic upper gut dysmotility. Dig Dis Sci. (1991) 36:616–20. doi: 10.1007/BF01297028
29. Moshiree, B, McDonald, R, Hou, W, and Toskes, PP. Comparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesis. Dig Dis Sci. (2010) 55:675–83. doi: 10.1007/s10620-009-1038-3
30. Onoma, M, Yogo, K, Ozaki, K, Kamei, K, Akima, M, Koga, H, et al. Oral mitemcinal (GM-611), an erythromycin-derived prokinetic, accelerates normal and experimentally delayed gastric emptying in conscious dogs. Clin Exp Pharmacol Physiol. (2008) 35:35–42. doi: 10.1111/j.1440-1681.2007.04744.x
31. Uchida, K, Otake, K, Inoue, M, Koike, Y, Matsushita, K, Araki, T, et al. Chronic intestinal pseudo-obstruction due to lymphocytic intestinal leiomyositis: case report and literature review. Intractable Rare Dis Res. (2012) 1:35–9. doi: 10.5582/irdr.2012.v1.1.35
32. Murtagh, K, Oldroyd, L, Ressel, L, and Batchelor, D. Successful management of intestinal pseudo-obstruction in a dog. Vet Rec Case Rep. (2013) 1:25. doi: 10.1136/vetreccr-2013-000025
33. Vandenberge, V, Paepe, D, Vercauteren, G, Daminet, S, Ducatelle, R, and Chiers, K. Chronic intestinal pseudo-obstruction in a Bernese Mountain dog. Vlaams Diergeneeskundig Tijdschrift. (2009) 78:117–20. doi: 10.21825/vdt.87506
34. Kopke, MA, Ruaux, CG, and Gal, A. Myenteric ganglionitis and intestinal leiomyositis in a Jack Russell terrier. J Small Anim Pract. (2020) 61:772–5. doi: 10.1111/jsap.12962
35. Jennes, E, Guggenberger, D, Zotz, R, Thompson, L, Brümmendorf, TH, Koschmieder, S, et al. Perioperative intravenous immunoglobulin treatment in a patient with severe acquired von Willebrand syndrome: case report and review of the literature. Clin Case Rep. (2017) 5:664–70. doi: 10.1002/ccr3.890
36. Arumugham, VB, and Rayi, A. Intravenous immunoglobulin (IVIG) [Updated 2023 Jul 3]. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing (2023).
37. Whelan, MF, O'Toole, TE, Chan, DL, Rozanski, EA, DeLaforcade, AM, Crawford, SL, et al. Use of human immunoglobulin in addition to glucocorticoids for the initial treatment of dogs with immune-mediated hemolytic anemia. J Vet Emerg Crit Care. (2009) 19:158–64. doi: 10.1111/j.1476-4431.2009.00403.x
38. Kazatchkine, MD, and Kaveri, SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. (2001) 345:747–55. doi: 10.1056/NEJMra993360
39. Kurtzberg, J, Friedman, HS, Chaffee, S, Falletta, JM, Kinney, TR, Kurlander, R, et al. Efficacy of intravenous gamma globulin in autoimmune-mediated pediatric blood dyscrasias. Am J Med. (1987) 83:4–9. doi: 10.1016/0002-9343(87)90544-4
40. Flores, G, Cunningham-Rundles, C, Newland, AC, and Bussel, JB. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia: results in 73 patients. Am J Hematol. (1993) 44:237–42. doi: 10.1002/ajh.2830440404
41. Bianco, D, Armstrong, PJ, and Washabau, RJ. A prospective, randomized, double-blinded, placebo-controlled study of human intravenous immunoglobulin for the acute management of presumptive primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med. (2009) 23:1071–8. doi: 10.1111/j.1939-1676.2009.0358.x
42. Norris, PA, Kaur, G, and Lazarus, AH. New insights into IVIg mechanisms and alternatives in autoimmune and inflammatory diseases. Curr Opin Hematol. (2020) 27:392–8. doi: 10.1097/MOH.0000000000000609
43. Spurlock, N, and Prittie, J. Use of human intravenous immunoglobulin therapy in Veterianry clinical practice. Vet Clin North Am. (2020) 50:1371–83. doi: 10.1016/j.cvsm.2020.07.015
44. Scott-Moncrieff, JC, Reagan, WJ, Snyder, PW, and Glickman, LT. Intravenous administration of human immune globulin in dogs with immune-mediated hemolytic anemia. J Am Vet Med Assoc. (1997) 210:1623–7. doi: 10.2460/javma.1997.210.11.1623
45. Kane, B, and Greer, RM. Human intravenous immunoglobulin use for hematological immune-mediated disease in dogs. J Am Vet Med Assoc. (2023) 261:1004–10. doi: 10.2460/javma.23.01.0043
46. Balog, K, Huang, A, Sum, S, Moore, GE, Thompson, C, and Scott-Moncrieff, JC. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med. (2013) 27:536–41. doi: 10.1111/jvim.12066
47. Stikeman, E, and Bianco, D. Use of human intravenous immunoglobulin for the treatment of 12 dogs with newly diagnosed malignant disease and presumed secondary immune-mediated thrombocytopenia. J Small Anim Pract. (2024) 65:338–45. doi: 10.1111/jsap.13700
48. Bianco, D, and Hardy, M. Treatment of Evans’ syndrome with human intravenous immunoglobulin and lefunomide in a diabetic dog. J Am Anim Hosp Assoc. (2009) 45:147–50. doi: 10.5326/0450147
49. Nuttall, T, and Malham, T. Successful intravenous human immunoglobulin treatment of drug-induced Stevens-Johnson syndrome in a dog. J Small Anim Pract. (2004) 45:357–61. doi: 10.1111/j.1748-5827.2004.tb00248.x
50. Trotman, T, Phillips, H, Fordyce, H, King, LG, Morris, DO, and Giger, U. Treatment of severe adverse cutaneous drug reactions with human intravenous immunoglobulin in two dogs. J Am Anim Hosp Assoc. (2006) 42:312–20. doi: 10.5326/0420312
51. Rahilly, LJ, Keating, JH, and O’Toole, TE. The use of intravenous human immunoglobulin in treatment of severe pemphigus foliaceus in a dog. J Vet Intern Med. (2006) 20:1483–6. doi: 10.1111/j.1939-1676.2006.tb00770.x
52. Abelson, A, Shelton, G, and Whelan, M. Use of mycophenolate mofetil as a rescue agent in the treatment of severe generalized myasthenia gravis in three dogs. J Vet Emerg Crit Care (San Antonio). (2009) 19:369–74. doi: 10.1111/j.1476-4431.2009.00433.x
53. Grozdanic, S, Harper, M, and Kecova, H. Antibody-mediated retinopathies in canine patients: mechanism, diagnosis, and treatment modalities. Vet Clin North Am Small Anim Pract. (2008) 38:361–87. doi: 10.1016/j.cvsm.2007.12.003
54. Nakane, S, Mukaino, A, Ihara, E, and Ogawa, Y. Autoimmune gastrointestinal dysmotility: the interface between clinical immunology and neurogastroenterology. Immunol Med. (2021) 44:74–85. doi: 10.1080/25785826.2020.1797319
55. Vilojoen, AD, Tamborini, A, Watson, PJ, and Bexfield, NH. Clinical characteristics and histology of cholecystectomised dogs with nongravity-dependent biliary sludge: 16 cases (2014-2019). J Small Anim Pract. (2021) 62:478–88. doi: 10.1111/jsap.13302
Keywords: intestinal leiomyositis, ileus, pseudo-obstruction, CIPO, intravenous immunoglobulin (IVIg), cholecystitis, T-cell activation
Citation: Olivarez MP, Williams J, Udomteerasuwat N, Corner S and Byers C (2024) Case report: Successful treatment of intestinal leiomyositis in a dog using adjunctive intravenous immunoglobulin. Front. Vet. Sci. 11:1373882. doi: 10.3389/fvets.2024.1373882
Edited by:
Muhammad Saqib, University of Agriculture, PakistanReviewed by:
Francesk Mulita, General University Hospital of Patras, GreeceAlyse Zacuto, Atlantic Veterinary Internal Medicine and Oncology, United States
Copyright © 2024 Olivarez, Williams, Udomteerasuwat, Corner and Byers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Patrick Olivarez, bWljaGVsbGUucGF0cmlja0B0aHJpdmVwZXQuY29t; Jarod Williams, amFyb2Qud2lsbGlhbXNAdGhyaXZlcGV0LmNvbQ==
 Michelle Patrick Olivarez
Michelle Patrick Olivarez Jarod Williams
Jarod Williams Nutnapong Udomteerasuwat3
Nutnapong Udomteerasuwat3 Sarah Corner
Sarah Corner Christopher Byers
Christopher Byers