- State Key Laboratory of Animal Nutrition, Beijing Engineering Technology Research Centre of Raw Milk Quality and Safety Control, College of Animal Science and Technology, China Agricultural University, Beijing, China
Introduction: Dairy industry growth faces challenges in China due to inadequate forage, leading to high-concentrate diets and potential rumen issues. Buffering agents, like sodium bicarbonate, play a crucial role in stabilizing rumen pH. Alkaline Mineral Complex (AMC), a liquid additive with a pH of 14, shows promise in supporting dairy cow health and mitigating heat stress through ionization.
Methods: This experiment was aimed to study the effect of adding AMC to total mixed ration (TMR) on in vitro ruminal fermentation and bacterial composition. AMCat 1, 2, 4, and 8 mL/kg was added to the substrate (0.5 g TMR). Nutrient digestibility was measured after 48 h fermentation, and fermentation parameters and microbial composition were measured after 48 h fermentation.
Results and discussion: The results of the experiment indicated that: The different concentrations of AMC showed a significant impact on time taken for gas production to reach 1/2 of the total gas production (HT) parameters (p < 0.05). Linear pH increase occurs at 6 and 24 h with rising AMC concentration (p < 0.05), showing a quadratic trend at 12 h (p < 0.05). The optimal buffering effect on rumen acid-base balance was observed at a 2 mL/kg concentration of AMC. Microbial diversity analysis indicated that there was no significant change in α-diversity with different AMC concentrations (p > 0.05). The microbial level demonstrated no significant difference in species diversity of rumen fluid bacteria among the various AMC concentration treatment groups compared to the control group, further supporting that the advantage of adding AMC in stabilizing the rumen environment without altering the structure of the rumen microbiota. Besides, the addition of AMC significantly increased the concentrations of acetate, propionate, total fatty acids (TVFA), and NH3-N, suggesting that AMC contributed to enhancing the energy and nitrogen utilization efficiency in ruminants. Based on the above detection indicators, we recommend that the most favorable concentration is 2 mL/kg.
1 Introduction
The expansion of the dairy industry has been aided by the increasing demand for dairy products. However, the lack of high-quality forage in China makes it difficult to measure the nutritional requirement of lactating ruminants. Many ranches have decided to increase the proportion of concentrate feed to meet this requirement. High-concentrate diets have a relatively low effective fiber content and can easily induce subacute rumen acidosis (SARA) in ruminants (1). SARA is a metabolic disorder in animals, and it is characterized by rumen fluid pH values that are consistently < 5.8 and persist for more than 4 h after feed consumption (2, 3). This phenomenon, which the rumen is unable to effectively neutralize, is mainly caused by an excessive intake of highly fermentable carbohydrates. It severely impairs the lactation performance of dairy cows, leading to substantial financial losses for the pasture. Additionally, it can also cause other diseases such as mastitis, which endangers animal health. Hence, it is critical to address the adverse effects of high-concentrate diets and emphasize the importance of balancing the physiological wellbeing and productivity of lactating dairy cows.
A buffer is a type of compound or mixture that enhances the acid-base buffering capacity of a solution. In ruminant animal production, it is important to maintain the pH in the rumen at a stable level of 5.8–6.2 to support the activity of rumen microorganisms (4). To maintain the normal rumen fermentation performance of cow-fed high-concentrate diets, strongly alkaline and weakly acidic salts are typically used as buffering agents to prevent rumen acidosis and improve their productivity. Some buffering agents, commonly used in ruminants both domestically and internationally, include sodium bicarbonate, magnesium oxide, sodium acetate, sodium butyrate, calcium carbonate, and other minerals. Composite buffering agents have more efficient pH regulation ability than single buffering agents. Neiderfer et al. revealed that the supplementation of the daily diet of lactating cows with CaCO3, MgO, and coated NaHCO3 effectively maintained their rumen fluid pH (5). Similarly, Snyder et al. observed that the addition of NaHCO3 and its composite buffering agent to the diet of lactating cows enhanced their milk production and milk fat percentage (6).
Alkaline mineral complex (AMC) is a colorless, tasteless, and non-toxic complex alkaline ion mixture with a pH of 14 (7–9). It is a liquid feed additive that helps cows maintain the acid-base balance of ruminal fluids, preserves the normal function of cellular ion pumps, and improves immunity. It activates immune cells by enhancing neuromuscular physiological information transmission and physiological regulatory functions, thereby alleviating heat stress in dairy cows. The ions generated by the ionization of AMC solution jointly regulate H+ in the rumen. Despite its limited application in dairy cows, this composite buffering agent has a promising potential (9).
Therefore, the objective of this study was to investigate the effects of different concentrations of AMC on fermentation characteristics and bacterial composition in vitro to establish the optimal additive concentration for large-scale feeding applications in dairy herds.
2 Materials and methods
2.1 Animals and their feeding management
The rumen fluid was collected from three healthy, mid-lactating, and rumen-cannulated Holstein dairy cows with similar milk yield (26 ± 1.63 kg/d) from Zhongdi Dairy Holdings Co., Ltd. (Beijing, China). The dairy cows had ad libitum access to feed and water. The total mixed ratio (TMR) was fed to the cows three times daily (07:00, 14:00, and 19:00), and the cows were milked three times a day at 06:30, 13:30, and 18:30. All the animal procedures were approved by the Institutional Animal Care and Use Committee of China Agricultural University (approval number: AW61902202-1-4).
2.2 Experimental design
2.2.1 Fermentation substrates
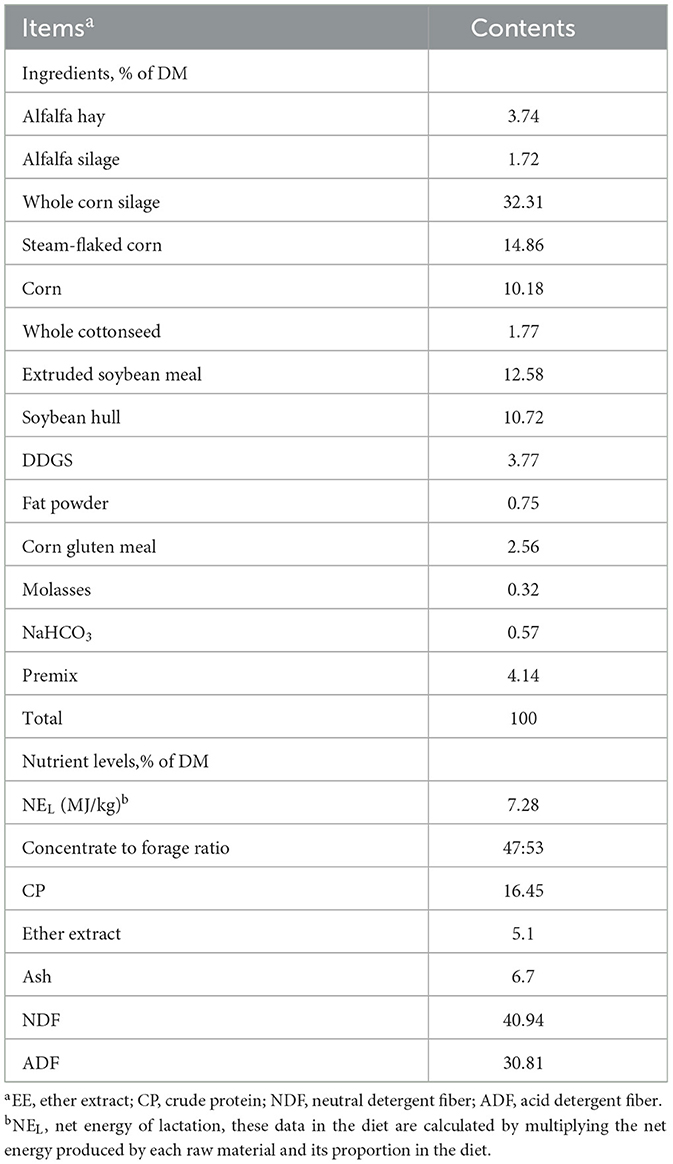
All fermentation substrates (donor cows' TMR) were crushed and kept in the oven at 65°C for 48 h (10, 11). After drying, the samples were crushed and sieved through a 1 mm screen for subsequent fermentation processes, and the chemical composition was determined using the Association of Official Analytical Chemists (AOAC) methods (12). The ingredients and nutrient compositions of all the fermentation substrates are shown in Table 1.
2.2.2 AMC
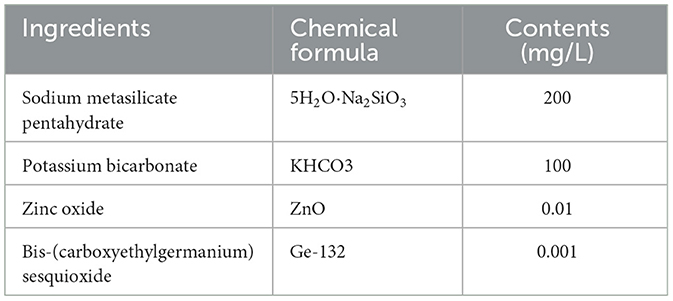
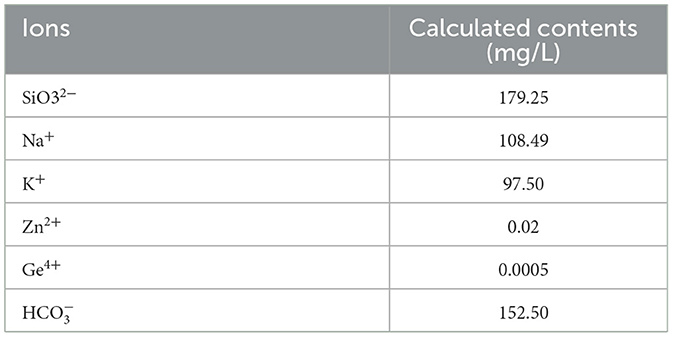
The AMC used in this study was provided by Beijing Jinaer Biotechnology Co., Ltd. The AMC utilizes zinc oxide and germanium compounds as cell activators in combination with sodium and potassium compounds. The elements, such as Si, Ge, K, and Zn, in the alkaline solution remain in ionic and water-soluble states, thereby maintaining a weak alkaline internal environment for the animals. The composition and mineral ion contents are shown in Tables 2, 3, respectively.
2.2.3 Rumen fluid collection
The rumen fluid was collected 2 h after morning feeding. The collected rumen fluid was filtered through four layers of gauze and placed in a thermos for quick return to the laboratory. It was then transferred into a 4 L beaker filled in advance with CO2 at 39°C in a water bath (11).
2.2.4 In vitro degradability
The test was divided into five groups with three replicates for measuring gas production and pH. The substrate degradation experiment was performed using five replicates per group. Except for the control group, AMC was added to each group at 1, 2, 4, and 8 mL/kg of the substrate.
For every in vitro gas production experiment, a total of 500 mg fermentation substrate, 25 mL of rumen fluid, and 50 mL of buffer (13) were added to a 120 mL anaerobic fermentation bottle. For the other fermentation bottles, 3 g samples from each treatment were individually placed into 250 mL glass bottles, which contained 150 mL of buffer solution and 75 mL of rumen fluid.
Each bottle was immediately sealed with butyl rubber stoppers and Hungate's screw caps after the addition of the experimental samples, and nitrogen was injected until oxygen was discharged.
The gas production bottles were placed inside a 39°C constant temperature incubator and were immediately connected to the corresponding gas channels of the AGRS-III system according to the pre-arranged inoculation order (14). Furthermore, the gas production (GP) was automatically recorded throughout the 48 h of fermentation. All the bottles were kept in a thermostatic incubator to ferment continuously for 6, 12, 24, 36, and 48 h. After 24 and 48 h of fermentation, the bottles were removed from the incubator. At every time point, the fermentation was halted by placing the bottles in a mixture of ice and water for 15 min.
2.3 Sample collection and measurement
The pH was measured five times at the five fermentation time points. After incubation, the contents of each bottle were filtered using a filter with 42 μm pores (sized 80 × 150 mm). As described in previous studies (12, 13), the volatile fatty acid concentration in the supernatant was determined using gas chromatography, and NH3-N was measured using a spectrophotometer. The remaining samples were kept at −80°C. One sample was used for further microbial community analysis, while others were used to measure microbial crude protein (MCP).
2.4 DNA extraction and determination
Total microbial genomic DNA was extracted using a kit from MP Biomedicals, Solon, OH, USA, and the NanoDrop® ND-2000 spectrophotometer (Thermo Scientific Co., Ltd., Waltham, Massachusetts, USA) was used to assess the DNA purity and concentration. Additionally, DNA integrity was assessed using 1% agarose gel electrophoresis.
The V3-V4 region of the 16S rRNA gene was amplified with forward primer 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACH VGGGTWTCTAAT-3′) through the polymerase chain reaction (PCR). For each sample, three PCR replicates were mixed, and 5 mL of the PCR product from each sample was detected using 2% agarose gel electrophoresis (15, 16). The PCR products were purified using an AxyPrep DNA Gel Extraction Kit (AP-GX-250, Axygen Biosciences, Union City, USA) and were quantified using a quantum fluorometer (E6150, Promega, WI, USA).
Finally, the amplicons were sequenced using a MiSeq pe300 platform (Illumina, Inc., San Diego, California, USA). Quality control (QC) and splicing of the original sequence and ASV representative sequences were clustered according to 97% similarity using UPARSE software (version7.0.1090,http://drive5.com/uparse/), and UCHIME software (version7.0, http://www.drive5.com/usearch/) was applied to eliminate the chimera (17).
The sequences containing more than 10% unknown nucleotides were excluded from the subsequent analysis. The paired-end clean tags were combined into raw tags using FLASH v. 1.2.11 software, following the methodology outlined by Magoč and Salzberg (18). The merging process had a minimum overlap of 10 bp, and a mismatch rate of 0.1 was used to generate Fasta sequences.
The sequencing data were saved in the form of a FASTQ file. The sequences were subjected to ASV clustering at a 97% similarity threshold using UPARSE 7.1 (19), and the chimeras were removed. The taxonomy annotations of ASV of species were classified and annotated using the Ribosomal Database Project (RDP) (http://rdp.cme.msu.edu/) (17) against the Silva 16S rRNA gene database (v138) with a confidence threshold of 70%.
2.5 Calculation and analyses
The corresponding cumulative gas production (GP, mL/g, dry matter basis) was fitted non-linearly with each fermentation time using the exponential model described by France et al. (20) as follows:
GPt: where GPt (mL) is the total gas production (mL/g dietary DM) over time t, A is the maximum gas production of the fermentation substrate at a gas production rate c (h−1) (mL), and lag is the delay time of fermentation gas production (h).
For the AGPR, the average gas production rate is as follows:
where A, c, and lag are the same as those in Equation (1).
AGPR, Average gas production rate when half of the ideal maximum gas production is achieved (mL/h).
The test data obtained were preliminarily collated using Excel 2020 and analyzed using the mixed model in SAS 9.4 (21). The standard error (SEM) of the least-squares mean of each measurement indicator was determined using LSMENAS statements, and multiple comparisons were performed using Duncan's test. The minimum significant difference method was used for comparisons when the difference was significant (p < 0.05), and 0.05 < p < 0.1 indicates that the data have a significant downward or upward trend.
The alpha diversity analysis at the ASV level was conducted using Mothur v1.30.1 (22) software. Differences in the α diversity index between different types were obtained using the Wilcoxon rank sum test.
In the beta diversity analysis, principal component analysis (PCA) based on the Bray-Curtis distance algorithm was used to test for discrepancies in microbial communities at the ASV level between different groups (23). The non-parametric Kruskal–Wallis rank sum test was used to detect the genera with significant differences in abundance between different groups, and the consistency of the differences in different genera was subjected to the Tukey–Kramer test in different subgroups between the groups. Additionally, hypothesis testing was performed to evaluate the genus abundance between multiple groups. These analyses revealed genus information that showed significant differences among the treatment groups.
The data were analyzed using the online platform Majorbio Cloud Platform (www.majorbio.com).
3 Results
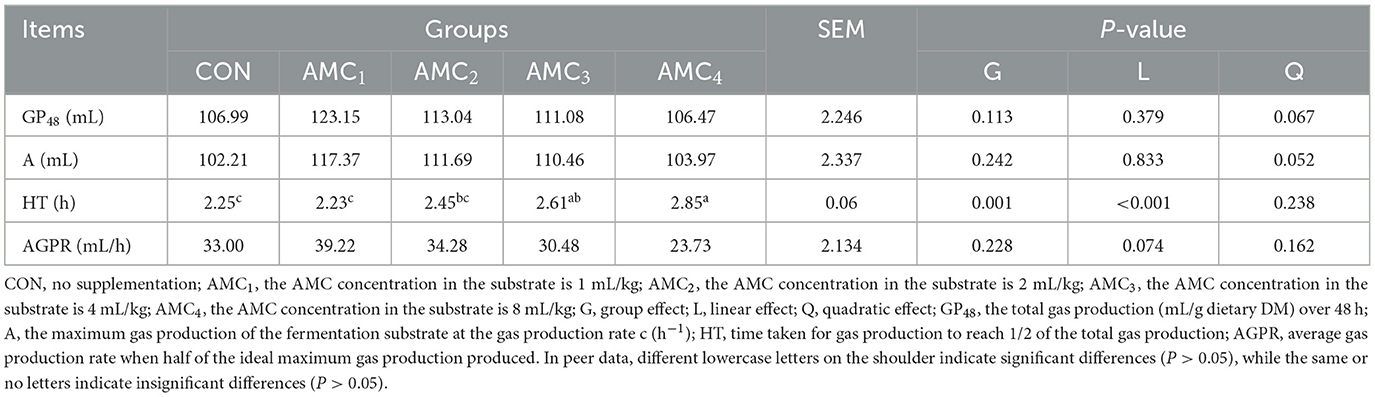
3.1 Gas production kinetics parameters
Table 4 presents an overview of the different concentrations of AMC in vitro gas production kinetics parameters. Through data analysis, it was found that HT had a linear trend of growth at different concentrations of AMC (p < 0.01), whereas there was no significant difference between the AMC1 and AMC2 groups and the control group. This indicates that the addition of AMC at a concentration of 1 and 2 mL/kg did not affect the rumen fermentation efficiency.
3.2 Fermentation parameters
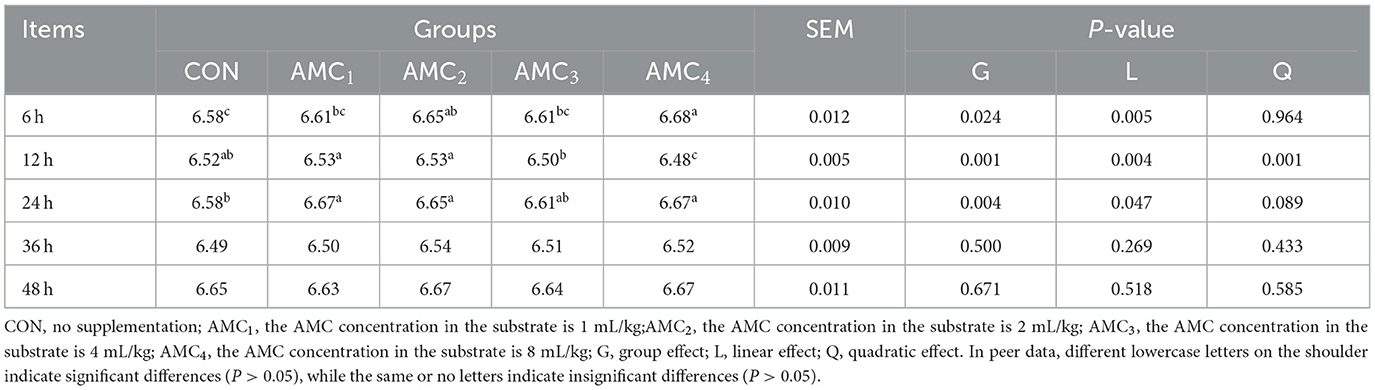
As shown in Table 5, with an increase in AMC concentrations, the pH of the rumen fluid increased linearly at 6 h and 24 h (p < 0.05), whereas the pH at 12 h showed a quadratic trend (p < 0.05). In addition, different concentrations of AMC had no significant effect on pH at 36 h and 48 h (p > 0.05). The data showed that AMC had a good buffering effect before 24 h, and this indicated a stabilizing effect on rumen pH. After 24 h, the rumen pH of both the control and treatment groups tended to stabilize.
Figure 1 shows the impact of the AMC concentration on the profiles of the fermentation parameters during the 48 h of fermentation. The data revealed a quadratic trend for all VFAs and TVFA at different concentrations of AMC, except for isobutyric acid (p < 0.05). With an increase in the concentration of AMC, the levels of acetate, propionate, butyrate, valerate, and isovaleric acid increased and subsequently decreased, with the highest value observed in AMC3. The ratio of acetate to propionate showed an initial decrease, followed by an increase with increasing concentrations of AMC. The AMC2 and AMC3 groups tended to exhibit more propionic acid-type fermentation (p < 0.05). The influence of different AMC concentrations on MCP was not significant (p > 0.05).
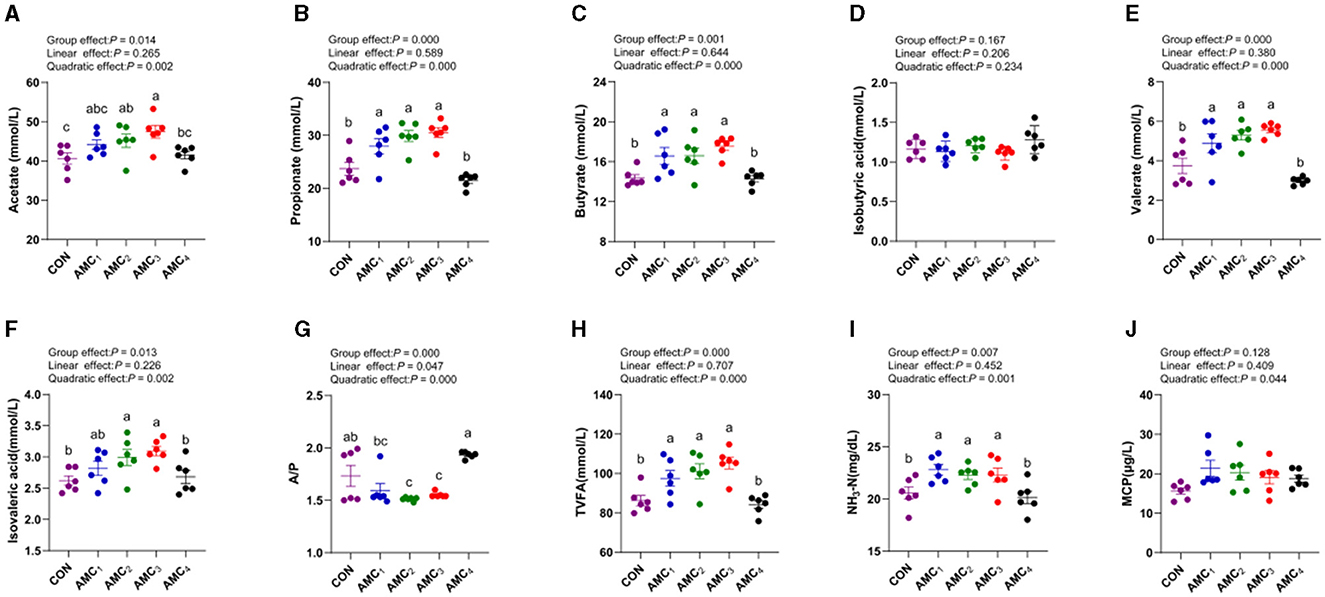
Figure 1. Effects of different concentrations of AMC on fermentation parameters. (A) Acetate content; (B) Propionate content; (C) Butyrate content; (D) Isbutyric content; (E) Valerate content; (F) Isovaleric acid content; (G) Acetate/Propionate; (H) TVFA-total volatile fatty acids; (I) NH3-N:Ammonia nitrogen; (J) MCP:Microbial protein. CON, no supplementation; AMC1, the AMC concentration in the substrate is 1 mL/kg; AMC2, the AMC concentration in the substrate is 2 mL/kg; AMC3, the AMC concentration in the substrate is 4 mL/kg; AMC4, the AMC concentration in the substrate is 8 mL/kg; G, group effect; L, linear effect; Q, quadratic effect; A/P, the ratio of Acetate and Propionate; TVFA, total volatile acids; NH3,-N, ammoniacal nitrogen; MCP, microbial crude protein.
3.3 In vitro degradability
Table 6 presents the effects of different AMC concentrations on nutrient degradability. The results showed that AMC has a negative effect in promoting ADF degradation during in vitro fermentation but has no significant effect on DM, NDF, or CP parameters. The acid detergent fiber (ADF) linearly increased with the concentration of AMC (p < 0.05).
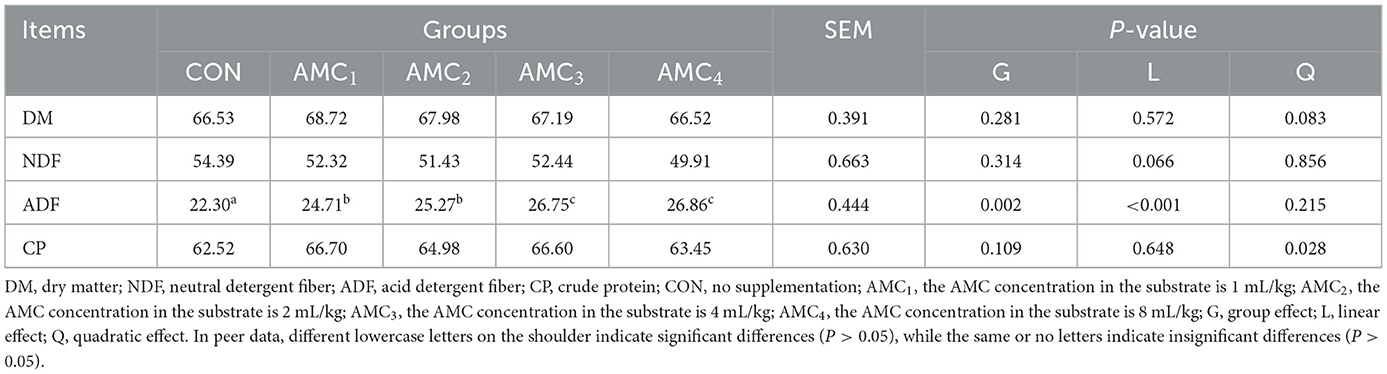
Table 6. Effects of different concentrations of AMC on the nutrient degradability in vitro fermentation.
3.4 Microbial diversity
Figure 2 shows the effects of different concentrations of AMC on the alpha diversity index in vitro fermentation. For the alpha diversity index, there was no significant difference between the treatment group and the CON group (p > 0.05).
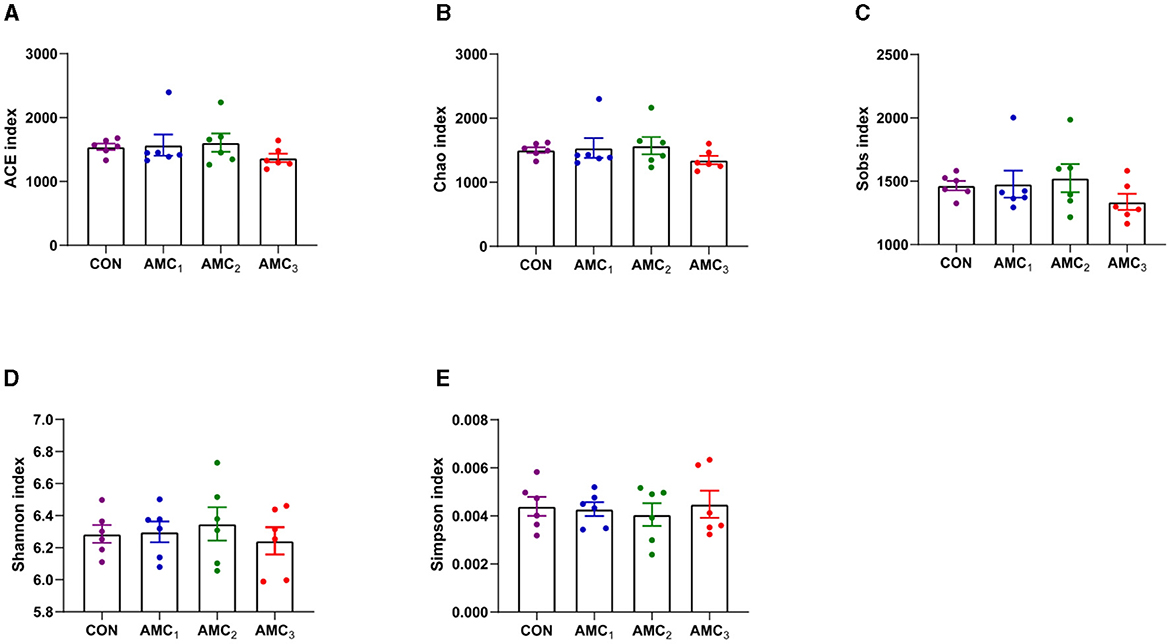
Figure 2. Effects of different concentrations of AMC on the nutrient degradability in vitro fermentation. (A) ACE diversity index; (B) Chao 1 diversity; (C) Sobs index; (D) Shannon diversity index; (E) Simpson index. CON, no supplementation; AMC1, the AMC concentration in the substrate is 1 mL/kg; AMC2, the AMC concentration in the substrate is 2 mL/kg; AMC3, the AMC concentration in the substrate is 4 mL/kg.
In addition, there was no distinct separation between the different supplementation groups and the CON group in the PCoA plot based on Bray–Curtis staining (Figure 3, p > 0.05). These results indicated that there were no significant differences in the species diversity of rumen fluid bacteria among the different concentrations of AMC.
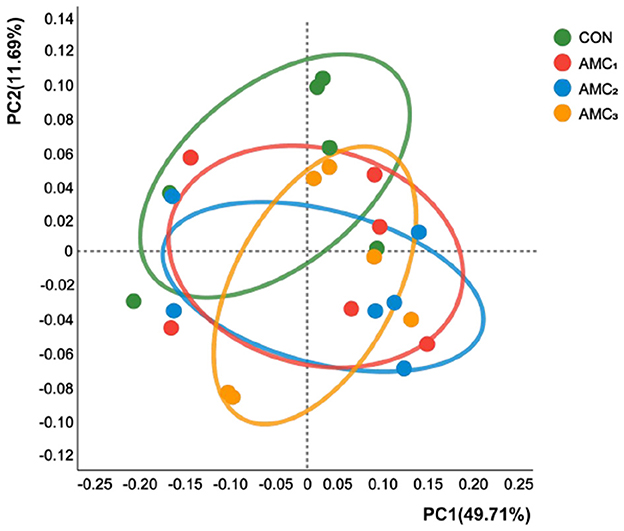
Figure 3. Principal coordinate analysis (PCoA) combined with permutational multivariate analysis of variance (PREANOVA) was calculated based on the ASV level and Bray–Curtis distances. CON, no supplementation; AMC1, the AMC concentration in the substrate is 1 mL/kg; AMC2, the AMC concentration in the substrate is 2 mL/kg; AMC3, the AMC concentration in the substrate is 4 mL/kg.
Figure 4 shows an overview of the genus composition of the microbiota. The abundances of Prevotella, Rikenellaceae-RC9-gut-group, and norank-f–F082 were found to be enriched in different groups.
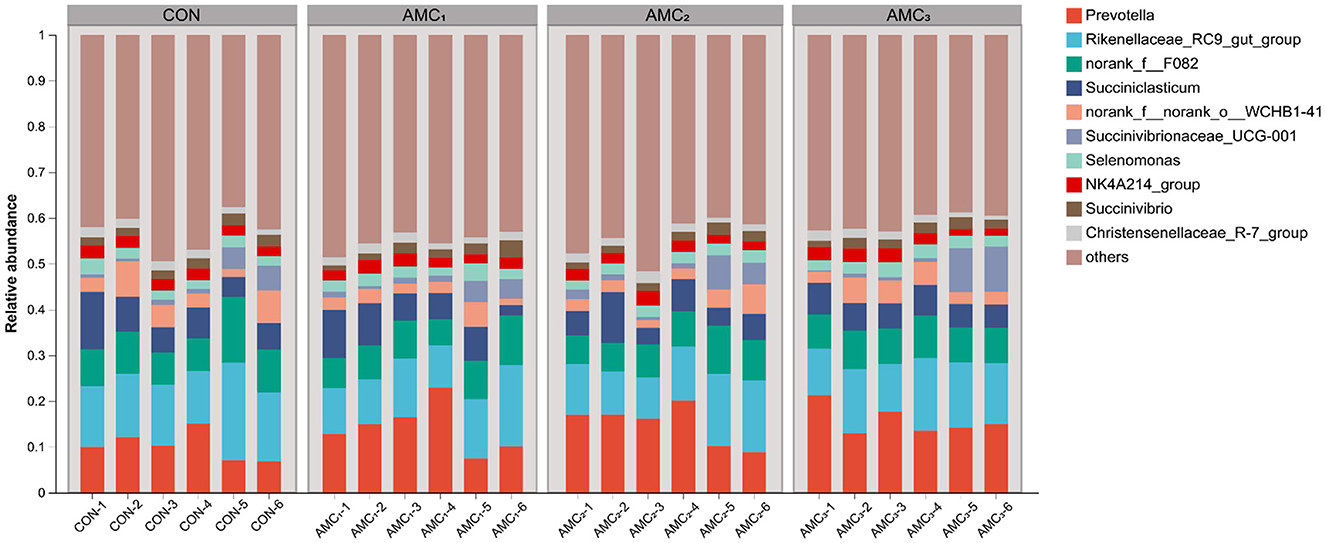
Figure 4. Genera composition of the microbiota from in vitro fermentation technique; CON, no supplementation; AMC1, the AMC concentration in the substrate is 1 mL/kg; AMC2, the AMC concentration in the substrate is 2 mL/kg; AMC3, the AMC concentration in the substrate is 4 mL/kg.
Figure 5 shows the microbial composition at the genus level under the different AMC treatments. As shown in Figure 5B, the relative abundance of Bacteroidales-RF16 in the AMC1 and AMC3 groups was significantly lower than that in the CON group (p < 0.05). Furthermore, the relative abundance of Lachnospiraceae UCG-008 in the AMC1 group was significantly lower than that in the CON group (Figure 5C, p < 0.05). Regarding Prevotellaceae-Ga6A1 and Lachnospira, different treatments revealed a significant impact on their abundance (Figure 5A, p < 0.05). However, the difference between the groups was not statistically significant (Figure 5D, p > 0.05). The relative abundance of the CON group was lower than that of the other treatment groups.
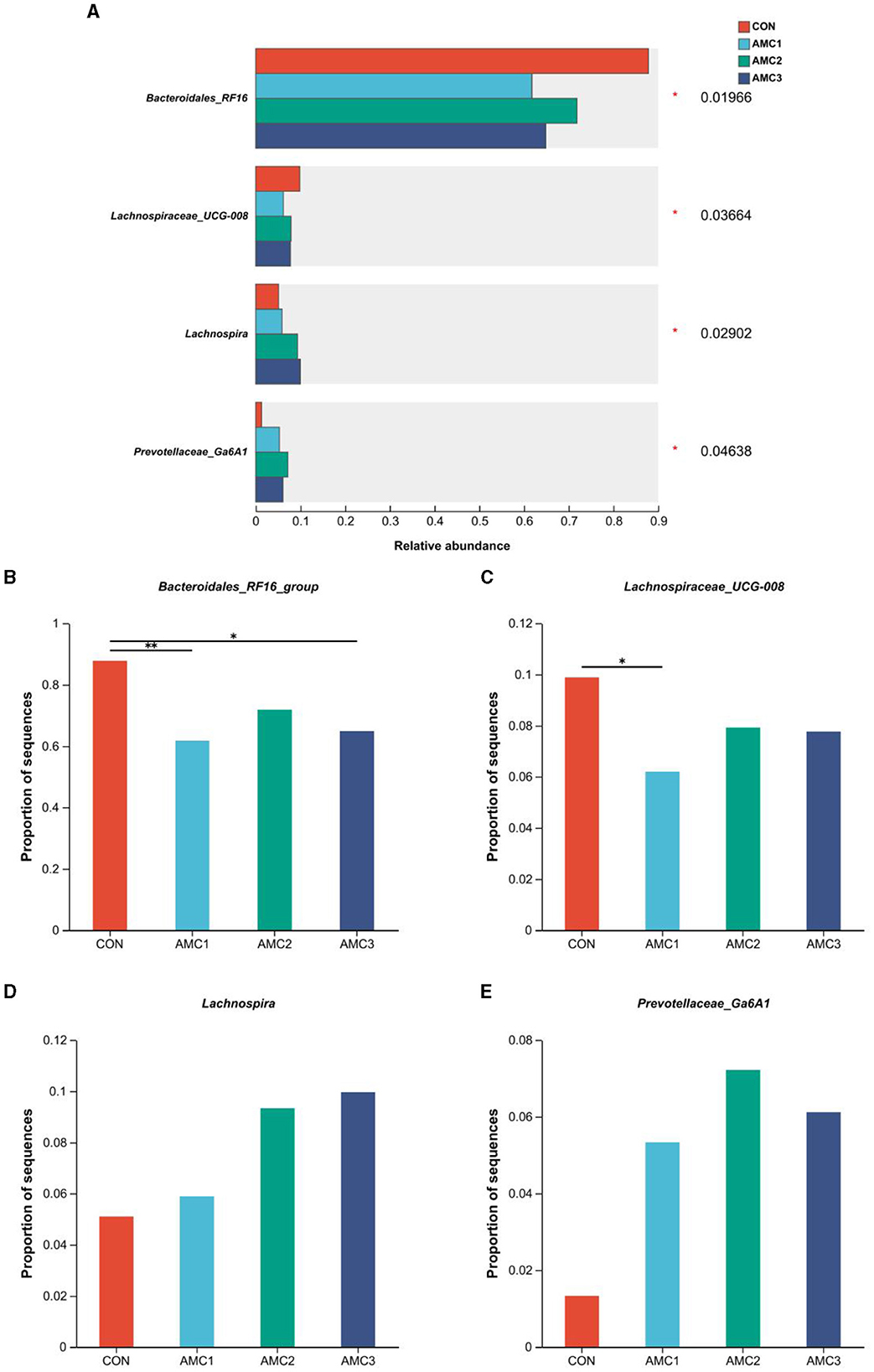
Figure 5. Bacterium with significant differences in species abundance at the genus level (A), relative abundance and p-value cut-off were <0.05; Analysis of differences in bacteria between any two groups (B–E): *0.01 < p ≤ 0.05, **0.001 < p ≤ 0.01, ***p ≤ 0.001; CON, no supplementation; AMC1, the AMC concentration in the substrate is 1 mL/kg; AMC2, the AMC concentration in the substrate is 2 mL/kg; AMC3, the AMC concentration in the substrate is 4 mL/kg.
4 Discussion
In this study, we focused on one of the prerequisites for normal rumen fermentation, which is the normal pH range of rumen fluid (5.5–7.5) (24). By adding AMC at different concentrations during in vitro fermentation of the rumen fluid, we observed that the pH of the rumen fluid, when fermented in vitro with different concentrations of AMC, ranged from 5.8 to 7.0, which is consistent with the optimal range of rumen pH. Notably, the addition of 1–2 mL/kg of AMC significantly elevated rumen pH at 6 and 24 h, suggesting that AMC had a significant buffering effect on rumen pH, particularly within the first 24 h of supplementation. Furthermore, the buffering effect of AMC stabilized after 24 h, as both the control and treatment groups exhibited a tendency toward stable rumen pH levels after this time point.
GP48 and HT are important indicators of rumen fermentation capacity and nutrient digestibility. Through our experiments, we found that AMC had a significant impact on the HT, consistent with the results of the pH. Specifically, when the rumen microbiota metabolizes to produce excess hydrogen ions, AMC can neutralize these ions, maintaining the solution's pH at a relatively stable level. This provides a suitable environment for promoting microbial activity and gas production processes.
VFAs, the main products of rumen fermentation, serve as the main energy sources and raw materials for synthetic and milk fats. Acetic and butyric acids are mainly used for milk fat synthesis, whereas propionic acid serves as a precursor for glucose synthesis and can competitively consume hydrogen to reduce methane production (14). Propionic acid is rapidly oxidized in the liver to produce energy. Similar to other short-chain fatty acids, propionic acid is a product of intestinal microbial fermentation of fiber and other indigestible carbohydrates, which is crucial for maintaining intestinal health and function. Valeric acid, isovaleric acid, and isobutyric acid, collectively categorized as short-chain VFAs with four to five carbon atoms, are referred to as branched-chain VFAs (25). The results in the present study indicated that all VFAs in the fermentation broth, except isobutyric acid, showed a quadratic change with the increasing concentration of AMC. As the AMC concentration increased, the acetic, propionic, butyric, valeric, and isovaleric acid contents increased and then decreased, and the A/P ratio first decreased and then increased. Although the ammonia nitrogen concentration remained unchanged, the total VFA concentration increased in the group supplemented with bicarbonate, indicating that the addition of a combination of buffer altered the liquid turnover and the rumen fermentation mode (26), which was beneficial for providing energy for ruminants. This trend can be attributed to an increase in Prevotellacea-Ga6A1. Previous studies have shown that Prevotella metabolizes hemicellulose, pectin, and proteins, with acetic and formic acids being the main fermentation products (27). The increase in these short-chain fatty acids led to a trend toward propionic acid-type fermentation in the rumen, indicating that more propionic acid provides energy through gluconeogenesis, which is particularly important for maintaining the energy balance in ruminants. High levels of propionic acid can inhibit milk fat synthesis, but this needs to be validated via in vitro experiments. These findings support the significant role of buffering agents in maintaining energy balance and promoting rumen health in ruminants.
The competitive dynamics observed between fiber-degrading bacteria in the phylum Bacillota and the genus Prevotella, with an increased relative abundance of Prevotellacea-Ga6A1, correspond to our results and suggest an inhibitory effect on the growth of Lachnospiraceae UCG-008. Lachnospira can also degrade polysaccharides and fiber contents to produce acetic acid. The observed discrepancies in ADF and VFA may be attributed to an increase in Lachnospira abundance (28). This indicated that AMC supplementation promoted the growth of beneficial bacteria in rumen microorganisms and the reproduction and metabolism of acetic acid-producing bacteria. AMC facilitated the fermentation and decomposition of carbohydrates, thereby promoting the metabolism and absorption of nutrients by dairy cattle.
NH3-N is produced by the fermentation of protein, non-protein nitrogen, and other nitrogenous compounds, and this can reflect the degree of rumen nitrogen metabolism (29). Inappropriate concentrations of NH3-N affect animal health. The appropriate range of rumen NH3-N concentration had been reported to be 6–30 mg/dL (30), and the result of the present study showed that the concentration of NH3-N are all within a reasonable range. MCP is the predominant nitrogen source for dairy cattle, contributing 60–80% of the required protein. This reflects the microbial utilization of NH3-N and indicates the abundance of microorganisms (31). Ample nitrogen sources, provision of VFAs as carbon scaffolds, and fermentation of organic matter have a collaborative effect on the synthesis efficiency and quantity of MCP (32). In this study, the addition of AMC significantly influenced the concentration of NH3-N. Additionally, NH3-N and MCP exhibited a quadratic trend with increasing AMC, indicating that AMC promoted rumen microorganisms to comprehensively utilize nutrients in the fermentation substrate.
The PCoA analysis revealed no significant differences in the microbial community structure between the treatment and control groups, suggesting the relatively mildness of the buffer. It is essential to emphasize that the subtle effects of the buffering agents do not imply a lack of impact on the microorganisms in all scenarios. These variations may be associated with the differences between individual samples, sample sizes, and the simulated environment of the in vitro experiments. It is imperative to conduct further in vivo experiments to validate the efficacy of this buffer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of China Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SL: Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. BX: Data curation, Formal Analysis, Methodology, Writing – review & editing. HJ: Formal Analysis, Methodology, Visualization, Writing – review & editing. SL: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by the earmarked fund for CARS36 and Science and Technology Project of the 12th Division of XPCC: Leading the Charge with Open Competition (SRS2022001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Humer E, Aschenbach JR, Neubauer V, Kröger I, Khiaosa-Ard R, Baumgartner W, et al. Signals for identifying cows at risk of subacute ruminal acidosis in dairy veterinary practice. J Anim Physiol Anim Nutr. (2018) 102:380–92. doi: 10.1111/jpn.12850
2. Humer E, Petri RM, Aschenbach JR, Bradford BJ, Penner GB, Tafaj M, et al. Invited review: practical feeding management recommendations to mitigate the risk of subacute ruminal acidosis in dairy cattle. J Dairy Sci. (2018) 101:872–88. doi: 10.3168/jds.2017-13191
3. Wetzels SU, Mann E, Metzler-Zebeli BU, Pourazad P, Qumar M, Klevenhusen F, et al. Epimural indicator phylotypes of transiently-induced subacute ruminal acidosis in dairy cattle. Front Microbiol. (2016) 7:274. doi: 10.3389/fmicb.2016.00274
4. Jackson JA, Akay V, Franklin ST, Aaron DK. The effect of cation-anion difference on calcium requirement, feed intake, body weight gain, and blood gasses and mineral concentrations of dairy calves. J Dairy Sci. (2001) 84:147–53. doi: 10.3168/jds.S0022-0302(01)74463-3
5. Neiderfer KP, Barnard AM, Moyer KZ, Trench AM, Taylor AE, Cronin SK, et al. Effects of calcium carbonate, magnesium oxide and encapsulated sodium bicarbonate on measures of post-ruminal fermentation. J Anim Physiol Anim Nutr. (2020) 104:802–11. doi: 10.1111/jpn.13331
6. Snyder TJ, Rogers JA, Muller LD. Effects of 12% sodium bicarbonate with two ratios of corn silage: grain on milk production, rumen fermentation, and nutrient digestion by lactating dairy cows. J Dairy Sci. (1983) 66:1290–7. doi: 10.3168/jds.S0022-0302(83)81937-7
7. Chen J, Xu YR, Kang JX, Zhao BC, Dai XY, Qiu BH, et al. Effects of alkaline mineral complex water supplementation on growth performance, inflammatory response, and intestinal barrier function in weaned piglets. J Anim Sci. (2022) 100:skac251. doi: 10.1093/jas/skac251
8. Chen J, Zhao B-C, Dai X-Y, Xu Y-R, Kang J-X, Li J-L. Drinking alkaline mineral water confers diarrhea resistance in maternally separated piglets by maintaining intestinal epithelial regeneration via the brain-microbe-gut axis. J Adv Res. (2023) 52:29–43. doi: 10.1016/j.jare.2022.12.008
9. Chen J, Xu X-W, Kang J-X, Zhao B-C, Xu Y-R, Li J-L. Metasilicate-based alkaline mineral water confers diarrhea resistance in maternally separated piglets via the microbiota-gut interaction. Pharmacol Res. (2023) 187:106580. doi: 10.1016/j.phrs.2022.106580
10. AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists. 19th ed. Arlington, VA: Association of Official Analytical Chemists, Inc. (2012). p. 1048–9.
12. Van Soest P, Robertson J, Lewis B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
13. Menke KH. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. (1988) 28:7–55.
14. Kohn RA, Dunlap TF. Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. J Anim Sci. (1998) 76:1702–9. doi: 10.2527/1998.7661702x
15. Liu C, Wu H, Liu S, Chai S, Meng Q, Zhou Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front Microbiol. (2019) 10:1116. doi: 10.3389/fmicb.2019.01116
16. Bai S, Cao Z-J, Cao B-B, Yang H-J, Li S-L, Liu J-X. Effects of different forage combinations in total mixed rations on in vitro gas production kinetics, ruminal and milk fatty acid profiles of lactating cows. Anim Sci J. (2018) 89:1261–70. doi: 10.1111/asj.13036
17. Ogunade I, Schweickart H, McCoun M, Cannon K, McManus C. Integrating 16S rRNA sequencing and LC–MS-based metabolomics to evaluate the effects of live yeast on rumen function in beef cattle. Animals. (2019) 9:28. doi: 10.3390/ani9010028
18. Magoč T, Salzberg SL. FLASH fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
19. Tonge DP, Pashley CH, Gant TW. Amplicon-based metagenomic analysis of mixed fungal samples using proton release amplicon sequencing. PLoS ONE. (2014) 9:e93849. doi: 10.1371/journal.pone.0093849
20. France J, Dijkstra J, Dhanoa MS, Lopez S, Bannink A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr. (2000) 83:143–50. doi: 10.1017/S0007114500000180
21. Institute, S. Base SAS 9.4 Procedures Guide: Statistical Procedures. Cary, NC: SAS Institute (2017).
22. Wang Y, Nan X, Zhao Y, Jiang L, Wang H, Hua D, et al. Dietary supplementation with inulin improves lactation performance and serum lipids by regulating the rumen microbiome and metabolome in dairy cows. Anim Nutr. (2021) 7:1189–204. doi: 10.1016/j.aninu.2021.09.007
23. Chong J, Liu P, Zhou G, Xia J. Using microbiome analyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc. (2020) 15:799–821. doi: 10.1038/s41596-019-0264-1
24. Mu Y, Qi W, Zhang T, Zhang J, Mao S. Multi-omics analysis revealed coordinated responses of rumen microbiome and epithelium to high-grain-induced subacute rumen acidosis in lactating dairy cows. mSystems. (2022) 7:e0149021. doi: 10.1128/msystems.01490-21
25. Roman-Garcia Y, Denton BL, Mitchell KE, Lee C, Socha MT, Firkins JL. Conditions stimulating neutral detergent fiber degradation by dosing branched-chain volatile fatty acids. I: Comparison with branched-chain amino acids and forage source in ruminal batch cultures. J Dairy Sci. (2021) 104:6739–55. doi: 10.3168/jds.2020-20054
26. Zhu Z, Difford GF, Noel SJ, Lassen J, Løvendahl P, Højberg O. Stability assessment of the rumen bacterial and archaeal communities in dairy cows within a single lactation and its association with host phenotype. Front Microbiol. 12:636223. doi: 10.3389/fmicb.2021.636223
27. Liang J, Fang W, Chang J, Zhang G, Ma W, Nabi M. Long-term rumen microorganism fermentation of corn stover in vitro for volatile fatty acid production. Bioresour Technol. 358:127447. doi: 10.1016/j.biortech.2022.127447
28. Gauly M, Ammer S. Review: challenges for dairy cow production systems arising from climate changes. Animal. (2020) 14:196–203. doi: 10.1017/S1751731119003239
29. Sannes RA, Messman MA, Vagnoni DB. Form of rumen-degradable carbohydrate and nitrogen on microbial protein synthesis and protein efficiency of dairy cows. J Dairy Sci. 85:900–8. doi: 10.3168/jds.S0022-0302(02)74148-9
30. Bertilsson J, Åkerlind M, Eriksson T. The effects of high-sugar ryegrass/red clover silage diets on intake, production, digestibility, and N utilization in dairy cows, as measured in vivo and predicted by the NorFor model. J Dairy Sci. (2017) 100:7990–8003. doi: 10.3168/jds.2017-12874
31. Lu Z, Xu Z, Shen Z, Tian Y, Shen H. Dietary energy level promotes rumen microbial protein synthesis by improving the energy productivity of the ruminal microbiome. Front Microbiol. (2019) 10:847. doi: 10.3389/fmicb.2019.00847
Keywords: lactating ruminants, rumen preference parameters, additive concentration, subacute rumen acidosis, dairy cows
Citation: Liu S, Xie B, Ji H and Li S (2024) Effects of dietary supplementation with alkaline mineral complex on in vitro ruminal fermentation and bacterial composition. Front. Vet. Sci. 11:1357738. doi: 10.3389/fvets.2024.1357738
Received: 18 December 2023; Accepted: 09 April 2024;
Published: 22 May 2024.
Edited by:
Xianwen Dong, Chongqing Academy of Animal Science, ChinaReviewed by:
Yuchao Zhao, Beijing University of Agriculture, ChinaYongjiang Wu, Chongqing University of Arts and Sciences, China
Copyright © 2024 Liu, Xie, Ji and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengli Li, c2hlbmdsaWNhdUAxNjMuY29t
†These authors have contributed equally to this work and share senior authorship
 Siyuan Liu
Siyuan Liu Biao Xie
Biao Xie Hongjin Ji
Hongjin Ji Shengli Li
Shengli Li