
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 18 June 2024
Sec. Veterinary Emergency and Critical Care Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1371781
Introduction: Cats with cardiomyopathy face an increased risk of arterial thromboembolism (ATE). Although clopidogrel is frequently utilized to mitigate this risk, feline responses to this therapy exhibit variability. This study evaluated 2 viscoelastic devices, thromboelastography (TEG) and Viscoelastic Coagulation Monitor (VCM), for monitoring clopidogrel in cats in comparison to light transmission aggregometry (LTA).
Methods: Twenty-eight healthy cats received clopidogrel for 7 days. Blood was collected at baseline and after treatment for analysis by TEG, VCM, and LTA.
Results: On LTA, maximum amplitude, slope, and area under the curve (AUC) significantly decreased after treatment (p < 0.0001). On VCM, maximum clot firmness (MCF) significantly increased after treatment (p = 0.002). On TEG, R-time significantly prolonged (p = 0.024), while K and alpha angle significantly changed (p = 0.0002 and p = 0.0014, respectively). There was a moderate negative correlation between TEG R-time and LTA AUC (r = −0.39, p = 0.042). Eight cats were identified as non-responders to clopidogrel. Of the 8 non-responders, 6 (75%) had shortened R time after treatment. VCM appeared to be less discriminatory in identifying non-responders.
Discussion: LTA remained the gold standard of monitoring clopidogrel treatment in cats. Unexpected changes on VCM and TEG were likely related to high interindividual and assay variability and increased sensitivity of feline platelets. R-time on TEG may have potential utility for point-of-care monitoring of clopidogrel response in cats.
Cats with cardiomyopathies have increased risk to develop arterial thromboembolism (ATE). The reported survival rate for ATE in the feline population ranges from 35 to 50% (1). Platelets contribute to the pathogenesis of ATE in cats affected by hypertrophic cardiomyopathy (HCM) through an increased expression of P-selectin, a biomarker of platelet activation (2). Clopidogrel is an antiplatelet drug that irreversibly inhibits P2Y12, a platelet ADP receptor, and is commonly prescribed in HCM cats to reduce the risk of intracardiac thrombosis and ATE. Although clopidogrel has been shown to be superior to aspirin in reducing ATE recurrence in cats that survived a primary ATE event, recurrence remains, which is likely caused by the highly variable response to clopidogrel in cats (3–5).
Similarly, in human medicine, great emphasis is placed on platelet function monitoring in patients at high risk of recurrent thrombotic events due to a high variability in the pharmacodynamic effect of clopidogrel among individuals (6, 7). In humans, this is largely due to polymorphisms in the CYP2C19 gene, responsible for encoding a member of the cytochrome P450 enzyme that mediates the biotransformation of the pro-drug into the active metabolite (8). A study by Ueda et al. demonstrated that clopidogrel resistance in a population of cats with HCM was due to a single nucleotide polymorphism in the P2RY1 gene, which encodes one of the ADP receptors, P2Y1. Cats with this variant consistently showed reduced response to clopidogrel measured by ADP-induced platelet aggregation (5). In addition, various ex vivo studies have shown variable response and possible resistance to clopidogrel in up to 15 to 35% of cats. Although no direct associations between clopidogrel resistance and ATE has been found in cats, it is well documented in human medicine that resistance to the drug is associated with recurrent thrombotic events after myocardial infarction (6, 7). This emphasizes the importance of optimizing antiplatelet therapy on an individualized approach. One way to overcome the variable pharmacodynamics of clopidogrel is to monitor on-treatment platelet function. In human medicine, many different tests are available to evaluate platelet function in patients receiving antiplatelet drugs (8, 9). Although light transmission or whole blood impedance aggregometry are considered the gold standard technology, these assays require dedicated equipment and specialized training to operate making their use in general practice impractical (9). In humans, several studies investigated the sensitivity of viscoelastic testing to assess platelet function such as platelet mapping with conflicting results (10). In veterinary medicine, a few diagnostic tests such as flow cytometry have been investigated for monitoring of clopidogrel induced platelet inhibition (11, 12). Unfortunately, due to the limited distribution outside of advanced laboratories and the requirement of trained personnel, platelet function monitoring remains an option exclusive for academic settings and advanced medical centers.
Viscoelastic Platelet Mapping on TEG, which utilizes separate assessments of clot formation due to thrombin, fibrin and platelet function, have been studied in dogs and humans to assess pharmacodynamic effects of antiplatelet therapy. However, increased platelet sensitization and inter assay variation in cats likely limit its ability to detect antiplatelet effects (13). VCM Vet (Entegrion, Inc.) is a novel portable, compact, and user-friendly viscoelastic diagnostic device. A small amount of whole blood without anticoagulant or activators can be processed at the point-of-care to assess global coagulation (14). The VCM has been validated in a healthy feline population, as well as, in various other animal species (15). Owing to the species differences in platelet physiology and the hyper-responsive nature of feline platelets, TEG or VCM may be able to assess changes in platelet function without any specialized equipment, agonists or modifications (15). Hence the VCM may be a viable option to monitor clopidogrel therapy in cats in a clinical setting.
The primary aim of this study was to compare the performance of the novel device, VCM Vet, to kaolin-activated thromboelastography (TEG), and light-transmission aggregometry (LTA), the gold standard, in monitoring clopidogrel treatment and detecting non-responders to clopidogrel in cats. Our secondary aim was to correlate changes between VCM Vet, TEG and the gold standard, LTA.
This prospective study was reviewed and approved by the University of California, Davis Institutional Animal Care and Use Committee (IACUC #20359).
Thirty healthy cats from a pathogen-free, university-owned colony of domestic shorthaired cats were enrolled in this study. Given a two-tailed design with an alpha level of 0.05 and an effect size of 15%, we expected to identify significant differences with an 80% power with a sample size of 22 cats. This calculation was based on standard deviation of the change in ADP-induced platelet aggregation in a previous study investigating the effect of clopidogrel in healthy cats by LTA (4). Given the likelihood of difficulties in sample collection and possible clotting of blood samples, additional enrollment of eight cats was allowed and the total number of cats enrolled was increased to 30. All cats were identified as healthy based on general physical examination, CBC and biochemistry performed before the study. None of the animals were on any medications for the duration of the study. All cats in the colony were housed in groups and had access to enrichment activities like daily social interactions with humans, toys, boxes and shelves. Drug administration and blood draw were timed so that blood collection could be performed only in 4 cats per day (2 in the morning and 2 in the afternoon). Blood collection was performed 5 to 15 min apart to minimize the variation of duration between sampling and assays.
All blood samples were obtained without sedation. Blood was drawn using a 21 Gage needle from the jugular vein or 22 Gage butterfly needle from the medial saphenous vein. Approximately 6 mL of blood was drawn from each animal on the day of the experiment. Whole blood was separated into aliquots of three vials of citrated blood containing 3.2% sodium citrate and 1 vial of nonanticoagulated blood. Citrated samples were placed on a rack in an insulated container at room temperature and delivered to the central laboratory within 30 min of collection.
The remaining nonanticoagulated whole blood (300 μL) in polypropylene syringes was placed immediately into a pre-warmed testing cartridge before insertion into the point-of-care viscoelastic test (VCM Vet, Durham, NC). One citrated tube was used for CBC analysis (HM5, Abaxis, Parsippany, NJ) and platelet count was adjusted due to the 10% dilution effect of citrate.
Thromboelastography was performed on citrated whole blood within 2 h of collection. TEG sample processing (ie, waiting time, temperature) was conducted according to our clinical laboratory standardized protocol. Upon arrival at the laboratory, samples were maintained at room temperature and gently inverted 3 to 5 times immediately prior to performing the test. After 1 mL of citrated blood was mixed with kaolin (cat. no. 6300, Haemonetics Corp, Boston, MA), an aliquot of 360 ul of kaolin-activated blood was added to pre-warmed plain cup with 20 μL of CaCl2 and analyzed (TEG 5000, Haemonetics Corp, Boston, MA). The analysis was interrupted after running the samples for 60 min.
Citrated blood was transferred to round-bottom polypropylene tubes and placed in 37° C bead bath for 30 min to facilitate sedimentation of erythrocytes. Platelet rich plasma (PRP) was then generated by centrifugation at 200 × g for 5 min (no brakes, 25–27° C). Complete blood count of PRP was performed using an automated analyzer (HM5 Hematology analyzer, Zoetis, Parsippany, NJ) and confirmed by blood smear evaluation, within 2 h after collection. Platelet count was adjusted to account for the 10% dilutional effect of citrate.
Platelet rich plasma generated from citrated whole blood was diluted to 1.5 × 108 /mL using Tyrodes-HEPES (pH 7.2, 5 mM dextrose, no divalent cations) as described4. Platelet poor plasma, generated by centrifugation at 10,000 × g for 5 min, from each cat served as the control light transmission. PRP was aliquoted to prewarmed siliconized cuvettes (Chronolog, Havertown, PA) containing a magnetic stir bar set at a constant stir speed of 1,200 rpm. Aggregation was then recorded for 1 min as baseline before the addition of 40 μM ADP or 1 U/mL bovine α-thrombin (Haematologic Technologies, Essex Junction, VT), utilized as positive control. Aggregation was recorded for an additional 5 min and measured as percent (%) of maximum aggregation, slope, and area under the curve (AUC) transmission using commercially available software (Chronolog, Havertown, PA). The remaining plasma was used for biochemistry analysis (total protein, albumin, blood urea nitrogen, and creatinine) using automated biochemical analyzer (Vetscan 2, Abaxis, Parsippany, NJ).
After baseline measurements (Day 0), each cat received 18.75 mg clopidogrel bisulfate (Plavix, Britol-Myers Squibb/Sanofi Pharmaceuticals, Bridgewater, NJ) every 24 h by mouth for seven consecutive days. The tablet was administered using a pilling device, and the administration was confirmed by observing the cat swallowing. During the study period, all cats were monitored closely for adverse effects such as vomiting, lethargy and bleeding diathesis.
On Day 8, approximately 18 h after the last dose of clopidogrel, another 6 mL of blood was drawn for each cat. The obtained blood was separated into aliquots identical to Day 0 and was utilized to repeat CBC, VCM Vet, TEG and LTA as described above.
For each analyte obtained from each tested device VCM Vet (CT, clot time; CFT, clot formation time; AA, alpha-angle; MCF, maximum clot formation), TEG (R, reaction time; K, kinetics; Alpha, alpha angle; MA, maximum amplitude; G-value), and LTA (maximum amplitude, AUC, area under the curve, slope), clopidogrel-induced platelet inhibition (%) was standardized based on the formula:
Percent Inhibition (%) = [(Analyte Day0 – Analyte Day8)/ Analyte Day0] x 100.
For the purpose of this study, cats with percent inhibition <80% on any variables on LTA were considered non- responders (NR) (4).
Data analysis was performed using commercially available software (Prism version 10.0a, La Jolla CA, United States). General column statistics were calculated for each method of assessment. Data were tested for normality using a D’Agostino-Pearson omnibus normality test. Parametric data were reported as mean and standard deviation while non-parametric data were reported as median with interquartile range. Normally distributed and paired data were analyzed using paired t-tests while nonparametric and paired data were analyzed using Wilcoxon signed-rank test. Correlation of standardized response to clopidogrel between LTA parameters, VCM Vet and TEG analytes was calculated with Spearman’s rank. Interindividual variability was calculated as the ratio between the standard deviation of a group and its means and expressed as coefficient of variation (CV). Statistical significance was set at p < 0.05.
No adverse event was recorded during the study period. Two cats were excluded from the final analysis due to lipemic serum which resulted in inaccurate measurements of LTA. A total of 28 cats were included in the final analysis.
On Day 0, CBC and biochemistry parameters were all within our laboratory reference ranges. On day 8, platelet count on citrated blood (Day 0: 234.9 × 106/ ml ± 91.6 vs. Day 8: 350.3 × 106/ ml ± 92.4, p < 0.0001) and PRP [Day 0: 239 × 106/ ml (169–370.5) vs. Day 8: 401 × 106/ ml (237.5–509), p = 0.003] was significantly higher compared to day 0 (Table 1). No other significant difference on CBC parameters was noted on Day 8 compared to Day 0.
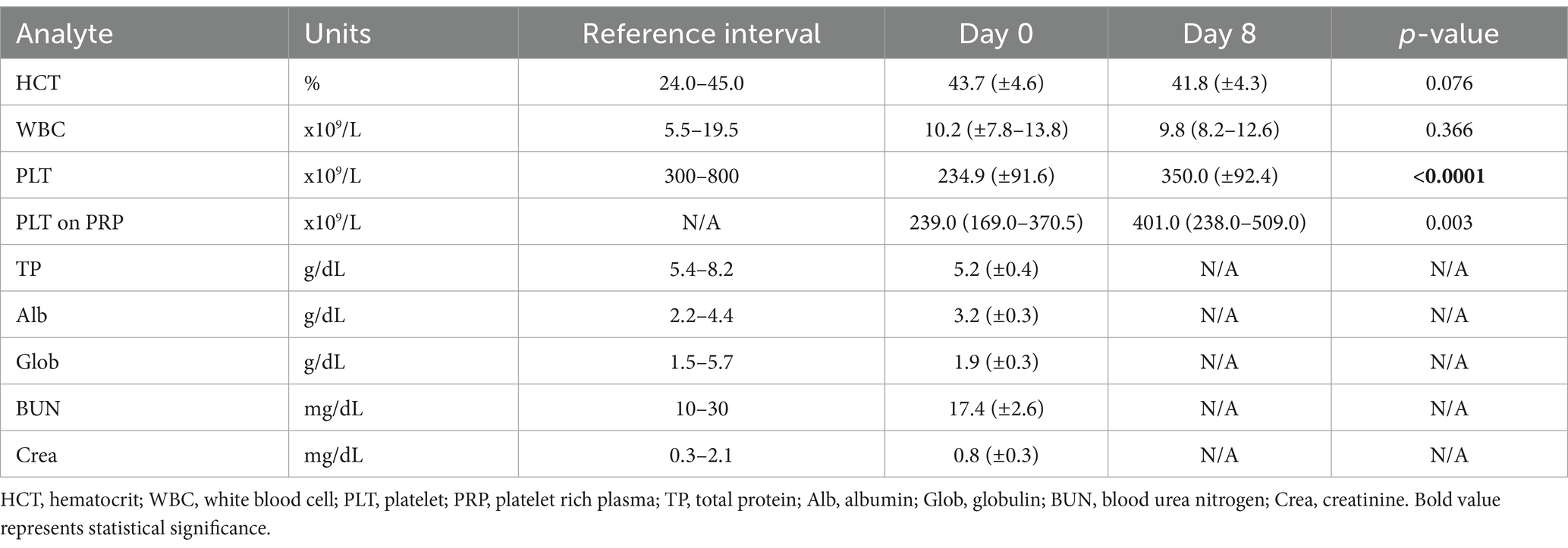
Table 1. Comparative analysis of CBC and biochemistry variables in 28 cats before (day 0) and after 7 (day 8) days of clopidogrel treatment.
All VCM Vet and TEG variables are reported in Table 2 with reference ranges generated by our institution reference laboratory (15). VCM Vet analysis demonstrated a significant increase in MCF after 7 days of clopidogrel treatment (Day 0: 36.2 ± 6.5 AU vs. Day 8: 40.1 ± 5.3 AU, p = 0.002) (Figure 1A). On TEG, R-time was significantly prolonged after clopidogrel treatment (Day 0: 2.6 min ± 0.5 vs. Day 8: 3.0 min ± 0.7, p = 0.024). In contrast, K became shorter [Day 0: 1.2 min (0.9–1.4) vs. 0.9 min (0.8–1.0), p = 0.0002], and Alpha angle increased [Day 0: 72.8° (70.8–75.9) vs. 75.9° (74.7–77) 0.0, p = 0.0014] after clopidogrel treatment (Figure 1B).
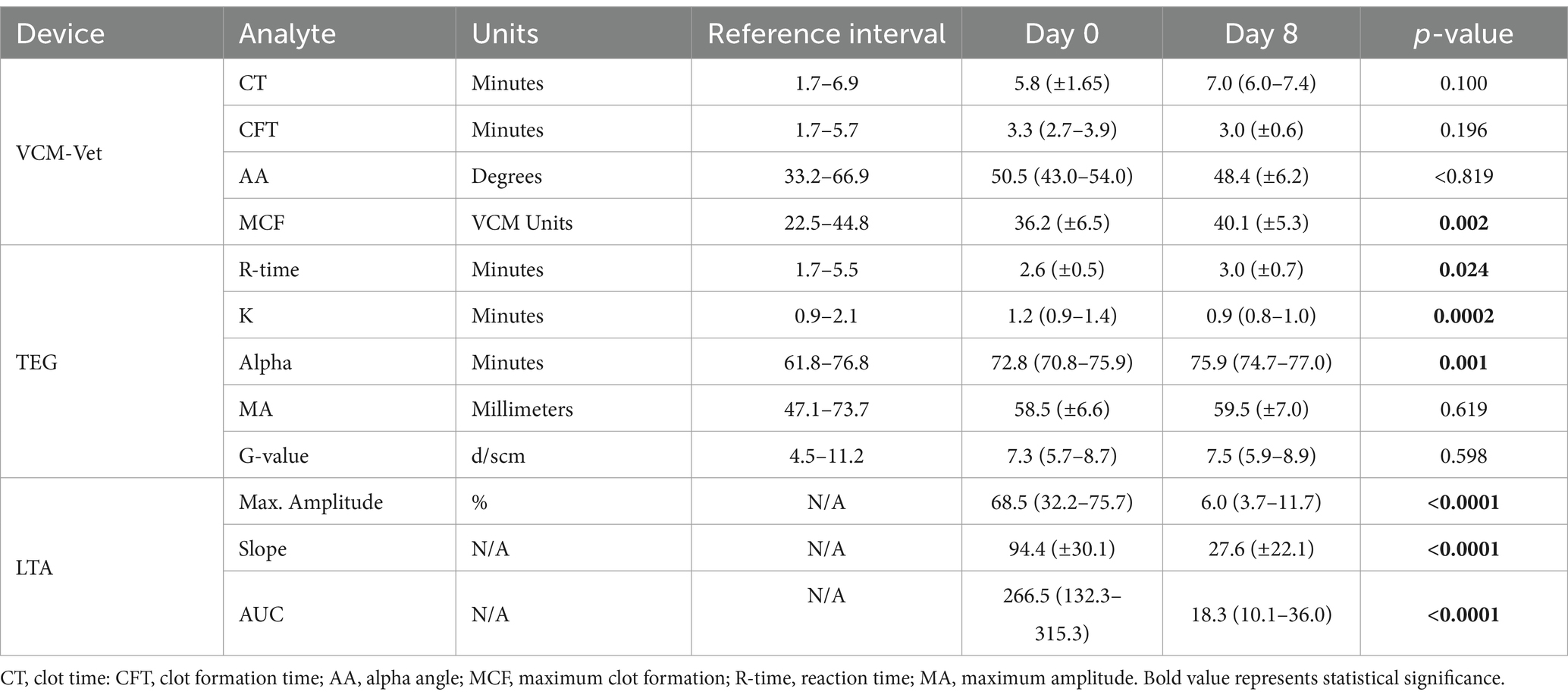
Table 2. Comparisons of viscoelastic measurements by VCM Vet, kaolin-activated thromboelastography (TEG), and light transmission aggregometry (LTA) in 28 cats before and after 7 days (Day 0 vs. Day 8) of clopidogrel treatment.
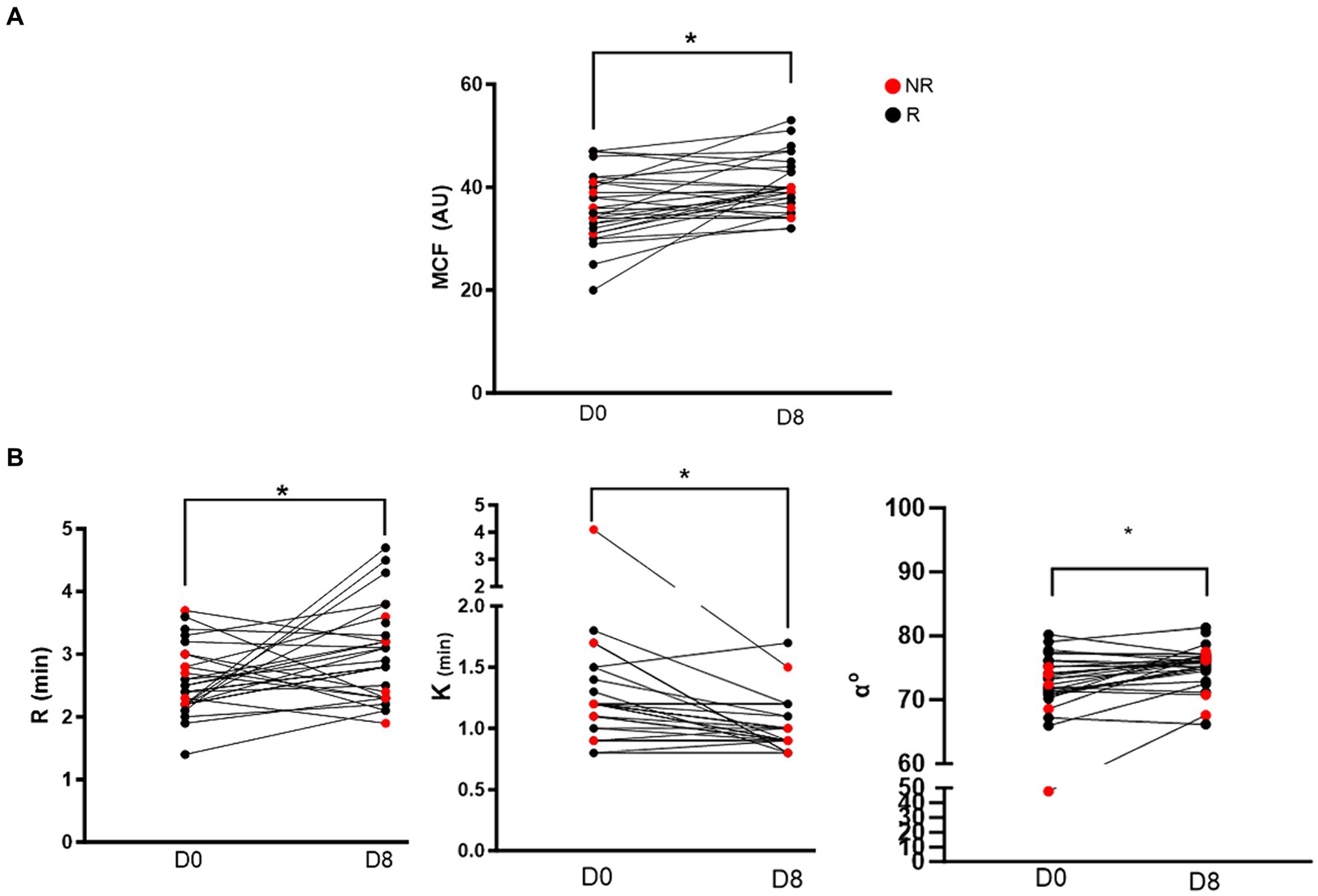
Figure 1. Before-and-after dot plots showing selected viscoelastic variables in 28 cats before (day 0) and after 7 days (day 8) of clopidgrel treatment. Red dots represent non-responders and black dogs represent responders. (A) Of the 4 VCM Vet variables, only maximum clot formation (MCF) was significantly increased on day 8 compared to baseline. (B) Three of the 5 TEG variables were significantly different after clopidogrel treatment. R-time (min) was prolonged after 7 days of clopdigrol treatment. However, K (min) and alpha angle (α°) were significantly decreased and increased, respectively. *p < 0.05.
On Day 0, the mean maximum amplitude measured by LTA was 68.5% (32.2–75.7) and decreased significantly after 7 days of clopidogrel treatment [6.0% (3.7–11.7), p < 0.0001]. Similarly, AUC [Day 0: 266.5 (132.2–315.3) vs. Day 18.3 (10.1–36.0), p < 0.0001] and slope (Day 0: 94.4 ± 30.1 vs. Day 8: 27.4 ± 22.1 p < 0.0001) were significantly decreased on day 8 (Figure 2A).
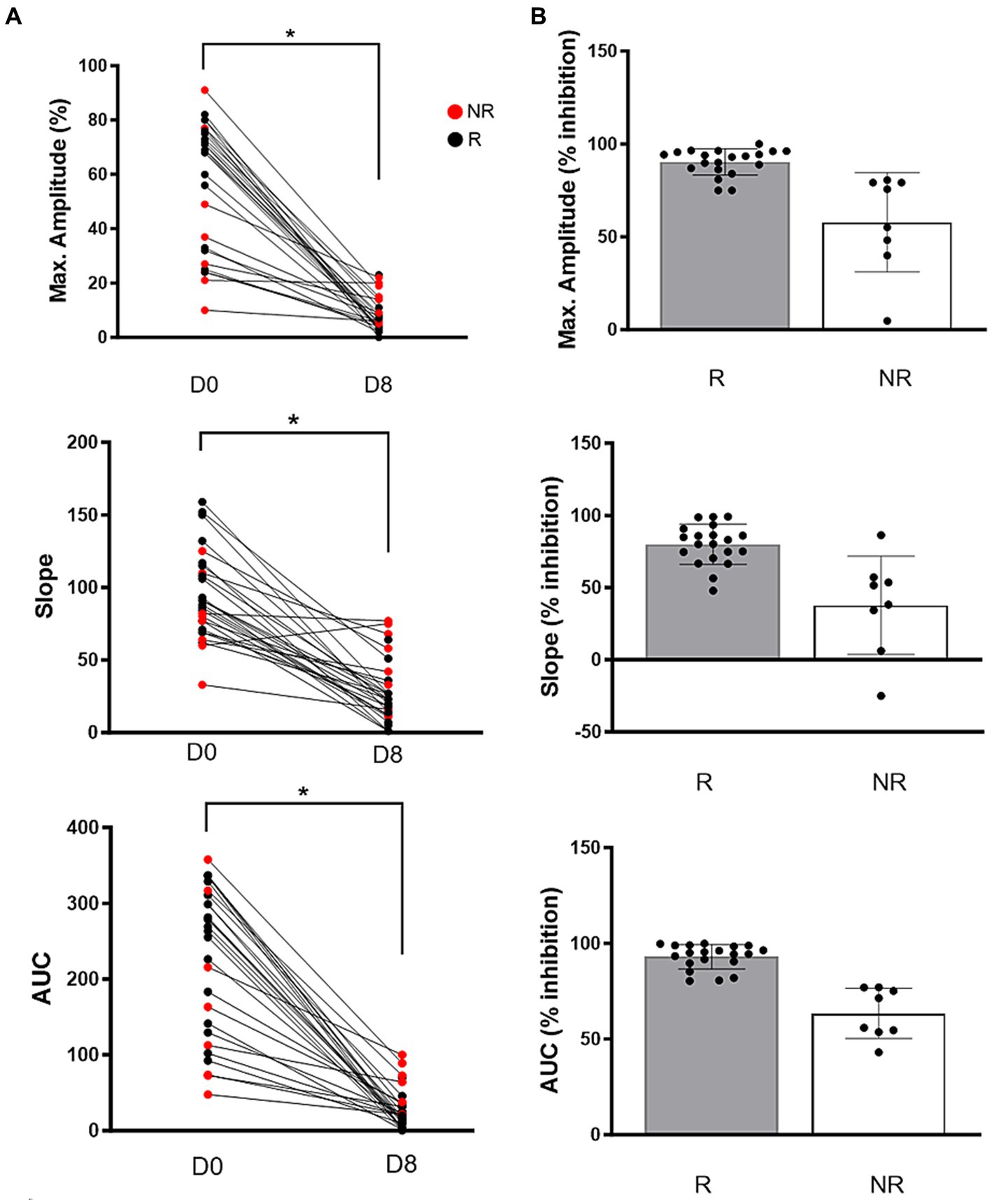
Figure 2. (A) Before-and-after dot pots in adenosine diphosphate-induced light transmission aggregometry in 28 cats after 7 days of clopidogrel treatment. Compared to baseline (D0), all variables including maximum amplitude (%), slope and area under the curve (AUC) were significantly decreased following treatment on day 8 (D8). Red dots represent non-responders and black dogs represent responders. (B) Clopidogrel response, measured as percent inhibition, is shown on scattered dot pots among responders (R) and non-responders (NR). Bar represents median and error bars show the interquartile ranges p < 0.05.
We identified a moderate negative correlation between the standardized percentages of inhibition of R-time (TEG) and AUC (LTA) (R2 = −0.39 p = 0.042). No other standardized parameter (TEG and VCM Vet analytes) included in the developed correlation matrix showed a significant correlation with the standardized LTA parameters (Table 3).

Table 3. Correlations between clopidogrel-induced platelet inhibition on light transmission aggregometry, VCM Vet and TEG in 28 healthy cats after 7 days of clopidogrel administration.
Although the overall platelet count was increased in cats after clopidogrel treatment, the degree of platelet increase did not correlate with changes in R (r = −0,03, p = 0.9), alpha-angle (r = −0.06, p = 0.75), and K (r = −0.03, p = 0.90) on TEG. Only MA was significantly and negatively correlated with changes in platelet count (r = −0.40, p = 0.04). Similarly, of the VCM variables, only change in MCF was significantly correlated with platelet count (r = −0.39, p = 0.036).
Of the 28 cats, 8 (28.6%) were classified as NRs to clopidogrel based on ADP-induced LTA. Variability in the degree of inhibition was high among NRs (Amplitude: inhibition = 4.8 to 75.8%, CV = 46.1%; Slope: inhibition = −25 to 86.3%, CV = 90.2%; AUC: inhibition = 43.1 to 77%, CV = 20.7%) (Figure 2B). Of the 8 cats, 5 cats (62.5%) had low aggregation (< 40% maximum amplitude) in response to ADP on Day 0 (Figure 2A).
Figure 3 summarizes the changes of TEG and VCM Vet variables among Rs and NRs, identified based on LTA. Of the 8 NRs, 6 (75%) cats had decreased R time on TEG compared to 2 cats in the responder group (10%) (Figure 3A). All NRs (100%) had MA that trended toward more hypercoagulable compared to 10 (50%) responders that had increased MA after clopidogrel treatment. VCM Vet appeared to be less discriminatory in identifying clopidogrel NRs (Figure 3B).
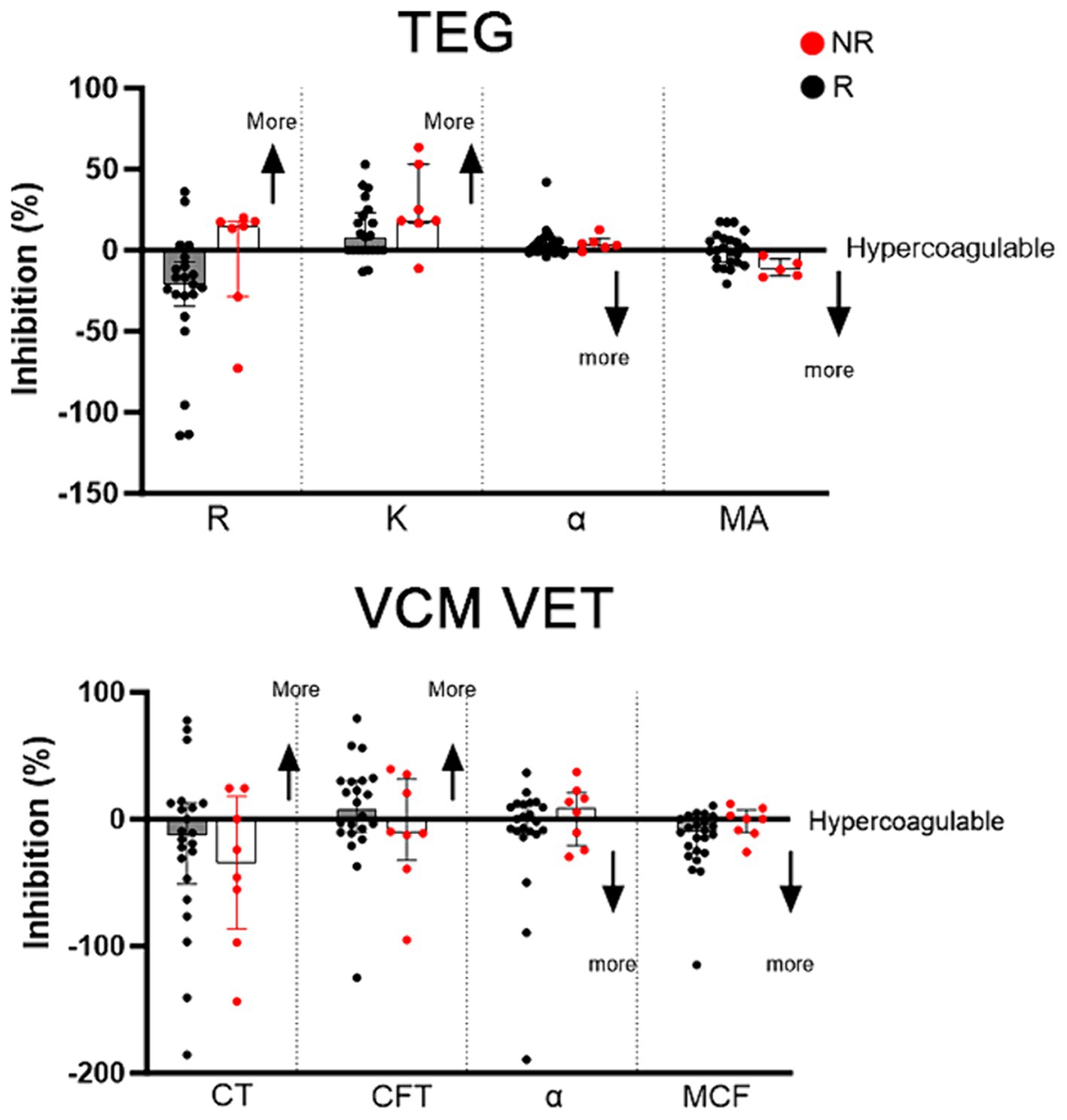
Figure 3. Clopidogrel response, measured as percent inhibition, on kaolin-activated thromboelastography (TEG) and VCM Vet after 7 days of clopdigorel treatment in 28 cats. Red dots represent non-responders and black dogs represent responders. Increased inhibition on R, K, CT and CFT indicates a more hypercoagulable tracing, whereas decreased inhibition on alpha, MA and MCF indicates a more hypercoagulable tracing.
This study aimed to assess the diagnostic utility of two distinct viscoelastic devices in monitoring clopidogrel response in cats. The necessity to perform platelet monitoring following clopidogrel administration is supported by recent research findings in which cats showed a reduced response to clopidogrel, as measured by ADP-induced aggregometry (5). In agreement with previously reported results, in our study population, we identified that 8 out of the 28 cats (28.6%) had decreased response to clopidogrel. Interestingly, a majority of these cats showed a decreased response to ADP prior to clopidogrel treatment and continued to have a modulated response after treatment. One plausible explanation for this finding is that the known non-synonymous single nucleotide polymorphism of the gene, P2RY1, affects the ADP receptor, P2Y1, which is not targeted by clopidogrel. This suggests that poor response to ADP may lead to suboptimal levels of inhibition by clopidogrel. This was further confirmed by Ueda et al., who demonstrated that intracellular levels of phosphorylation of vasodilator-stimulated phosphoprotein, which is an indicator of P2Y12 inhibition, in cats treated with clopidogrel were not associated with the pharmacogenomic effect of clopidogrel (5). Another explanation is in vitro platelet activation, which would compromise platelet response to ADP.
Our research identified that, of all VCM and TEG analytes, only one showed a moderate correlation with the gold standard LTA. According to our analysis, persistent hypercoagulable tracings on kaolin-activated TEG after clopidogrel treatment may identify cats that were poor responders.
Given the high variability in platelet response to ADP, it is, therefore, desirable to identify a platelet function monitoring assay that could be implemented in clinical practice to assess individual response to ADP and clopidogrel therapy. Light transmission aggregometry and flow cytometry have traditionally been considered the gold standard of assessing the efficacy of antiplatelet therapy. These techniques, however, are strictly limited to research settings because they require sophisticated equipment and technical expertise. In recent years, the application of viscoelastic testing has gained popularity in the veterinary field. Until now, TEG has primarily been confined to specialty hospitals and research centers (16). A proprietary modified TEG protocol, TEG Platelet Mapping™ assay (TEG-PM), has been validated in human patients to specifically monitor platelet inhibition following clopidogrel therapy (17). Although a recent publication also demonstrated detectable platelet inhibitory effect in dogs treated with clopidogrel, TEG-PM was found to be unreliable in healthy cats (13, 18). Specifically, when reptilase was used to isolate the contribution of fibrin from platelets to clot formation, increased pre-analytical platelet activation in cats resulted in indistinct differences in MA (13). This finding indicates that TEG-PM using the manufacturer’s protocol may not be able to monitor clopidogrel response in cats. The point-of-care VCM device has recently been introduced to the veterinary market, offering a more readily accessible and widely available viscoelastic testing option (14, 15). In contrast to TEG, VCM analysis is performed on non-anticoagulated blood and does not require any reagents. These factors reduce the technical expertise required but also limit the ability to evaluate the role of individual contributors such as platelets, hematocrit, and fibrin in clot formation.
In our study, only a limited number of variables from the viscoelastic assays showed statistically significant differences after clopidogrel treatment. The prolongation of R on TEG, a measurement of thrombin generation, may be caused by modulation in platelet activation by clopidogrel. During the propagation phase of coagulation, platelets play a central role in facilitating the formation of prothrombinase complex (factors Xa and Va) by externalizing their electronegative phospholipids such as phosphatidylserine (19, 20). This process was previously shown to be further suppressed when cats were treated with dual agent therapy consisting of clopidogrel and rivaroxaban, a direct factor Xa inhibitor (21). An in vitro study in humans, however, found that inhibition of platelet integrin did not prolong R on TEG (22). This further highlights the significance of species differences in coagulation. Based on our preliminary findings, 90% of responders were observed to have increased R-time on TEG after clopidogrel treatment compared to a majority of NRs which had decreased R time after treatment. This suggests that R time on TEG may have diagnostic significance in assessing response to clopidogrel. Further studies in clinical cats are needed to verify this finding.
Clot kinetics, while not influenced by platelet count, have been shown to decrease with integrin inhibition in human platelets (22, 23). Notably, some of the observed variables showed a trend toward hypercoagulability after clopidogrel treatment in cats as indicated by an increase in MCF on VCM Vet, increased alpha angle and shortened K on TEG. In TEG, K and alpha angle are measurements of clot kinetics reflecting the rate at which fibrin emerges and polymerizes to form fibrin filaments (24). The underlying mechanism of these unexpected findings is unclear. One possibility is the high inter-assay or operator variability associated with viscoelastic testing and the duration between sampling and testing, which may cause in vitro activation of tissue factor or factor XIIa. However, every effort was made to standardize our methods and duration between sampling and assays. Another plausible explanation is that clopidogrel treatment may have prevented in vitro activation, allowing platelets to be rested and, thereby, augmenting their response to physiologic agonists during viscoelastic testing. This hyperresponsive nature of feline platelets has previously been shown in cats with increased thrombin generation kinetics in the presence of tissue factor after clopidogrel treatment (21).
After 7 days of clopidogrel treatment, a significant increase in platelet count was observed on CBC. This unexpected finding suggests a potential influence of the administered treatment on platelet dynamics, possibly related to its impact on in vitro platelet activation and platelet aggregation. It is plausible that by inhibiting the platelet aggregation process, clopidogrel limits the in vitro formation of platelet aggregates, potentially leading to a higher number of platelets detected by automatic counters. However, it is crucial to note that this phenomenon might not necessarily translate to a physiological increase in functional platelets. Careful monitoring is essential to differentiate between the apparent rise in platelet count and any actual changes in platelet physiology or clotting tendencies.
VCM analytes are obtained from non-anticoagulated blood. Without the presence of strong activators like tissue factor or platelet agonists, this assay may not be able to detect changes in platelet function given the variability of the assay and the hyperresponsiveness nature of feline platelets (15). Performing the VCM in series or duplicates could also reduce the inherent variability due to blood draws and platelet clumping. Further studies are needed to investigate if the addition of in vitro activators or platelet agonists like ADP in whole blood prior to analysis would augment the ability of the VCM to detect changes in platelet function due to clopidogrel treatment in cats.
The present study has several limitations that should be considered when interpreting the results. First, our study consisted exclusively of healthy colony cats, which may not be entirely representative of the general feline population. The inclusion of only healthy cats may limit the generalizability of our findings to those with underlying health conditions such as cardiomyopathy. In addition, resistance to clopidogrel was not confirmed by genetic testing. Second, the transportation of the blood samples from the cat colony to the central laboratory for analysis is a notable limitation. The handling and transportation of these samples may have introduced variability in sample conditions, which might have caused in vitro PLT activation, further confounding the interpretation of our results. An additional limitation of this study is that plasma fibrinogen and coagulation proteins were not measured hence the underlying causes of increased clot kinetics and clot strength on viscoelastic assays could not be determined. Although the collected blood samples were routinely screened for platelet clumping, the detailed information regarding clumping events was not consistently annotated, as the primary focus was on confirming adequate platelet counts in presumably thrombocytopenic samples. This omission restricts our understanding of the full pharmacological effects of clopidogrel in our study population. In light of these limitations, future research should consider a more diverse and representative sample, meticulous sample handling, and the evaluation of active metabolite levels to enhance the comprehensiveness and reliability of the findings.
In conclusion, our study found that while LTA remains the gold standard of monitoring clopidogrel treatment, R-time on TEG has the potential to serve as an alternative monitoring tool. Further clinical studies are needed to confirm these preliminary findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was approved by University of California, Davis Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
TR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. KJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. NN: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. RL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Formal analysis, Validation, Visualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Center for Companion Animal Health (#2017-50-R).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Smith, SA, Tobias, AH, Jacob, KA, Fine, DM, and Grumbles, PL. Arterial thromboembolism in cats: acute crisis in 127 cases (1992-2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med. (2003) 17:73–83. doi: 10.1892/0891-6640(2003)017<0073:aticac>2.3.co;2
2. Tablin, F, Schumacher, T, Pombo, M, Marion, CT, Huang, K, Norris, JW, et al. Platelet activation in cats with hypertrophic cardiomyopathy. J Vet Intern Med. (2014) 28:411–8. doi: 10.1111/jvim.12325
3. Hogan, DF, Fox, PR, Jacob, K, Keene, B, Laste, NJ, Rosenthal, S, et al. Secondary prevention of cardiogenic arterial thromboembolism in the CAT: the double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J Vet Cardiol. (2015) 17:S306–17. doi: 10.1016/j.jvc.2015.10.004
4. Li, RH, Stern, JA, Ho, V, Tablin, F, and Harris, SP. Platelet activation and Clopidogrel effects on ADP-induced platelet activation in cats with or without the A31P mutation in MYBPC3. J Vet Intern Med. (2016) 30:1619–29. doi: 10.1111/jvim.14568
5. Ueda, Y, Li, RHL, Nguyen, N, Ontiveros, ES, Kovacs, SL, Oldach, MS, et al. A genetic polymorphism in P2RY 1 impacts response to clopidogrel in cats with hypertrophic cardiomyopathy. Sci Rep. (2021) 11:12522. doi: 10.1038/s41598-021-91372-3
6. Matetzky, S, Shenkman, B, Guetta, V, Shechter, M, Beinart, R, Goldenberg, I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. (2004) 109:3171–5. doi: 10.1161/01.CIR.0000130846.46168.03
7. Collette, SL, Bokkers, RPH, Dierckx, RAJO, van der Laan, MJ, Zeebregts, CJ, and Uyttenboogaart, M. Clinical importance of testing for clopidogrel resistance in patients undergoing carotid artery stenting-a systematic review. Ann Transl Med. (2021) 9:1211. doi: 10.21037/atm-20-7153
8. Harper, AR, and Price, MJ. Platelet function monitoring and clopidogrel. Curr Cardiol Rep. (2013) 15:321. doi: 10.1007/s11886-012-0321-7
9. Sambu, N, and Curzen, N. Monitoring the effectiveness of antiplatelet therapy: opportunities and limitations. Br J Clin Pharmacol. (2011) 72:683–96. doi: 10.1111/j.1365-2125.2011.03955.x
10. Ranucci, M, and Baryshnikova, E. Sensitivity of viscoelastic tests to platelet function. J Clin Med. (2020) 9:189. doi: 10.3390/jcm9010189
11. Ho, KK, Abrams-Ogg, AC, Wood, RD, O’Sullivan, ML, Kirby, GM, and Blois, SL. Assessment of platelet function in healthy cats in response to commonly prescribed antiplatelet drugs using three point-of-care platelet function tests. J Feline Med Surg. (2016) 19:638–47. doi: 10.1177/1098612X16648182
12. Li, RHL, Nguyen, N, Rosati, T, and Jandrey, K. Assessment of P2Y12 inhibition by Clopidogrel in feline platelets using flow cytometry quantification of vasodilator-stimulated phosphoprotein phosphorylation. Front Vet Sci. (2020) 7:267. doi: 10.3389/fvets.2020.00267
13. Blois, SL, Banerjee, A, and Wood, RD. Evaluation of thrombelastographic platelet-mapping in healthy cats. Vet Clin Pathol. (2012) 41:223–7. doi: 10.1111/j.1939-165X.2012.00419.x
14. Hennink, I, Peters, L, van Geest, G, and Adamik, KN. Evaluation of a viscoelastic coagulation monitoring system (VCM vet). Animals (Basel). (2023) 13:405. doi: 10.3390/ani13030405
15. Rosati, T, Jandrey, KE, Burges, JW, and Kent, MS. Establishment of a reference interval for a novel viscoelastic coagulometer and comparison with thromboelastography in healthy cats. Vet Clin Pathol. (2020) 49:660–4. doi: 10.1111/vcp.12916
16. Burton, AG, and Jandrey, KE. Use of Thromboelastography in clinical practice. Vet Clin North Am Small Anim Pract. (2020) 50:1397–409. doi: 10.1016/j.cvsm.2020.08.001
17. Volod, O, Arabia, FA, Lam, LD, Runge, A, Cheng, C, and Czer, LSC. Platelet mapping by Thromboelastography and whole blood Aggregometry in adult patients supported by mechanical circulatory support device on aspirin therapy. J Extra Corpor Technol. (2020) 52:13–21. doi: 10.1051/ject/202052013
18. Griebsch, C, Hall, E, and Barrs, VR. Effectiveness of aspirin vs. clopidogrel in dogs with immune mediated haemolytic anaemia evaluated by serial thromboelastography and platelet mapping. Vet J. (2022) 287:105882. doi: 10.1016/j.tvjl.2022.105882
19. Nagata, S, Sakuragi, T, and Segawa, K. Flippase and scramblase for phosphatidylserine exposure. Curr Opin Immunol. (2020) 62:31–8. doi: 10.1016/j.coi.2019.11.009
20. Smith, SA . The cell-based model of coagulation. J Vet Emerg Crit Care (San Antonio). (2009) 19:3–10. doi: 10.1111/j.1476-4431.2009.00389.x
21. Lo, ST, Li, RHL, Georges, CJ, Nguyen, N, Chen, CK, Stuhlmann, C, et al. Synergistic inhibitory effects of clopidogrel and rivaroxaban on platelet function and platelet-dependent thrombin generation in cats. J Vet Intern Med. (2023) 37:1390–400. doi: 10.1111/jvim.16727
22. Bowbrick, VA, Mikhailidis, DP, and Stansby, G. Value of thromboelastography in the assessment of platelet function. Clin Appl Thromb Hemost. (2003) 9:137–42. doi: 10.1177/107602960300900208
23. Lynch, AM, Ruterbories, L, Jack, J, Motsinger-Reif, AA, and Hanel, R. The influence of packed cell volume versus plasma proteins on thromboelastographic variables in canine blood. J Vet Emerg Crit Care (San Antonio). (2020) 30:418–25. doi: 10.1111/vec.12979
Keywords: clopidogrel resistance, arterial thromboembolic event (ATE), monitoring, light-transmission aggregometry, hypertrophic cardiomyopathy
Citation: Rosati T, Jandrey KE, Stern JA, Nguyen N and Li RHL (2024) Evaluation of clopidogrel response in healthy cats using a novel viscoelastic test and thromboelastography. Front. Vet. Sci. 11:1371781. doi: 10.3389/fvets.2024.1371781
Received: 17 January 2024; Accepted: 17 May 2024;
Published: 18 June 2024.
Edited by:
Gabriele Rossi, Murdoch University, AustraliaReviewed by:
Benjamin M. Brainard, University of Georgia, United StatesCopyright © 2024 Rosati, Jandrey, Stern, Nguyen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronald H. L. Li, cmhsaTJAbmNzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.