- 1School of Animal Technology and Innovation, Institute of Agricultural Technology, Suranaree University of Technology, Nakhon Ratchasima, Thailand
- 2Program in Agriculture, Faculty of Science and Technology, Nakhon Ratchasima Rajabhat University, Nakhon Ratchasima, Thailand
- 3Guizhou University of Traditional Chinese Medicine, Guiyang, China
Lycopene is a kind of natural carotenoid that could achieve antioxidant, anti-cancer, lipid-lowering and immune-improving effects by up-regulating or down-regulating genes related to antioxidant, anti-cancer, lipid-lowering and immunity. Furthermore, lycopene is natural, pollution-free, and has no toxic side effects. The application of lycopene in animal production has shown that it could improve livestock production performance, slaughter performance, immunity, antioxidant capacity, intestinal health, and meat quality. Therefore, lycopene as a new type of feed additive, has broader application prospects in many antibiotic-forbidden environments. This article serves as a reference for the use of lycopene as a health feed additive in animal production by going over its physical and chemical characteristics, antioxidant, lipid-lowering, anti-cancer, and application in animal production.
1 Introduction
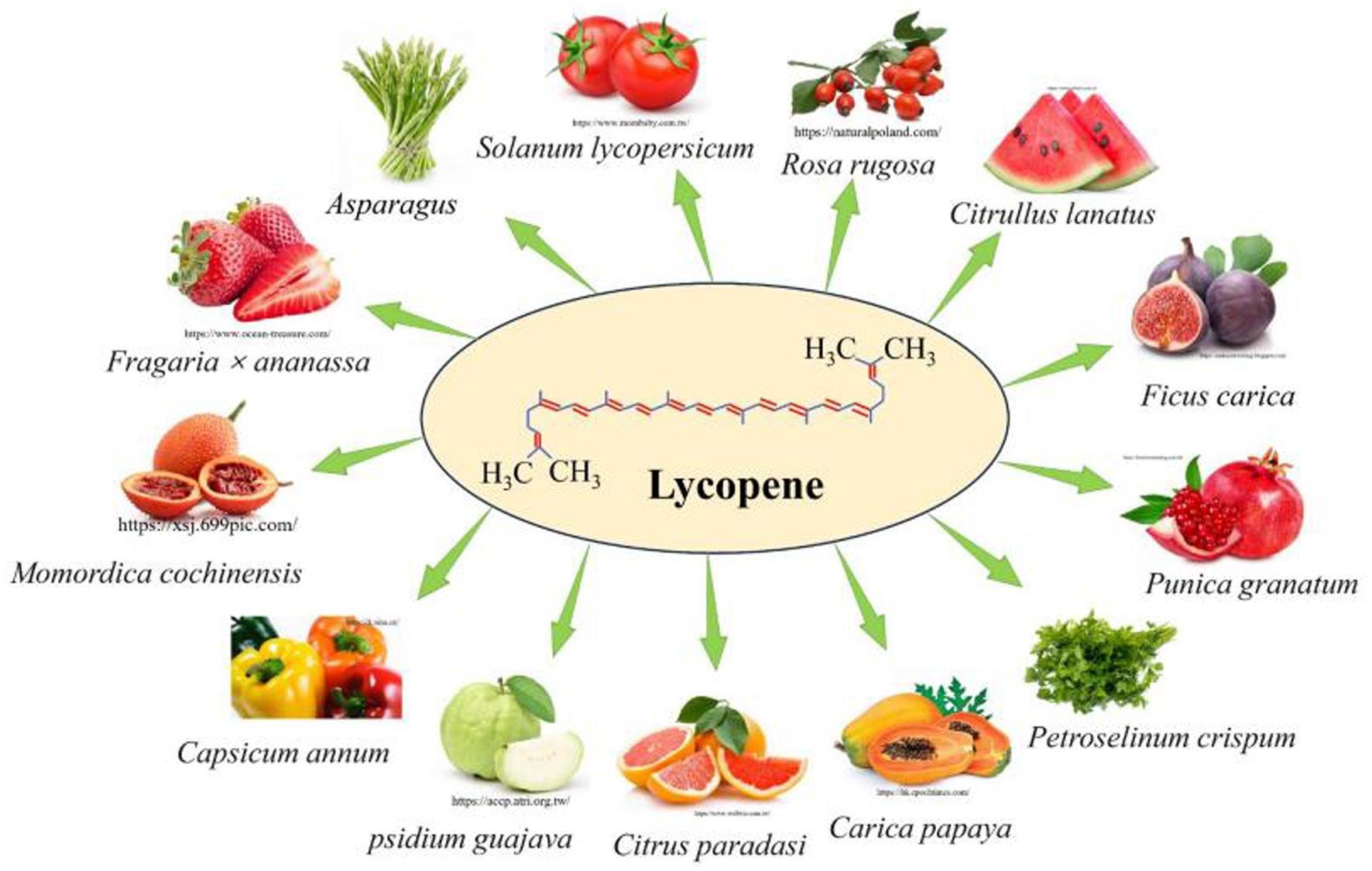
Lycopene was an acyclic isomer of β-carotene and a natural, non-polluting, non-toxic pigment, which found primarily in red or orange fruits and vegetables, such as Solanum lycopersicum, Carica papaya, Psidium guajava, Citrullus lanatus, and Punica granatum (1) (Figure 1). Furthermore, lycopene also found in certain non-red and orange foods, such as Asparagus and Petroselinum crispum (2) and marine halophilic archaea can also produce lycopene (3). However, the human body cannot synthesize lycopene by itself, and 85% of the lycopene needed by the human body mostly comes from tomatoes or tomato-based products (4). The content of lycopene in fresh tomatoes and tomato products is shown in Table 1. The absorption efficiency of the human body for lycopene was 10–30%, and excessive intake will be excreted from the body (6–8). The current methods for extracting lycopene mainly include classic organic solvent extraction, pulsed electric field, enzyme-assisted extraction, supercritical fluid extraction, ultrasonic-assisted extraction, microwave-assisted extraction, and Water-Induced hydro colloidal complexation (9). Lycopene had anti-cancer (10), antioxidant (11), anti-inflammatory (12), regulating body metabolism (13, 14), and immunity (15), and so on. Currently, reports about lycopene are gradually increasing. Previous research has shown that lycopene improved animal production performance (16), maintained intestinal health (17), ameliorated meat quality (18), ameliorated immunity (19) and regulated body metabolism (20), and has good application prospects.
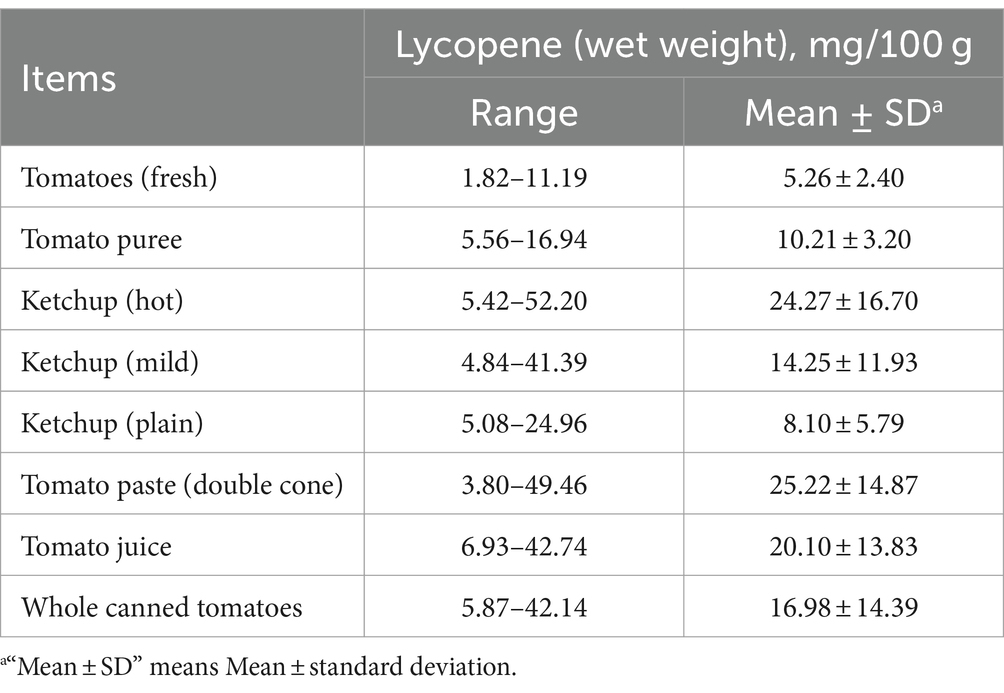
Table 1. Lycopene content in common fresh tomatoes and tomato products (5).
The importance of lycopene has been a research hotspot in recent decades. This article will focus on a more comprehensive review of the physiological functions, mechanisms of action, and application of lycopene in animal production. Therefore, this latest review will ultimately provide more useful information and the latest research perspectives for animal husbandry researchers for animal production, to provide the latest reference for the functional utilization of lycopene as a feed additive.
2 Physicochemical properties and safety of lycopene
2.1 Physicochemical properties of lycopene
Lycopene was an isoprenoid unsaturated olefin compound and a fat-soluble carotenoid. Lycopene consists of 40 carbon chains, including 11 conjugated double bonds and 2 non-conjugated double bonds (Figure 2). Its molecular formula is C40H56, its relative molecular mass is 536.85, and its melting point ranges from 172 to 175°C. Lycopene has obvious lipophilicity and is easily soluble in organic solvents, such as Hexane (C6H14), chloroform (CHCl3), carbon disulfide (CS2), petroleum ether, acetone (C3H6O) and benzene (C6H6), and so on. However, Lycopene is insoluble in water, and methanol, ethanol, and is sensitive to light, acid, catalysts, high temperatures, and metal ions (22). In nature, lycopene has a high degree of unsaturation and mainly appears in the form of the all-trans isomer, which easily oxidatively degrades and undergoes an isomerization reaction under the influence of light, temperature, or chemical reactions, converting from all-trans isomers to mono-cis or poly-cis isomers (23). The most stable form of lycopene among all isomers is said to be 5-cis, which is followed by all-trans, 9-cis, 13-cis, 15-cis, 7-cis, and 11-cis (24). Consequently, all-trans isomers comprise 90% of the lycopene found in tomatoes, whereas most of the processed tomato products exist in the form of the cis isomer.
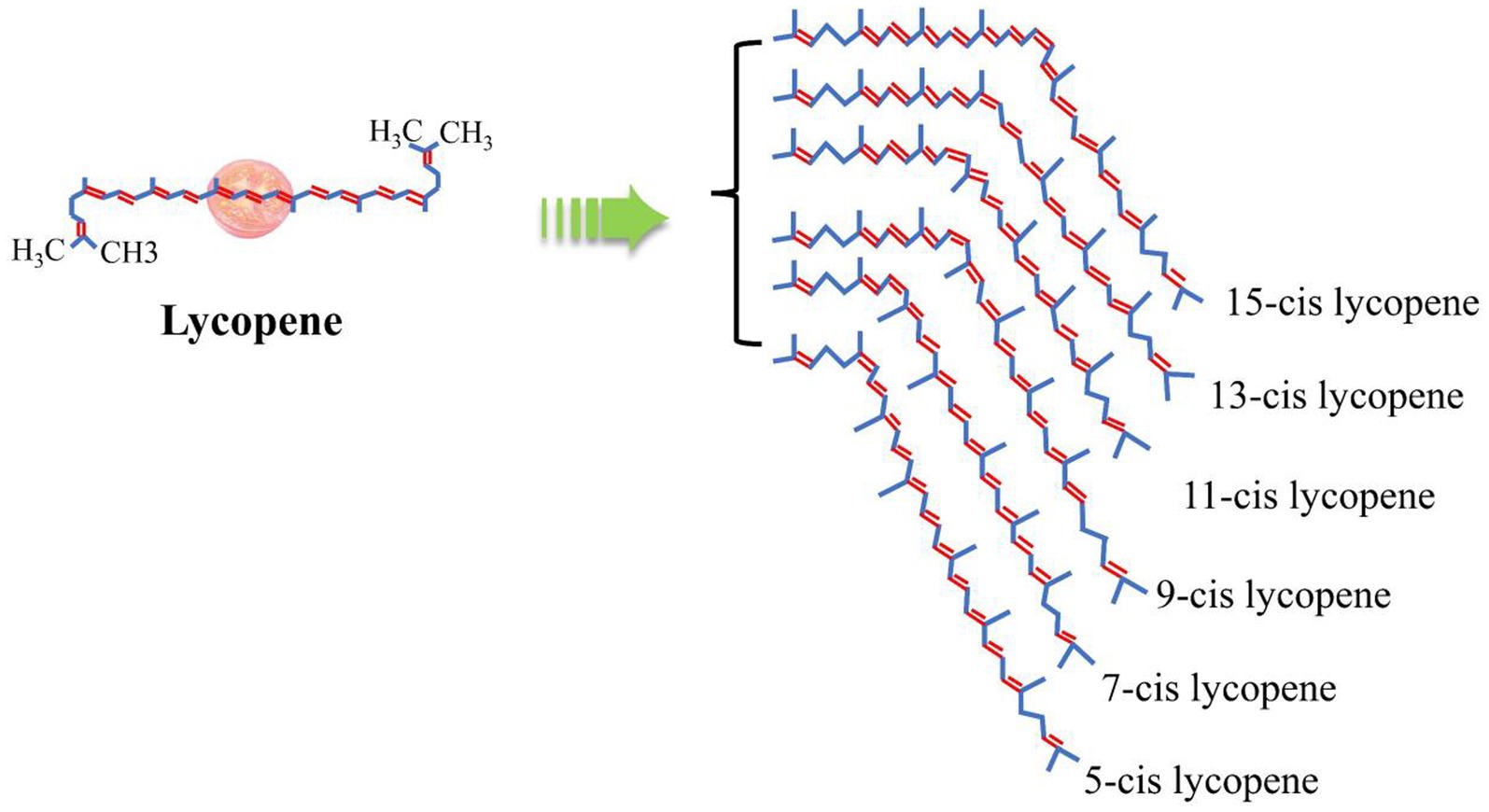
Figure 2. Chemical structure of lycopene (21).
2.2 Safety of lycopene
Lycopene is harmless to animals and humans and has certain benefits, which whether in the form of preparation or crystallization, was not genotoxic under stable conditions (25), Lycopene were not toxic to rabbit lymphocytes and did not produce any mutagenic activity (26). Similar results have been obtained in subchronic and chronic safety studies in mice (27). Additionally, it was concluded by Rao et al. (7) that healthy individuals who consume 5 to 75 mg of lycopene daily will not experience any negative effects. At the same time, Kong et al. (28) indicated that a daily intake of 3 g/kg of lycopene from food will not harm the human body. Therefore, lycopene is considered a safe Non-toxic substance.
3 Physiological functions and mechanisms of action of lycopene
3.1 Antioxidant function
Under normal physiological conditions, the generation and elimination of free radicals were always in a state of dynamic balance. When oxidative stress occurs, this dynamic balance will be broken, and fat, protein, and DNA in the body will be damaged, leading to a variety of diseases (29). The advancement of animal husbandry is gravely threatened by oxidative stress, which brings huge economic losses to the industry (30). Antibiotics were once useful antioxidant medications that might enhance animal immunity, growth performance, and disease prevention. However, animal production, human health, and environmental sustainability are threatened by antibiotic resistance and residues (31). Since the European Union (EU) banned the use of antibiotics in animal feed in 2006, and researchers have been trying to find plant-based feed supplements as safe antibiotic alternatives (32). In July 2020, China announced that livestock farming had officially entered a new era of banning the use of antibiotics in feed (33). Therefore, developing natural, healthy, and safe antibiotic alternatives for animal husbandry is one of the hot spots in modern feed research.
Relevant reports indicated that lycopene due to its polyunsaturated structure, not only directly scavenged free radicals, but also indirectly scavenged free radicals by regulating the enzymatic antioxidant defense system and enzymatic oxidative damage system (34). Therefore, lycopene protects proteins, DNA, and lipids in the body from oxidative damage, thereby reducing oxidative stress in the body and maintaining animal health.
3.1.1 Directly scavenge free radicals
The unique 11 conjugated double bonds in lycopene are highly reactive towards oxygen and free radicals (35). Furthermore, in different carotenoids, lycopene has an antioxidant capacity second only to astaxanthin and is an important inhibitor of reactive oxygen species (ROS). Its effectiveness in scavenging singlet oxygen is 100 times greater than vitamin E, 10 times greater than that of α-tocopherol , and double that of β-carotene (36, 37). Lycopene scavenged hydroxyl radicals through an addition reaction (38) and could also react with peroxynitrite, thus effectively functioning as a nitrite scavenger (39, 40). Moreover, lycopene also has the function of scavenging sulfuryl, nitrogen dioxide, and sulfuryl free radicals (41). Whether under polar or non-polar conditions, lycopene has a higher rate of scavenging hydrogen peroxide (H2O2) free radicals than β-carotene (42).
3.1.2 Lycopene antioxidant system activity
The antioxidant mechanism of lycopene is shown in Figure 3. Lycopene indirectly acts on free radicals by regulating the enzymatic antioxidant defense system and enzymatic oxidative damage system, protecting the body from oxidative damage (34, 44). Shen et al. (45) have shown that lycopene promoted mouse cardiac glutathione peroxidase (GSH-Px) activity and increased cardiac glutathione (GSH) levels; at the same time, cardiac myeloperoxidase (MPO), H2O2 levels and glutathione S-transferase (GST) activity shown a decreasing trend. Therefore, their experiment proved that lycopene inhibiting di (2-ethylhexyl) phthalate-induced oxidative stress response. On the other hand, lycopene promoted the activities of GST and catalase (CAT) in the liver of ducklings and increased the total antioxidant capacity (T-AOC) of the liver, which reduced the liver malondialdehyde (MDA) content and the residual aflatoxin in the liver of ducklings obviously of the body (46). Wen et al. (47) indicated that the incorporation of 200 mg/kg lycopene in diets of swine diets increased antioxidant indices superoxide dismutase (SOD), T-AOC, GSH-Px, and CAT in serum, and reduced the levels of MDA. Relevant records have shown that lycopene increased the levels of peroxisome proliferator-activated receptor γ (PPAR-γ) and typical antioxidant biomarkers in rats fed a high-fat diet (48). Similarly, Li et al. (49) obtained similar results in a study on a mouse model of ulcerative colitis induced by dextran sulfate sodium. Moreover, lycopene combined with metformin increased the activity of the antioxidant enzyme paraoxonase-1 ( PON-1 ), enhanced the endogenous oxidative defense ability of obese mice, and protected the health of the kidneys and liver (50). A previous study by Aboubakr et al. (51) indicated that lycopene effectively reduced cardiac antioxidant damage and apoptosis caused by methotrexate. Lee et al. (52) indicated that lycopene also reduced reactive ROS production by inhibiting nicotinamide adenine dinucleotide phosphate oxidase ( NOX ) activity, which in turn ameliorates oxidative damage to acinous cell induced by ethanol and the fatty palmitoleic acid.
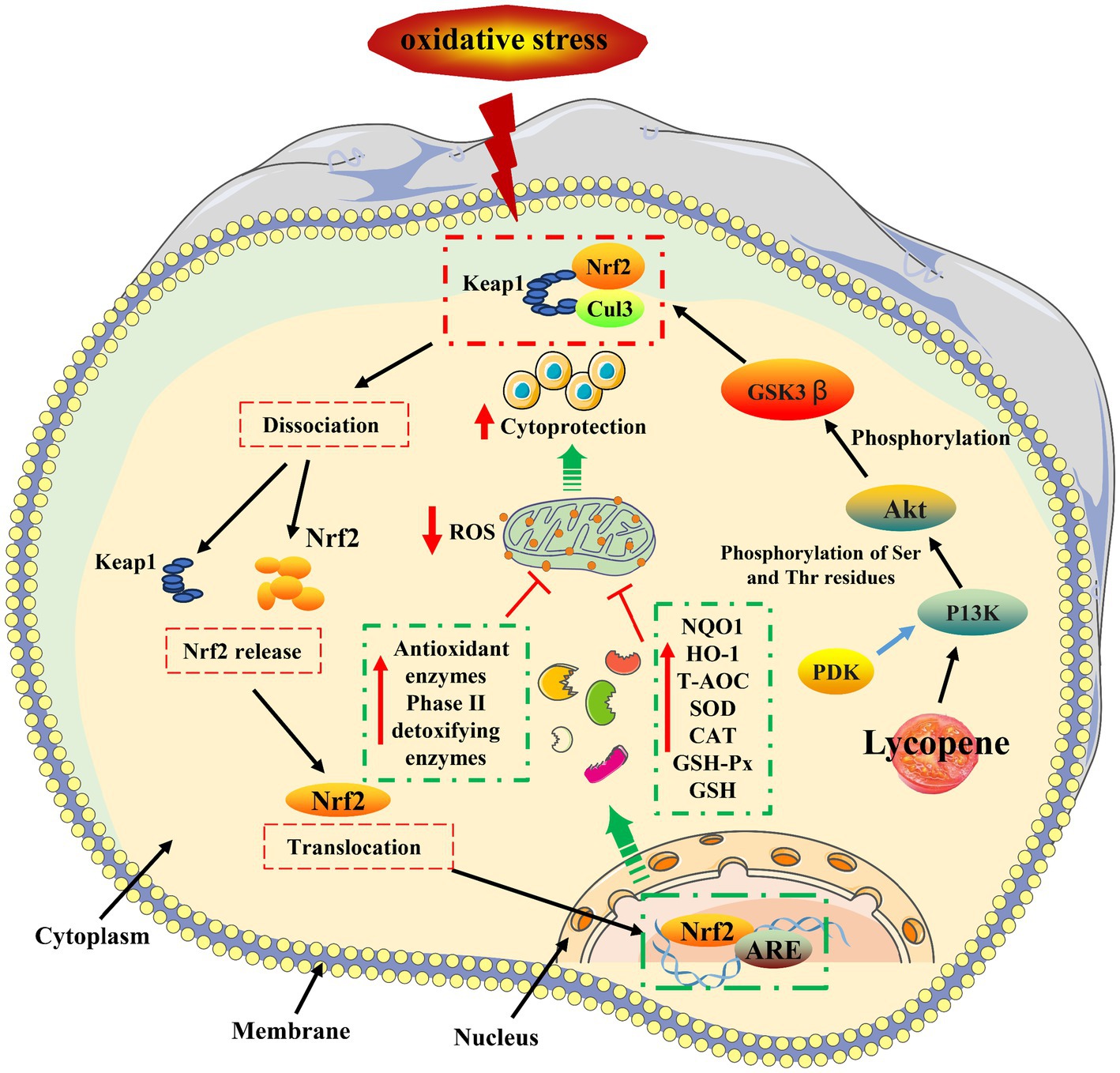
Figure 3. Lycopene antioxidant mechanism (10, 43). HNF1-α, Hepatocyte nuclear factor1-α; LDL-R, Hepatic LDL receptors.
The antioxidant mechanism of lycopene was shown in Figure 4. Nuclear factor erythroid 2-related factor 2 ( Nrf2 ) is an important transcription factor that affects the activities of CAT, SOD, GST, and GSH-Px in the oxidative system, (58, 59). Normally, the cytoplasmic kelch-like ECH-associated protein 1 (Keap1) is bound by the Neh2 (Nrf2-ECH homology) domain at the end of Nrf2, negatively regulating Nrf2 and usually preventing Nrf2 from translocating to the nucleus (60, 61). In responding to oxidative stress, the binding of Keap1 and Nrf2 will be rapidly dissociated, prompting Nrf2 to enter the nucleus and bind to the antioxidant response element (ARE) sequence in the nucleus (62). Consequently, this process transcriptionally upregulates the expression of ARE and Nrf2, stimulating the production of phase II detoxifying antioxidant enzymes and upregulating the expression of antioxidant-related stress genes (63). Keap1/Nrf2/ARE pathway regulates NAD (P)H: quinone receptor oxidoreductase 1 ( NQO1 ) and heme oxidase 1 ( HO-1 ), induces the expression of various antioxidant protection genes, reduces the damage of ROS to cells, and effectively reduces the occurrence of liver diseases caused by oxidative stress (64, 65). Relevant research has shown that lycopene could act on the Nrf2/HO-1 and protein kinase B(Akt)/Nrf2 signaling pathway, induce Nrf2 gene transcription, upregulate the mRNA expression levels of NQO1 and HO-1, improve the activities of SOD, CAT, and GSH-Px, and reduce the accumulation of ROS and MDA oxidative stress end-products (66–70). Furthermore, it has been documented that lycopene inhibited β-secretase ( BACE ) activity by triggering the PI3K/Akt/Nrf2 signaling pathway, which then attenuates the damage of oxidative stress and apoptosis in M146L cells (43).
In conclusion, there are two basic ways that lycopene might increase the body’s antioxidant capacity. On the one hand, it can directly act on free radicals to achieve the scavenging effect. On the other hand, it can improve the mRNA expression level and activity of related antioxidant enzymes by activating the Nrf2 antioxidant signaling pathway to achieve the purpose of scavenging free radicals and maintaining the oxidation balance of cells or the body.
3.2 Lycopene’s lipid-lowering function
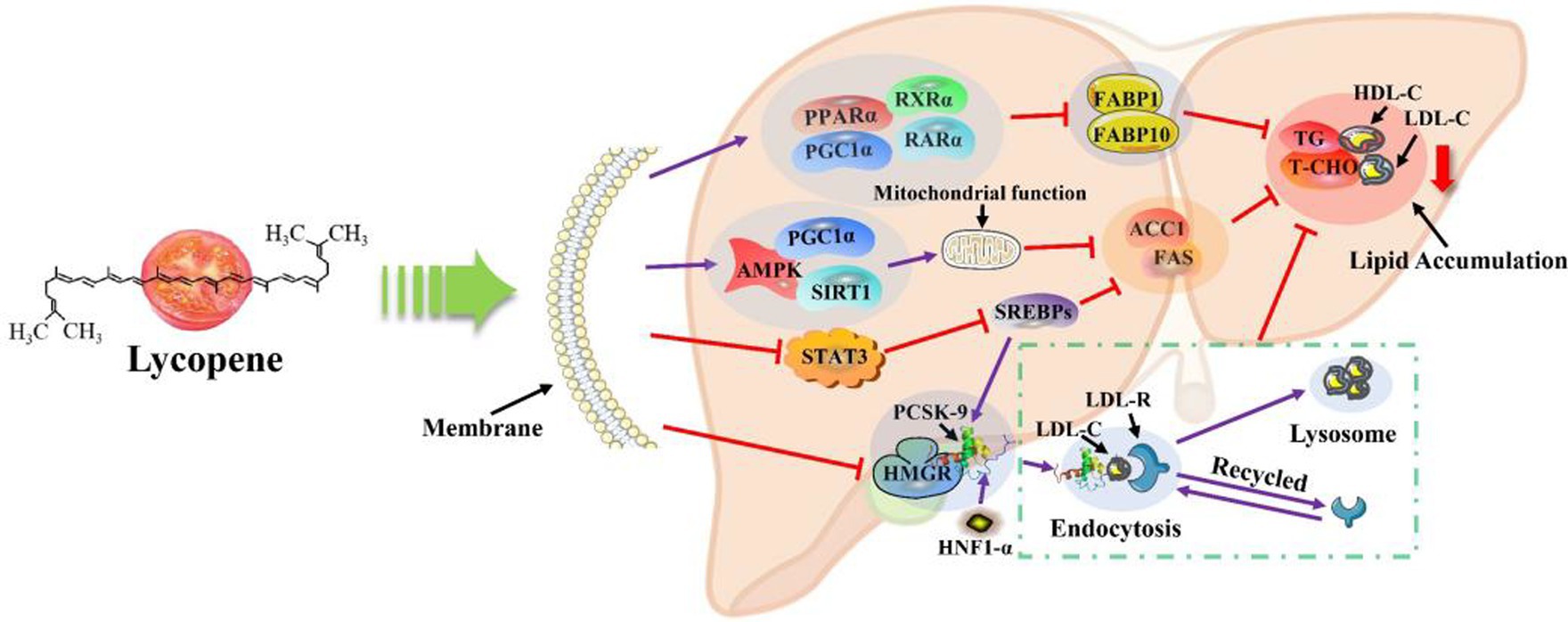
The lipid-lowering mechanism of lycopene was shown in Figure 4. Lycopene could inhibit lipid synthesis in the body through multiple pathways of action, and at the same time accelerate the rate of lipid transport and mobilization efficiency, thereby achieving lipid-lowering effects. Peroxisome proliferator-activated receptor α ( PPARα ), as the most important factor in the lipid-lowering mechanism of lycopene, plays a decisive role in the lipid-lowering function of lycopene. Relevant research has shown that PPARα could activate β-oxidation-related genes, thereby increasing the expression of mRNA of related genes, and promote the uptake of lipid mitochondria in body cells, accelerate lipolysis, and then play an important role in lipid metabolism in the body. PPARα not only orchestrates the coordination of lipoprotein metabolism but also directly or indirectly controls the lipogenesis pathway in the liver (71, 72). In another study, lycopene was found to possess another important mechanism of lipid metabolism. Lycopene increased the gene expression of PPARγ coactivator 1α ( PGC1α ), PPARα , retinoic X receptor α ( RXRα ) and retinoic acid receptor α ( RARα ) in the liver of breeding hens, which downregulated the expression of liver fatty acid binding protein 1 ( FABP1 ), liver fatty acid binding protein 10 ( FABP10 ) and jejunum fatty acid transporter 4 ( FATP4 ) genes. Moreover, lycopene also upregulated the expression of PPARγ , RXRγ , RXRα , and duodenal RXRα genes in the jejunum. Therefore, lycopene accelerates the fat metabolism rate in the body and reduces fat deposition through this pathway (55). Lycopene could activate the AMP-activated protein kinase/ Sirtuin 1/peroxisome proliferator-activated receptor-γ-coactivator 1α (AMPK/SIRT1/PGC1α) or inhibit signal transducer and activator of transcription 3 (STAT3) biosynthetic signaling pathway. and then lycopene upregulated the mRNA expression levels of SIRT1 and inhibited the mRNA expression levels of sterol regulatory element binding proteins (SREBPs), fatty acid synthase ( FAS ), and acetyl-CoA carboxylase 1 ( ACC1 ) genes, thereby inhibiting the synthesis of lipids, and ultimately reducing the levels of total cholesterol (T-CHO), triglycerides (TG), and low-density lipoprotein (LDL-C) in the serum of mouse livers and broiler chickens (13, 14, 56). Moreover, lycopene reduced lipid synthesis and prevented mitochondrial dysfunction induced by palmitate, thereby reducing the risk of nonalcoholic fatty liver disease (NAFLD) in mice (13, 14, 56, 57). In addition, it was concluded by Huang et al. (73) and Albrahim et al. (48) that lycopene could improve liver damage caused by a high-fat diet in mice. Moreover, Lu et al. (74) also reported that lycopene reduced TG in HepG2 cells caused by oleic acid and palmitoleic acid. Alvi et al. (53) demonstrated that lycopene inhibits the activity of HMG-CoA reductase (HMGR) and proprotein convertase subtilisin / kexin type 9 (PCSK-9) transcription. Therefore, lycopene reduced the synthesis of T-CHO and the endocytosis of LDL-C by inhibiting the activity of HMGR and LDL-C receptors, thus achieving the purpose of lipid-lowering (54). Moreover, lycopene enhanced the activity of AMP-activated protein kinase ( AMPK-P ) and downregulating ATP citrate-raising lyase ( ACLY ). Ultimately, the levels of MDA, HMG-CoA reductase , FAS , ACLY , and TNF-α were reduced and the levels of AMPK-P and GSH were increased in diabetic hyperlipidemia rats (75).
In conclusion, through a review of many existing studies, it was found that there are four main pathways for the lipid-lowering mechanism of lycopene. Lycopene activates the PPARα and AMPK/SIRT1/PGC1α pathways, thereby downregulating the expressions of FABP1 , FABP10 , ACC1 and FAS . In addition, lycopene could also downregulate the expression of ACC1 and FAS and LDL-C endocytosis by inhibiting the STAT3 biosynthetic signaling pathway and the HMGR/PCSK-9 transcriptional activity pathway, ultimately achieving a lipid-lowering effect.
3.3 Lycopene anti-cancer function
The anti-cancer mechanism of lycopene was shown in Figure 5. There were in vitro and in vivo research has shown that lycopene has an important anticancer effect, which inhibited the proliferation of cancer cells through antioxidant activity, regulation of anti-inflammation, growth factor signaling, apoptosis induction, and cell arrest, such as anterior prostate cancer, cervical cancer, breast cancer, melanoma, ovarian cancer, oral cancer, hepatocellular carcinoma, lung cancer, and other cancer cells (13, 14, 80–88). The content of Serum lycopene and lycopene intake was inversely proportional to the probability of cancer (10). Inducing cancer cell apoptosis is one of the most important anti-cancer mechanisms of lycopene. The main pathway of apoptotic decomposition of cells is the activation of BH3-only protein (BH3) by DNA damage, which directly activated B⁃cell lymphoma, leukaemia⁃2⁃associated X protein (BAX), and B⁃cell lymphoma, leukaemia⁃2⁃associated K protein (BAK) and made them become homologous oligosaccharides. At this time, due to the penetration of outer mitochondrial members, mitochondrial intermembrane proteins are released in the cytoplasm and combine with Apaf-1 to form procaspase-9-activating a heptameric protein complex called the apoptosome, which ultimately activates caspase-3 and caspase-7 cleaves the target protein, led to apoptotic breakdown (77). Moreover, in “type II” cells, such as hepatocytes, the mitochondrial amplifying loop activates the effector caspase and then releases SMAC to downregulate XIAP expression to mitigate its mediated caspase inhibition (77). Previous studies have shown that lycopene reduced carcinogenesis by inhibiting the phosphatidyl-inositol 3-kinase/serine–threonine kinase (PI3K-AKT) signaling pathway, similarly, lycopene induced apoptosis by downregulating B, cell CLL/lymphoma-2 (BCL-2), and upregulating BAX (79, 89). Previous records have shown that lycopene might increase the levels of Bax and E-cadherin and downregulate the levels of N-cadherin, phosphatidylinositol 3-kinase (p-PI3K), protein Kinase B (p-AKT), BCL-2, and phosphatidylinositol 3-kinases (PI3K)/AKT/mammalian target of rapamycin (p-m-TOR). The inactivation of PI3K/AKT/m-TOR signaling inhibits epithelial-to-mesenchymal transition apoptosis in oral cancer cells (13, 14). Moreover, lycopene could inhibit the proliferation of human breast cancer MCF-7 cells and promote their apoptosis by upregulating the gene expression of p53 protein (p53) and Bax (78).
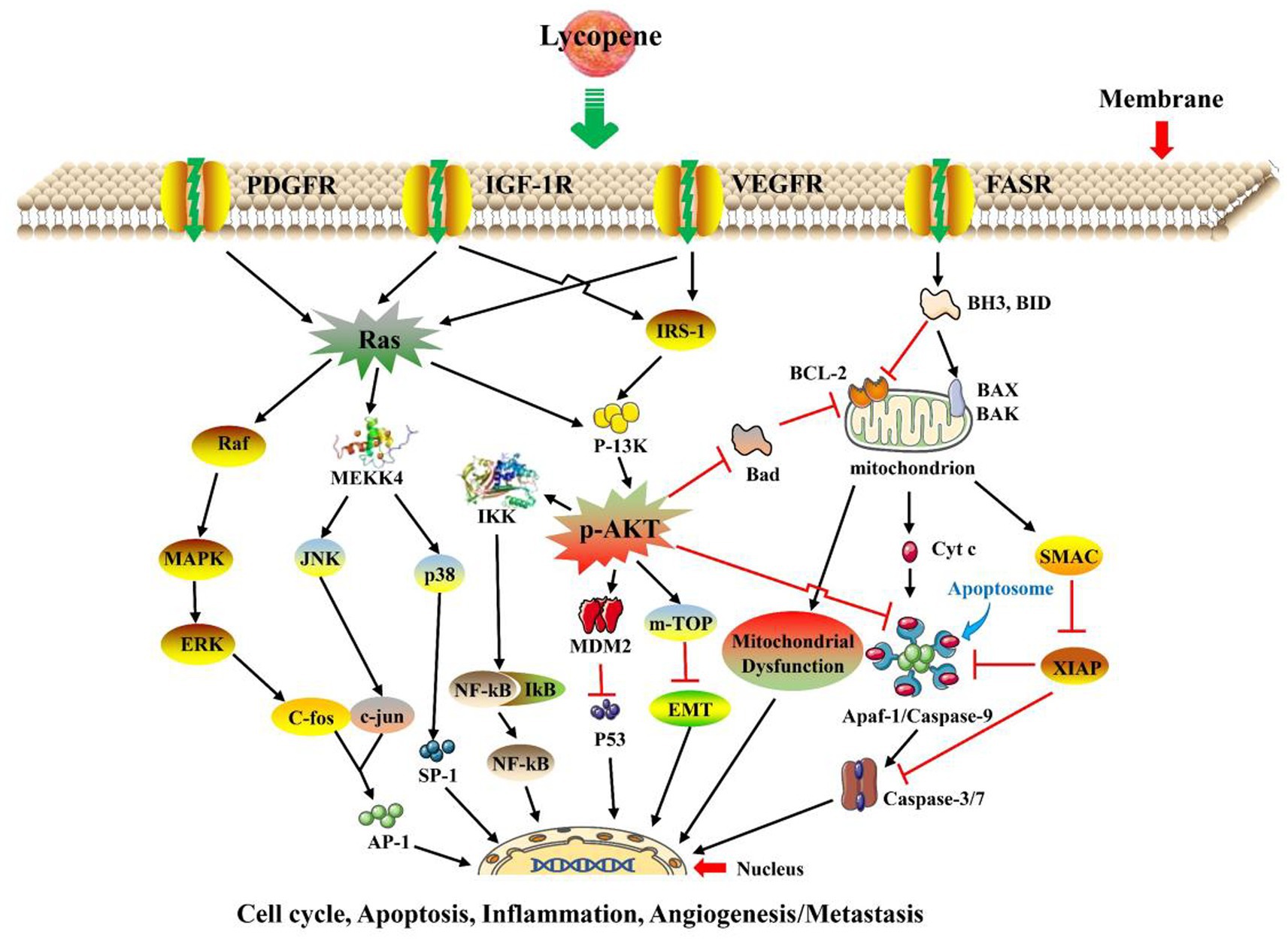
Figure 5. The main anti-cancer mechanism of lycopene (14, 46, 48, 76–79). MAO-A, Monoamine oxidase-A; ADA, Adenosine deaminase; NTPDase, Nucleotide triphosphatase; AchE, Acetylcholine esterase.
It was concluded by Ozkan et al. (10) that that lycopene could play an anti-inflammatory and antioxidant role in ovarian cells by upregulating Nrf2 or downregulating nuclear factor-kB ( NF-kB ) and STAT3 signals, thus inhibiting the occurrence of spontaneous ovarian cancer in laying hens. Furthermore, lycopene also downregulated the mRNA expression of integrin β1 ( ITGB1 ), integrin α5 ( ITGA5 ), focal adhesion kinase ( FAK ), integrin-linked kinase ( ILK ), matrix metalloproteinase 9 ( MMP9 ) and the expression of epithelial to mesenchymal transition (EMT) inhibits the activity of mitogen-activated protein kinase ( MAPK ) and reduced the mRNA expression level of the ovarian cancer biomarker CA125, thereby inhibited the proliferation of primary ovarian cancer cells and metastatic cells in mice (76). Cheng et al. (90) concluded that low-dose lycopene increased the expression level of 8-oxoguanine DNA glycosylase ( OGG1 ), Nei-like DNA glycosylase ( NEIL1 , NEIL2 , NEIL3 ), and connexin 43 (Cx43). Similarly, it also upregulated the expression level of scavenger receptor (SRB) protein SR-B1, thereby inhibiting lung cancer by improving gene stability and inhibiting smoke-induced oxidative stress. Lycopene inhibited the proliferation of ferret liver cancer and lung cancer cells induced by tobacco carcinogens by inhibiting the protein expression levels of lung α7 nicotinic acetylcholine receptor or NF-kB and cytochrome P450E1 (80). Additionally, it was concluded by Jhou et al. (91) that 2.5 μM lycopene could significantly downregulate the activities of NADPH oxidase 4 ( NOX4 ), matrix metalloproteinase 2 ( MMP-2 ) and matrix metalloproteinase 9 ( MMP-9 ), and then inhibited the migration of human hepatic adenocarcinoma SK-Hep-1 cells. Moreover, inhibiting the migration of human umbilical vein endothelial cells and the activity of vascular endothelial growth factor are also two ways in which lycopene could effectively prevent cancer (92, 93).
In conclusion, Lycopene could induce mitochondrial apoptosis induced by the inactivation of the growth factor ( FASR , VEGFR , IGF-1R , and PDGFR ), mainly inducing BH3 dependent and independent activation of BAX and BAK, Ras/RAF/MAPK, and PI3K/AKT/PKB signaling pathways to achieve anticancer effects. When these pathway pathways are activated. Many related anti-cancer factors will be activated, thereby regulating the cell cycle, inflammation, apoptosis, metastasis, angiogenesis, and so on, ultimately achieving the purpose of anti-cancer.
3.4 Anti-inflammatory and immunomodulatory functions of lycopene
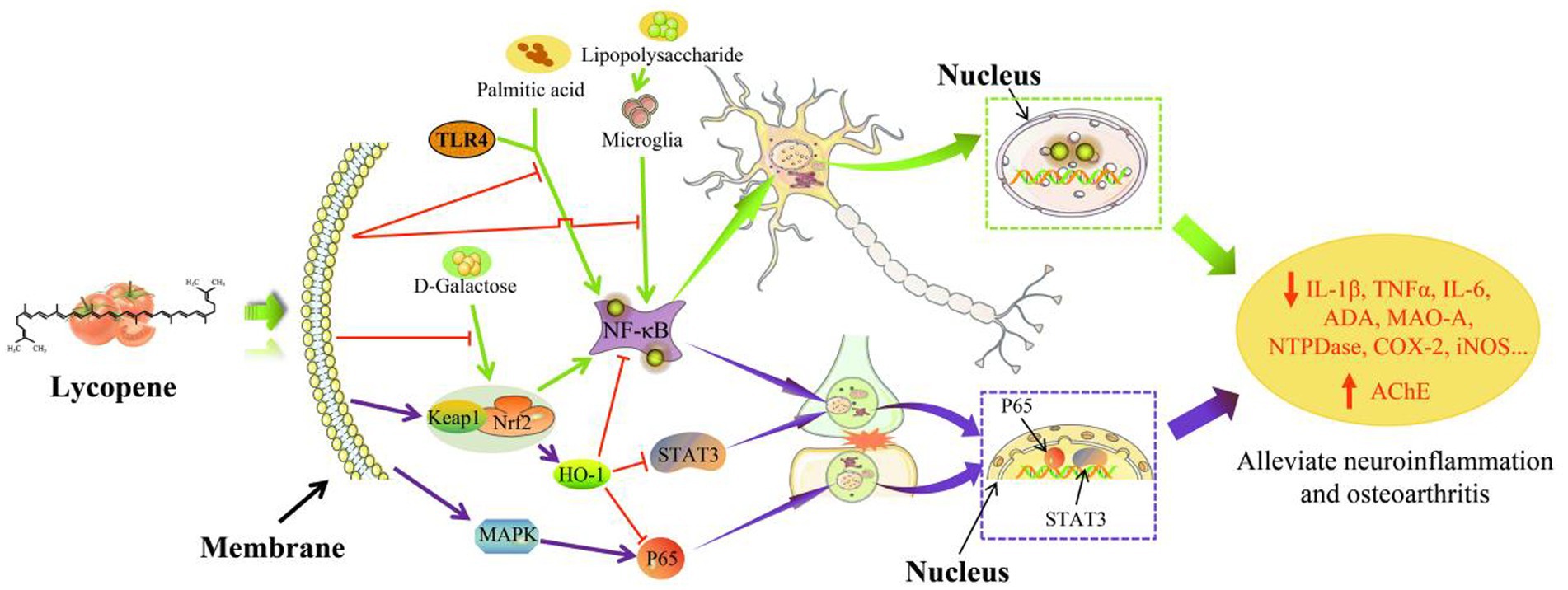
The anti-inflammatory mechanism of lycopene was shown in Figure 6. Lycopene improved inflammation in the body by upregulating and downregulating many signaling channels and limited the transcription and expression of inflammatory mediator-related factors (34). As a key transcription factor involved in body inflammation and cellular immunity, NF-kB will participate in the transcription and synthesis of inflammatory cytokines and chemokines in acute inflammatory responses after activation and has powerful anti-apoptotic and endothelial anti-inflammatory effects. Therefore, it plays an important role in the body’s anti-inflammatory and immune regulation (99, 100). A previous study by Ugbaja et al. (101) indicated that lycopene reduced oxidative stress and downregulated the toll-like receptor-4 (TLR4) / NF- ĸ B-p65 axis in female Wistar rats, thereby mitigating the neuroinflammation caused by palmitic acid (PA). Furthermore, the reduction of lipopolysaccharide (LPS) induced neuroinflammation in male C57BL/6 J mice is affected by lycopene through the regulation of MAPK , NF-κB , and Nrf2 signaling pathways (95). It was concluded by Zhao et al. (102) that lycopene mediated the Nrf2/NF- ĸ B pathway in CD-1 mice, which reduced neuroinflammation brought on by oxidative stress. Lycopene could activate the Nrf2/HO-1 signaling pathway, thereby inhibited the activation of the NF - κB and STAT3 pathways caused by interleukin-1β (IL-1β), limited tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase ( iNOS ) gene transcription, and then reduced TNF-α, IL-6 and prostaglandin content, and slow down arthritis in mice (98). Moreover, lycopene also reduced arthritis by activating the Kelch-like epichlorohydrin-related protein-1 ( Keap1 )-Nrf2 signaling pathway (97). In improving cellular inflammation, lycopene has been reported to stimulate PPARγ gene transcription by inhibiting MAPK and NF - κB signaling, on the other hand, lycopene also activated the Nrf2/HO-1 pathway, enhanced autophagy in liver macrophages, inhibited the expression of nucleotide-binding oligomerization domain-like receptor protein 3 ( NLRP3 ), and reduced the secretion of IL-1β, IL-6, interleukin-8 (IL-8), and TNF-α ultimately achieves the purpose of improving macrophage inflammation (94, 96). Previous research has shown that the combination of quercetin and lycopene reduced the expression levels of modulatory metalloproteinase 7 ( MMP7 ), MDA, MPO, and hydroxyproline, while the expression levels of SOD and GSH was elevated and has the potential to resolve inflammation induced by ochratoxin A ( OTA ) in rats (103). Notably, lycopene inhibited monocyte adhesion and migration induced by high mobility group protein box 1 (HMGB1) produced by necrotic cells and immune cells exposed to pro-inflammatory signals by decreasing the gene expression of cell adhesion molecules. Consequently, it reduced LPS-induced HMGB1 release and HMGB1-mediated secretion of TNF-α and secretory phospholipase A2, and the body finally exerts its anti-inflammatory function (104, 105).
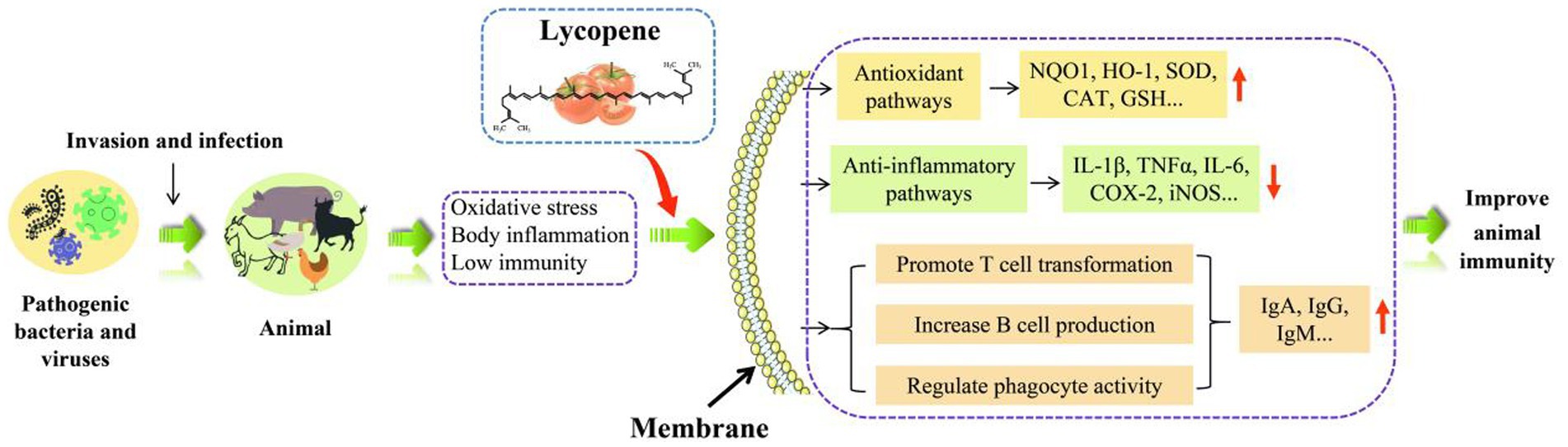
In addition to inhibiting inflammation in the body, lycopene also has the function of enhancing cellular immunity and humoral immunity. The immunomodulatory of lycopene was shown in Figure 7. Furthermore, lycopene also has the function of protecting lymphocytes, promoting the transformation of T lymphocytes, increasing the CD4+/CD8+ ratio, and enhancing the activity of natural killer cells (109–111). It was concluded by Sarker et al. (112, 113) that lycopene reduced the damage aflatoxin B1 (AFB1) caused to broiler intestinal immune function by upregulating the production of claudin-1 (CLDN-1) mRNA and lowered the levels of the inflammatory cytokine interferon-γ (IFN-γ) and IL-1β. Similarly, Hidayat et al. (19) also obtained the same result. Lycopene might work by encouraging T-cell transition, raising the CD4+/CD8+ ratio, and concurrently lowering inflammatory cytokine levels, all of which would enhance rats’ immunity (106). Moreover, lycopene could also ensure the health of the sow placenta by reducing the expression level of placental inflammatory factors and improving immunoglobulin content, antioxidant capacity, placental lipid transport, and lipid metabolism (16). Other studies have found that lycopene could regulate the efficiency of IFN-γ and IL-2 production by mouse T lymphocytes or indirectly activate T cells (108). Furthermore, lycopene could increase the production of spleen B lymphocytes and serum immunoglobulin G content in mice, thereby improving the immune function of mice (107).
In conclusion, lycopene has potent anti-inflammatory and immunoregulatory properties. It could promote the development of B and T lymphocytes, stimulate the normal differentiation of T cells, along regulate the production and release of factors linked to inflammation.
4 Application of lycopene in poultry and ruminant production
As a new type of health additive and dietary supplement, lycopene has many functions in animal production, such as improving production performance, intestinal health, meat quality, and increasing animal reproduction rate, and has good application potential.
4.1 Application of lycopene in poultry production
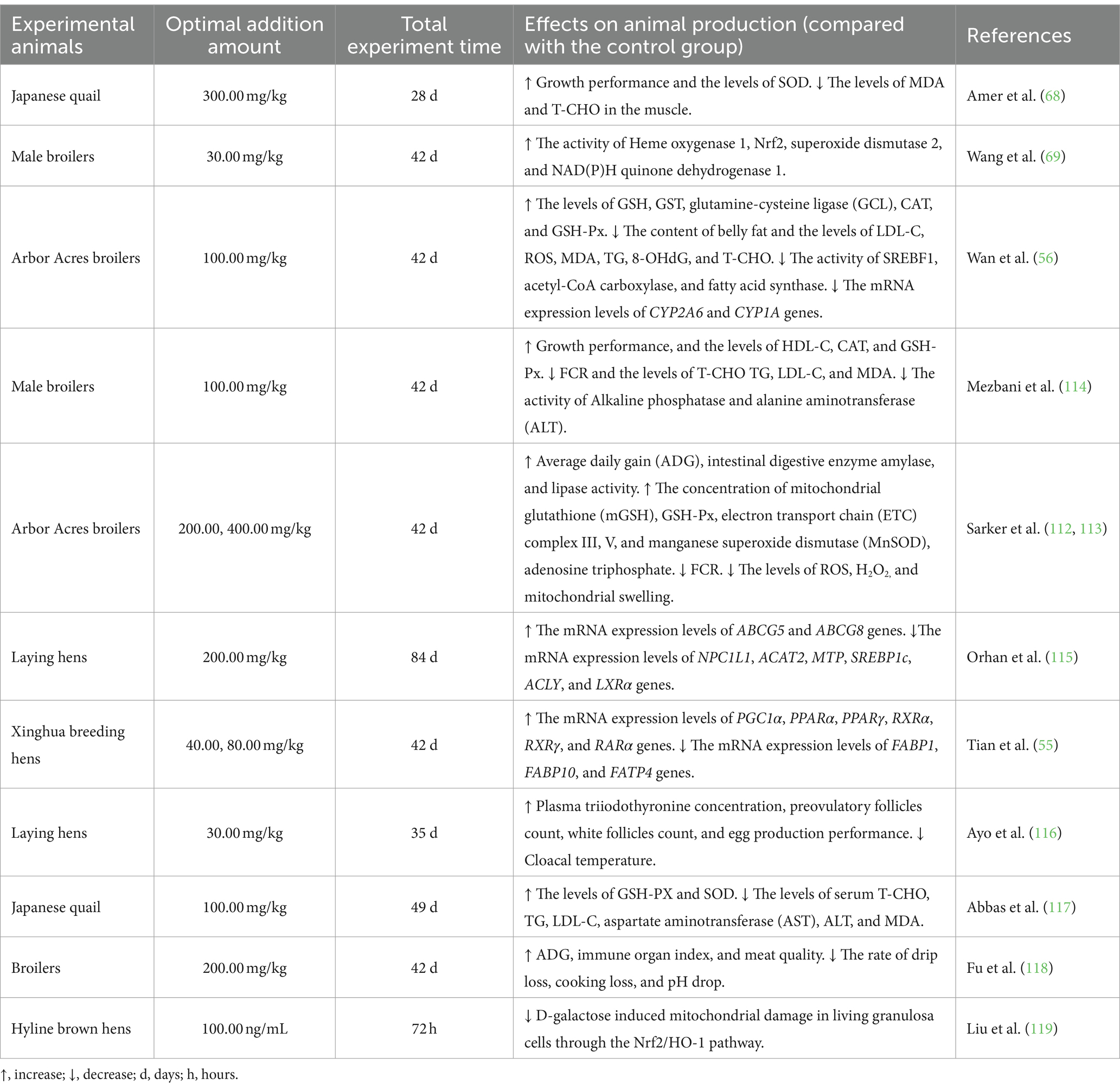
Lycopene was widely used in poultry production because of its strong antioxidant, anti-inflammatory, and immune-regulating effects. In the current poultry research reports, lycopene was mainly used in poultry feed as a feed additive. It could be concluded from Table 2 that lycopene could reduce the feed conversion ratio (FCR), belly fat weight, and the levels of MDA, LDL-, TG, T-CHO, IFN-γ, IL-1β, ROS, and H2O2 in poultry. In addition, lycopene reduced the rate of drip loss, cooking loss, and pH drop in broilers. The incorporation of lycopene in poultry diets could improve the growth performance, feed intake, and meat quality of poultry. Moreover, lycopene could also improve the GSH-PX, GSH, GST, SOD, immune organ index, and HDL-C indicators of poultry. In gene expression, lycopene increased the mRNA expression levels of Nrf2, ABCG5, ABCG8, Cludin-1, PGC1α, PPARα, RXRα, RARα, and RXRγ. Furthermore, lycopene reduced the activity of acetyl-CoA carboxylase, sterol regulatory element binding protein 1 (SREBF1), fatty acid synthase, microsomal triglyceride transfer protein (MTP), Niemann-Pick C1Like1 ( NPC1L1 ), cholesterol O-acyltransferase (ACAT) 2 ( ACAT2 ), Which lycopene also reduced the mRNA expression levels of SREBP1c, LXRα, ACLY, FABP1, FABP10 and FATP4. Lycopene increased the activity of intestinal digestive enzymes amylase, lipase, glutamine-cysteine ligase, mitochondrial glutathione, electron transport chain complex III, V, and manganese superoxide dismutase (MnSOD) in poultry. A previous study by Ayo et al. (116) indicated that lycopene could increase the concentration of plasma triiodothyronine concentration and the count of preovulatory follicles and white follicles. Furthermore, the temperature of the cloacal is reduced, thus increasing the reproductive rate of laying hens.
4.2 Application of lycopene in ruminant production
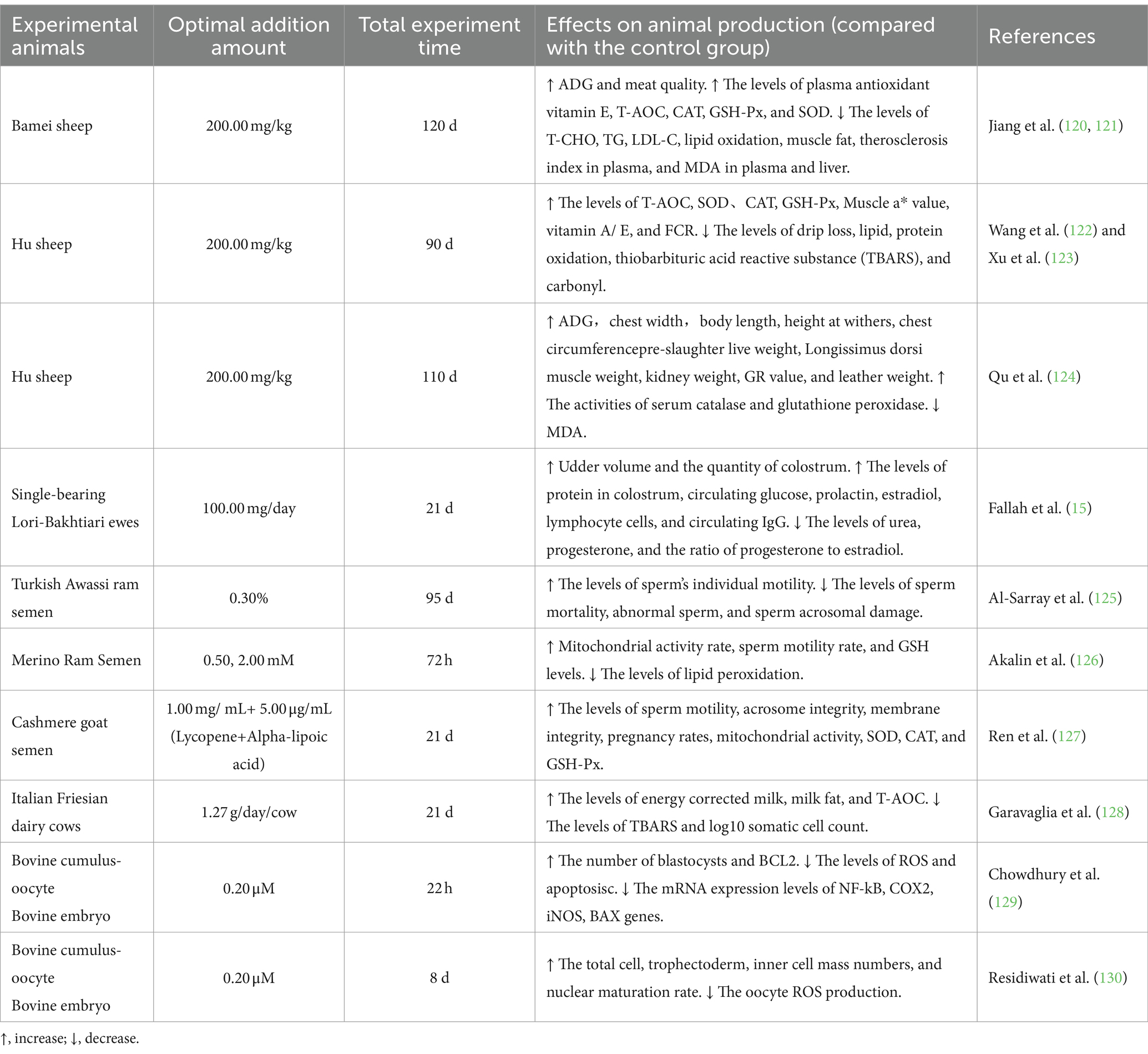
Lycopene has been widely used in animal production because of its special beneficial functions to animals. There are surprisingly few reported lycopene-related outcomes in ruminant production, according to the most recent published reports. It could be concluded from Table 3 that supplementing lycopene to sheep and goat diets could improve T-AOC, CAT, GSH-Px, SOD, vitamin E, production performance, slaughter performance, and meat quality. Additionally, it was concluded by Fallah et al. (15) that lycopene could enhance immunological markers, sex hormones, and milk quality, which promote the breast development of ewes. The incorporation of lycopene in diets reduced the levels of T-CHO, TG, LDL-C, and MDA, which reduced FCR, Muscle L* value, the content of muscle fat, lipid oxidation, and protein oxidation (including thiobarbituric acid-reactive substance and carbony l) in sheep and goats. In addition, in an experiment on the effect of lycopene on ram semen, it was concluded that supplementing lycopene improved the individual motility of goat sperm and reduced sperm mortality and abnormal sperm content. The application of lycopene in cattle diets production is rarely reported. Lycopene improved the milk quality, embryo development, and quantity of cattle, and reduced ROS levels and the expression of genes such as NF-kB, iNOS, BCL-2 and COX2.
In conclusion, we have reviewed many existing literature reports and found that the incorporation of lycopene in diets of poultry and ruminant feeding could improve growth performance, meat quality, and reproductive performance, but the optimal amount of lycopene has a large span. Existing reports have found that the optimal amount of lycopene added to poultry feed ranges from 30.00 mg/kg to 400.00 mg/kg. Additionally, in the feeding of ruminants, the amount of lycopene added to the feed should not exceed 200.00 mg/kg. From the data obtained, it can be concluded that the study of the optimal addition amount of lycopene in poultry and ruminant feed is still a hot topic, which needs to be further verified in the future.
5 Conclusion
Lycopene is a new type of healthy feed additive and has been gradually applied in animal production because of its natural, pollution-free, non-toxic, and side effects, and its physiological functions such as anti-inflammatory, antioxidant, and immune regulation. Lycopene could be regulated through many mechanisms to improve the antioxidant, immunity, meat quality, and reproductive rate of animals. However, the existing research reports found that lycopene is mainly used in poultry research in animal production research, while research reports on ruminants are relatively rare and many studies are still in their infancy.
In conclusion, the current research on lycopene in animal production is still relatively limited, not systematic and in-depth enough, and the amount of lycopene added to different types of animal feed is not clear enough, and its mechanism of action and targets need to be further explored. Therefore, research on the application of lycopene in different physiological conditions and growth stages of animals can be strengthened in the future. Notably, explore the appropriate addition amount of lycopene in feed and reveal its specific mechanism of action. This is of great significance to promote the development of lycopene feed and the ecological and healthy breeding of livestock.
Author contributions
YL: Data curation, Writing – original draft, Writing – review & editing. SP: Investigation, Methodology, Visualization, Writing – review & editing. SL: Data curation, Writing – review & editing. XN: Formal analysis, Supervision, Validation, Writing – review & editing. ST: Data curation, Investigation, Methodology, Writing – review & editing. NT: Conceptualization, Methodology, Writing – review & editing. YH: Investigation, Project administration, Writing – review & editing. PP: Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Suranaree University of Technology (SUT; contract no. Fulltime 61/28/2564 and Full-time 66/07/2567), Thailand Science Research and Innovation (TSRI), National Science Research and Innovation Fund (NSRF; project codes: 90464; 160368; FF3-303-65-36-17(B)), National Research Council of Thailand (NRCT; project code: 900105), National Research Council of Thailand (NRCT) and Suranaree University of Technology (SUT) project code NRCT5-RSA63009-01.
Acknowledgments
Gratefully recognizes the Suranaree University of Technology scholarship for External Grants and Scholarships for Graduate Students (SUT-OROG scholarship).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saini, RK, A Bekhit, AE, Roohinejad, S, Rengasamy, KRR, and Keum, YS. Chemical stability of lycopene in processed products: a review of the effects of processing methods and modern preservation strategies. J Agric Food Chem. (2019) 68:712–26. doi: 10.1021/acs.jafc.9b06669
2. Hedayati, N, Naeini, MB, Nezami, A, Hosseinzadeh, H, Wallace Hayes, A, Hosseini, S, et al. Protective effect of lycopene against chemical and natural toxins: A review. Biofactors. (2019) 45:5–23. doi: 10.1002/biof.1458
3. Rodrigo-Baños, M, Garbayo, I, Vílchez, C, Bonete, MJ, and Martínez-Espinosa, RM. Carotenoids from Haloarchaea and their potential in biotechnology. Mar Drugs. (2015) 13:5508–32. doi: 10.3390/md13095508
4. Maiani, G, Periago Castón, MJ, Catasta, G, Toti, E, Cambrodón, IG, Bysted, A, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. (2009) 53:S194–218. doi: 10.1002/mnfr.200800053
5. Marković, K, Hruškar, M, and Vahčić, N. Lycopene content of tomato products and their contribution to the lycopene intake of Croatians. Nutr Res. (2006) 26:556–60. doi: 10.1016/j.nutres.2006.09.010
6. Bramley, PM . Is lycopene beneficial to human health? Phytochemistry. (2000) 54:233–6. doi: 10.1016/S0031-9422(00)00103-5
7. Rao, A, and Agarwal, S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr Cancer. (1998) 31:199–203. doi: 10.1080/01635589809514703
8. Stahl, W, and Sies, H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. (1996) 336:1–9. doi: 10.1006/abbi.1996.0525
9. Madia, VN, De Vita, D, Ialongo, D, Tudino, V, De Leo, A, Scipione, L, et al. Recent advances in recovery of lycopene from tomato waste: A potent antioxidant with endless benefits. Molecules. (2021) 26:4495. doi: 10.3390/molecules26154495
10. Ozkan, G, Günal-Köroğlu, D, Karadag, A, Capanoglu, E, Cardoso, SM, Al-Omari, B, et al. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed Pharmacother. (2023) 161:114428. doi: 10.1016/j.biopha.2023.114428
11. Imran, M, Ghorat, F, Ul-Haq, I, Ur-Rehman, H, Aslam, F, Heydari, M, et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants. (2020) 9:706. doi: 10.3390/antiox9080706
12. Saini, RK, Rengasamy, KR, Mahomoodally, FM, and Keum, Y-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol Res. (2020) 155:104730. doi: 10.1016/j.phrs.2020.104730
13. Wang, J, Geng, T, Zou, Q, Yang, N, Zhao, W, Li, Y, et al. Lycopene prevents lipid accumulation in hepatocytes by stimulating PPARα and improving mitochondrial function. J Funct Foods. (2020) 67:103857. doi: 10.1016/j.jff.2020.103857
14. Wang, R, Lu, X, and Yu, R. Lycopene inhibits epithelial–mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR signal pathway. Drug Des Dev Ther. (2020) 14:2461–71. doi: 10.2147/DDDT.S251614
15. Fallah, R, Kiani, A, and Khaldari, M. Supplementing lycopene combined with corn improves circulating IgG concentration in pregnant ewes and their lambs. Trop Anim Health Prod. (2021) 53:360. doi: 10.1007/s11250-021-02802-3
16. Sun, S, Meng, Q, Bai, Y, Cao, C, Li, J, Cheng, B, et al. Lycopene improves maternal reproductive performance by modulating milk composition and placental antioxidative and immune status. Food Funct. (2021) 12:12448–67. doi: 10.1039/D1FO01595H
17. Nikiforov-Nikishin, A, Smorodinskaya, S, Kochetkov, N, Nikiforov-Nikishin, D, Danilenko, V, Bugaev, O, et al. Effects of three feed additives on the culturable microbiota composition and histology of the anterior and posterior intestines of zebrafish (Danio rerio). Animals. (2022) 12:2424. doi: 10.3390/ani12182424
18. Danuta, J, Marian, C, Wiesław, P, and Anna, R. The effect of fish oil, lycopene and organic selenium as feed additives on rabbit meat quality. J Appl Anim Res. (2020) 48:476–83. doi: 10.1080/09712119.2020.1828893
19. Hidayat, DF, Mahendra, MYN, Kamaludeen, J, and Pertiwi, H. Lycopene in feed as antioxidant and Immuno-modulator improves broiler Chicken’s performance under heat-stress conditions. Vet Med Int. (2023) 2023:1–7. doi: 10.1155/2023/5418081
20. Shevchenko, L, Iakubchak, O, Davydovych, V, Honchar, V, Ciorga, M, Hartung, J, et al. Influence of lycopene and astaxanthin in feed on metabolic parameters of laying hens, yolk color of eggs and their content of carotenoids and vitamin A when stored under refrigerated conditions. Pol J Vet Sci. (2021) 24:525–35. doi: 10.24425/pjvs.2021.139977
21. Bin-Jumah, MN, Nadeem, MS, Gilani, SJ, Mubeen, B, Ullah, I, Alzarea, SI, et al. Lycopene: A natural arsenal in the war against oxidative stress and cardiovascular diseases. Antioxidants. (2022) 11:232. doi: 10.3390/antiox11020232
22. Shi, J., Mazza, G., and Le Maguer, M. (2002) Lycopene from tomatoes. In J. Shi, G. Mazza, and M. MaguerLe (eds.) Functional foods: biochemical and processing aspects, vol. 2. Boca Raton, FL: CRC Press: 135–167.
23. SHI, J, le MAGUER, MARC, BRYAN, M, and KAKUDA, Y. Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J Food Process Eng. (2003) 25:485–98. doi: 10.1111/j.1745-4530.2003.tb00647.x
24. Rao, AV, and Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. (2000) 19:563–9. doi: 10.1080/07315724.2000.10718953
25. Ascenso, A. P. H. (2012). Carrier-mediated dermal delivery for prevention or treatment of skin disorders [D]. Universidade de Lisboa (Portugal).
26. Perucatti, A, Genualdo, V, Pauciullo, A, Iorio, C, Incarnato, D, Rossetti, C, et al. Cytogenetic tests reveal no toxicity in lymphocytes of rabbit (Oryctolagus cuniculus, 2n= 44) feed in presence of verbascoside and/or lycopene. Food Chem Toxicol. (2018) 114:311–5. doi: 10.1016/j.fct.2018.02.053
27. McClain, RM, and Bausch, J. Summary of safety studies conducted with synthetic lycopene. Regul Toxicol Pharmacol. (2003) 37:274–85. doi: 10.1016/S0273-2300(03)00004-7
28. Kong, K-W, Khoo, H-E, Prasad, KN, Ismail, A, Tan, C-P, and Rajab, NF. Revealing the power of the natural red pigment lycopene. Molecules. (2010) 15:959–87. doi: 10.3390/molecules15020959
29. Halliwell, B . Biochemistry of oxidative stress. Biochem Soc Trans. (2007) 35:1147–50. doi: 10.1042/BST0351147
30. Chen, J, Huang, Z, Cao, X, Zou, T, You, J, and Guan, W. Plant-derived polyphenols in sow nutrition: An update. Anim Nutr. (2023) 12:96–107. doi: 10.1016/j.aninu.2022.08.015
31. Ghimpețeanu, OM, Pogurschi, EN, Popa, DC, Dragomir, N, Drăgotoiu, T, Mihai, OD, et al. Antibiotic use in livestock and residues in food—A public health threat: A review. Food Secur. (2022) 11:1430. doi: 10.3390/foods11101430
32. Kobayashi, Y, Oh, S, Myint, H, and Koike, S. Use of Asian selected agricultural byproducts to modulate rumen microbes and fermentation. J Anim Sci Biotechnol. (2016) 7:70–10. doi: 10.1186/s40104-016-0126-4
33. Zeng, Y, Wang, Z, Zou, T, Chen, J, Li, G, Zheng, L, et al. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front Vet Sci. (2021) 8:623899. doi: 10.3389/fvets.2021.623899
34. Mei, HD, Li, YF, Ma, XY, and Yu, M. Physiological function of lycopene and its application in swine and chicken production. Chinese J Anim Nutr. (2023) 35:727–37. doi: 10.12418/CJAN2023.070
35. Krinsky, NI . The antioxidant and biological properties of the carotenoids a. Ann N Y Acad Sci. (1998) 854:443–7. doi: 10.1111/j.1749-6632.1998.tb09923.x
36. Przybylska, S . Lycopene–a bioactive carotenoid offering multiple health benefits: a review. Int J Food Sci Tech. (2020) 55:11–32. doi: 10.1111/ijfs.14260
37. Sies, H, and Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. (1995) 62:1315S–21S. doi: 10.1093/ajcn/62.6.1315S
38. Prasad, AK, and Mishra, PC. Modeling the mechanism of action of lycopene as a hydroxyl radical scavenger. J Mol Model. (2014) 20:2233–10. doi: 10.1007/s00894-014-2233-5
39. Muzandu, K, Ishizuka, M, Sakamoto, KQ, Shaban, Z, El Bohi, K, Kazusaka, A, et al. Effect of lycopene and β-carotene on peroxynitrite-mediated cellular modifications. Toxicol Appl Pharm. (2006) 215:330–40. doi: 10.1016/j.taap.2006.03.006
40. Panasenko, OM, Sharov, VS, Briviba, K, and Sies, H. Interaction of peroxynitrite with carotenoids in human low density lipoproteins. Arch Biochem Biophys. (2000) 373:302–5. doi: 10.1006/abbi.1999.1424
41. Mortensen, A, and Skibsted, LH. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy. FEBS Lett. (1997) 417:261–6. doi: 10.1016/S0014-5793(97)01297-0
42. Galano, A, and Francisco-Marquez, M. Reactions of OOH radical with β-carotene, lycopene, and torulene: hydrogen atom transfer and adduct formation mechanisms. J Phys Chem B. (2009) 113:11338–45. doi: 10.1021/jp904061q
43. Fang, Y, Ou, S, Wu, T, Zhou, L, Tang, H, Jiang, M, et al. Lycopene alleviates oxidative stress via the PI3K/Akt/Nrf2pathway in a cell model of Alzheimer’s disease. PeerJ. (2020) 8:e9308. doi: 10.7717/peerj.9308
44. Zuo, ZY . Advances in physiological functions of lycopene and its application in animal production. Chinese J Anim Sci. (2021) 57:40–5. doi: 10.19556/j.0258-7033.20200331-02
45. Shen, Y, Liu, L, Li, M-Z, Wang, H-R, Zhao, Y, and Li, J-L. Lycopene prevents Di-(2-ethylhexyl) phthalate-induced mitophagy and oxidative stress in mice heart via modulating mitochondrial homeostasis. J Nutr Biochem. (2023) 115:109285. doi: 10.1016/j.jnutbio.2023.109285
46. El-Sheshtawy, SM, El-Zoghby, AF, Shawky, NA, and Samak, DH. Aflatoxicosis in Pekin duckling and the effects of treatments with lycopene and silymarin. Vet World. (2021) 14:788–93. doi: 10.14202/vetworld.2021.788-793
47. Wen, W, Chen, X, Huang, Z, Chen, D, Yu, B, He, J, et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim Nutr. (2022) 8:256–64. doi: 10.1016/j.aninu.2021.06.012
48. Albrahim, T, and Alonazi, MA. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed Pharmacother. (2021) 141:111831. doi: 10.1016/j.biopha.2021.111831
49. Li, Y, Pan, X, Yin, M, Li, C, and Han, L. Preventive effect of lycopene in dextran sulfate sodium-induced ulcerative colitis mice through the regulation of TLR4/TRIF/NF-κB signaling pathway and tight junctions. J Agric Food Chem. (2021) 69:13500–9. doi: 10.1021/acs.jafc.1c05128
50. Motta, BP, Pinheiro, CG, Figueiredo, ID, Cardoso, FN, Oliveira, JO, Machado, RTA, et al. Combined effects of lycopene and metformin on decreasing oxidative stress by triggering endogenous antioxidant defenses in diet-induced obese mice. Molecules. (2022) 27:8503. doi: 10.3390/molecules27238503
51. Aboubakr, M, Farag, A, Elfadadny, A, Alkafafy, M, Soliman, A, and Elbadawy, M. Antioxidant and anti-apoptotic potency of allicin and lycopene against methotrexate-induced cardiac injury in rats. Environ Sci Pol. (2023) 30:88724–33. doi: 10.1007/s11356-023-28686-4
52. Lee, J, Lim, JW, and Kim, H. Lycopene inhibits oxidative stress-mediated inflammatory responses in ethanol/palmitoleic acid-stimulated pancreatic acinar AR42J cells. Int J Mol Sci. (2021) 22:2101. doi: 10.3390/ijms22042101
53. Alvi, SS, Ansari, IA, Khan, I, Iqbal, J, and Khan, MS. Potential role of lycopene in targeting proprotein convertase subtilisin/kexin type-9 to combat hypercholesterolemia. J Free Radic Biol Med. (2017) 108:394–403. doi: 10.1016/j.freeradbiomed.2017.04.012
54. Fuhrman, B, Elis, A, and Aviram, M. Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun. (1997) 233:658–62. doi: 10.1006/bbrc.1997.6520
55. Tian, H, Liu, G, Guo, Y, Li, Y, Deng, M, Liu, D, et al. Lycopene supplementation regulates the gene expression profile and fat metabolism of breeding hens. J Anim Physiol Anim Nutr. (2020) 104:936–45. doi: 10.1111/jpn.13344
56. Wan, X, Yang, Z, Ji, H, Li, N, Yang, Z, Xu, L, et al. Effects of lycopene on abdominal fat deposition, serum lipids levels and hepatic lipid metabolism-related enzymes in broiler chickens. Anim Biosci. (2021) 34:385–92. doi: 10.5713/ajas.20.0432
57. Zeng, Z, He, W, Jia, Z, and Hao, S. Lycopene improves insulin sensitivity through inhibition of STAT3/Srebp-1c-mediated lipid accumulation and inflammation in mice fed a high-fat diet. Exp Clin Endocrinol Diabetes. (2017) 125:610–7. doi: 10.1055/s-0043-101919
58. Chen, S, Zou, L, Li, L, and Wu, T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. (2013) 8:e53662. doi: 10.1371/journal.pone.0053662
59. Xu, M, Chen, X, Huang, Z, Chen, D, Li, M, He, J, et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. (2022) 367:130781. doi: 10.1016/j.foodchem.2021.130781
60. Itoh, K, Wakabayashi, N, Katoh, Y, Ishii, T, Igarashi, K, Engel, JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. (1999) 13:76–86. doi: 10.1101/gad.13.1.76
61. Liang, M, Wang, Z, Li, H, Cai, L, Pan, J, He, H, et al. L-arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. (2018) 115:315–28. doi: 10.1016/j.fct.2018.03.029
62. De Haan, JB . Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes. (2011) 60:2683–4. doi: 10.2337/db11-1072
63. Palozza, P, Catalano, A, Simone, R, and Cittadini, A. Lycopene as a guardian of redox signalling. Acta Biochim Pol. (2012) 59:21–25. doi: 10.18388/abp.2012_2163
64. Dinkova-Kostova, AT, and Talalay, P. NAD (P) H: quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. (2010) 501:116–23. doi: 10.1016/j.abb.2010.03.019
65. Zhou, J, Zheng, Q, and Chen, Z. The Nrf2 pathway in liver diseases. Front Cell Dev Biol. (2022) 10:826204. doi: 10.3389/fcell.2022.826204
66. Ramadan, SS, Almeer, R, Albasher, G, and Abdel Moneim, AE. Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin Rev. (2022) 41:446–56. doi: 10.1080/15569543.2021.1891938
67. Sun, N, Yang, T, Tang, Y, Zhao, Y, Wang, H, Zhao, S, et al. Lycopene alleviates chronic stress-induced liver injury by inhibiting oxidative stress-mediated endoplasmic reticulum stress pathway apoptosis in rats. J Agri Food Chem. (2022) 70:14414–26. doi: 10.1021/acs.jafc.2c06650
68. Amer, SA, Kishawy, AT, Osman, A, Mahrose, KM, Hassanine, E-SI, Rehman, ZU, et al. Influence of dietary graded levels of lycopene on the growth performance, muscle cholesterol level and oxidative status of Japanese quail fed high-fat diet. An Acad Bras Ciênc. (2020) 92:e20190065. doi: 10.1590/0001-3765202020190065
69. Wang, Y, Liu, Z, Ma, J, Xv, Q, Gao, H, Yin, H, et al. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. (2022) 57:102494. doi: 10.1016/j.redox.2022.102494
70. Zheng, S, Deng, Z, Chen, F, Zheng, L, Pan, Y, Xing, Q, et al. Synergistic antioxidant effects of petunidin and lycopene in H9c2 cells submitted to hydrogen peroxide: role of Akt/Nrf2 pathway. J Food Sci. (2020) 85:1752–63. doi: 10.1111/1750-3841.15153
71. Pawlak, M, Lefebvre, P, and Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. (2015) 62:720–33. doi: 10.1016/j.jhep.2014.10.039
72. Reynés, B, Palou, M, and Palou, A. Gene expression modulation of lipid and central energetic metabolism related genes by high-fat diet intake in the main homeostatic tissues. Food Funct. (2017) 8:629–50. doi: 10.1039/C6FO01473A
73. Huang, T, Yu, J, Ma, Z, Fu, Q, Liu, S, Luo, Z, et al. Translatomics probes into the role of lycopene on improving hepatic steatosis induced by high-fat diet. Front Nutr. (2021) 8:727785. doi: 10.3389/fnut.2021.727785
74. Lu, Y, Shao, M, Xiang, H, Zheng, P, Wu, T, and Ji, G. Integrative transcriptomics and metabolomics explore the mechanism of kaempferol on improving nonalcoholic steatohepatitis. Food Funct. (2020) 11:10058–69. doi: 10.1039/D0FO02123G
75. Elseweidy, MM, Elawady, AS, Sobh, MS, and Elnagar, GM. Lycopene ameliorates hyperlipidemia via potentiation of AMP-activated protein kinase and inhibition of ATP-citrate lyase in diabetic hyperlipidemic rat model. Life Sci. (2022) 308:120934. doi: 10.1016/j.lfs.2022.120934
76. Holzapfel, NP, Shokoohmand, A, Wagner, F, Landgraf, M, Champ, S, Holzapfel, BM, et al. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am J Cancer Res. (2017) 7:1322–36.
77. Jeng, PS, Inoue-Yamauchi, A, Hsieh, JJ, and Cheng, EH. BH3-dependent and independent activation of BAX and BAK in mitochondrial apoptosis. Curr Opin Physio. (2018) 3:71–81. doi: 10.1016/j.cophys.2018.03.005
78. Peng, S, Li, J, Zhou, Y, Tuo, M, Qin, X, Yu, Q, et al. In vitro effects and mechanisms of lycopene in MCF-7 human breast cancer cells. Genet Mol Res. (2017) 16:13. doi: 10.4238/gmr16029434
79. Trejo-Solís, C, Pedraza-Chaverrí, J, Torres-Ramos, M, Jiménez-Farfán, D, Cruz Salgado, A, Serrano-García, N, et al. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid-based Complement Alternat Med. (2013) 2013:1–17. doi: 10.1155/2013/705121
80. Aizawa, K, Liu, C, Tang, S, Veeramachaneni, S, Hu, KQ, Smith, DE, et al. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int J Cancer. (2016) 139:1171–81. doi: 10.1002/ijc.30161
81. Bano, S, Ahmed, F, Khan, F, Chaudhary, SC, and Samim, M. Targeted delivery of thermoresponsive polymeric nanoparticle-encapsulated lycopene: in vitro anticancer activity and chemopreventive effect on murine skin inflammation and tumorigenesis. RSC Adv. (2020) 10:16637–49. doi: 10.1039/C9RA10686C
82. Di Sano, C, Lazzara, V, Durante, M, D’Anna, C, Bonura, A, Dino, P, et al. The protective anticancer effect of natural lycopene supercritical CO2 watermelon extracts in adenocarcinoma lung cancer cells. Antioxidants. (2022) 11:1150. doi: 10.3390/antiox11061150
83. Elgass, S, Cooper, A, and Chopra, M. Lycopene treatment of prostate cancer cell lines inhibits adhesion and migration properties of the cells. Int J Med Sci. (2014) 11:948–54. doi: 10.7150/ijms.9137
84. Jain, A, Sharma, G, Ghoshal, G, Kesharwani, P, Singh, B, Shivhare, U, et al. Lycopene loaded whey protein isolate nanoparticles: An innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int J Pharm. (2018) 546:97–105. doi: 10.1016/j.ijpharm.2018.04.061
85. Rowles, JL III, Ranard, KM, Applegate, CC, Jeon, S, An, R, and Erdman, JW Jr. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose–response meta-analysis. Prostate Cancer Prostatic Dis. (2018) 21:319–36. doi: 10.1038/s41391-017-0005-x
86. Sahin, K, Yenice, E, Tuzcu, M, Orhan, C, Mizrak, C, Ozercan, IH, et al. Lycopene protects against spontaneous ovarian cancer formation in laying hens. J Cancer Prev. (2018) 23:25–36. doi: 10.15430/JCP.2018.23.1.25
87. Sharma, P, Saxena, P, Jaswanth, A, Chalamaiah, M, Tekade, K, and Balasubramaniam, A. Novel encapsulation of lycopene in niosomes and assessment of its anticancer activity. J bioequiv Bioavailab. (2016) 8:224–32. doi: 10.4172/jbb.1000300
88. Zhu, J, Hu, Q, and Shen, S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J Liposome Res. (2020) 30:37–44. doi: 10.1080/08982104.2019.1580720
89. Ataseven, D, Öztürk, A, Özkaraca, M, and Joha, Z. Anticancer activity of lycopene in HT-29 colon cancer cell line. Med Oncol. (2023) 40:127. doi: 10.1007/s12032-023-02001-0
90. Cheng, J, Miller, B, Balbuena, E, and Eroglu, A. Lycopene protects against smoking-induced lung cancer by inducing base excision repair. Antioxidants. (2020) 9:643. doi: 10.3390/antiox9070643
91. Jhou, B-Y, Song, T-Y, Lee, I, Hu, M-L, and Yang, N-C. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. J Agric Food Chem. (2017) 65:6893–903. doi: 10.1021/acs.jafc.7b03036
92. Elgass, S, Cooper, A, and Chopra, M. Lycopene inhibits angiogenesis in human umbilical vein endothelial cells and rat aortic rings. Int J Med Sci. (2012) 108:431–9. doi: 10.1017/S0007114511005800
93. Şahin, M, Şahin, E, and Gümüşlü, S. Effects of lycopene and apigenin on human umbilical vein endothelial cells in vitro under angiogenic stimulation. Acta Histochem. (2012) 114:94–100. doi: 10.1016/j.actis.2011.03.004
94. Palozza, P, Simone, R, Catalano, A, Monego, G, Barini, A, Mele, MC, et al. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. J Nutr Biochem. (2011) 22:259–68. doi: 10.1016/j.jnutbio.2010.02.003
95. Wang, J, Li, L, Wang, Z, Cui, Y, Tan, X, Yuan, T, et al. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutr Biochem. (2018) 56:16–25. doi: 10.1016/j.jnutbio.2018.01.009
96. Xue, R, Qiu, J, Wei, S, Liu, M, Wang, Q, Wang, P, et al. Lycopene alleviates hepatic ischemia reperfusion injury via the Nrf2/HO-1 pathway mediated NLRP3 inflammasome inhibition in Kupffer cells. Ann Transl Med. (2021) 9:631. doi: 10.21037/atm-20-7084
97. Yang, J, Song, X, Feng, Y, Liu, N, Fu, Z, Wu, J, et al. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway. Free Radical Bio Med. (2020) 152:854–64. doi: 10.1016/j.freeradbiomed.2020.01.185
98. Zhan, J, Yan, Z, Kong, X, Liu, J, Lin, Z, Qi, W, et al. Lycopene inhibits IL-1β-induced inflammation in mouse chondrocytes and mediates murine osteoarthritis. J Cell Mol Med. (2021) 25:3573–84. doi: 10.1111/jcmm.16443
99. Cao, L, Zhao, J, Ma, L, Chen, J, Xu, J, Rahman, SU, et al. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol Environ Saf. (2021) 225:112737. doi: 10.1016/j.ecoenv.2021.112737
100. Liao, QJ, Ye, LB, Timani, KA, Zeng, YC, She, YL, Ye, L, et al. Activation of NF-κB by the full-length nucleocapsid protein of the SARS coronavirus. Acta Biochim Biophys Sin. (2005) 37:607–12. doi: 10.1111/j.1745-7270.2005.00082.x
101. Ugbaja, RN, James, AS, Ugwor, EI, Akamo, AJ, Thomas, FC, and Kosoko, AM. Lycopene suppresses palmitic acid-induced brain oxidative stress, hyperactivity of some neuro-signalling enzymes, and inflammation in female Wistar rat. Sci Rep. (2021) 11:15038. doi: 10.1038/s41598-021-94518-5
102. Zhao, B, Ren, B, Guo, R, Zhang, W, Ma, S, Yao, Y, et al. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol. (2017) 109:505–16. doi: 10.1016/j.fct.2017.09.050
103. Yin, Z, Wang, Q, and Cheng, H. Synergistic protective effect of interactions of quercetin with lycopene against Ochratoxin A-induced ulcerative colitis. Appl Biochem Biotech. (2023) 195:5253–66. doi: 10.1007/s12010-022-04287-8
104. Lee, W, Ku, S-K, Bae, JW, and Bae, J-S. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem Toxicol. (2012) 50:1826–33. doi: 10.1016/j.fct.2012.03.003
105. Mozos, I, Stoian, D, Caraba, A, Malainer, C, Horbańczuk, JO, and Atanasov, AG. Lycopene and vascular health. Front Pharmacol. (2018) 9:521–535. doi: 10.3389/fphar.2018.00521
106. Chai, JB, Li, P, and Pei, ZP. The effects and mechanism of lycopene on immune function of ovarian cancer rats. Pract Oncol J. (2017) 31:13–7. doi: 10.11904/j.issn.1002-3070.2017.01.003
107. Garcia, AL, Rühl, R, Herz, U, Koebnick, C, Schweigert, FJ, and Worm, M. Retinoid-and carotenoid-enriched diets influence the ontogenesis of the immune system in mice. Immunology. (2003) 110:180–7. doi: 10.1046/j.1365-2567.2003.01734.x
108. Yamaguchi, M, Hasegawa, I, Yahagi, N, Ishigaki, Y, Takano, F, and Ohta, T. Carotenoids modulate cytokine production in Peyer's patch cells ex vivo. J Agric Food Chem. (2010) 58:8566–72. doi: 10.1021/jf101295y
109. Kobayashi, T, Iijima, K, Mitamura, T, Toriizuka, K, Cyong, J, and Nagasawa, H. Effects of lycopene, a carotenoid, on intrathymic T cell differentiation and peripheral CD4/CD8 ratio in a high mammary tumor strain of SHN retired mice. Anti-Cancer Drugs. (1996) 7:195–8. doi: 10.1097/00001813-199602000-00008
110. Riso, P, Pinder, A, Santangelo, A, and Porrini, M. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? Am J Clin Nutr. (1999) 69:712–8. doi: 10.1093/ajcn/69.4.712
111. Watzl, B, Bub, A, Blockhaus, M, Herbert, BM, Luhrmann, PM, Neuhauser-Berthold, M, et al. Prolonged tomato juice consumption has no effect on cell-mediated immunity of well-nourished elderly men and women. J Nutr. (2000) 130:1719–23. doi: 10.1093/jn/130.7.1719
112. Sarker, MT, Wan, X, Yang, H, and Wang, Z. Dietary lycopene supplementation could alleviate aflatoxin b1 induced intestinal damage through improving immune function and anti-oxidant capacity in broilers. Animals. (2021) 11:3165. doi: 10.3390/ani11113165
113. Sarker, MT, Wang, ZY, Yang, H, Wan, X, and Emmanuel, A. Evaluation of the protective effect of lycopene on growth performance, intestinal morphology, and digestive enzyme activities of aflatoxinB1 challenged broilers. Anim Sci J. (2021) 92:e13540. doi: 10.1111/asj.13540
114. Mezbani, A, Kavan, BP, Kiani, A, and Masouri, B. Effect of dietary lycopene supplementation on growth performance, blood parameters and antioxidant enzymes status in broiler chickens. Livest Res Rural Dev. (2019) 31
115. Orhan, C, Kucuk, O, Sahin, N, Tuzcu, M, and Sahin, K. Lycopene supplementation does not change productive performance but lowers egg yolk cholesterol and gene expression of some cholesterol-related proteins in laying hens. Brit Poultry Sci. (2021) 62:227–34. doi: 10.1080/00071668.2020.1839017
116. Ayo, JO, Obidi, JA, Rekwot, PI, and Onyeanusi, BI. Modulatory effects of lycopene and vitamin E on cloacal temperature, thyroid hormonal and reproductive performance responses in laying hens during the hot-dry season. J Therm Biol. (2022) 104:103105. doi: 10.1016/j.jtherbio.2021.103105
117. Abbas, RJ, and AL-Jrrah, IAT. Effects of natural and synthetic lycopene dietary supplementation on selected biochemical traits and antioxidant status of Japanese quail (Coturnix japonica). Anim Sci Paper Rep. (2020) 38:275–86.
118. Fu, XZ, Lu, ZF, and Shen, HY. Effects of lycopene compound additive on growth performance, immune organ index and meat quality of broiler chickens. Jiangsu Agric Sci. (2018) 46:202–5. doi: 10.15889/j.issn.1002-1302.2018.19.054
119. Liu, X, Lin, X, Zhang, S, Guo, C, Li, J, Mi, Y, et al. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging (Albany NY). (2018) 10:2016–36. doi: 10.18632/aging.101526
120. Jiang, H, Wang, Z, Ma, Y, Qu, Y, Lu, X, Guo, H, et al. Effect of dietary lycopene supplementation on growth performance, meat quality, fatty acid profile and meat lipid oxidation in lambs in summer conditions. Small Rumin Res. (2015) 131:99–106. doi: 10.1016/j.smallrumres.2015.08.017
121. Jiang, H, Wang, Z, Ma, Y, Qu, Y, Lu, X, and Luo, H. Effects of dietary lycopene supplementation on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot Bamei lamb. Asian-Australasian J Anim Sci. (2015) 28:958–65. doi: 10.5713/ajas.14.0887
122. Wang, B, Xu, C-C, Liu, C, Qu, Y-H, Zhang, H, and Luo, H-L. The effect of dietary lycopene supplementation on drip loss during storage of lamb meat by iTRAQ analysis. Antioxidants. (2021) 10:198. doi: 10.3390/antiox10020198
123. Xu, C, Qu, Y, Hopkins, DL, Liu, C, Wang, B, Gao, Y, et al. Dietary lycopene powder improves meat oxidative stability in Hu lambs. J Sci Food Agric. (2019) 99:1145–52. doi: 10.1002/jsfa.9282
124. Qu, Y, Luo, H, Liu, C, Guo, P, Ma, Y, and Xu, C. Effects of lycopene supplementation on growth development, slaughter performance and serum antioxidant indices of sheep. Chinese J Anim Nutr. (2017) 29:1257–64. doi: 10.3969/j.issn.1006-267x.2017.04.022
125. Al-Sarray, MAR, Hobi, A-R, Abd, AA-K, Al-Ani, E-DT, and Alaa, A. Effect of adding lycopene to the Tris extender on Awassi rams semen quality preseveded at cooling and freezing storage. Plant Arch. (2019) 9725210:3971–6.
126. Peker Akalin, P, Bucak, MN, Güngör, Ş, Başpinar, N, Çoyan, K, Dursun, Ş, et al. Influence of lycopene and cysteamine on sperm and oxidative stress parameters during liquid storage of ram semen at 5 C. Small Rumin Res. (2016) 137:117–23. doi: 10.1016/j.smallrumres.2016.03.017
127. Ren, F, Feng, T, Dai, G, Wang, Y, Zhu, H, and Hu, J. Lycopene and alpha-lipoic acid improve semen antioxidant enzymes activity and cashmere goat sperm function after cryopreservation. Cryobiology. (2018) 84:27–32. doi: 10.1016/j.cryobiol.2018.08.006
128. Garavaglia, L, Galletti, S, and Tedesco, D. Silymarin and lycopene administration in periparturient dairy cows: effects on milk production and oxidative status. N Z Vet J. (2015) 63:313–8. doi: 10.1080/00480169.2015.1047911
129. Chowdhury, M, Choi, B-H, Khan, I, Lee, K-L, Mesalam, A, Song, S-H, et al. Supplementation of lycopene in maturation media improves bovine embryo quality in vitro. Theriogenology. (2017) 103:173–84. doi: 10.1016/j.theriogenology.2017.08.003
Keywords: antioxidant, anti-cancer, additive, lipid-lowering, lycopene
Citation: Long Y, Paengkoum S, Lu S, Niu X, Thongpea S, Taethaisong N, Han Y and Paengkoum P (2024) Physicochemical properties, mechanism of action of lycopene and its application in poultry and ruminant production. Front. Vet. Sci. 11:1364589. doi: 10.3389/fvets.2024.1364589
Edited by:
Jesus R. Requena, University of Santiago de Compostela, SpainReviewed by:
Sameh A. Abdelnour, Zagazig University, EgyptFeng Ji, Beijing Academy of Agriculture and Forestry Sciences, China
Copyright © 2024 Long, Paengkoum, Lu, Niu, Thongpea, Taethaisong, Han and Paengkoum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pramote Paengkoum, cHJhbW90ZUBzdXQuYWMudGg=
 Yong Long
Yong Long Siwaporn Paengkoum
Siwaporn Paengkoum Shengyong Lu1
Shengyong Lu1 Pramote Paengkoum
Pramote Paengkoum