- 1College of Animal Science and Technology, Heilongjiang Bayi Agricultural University, Daqing, Heilongjiang, China
- 2Heilongjiang Key Laboratory of Efficient Utilization of Feed Resources and Nutrition Manipulation in Cold Region, Heilongjiang Bayi Agricultural University, Daqing, Heilongjiang, China
Objective: Orchitis is a common reproductive disease of male animals, which has serious implications to human and animal reproduction. Additionally, phlorizin (PHN), a common polyphenol in apples and strawberries, has a variety of biological activities, including antioxidant, anti-inflammatory, anti-diabetic, and anti-aging activities. We aimed to determine the protective effects and potential mechanisms of PHN in lipopolysaccharide (LPS)-induced acute orchitis in mice.
Method: After 21 days of PHN pretreatment, mice were injected with LPS to induce testicular inflammation, and then the changes of testicular tissue structure, expression of inflammatory factors, testosterone level, expression of testosterone-related genes, adhesion gene and protein expression were detected, and the structural changes in the intestinal flora after PHN treatment were further detected by 16SRNA.
Result: Our results demonstrated that PHN treatment reduced LPS-induced testicular injury and body and testicular weight losses. The mRNA expression levels of pro-inflammatory cytokines-related genes and antioxidant enzyme activity were also decreased and elevated, respectively, by PHN administration; however, PHN treatment also reduced the LPS-induced decrease in testosterone levels in the testes. Additionally, further studies found that PHN increased the expression of marker proteins zonula occludens-1 (ZO-1) and occludin associated with the blood testosterone barrier compared with that in LPS treatment groups. To further examine the potential mechanisms of the protective effect of PHN on LPS-induced testicular injury, we compared the differences of gut microbiota compositions between the 100 mg/kg PHN treatment group and the control group using 16SRNA. Metagenomic analyses indicated that the abundances of Bacteroidetes, Muribaculaceae, Lactobacillaceae, uncultured bacterium f Muribaculaceae, and Lactobacillus in the PHN treatment group improved, while potential microbes that can induce intestinal diseases, including Verrucomicrobia, Epsilonbacteraeota, Akkermansiaceae, and Akkermansia decreased in the PHN treatment group.
Conclusion: Our results indicate that PHN pretreatment might alleviate orchitis by altering the composition of gut microflora, which may provide a reference for reducing the occurrence of acute orchitis in male animals.
1 Introduction
Male animals reproductive tract inflammation and infections are closely related to infertility, among which orchitis is an important reproductive disease in male animals. Orchitis can reduce spermatogenesis and sperm quality, thereby causing serious damage to human and animal reproduction and serious economic damage to animal breeding (1, 2). Many factors contribute to orchitis in both humans and animals, including bacterial and viral infections and several diseases, including autoimmune diseases (3), cryptorchidism (4), and obesity (5); therefore, developing new methods for orchitis prevention and treatment is important.
Spermatogenic cells at different stages of development, Sertoli cells, and Leydig cells play the most important roles in testicular function. Leydig cells are in the interstitial compartments of the testes and are responsible for testosterone production, which is crucial for the normal development of male sex organs, spermatogenesis, and sperm maturation (6). Additionally, the blood-testis barrier (BTB) is important for normal spermatogenesis. The BTB in the testes, which consists of adjacent Sertoli cells near the basal membrane of the seminiferous tubules, peritubular tissue encircling seminiferous tubules, and interstitial capillary endothelium are crucial to maintaining the microenvironment necessary for testicular function (7). It can isolate spermatogenic cells at all developmental stages from the circulatory system, thereby preventing the diffusion of various endogenous and exogenous toxic chemicals in mammals (8, 9). The main structural component of the BTB is the Sertoli intercellular junction complex, which is formed by the coexistence of several proteins, including gap junctions (GJ), tight junctions (TJ), and basic ectoplasmic specializations (ES) (10). Years of research has shown that the pathological pathway of testicular inflammation predominantly includes inflammatory cytokine imbalance (11), testosterone synthesis disruption (12), oxidative stress (11), and BTB disruption (13), which leads to the apoptosis of spermatocytes and spermatids.
Phlorizin (PHN; Figure 1A) is a glucoside of phloetin, chemically named 1-[2-(beta.D-glucopyranosyloxy)-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)-1-propanone, that belongs to the dihydrochalcone family of flavonoids (14, 15). It is found predominantly in the root bark, stem, young leaves, and fruits of apple trees (16, 17). PHN has many important biological activities, including antioxidant activity (18), blood sugar regulation (19), memory improvement (20), and anti-allergy (21) and anti-cancer activities (22) and has potential application value in the health product industry (23). Additionally, studies on obese mice induced by high-fat diets showed that dietary PHN can ameliorate the redox state, and its main mechanism is closely related to gut microbiota variations (24). PHN can also reduce blood lipopolysaccharide (LPS) level and increase insulin sensitivity in obese mice and mice with type 2 diabetes by regulating gut microbiota variations (25); however, orchitis can be induced by bacterial LPS, which can result in failure of spermatogenesis and damage to the BTB (26, 27). Therefore, we used an LPS-induced acute orchitis mouse model to study whether PHN can prevent acute orchitis and to explore its potential mechanisms.
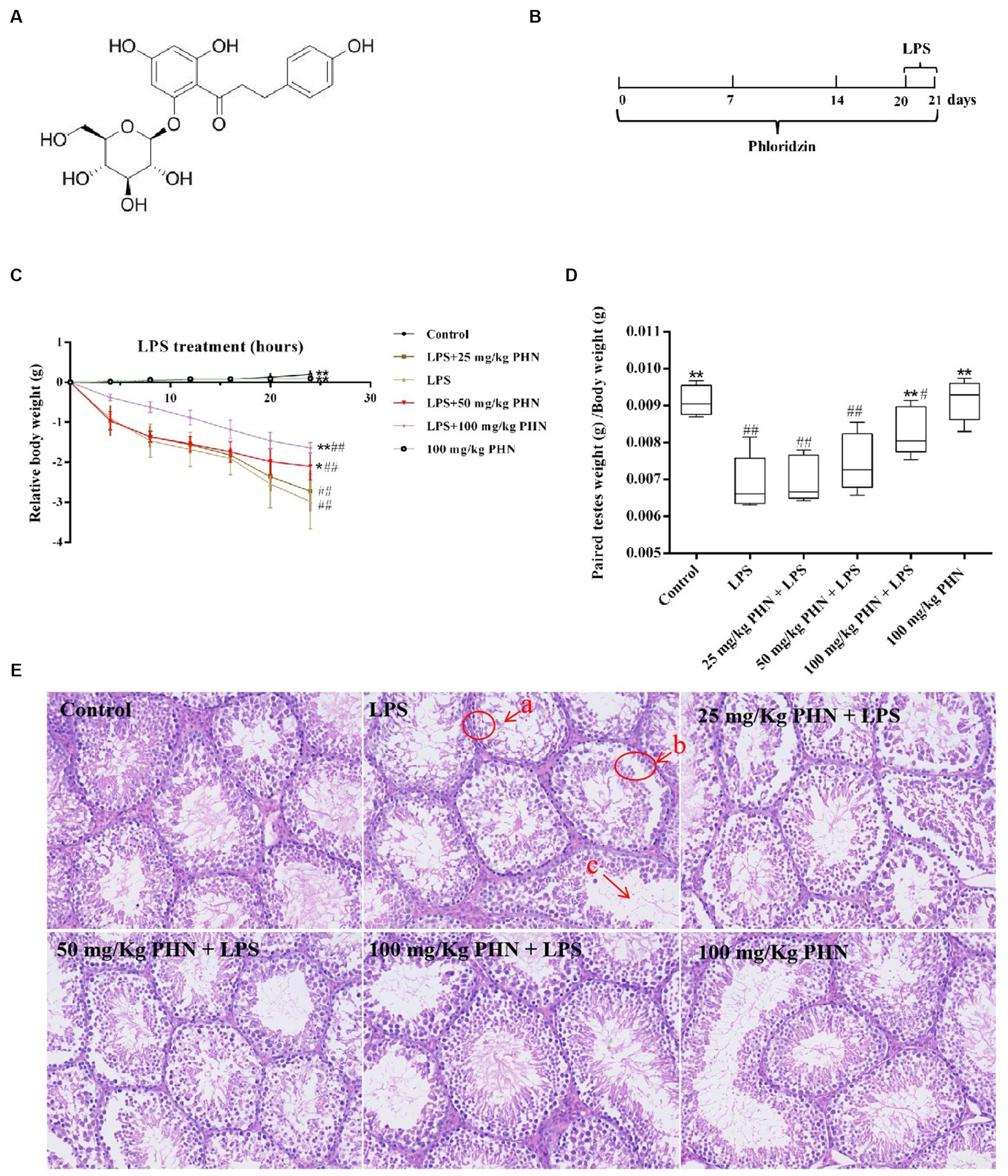
Figure 1. PHN decreased LPS-induced orchitis. (A) PHN chemical structure. (B) The experimental treatment protocol with PHN or LPS. (C) Relative changes in body weight. (D) Paired testes weight (g) /Body weight (g). (E) H&E staining, 200×. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the control group.
2 Methods
2.1 Chemicals
PHN (>98% HPLC) was purchased from Chengdu Preferred Biotechnology Co., Ltd. (Chengdu, China). Glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals used in this study were of analytical reagent grade. Unless otherwise stated, all chemicals used in this study were purchased from Sigma Chemical (St Louis, MO, United States).
2.2 Animals
Male C57BL/6 (n = 72) mice with similar body weight (21–23 g) were purchased from the Laboratory Animal Department of the Harbin Medical University (Harbin, China) and housed at 24 ± 1°C and received food and water ad libitum. The mice were housed in a clean environment to strict ensure animal welfare.
2.3 Testitis induction and evaluations
In this study, LPS (5 mg/kg) was intraperitoneally injected to induce acute orchitis in mice (11). Mice were randomly divided into six equal groups, that is, the control, LPS, PHN (25, 50, and 100 mg/kg) with LPS, and PHN (100 mg/kg) groups. Phloridzin was dissolved in distilled water and orally administered to animals at dosages of 25, 50 and 100 mg/kg body weight daily throughout the experimental period. The experimental timelines for the animal models are shown in Figure 1B. In the PHN (25, 50, and 100 mg/kg) + LPS groups, mice were intragastrically administered their respective PHN dose 21 d before LPS treatment daily. Mouse orchitis was induced by intraperitoneal LPS injection at 5 mg/kg (PHN weight/mouse body weight) for 24 h (from day 20 to 21). Twenty-four hours after injection of LPS, mice were euthanized and testes were collected to detect tissue structure, expression of factors associated with inflammation and testosterone synthesis, and integrity of the blood-testosterone barrier. In addition, blood is collected and then centrifuged to obtain serum, which is assayed for testosterone content. Moreover, mice in the PHN groups were continuously treated with their respective PHN doses during LPS treatment.
2.4 Oxidative stress and myeloperoxidase assay
Glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), and myeloperoxidase (MPO) activity in the teste tissues from different groups was examined using their corresponding kits (Nanjing Jiancheng Bioengineering Institute) in accordance with the manufacturer protocols. The enzymatic activity was measured by a microplate reader (Bio-Rad, United States of America) according to the respective absorbance.
2.5 Testosterone assay by ELISA tests
ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to measure testosterone levels in the serum samples. Approximately 500 μL of blood samples were obtained from each mouse, and serum samples were collected by centrifugation at 1000 g for 12 min at 4°C. Serum testosterone levels were measured using kits according to the manufacturer protocols by a microplate reader (Bio-Rad, United States of America).
2.6 Quantitative real-time polymerase chain reaction
Total RNA was isolated from teste samples from different treatment groups using TRIzol reagent (Invitrogen), according to the manufacturer instructions. The RNA concentration was measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific), and complementary DNA (cDNA) was synthesized using a SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, United States). Real-time fluorescence quantitative PCR was performed using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, United States) and SYBR Green Plus reagent kit (TransGen Biotech, AQ141, Beijing, China). Primer sequences used in this study are listed in Table 1 with β-Actin was used as the reference gene. Quantitative RT-PCR was conducted thrice and normalized to the expression of the reference gene (i.e., β-actin). The relative gene expression levels were calculated using the 2-ΔΔCT method.
2.7 Hematoxylin–eosin staining
Teste tissues obtained from different treatment groups and were fixed in 4% (v/v) paraformaldehyde for 24 h, embedded in paraffin, and cut into 5 μm sagittal sections. The sections were de-paraffinized with xylene and ethanol, washed with phosphate-buffered saline (PBS), and permeabilized with 0.1 M citrate and 0.1% Triton X-100 permeabilization solution. The deparaffinized sections were stained with hematoxylin and eosin (H&E), and images were captured using an OLYMPUS BX53 microscope to examine pathological structural changes in the testes.
2.8 Immunofluorescence
Immunofluorescence staining was performed on paraffin-embedded sections of testicular tissue. Tissue slices were deparaffinized, rehydrated, and washed with 1% PBS-Tween. Sections were deparaffinized in xylene for 24 h and rehydrated. After washing with 1% PBS-Tween, the sections were treated with 3% hydrogen peroxide, permeabilized with 0.3% Triton X-100, and blocked with 3% BSA. Next, the sections were incubated for 1 h at 37°C with primary antibodies directed against Zona occludens 1 (ZO-1) or occludin (1:200; Beijing Biosynthesis Biotechnology Co., LTD, Beijing, China). After washing with PBS three times, the slides were incubated with species-specific fluorescent secondary antibodies (1:200; Beijing Biosynthesis Biotechnology Co., LTD, Beijing, China) for 1 h at 23°C and stained with Hoechst 33342. Finally, the cover slips were mounted, and images were captured using a light microscope (Olympus, Tokyo, Japan).
2.9 Gut microbiota analysis
Before the experiment began, each mouse was marked for subsequent experiments, and fresh fecal pellets were obtained after 20 d. Total DNA was extracted from the samples, primers were designed and synthesized in accordance with the conserved block, and the ends of the primers were connected with sequencing connectors to conduct PCR amplification. A sequencing library was established using product purification, quantification, and homogenization. The original data from high-throughput sequencing were analyzed and converted into sequence readings through base calling. The same operational taxonomic unit (OTU) was defined as a sequence greater than or equal to 97% for bacterial classification.
2.10 Statistical analysis
Statistical analysis of experimental data was performed using SPSS 17.0. The values are presented as mean ± standard deviation (SD), and multiple comparisons were analyzed using one-way ANOVA followed by Tukey’s multiple-comparison test. The differences between the two groups of data were assessed using an unpaired two-tailed Student t-test. Significant differences are represented by p < 0.05 and p < 0.01.
3 Results
3.1 Phlorizin alleviated LPS-induced orchitis
Excessive LPS in the body can cause systemic sepsis in animals, which can cause serious weight loss over a short period; therefore, we measured body weight variations of the different treatment groups every 4 h after LPS injection, and our results showed a significant decrease in the LPS treatment group compared to that in the control group; however, those treated with PHN (50 and 100 mg/kg) significantly decreased the LPS-induced weight loss (Figure 1C). Additionally, testicular weight loss is also an important marker of orchitis; therefore, we examined the relative changes in testicular and body weights. The results showed that LPS treatment significantly reduced testicular weight compared with the control group, while 100 mg/kg PHN treatment significantly restored this change (Figure 1D). Moreover, from histopathological observations, we found that the number of spermatogenic cells were decreased (Figures 1E-a), most spermatogenic cells were exfoliated (Figures 1E-b), and the mature sperm were rare (Figures 1E-c) in the LPS treatment group; however, PHN treatment partially reversed these changes in a dose-dependent manner.
3.2 Inhibition of MPO activity
Myeloperoxidase is abundant in neutrophils and is closely related to inflammation. Here, we examined the effect of PHN pretreatment on LPS-induced MPO activity in the testes using ELISA. As shown in Figure 2, LPS treatment increased a nearly three-fold MPO activity in the testes compared with the control group; however, compared with the LPS group, different concentrations of PHN significantly reduced MPO activity in a dose-dependent manner (Figure 2).
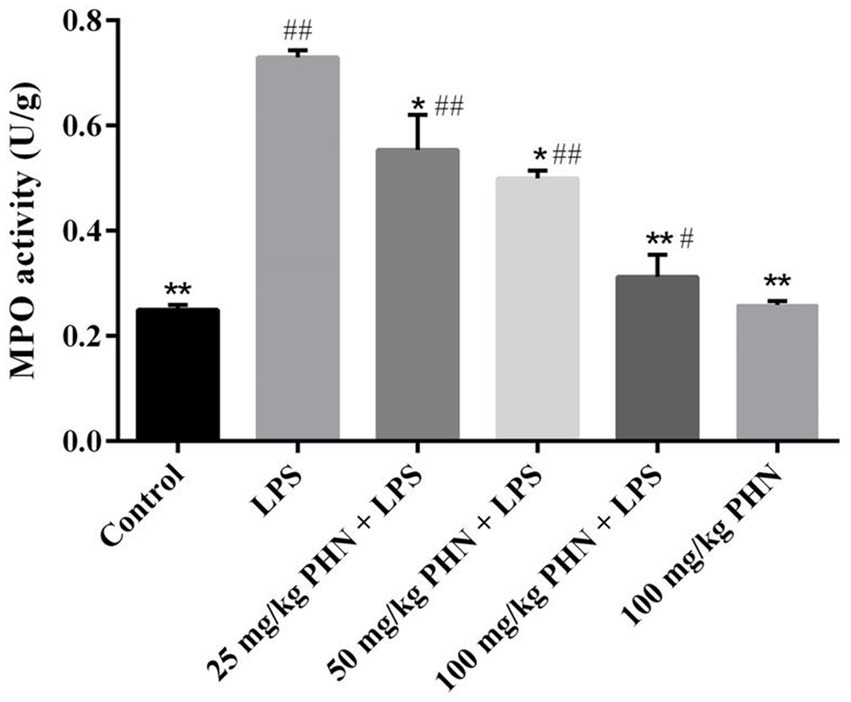
Figure 2. Decrease activity of MPO. Samples were collected from the testes homogenate of the experimental mice. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the control group.
3.3 Inhibition of pro-inflammatory cytokines
Changes in pro-inflammatory cytokines damaged the Leydig cells and BTB, thereby resulting in decreased testosterone synthesis and spermatogenesis. Here, we examined the effect of PHN pre-treatment on LPS-induced inflammatory cytokine production in the testes. Our results indicated that LPS treatment significantly increased the mRNA expressions of Tumor necrosis factor α (TNF-α), Interleukin-17A (IL-17A),Interleukin-1β (IL-1β), and Interleukin-2 (IL-2) compared with those in the control (Figures 3A–D); however, different PHN concentrations significantly reduced the mRNA expressions of TNF-α, IL-17A, and IL-1β compared with those in the LPS treatment group (Figures 3A–C). Additionally, 25 mg/kg and 50 mg/kg PHN did not affect the mRNA expression of IL-2, while 100 mg/kg PHN significantly reduced the mRNA expression of IL-2 compared with that in the LPS treatment group (Figure 3D).
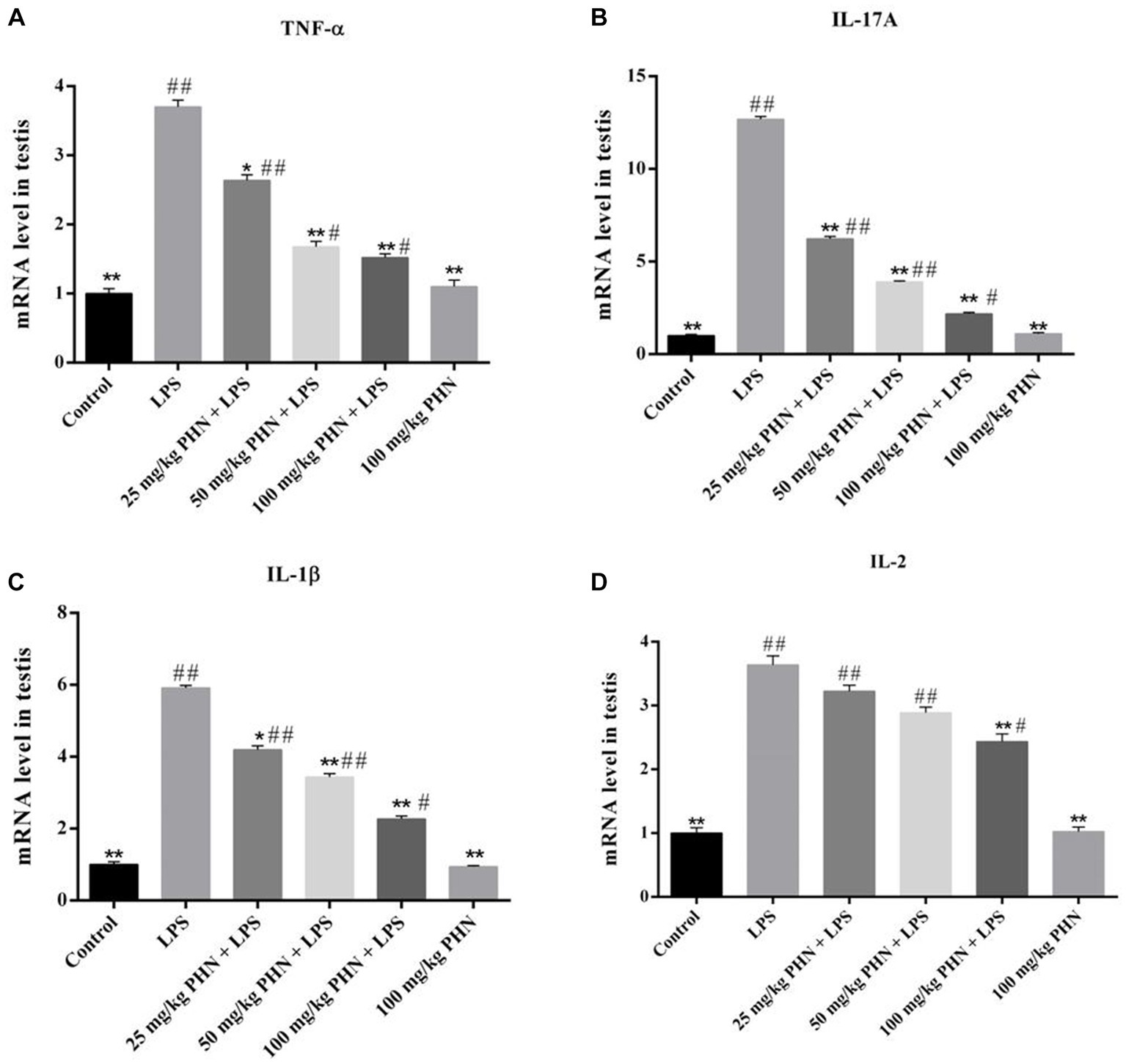
Figure 3. Reduction of pro-inflammatory cytokines. The mRNA expression levels of TNF-α (A), IL-17A (B), IL-1β (C) and IL-2 (D) in testes tissues were detected by qRT-PCR. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the control group.
3.4 Improvement of oxidative stress
To test whether PHN treatment has an antioxidant function in LPS-induced orchitis, we tested three oxidative stress markers. Our results showed that the expression levels of SOD (25.5 vs. 50.0, p = 0.002) and GSH (7.5 vs. 26.1, p = 0.006) in the LPS-induced acute orchitis group were significantly lower than those in the control group (Figures 4A,B). In contrast, the mice in the LPS group showed a significant increase in MDA (1.4 vs. 8.4, p = 0.015) concentration (Figure 4C). Compared with LPS group, after 50 and 100 mg/kg PHN treatment, the activity of SOD was significantly up-regulated (25.5 vs. 34.9, p = 0.049; 25.5 vs. 42.5, p = 0.017; Figure 4B). Moreover 100 mg/kg PHN treatment, GSH and MDA were significantly up-regulated (7.5 vs. 16.8, p = 0.042; Figure 4A) and down-regulated (8.4 vs. 5.3, p = 0.044; Figure 4C) compared with LPS group, respectively.
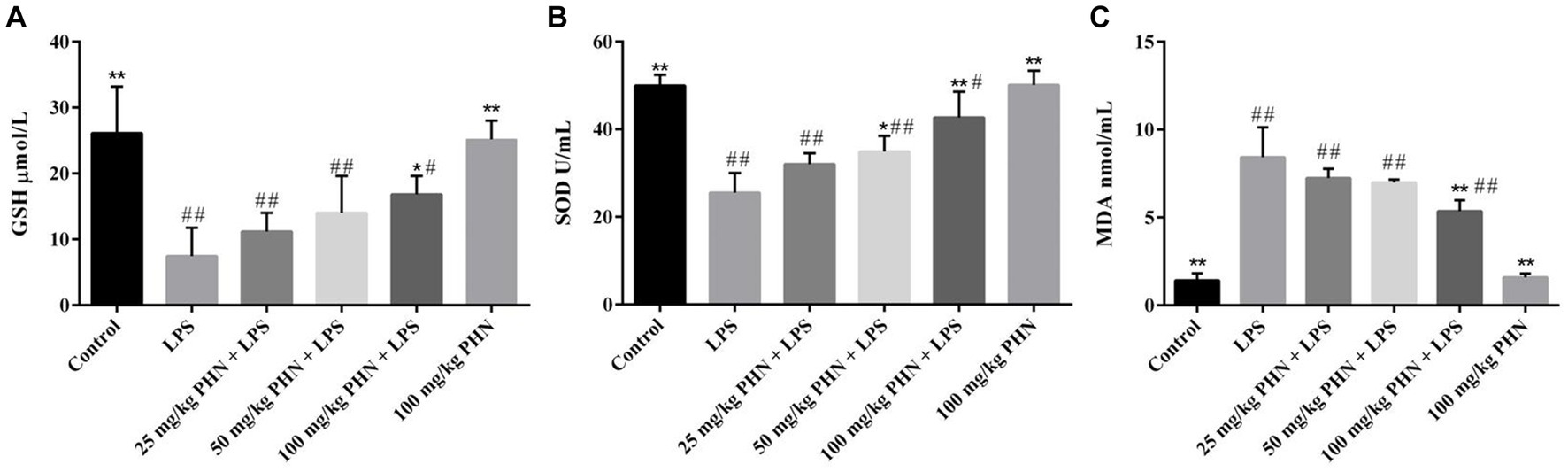
Figure 4. Amelioration of oxidative stress. (A) The activity of GSH. (B) The activity of SOD. (C)The production of MDA. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the control group.
3.5 Enhancement testosterone levels
Testosterone plays an important role in spermatogenesis and maintenance of sperm motility in mammals; however, Leydig cells damaged during orchitis result in lower testosterone levels. As shown in Figure 5, our results indicate that the mRNA expression of genes associated with testosterone synthesis (3beta-hydroxysteroid dehydrogenase, 3β-HSD and steroidogenic acute regulatory protein, stARF) in the LPS group was significantly lower than that in the control group. Consistent with these results, the testosterone levels in the blood were also significantly decreased in the LPS group compared with that in the control group (2.8 vs. 8.6, p = 0.002; Figure 5A). However, these changes were significantly reversed after PHN treatment in a dose-dependent manner (2.8 vs. 3.9, p = 0.023; 2.8 vs. 4.7, p = 0.011; 2.8 vs. 6.9, p = 0.008; Figure 5A).
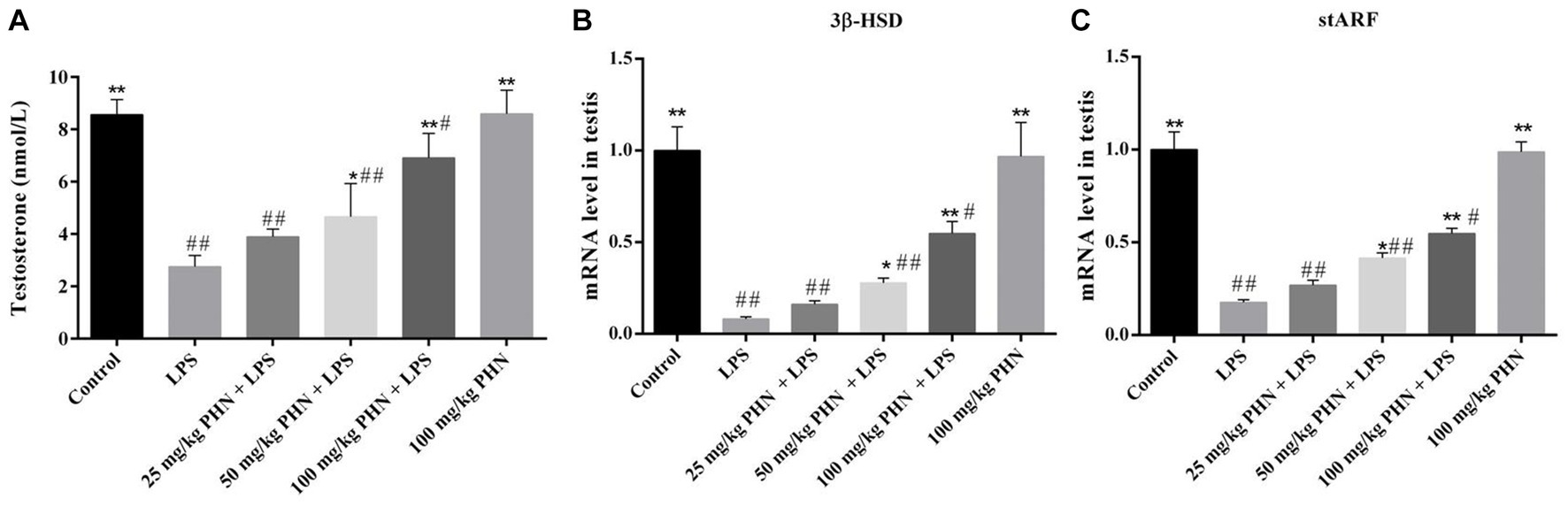
Figure 5. Improvement of testosterone production. (A) Testosterone levels in serum were detected by ELISA. The mRNA expression levels of 3β-HSD (B) and stARF (C) in testes tissues were detected by qRT-PCR. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the control group.
3.6 Enhance of the expression of ZO-1 and occludin
The BTB is important in ensuring the microenvironment for independent spermatogenesis in the testes. Therefore, we examined the mRNA and protein expression of the BTB-associated tight junction proteins ZO-1 and occludin and found that the mRNA expression levels of ZO-1 and occludin in of the LPS-treated mice testes were significantly decreased (Figures 6A–B), and the protein content was also significantly decreased by immunofluorescence (Figures 6C–E). However, the mRNA and protein expression levels of ZO-1 and oocludin significantly recovered after PHN treatment in a dose-dependent manner (Figures 6C–E).
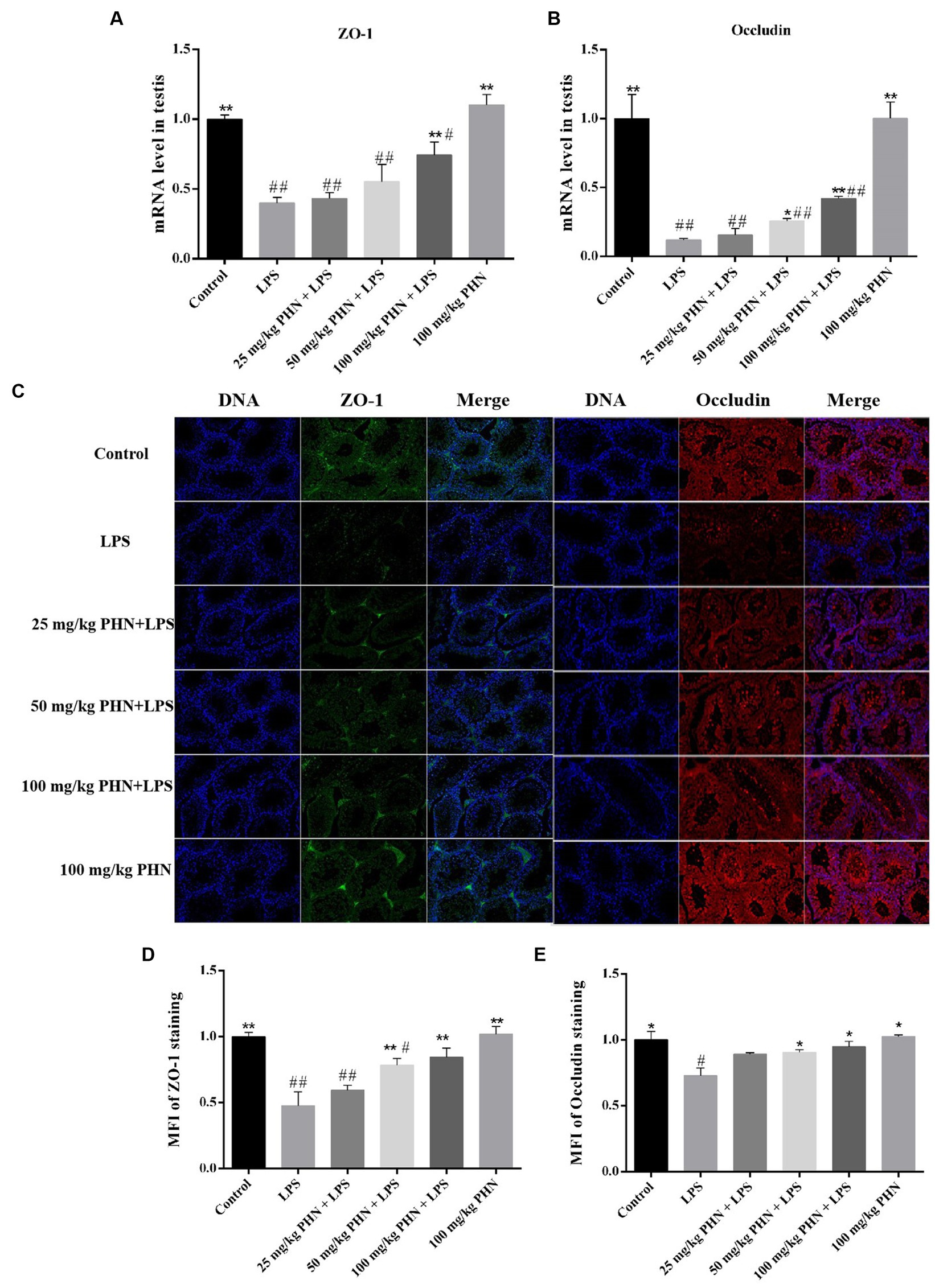
Figure 6. Enhance of the expression of ZO-1 and occludin. The mRNA expression of ZO-1 (A) and occludin (B) were measured by qRT-PCR in testes tissues. (C) The protein expression of ZO-1 and occludin were measured by immunofluorescence in testes tissues. 200×. Mean fluorescence intensity (MFI) of ZO-1 (D) and Occludin (E) staining as determined using Nikon NIS element software. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the control group.
3.7 Effects of PHN treatment on gut microbiota at the phylum level
The changes in microbial species, a clone library of the 16S rRNA gene, were established and sequenced to study the potential mechanism of PHN against LPS-induced orchitis in mice. For beta diversity analysis, principal coordinates analysis (PCoA) was used to analyze the microbiota communities. The data showed significant and distinct clustering of microbiota composition between the control and PHN treatment groups (Figure 7A), indicating that significant differences in microbial composition between the control and PHN treatment groups. Each sample contained 10 different phyla, including Bacteroidetes, Firmicutes, Verrucomicrobia, Proteobacteria, Epsilonbacteraeota, Patescibacteria, Actinobacteria, Tenericutes, Cyanobacteria, and Deferribacteres in descending order (Figure 7A). The four phyla with the highest abundances were Bacteroidetes, Firmicutes, Verrucomicrobia, and Epsilonbacteraeota. After PHN treatment, Firmicutes and proteobacteria did not change significantly (Figures 7C,F), but the Bacteroidetes abundance was significantly higher than that in the control group (Figure 7B). However, the ratio of Firmicutes to Bacteroidetes in the PHN-treated group was significantly lower than those in the control group (Figure 8D). Additionally, the Epsilonbacteraeota and Verrucomicrobia abundances in the PHN group were significantly lower than those in the control group (Figures 7E,G). Moreover, further analysis by LDA found that Bacteroidetes and Verrucomicrobia also showed significantly increase and decrease at phylum level (Supplementary Figure S1).
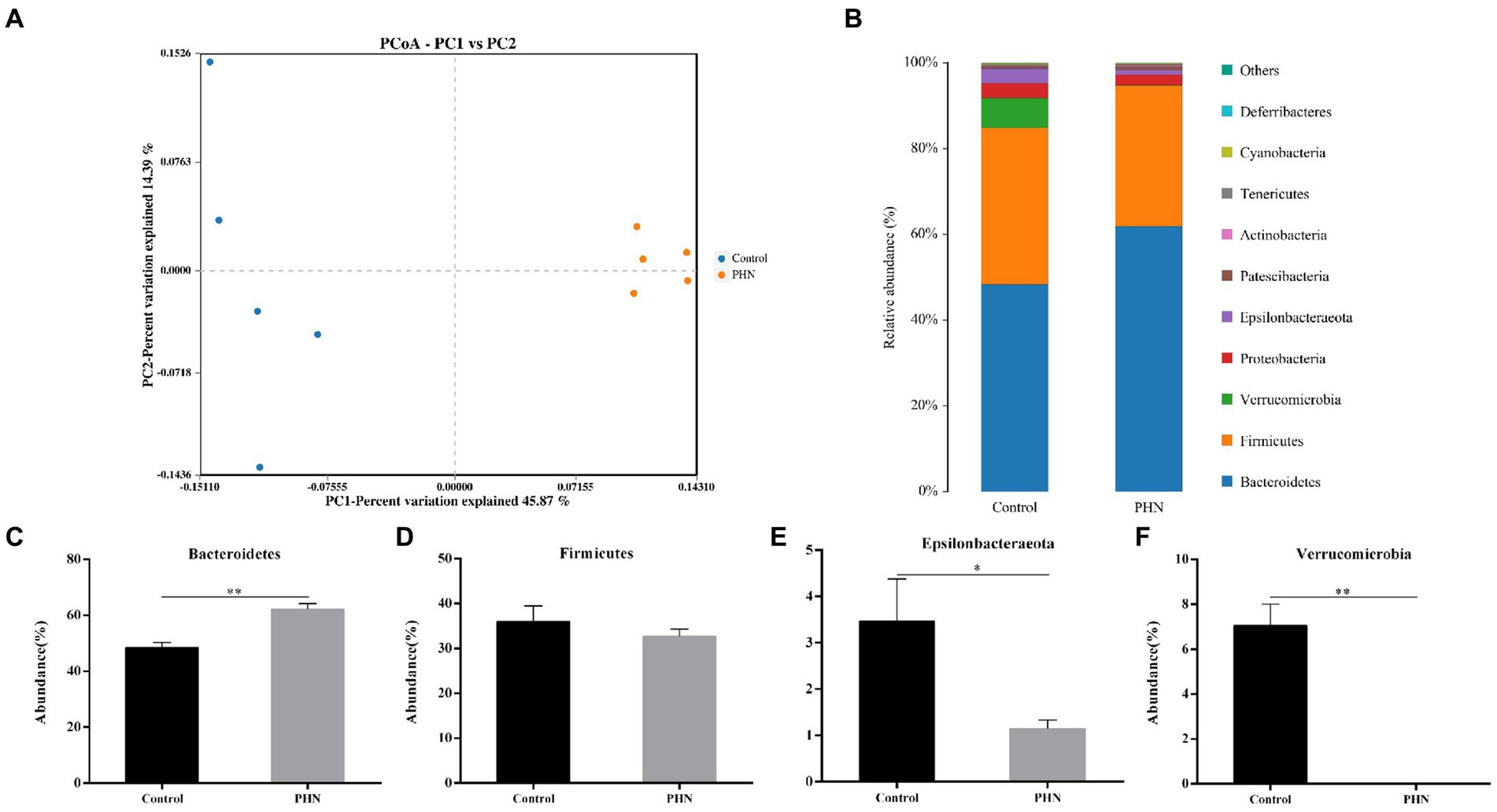
Figure 7. The relative abundance of gut microbiota after PHN treatment. (A) Beta diversity changes calculated by PCoA based on the OTU abundance. Each point represents the fecal microbiota of a mouse. (B) Relative abundance of the top 10 phyla were demonstrated as Bar graph. (C–F) Relative abundance of major phyla in the feces. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group.
3.8 Effects of PHN treatment on intestinal microbiome abundance at the family and genus levels
At the family level, Muribaculaceae was the most abundant in all fecal samples (Figure 8A). The proportion of Muribaculaceae in the PHN group was 50.52%, which was significantly higher than that in the control group (37.72%; Figure 8B). The second most abundant family in the PHN groups was Lachnospiraceae (9.20%),which was significantly lower than that in the control group (19.38%; Figure 8C). The third most abundant family in the PHN group was Lactobacillaceae (13.36%), which was significantly higher than that in the control group (5.40%; Figure 8D). The relative abundance of Ruminococcaceae (Figure 8E) did not change, but the relative Prevotellaceae and Akkermansiaceae abundances significantly increased and decreased (Figures 8F–G). At the genus level, uncultured bacterium f Muribaculaceae and Lactobacillus were significantly increased, and Akkermansia were significantly decreased in the PHN group compared with those of the control group (Figures 9A–E). Additionally, LDA analysis showed that similar results at the family and genus levels (Supplementary Figure S1).
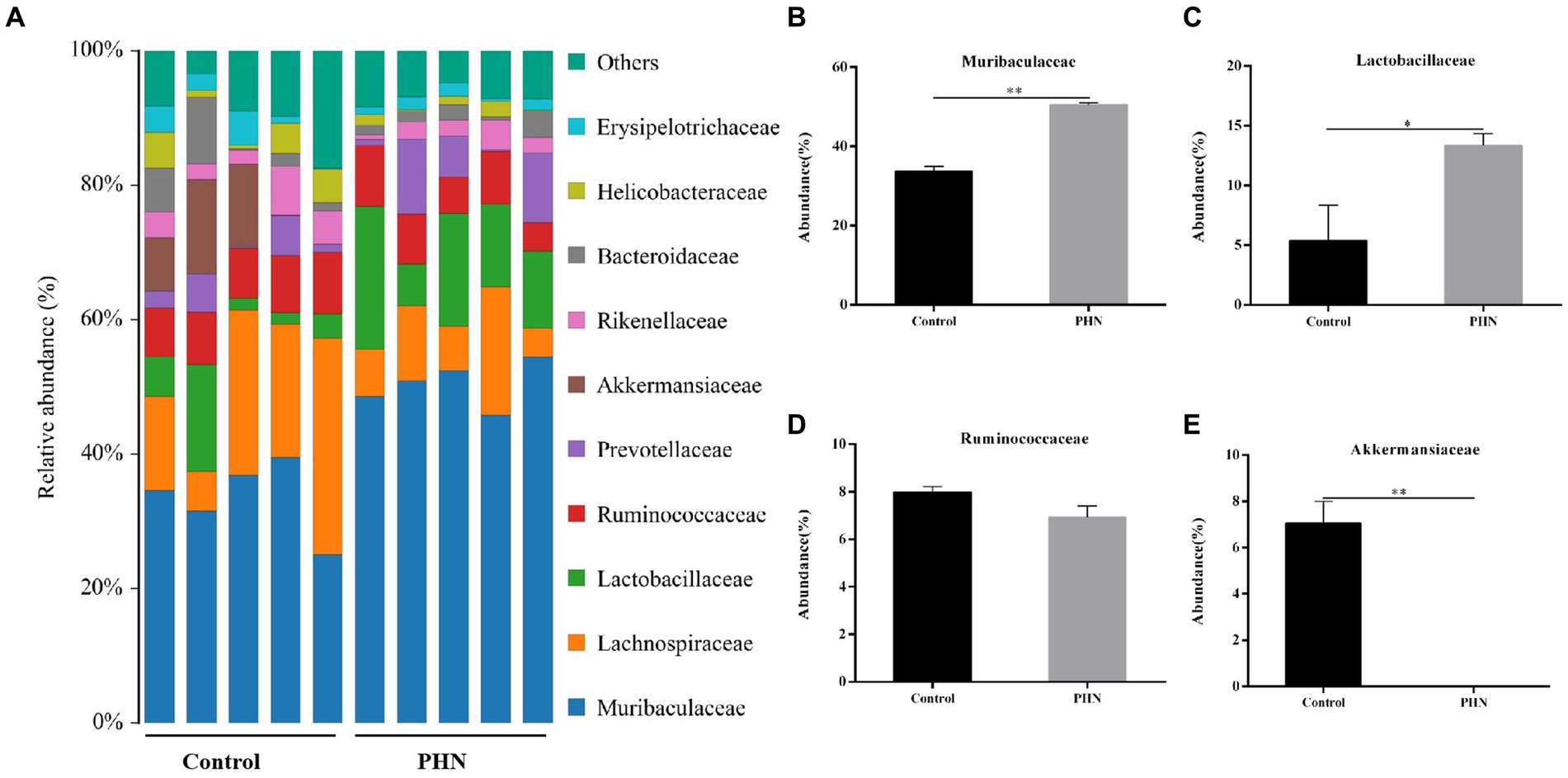
Figure 8. The relative abundance of gut microbiota at family level after PHN treatment. (A) Relative abundance of the top 10 family were demonstrated as Bar graph. (B–E) Relative abundance of major family in the feces. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group.
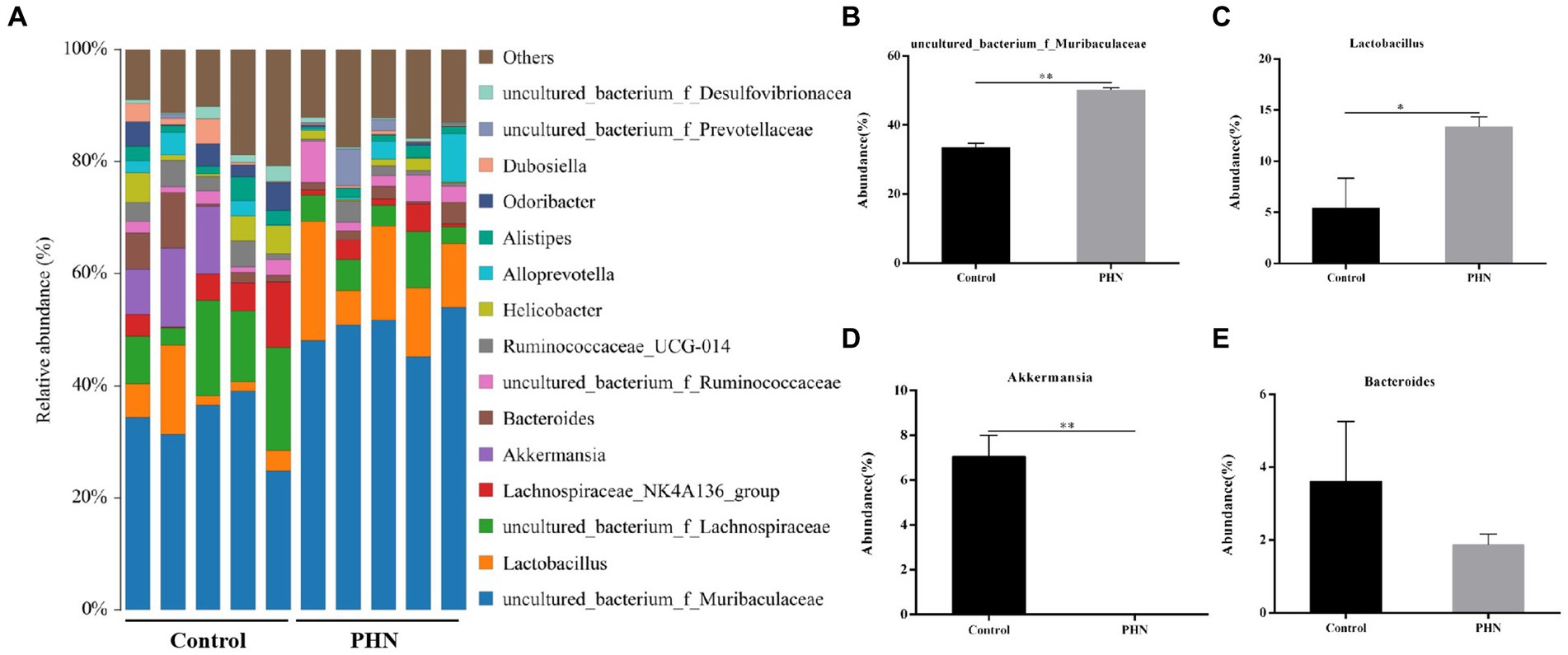
Figure 9. The relative abundance of gut microbiota at genus level after PHN treatment. (A) Relative abundance of the top 10 genus were demonstrated as Bar graph. (B–E) Relative abundance of major genus in the feces. Data are demonstrated as means ± SD (n = 5). *p < 0.05 and **p < 0.01 vs. the LPS group.
4 Discussion
Orchitis is a complex infectious disease affecting the reproductive tract of male animals that has serious impacts on reproduction and reduces the quality of life. Damage to the BTB is difficult to repair; therefore, orchitis treatment is particularly difficult. PHN is a component of functional foods, primarily in apple peels, and has many biological activities, including antioxidative and anti-inflammatory activities; however, the preventive effect of PHN against LPS-induced orchitis in mice has not yet been reported. In this study, we examined the effects of different concentrations of PHN on orchitis and the underlying mechanisms. Similar to previous studies, body weight loss, testicular to body weight ratio decline, and testicular tissue structure damage were induced by LPS; however, we found that PHN significantly reduced LPS-induced changes as direct and indirect indicators of the severity of orchitis. These preliminary results suggest that PHN exerts a protective effect against LPS-induced orchitis.
LPS exposure of the testes promoted the secretion of TNF-α from Leydig cells (2). TNF-α is the earliest endogenous mediator of the inflammatory processes, while IL-17 is produced by highly differentiated Th17 cells and is an effective mediator of inflammation (28). Many studies have also shown that an imbalance between pro- and anti-inflammatory molecules, including TNF-α and IL-1β, in the testes can lead to orchitis (29, 30). In this study, the mRNA expressions of IL-2, TNF-α, IL-17A, and IL-1β, which have essential roles in inflammatory processes, were elevated in LPS-induced orchitis; however, PHN have been found to reduce their expression.
Testosterone is a steroid hormone secreted by Leydig cells that is important for sperm spermatogenesis and the maintenance of sperm motility (31). Inhibition of testosterone production disrupts spermatogenesis in humans and animals, leading to infertility in males (32). Additionally, Allen et al. demonstrated that LPS can damage mitochondria in Leydig cells by inducing an increase in Reactive Oxygen Species (ROS), inhibiting the synthesis and secretion of steroid hormones in Leydig cells, and reducing the expression of StAR and 3β-HSD genes (33). StAR-mediated cholesterol transport from the outer to inner mitochondrial membrane is a critical step during steroid formation (34–36). 3β-HSD is a steroid synthase that plays an important role in catalyzing the conversion of cholesterol steroid substrates to testosterone (37). Our study demonstrated that PHN could significantly restore LPS-induced decrease in testosterone content by increasing the mRNA expression levels of genes related to testosterone synthesis.
Furthermore, an increase in these pro-inflammatory cytokines causes an inflammatory response, which damages the structure of the BTB (2). The BTB divides the vas deferens into a basal compartment and lumen, which mainly includes basic ES, GJ, and TJ between Sertoli cells, providing a stable biochemical microenvironment for spermatogenesis (38, 39). Various TJ proteins, such as the ZO-1 complex, are involved in BTB formation. Moreover, another study reported that LPS elevation induced occludin downregulation and increased BTB permeability (27). Our study demonstrated that PHN reduced LPS-induced BTB damage in testes by increasing the expression of ZO-1 and occludin proteins.
The intestinal microbe is a large and diverse community of microbes, which is a substantial and complex ecosystem with mutual dependence on and restriction of the host. Therefore, the structure of the gut microbiota has an important impact on the health of the host. Recently, many studies have shown that gut microbiota is crucial to regulating male animal reproduction, including spermatogenesis and testosterone secretion (40, 41). Therefore, we examined whether PHN treatment could cause changes in the intestinal microflora structure, leading to reduced LPS sensitivity in mice. Previous studies have shown that PHN-treated mice showed no significant changes in Bacteroidetes and Firmicutes abundances at the phylum level in their feces (42); however, our study indicated that PHN treatment can lead to a significant increase in the relative abundance of Bacteroidetes. Short-chain fatty acids (SCFAs) concentrations were positively correlated with Bacteroidetes count (43). The phylum Verrucomicrobia is negatively correlated with obesity through the degradation of intestinal mucin (44). In this study, PHN treatment significantly increased and decreased the colonization of Bacteroides and Verrucomicrobia in the intestinal tract, which may be an important reason for PHN reducing LPS-induced orchitis in mice. Epsilonbacteraeota is harmful to the intestinal tract, which is significantly increased in dextran sulfate sodium (DSS)-induced colitis in mice (45). The relative abundance of Epsilonbacteraeota was significantly reduced, suggesting that PHN treatment could improve intestinal flora and thus enhance LPS resistance in mice. At the family level, Muribaculaceae is known as a short-chain fatty acid (SCFAs) producer with beneficial effects on intestinal homeostasis and health (46), and is also associated with the formation of the inner mucus layer in the colon and barrier function (47). Furthermore, propionate produced by Muribaculaceae plays an important role in the anti-inflammatory effects and maintenance of intestinal barrier function (47). In the present study, Muribaculaceae was important improve in PHN treatment compared with control group. Ruminococcaceae was also related to testicular function (48), but there was no significant change in its relative abundance. Furthermore, Lactobacillaceae, of the Firmicutes phylum, are well known for their role in digesting carbohydrates and their probiotic properties (49). The increased relative abundances of Muribaculaceae and Lactobacillaceae may be another important reason for the reduction in LPS-induced orchitis. Meanwhile, Akkermansiaceae, a group of mucin-degrading bacteria in the gut, decreased the integrity of the intestinal barrier function and led to increased permeability of the intestinal epithelium (50). In our study, the relative abundance of Akkermansiaceae decreased in PHN treatment than control group. These findings provide evidence that PHN treatment may enhance intestinal epithelial integrity, resulting in a decrease of LPS in the blood. The genus level data showed that PHN treatment significantly increased uncultured bacterium f Muribaculaceae and Lactobacillus, but decreased Akkermansia. Uncultured bacterium f Muribaculaceae could produce succinic acid, an important intermediate in the synthesis of propionic acid, through the degradation of polysaccharides (51). Furthermore, Lactobacillus is a beneficial bacterium in the intestinal tract that plays an important role in regulating health. Previous studies have shown that different types of Lactobacillus have anti-inflammatory properties (52), improve carbohydrate and fatty acid metabolism, and reduce lithocholic acid levels (53). Hao et al. reported that alginate oligosaccharide increased the sperm quality of mice with type 1 diabetes by increasing the Lactobacillus abundance (54). Similar to previous studies, PHN treatment significantly increased the abundance of Lactobacillus and uncultured bacterium f Muribaculaceae, which may explain PHN protection against LPS-induced testicular injury in mice. The relative abundances of Akkermansia were significantly lower in the PHN treatment group than in the control group. Akkermansia had anti-obesity effects, but a higher relative abundance of Akkermansia in the host intestinal tract could destroy intestinal mucins, which can promote colonic tumorigenesis and lead to intestinal inflammation (55, 56). These results indicate that PHN treatment significantly improved the intestinal microflora, which may be another important factor in the reduction of LPS-induced acute orchitis in mice.
In conclusion, our results indicate that PHN pretreatment might alleviate orchitis by altering the composition of gut microflora, which may provide a reference for reducing the incidence of acute orchitis.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/bioproject/; PRJNA1105222.
Ethics statement
The animal study was approved by Heilongjiang Bayi Agricultural University Committee of Ethics for Animal Welfare and Research. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
QG: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. T-FL: Methodology, Writing – original draft. JH: Methodology, Writing – original draft. J-CL: Methodology, Writing – original draft. Z-CZ: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Y-LQ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Doctoral Starting Up Foundation of the Heilongjiang Bayi Agricultural University (XYB201910), Natural Science Foundation of Heilongjiang Province of China (LH2023C080), China Postdoctoral Science Foundation (2023 M731028), Heilongjiang Province “Hundred Million” Project Science and Technology Major project (2021ZX12B03), National Natural Science Foundation of China (Grant No.32072758), and Heilongjiang Postdoctoral Found (LBH-Q20054).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1340591/full#supplementary-material
References
1. Hedger, MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. (2011) 32:625–40. doi: 10.2164/jandrol.111.012989
2. Theas, MS, Jacobo, PV, Pérez, CV, Guazzone, VA, and Lustig, L. Inflammation and spermatogenesis 4. Spermatogenesis: Biol Clin Implicat. (2018):63–72. doi: 10.1201/9780429488634-4
3. Klein, B, Haggeney, T, Fietz, D, Indumathy, S, Loveland, KL, Hedger, M, et al. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum Reprod. (2016) 31:2192–202. doi: 10.1093/humrep/dew211
4. Nistal, M, Riestra, ML, and Paniagua, R. Focal orchitis in undescended testes: discussion of pathogenetic mechanisms of tubular atrophy. Arch Pathol Lab Med. (2002) 126:64–9. doi: 10.5858/2002-126-0064-FOIUT
5. Leisegang, K, Henkel, R, and Agarwal, A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am J Reprod Immunol. (2019) 82:e13178. doi: 10.1111/aji.13178
6. Liu, Y, Li, X, Xiao, S, Liu, X, Chen, X, Xia, Q, et al. The effects of gold nanoparticles on Leydig cells and male reproductive function in mice. Int J Nanomedicine. (2020) 15:9499–514. doi: 10.2147/IJN.S276606
7. Zhao, S, Zhu, W, Xue, S, and Han, D. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol. (2014) 11:428–37. doi: 10.1038/cmi.2014.38
8. Mruk, DD, and Cheng, CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. (2004) 25:747–806. doi: 10.1210/er.2003-0022
9. Setchell, BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol. (2008) 636:212–33. doi: 10.1007/978-0-387-09597-4_12
10. Cheng, CY, Wong, EW, Lie, PP, Li, MW, Mruk, DD, Yan, HH, et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: an unexpected turn of events. Spermatogenesis. (2011) 1:105–15. doi: 10.4161/spmg.1.2.15745
11. Ok, F, Kaplan, HM, Kizilgok, B, and Demir, E. Protective effect of alpha-linolenic acid on lipopolysaccharide-induced Orchitis in mice. Andrologia. (2020) 52:e13667. doi: 10.1111/and.13667
12. Deng, SL, Zhang, BL, Reiter, RJ, and Liu, YX. Melatonin ameliorates inflammation and oxidative stress by suppressing the p38MAPK signaling pathway in LPS-induced sheep orchitis. Antioxidants. (2020) 9:1277. doi: 10.3390/antiox9121277
13. Nicolas, N, Muir, JA, Hayward, S, Chen, JL, Stanton, PG, Gregorevic, P, et al. Induction of experimental autoimmune orchitis in mice: responses to elevated circulating levels of the activin-binding protein, follistatin. Reproduction. (2017) 154:293–305. doi: 10.1530/REP-17-0010
14. Jones, O. Effect of phloridzin and phloroglucinol on apple shoots. Nature. (1976) 262:392–3. doi: 10.1038/262392a0
15. Ehrenkranz, JR, Lewis, NG, Ronald Kahn, C, and Roth, J. Phlorizin: a review. Diabetes Metab Res Rev. (2005) 21:31–8. doi: 10.1002/dmrr.532
16. Lu, Y, and Foo, LY. Constitution of some chemical components of apple seed. Food Chem. (1998) 61:29–33. doi: 10.1016/S0308-8146(97)00123-4
17. Crespy, V, Aprikian, O, Morand, C, Besson, C, Manach, C, Demigné, C, et al. Bioavailability of phloretin and phloridzin in rats. J Nutr. (2001) 131:3227–30. doi: 10.1093/jn/131.12.3227
18. Rupasinghe, HV, and Yasmin, A. Inhibition of oxidation of aqueous emulsions of omega-3 fatty acids and fish oil by phloretin and phloridzin. Molecules. (2010) 15:251–7. doi: 10.3390/molecules15010251
19. Ikumi, Y, Kida, T, Sakuma, S, Yamashita, S, and Akashi, M. Polymer–phloridzin conjugates as an anti-diabetic drug that inhibits glucose absorption through the Na+/glucose cotransporter (SGLT1) in the small intestine. J Control Release. (2008) 125:42–9. doi: 10.1016/j.jconrel.2007.10.001
20. Kamdi, SP, Badwaik, HR, Raval, A, and Nakhate, KT. Ameliorative potential of phloridzin in type 2 diabetes-induced memory deficits in rats. Eur J Pharmacol. (2021) 913:174645. doi: 10.1016/j.ejphar.2021.174645
21. Nozu, T, Miyagishi, S, Ishioh, M, Takakusaki, K, and Okumura, T. Phlorizin attenuates visceral hypersensitivity and colonic hyperpermeability in a rat model of irritable bowel syndrome. Biomed Pharmacother. (2021) 139:111649. doi: 10.1016/j.biopha.2021.111649
22. Mantso, T, Trafalis, DT, Botaitis, S, Franco, R, Pappa, A, Rupasinghe, HV, et al. Novel docosahexaenoic acid ester of phloridzin inhibits proliferation and triggers apoptosis in an in vitro model of skin cancer. Antioxidants. (2018) 7:188. doi: 10.3390/antiox7120188
23. Niederberger, KE, Tennant, DR, and Bellion, P. Dietary intake of phloridzin from natural occurrence in foods. Br J Nutr. (2020) 123:942–50. doi: 10.1017/S0007114520000033
24. Liu, D, Ji, Y, Guo, Y, Wang, H, Wu, Z, Li, H, et al. Dietary supplementation of apple phlorizin attenuates the redox state related to gut microbiota homeostasis in c57bl/6j mice fed with a high-fat diet. J Agric Food Chem. (2020) 69:198–211. doi: 10.1021/acs.jafc.0c06426
25. Mei, X, Zhang, X, Wang, Z, Gao, Z, Liu, G, Hu, H, et al. Insulin sensitivity-enhancing activity of phlorizin is associated with lipopolysaccharide decrease and gut microbiota changes in obese and type 2 diabetes (db/db) mice. J Agric Food Chem. (2016) 64:7502–11. doi: 10.1021/acs.jafc.6b03474
26. Pan, Y, Liu, Y, Wang, L, Xue, F, Hu, Y, Hu, R, et al. MKP-1 attenuates LPS-induced blood-testis barrier dysfunction and inflammatory response through p38 and IκBα pathways. Oncotarget. (2016) 7:84907–23. doi: 10.18632/oncotarget.12823
27. Metukuri, MR, Reddy, CMT, Reddy, P, and Reddanna, P. Bacterial LPS mediated acute inflammation-induced spermatogenic failure in rats: role of stress response proteins and mitochondrial dysfunction. Inflammation. (2010) 33:235–43. doi: 10.1007/s10753-009-9177-4
28. Rovedatti, L, Kudo, T, Biancheri, P, Sarra, M, Knowles, C, Rampton, DS, et al. Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut. (2009) 58:1629–36. doi: 10.1136/gut.2009.182170
29. O’Bryan, MK, Schlatt, S, Phillips, DJ, de Kretser, DM, and Hedger, MP. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. (2000) 141:238–46. doi: 10.1210/endo.141.1.7240
30. Chen, Y, Wang, J, Zhang, Q, Xiang, Z, Li, D, and Han, X. Microcystin-leucine arginine exhibits immunomodulatory roles in testicular cells resulting in orchitis. Environ Pollut. (2017) 229:964–75. doi: 10.1016/j.envpol.2017.07.081
31. Smith, LB, and Walker, WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. (2014) 30:2–13. doi: 10.1016/j.semcdb.2014.02.012
32. Ohlander, SJ, Lindgren, MC, and Lipshultz, LI. Testosterone and male infertility. Urol Clin North Am. (2016) 43:195–202. doi: 10.1016/j.ucl.2016.01.006
33. Allen, JA, Diemer, T, Janus, P, Hales, KH, and Hales, DB. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine. (2004) 25:265–76. doi: 10.1385/ENDO:25:3:265
34. Zirkin, BR, and Papadopoulos, V. Leydig cells: formation, function, and regulation. Biol Reprod. (2018) 99:101–11. doi: 10.1093/biolre/ioy059
35. Stocco, DM, and Clark, BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. (1996) 17:221–44. doi: 10.1210/edrv-17-3-221
36. Hasegawa, T, Zhao, L, Caron, KM, Majdic, G, Suzuki, T, Shizawa, S, et al. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. (2000) 14:1462–71. doi: 10.1210/mend.14.9.0515
37. Hu, GX, Zhao, BH, Chu, YH, Zhou, HY, Akingbemi, BT, Zheng, ZQ, et al. Effects of genistein and equol on human and rat testicular 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase 3 activities. Asian J Androl. (2010) 12:519–26. doi: 10.1038/aja.2010.18
38. Cheng, CY, and Mruk, DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. (2012) 64:16–64. doi: 10.1124/pr.110.002790
39. Patterson, E, Ryan, PM, Cryan, JF, Dinan, TG, Ross, RP, Fitzgerald, GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. (2016) 92:286–300. doi: 10.1136/postgradmedj-2015-133285
40. Zhang, P, Feng, Y, Li, L, Ge, W, Yu, S, Hao, Y, et al. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut. (2021) 70:222–5. doi: 10.1136/gutjnl-2020-320992
41. Hou, X, Zhu, L, Zhang, X, Zhang, L, Bao, H, Tang, M, et al. Testosterone disruptor effect and gut microbiome perturbation in mice: early life exposure to doxycycline. Chemosphere. (2019) 222:722–31. doi: 10.1016/j.chemosphere.2019.01.101
42. Zhang, XY, Chen, J, Yi, K, Peng, L, Xie, J, Gou, X, et al. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes. (2020) 12:1842990–18. doi: 10.1080/19490976.2020.1842990
43. Al-Asmakh, M, Sohail, MU, Al-Jamal, O, Shoair, BM, Al-Baniali, AY, Bouabidi, S, et al. The effects of gum acacia on the composition of the gut microbiome and plasma levels of short-chain fatty acids in a rat model of chronic kidney disease. Front Pharmacol. (2020) 11:569402. doi: 10.3389/fphar.2020.569402
44. Depommier, C, Everard, A, Druart, C, Plovier, H, Van Hul, M, Vieira-Silva, S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. (2019) 25:1096–103. doi: 10.1038/s41591-019-0495-2
45. Wang, J, Zhu, G, Sun, C, Xiong, K, Yao, T, Su, Y, et al. TAK-242 ameliorates DSS-induced colitis by regulating the gut microbiota and the JAK2/STAT3 signaling pathway. Microb Cell Fact. (2020) 19:158–17. doi: 10.1186/s12934-020-01417-x
46. Ramírez-Acosta, S, Selma-Royo, M, Collado, MC, Navarro-Roldán, F, Abril, N, and García-Barrera, T. Selenium supplementation influences mice testicular selenoproteins driven by gut microbiota. Sci Rep. (2022) 12:4218. doi: 10.1038/s41598-022-08121-3
47. Volk, JK, Nyström, EE, van der Post, S, Abad, BM, Schroeder, BO, Johansson, Å, et al. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. J Exp Med. (2019) 216:2602–18. doi: 10.1084/jem.20190679
48. Tian, X, Yu, Z, Feng, P, Ye, Z, Li, R, Liu, J, et al. Lactobacillus plantarum TW1-1 alleviates diethylhexylphthalate-induced testicular damage in mice by modulating gut microbiota and decreasing inflammation. Front Cell Infect Microbiol. (2019) 9:221. doi: 10.3389/fcimb.2019.00221
49. Rossi, M, Johnson, DW, Morrison, M, Pascoe, EM, Coombes, JS, Forbes, JM, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. (2016) 11:223–31. doi: 10.2215/CJN.05240515
50. Geerlings, SY, Kostopoulos, I, De Vos, WM, and Belzer, C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms. (2018) 6:75. doi: 10.3390/microorganisms6030075
51. Wang, Z, Li, X, Zhang, L, Wu, J, Zhao, S, and Jiao, T. Effect of oregano oil and cobalt lactate on sheep in vitro digestibility, fermentation characteristics and rumen microbial community. Animals. (2022) 12:118. doi: 10.3390/ani12010118
52. Malik, M, Suboc, TM, Tyagi, S, Salzman, N, Wang, J, Ying, R, et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. (2018) 123:1091–102. doi: 10.1161/CIRCRESAHA.118.313565
53. Wang, Q, Lv, L, Jiang, H, Wang, K, Yan, R, Li, Y, et al. Lactobacillus helveticus R0052 alleviates liver injury by modulating gut microbiome and metabolome in D-galactosamine-treated rats. Appl Microbiol Biotechnol. (2019) 103:9673–86. doi: 10.1007/s00253-019-10211-8
54. Hao, Y, Feng, Y, Yan, X, Chen, L, Zhong, R, Tang, X, et al. Gut microbiota-testis axis: FMT improves systemic and testicular micro-environment to increase semen quality in type 1 diabetes. Mol Med. (2022) 28:1–17. doi: 10.1186/s10020-022-00473-w
55. Li, Y, Li, J, Su, Q, and Liu, Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. (2019) 10:3637–49. doi: 10.1039/c9fo00195f
Keywords: phlorizin, orchitis, blood testosterone barrier, testosterone, gut microbiota
Citation: Guo Q, Li T-F, Huang J, Li J-C, Zhang Z-C and Qu Y-L (2024) The protective role of phlorizin against lipopolysaccharide-induced acute orchitis in mice associated with changes in gut microbiota composition. Front. Vet. Sci. 11:1340591. doi: 10.3389/fvets.2024.1340591
Edited by:
Rao Zahid Abbas, University of Agriculture, PakistanReviewed by:
Jianzhong Wang, Shanxi Agricultural University, ChinaJinming Wang, Shanxi Agricultural University, China
Copyright © 2024 Guo, Li, Huang, Li, Zhang and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ze-Cai Zhang, emN6aGFuZzg5QDEyNi5jb20=; Yong-Li Qu, WWxxdTAwN0AxMjYuY29t
†These authors have contributed equally to this work
 Qing Guo
Qing Guo Tian-Feng Li2†
Tian-Feng Li2† Ze-Cai Zhang
Ze-Cai Zhang