
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 14 March 2024
Sec. Veterinary Infectious Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1373178
Background: Dogs and cats are the hosts of many vector-borne human pathogens that can be transmitted to humans. Given their direct and intimate contact with humans, companion dogs and cats are considered direct sentinels of vector-borne human pathogens. However, limited information is currently available regarding canine and feline zoonotic pathogens in China. This study detected canine and feline vector-borne human pathogens to better understand the potential risk to humans.
Methods: Blood samples were collected from 275 domestic companion animals (117 dogs and 158 cats) living in Tianjin city, China, and the presence of DNA from Anaplasma, Babesia, Bartonella, and Rickettsia was detected by semi-nested polymerase chain reaction (PCR). The PCR products of the expected size were sequenced, and these newly generated sequences were subjected to BLASTN, nucleotide identity, and phylogenetic analyses.
Results: A total of 24 blood samples tested positive for vector-borne pathogens in companion dogs and cats in Tianjin city, China, with a relatively low positive rate of 8.7%. Specifically, seven human pathogens, including Rickettsia raoultii, Candidatus Rickettsia jingxinensis, Rickettsia sibirica, Rickettsia felis, Babesia venatorum, Bartonella tribocorum, and Bartonella Henselae, were identified. In addition, Anaplasma ovis with zoonotic potential and Candidatus A. cinensis were detected.
Conclusion: Our results indicate substantial genetic diversity in the vector-borne human pathogens circulating in companion dogs and cats. Interventions based on “One Health” should be taken to reduce the potential risks of contracting infection from companion dogs and cats in Tianjin, China.
Companion dogs and cats are considered good friends of humans, and they are treated like family and experience close contact with humans, sharing their living environment. Despite the benefits of companion cats and dogs for humans, they are actually important sources of many neglected infectious diseases, such as the rabies virus responsible for the infamous rabies. In addition, pet dogs and cats are the reservoir hosts of several vector-borne pathogens, such as Anaplasma phagocytophilum, Anaplasma capra, Ehrlichia chaffeensis, and Bartonella henselae (1–3). Furthermore, these pathogens can be transmitted from companion cats and dogs to humans. Therefore, canine and feline vector-borne zoonotic diseases can be prevented and controlled under the “One Health” concept in all aspects, including epidemiology (4).
The vector-borne pathogens of major concern in companion cats and dogs that can infect humans are the genera Anaplasma, Ehrlichia, Rickettsia, Borrelia, and Bartonella (4, 5). The common pathogens are A. phagocytophilum, which causes human granulocytic anaplasmosis (HGA); E. chaffeensi, which causes human monocytic ehrlichiosis (HME); Ehrlichia ewingii, which causes human granulocytic ehrlichiosis (HGE); Bar. henselae, which causes cat scratch disease; and spotted fever group rickettsiae (SFGR), which causes rickettsiosis. Many vector-borne causative agents can infect companion cats and dogs through blood-sucking arthropods (including ticks, fleas, and mites). Humans can be infested by fleas or ticks obtained in the wild and brought home by pet dogs and cats, serving as bridging hosts. Therefore, dogs and cats that are in direct contact with their owners can be considered direct sentinels for human infections, as confirmed in previous studies (2, 6–12).
In China, limited studies have been performed to determine the presence of vector-borne zoonotic pathogens in companion cats and dogs. Regarding SFGR, Rickettsia felis (13, 14), Rickettsia massiliae (15), Rickettsia conorii (16), Rickettsia raoultii, and Candidatus Rickettsia tarasevichiae (13) have been identified in dogs. Regarding Bartonella, Bar. henselae (17–20) and Bartonella clarridgeiae (20) have been detected in cats. Regarding Anaplasma, Anaplasma platys, A. phagocytophilum, Anaplasma ovis, Anaplasma bovis, and Anaplasma capra have been found in dogs (3, 21–23). To date, no human-pathogenic Babesia species have been identified in cats or dogs. More importantly, human cases caused by R. felis (24), R. raoultii (25), Candidatus R. tarasevichiae (26), Bar. henselae (27), A. phagocytophilum (28), and A. capra (29) have been found in China.
Tianjin city is the largest port city in northern China, with a large population and numerous pet stores. A previous study in Tianjin city showed that Ixodes persulcatus and Haemaphysalis longicornis were the predominant species, while no human pathogens were identified in them using molecular methods (30). In addition, a seroepidemiological survey indicated that antibodies against A. phagocytophilum, Rickettsia sibirica, and Ehrlichia chaffeensis were identified in humans (31). To date, Bartonella and Babesia have not been identified in any samples. Furthermore, no study has been performed to reveal the presence of vector-borne human pathogens in companion cats or dogs. As these animals are direct sentinels for human infections, here, a comprehensive molecular survey was conducted to assess the potential risk to humans of agents belonging to the genera Anaplasma, Babesia, Bartonella, and Rickettsia in dogs and cats in Tianjin, China.
From March to October 2021, 275 pets (i.e., 117 dogs and 158 cats) living in urban areas (Hexi District) of Tianjin city were randomly enrolled at a veterinary medical center in Tianjin city after presentation for a general inspection. These pets were selected to detect vector-borne bacteria, and all of them were clinically healthy with no clinical signs. In addition, all of the animals were born in Tianjin city and had no traveling history outside Tianjin city in the past year. A volume of 1 mm of the EDTA-anticoagulated whole blood sample was collected from each animal by venipuncture of the jugular vein with the help of veterinarians, immediately stored in a −80°C freezer, and subsequently transported on dry ice to the laboratory of the College of Basic Medicine, Chengde Medical University. This study was approved by the Scientific Ethics Committee of Chengde Medical University (number 202004). Oral consent for blood collection was obtained from all the owners of the companion dogs and animals.
The frozen blood sample was thawed, and 200 μL was used for DNA extraction using the OMEGA Blood DNA Kit (OMEGA, Norcross, GA, United States) as per the manufacturer’s instructions. The DNA was eluted in 80 μL of elution buffer and stored at −20°C until further pathogen detection.
Vector-borne pathogens were identified by detecting their DNA using semi-nested PCR. The genus Rickettsia was identified by amplifying the partial ompA gene using the primer pairs Rr190k.70p/Rr190k.720n and Rr190k.70p/Rr190k.602n (32). The genus Babesia was detected by amplifying the partial 18S rRNA gene using the primer pairs BS1/PiroC and PiroA/PiroC (33). The primer pairs F/R1 and F/R2 targeting the gltA gene were used to detect Anaplasma ovis (34). The primer pairs Pglt-F/Pglt-R1 and Pglt-F/Pglt-R2 targeting the gltA gene were used to detect Candidatus A. cinensis (35). The primer pairs Bar-ftsz-F1/Bar-ftsz-R (Bar-ftsz-RM) and Bar-ftsz-F2/Bar-ftsz-R (Bar-ftsz-RM) targeting the ftsz gene were used to detect the genus Bartonella (36). The gltA gene was also amplified using the primer pairs Bar-gltA-F/Bar-gltA-R1 and Bar-gltA-F/Bar-gltA-R2, as described by Jian et al. (36). All the primer sequences used in the present study are shown in Table 1. The PCR procedures were the same as those used in previous studies (32–36). In addition, to prevent contamination, the PCR mixture preparation, template addition, and agarose gel electrophoresis were performed in a fume hood in three separate rooms, and filter tips were also used in each assay. Furthermore, ddH2O was used as a negative control.
The PCR products were examined by electrophoretic analysis on a 1.0% agarose gel. The amplicons of the expected size were purified from agarose gels using the Takara MiniBEST Agarose Gel DNA Extraction Kit Version 4.0 (Takara, Dalian, China) and sequenced bidirectionally with the PCR primers using the ABI-PRISM Dye Termination Sequencing Kit and the ABI 3730 Genetic Analyzer.
All the sequences recovered in this study were subjected to BLASTN against the GenBank database to determine the similarity with known sequences. The MegAlign program in Lasergene was used to calculate the nucleotide sequence identities between the sequences in this study and reference sequences (37). PhyML 3.0 was used to reconstruct the maximum likelihood (ML) tree (38). The most adequate nucleotide substitution model (GTR + Γ + I) used for phylogenetic analysis was estimated by MEGA 6.0.6 (39). The bootstrap analysis with 1,000 replicates was performed to evaluate the reliability of the trees. All the sequences in this study have been deposited in GenBank, and the accession numbers corresponding to each sequence are shown in Supplementary Table S1.
A Chi-square test was used to compare the positive rates of vector-borne pathogens infection in cats and dogs using SPSS software version 24.0 (Armonk, New York, United States), and a p < 0.05 was considered to indicate statistical significance.
The BLASTN analysis of sequences obtained using PCR showed that 24 blood samples tested positive for a diverse range of vector-borne pathogens, with a total positive rate of 8.7% (24/275). In total, seven causative agents, including R. raoultii, Candidatus R. jingxinensis, R. sibirica, R. felis, Bab. venatorum, Bar. Tribocorum, and Bar. henselae, were identified, as were A. ovis with zoonotic potential and Candidatus A. cinensis. Of the 24 positive samples, nine were from cats, and 15 were from dogs, with positive rates of 5.7% (9/158) and 12.8% (15/117), respectively, presenting no significant difference (p = 0. 38).
Specifically, 13 tested positive for Rickettsia, with a total positive rate of 4.7%. In detail, the positive rates of Rickettsia infection in cats and dogs were 3.8% (6/158) and 6.0% (7/117), respectively, showing no significant difference (p = 0.398). Based on the BLASTN analysis, R. raoultii, Candidatus R. jingxinensis, and R. sibirica were identified in dogs, and R. raoultii, Candidatus R. jingxinensis, R. sibirica, and R. felis were identified in cats. In addition, Anaplasma was identified in one cat (0.6%, 1/158) and three dogs (2.6%, 3/117), with no significant difference in the positive rate (p = 0.186). The BLASTN analysis revealed A. ovis infection in one cat and two dogs, as well as Candidatus A. cinensis infection in one dog. Furthermore, the positive rate of dogs infected with Bab. venatorum (1.9%, 3/158) was similar to that of cats (1.7%, 2/117). Interestingly, Bartonella species, including Bar. tribocorum and Bar. henselae, were detected only in dogs (1.7%, 2/117). Co-infection with different pathogens was not observed in any of the animals.
Regarding Bab. venatorum, five partial 18S rRNA gene sequences, including two from cats and three from dogs, shared 100% nucleotide identity. Furthermore, all five of these sequences presented the highest nucleotide identity of 99.7% with the isolate Weichang-HcBv3 (MG869297), which was identified in a tick from Weichang County of China, and more than 99.3% nucleotide identity with other Bab. venatorum isolates in GenBank. Consistently, these five variants clustered with other Bab. venatorum isolates in the phylogenetic tree (Figure 1A).
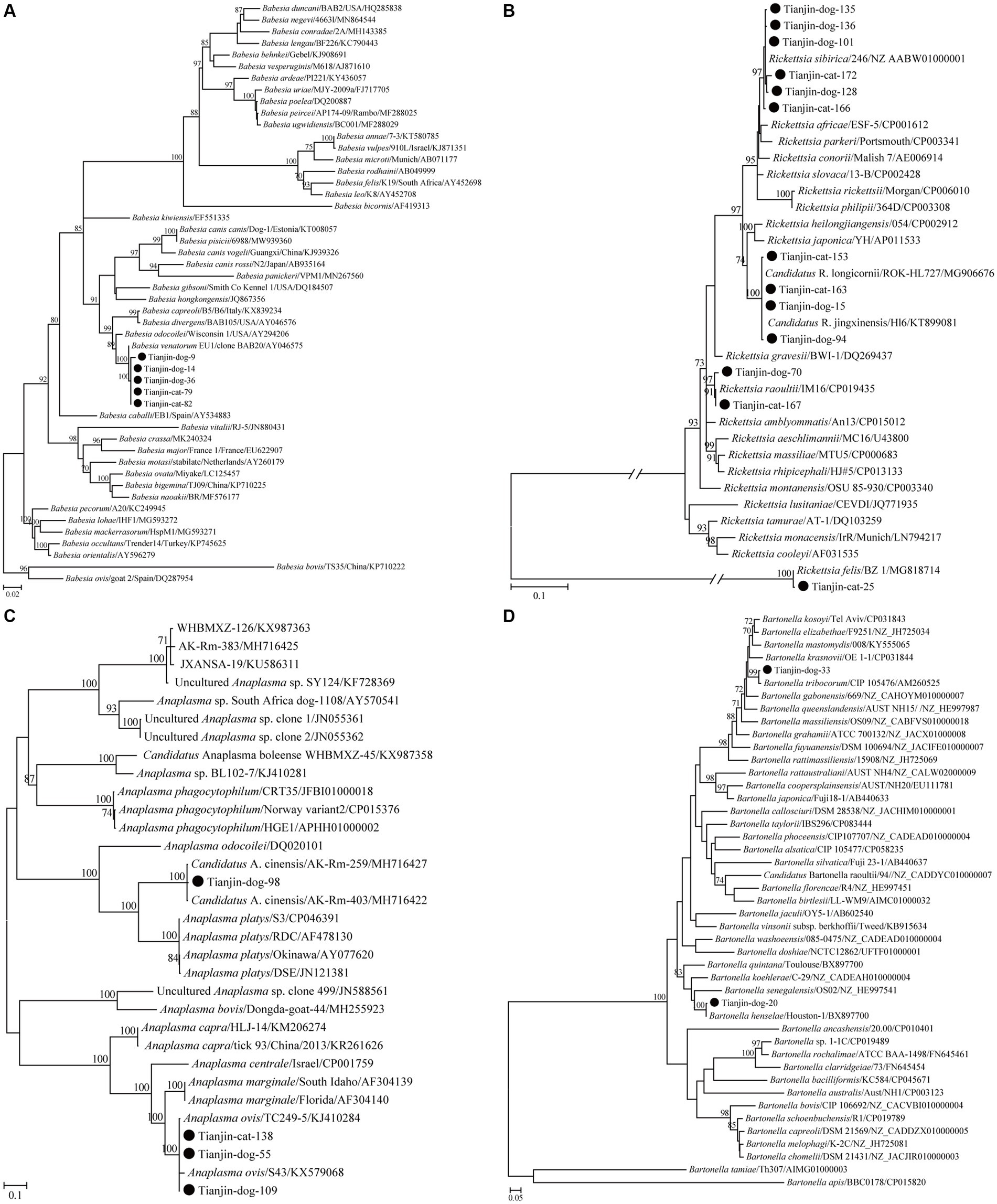
Figure 1. ML trees reconstructed based on the partial 18S rRNA (376 bp) gene sequences of Babesia (A), partial ompA (532 bp) gene sequences of Rickettsia (B), partial gltA (355 bp) gene sequences of Anaplasma (C), and partial ftsz (575 bp) gene sequences of Bartonella (D). Bootstrap values were calculated with 1,000 replicates and only >70% are shown. Sequences determined herein are marked with a black circle. The tree was mid-point rooted, and the scale bar represents the number of nucleotide substitutions per site.
Regarding the genus Rickettsia, all 13 partial ompA gene sequences were classified into four groups in the phylogenetic tree and corresponded to R. felis, Candidatus R. jingxinensis, R. raoultii, and R. sibirica (Figure 1B). The first group contained only one sequence detected in cats that shared 100% nucleotide identity with the reference sequence of R. felis URRWXCal2 (CP000053) and more than 97.4% nucleotide identity with others in GenBank. The second consisted of four sequences, including two from cats and another two from dogs, presented 99.6–100% nucleotide identities with each other, and shared the highest nucleotide identities of 98.3–99.8% with those of Candidatus R. longicornii in GenBank. The third group contained two sequences, including one from a cat and the other from a dog, presented 99.4% nucleotide identity with each other, and shared the highest nucleotide identities of 98.6–100% with those of R. raoultii in GenBank. The last group comprised six sequences, including two from cats and four from dogs, and exhibited 99.6 to 100% nucleotide identities with each other and 99.2 to 100% with those of R. sibirica in GenBank.
Regarding the genus Anaplasma, two species, namely, A. ovis and Candidatus A. cinensis, were identified based on the similarity of nucleotide sequences. In the phylogenetic tree, the Candidatus Anaplasma cinensis isolates in this study clustered with known Candidatus A. cinensis variants, separated from A. platys (Figure 1C). A partial gltA gene sequence from one dog herein shared 98.6–99.5% nucleotide identities with those of Candidatus A. cinensis in GenBank. All three partial gltA gene sequences of A. ovis in this study clustered with those of A.ovis in the gltA-based tree (Figure 1C). All of them shared 100% nucleotide identity with each other and 99.6–100% nucleotide identities with those of A. ovis in GenBank.
Regarding the genus Bartonella, two partial sequences identified from dogs but not cats clustered with those of Bar. tribocorum and Bar. henselae in the ftsZ-based tree (Figure 1D). The partial ftsZ gene sequence of Bar. tribocorum obtained in this study presented 99.6–100% nucleotide identities with known ones of Bar. tribocorum in GenBank. Regarding Bar. henselae, the partial gltA and ftsZ gene sequences in the present study had 99.7–100% and 99.1–99.8%, respectively, nucleotide identities with those of Bar. henselae in GenBank.
In terms of “One health,” companion animals, including dogs and cats, play a key role in the natural transmission and maintenance of some vector-borne pathogens as hosts (40). In this study, blood samples from companion dogs and cats were collected to identify vector-borne pathogens in the urban areas of Tianjin municipality in China. The results showed nine species, namely R. raoultii, Candidatus R. jingxinensis, R. sibirica, R. felis, Bab. venatorum, Bar. tribocorum, Bar. henselae, A. ovis, and Candidatus A. cinensis, were identified, exhibiting substantial genetic diversity of vector-borne bacteria and protozoans locally. Because these companion cats and dogs had no traveling history outside Tianjin city in the past 1 year, these pathogens identified in this study may have originated from Tianjin, China. Alternatively, these pathogens may have come from vectors that were brought back to Tianjin city by owners traveling outside Tianjin city, although this possibility is very small. Therefore, vectors, mainly including ticks, should be collected to determine their associated pathogens in Tianjin city in future studies. The total positive rate of vector-borne pathogens infection in dogs was higher than that in cats, and dogs were also more frequently infected with Rickettsia. However, similar positive rates of other agents were observed in dogs and cats. Furthermore, R. raoultii, Candidatus R. jingxinensis, R. sibirica, R. felis, Bab. venatorum, Bar. tribocorum, and Bar. henselae were pathogenic to humans. Human infections with all these pathogens have been found in China, except for Bar. tribocorum (41). In addition, A. ovis has demonstrated zoonotic potential because it has been detected in a human case (42).
Although several Babesia species have been identified in cats and dogs, most are not pathogenic to humans except for Bab. microti (43). Furthermore, rodents are considered reservoir hosts, although Bab. microti has been identified in cats in several previous studies (44). Babesia species only infecting cats and dogs were not identified in this study, while Bab. venatorum, which is also an emerging human pathogen, was detected in both dogs and cats. In China, Bab. venatorum has been identified in Ixodes persulcatus in Heilongjiang Province and has caused human infections in Heilongjiang and Xinjiang Provinces (41). In addition, Bab. venatorum was also found in I. persulcatus and Haemaphysalis concinna removed from humans in Hebei, Chian (45). In Tianjin city, I. persulcatus has been found (46), and whether Bab. venatorum infection occurs in this tick species should be determined locally. Importantly, the risk of Bab. venatorum to the local population should be evaluated in future studies.
In China, more than 20 Rickettsia species have been identified among diverse ticks (41) as hosts of ticks, dogs, and cats are naturally at risk for rickettsial infection. To date, several investigations have been performed, and at least six Rickettsia species, R. felis (13, 14), R. massiliae, Candidatus R. barbariae (15), R. conorii (16), R. raoultii, and Candidatus R. tarasevichiae (13) have been identified in dogs. In addition, the positive rate of Rickettsia infection in dogs ranged from 0.8 to 8.0%. Although reactive antibodies against Rickettsia have been found in cats, DNA was not identified in any of the blood samples (14). In this study, R. raoultii, Candidatus R. jingxinensis, R. sibirica, and R. felis were identified in cats, and the former three were identified in dogs in Tianjin, China. Moreover, the positive rates in this study were similar to those in previous studies (13, 14). Interestingly, this is the first report of Candidatus R. jingxinensis and R. sibirica in cats and dogs. In addition, R. felis was not identified in dogs in this study, although such infections have been reported previously (14). Given that the above-mentioned four Rickettsia species can infect humans, more attention should be paid to investigating human infections despite the relatively low positive rate of Rickettsia infection in companion cats and dogs in Tianjin, China. In addition, investigations should be performed to determine the prevalence of R. raoultii, Candidatus R. jingxinensis, and R. sibirica in ticks and of R. felis in cat flea (Ctenocephalides felis), which will be helpful for control and prevention of rickettsial infection in humans.
To date, eight validated species have been found in the genus Anaplasma; two have been confirmed to be pathogenic to humans and three possess zoonotic potential. Among the genus Anaplasma, A. phagocytophilum, A. platys, A. bovis, and Candidatus A. turritanum have been detected in cats (2). A. phagocytophilum, A. platys, A. bovis, A. ovis, and A. capra have been found in dogs (3, 23, 47). In this study, Candidatus A. cinensis was identified in dogs, and A. ovis was identified in both dogs and cats. To the best of our knowledge, this is the first report on A. ovis and Candidatus A. cinensis identified in cats and dogs, respectively. Given the close relationship between Candidatus A. cinensis and A. platys and the zoonotic potential of A. platys, the pathogenicity of Candidatus A. cinensis should be evaluated in the future studies.
Bartonella spp. are emerging vector-borne human pathogens, and a great number of mammals, including cats and dogs, are considered to be reservoir hosts (1). To date, Bar. henselae, Bar. quintana, Bar. koehlerae, Bar. Bovis, and Bar. clarridgeiae have been detected in both cats and dogs. In addition, Bar. vinsonii, Bar. elizabethae, Bar. washoensis, Bar. Rochalimae, and Bar. vinsonii have been found in dogs (48). More importantly, all these Bartonella species are pathogenic to humans. Therefore, these Bartonella species carried by cats and dogs pose a great potential threat to human health. Cats are the primary reservoir hosts for Bar. henselae, which is the predominant Bartonella species identified in cats (49). However, dogs may act as accidental hosts for Bar. Henselae, although it was detected occasionally in dogs (19, 50). In this study, Bar. henselae was detected in one dog and not in cats, with a low positive rate, suggesting a low potential risk of Bartonella infection in humans. However, Bar. henselae has been circulating in Tianjin city, and additional studies should be conducted in the future to determine its prevalence in cats and dogs in Tianjin, China. In addition to Bar. henselae, Bar. tribocorum, an emerging human pathogen hosted by rodents, was found in one dog. To the best of our knowledge, this is the first report on Bar. tribocorum infections in dogs. Given the direct transmission of Bar. henselae from cats to humans by scratch, whether Bar. tribocorum can be transmitted in the same way should be concerned.
One limitation of our study is that information on the gender, age, and breed of the cats and dogs was not collected; therefore, the correlations between the positive rate and gender, age, and breed cannot be determined. In this study, another limitation is that ticks, fleas, and other arthropod vectors were not collected from the companion cats and dogs. This limitation resulted in an inability to determine the transmission risks of the pathogens identified in this study. The real risks of these pathogens identified in this study to humans should be evaluated by identifying human cases in the future studies.
In conclusion, a molecular survey of vector-borne pathogens was conducted in companion cats and dogs in Tianjin, China. Our results revealed that seven human pathogens, namely R. raoultii, Candidatus R. jingxinensis, R. sibirica, R. felis, Bab. venatorum, Bar. tribocorum, and Bar. henselae, are circulating in Tianjin city, but the positive rate is low. In addition, A. ovis with zoonotic potential and Candidatus A. cinensis were identified. Considering the close and direct contact between companion cats and dogs and their owners, greater efforts are needed to prevent fleas or ticks from parasitizing these animals and decrease the transmission of the above-mentioned pathogens from wild animals to companion cats and dogs and further to humans.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The animal studies were approved by Ethics Committee of Chengde Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was not obtained from the owners for the participation of their animals in this study because only blood samples were collected with the help of veterinarians, and we did not conduct any other experiments on these animals. Oral permission was granted by the owners of the pet dogs and animals for blood collection.
RJ: Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. JX: Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Z-YX: Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. S-SC: Data curation, Investigation, Writing – original draft, Writing – review & editing. F-NW: Data curation, Investigation, Writing – original draft, Writing – review & editing. LD: Data curation, Supervision, Validation, Writing – original draft, Writing – review & editing. G-CX: Data curation, Supervision, Validation, Writing – original draft, Writing – review & editing. W-PG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Resources, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Hebei Natural Science Foundation (no. C2022406003), the Young Talent Program of Higher School in Hebei Province (no. BJ2020024), and the Scientific Research Foundation for High-level Talents of Chengde Medical University (no. 202001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1373178/full#supplementary-material
1. Breitschwerdt, EB, Maggi, RG, Chomel, BB, and Lappin, MR. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care (San Antonio). (2010) 20:8–30. doi: 10.1111/j.1476-4431.2009.00496.x
2. Pennisi, MG, Hofmann-Lehmann, R, Radford, AD, Tasker, S, Belák, S, Addie, DD, et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg. (2017) 19:542–8. doi: 10.1177/1098612X17706462
3. Shi, K, Li, J, Yan, Y, Chen, Q, Wang, K, Zhou, Y, et al. Dogs as new hosts for the emerging zoonotic pathogen Anaplasma capra in China. Front Cell Infect Microbiol. (2019) 9:394. doi: 10.3389/fcimb.2019.00394
4. Little, SE. Future challenges for parasitology: vector control and one health in the Americas. Vet Parasitol. (2013) 195:249–55. doi: 10.1016/j.vetpar.2013.04.006
5. Ceylan, O, Xuan, X, and Sevinc, F. Primary tick-borne protozoan and Rickettsial infections of animals in Turkey. Pathogens. (2021) 10:231. doi: 10.3390/pathogens10020231
6. Beall, MJ, Alleman, AR, Breitschwerdt, EB, Cohn, LA, Couto, CG, Dryden, MW, et al. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors. (2012) 5:29. doi: 10.1186/1756-3305-5-29
7. Bowman, D, Little, SE, Lorentzen, L, Shields, J, Sullivan, MP, and Carlin, EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet Parasitol. (2009) 160:138–48. doi: 10.1016/j.vetpar.2008.10.093
8. Grasperge, BJ, Wolfson, W, and Macaluso, KR. Rickettsia parkeri infection in domestic dogs, southern Louisiana, USA, 2011. Emerg Infect Dis. (2012) 18:995–7. doi: 10.3201/eid1806.120165
9. Hamer, SA, Tsao, JI, Walker, ED, Mansfield, LS, Foster, ES, and Hickling, GJ. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Res. (2009) 70:49–56. doi: 10.2460/ajvr.70.1.49
10. Iannino, F, Salucci, S, Di Provvido, A, Paolini, A, and Ruggieri, E. Bartonella infections in humans dogs and cats. Vet Ital. (2018) 54:63–72. doi: 10.12834/VetIt.398.1883.2
11. Ortuño, A, Pons, I, Nogueras, MM, Castellà, J, and Segura, F. The dog as an epidemiological marker of Rickettsia conorii infection. Clin Microbiol Infect. (2009) 15:241–2. doi: 10.1111/j.1469-0691.2008.02158.x
12. Regier, Y, Komma, K, Weigel, M, Kraiczy, P, Laisi, A, Pulliainen, AT, et al. Combination of microbiome analysis and serodiagnostics to assess the risk of pathogen transmission by ticks to humans and animals in Central Germany. Parasit Vectors. (2019) 12:11. doi: 10.1186/s13071-018-3240-7
13. Shao, JW, Yao, XY, Song, XD, Li, WJ, Huang, HL, Huang, SJ, et al. Molecular detection and genetic diversity of Rickettsia spp. in pet dogs and their infesting ticks in Harbin, northeastern China. BMC Vet Res. (2021) 17:113. doi: 10.1186/s12917-021-02823-y
14. Zhang, J, Lu, G, Kelly, P, Zhang, Z, Wei, L, Yu, D, et al. First report of Rickettsia felis in China. BMC Infect Dis. (2014) 14:682. doi: 10.1186/s12879-014-0682-1
15. Guo, J, Song, S, Cao, S, Sun, Z, Zhou, Q, Deng, X, et al. Molecular detection of zoonotic and veterinary pathogenic Bacteria in pet dogs and their parasitizing ticks in Junggar Basin, North-Western China. Front Vet Sci. (2022) 9:895140. doi: 10.3389/fvets.2022.895140
16. Liang, CW, Zhao, JB, Li, J, Chang, LT, Yu, HL, Zhang, LX, et al. Spotted fever group Rickettsia in Yunnan Province, China. Vector Borne Zoonotic Dis. (2012) 12:281–6. doi: 10.1089/vbz.2011.0835
17. Huang, K, Kelly, PJ, Zhang, J, Yang, Y, Liu, W, Kalalah, A, et al. Molecular detection of Bartonella spp. in China and St. Kitts. Can J Infect Dis Med Microbiol. (2019) 2019:3209013. doi: 10.1155/2019/3209013
18. Yuan, C, Zhu, C, Wu, Y, Pan, X, and Hua, X. Bacteriological and molecular identification of Bartonella species in cats from different regions of China. PLoS Negl Trop Dis. (2011) 5:e1301. doi: 10.1371/journal.pntd.0001301
19. Zhang, XL, Li, XW, Li, WF, Huang, SJ, and Shao, JW. Molecular detection and characterization of Bartonella spp. in pet cats and dogs in Shenzhen, China. Acta Trop. (2019) 197:105056. doi: 10.1016/j.actatropica.2019.105056
20. Zhang, Y, Zhang, Z, Lou, Y, and Yu, Y. Prevalence of hemoplasmas and Bartonella species in client-owned cats in Beijing and Shanghai, China. J Vet Med Sci. (2021) 83:793–7. doi: 10.1292/jvms.20-0681
21. Cui, Y, Yan, Y, Wang, X, Cao, S, Zhang, Y, Jian, F, et al. First molecular evidence of mixed infections of Anaplasma species in dogs in Henan, China. Ticks Tick Borne Dis. (2017) 8:283–9. doi: 10.1016/j.ttbdis.2016.12.001
22. Hussain, S, Hussain, A, Aziz, MU, Song, B, Zeb, J, Hasib, FMY, et al. First molecular confirmation of multiple zoonotic vector-borne diseases in pet dogs and cats of Hong Kong SAR. Ticks Tick Borne Dis. (2023) 14:102191. doi: 10.1016/j.ttbdis.2023.102191
23. Yang, B, Ye, C, Sun, E, We, NY, Qian, D, and Sun, H. First molecular evidence of Anaplasma spp. co-infection in stray dogs from Anhui, China. Acta Trop. (2020) 206:105453. doi: 10.1016/j.actatropica.2020.105453
24. Teng, Z, Zhao, N, Ren, R, Zhang, X, Du, Z, Wang, P, et al. Human Rickettsia felis infections in mainland China. Front Cell Infect Microbiol. (2022) 12:997315. doi: 10.3389/fcimb.2022.997315
25. Li, H, Zhang, PH, Huang, Y, Du, J, Cui, N, Yang, ZD, et al. Isolation and identification of Rickettsia raoultii in human cases: a surveillance study in 3 medical centers in China. Clin Infect Dis. (2018) 66:1109–15. doi: 10.1093/cid/cix917
26. Liu, W, Li, H, Lu, QB, Cui, N, Yang, ZD, Hu, JG, et al. Candidatus Rickettsia tarasevichiae infection in eastern Central China: a case series. Ann Intern Med. (2016) 164:641–8. doi: 10.7326/M15-2572
27. Liu, Q, Eremeeva, ME, and Li, D. Bartonella and Bartonella infections in China: from the clinic to the laboratory. Comp Immunol Microbiol Infect Dis. (2012) 35:93–102. doi: 10.1016/j.cimid.2012.01.002
28. Zhang, L, Liu, Y, Ni, D, Li, Q, Yu, Y, Yu, XJ, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA. (2008) 300:2263–70. doi: 10.1001/jama.2008.626
29. Li, H, Zheng, YC, Ma, L, Jia, N, Jiang, BG, Jiang, RR, et al. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect Dis. (2015) 15:663–70. doi: 10.1016/S1473-3099(15)70051-4
30. Wu, TY, Wang, W, Chen, SB, Qin, N, Zhang, J, Li, PY, et al. Preliminary survey of ticks and tick⁃borne pathogens in Tianjin, China. Chin J Vector Biol Control. (2013) 24:246–8.
31. Liang, CW, Zhang, Y, Zhao, JB, Zhang, ZL, Yu, HL, Yin, JY, et al. Seroepidemiological survey of tickborne rickettsial diseases among high-risk population in Tianjin. Chin J Public Health. (2011) 27:719–20.
32. Ishikura, M, Ando, S, Shinagawa, Y, Matsuura, K, Hasegawa, S, Nakayama, T, et al. Phylogenetic analysis of spotted fever group rickettsiae based on gltA, 17-kDa, and rOmpA genes amplified by nested PCR from ticks in Japan. Microbiol Immunol. (2003) 47:823–32. doi: 10.1111/j.1348-0421.2003.tb03448.x
33. Armstrong, PM, Katavolos, P, Caporale, DA, Smith, RP, Spielman, A, and Telford, SR 3rd. Diversity of Babesia infecting deer ticks (Ixodes dammini). Am J Trop Med Hyg. (1998) 58:739–42. doi: 10.4269/ajtmh.1998.58.739
34. Guo, WP, Huang, B, Zhao, Q, Xu, G, Liu, B, Wang, YH, et al. Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an, China. PLoS Negl Trop Dis. (2018) 12:e0006916. doi: 10.1371/journal.pntd.0006916
35. Guo, WP, Zhang, B, Wang, YH, Xu, G, Wang, X, Ni, X, et al. Molecular identification and characterization of Anaplasma capra and Anaplasma platys-like in Rhipicephalus microplus in Ankang, Northwest China. BMC Infect Dis. (2019) 19:434. doi: 10.1186/s12879-019-4075-3
36. Jian, R, Ren, Q, Xue, J, Xie, GC, Wang, J, Chen, GQ, et al. Genetic diversity of Bartonella infection in residential and field rodents in Hebei, China. Front Microbiol. (2022) 13:1039665. doi: 10.3389/fmicb.2022.1039665
37. Burland, TG. DNASTAR’s Lasergene sequence analysis software. Methods Mol Biol. (2000) 132:71–91. doi: 10.1385/1-59259-192-2:71
38. Guindon, S, Dufayard, JF, Lefort, V, Anisimova, M, Hordijk, W, and Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. (2010) 59:307–21. doi: 10.1093/sysbio/syq010
39. Tamura, K, Stecher, G, Peterson, D, Filipski, A, and Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. (2013) 30:2725–9. doi: 10.1093/molbev/mst197
40. Skotarczak, B. The role of companion animals in the environmental circulation of tick-borne bacterial pathogens. Ann Agric Environ Med. (2018) 25:473–80. doi: 10.26444/aaem/93381
41. Zhao, GP, Wang, YX, Fan, ZW, Ji, Y, Liu, MJ, Zhang, WH, et al. Mapping ticks and tick-borne pathogens in China. Nat Commun. (2021) 12:1075. doi: 10.1038/s41467-021-21375-1
42. Chochlakis, D, Ioannou, I, Tselentis, Y, and Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg Infect Dis. (2010) 16:1031–2. doi: 10.3201/eid1606.090175
43. Penzhorn, BL, and Oosthuizen, MC. Babesia species of domestic cats: molecular characterization has opened Pandora’s box. Front Vet Sci. (2020) 7:134. doi: 10.3389/fvets.2020.00134
44. Zhou, X, Xia, S, Huang, JL, Tambo, E, Zhuge, HX, and Zhou, XN. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasit Vectors. (2014) 7:509. doi: 10.1186/s13071-014-0509-3
45. Xue, J, Ren, Q, Yang, XL, Wang, J, Xie, G, Du, L, et al. Human pathogens in ticks removed from humans in Hebei. China Heliyon. (2023) 9:e13859. doi: 10.1016/j.heliyon.2023.e13859
46. Hou, XX, Liu, Y, Hao, Q, Chen, JY, Geng, Z, Song, CY, et al. Investigation on primary vectors of Borrelia burgdorferi in Ji County of Tainjin city. Chin Prev Med. (2008) 9:358–9.
47. Fukui, Y, and Inokuma, H. Subclinical infections of Anaplasma phagocytophilum and Anaplasma bovis in dogs from Ibaraki, Japan. Jpn J Infect Dis. (2019) 72:168–72. doi: 10.7883/yoken.JJID.2018.470
48. Cheslock, MA, and Embers, ME. Human Bartonellosis: an underappreciated public health problem? Trop Med Infect Dis. (2019) 4:69. doi: 10.3390/tropicalmed4020069
49. Breitschwerdt, EB. Feline bartonellosis and cat scratch disease. Vet Immunol Immunopathol. (2008) 123:167–71. doi: 10.1016/j.vetimm.2008.01.025
Keywords: companion cats and dogs, Anaplasma, Babesia, Bartonella, Rickettsia, pathogens
Citation: Jian R, Xue J, Xu Z-Y, Chen S-S, Wang F-N, Du L, Xie G-C and Guo W-P (2024) Genetic diversity of vector-borne zoonotic pathogens in companion dogs and cats, Tianjin, China. Front. Vet. Sci. 11:1373178. doi: 10.3389/fvets.2024.1373178
Received: 19 January 2024; Accepted: 28 February 2024;
Published: 14 March 2024.
Edited by:
Hua-Ji Qiu, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Lanjing Wei, University of Kansas, United StatesCopyright © 2024 Jian, Xue, Xu, Chen, Wang, Du, Xie and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Ping Guo, Z3Vvd2VucGluZ0Bud3N1YWYuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.