- 1Linxia Animal Husbandry Technology Extension Station, Linxia, China
- 2Linxia Animal Quarantine Station, Linxia, China
- 3Key Laboratory of Environmental Ecology and Population Health in Northwest Minority Areas, Medicine of Northwest Minzu University, Lanzhou, China
Postpartum blood calcium (Ca) concentration is related to the reproduction and health of cattle. Oral calcium supplements were given to dairy cows after calving to increase blood Ca concentration and reduce the risk of hypocalcemia. However, studies have shown that oral Ca has different effects in preventing disease. The purposes of this study were (i) to conduct a meta-analysis to evaluate the expected effect of oral Ca on incidence of calving-related diseases, pregnancy risk and milk yield in dairy cows, and (ii) to make a quality assessment of these related studies. In total, 22 eligible studies were included in this review. Meta-analysis showed that oral Ca could significantly reduce the incidence of hypocalcemia (clinical hypocalcemia: relative risk (RR) = 0.67, 95% confidence interval (CI) = [0.52, 0.87]; subclinical hypocalcemia: RR = 0.81, CI = [0.72, 0.91]), and incidence of retained placenta (RR = 0.77, CI = [0.62, 0.95]), improved blood Ca concentrations: mean difference (MD) = 0.08; 95% CI = [0.04, 0.11]. For other results, the meta-analysis revealed a lack of evidence of the correlation between oral Ca and serum magnesium (Mg) / phosphorus (P) concentration (Mg: MD = −0.04; 95% CI = [−0.10, 0.02]; P: MD = 0.05; 95% CI = [−0.10, 0.21]) or incidence of other calving-related disorders (metritis: RR = 1.06, CI = [0.94, 1.19]; ketosis: RR = 1.04, CI = [0.91, 1.18]; mastitis: RR = 1.02, CI = [0.86, 1.21]; displacement of the abomasum: RR = 0.81, CI = [0.57, 1.16]) or pregnancy risk (pregnancy risk at first service: RR = 0.99, CI = [0.94, 1.05]; overall pregnancy rate: RR = 1.03, CI = [0.98, 1.08]) or milk yield (MD = 0.44; 95% CI = [−0.24, 1.13]). The distribution of the funnel plot formed by the included studies was symmetrical, and the Egger’s test had a p > 0.05, indicating that there was no significant publication bias. Sensitivity analyses results suggested that the results of meta-analysis are robust. Quality assessment of the included studies revealed that the risk of bias was focused on selection bias, performance bias, detection bias and other sources of bias, and the future research should focus on these aspects.
Introduction
Clinical hypocalcemia (also known as milk fever or parturient paresis) and subclinical hypocalcemia are common perinatal health disorders in dairy cows, which can lead to other clinical diseases or death (1–3). Postpartum milk production of dairy cows will rapidly consume circulating calcium, leading to a decrease in blood calcium (Ca) concentration (4, 5). When the total blood calcium <1.4 mmol/L, or serum total Ca < 2.0 mmol/L with clinical signs, it is diagnosed clinical hypocalcemia (6, 7). There are no obvious clinical symptoms in subclinical hypocalcemia. In published studies, subclinical hypocalcemia was defined using thresholds that varied from 1.88 to 2.35 mmol/L (8).
Ca metabolism is interrelated with other minerals. When the blood calcium concentration decreases, the blood magnesium (Mg) concentration initially increases and then decreases until it stabilizes 3–4 weeks postpartum (9, 10). Some studies have determined that found that prenatal feeding with P-deficient diet had a positive effect on Ca homeostasis of perinatal dairy cows (11, 12). In feedstuffs, sodium (Na), potassium (K), chlorine (Cl) and sulfur (S) are ionic variables in the dietary cation-anion differences (DCAD) equation, and controlling their content in the diet is the key to reducing hypocalcemia in cows (13, 14). Milk fever have effects on decreased reproductive performance, and increased prevalence of other diseases during early lactation (6, 15, 16). Ducusin et al. pointed out that the hypocalcemic condition of parturient paretic cows in vivo causes decreased phagocytosis and resting [Ca2+]i in polymorphonuclear leukocytes, which may partly contribute to greater susceptibility to infection (17). Milk fever can reduce tension and contractility of the uterine muscles, leading to dystocia or retained fetal membranes (18). Although study have pointed out that the occurrence of displaced abomasum was 3.7 times more likely in cows that had subclinical hypocalcemia than in cows with normocalcemia (19), Zurr et al. have found that calcium has negative effects on abomasal motility only at extremely low levels (20). Research shows that postpartum cows will experience three different forms of subclinical hypocalcemia: transient, persistent or delayed, of which persistent and delayed hypocalcemia have harmful effects on health and milk production (5).
Postpartum Ca supplementation is performed by oral, intravenous injection and subcutaneous injection. Owing to convenience, Ca is often orally administered to dairy cows after calving to increase their postpartum blood calcium concentration and reduce the incidence of hypocalcemia (21–23). At present, most farms use the commercial product Ca bolus as a supplement, and its main components are CaCl2 and CaSO4. In addition, there are other forms of calcium supplements, such as Ca gel, Ca tubes and Ca fluid preparation (8, 24–31). Although Valldecabres et al. conducted a meta-analysis, it was revealed that oral Ca supplements were not associated with milk yield or pregnancy rate at first service (32). However, the meta-analysis included a small number of outcome indicators and did not evaluate the quality of the included studies (32). The purposes of this study were (i) to conduct a meta-analysis to evaluate the effectiveness of oral Ca on improving calving-related diseases (hypocalcemia, retained placenta, metritis, ketosis, mastitis, displacement of the abomasum), pregnancy risk and milk yield in dairy cows, and (ii) to make a quality assessment of these related studies, so as to provide references for scientific design and implementation of animal research in the future.
Materials and methods
Search strategies
Publications from its establishment to October 19, 2023 were searched in PubMed, Embase and Web of Science databases, and the search was not limited by language. In addition, reference tracks (Federal Bureau of Agriculture), abstracts,1 and Google Scholar2 were used to supplement the search. The search strategies used were: (hypocalcemia OR hypocalcemias OR parturient paresis OR parturient pareses OR animal milk fever) AND (cattle OR Bos indicus OR zebu OR zebus OR Bos taurus OR domestic cow OR domestic cows OR Bos grunniens OR yak OR yaks) AND (calcium OR calcium 40).
Study selection criteria
According to the PRISMA guidelines for Systematic Reviews and Meta-Analysis (33), following the principles of PICO (Problem/Patient/Population, Intervention/Indicator, Comparison, Outcome), the inclusion standards are pre-defined as follows: (i) question: healthy postpartum cows; (ii) intervention: oral calcium; (iii) comparison: standard interventions except for oral calcium; (iv) results: production-related diseases and blood indicators, including clinical and subclinical hypocalcemia, and Ca, Mg, and P concentrations in the blood, pregnancy risk and milk yield; and (v) study design: control in vivo experiment. In contrast, the exclusion criteria were as follows: (i) there are no restrictions on specific cow breed, parity; (ii) no limit to the sources, doses, forms and frequency of oral calcium; (iii) case reports, review articles, and letters; (iv) unreliable or incomplete data.
Data extraction
Two authors collected the details of included studies independently, and a third person adjudicated when they disagreed. These details include: (i) first author name and year of publication; (ii) characteristics of animal models (breed, parity, milk production and feed); (iii) information on treatment/control group, including the treatment drug and control, sample size, calcium form, dosage, time, and number of administrations; and (iv) outcome indicators (incidence of clinical and subclinical hypocalcemia, blood Ca concentrations; Mg and P serum concentrations; the incidence of ketosis, mastitis, metritis, displacement of the abomasum, retained placenta; pregnancy risk at first service, overall pregnancy rate and milk yield). If there are multiple groups of experimental group data with the same intervention in the literature, each group will be considered independent data and included in the analysis. In addition, the data in the graph were measured by using a digital ruler software (Getdata Graph Digitizer, version 2.25, Russia).
Quality evaluation of included studies
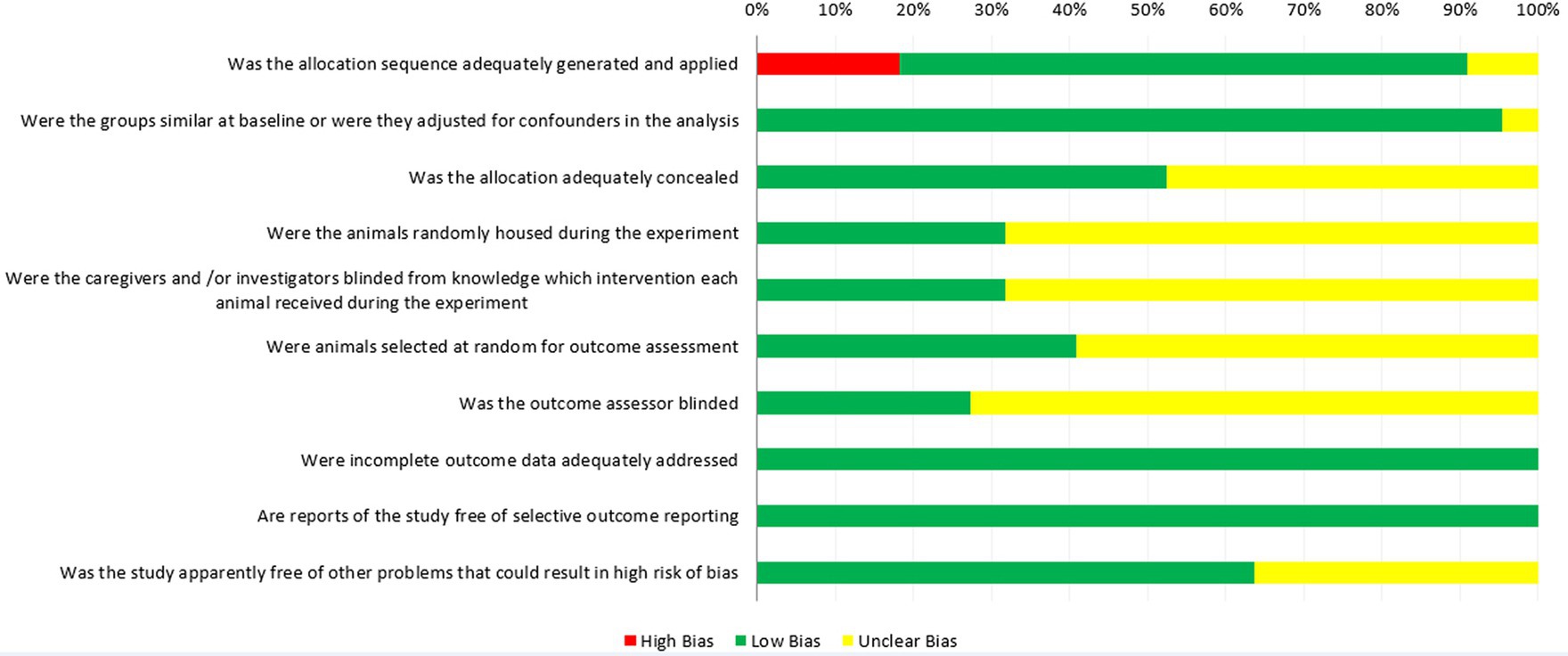
The Cochrane Collaboration developed the RoB tool to assess the quality of Randomized Controlled Trial (RCT) to avoid the risk of bias in animal experiments, and the SYRCLE RoB tool is an adapted version of the Cochrane RoB tool. The SYstematic Review Center for Laboratory Animal Experimentation (SYRCLE) RoB tool was used to assess the risk of bias in animal studies by using the 10 items: sequence generation (selection bias), baseline characteristics (selection bias), allocation concealment (selection bias), random housing (performance bias), blinding (performance bias), random outcome assessment (detection bias), blinding (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), other sources of bias (other). The evaluation results of each project classify the research into high risk, low risk or unclear risk according to PRISMA guidelines. In the result chart, red, green and yellow represent “high risk,” “low risk” and “unclear risk” respectively (34).
Statistical analysis
This study, used RStudio software (version 5701.9.1.0) for data analysis. The mean difference (MD) with 95% confidence interval (CI) for continuous variables and relative risk (RR) with 95% CI for dichotomous variables were calculated. Heterogeneity was assessed using I2 and Π2 statistical tests, and heterogeneity was considered high, if I2 was greater than 50%. Sensitivity analyses were performed for outcome measures with moderate heterogeneity (heterogeneity I2 of nearly 50%) to assess the robustness of the results. Statistical significance was set at p < 0.05. Publication bias assessment was performed using Egger’s test.
Results
Literature screening
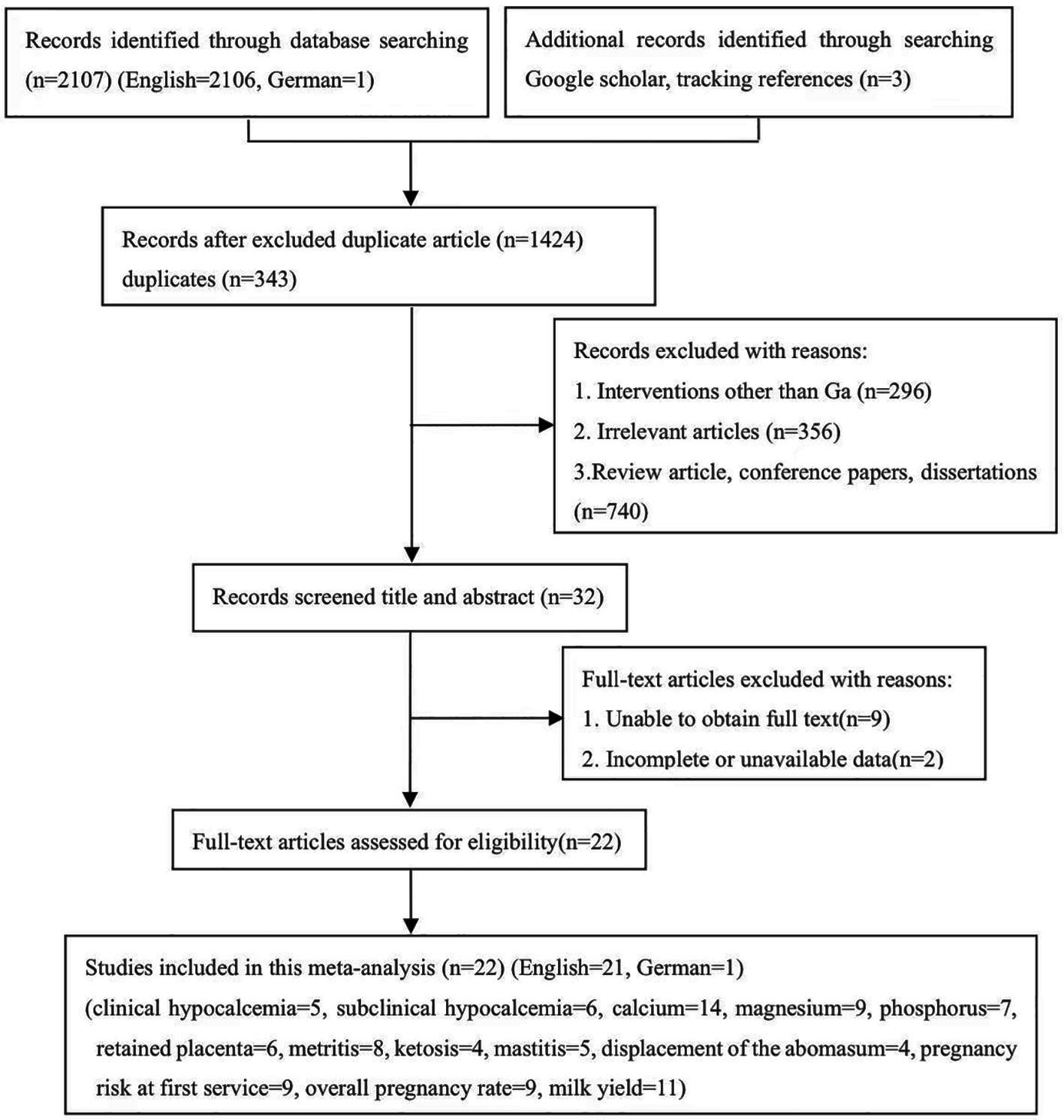
A total of 2,110 potentially relevant studies were identified through a database searching and supplementary searches. 343 studies were considered duplicated, while 1,402 studies were excluded since they did not meet the inclusion criteria. Finally, 22 eligible studies were included, as shown in Figure 1 (English = 21, German = 1).
Characteristics of the included studies
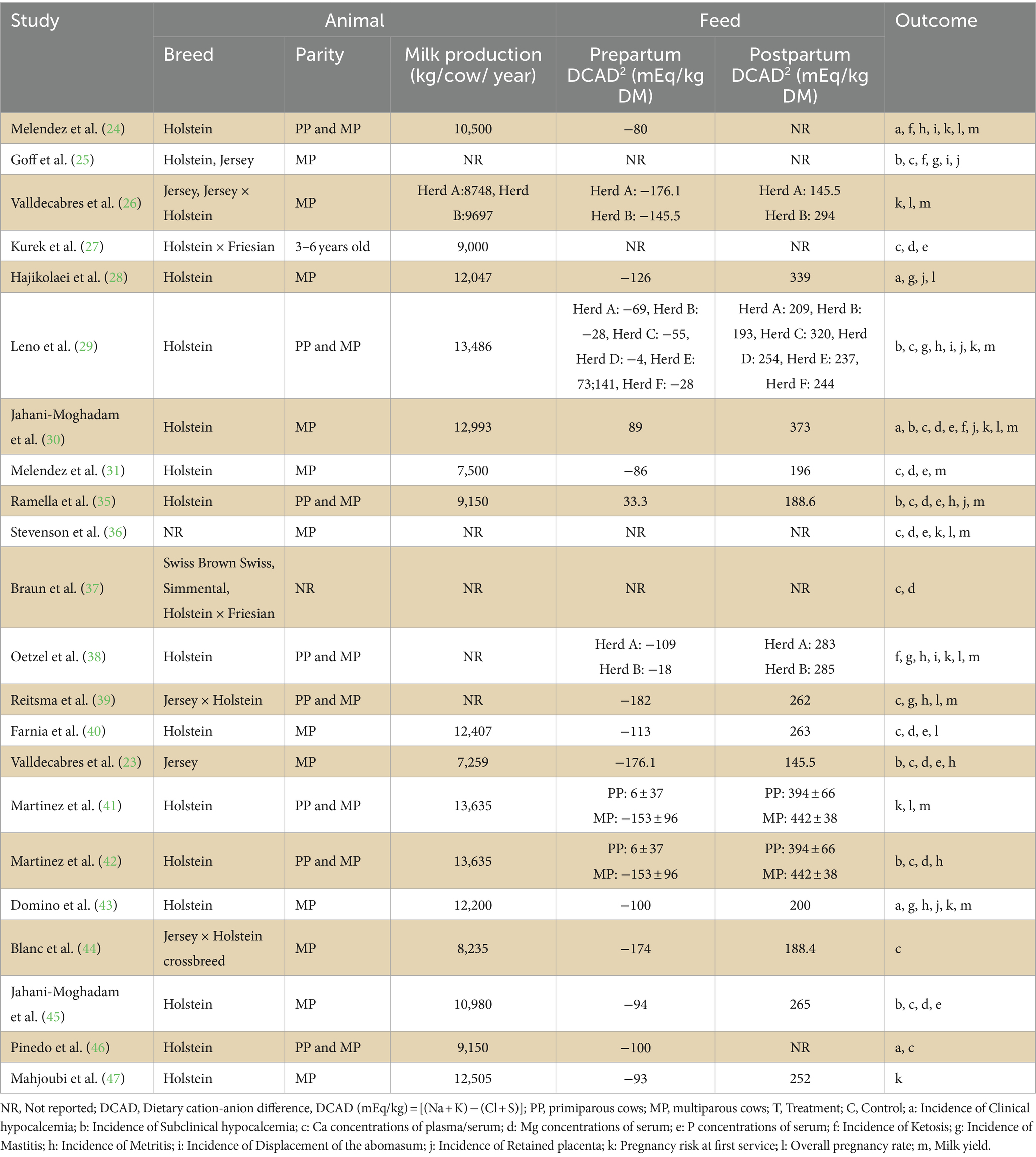
Of the 22 eligible studies, 15 studies used Holstein cows, three studies used Jersey cows, and five studies adopted crossbred cows. One study did not report the breed used for their experiment. Regarding parity, 12 studies used multiparous cows, eight used primiparous and multiparous cows, and two did not report parity. Seventeen studies reported milk production in cows, and 18 reported DCAD. The major outcome indicators were the incidence of clinical and subclinical hypocalcemia. Secondary outcome indicators include the concentration of Ca, Mg, and P in the blood; incidence of ketosis, mastitis, metritis, displacement of the abomasum, retained placenta; pregnancy risk at first service, overall pregnancy rate and milk yield. The overall characteristics of the included studies are summarized in Table 1.
The definitions of clinical diseases in the included studies are not consistent. Clinical hypocalcemia is defined as lying flat within 24 h after delivery, corrected by intravenous injection of calcium, or as the symptoms of lying weak or cold limbs without signs of physical injury within 72 h after delivery (28, 29). Subclinical hypocalcemia is defined as serum Ca ≤ 2.12 mmol/L, or blood total Ca < 2.125 mM, or ≤ 1.00 mmol/L on calving day (23, 35, 38). Ketosis is defined as a sharp drop in milk production, with acetoacetic acid in urine, or BHBA ≥1.2 mmol/L on BHBA test (24, 25, 38). Metritis is defined as foul-smelling uterine secretions that are red to brown within 14 days after delivery, or body temperature ≥ 39.5\u00B0C, lethargy, anorexia and vaginal discharge (29, 35). Displacement of the abomasum is defined as the decrease of milk production, and at the same time, when tapping and auscultating the left or right abdominal wall between the ninth and twelfth rib spaces, high-pitched tympanic resonance can be heard, with or without colic (24). Retained placenta is defined as the fetal membranes or placenta were not discharged within 24 h after delivery (28, 29, 35).
Characteristics of the treatment
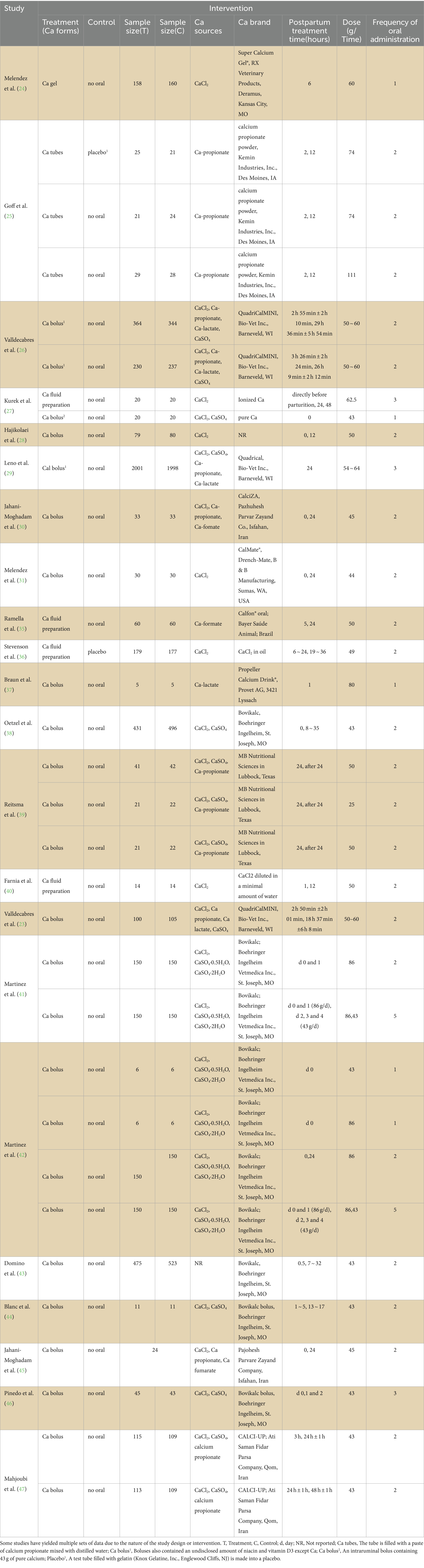
The characteristics of the interventions are presented in Table 2. Table 2 is as simple as possible, but due to the characteristics of study design or intervention, some studies have extracted multiple sets of data. The sample sizes of the included studies varied widely, ranging from 10 to 3,999 animals, with only 10 studies having the same sample size for the experimental and control groups. Accurate data of cows assigned to each experimental group could not be obtained from one study after contacting the authors (46). The treatment group cows in the included studies used different forms of Ca, including Ca gel, Ca tube, Ca bolus or Ca liquid preparation, among which Ca bolus was the most commonly used (18 studies). Cows in the control group usually have no calcium supplement, and only one study used a placebo made of test tubes filled with gelatin. There are varying many sources of Ca, and in most studies, calcium chloride (CaCl2) was used as the primary calcium source, one study did not report Ca sources. Eighteen studies used commercial brands of calcium. Moreover, the treatment times and frequencies in the included studies were inconsistent. In most studies, the time for oral calcium administration was 0–6, 12, and 24 h after calving. The times for the treatment regimen was 1 to 3, only two articles administer oral calcium 5 times. The dose was also inconsistent (25–110 g).
Quality evaluation of the included studies
The quality assessment of the included studies using SYRCLE’s RoB tool is show in Figure 2, in which all 22 studies were considered as controlled studies. Sixteen studies used random methods to generate sequences (low risk, green), however in four studies, cows were alternately assigned to treatment and control groups, based on the calving date or lactation order (high risk, red). Two studies did not describe the grouping method (unclear risk, yellow). The number of animals in the experimental and control groups in the 12 studies was also inconsistent; therefore, the risk of bias assessment of their “baseline characteristics” was unclear (unclear risk, yellow), and 19 studies adjusted for confounders in the analysis (low risk, green). Eleven studies described the allocation concealment method (low risk, green), and during the experiment implementation stage, seven studies mentioned random housing of the animals (low risk, green), while, 15 studies did not describe them (unclear risk, yellow). Lighting, humidity, temperature and other housing conditions are known to affect the research results, so it is necessary to randomize the feeding of these animals to reduce the performance bias (48). Only seven studies mentioned blinding measures used to blind trial investigators, study designers, and the outcome assessors from avoiding know which intervention each cow had received (low risk, green). Moreover, nine studies mentioned random selection of animals for assessment (low risk, green). Twenty-two studies described complete data for each primary outcome (low risk, green). In the assessment of “Is the study report irrelevant to selective outcome reporting,” all studies were considered low risk of bias (low risk, green). Eight studies mentioned other problems that could have resulted in an unclear risk of bias (low risk, green).
Incidence of clinical and subclinical hypocalcemia
A meta-analysis of the five studies showed that oral Ca significantly reduced the incidence of clinical hypocalcemia, compared to the control (n = 1,629; RR = 0.67; 95% CI = [0.52, 0.87], p = 0.003; heterogeneity I2 = 49%; Figure 3A), and the difference between the two groups was statistically significant. The meta-analysis of six studies shown that oral Ca significantly reduced the incidence of subclinical hypocalcemia (n = 4,903; RR = 0.81; 95% CI = [0.72, 0.91], p = 0.0005; heterogeneity I2 = 66%; Figure 3B). The difference between the two groups was statistically significant.
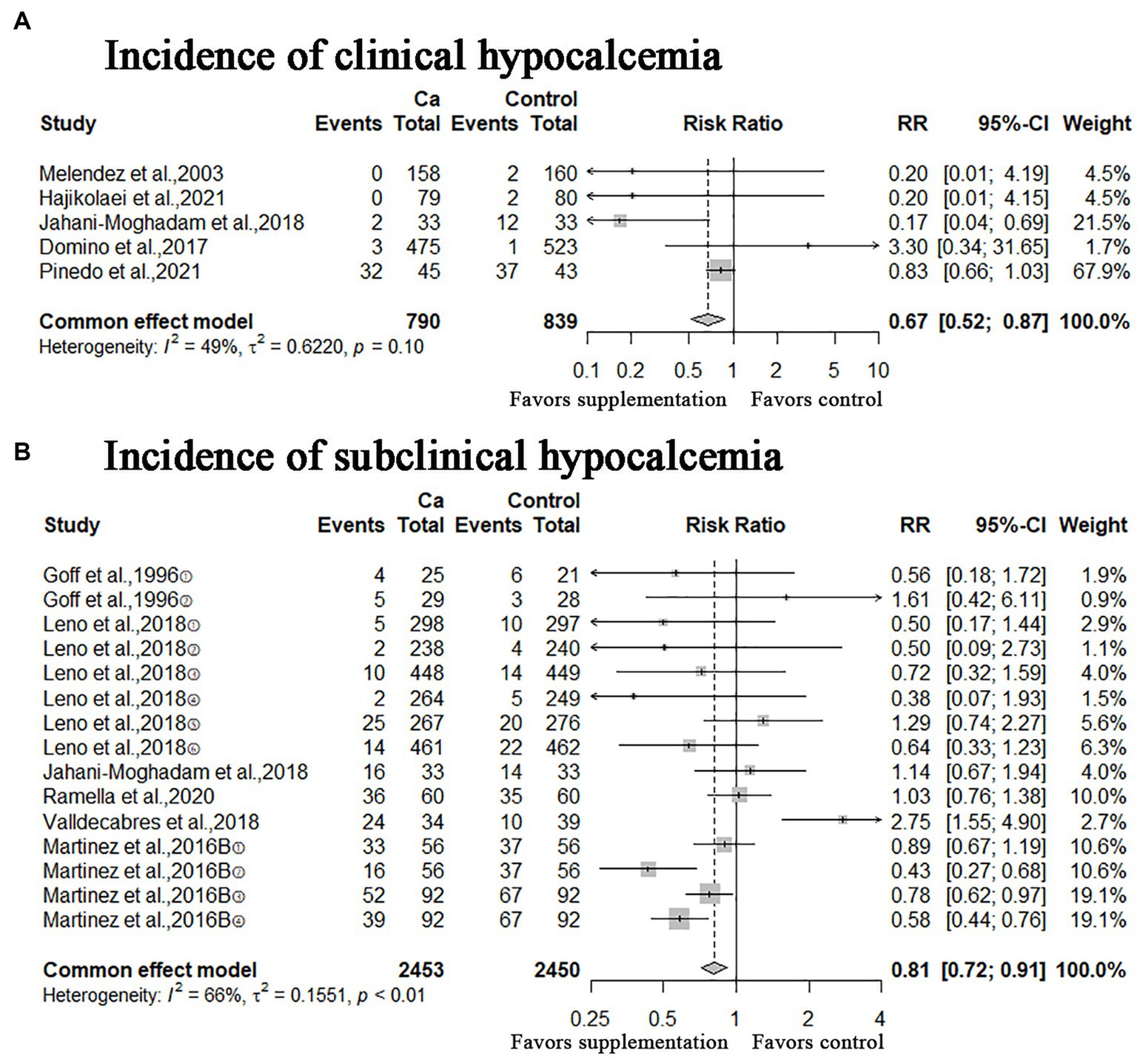
Figure 3. Meta-analysis of incidence of clinical hypocalcemia, subclinical hypocalcemia. Ca: Oral calcium supplements.
Blood indicators
A meta-analysis of 14 studies showed that oral Ca significantly improves Ca concentration in blood compared to the control (n = 1797; MD = 0.08; 95% CI = [0.04, 0.11]; p < 0.0001; heterogeneity I2 = 73%; Figure 4A), the difference between the two groups was statistically significant. The meta-analysis of serum Mg and P concentrations illustrated in Figures 4B,C, the MD of serum Mg concentrations was −0.04 (95% CI = [−0.10, 0.02]; p = 0.2068; heterogeneity I2 = 87%), the result was not statistically significant; the MD of serum P concentrations was 0.05 (95% CI = [−0.10, 0.21]; p = 0.4944; heterogeneity I2 = 79%), this result was not statistically significant.
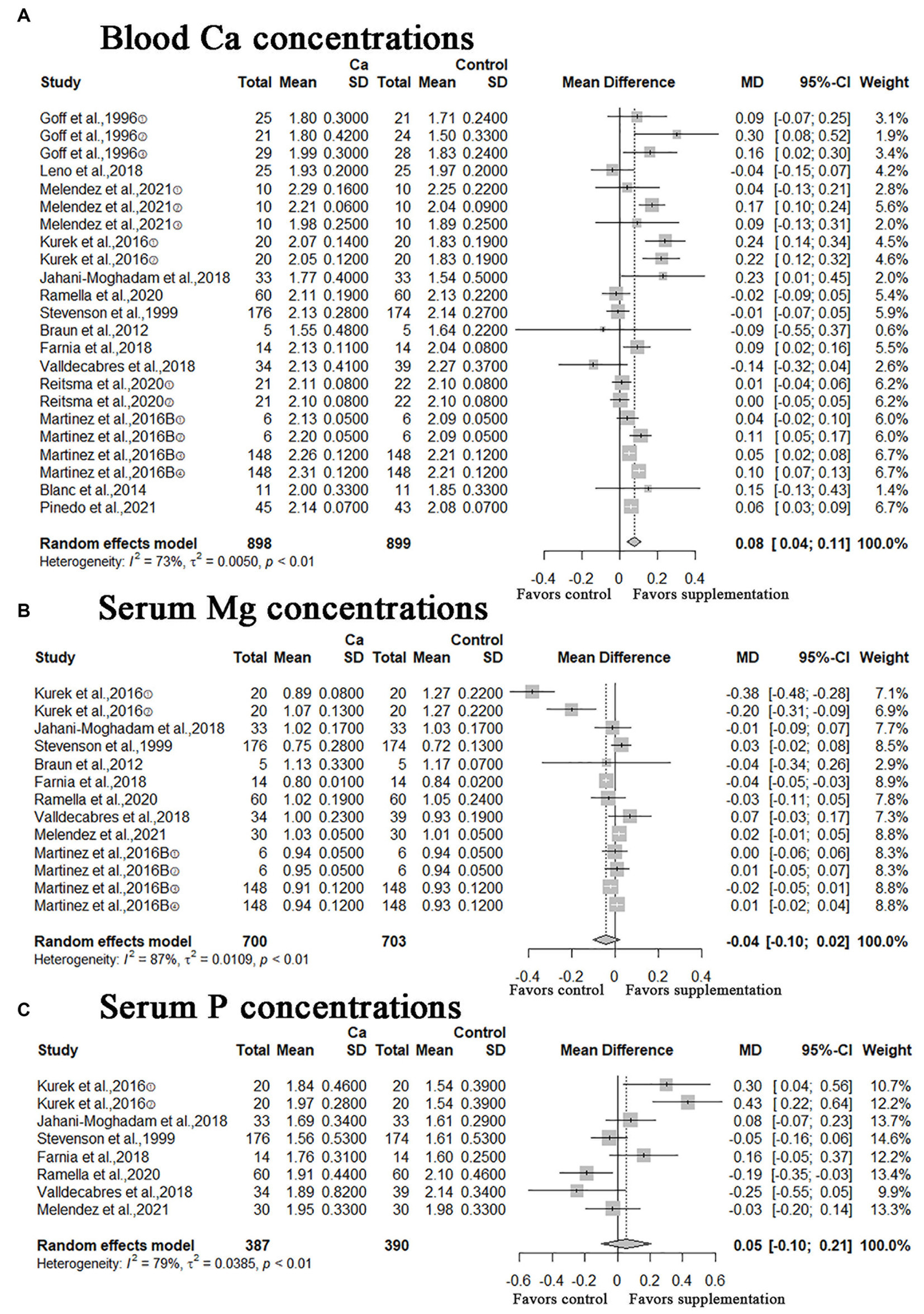
Figure 4. Meta-analysis of blood Ca concentrations, serum Mg and P concentrations. Ca Oral calcium supplements.
Incidence of calving-related clinical disorders
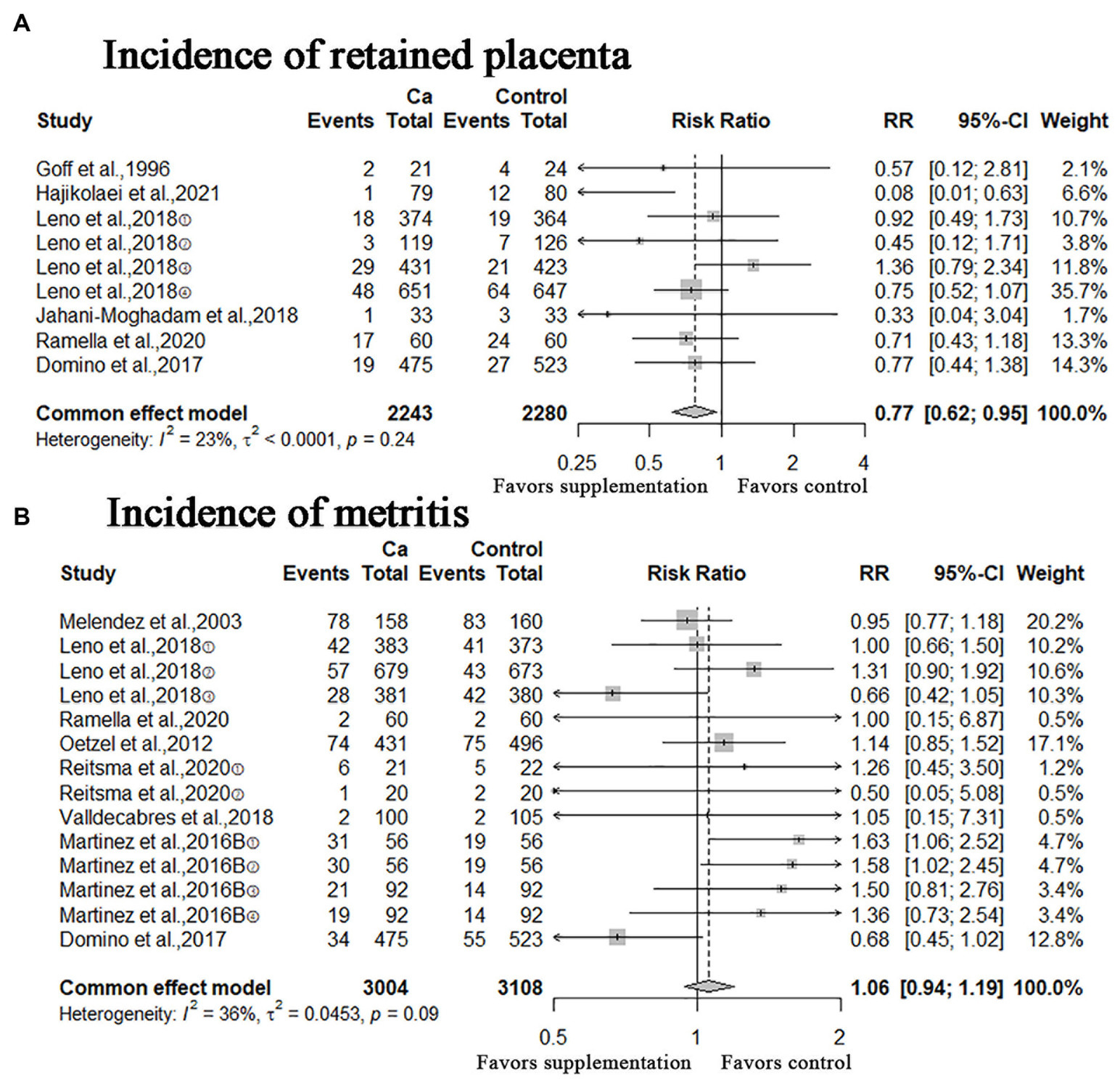
In this study, other calving-related clinical disorders including: retained placenta, metritis, ketosis, mastitis, displacement of the abomasum were included. The results of meta-analysis are shown in Figures 5, 6. A meta-analysis of the six studies showed that oral Ca significantly reduced the incidence of retained placenta, compared to the control (n = 4,523; RR = 0.77; 95% CI = [0.62, 0.95], p = 0.0151; heterogeneity I2 = 23%; Figure 5A), the difference between the two groups was statistically significant. The RR of incidence of metritis was 1.06 (n = 6,112; 95% CI = [0.94, 1.19]; p = 0.3567; heterogeneity I2 = 36%; Figure 5B); the RR of incidence of ketosis was 1.04 (n = 1,053, 95% CI = [0.91, 1.18]; p = 0.5933; heterogeneity I2 = 0%; Figure 6A); the RR of incidence of mastitis was 1.02 (n = 5,854; 95% CI = [0.86, 1.21]; p = 0.8436; heterogeneity I2 = 1%; Figure 6B); the RR of incidence of displacement of the abomasum was 0.81 (n = 3,357; RR = 0.81; 95% CI = [0.57, 1.16]; p > 0.2573; heterogeneity I2 = 28%; Figure 6C); however, these results are not statistically significant (incidence of metritis, ketosis, mastitis and displacement of the abomasum).
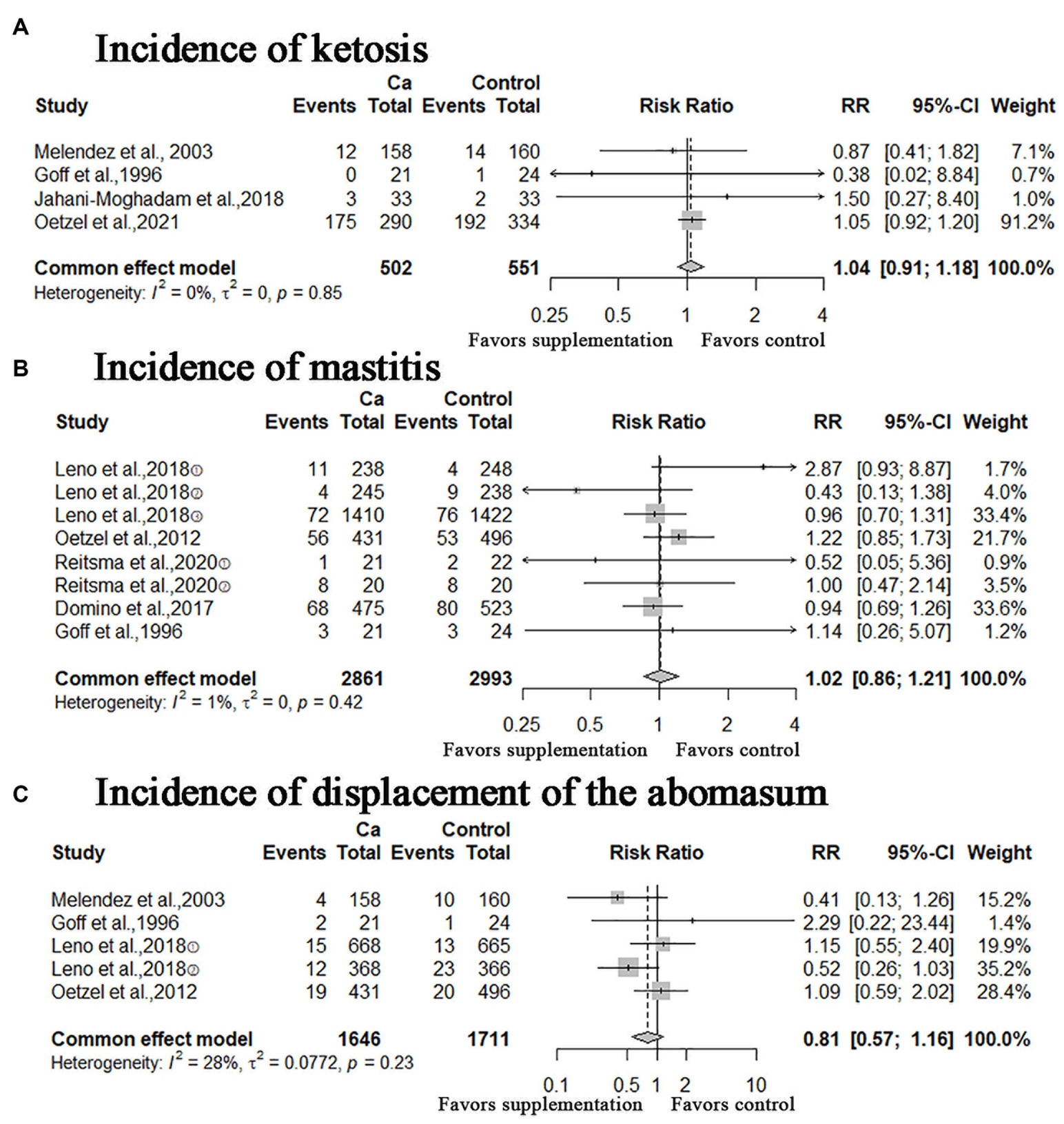
Figure 6. Meta-analysis of incidence of ketosis, mastitis, and displacement of the abomasum. Ca: Oral calcium supplements.
Pregnancy risks and milk yield
In addition, we performed a meta-analysis of the number of pregnancies and milk yield, the RR of pregnancy risk at first service was 0.99 (n = 7,481; 95% CI = [0.94, 1.05]; p = 0.8309; heterogeneity I2 = 42%; Figure 7A), the result was not statistically significant; the RR of overall pregnancy rate was 1.03 (n = 3,411; 95% CI = [0.98, 1.08]; p > 0.2030; heterogeneity I2 = 7%; Figure 7B), this result was not statistically significant; the MD of milk yield was 0.44 (95% CI = [−0.24, 1.13]; p = 0.2022; heterogeneity I2 = 99%; Figure 7C), the result was still not statistically significant.

Figure 7. Meta-analysis of pregnancy risk at first service, overall pregnancy rate and milk yield. Ca: Oral calcium supplements.
Publication bias and sensitivity analyses
Publication bias was analyzed using funnel plots and Egger’s test. The distribution of the funnel plot was symmetrical (Supplementary Figure S1) the p-values of incidence of subclinical hypocalcemia, Ca concentration in blood, incidence of metritis, and number of pregnant cows at first service in Egger’s test were 0.8667, 0.5329, 0.7050, and 0.8959 respectively, and the results suggested that there was no publication bias between oral Ca and the incidence of these diseases. Sensitivity analyses were performed for meta-analysis of the incidence of clinical hypocalcemia, subclinical hypocalcemia and number of pregnant cows at first service to assess the robustness of the results, and the results suggested that the results of meta-analysis are robust (Supplementary Figures S2–S6).
Discussion
Due to the increase in the cost of veterinary services and medicines and the decline in milk production, postpartum diseases of dairy cows have affected the profitability of farms (49–51). Farmers pay more and more attention to reducing the incidence of postpartum diseases in dairy cows. This meta-analysis aimed to evaluate the expected effect of oral Ca on postpartum hypocalcemia, calving-related diseases, fertility and milk yield in dairy cows. In this meta-analysis, the incidence of some calving-related diseases was significantly reduced after oral calcium administration, which include clinical hypocalcemia, subclinical hypocalcemia and retained placenta. The Ca concentrations of blood also significantly improved after Ca treatment. Clinical hypocalcemia, subclinical hypocalcemia and retained placenta are common postpartum reproductive disease. In addition, cows suffering from metabolic disorders in the early lactation period usually suffer from impaired fertility later, and may incur additional treatment costs due to delayed conception (51). Reducing the incidence of these diseases has important economic implications. Valldecabres et al. conducted a meta-analysis of the relationship between oral calcium supplements and milk production or first pregnancy rate, and found no evidence of the relationship between these outcome indicators and oral calcium (32). Our meta-analysis obtained similar results. In order to ensure the authenticity of the research evidence, we added the outcome indicators of other clinical diseases related to calving, which is more meaningful to clinical practice. However, these results were not statistically significant. Therefore, there is no evidence that oral calcium can reduce the incidence of metritis, ketosis, mastitis and abomasum displacement, or improve serum Mg and P concentrations and milk yield. According to our study selection criteria, the sources, forms or doses of oral Ca are not limited. Due to the limited number of clinical studies, it is impossible to find the relationship between various factors (Ca sources, doses and forms) and oral Ca by meta-regression, and it is also impossible to explain the influence of different calcium forms on the outcome index.
In addition, the long-term effects of oral calcium may be underestimated because cows that became sick immediately after calving (before the intervention) were removed from the herd, which may explain why some studies did not report the effect of oral calcium on the incidence of clinical hypocalcemia and its effects on blood Ca, Mg, and P concentrations. However, meta-analyses of serum Ca, Mg, P, the incidence of subclinical hypocalcemia, and milk yield were highly heterogeneous (> 50%) and remained high after sensitivity analyses. The results indicated that heterogeneity was clinical; hence we analyzed the possible sources of heterogeneity: (i) the cattle used in the included studies varied in breed, parity, and sample size. For example, some studies used Holstein cows, whereas others used crossbred cows. It is well known that the biological characteristics of dairy cows of different ages and parities (primiparous and multiparous cows) differ, inevitably affecting the experimental results; (ii) feed: prepartum and postpartum DCAD. In a meta-analysis, Santos et al., concluded that prepartum DCAD increased the Ca and P concentrations on the day of calving, as well as Ca concentrations after calving, and reduced the risk of perinatal clinical hypocalcemia, retained placenta, and metritis after calving (10, 24), therefore, diet differences could be a reason for the high heterogeneity observed in the meta-analysis results; (iii) different calcium forms, sources and brand may affect the absorption rate of animals, and thus the experimental effect; (iv) specific interventions (including interventions time, dose, and number of oral interventions) in the experimental and control groups were also not uniform; (v) the effect of confounders, such as inconsistent baselines between experimental and control groups. These differences directly affected the observed results.
The quality assessment of the results of the included studies is shown in Figure 2, the risk of bias in most animal experiments was mainly concentrated in the following aspects: (i) selection bias: cows were alternately assigned to treatment and control groups based on calving date or lactation order in four studies; (ii) performance biases: most studies did not report the random placement of cattle in the free-stall barn, and it is uncertain whether farm personnel and investigators were unaware of the interventions that each animal received during the experiment; (iii) detection biases: random selection of animals during the result evaluation process was not clearly stated; (iv) other sources of bias, inappropriate research influence by funders. Finally, the following factors should be considered in future research to avoid bias: (i) the study design should be more standardized, such as the grouping method of animals (random average grouping); (ii) the animals used in the experiments should have consistent baseline characteristics, including breed and parity; (iii) the details of the experimental method should also be consistent, including the Ca form, Ca sources, the intervention time, dose, and oral frequency; (iv) blind method is applicable to researchers, farm workers, data collectors and statistical analysts to ensure the authenticity and reliability of the results; (iv) research reports should be more detailed and provide complete data reports.
Conclusion
This study used meta-analysis to evaluate the efficacy of oral Ca in preventing hypocalcemia or calving-related diseases, and improving pregnancy risk or milk yield in dairy cows. All analyses showed that compared with the control treatments, oral Ca administration significantly reduced the incidence of postpartum hypocalcemia (include clinical and subclinical hypocalcemia) and retained placenta, and increased blood Ca concentrations of dairy cows. However, there is no evidence that oral Ca can reduce the incidence of postpartum metritis, ketosis, mastitis and abomasum displacement, or improve serum Mg/P concentrations, pregnancy risk and milk yield. The quality evaluation of the included studies shows that the risk of bias in most animal experiments mainly center on the selection bias, performance bias, detection bias and other sources of bias. We suggest that future research should focus on these aspects and follow standardized research designs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZR-M: Conceptualization, Data curation, Methodology, Writing – original draft. L-LM: Data curation, Formal analysis, Writing – original draft. FZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YB: Writing – review & editing, Data curation, Methodology, Supervision, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation (NSFC) of China (81860716) and the Gansu Province Natural Science Fund Project (22JR11RA237).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1357640/full#supplementary-material
SUPPLEMENTARY Figure S1 | Funnel plot of incidence of subclinical hypocalcemia, Ca concentration in plasma/serum, incidence of metritis, and number of pregnant cows at first service.
SUPPLEMENTARY Figure S2 | Sensitivity analyses of incidence of clinical hypocalcemia, incidence of subclinical hypocalcemia.
SUPPLEMENTARY Figure S3 | Sensitivity analyses of blood Ca concentrations, serum Mg and P concentrations.
SUPPLEMENTARY Figure S4 | Sensitivity analyses of incidence of retained placenta, metritis.
SUPPLEMENTARY Figure S5 | Sensitivity analyses of incidence of ketosis, mastitis, and displacement of the abomasum.
SUPPLEMENTARY Figure S6 | Sensitivity analyses of pregnancy risk at first service, overall pregnancy rate and milk yield.
Footnotes
References
1. Roberts, K, Bennison, J, and Mcdougall, S. Effect of treatment with oral ca boluses following calving on concentrations of ca in serum in pasture-based dairy cows. N Z Vet J. (2018) 67:20–6. doi: 10.1080/00480169.2018.1520654
2. Roche, JR. Hypocalcaemia and DCAD for the pasture-based transition cow - a review. Acta Vet Scand Suppl. (2003) 97:65–74. doi: 10.1186/1751-0147-44-203
3. Martín-Tereso Javier Martens, H. Calcium and magnesium physiology and nutrition in relation to the prevention of Milk fever and tetany (dietary Management of Macrominerals in preventing disease). Vet Clin N Am Food Anim Pract. (2014) 30:643–70. doi: 10.1016/j.cvfa.2014.07.007
4. Goff, JP, Hohman, A, and Timms, LL. Effect of subclinical and clinical hypocalcemia and dietary cation-anion difference on rumination activity in periparturient dairy cows. J Dairy Sci. (2020) 103:2591–601. doi: 10.3168/jds.2019-17581
5. McArt, JAA, and Neves, RC. Association of transient, persistent, or delayed subclinical hypocalcemia with early lactation disease, removal, and milk yield in Holstein cows. J Dairy Sci. (2020) 103:690–701. doi: 10.3168/jds.2019-17191
6. Degaris, PJ, and Lean, IJ. Milk fever in dairy cows: a review of pathophysiology and control principles. Vet J. (2008) 176:58–69. doi: 10.1016/j.tvjl.2007.12.029
7. Venjakob, PL, Pieper, L, Heuwieser, W, and Borchardt, S. Ssociation of postpartum hypocalcemia with early-lactation milk yield, reproductive performance, and culling in dairy cows. J Dairy Sci. (2018) 101:9396–405. doi: 10.3168/jds.2017-14202
8. Serrenho, RC, DeVries, TJ, Duffield, TF, and LeBlanc, SJ. Graduate student literature review: what do we know about the effects of clinical and subclinical hypocalcemia on health and performance of dairy cows? J Dairy Sci. (2021) 104:6304–26. doi: 10.3168/jds.2020-19371
9. Rodney, RM, Martinez, N, Block, E, Hernandez, LL, Celi, P, Nelson, CD, et al. Effects of prepartum dietary cation-anion difference and source of vitamin D in dairy cows: vitamin D, mineral, and bone metabolism. J Dairy Sci. (2018) 101:2519–43. doi: 10.3168/jds.2017-13737
10. Santos, JEP, Lean, IJ, Golder, H, and Block, E. Meta-analysis of the effects of prepartum dietary cation-anion difference on performance and health of dairy cows. J Dairy Sci. (2019) 102:2134–54. doi: 10.3168/jds.2018-14628
11. Cohrs, I, Wilkens, MR, and Grünberg, W. Short communication: effect of dietary phosphorus deprivation in late gestation and early lactation on the calcium homeostasis of periparturient dairy cows. J Dairy Sci. (2018) 101:9591–8. doi: 10.3168/jds.2018-14642
12. Wächter, S, Cohrs, I, Golbeck, L, Scheu, T, Eder, K, and Grünberg, W. Effects of restricted dietary phosphorus supply during the dry period on productivity and metabolism in dairy cows. J Dairy Sci. (2022) 105:4370–92. doi: 10.3168/jds.2021-21246
13. Melendez, P, and Poock, S. A dairy herd case investigation with very low dietary cation-anion difference in prepartum dairy cows. Front Nutrition. (2017) 4:26. doi: 10.3389/fnut.2017.00026
14. Goff, JP. Calcium and magnesium disorders. Vet Clin North Am Food Anim Pract. (2014) 30:359–81. doi: 10.1016/j.cvfa.2014.04.003
15. Chapinal, N, LeBlanc, SJ, Carson, ME, Leslie, KE, Godden, S, Capel, M, et al. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J Dairy Sci. (2012) 95:5676–82. doi: 10.3168/jds.2011-5132
16. Martinez, N, Sinedino, L, Bisinotto, RS, Ribeiro, ES, Gomes, GC, and Lima, FS. Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. J Dairy Sci. (2014) 97:874–87. doi: 10.3168/jds.2013-7408
17. Ducusin, R, Uzuka, Y, Satoh, E, Otani, M, Nishimura, M, and Tanabe, S. Effects of extracellular Ca2+ on phagocytosis and intracellular Ca2+ concentrations in polymorphonuclear leukocytes of postpartum dairy cows. Res Vet Sci. (2003) 75:27–32. doi: 10.1016/s0034-5288(03)00038-9
18. Borsberry, S, and Dobson, H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet Rec. (1989) 124:217–9. doi: 10.1136/vr.124.9.217
19. Rodríguez, EM, Arís, A, and Bach, A. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J Dairy Sci. (2017) 100:7427–34. doi: 10.3168/jds.2016-12210
20. Zurr, L, and Leonhard-Marek, S. Effects of β-hydroxybutyrate and different calcium and potassium concentrations on the membrane potential and motility of abomasal smooth muscle cells in cattle. J Dairy Sci. (2012) 95:5750–9. doi: 10.3168/jds.2012-5479
21. Goff, JP, and Koszewski, NJ. Comparison of 0.46% calcium diets with and without added anions with a 0.7% calcium anionic diet as a means to reduce periparturient hypocalcemia. J Dairy Sci. (2018) 101:5033–45. doi: 10.3168/jds.2017-13832
22. Dhiman, TR, and Sasidharan, V. Effectiveness of calcium chloride in increasing blood calcium concentrations of periparturient dairy cows. J Anim Sci. (1999) 77:1597–605. doi: 10.2527/1999.7761597x
23. Valldecabres, A, Pires, J, and Silva-Del-Río, N. Effect of prophylactic oral calcium supplementation on postpartum mineral status and markers of energy balance of multiparous Jersey cows. J Dairy Sci. (2018) 101:4460–72. doi: 10.3168/jds.2017-12917
24. Melendez, P, Donovana, GA, Riscoa, CA, Littellb, R, and Goff, JP. Effect of calcium-energy supplements on calving-related disorders, fertility and milk yield during the transition period in cows fed anionic diets. Theriogenology. (2003) 60:843–54. doi: 10.1016/s0093-691x(03)00103-1
25. Goff, JP, Horst, RL, Jardon, PW, Borelli, C, and Wedam, J. Field trials of an oral calcium propionate paste as an aid to prevent milk fever in periparturient dairy cows. J Dairy Sci. (1996) 79:378–83. doi: 10.3168/jds.S0022-0302(96)76375-0
26. Valldecabres, A, and Silva-Del-Río, N. Effects of postpartum oral calcium supplementation on milk yield, milk composition, and reproduction in multiparous Jersey and Jersey × Holstein crossbreed cows. J Dairy Sci. (2021) 104:795–805. doi: 10.3168/jds.2020-19079
27. Kurek, L., Lutnicki, K., Olech, M., Brodzki, P., and Golynski, M. J Elem. (2016) 21, 77–87. doi: 10.5601/jelem.2015.20.2.870
28. Hajikolaei, MRH, Nouri, M, Amirabadi, SH, Shariari, A, and Constable, PD. Effect of antepartum vitamin D3 (cholecalciferol) and postpartum oral calcium administration on serum total calcium concentration in Holstein cows fed an acidogenic diet in late gestation. Res Vet Sci. (2021) 136:239–46. doi: 10.1016/j.rvsc.2021.02.017
29. Leno, BM, Neves, RC, Louge, IM, Curler, MD, Thomas, MJ, Overton, TR, et al. Differential effects of a single dose of oral calcium based on postpartum plasma calcium concentration in Holstein cows. J Dairy Sci. (2018) 101:3285–302. doi: 10.3168/jds.2017-13164
30. Jahani-Moghadam, M, Chashnidel, Y, Teimouri-Yansari, A, Mahjoubi, E, and Dirandeh, E. Effect of oral calcium bolus administration on milk production, concentrations of minerals and metabolites in serum, early-lactation health status, and reproductive performance of Holstein dairy cows. N Z Vet J. (2018) 66:132–7. doi: 10.1080/00480169.2018.1432427
31. Melendez, P, Roeschmann, C, Arevalo, A, and Moller, J. The effect of oral calcium boluses at parturition on blood metabolites and milk yield in grazing Holstein cattle. Livest Sci. (2021) 248:104510. doi: 10.1016/j.livsci.2021.104510
32. Valldecabres, A, Branco-Lopes, R, Bernal-Córdoba, C, and Silva-Del-Río, N. Production and reproduction responses for dairy cattle supplemented with oral calcium bolus after calving: systematic review and meta-analysis. JDS Commun. (2022) 4:9–13. doi: 10.3168/jdsc.2022-0235
33. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. British Medical J. (2009) 339:b2700. doi: 10.1136/bmj.b2700
34. Hooijmans, CR, Rovers, MM, de Vries, RB, Leenaars, M, Ritskes-Hoitinga, M, and Langendam, MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
35. Ramella, KDCL, Santos, LGC, Patelli, THC, Flaiban, KKMC, and Lisboa, JAN. Effects of postpartum treatment with oral calcium formate on serum calcium, serum metabolites, and the occurrence of diseases at the beginning of lactation of high-producing dairy cows. Preventive Vet Med. (2020) 185:105180. doi: 10.1016/j.prevetmed.2020.105180
36. Stevenson, MA, Williamson, NB, and Hanlon, DW. The effects of calcium supplementation of dairy cattle after calving on milk, milk fat and protein production, and fertility. N Z Vet J. (1999) 47:53–60. doi: 10.1080/00480169.1999.36111
37. Braun, U, Blatter, M, and Hässig, M. Untersuchungen zur Wirkung von Kalziumlaktatbei Kühen postpartum. Schweiz Arch Tierheilkd. (2012) 154:233–8. doi: 10.1024/0036-7281/a000338
38. Oetzel, GR, and Miller, BE. Effect of oral calcium bolus supplementation on early-lactation health and milk yield in commercial dairy herds. J Dairy Sci. (2012) 95:7051–65. doi: 10.3168/jds.2012-5510
39. Reitsma, LM, Batchelder, TA, Davis, EM, Machado, VS, and Ballou, MA. Effects of oral calcium bolus supplementation on intracellular polymorphonuclear leukocyte calcium levels and functionality in primiparous and multiparous dairy cows. J Dairy Sci. (2020) 103:11876–88. doi: 10.3168/jds.2020-18835
40. Farnia, SA, Rasoolia, A, Nouria, M, Shahryaric, A, Khosravi Bakhtiaryd, M, and Constable, PD. Effect of postparturient oral calcium administration on serum total calcium concentration in Holstein cows fed diets of different dietary cation-anion difference in late gestation. Res Vet Sci. (2018) 117:118–24. doi: 10.1016/j.rvsc.2017.11.017
41. Martinez, N, Sinedino, LD, Bisinotto, RS, Daetz, R, Risco, CA, Galvão, KN, et al. Effects of oral calcium supplementation on productive and reproductive performance in Holstein cows. J Dairy Sci. (2016) 99:8417–30. doi: 10.3168/jds.2015-10529
42. Martinez, N, Sinedino, LD, Bisinotto, RS, Daetz, R, Lopera, C, Risco, CA, et al. Effects of oral calcium supplementation on mineral and acid-base status, energy metabolites, and health of postpartum dairy cows. J Dairy Sci. (2016) 99:8397–416. doi: 10.3168/jds.2015-10527
43. Domino, AR, Korzec, HC, and McArt, JAA. Field trial of 2 calcium supplements on early lactation health and production in multiparous Holstein cows. J Dairy Sci. (2017) 100:9681–90. doi: 10.3168/jds.2017-12885
44. Blanc, CD, Van, M, der List Aly, SS, Rossow, HA, and Silva-del-Río, N. Blood calcium dynamics after prophylactic treatment of subclinical hypocalcemia with oral or intravenous calcium. J Dairy Sci. (2014) 97:6901–6. doi: 10.3168/jds.2014-7927
45. Jahani-Moghadam, M, Teimouri-Yansari, A, Chashnidel, Y, Dirandeh, E, and Mahjoubi, E. Short- and long-term effects of postpartum oral bolus v. subcutaneous ca supplements on blood metabolites and productivity of Holstein cows fed a prepartum anionic diet. Animal. (2020) 14:983–90. doi: 10.1017/S175173111900257X
46. Pinedo, P, Manríquez, D, Marota, N, Mongiello, G, and Velez, J. Effect of oral calcium administration on metabolic status and uterine health of dairy cows with reduced postpartum rumination and eating time. BMC Vet Res. (2021) 17:178. doi: 10.1186/s12917-021-02881-2
47. Mahjoubi, E, Mousaviara, SA, Hossein Yazdi, M, Hosseinzadehakandi, M, and McArt, JAA. A randomized field trial assessing the timing of postpartum calcium bolus administration on milk yield of multiparous Holstein cows. J Dairy Sci. (2023) 106:7320–8. doi: 10.3168/jds.2022-22671
48. Beynen, ACGK, and van Zutphen, LFM. Standardization of the animal and its environment. In principles of laboratory animal science. Revised Edition. Eds. van Zutphen LFMB V, Beynen AC. Amsterdam and New York: Elsevier BV (2001).
49. Hazel, AR, Heins, BJ, and Hansen, LB. Health treatment cost, stillbirth, survival, and conformation of Viking red-, Montbéliarde-, and Holstein-sired crossbred cows compared with pure Holstein cows during their first 3 lactations. J Dairy Sci. (2020) 103:10917–39. doi: 10.3168/jds.2020-18604
50. Hardie, LC, Heins, BJ, and Dechow, CD. Genetic parameters for Stayability of Holsteins in US organic herds. J Dairy Sci. (2021) 104:4507–15. doi: 10.3168/jds.2020-19399
Keywords: oral calcium, cattle, clinical hypocalcemia, meta-analysis, quality assessment
Citation: Ma Z-R, Ma L-L, Zhao F and Bo Y (2024) Effects of oral calcium on reproduction and postpartum health in cattle: a meta-analysis and quality assessment. Front. Vet. Sci. 11:1357640. doi: 10.3389/fvets.2024.1357640
Edited by:
Micaela Sgorbini, University of Pisa, ItalyCopyright © 2024 Ma, Ma, Zhao and Bo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Zhao, cnVuZm9yNzEwQDE2My5jb20=; Yan Bo, dGhvc2Vmb3JteWxvdmVkcGVvcGxlQG91dGxvb2suY29t
 Zheng-Ren Ma1
Zheng-Ren Ma1 Fei Zhao
Fei Zhao