
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 10 April 2024
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1367843
This article is part of the Research Topic Nutrition Regulation and Stress in Ruminant View all 18 articles
Introduction: The aim of this experiment is to explore the effects of salvia sclarea extract on the growth performance, apparent nutrient digestibility, antioxidant capacity, and immune function of lambs. Sixty female lambs (Chinese Merino sheep) aged 2 months and weighing 20 ± 2 kg were selected and randomly divided into five groups of twelve lambs in each. While the control group (CK) received only basal feed, the experimental group was supplemented with different concentrations of salvia sclarea extract in the basal feed at 0.04 mL/kg (group CL1), 0.08 mL/kg (group CL2), 0.12 mL/kg (group CL3), and 0.16 mL/kg (group CL4). The feeding period was 85 days, including 15 days of pre-feeding and 70 days of regular feeding. Body weight and feed intake were recorded during the test period, and blood was collected at the end of the test for the determination of immune and antioxidant indices. The results showed that the average daily gain and average daily feed intake of lambs were significantly increased in CL3 group compared to CK group (p < 0.05). Also, the apparent nutrient digestibility of crude protein and neutral detergent fiber was significantly increased (p < 0.05). The Dry matter, acid detergent fiber and Ether extract were not significantly different (p > 0.05). The serum levels of superoxide dismutase, catalase, glutathione peroxidase, and antioxidant capacity were significantly higher in the CL2, CL3, and CL4 groups compared to CK group, while malondialdehyde levels were significantly lower (p < 0.05). The serum levels of immune globulin A, immune globulin G, immune globulin M, interferon-γ, and interleukin-10 were significantly higher and the levels of tumor necrosis factor-α and interleukin-1β were significantly lower in the CL2, CL3, and CL4 groups (p < 0.05). In conclusion, the addition of salvia sclarea extract to the ration promotes growth performance and nutrient digestion in lambs. Improvement of immune response by increasing immunoglobulin and cytokine concentrations. And it enhances the antioxidant status by increasing the antioxidant enzyme activity in lambs.
This study aimed to explore the effects of Salvia sclarea extract on the growth performance, apparent nutrient digestibility, antioxidant capacity, and immune function of the lambs.
Methods: Sixty female lambs (Chinese Merino sheep) aged 2 months and weighing 20 ± 2 kg were selected and randomly divided into five groups of 12 lambs each. The control group (CK) received only basal feed, whereas the experimental group was supplemented with different concentrations of salvia sclarea extract in the basal feed at 0.04, 0.08, 0.12, and 0.16 mL/kg (CL1, CL2, CL3, and CL4, respectively). The feeding period was 85 days, including 15 days of pre-feeding and 70 days of regular feeding. Body weight and feed intake were recorded during the test period, and blood was collected at the end of the test to determine immune and antioxidant indices.
Results: The results showed that the average daily weight gain and feed intake of the lambs were significantly higher in the CL3 group than in the CK group (p < 0.05). In addition, the apparent nutrient digestibility of crude protein and neutral detergent fiber increased significantly (p < 0.05). The dry matter, acid detergent fiber, and ether extract were not significantly different (p > 0.05). Serum levels of superoxide dismutase, catalase, and glutathione peroxidase and antioxidant capacity were significantly higher in the CL2, CL3, and CL4 groups than in the CK group, whereas malondialdehyde levels were significantly lower (p < 0.05). The serum levels of immune globulin immune globulin A, immune globulin G, immune globulin M, interferon-γ, and interleukin-10 were significantly higher and the levels of tumor necrosis factor-α and interleukin-1β were significantly lower in the CL2, CL3, and CL4 groups (p < 0.05).
Discussion: In conclusion, the addition of the S. sclarea extract to the diet promoted growth performance and nutrient digestion in lambs. Immune response was improved by increasing Ig and cytokine concentrations. It enhances antioxidant status by increasing antioxidant enzyme activity in lambs.
Early postweaning stressors in lambs release glucocorticoids and coincide with a decrease in growth hormones, which suppress the immune system and reduce growth performance (1). A dietary change from milk to solid feed is considered a major stressor after weaning. The lack of a fully functional rumen in recently weaned ruminants reduces nutrient digestibility (2). Nutritional strategies have recently emerged. It has been proposed as a key factor in improving animal health and welfare as well as increasing livestock productivity (3). Dietary composition has long been recognized as an important factor in animal health, with significant effects on the acquired and innate immune systems, especially on inflammation (4). A number of compounds and products derived from plant-derived byproducts have been reported to show proinflammatory or anti-inflammatory effects and therapeutic responses and can trigger the expected response of the animal body to production parameters (5).
Salvia sclarea L. is a native Asian plant that is widely used in various fields including food, medicine, oils, and landscaping (6). It originated in Europe and is now primarily cultivated in France, Russia, and other countries. This plant was introduced into China in the early 1970s and cultivated in the Shaanxi and Henan provinces. In 2011, it was introduced into the Zhaosu area of Xinjiang, which has a cultivated area of approximately 1,000 ha per year. The plant produces 30 t of raw material and 60 kg of essential oil per ha (7). Previous phytochemical studies have identified and isolated various bioactive compounds, including flavonoids, volatile oils, fatty acids, triterpenes, and phenolic compounds, from S. sclarea frutescens (8). Owing to these biological components, pharmacological effects have been shown, including anti-inflammatory, antioxidant, and antibacterial effects (9). Previous studies have shown that dietary supplementation with S. sclarea improves meat quality without adversely affecting growth performance or carcass characteristics (10). However, few studies have investigated the effects of S. sclarea extract on lambs. Based on this, this study aimed to provide valuable scientific insights and powerful references for the rational application of S. sclarea extract in lamb production by adding different levels of S. sclarea extract to the growth performance, apparent nutrient digestibility, serum immunity, and antioxidant indices of lambs.
All experimental procedures were performed in strict accordance with guidelines and were reviewed and approved by the Institutional Animal Bioethics Committee of Shihezi University (Xinjiang, China).
The S. sclarea extract (essential oil) used in the experiment appeared to be a light-yellow liquid. It was prepared by hydrodistillation of the flowers, leaves, and stems of S. sclarea. The active ingredients were linalyl acetate (54.79%) and linalool (30.22%), as detected using LC–MS. The purity of the S. sclarea extract was 85%. It was produced in the East Industrial Park of Zhaosu County, Xinjiang, China.
Sixty female lambs (Chinese Merino sheep) aged 2 months and weighing 20 ± 2 kg were selected and randomly divided into five groups of 12 lambs each. The control group (CK) received basal feed alone, whereas the experimental group was supplemented with different gradients of S. sclarea extract in the basal feed at 0.04, 0.08, 0.12, and 0.16 mL/kg (CL1, CL2, CL3, and CL4, respectively). The feeding period was 85 days, including 15 days of pre-feeding and 70 days of normal feeding, and the sheep were fed daily at 06:00 h and 18:00 h. Salvia sclarea extract was precisely and uniformly mixed with the ration in strict accordance with the experimental design additive ratios. The sheep were housed in single-cage enclosures (1.5 × 1 × 1 m) with free access to water, and the sheds, water troughs, and troughs were cleaned and sterilized regularly to record daily feed intake and body weight. The composition and nutritional levels of the basal rations are listed in Table 1.
The lambs were weighed on the 1st and lower 70th days of the positive trial period and recorded as the initial body weight (IBW) and final body weight (FBW) of the test lambs, respectively, both on an empty stomach before the morning feeding. During the trial period, the amount of feed and leftovers were recorded in detail for each bureau pen per day, which was used as the basis for calculating the average daily feed intake (ADFI, ADFI = total feed intake/experimental days), average daily gain (ADG, ADFI = total feed intake/experimental days), and feed to weight ratio (F/G, F/G = ADFI/ADG) of the lambs.
Ration and fecal samples were collected during the last 3 days of the positive trial period. Samples were collected using the partial collection method, in which the ewes in each group were placed in homemade collection bags at 09:00 h and feces were recovered at 17:00 h each day. A sample of 200 g of feces was added to 10 mL of 10% sulfuric acid for nitrogen fixation and stored at −20°C for measurement. The collected grain and manure samples were placed in an oven at 65°C for 48 h, weighed after 24 h of moisture return, and prepared as analytical samples by crushing through an 80 mesh sieve. The dry matter (DM), crude protein (CP), crude ash (Ash), ether extract (EE), Ca, and P contents were determined using the methods of AOAC (2010) (12). The content of neutral and acid detergent fibers (NDF and ADF, respectively) was determined according to the method described by Van Soest et al. (13). The apparent digestibility of nutrients in lambs was determined using the acid-insoluble ash (AIA) endogenous indicator method (14).
At the end of the experiment, 10 mL of blood was collected from the jugular vein of the sheep using a disposable syringe, and the serum was separated statically and stored frozen (−20°C) for subsequent experiments.
Serum immunity indicators, including antibodies: immunoglobulin G (IgG), immunoglobulin A (IgA) and immunoglobulin M (IgM), cytokines: tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-2 (IL-2), were measured using enzyme-linked immunosorbent assay (ELISA) method according to the instructions of the kit, Interleukin-4 (IL-4), Interleukin-6 (IL-6), Interleukin-8 (IL-8) and Interleukin-10 (IL-10) and antioxidant indexes: total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA). The kits were purchased from Nanjing Jianjian Bioengineering Institute. Each sample was tested using an enzyme-labeling analyzer to detect the corresponding absorbance and a standard curve was established according to the manufacturer’s instructions to determine the concentration of the target factor.
All data were analyzed using one-way ANOVA, linear analysis, and quadratic correlation using the SPSS software (version 22.0; SPSS, Inc., Chicago, IL, United States). Duncan’s method was used for multiple comparisons, and the experimental data were expressed as the mean and standard error of the mean (SEM). p < 0.05 indicated a significant difference, p < 0.01 indicated highly significant difference, and 0.05 ≤ p < 0.10 indicated a trend.
No significant difference (p > 0.05) was observed in the initial weight of lambs in each group. The final weight of lambs in the CL4 group was significantly higher than that in the CK group (p < 0.05), and there was no significant difference (p > 0.05) among the CL2, CL3, and CL4 groups. Compared to the CK group, the ADG and ADFI of lambs in the CL3 and CL4 groups supplemented with Salvia sclarea extract were significantly higher, and the F/G was significantly lower (p < 0.05). The ADG of CL1, CL2, CL3, and CL4 increased by 7.97, 16.56, 23.31, and 34.35%, respectively, compared to that of the CK group (Table 2).
The apparent digestibility of DM, ADF, and EE in the four experimental groups CL1, CL2, CL3 and CL4 with the addition of S. sclarea extract was higher than that in the CK group, but the difference was not significant (p > 0.05). The apparent digestibility of NDF in the CL2 group was significantly higher than that in the CK group (p < 0.05); however, there was no significant difference among the CL1, CL3, and CL4 groups (p > 0.05). The apparent digestibility of CP and NDF in the CL3 group was significantly higher than that in the CK group (p < 0.05); however, there was no significant difference between the CL1 and CL3 groups (p > 0.05) (Table 3).
Compared with the CK group, the levels of SOD, CAT, GSH-Px, and T-AOC in the serum of lambs in the CL2, CL3, and CL4 groups treated with S. sclarea extract were significantly higher, whereas the MDA content was significantly lower (p < 0.05). Moreover, with an increase in the amount of S. sclarea extract, the content of SOD, CAT, GSH-Px, and T-AOC in the four experimental groups first increased and then decreased, whereas the MDA content showed the opposite trend. The CL1 group with 0.04 mL/kg S. sclarea extract had significantly higher CAT and GSH-Px content than the CK group (p < 0.05), whereas the content of MDA, SOD, and T-AOC was not significantly affected (p > 0.05). The SOD content was significantly higher (p < 0.05) in the CL3 group with the addition of 0.12 mL/kg S. sclarea extract than in the other four groups (Figure 1).
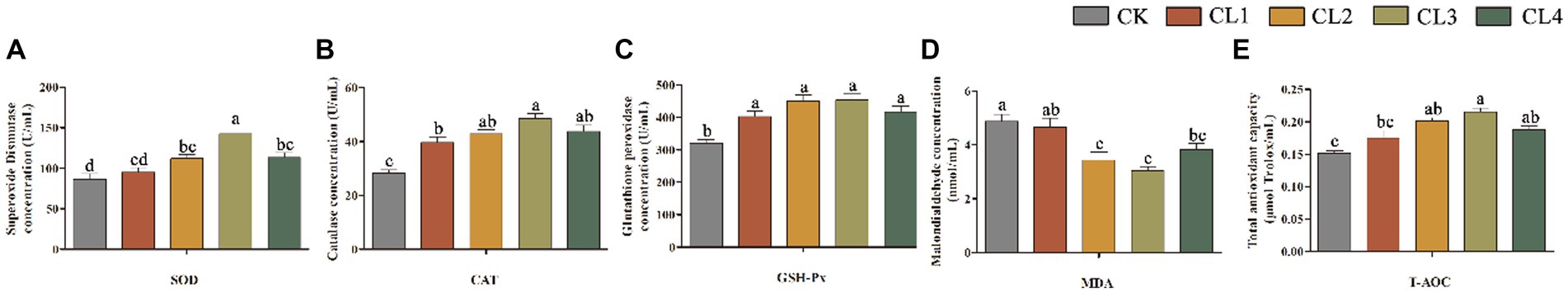
Figure 1. Effect of salvia sclarea extract on serum antioxidant indexes in lambs. (A) SOD; (B) CAT; (C) GSH-Px; (D) MDA; (E) T-AOC, CK, control group; CL1: 0.04 mL/kg; CL2: 0.08 mL/kg; CL3: 0.12 mL/kg; CL4: 0.16 mL/kg. Lower case letters in each bar chart indicate significant difference, the same letter indicates no significant difference (p > 0.05), and different lower case letters indicate significant difference (p < 0.05).
Compared with the CK group, the content of IgA, IgG, IgM, IFN-γ, and IL-10 in the serum of lambs in the CL2, CL3, and CL4 groups was significantly higher (p < 0.05); that of TNF-α, and IL-1β was significantly lower (p < 0.05); and that of IL-2 and IL-6 showed no significant difference (p > 0.05). The IL-4 content decreased in the test groups CL1, CL2, CL3, and CL4 with the addition of S. sclarea extract; however, the difference between the CL2 and CK groups was significant (p < 0.05), whereas the rest were not significantly different (p > 0.05). The content of IgA, IgG, IgM, IFN-γ, and IL-10 in the serum of lambs in the CL3 group was higher than that in the other three test groups (Figure 2).
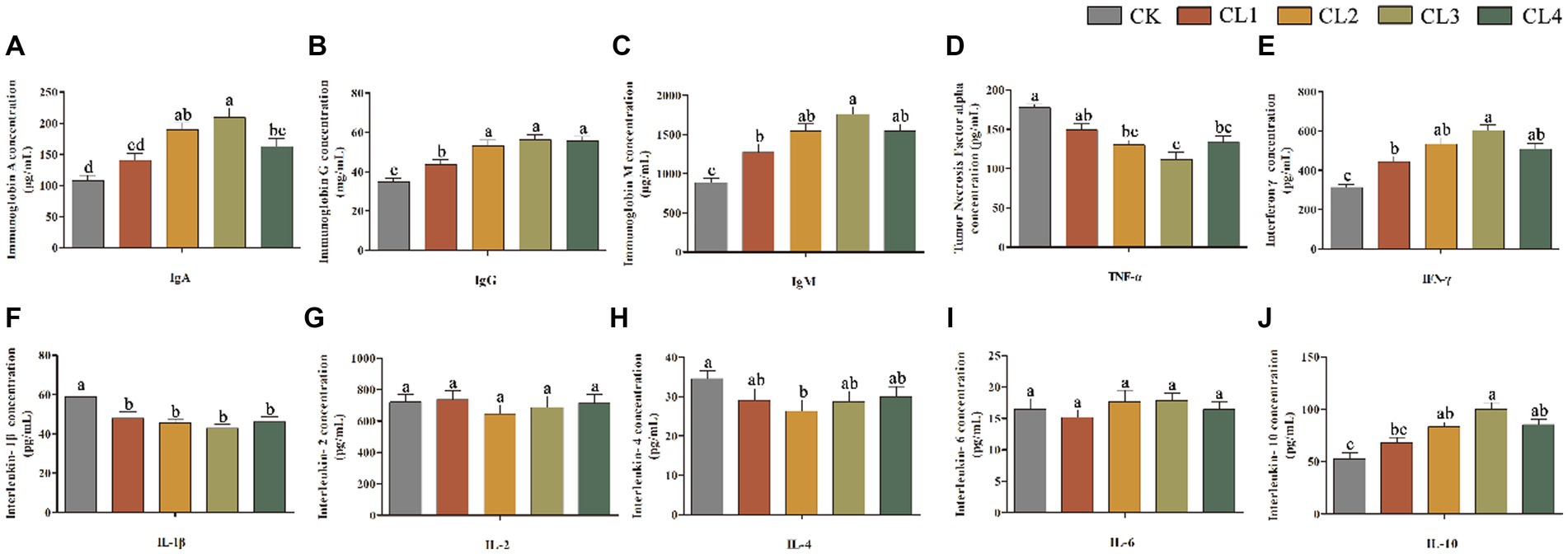
Figure 2. Effect of salvia sclarea extract on serum immunity indexes in lambs. (A) IgA; (B) IgG; (C) IgM; (D) TNF-α; (E) IFN-γ; (F) IL-1β; (G) IL-2; (H) IL-4; (I) IL-6; (J) IL-10, CK, control group; CL1: 0.04 mL/kg; CL2: 0.08 mL/kg; CL3: 0.12 mL/kg; CL4: 0.16 mL/kg. Lower case letters in each bar chart indicate significant difference, the same letter indicates no significant difference (p > 0.05), and different lower case letters indicate significant difference (p < 0.05).
Salvia sclarea has long been grown in China as a medicinal and food plant, and has attracted attention for its unique active substances (9). Studies have shown that the aromatic S. sclarea is rich in phenolic acids (caffeic and rosemarinic acids), flavonoids, and volatiles (monoterpenes and sesquiterpenes) (15), among other active ingredients that enhance the immunity and antioxidant capacity of the animal and promote its growth and development (8). In this study, we found that the addition of S. sclarea frutescens extract to lamb rations had a positive effect on the growth performance of lambs by improving feed conversion efficiency and optimizing the nutrient utilization. Moreover, the feed intake of lambs increased with an increase in S. sclarea extract addition, and the feed intake significantly increased by 10.24% when the addition reached 0.12 mL/kg. This indicates that the unique aromatic odor due to the richness of terpenes, alkenes, and aromatic compounds improved the palatability of the feed and induced appetite in the lambs (16). Feed palatability can effectively increase the feed intake of lambs, increase their dietary intake, and improve their daily weight gain and feed utilization. Another study showed that phenolics in S. sclarea extracts could increase the activity of digestive enzymes by entering the intestinal tract (17). This reduces the number of harmful bacteria in the intestinal tract including Escherichia coli, Staphylococcus aureus, and Bacillus subtilis (18). Beneficial bacteria colonize the intestine, consume free oxygen, create a low-oxygen environment, and inhibit the growth of harmful aerobic bacteria and spoilage microorganisms. The joint action of the two reduces harmful bacteria on the consumption of nutrients to promote growth (19).
The maintenance of good production performance in animals is closely related to the digestion and metabolism of various nutrients in the body. Increasing nutrient digestibility can promote the absorption of nutrients such as DM and CP, thus promoting growth performance to a certain extent. The test group of lambs fed the S. sclarea extract had a higher apparent digestibility of CP and NDF than the CK group. No differences in the apparent digestibility of DM, EE, or NDF were found between the diets. In the present study, the concentrations of ingredients in the experimental TMR diet were similar. Therefore, these differences were attributed to the incorporation of the S. sclarea extract. There were some differences in the chemical compositions and bioactivities of the diets. The variable response of apparent digestibility in the diet treatment groups may be partly due to the higher CP of these lambs, as these factors positively influenced the degree of digestibility of the rations (20).
A positive correlation between oxidative stress and disease has been widely documented in animals. Antioxidant capacity reflects the body’s ability to scavenge free radicals accumulated in cells and tissues and protect the structure and function of cell membranes from damage by peroxides (21). MDA is a product of the lipid peroxidation reaction, and its content reflects the degree of lipid peroxidation in the body, which in turn reflects the degree of cellular attack by free radicals. The results of our study showed that the addition of S. sclarea extract to the ration increased serum CAT, SOD, GSH-Px activity, and T-AOC and decreased serum MDA content in lambs, with the best effect in the 0.12 mL/kg addition group. This indicated that the S. sclarea extract improved the performance and health of lambs by effectively reducing oxidative stress. Similar to the results of Deng et al. (22), the addition of S. sclarea to the diet of lambs significantly increased T-AOC activity and MDA content in the liver and improved the oxidative status of muscle and meat quality.
Li et al. (23) found that the dietary addition of S. sclarea extract increased serum SOD activity and decreased MDA content in the liver, spleen, and jejunal mucosa of piglets. The S. sclarea extract used in this study is rich in phenolic acids, flavonoids, and other bioactive components. Phenolic acids can combine with peroxyl radicals to reduce or eliminate free radicals. Phenolic hydroxyl groups can chelate transition metal ions to block biological oxidation (24). Terpenoids can increase the activity of antioxidant enzymes in animals and thus exert their antioxidant capacity (25). Flavonoids such as lignans, rosmarinic acid, and apigenin have powerful antioxidant properties (26). Based on this, we hypothesized that S. sclarea extract enhances serum CAT, SOD, and GSH-Px activities in lambs by competitively scavenging reactive oxygen species. Therefore, it has great potential as an antioxidant to prevent oxidative damage during livestock production.
Immunoglobulins are humoral immune effector molecules that play an important role in the immune system of young animals, and serum immunoglobulin levels can be used to evaluate the health status of an organism (27). IgA, IgM, and IgG, play a major role in the immune response. As a class of immunologically active molecules, they specifically bind to antigens and remove them via sedimentation and phagocytosis (28). They mediate immune and inflammatory responses when an animal is infested with bacteria and viruses and play an important role in the body’s immune response and immunoregulation (29). In this study, the S. sclarea extract affected the immune response in lambs by inducing IgG and IgM production. These responses protect lambs from pathogenic and nonpathogenic immune attacks. Linoleic and linolenic acids are “essential fatty acids” converted into eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Most studies have shown that EPA and DHA can clinically attenuate T cell immune-mediated inflammatory diseases. And α-linolenic acid enhances the immunity of the body (30). Previous studies demonstrated that essential fatty acids exert anti-inflammatory effects. When the animal body suffers from acute or chronic inflammation, as well as infection, it inhibits the production of prostaglandin 2 by secreting inflammatory factors and plays a role in regulating immune function (31). Liu et al. injected different doses of linolenic acid into the duodenum of dairy cows through a fistula and found that serum IgG levels increased significantly and prostaglandin 2 levels decreased in the 200 g/day dose group (32). Moreover, S. sclarea extract contains phenolics that increase leukocyte phagocytosis and can increase the release of IgA, IgM, and IgG. Regulating the expression of cytokines and increasing the expression of antibodies in the serum enhances the ability of the body to clear pathogens, thus strengthening its immune function (33).
As important components of cellular immunity, cytokines are critical for lymphocyte development and the subsequent functional activity of the peripheral immune system (34). Cytokines are produced by immune and non-immune cells and can be categorized into proinflammatory cytokines produced by a variety of immune cells (such as IL-1β, IL-6, and TNF-α) and anti-inflammatory cytokines (such as IL-4, IL-10) (35). Among them, IL-1β can induce cells to secrete inflammatory factors and inflammatory transmitters, triggering the body’s inflammatory response (36). IL-6 promotes immune cell differentiation and enhances their functional activity. IL-1β secretes IL-2 via T cells, whereas IL-6 induces IL-2 production by activating the NF-κB signaling pathway through signal transduction. IL-2 is a core substance in the immunoregulatory network of the body, reflecting the initiation of the immune response and playing an important regulatory role in both cellular and humoral immunity (37). IL-10 is an important anti-inflammatory factor secreted by Tregs and is regulated by the immunomodulatory factor IL-4, which can be used to determine the level of immunity in lambs (38). In this study, the addition of S. sclarea extract to the ration decreased the levels of proinflammatory cytokines IL-1β and TNF-α and increased the serum anti-inflammatory cytokine IL-10, thereby modulating the immune response. Lignans, a constituent of S. sclarea extract, inhibited the production of TNF-α in mouse serum (39). Moreover, linolenic acid, an anti-inflammatory agent in a mouse model of colonic inflammation, had a protective effect against TNBS-induced colitis through the Th1/Th2/Th17 pathway with inflammation-reducing efficacy (40). In addition, a previous study demonstrated that dietary supplementation with phenolic compounds inhibited LPS-induced expression of proinflammatory cytokines IL-1β and IFN-γ through NF-κB and MAPK signaling pathways (41). These results demonstrate the potential of S. sclarea extracts to reduce the infection load and inflammatory responses in vivo.
In conclusion, the addition of 0.12 mL/kg S. sclarea extract to the diet improves the growth performance of lambs by increasing feed intake and nutrient digestibility. It also improves the health status of lambs by increasing their serum antioxidant capacity and immune function. S. sclarea extract has great potential as a feed additive in livestock production. It is also worth exploring its potential use in various animal production environments.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal studies were approved by the Institutional Animal Bioethics Committee of Shihezi University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
XM: Writing – original draft. YN: Writing – review & editing. SN: Writing – review & editing. WZ: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Xinjiang Uygur Autonomous Region Key Research and Development Program, “Research on Nutritional Control and Intelligent Technology for Precision Feeding of Meat Sheep in Northern Xinjiang Pastoral Areas”, Project No. 2022B02029-2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Guilloteau, P, Zabielski, R, Hammon, HM, and Metges, CC. Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. J Physiol Pharmacol. (2009) 60:17–35. doi: 10.1002/jso.2930140314
2. Zhong, RZ, Yu, M, Liu, HW, Sun, HX, Cao, Y, and Zhou, DW. Effects of dietary Astragalus polysaccharide and Astragalus membranaceus root supplementation on growth performance, rumen fermentation, immune responses, and antioxidant status of lambs. Anim Feed Sci Technol. (2012) 174:60–7. doi: 10.1016/j.anifeedsci.2012.02.013
3. Rahimi-Tari, M, Sadeghi, AA, Motamedi-Sedeh, F, Aminafshar, M, and Chamani, M. Hematological parameters, antioxidant status, and gene expression of γ-INF and IL-1β in vaccinated lambs fed different type of lipids. Trop Anim Health Prod. (2023) 55:168. doi: 10.1007/s11250-023-03585-5
4. Zhang, R, Zhu, W, and Mao, S. High-concentrate feeding upregulates the expression of inflammation-related genes in the ruminal epithelium of dairy cattle. J Anim Sci Biotechnol. (2016) 7:42. doi: 10.1186/s40104-016-0100-1
5. Vinuesa, A, Pomilio, C, Gregosa, A, Bentivegna, M, Presa, J, Bellotto, M, et al. Inflammation and insulin resistance as risk factors and potential therapeutic targets for Alzheimer’s disease. Front Neurosci. (2021) 15:653651. doi: 10.3389/fnins.2021.653651
6. Sargi, SC, Silva, BC, Santos, HMC, Montanher, PF, Boeing, JS, Santos Júnior, OO, et al. Antioxidant capacity and chemical composition in seeds rich in omega-3: chia, flax, and perilla. Food Sci Technol. (2013) 33:541–8. doi: 10.1590/S0101-20612013005000057
7. Zhang, F. Cultivation technology of high yielding aromatic perilla in Zhaosu alpine dry farming area of Xinjiang with spot sowing on membrane. Agric Eng Technol. (2016) 36:62. doi: 10.16815/j.cnki.11-5436/s.2016.20.047
8. Chen, Z, Wu, K, Zhu, W, Wang, Y, Su, C, and Yi, F. Chemical compositions and bioactivities of essential oil from perilla leaf (Perillae folium) obtained by ultrasonic-assisted hydro-distillation with natural deep eutectic solvents. Food Chem. (2022) 375:131834. doi: 10.1016/j.foodchem.2021.131834
9. Yu, H, Qiu, J-F, Ma, L-J, Hu, Y-J, Li, P, and Wan, J-B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem Toxicol. (2017) 108:375–91. doi: 10.1016/j.fct.2016.11.023
10. Deng, K, Ma, T, Wang, Z, TanTai, W, Nie, H, Guo, Y, et al. Effects of perilla frutescens seed supplemented to diet on fatty acid composition and lipogenic gene expression in muscle and liver of Hu lambs. Livest Sci. (2018) 211:21–9. doi: 10.1016/j.livsci.2018.03.001
11. Mordenti, A, Bosi, P, Corino, C, Crovetto, GM, Casa, GD, Franci, O, et al. Nutrient requirements of sheep. Natl Acad Sci. (1975) 2:73–87. doi: 10.4081/ijas.2003.73
12. Aoac, I. Official methods of analysis of AOAC international, 18^ed. AOAC Int. (2010) 6:382. doi: 10.1016/0924-2244(95)90022-5
13. Van Soest, PJ, Robertson, JB, and Lewis, BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
14. Merchen, NR. Digestion, absorption and excretion in ruminants. Ruminant Animal Digestive Physiology & Nutrition (1988)
15. Zhou, P, Yin, M, Dai, S, Bao, K, Song, C, Liu, C, et al. Multi-omics analysis of the bioactive constituents biosynthesis of glandular trichome in Perilla frutescens. BMC Plant Biol. (2021) 21:277. doi: 10.1186/s12870-021-03069-4
16. Ahmed, HM, and Tavaszi-Sarosi, S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. (2019) 275:730–8. doi: 10.1016/j.foodchem.2018.09.155
17. Loo, YT, Howell, K, Chan, M, Zhang, P, and Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr Rev Food Sci Food Saf. (2020) 19:1268–98. doi: 10.1111/1541-4337.12563
18. Zhao, Y, Li, H, Zhang, Z, Ren, Z, and Yang, F. Extraction, preparative monomer separation and antibacterial activity of total polyphenols from Perilla frutescens. Food Funct. (2022) 13:880–90. doi: 10.1039/d1fo02282b
19. Abdallah, A, Zhang, P, Zhong, Q, and Sun, Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr Drug Metab. (2019) 20:54–64. doi: 10.2174/1389200219666180523102920
20. Soest, PJV. Nutritional ecology of the ruminant, Nutritional ecology of the ruminant. Cornell University Press (2018)
21. Lauridsen, C. From oxidative stress to inflammation: redox balance and immune system. Poult Sci. (2019) 98:4240–6. doi: 10.3382/ps/pey407
22. Deng, KP, Fan, YX, Ma, TW, Wang, Z, TanTai, WJ, Nie, HT, et al. Carcass traits, meat quality, antioxidant status and antioxidant gene expression in muscle and liver of Hu lambs fed perilla seed. J Anim Physiol Anim Nutr. (2018) 102:e828–37. doi: 10.1111/jpn.12841
23. Li, J, Zhang, Q, Zhuo, Y, Fang, Z, Che, L, Xu, S, et al. Effects of multi-strain probiotics and Perilla frutescens seed extract supplementation alone or combined on growth performance, antioxidant indices, and intestinal health of weaned piglets. Animals. (2022) 12:2246. doi: 10.3390/ani12172246
24. Hromádková, Z, Paulsen, BS, Polovka, M, Košťálová, Z, and Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydr Polym. (2013) 93:22–30. doi: 10.1016/j.carbpol.2012.05.021
25. Fan, J, Feng, H, Yu, Y, Sun, M, Liu, Y, Li, T, et al. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohydr Polym. (2017) 157:629–36. doi: 10.1016/j.carbpol.2016.10.040
26. Andrade, AWL, Machado, K d C, Figueiredo, DDR, David, JM, Islam, MT, Uddin, SJ, et al. In vitro antioxidant properties of the biflavonoid agathisflavone. Chem Cent J. (2018) 12:75. doi: 10.1186/s13065-018-0443-0
27. Salman, S, Khol-Parisini, A, Schafft, H, Lahrssen-Wiederholt, M, Hulan, HW, Dinse, D, et al. The role of dietary selenium in bovine mammary gland health and immune function. Anim Health Res Rev. (2009) 10:21–34. doi: 10.1017/S1466252308001588
28. Yin, X, Ji, S, Duan, C, Ju, S, Zhang, Y, Yan, H, et al. Rumen fluid transplantation affects growth performance of weaned lambs by altering gastrointestinal microbiota, immune function and feed digestibility. Animal. (2021) 15:100076. doi: 10.1016/j.animal.2020.100076
29. Hosoi, T, Honda, M, Oba, T, and Ozawa, K. ER stress upregulated PGE₂/IFNγ-induced IL-6 expression and down-regulated iNOS expression in glial cells. Sci Rep. (2013) 3:3388. doi: 10.1038/srep03388
30. Wang, Q, and Wang, X. The effects of a low linoleic acid/α-linolenic acid ratio on lipid metabolism and endogenous fatty acid distribution in obese mice. Int J Mol Sci. (2023) 24:12117. doi: 10.3390/ijms241512117
31. Kinsella, JE, Lokesh, B, Broughton, S, and Whelan, J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. (1990) 6:24–44.
32. Khas-Erdene, Q, Wang, JQ, Bu, DP, Wang, L, Drackley, JK, Liu, QS, et al. Short communication: responses to increasing amounts of free alpha-linolenic acid infused into the duodenum of lactating dairy cows. J Dairy Sci. (2010) 93:1677–84. doi: 10.3168/jds.2009-2681
33. Brenes, A, and Roura, E. Essential oils in poultry nutrition: main effects and modes of action. Anim Feed Sci Technol. (2010) 158:1–14. doi: 10.1016/j.anifeedsci.2010.03.007
34. Wang, Y, Wang, R, Hao, X, Hu, Y, Guo, T, Zhang, J, et al. Growth performance, nutrient digestibility, immune responses and antioxidant status of lambs supplemented with humic acids and fermented wheat bran polysaccharides. Anim Feed Sci Technol. (2020) 269:114644. doi: 10.1016/j.anifeedsci.2020.114644
35. Schroder, K, Hertzog, PJ, Ravasi, T, and Hume, DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. (2004) 75:163–89. doi: 10.1189/jlb.0603252
36. Jimenez-Del-Rio, M, and Velez-Pardo, C. The bad, the good, and the ugly about oxidative stress. Oxidative Med Cell Longev. (2012) 2012:163913. doi: 10.1155/2012/163913
37. Yang, Z, Liu, C, Zheng, W, Teng, X, and Li, S. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol Trace Elem Res. (2016) 169:341–51. doi: 10.1007/s12011-015-0407-3
38. Al-Sadi, R, Boivin, M, and Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. (2009) 34:2765–78. doi: 10.2741/3413
39. Wang, X-F, Li, H, Jiang, K, Wang, Q-Q, Zheng, Y-H, Tang, W, et al. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells. Fitoterapia. (2018) 130:61–5. doi: 10.1016/j.fitote.2018.08.006
40. Wen, J, Khan, I, Li, A, Chen, X, Yang, P, Song, P, et al. Alpha-linolenic acid given as an anti-inflammatory agent in a mouse model of colonic inflammation. Food Sci Nutr. (2019) 7:3873–82. doi: 10.1002/fsn3.1225
Keywords: Salvia sclarea L. extract, lambs, growth performance, immune function, antioxidant capacity
Citation: Ma X, Niu Y, Nan S and Zhang W (2024) Effect of Salvia sclarea L. extract on growth performance, antioxidant capacity, and immune function in lambs. Front. Vet. Sci. 11:1367843. doi: 10.3389/fvets.2024.1367843
Received: 09 January 2024; Accepted: 01 April 2024;
Published: 10 April 2024.
Edited by:
Xianwen Dong, Chongqing Academy of Animal Science, ChinaReviewed by:
Adham Al-Sagheer, Zagazig University, EgyptCopyright © 2024 Ma, Niu, Nan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenju Zhang, emhhbmd3ajEwMjJAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.