
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 23 February 2024
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1337698
Introduction: Yupingfeng polysaccharide (YPF-P) is the main substance of alcohol deposition in Yupingfeng powder, which has many biological functions such as enhancing immunity, repairing intestinal barrier and enhancing antioxidant ability. This study employed in vitro growth-promoting drug feed additives and animal experiments to comprehensively evaluate the use of YPF-P in broiler production.
Methods: A total of 1,296 151 days-old Qingyuan Partridge chickens were randomly divided into four groups with six replicates and 54 hens per replicate: the control group was fed basal diet, and the experimental groups were fed diets supplemented with 4 g/kg, 8 g/kg, and 12 g/kg YPF-P for 14 days. Broilers were weighed before and at the end of the experiment to calculate total weight gain (GW), average daily gain (ADG), and feed compensation. At the end of the experiment, six chickens from each group were randomly selected for subwing vein blood sampling, which was used to measure serum biochemical indicators GHRH, GH, and IGF-1 by ELISA method. Randomly select chickens from control group and 8 g/kg group for slaughter, and cecal contents were collected for 16S high-throughput sequencing.
Results: Dietary supplementation of 8 g/kg YPF-P can significantly increase the final body weight, total weight gain, average daily gain and decrease the feed to gain ratio of chickens. During 151–165 days, serum IGF-1 concentrations increased significantly (p < 0.05). There were no significant changes in serum GH concentration (p > 0.05). In terms of gut microbiota, there was no significant difference between control group and test group in Shannon index and Simpson index. Compared with the control group,the addition of 8 g/kgYPF-P significantly increased the abundance of Firmicutes and significantly decreased the abundance of Bacteroides at the phylum level.At the genus level, the relative abundance of unclassified_Oscillospiraceae was significantly increased and the unclassified_Muribaculaceae, uncultured_Bacteroidales_bacterium, Lactobacillus, Alloprevotella, Ligilactobacillus, Prevotellaceae_UCG_001, and unclassified_Atopobiaceae was significantly decreased.
Conclusion: The above results showed that adding 8 mg/kg of YPF-P could increase the average daily gain of Qingyuan Partridge chickens, reduce the ratio of feed to meat, and affect the distribution proportion of intestinal microflora in chickens to some extent.
Antibiotics have bacteriostatic or bactericidal effects. Studies have shown that using appropriate doses of antibiotics in the feed of food animals can prevent animal diseases and improve production performance (1–3). This has been common practice in modern animal husbandry for decades. However, the irrational use of antibiotics has led to the emergence of more and more drug-resistant bacteria. These antibiotic-resistant bacteria remain in animals and are easily passed to humans through the food chain (4), and these antibiotic-resistant bacteria can be pathogenic to humans and widely spread in the environment through animal feces (5), thus affecting animal and human health and food safety. This has raised concerns, prompting efforts to develop so-called alternatives to antibiotics.
At present, the natural antibiotic substitutes found by people are mainly beneficial prebiotics, probiotics, alkaloids, antimicrobial peptides, essential oils, and plant substances, etc. (4, 6–10), the most common feed additives have been widely used in the animal husbandry industry after antibiotic growth promoters were banned. Plant-derived feed additives include herbs, plant extracts (essential oils, oil-based resins, tannins, saponins, polysaccharides, phenols, flavonoids, etc.), and their active ingredients (11–13). As feed additives, these substances can promote the growth of livestock and poultry, increase the feed reward, and improve the immune capacity and antioxidant capacity of the animal body. Olawuwo’s et al. research shows that (14), the powder of Morinda lucida, Acalypha wilkesiana, and Ficus exasperata is higher in macronutrients and micronutrients, such as K, Ca2+, Na+, Mg2+, and P, than the standard broiler feed. It is also rich in organic acids and sugars required for livestock nutrition and can effectively maintain antioxidant defenses. To improve animal health and promote the effect of growth performance. Studies have also shown that the addition of Artemisia lordosica polysaccharide can play an anti-inflammatory and antioxidant role, improve the protein digestibility of broilers, reduce the content of serum stress hormones and pro-inflammatory cytokines, and alleviate the growth inhibition and intestinal damage induced by lipopolysaccharides (LPS) (15). Dietary supplementation of Origanum vulgare and Andrographis paniculata extract (FOA) (16) can reduce feed intake, increase the ratio of beneficial and harmful bacteria in the gut, reduce coccidium oocysts and alleviate intestinal lesions of broilers. To sum up, the plants extract substances added can promote the growth of poultry and intestinal health, improve the body, and improve anti-inflammatory and antioxidant function.
Yupingfeng powder, a traditional Chinese medicine formula, is composed of Astragalus membranaceus, Atractylodes macrocephala Koidz. and Saposhnikovia divaricata (Turcz.) Schischk (17). It has the functions of immune regulation (18), anti-virus (19), anti-tumor (20), and anti-aging (21). Yupingfeng polysaccharide is the main substance in the alcohol-precipitation part of Yupingfeng powder, which has many biological functions such as enhancing the body’s immunity (22), repairing the intestinal barrier (23), and enhancing the body’s antioxidant capacity (24). For example, YPF-P significantly increased the thymus index and promoted the secretion of total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), immunoglobulin A (IgA), immunoglobulin A (IgG) and immunoglobulin M (IgM) and other immune factors and antioxidant factors in the blood (25). The study by Chen et al. also showed that dietary Yupingfeng polysaccharide supplementation could significantly increase the expression of immune-related genes in blood cells and the intestinal tract; In addition, it can also reduce the cumulative mortality of Litopenaeus vannamei after Vibrio harveyi attack (26). The results showed that YPF-P is beneficial to animals, and can play a certain role in immune response, oxidative stress, and intestinal homeostasis of animals. YPF-P is a worthwhile green plant additive.
The gut is the largest body interface in contact with the environment (27), its primary function is to digest and absorb nutrients while forming a barrier against luminal pathogens such as antigenic peptides, bacteria, toxins, parasites, allergens and carcinogens (28, 29). The intestinal tract is rich in microorganisms [~1012–1013, (30)], mainly composed of anaerobic bacteria, and it is challenging to explore the microbial diversity of healthy intestinal flora using traditional culture methods (31). Guo et al. showed that Lentinus edodes extract (LenE), Tremella fuciformis extract, and Astragalus membranaceus Radix extract could increase the number of potentially beneficial bacteria (Bifidobacterium and Lactobacillus) in chicken cecum microflora while decreasing the number of potentially harmful bacteria (Bacteroides and Escherichia coli) (32), these polysaccharides extracts can be used as regulators of intestinal microorganisms in broilers to promote body health. More recently, natural plant polysaccharides, which are considered quality prebiotics, have been shown to reach the large intestine, where they can interact with the gut microbiota (GM) (33), where they can promote the proliferation of beneficial bacteria and inhibit the overgrowth and reproduction of foreign bacteria and potentially pathogenic bacteria, thereby regulating gut health (34). This study investigated the effects of different concentrations of Yupingfeng polysaccharide as a feed additive on growth performance and intestinal flora of Qingyuan Partridge chickens, aiming to provide the theoretical basis for Yupingfeng polysaccharide as a green and healthy feed additive for the poultry production industry.
Yupingfeng polysaccharide were provided by Foshan Dezhong Pharmaceutical Co., LTD. In the preparation process of Yupingfeng polysaccharide, Astragalus membranaceus, Atractylodes macrocephala Koidz. and Saposhnikovia divaricata (Turcz.) Schischk. were weighed according to the weight ratio of 3:1:1. The herbal mixture was boiled for 2 h in 8 volumes of water (v/w) and again for 1 h in 6 volumes of water. The two extracts were mixed and filtered, lyophilized and stored at 4°C.
A total of 1,296 Qingyuan Partridge chickens (151 days-old, female) with similar initial body weight were randomly divided into four groups, with six replicates per group and 54 chickens per replicate, every three chickens are raised in a cage. Qingyuan Partridge chicken is raised in layer cage. During the whole test period, the temperature of the room in which the chickens were kept was maintained between 20°C and 26°C, with a light duration of 16 h per day and a light intensity of 20 to 30 lx. The diets were as follows: (1) control group, basal diet; (2) YC1, basal diet +4 g/kg YPF-P; (3) YC2, basal diet +8 g/kg YPF-P; (4) YC3, basal diet +12 g/kg YPF-P. The period of 65 days was carried out in the last 2 weeks before the Column quantity. Qingyuan Partridge chickens and standard feed used in this experiment were purchased from Guangdong Tiannong Food Co., LTD. (Guangdong, China). This experiment was approved by the Institutional Experimental Animal Ethics Committee of Foshan University (FOSU 2022-292).
The basic diets of this experiment are shown in Table 1. The Yupingfeng polysaccharide was provided in powder form and added to the base diet, and was fed once a day at an average of 100 g/piece at 8:00 am. During the experiment, the free drinking water of Qingyuan polysaccharide chicken was guaranteed. At the end of the experiment, a chicken with a body weight close to the average was selected from each replicate, weighed and recorded the weight of the broiler, and blood samples were collected from the subwing vein. Finally, the chickens were killed and the cecal contents were collected, and the above samples were sent to Beijing Baimaike Biotechnology Co., LTD for 16S sequencing.
The initial body weight (IW) of broilers was recorded before the start of the experiment. On the 14th day of the experiment, broilers were fasted for 12 h, then the body weight (FW) of broilers was measured, and the growth was measured based on the average weight gain (DW) of surviving broilers in each group. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion (ADFI/ADG) were calculated.
At the end of the experimental period, six chickens were randomly selected from each group (one bird per replicate). After fasting for 12 h (taking water at will), a blood sample was obtained from the broiler by puncturing the wing vein of the broiler and the sample was transferred to a 1.5 mL Eppendorf tube. The tubes were centrifuged at 4°C at 3,000 × g for 15 min to isolate the serum, which was then stored at −20°C for analysis of serum parameters. According to the manufacturer’s instructions, the contents of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) were determined by ELISA kit (Shanghai enzyme-linked Biotechnology Co., Ltd., Shanghai, China).
Cecal contents isolated after slaughter. Total genomic DNA was extracted from Cecal contents samples using the TGuide S96 Magnetic Soil/Stool DNA Kit Tiangen Biotech (Beijing Co., Ltd.) according to the manufacturer’s instructions. The hypervariable region V3–V4 of the bacterial 16S rRNA gene were amplified with primer pairs 338F: 5′-ACTCCTACGGGAGGCAGCA-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′. PCR products were checked on agarose gel and purified through the Omega DNA purification kit (Omega Inc., Norcross, GA, United States). The purified PCR products were collected and the paired ends (2 × 250 bp) were performed on the Illumina Novaseq 6,000 platform.
The qualified sequences with more than 97% similarity thresholds were allocated to one operational taxonomic unit (OTU) using USEARCH (version 10.0). Taxonomy annotation of the OTUs/ASVs was performed based on the Naive Bayes classifier in QIIME2 using the SILVA database (35) (release 138.1) with a confidence threshold of 70%. Alpha was performed to identify the complexity of species diversity of each sample utilizing QIIME2 software. Beta diversity calculations were analyzed by principal coordinate analysis (PCoA) to assess the diversity in samples for species complexity. One-way analysis of variance was used to compare bacterial abundance and diversity. Linear discriminant analysis (LDA) coupled with effect size (LEfSe) was applied to evaluate the differentially abundant taxa. The online platform BMKCloud1 was used to analyze the sequencing data.
SPSS statistical software (Version 27 for windows, SPSS Inc., Chicago, IL, United States) was used for one-way analysis of variance (ANOVA). Duncan’s multirange test was used to examine the differences between treatments. All results are expressed in mean and standard error (SEM) of mean unless otherwise stated, p < 0.05 was considered statistically significant.
At the end of the experiment, compared with the control group, the final body weight, total weight gain and average daily gain of 8 g/kg Qingyuan Partridge chickens were significantly higher (p < 0.05), while the feed-meat ratio was significantly lower (p < 0.05) (see Table 2).
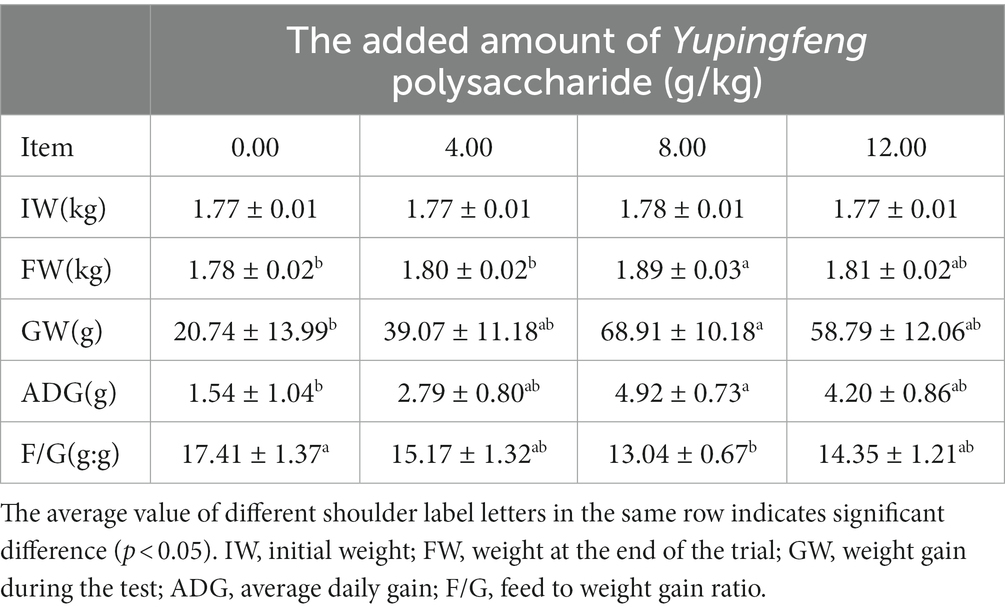
Table 2. Effects of adding different concentrations of Yupingfeng polysaccharide on production performance of Qingyuan Partridge chicken.
As shown in Figure 1, there were significant increases in serum GHRH and IGF-1 concentrations during 151–165d (p < 0.05). In addition, YPF-P supplementation did not affect serum GH concentration (p > 0.05).
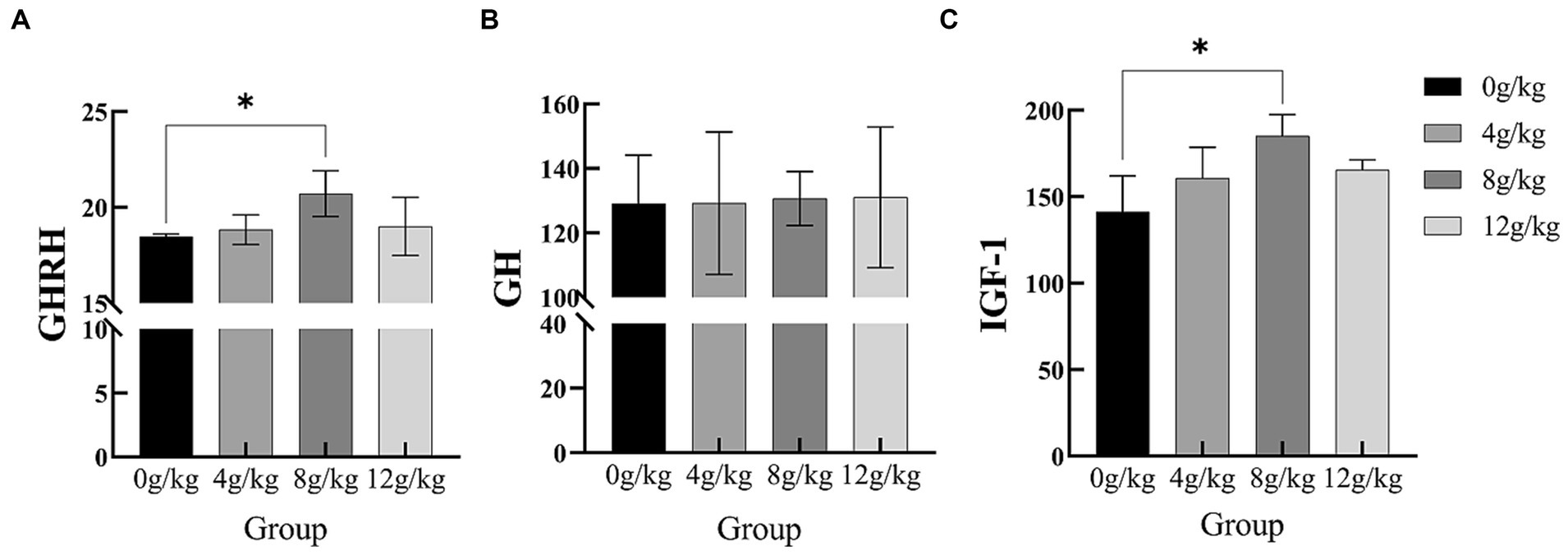
Figure 1. Effect of Yupingfeng polysaccharide on serum parameters of Qingyuan Partridge chickens. (A) Growth hormone releasing hormone (GHRH); (B) Growth hormone (GH); (C) Insulin-like growth factor-1 (IGF-1).
The original data was spliced (FLASH, version 1.2.11), the spliced sequences were qualitatively filtered (Trimmomatic, version 0.33), and the illusion was removed (UCHIME, version 8.1) to obtain high-quality Tags sequences. The collected data were analyzed, and draw the dilution curve (Figure 2A). The curve is asymptotic to the X-axis, indicating that the species in this environment will not increase significantly with the number of sequenced, and it is close to saturation. Sequences were clustered at the similarity level of 97% (USEARCH, version 10.0), and 0.005% of all sequences sequenced were filtered as a threshold. The filtered sequences became outs, and similar OTUs were intersected in the Venn diagram. Results as shown in Figures 2B,C, a total of 4,748 outs were aggregated in the two groups, of which 1,688 were unique to the control group, and 2,315 were unique to the test group.
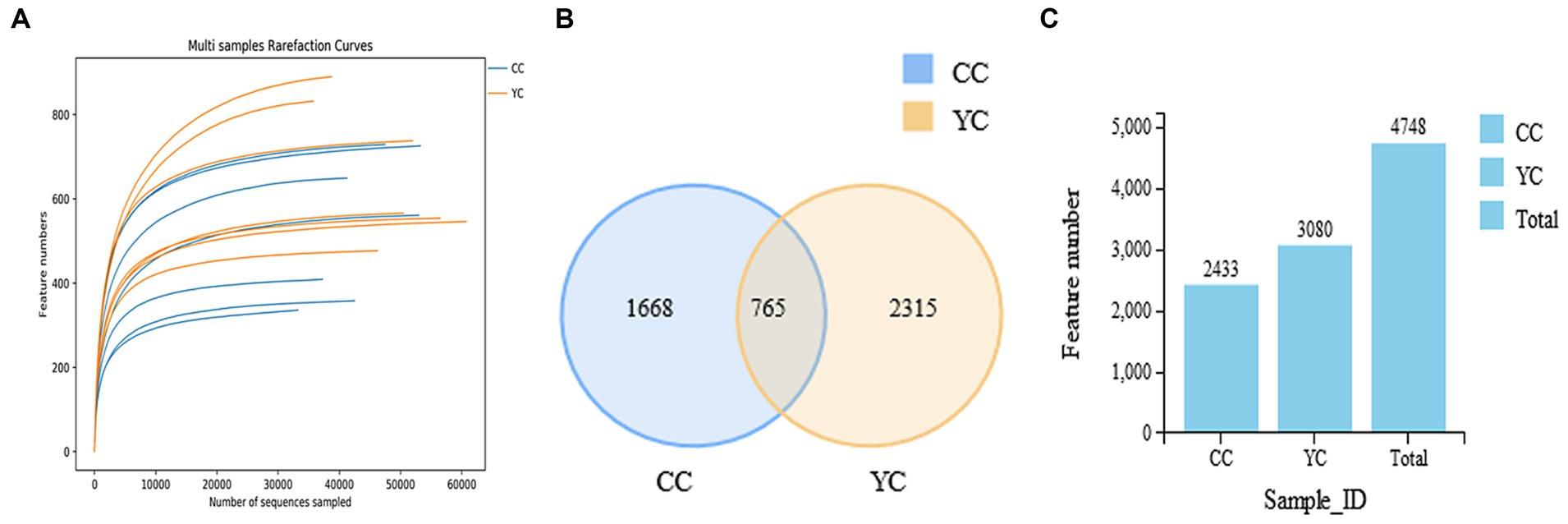
Figure 2. Dilution curve, number of OUT distributions and Venn diagram. (A) Dilutive curve of gut microbes. Abscissa: number of sequencing data; Ordinate: number of species observed. (B) Two groups of OTU distribution histogram; (C) Veen. CC, control group; YPF-P, supplemental level was 0 g/kg; YC, YPF-P supplemental amount is 8 g/kg.
Shannon index and Simpson index were used to measure the α diversity of species, and the β diversity of cecal flora between different groups was displayed by principal coordinate analysis (PCoA) results. The results of cecal microbial α diversity analysis between the two groups are shown in Figures 3A,B. Compared with the control group, there were no significant differences in Shannon index and Simpson index in YC polysaccharide addition group (YC group). However, all of them were higher than those in the control group, indicating that the addition of polysaccharide had a tendency to increase the diversity of cecal flora. The results of PCoA analysis (Figure 3C) showed that the microbial communities of the control group and the Yupingfeng polysaccharide addition group were significantly separated, and the contribution rates of the first two principal components PCoA1 and PCoA2 were 20.71% and 12.41%, respectively, which revealed certain changes in intestinal flora. Correlation network analysis based on relative abundance at the genus level showed that both CC group and YC group had 49 nodes and 100 edges, but the core genera of CC group and YC group contained 10 phylum and 9 phylum, respectively, indicating that YPF-P could change the distribution of cecal microflora of Qingyuan Partridge Chicken (Figures 3D,E).
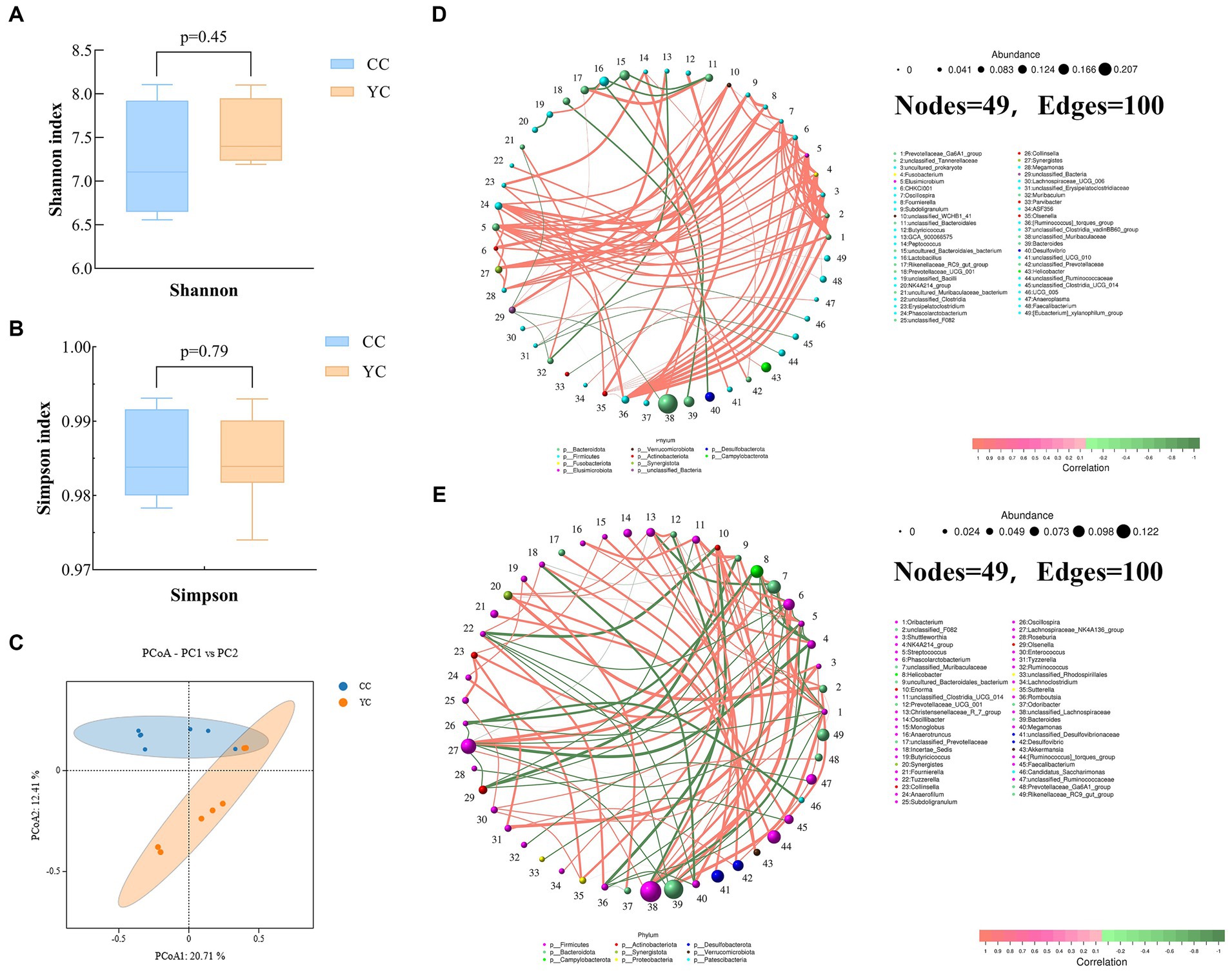
Figure 3. Effects of Yupingfeng polysaccharide on intestinal flora diversity of Qingyuan Partridge chickens. (A) The Simpson index results of alpha diversity of cecal microbiota in different groups. Data were analyzed by Student’s t test; (B) The Simpson index results of alpha diversity of cecal microbiota in different groups. Data were analyzed by Student’s t test; (C) Principal coordinate analysis (PCoA) of cecal microbiota in different groups. PCoA plotswere generated using OUT abundance data according to the Binary–Jaccard distance algorithm; (D) Correlation network analysis of cecal microorganisms based on core genera of CC group. Different colors of nodes indicate different phylum levels. Edges represent significant correlations (p < 0.05); (E) Correlation network analysis of cecal microorganisms based on core genera of YC group. Different colors of nodes indicate differentphylum levels. Edges represent significant correlations (p < 0.05).
Using SILVA as a reference database, the naive Bayes classifier was used to annotate the feature sequences, and the species classification information corresponding to each feature could be obtained. Then the sample community composition could be counted at various levels (phylum, class, order, family, genus, species). QIIME software was used to generate species abundance tables at different taxonomic levels, and R were used to draw community structure histogram at different taxonomic levels. Species distribution histogram at phylum level and genus level was selected to display the results, as shown in Figure 4. The analysis of species annotations at the phylum classification level (Figure 4A) showed that the top 10 intestinal flora included Firmicutes, Bacteroideta, Desulfobacterota, Campylobacterota, Actinobacteriota, Proteobacteria, Fusobacteriota, and Synergistota, among which Firmicutes and Bacteroidetes accounted for the highest content, which could reach more than 80% of the relative abundance. There were Firmicutes (43.18%) and Bacteroidetes (41.94%) in the control group and Firmicutes (55.45%) and Bacteroidetes (25.30%) in the YC group. Compared with the control group, the Firmicutes were significantly increased (p < 0.05) and Bacteroidetes were significantly decreased (p < 0.05) in the YC group (Figure 4C). The species annotation analysis at the genus classification level (Figure 4B) showed that the dominant flora were: unclassified_Muribaculaceae, unclassified_Lachnospiraceae, Bacteroides, Lachnospiraceae_NK4A136_group, unclassified_Oscillospiraceae, Helicobacter, [Ruminococcus]_torques__group, Desulfovibrio, Rikenellaceae_Rc9_gut_group, unclassified_Bacteroidales, et al. Among them, there was a significant difference between the two groups in unclassified_Muribaculaceae, uncultured_Bacteroidales_bacterium, unclassified_Oscillospiraceae, Lactobacillus, Alloprevotella, Ligilactobacillus, Prevotellaceae_UCG_001, and unclassified_Atopobiaceae (Figure 4D), then unclassified_Oscillospiraceae was significantly increased, while several other differential genera were significantly decreased.
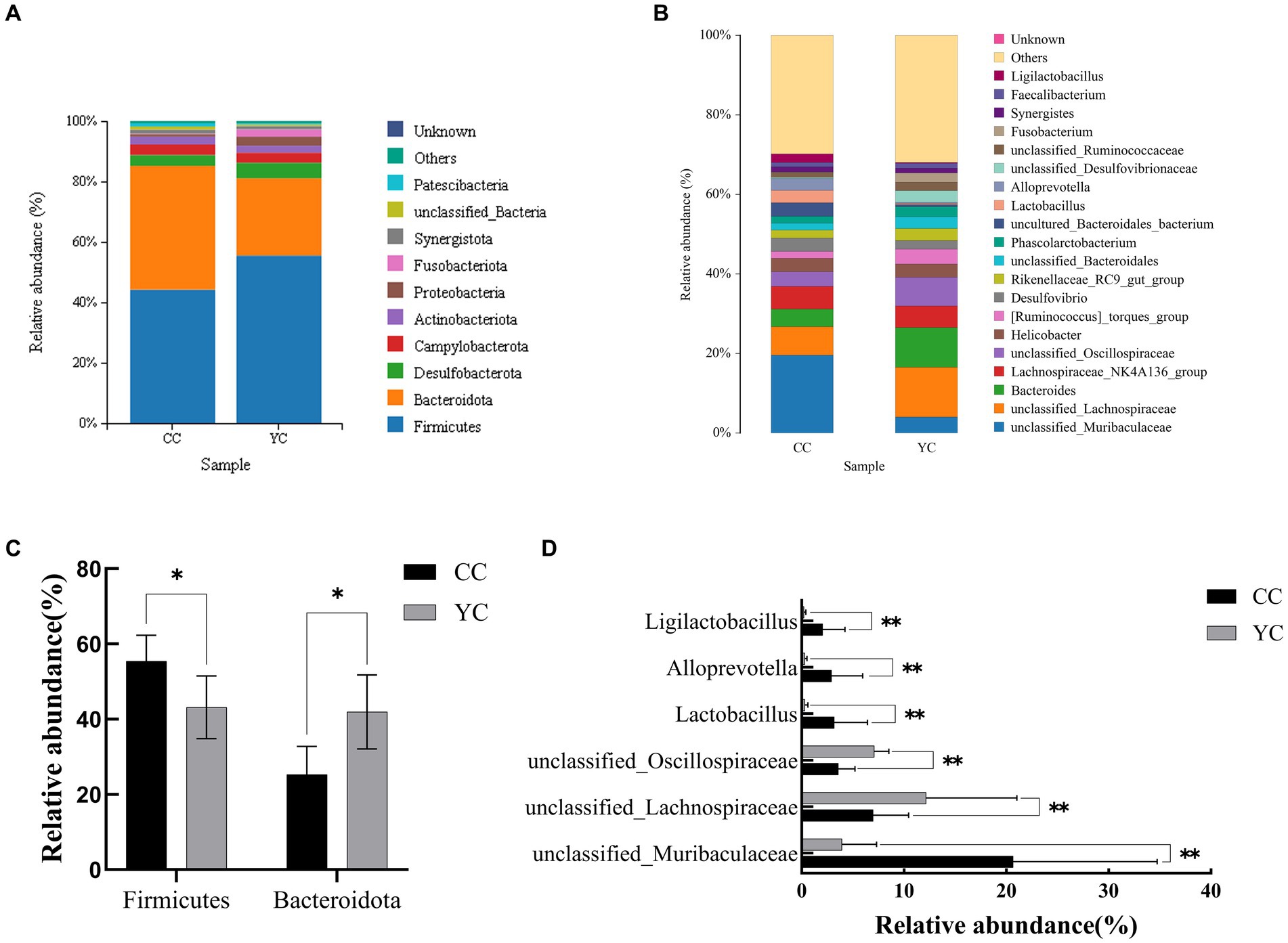
Figure 4. Effects of Yupingfeng polysaccharide on intestinal microflora phylum and genus level of Qingyuan Partridge chickens. (A) Phylum-level species distribution bar chart, showing only the top 10 species; (B) Genus level species distribution bar chart, showing only the top 20 species; (C) Bacterialphyla with significant differences at phylum level; (D) Bacterial genera with significant differences at the genus level.
Picrust2(2.3.0) software was used to compare the sample 16SrDNA with the reference sequence of the Integrated Microbial Genome (IMG) database, build the species evolutionary tree, and identify the closest species. Corresponding to the family information in KEGG and COG databases, the analysis results of KEGG metabolic pathway differences between groups and the statistical results of COG functional classification are shown in Figure 5. Figure 5A shows differences in metabolic pathways between control and experimental groups, including cell growth and death, signaling molecules and interactions interaction, translation, and metabolism of other amino acids pathway. Functional differences between the two groups were translation, ribosomal structure and biogenesis, post-translation modification, protein turnover, and chaperones (Figure 5B).

Figure 5. Functional predictive analysis. (A) Difference analysis diagram of KEGG metabolic pathway at the second level. Different colors in the diagram represent different groups. (B) Statistical results of COG functional classification in the second level. The figure on the left shows the abundance ratio of different functions in two samples or two groups of samples, the middle shows the difference ratio of functional abundance in the 95% confidence interval, and the value on the far right is the p-value.
In recent years, studies have shown that the extracts of medicinal plant active ingredients, including Chinese herbal polysaccharides, have been playing a positive role in livestock production and disease prevention and treatment (12). Chinese herbal polysaccharide can play a variety of biological functions (36, 37), such as anti-inflammatory (38), anti-tumor (39), antioxidant, lowering blood sugar, lowering blood lipids (40), immune regulation (41), and regulating intestinal flora (42), etc. Chen et al. found that the basal diet supplemented with 1,000 mg/kg Lonicera fulvotomentosa extract could significantly increase the final weight of weaned piglets and the diversity and richness of intestinal flora in the jejunum and rectum. With the increase inf extract concentration (600 mg/kg, 800 mg/kg, 1,000 mg/kg), the diarrhea index of piglets was decreased [p < 0.05, (43)]. Appropriate doses of Piper betel (PB) (4 g/kg) and Persicaria odorata (PO) (8 g/kg) can also be used as growth promoters to significantly increase weight gain and reduce feed reward in broilers while positively regulating intestinal structure (44). Reda observed that adding 750 and 1,000 mg/kg of Licorice (Glycyrrhiza glabra) to the diet of Japanese quails enhanced the growth performance of the animals and reduced intestinal pathogens (45). These results are consistent with our experimental results. In this experiment, when the added amount of Yupingfeng polysaccharide is 8 g/kg, the final body weight, the total weight gain and the average daily gain of Qingyuan Partridge chickens can be significantly improved (p < 0.05), and the feed-to-gain ratio can be significantly reduced (p < 0.05). Therefore, appropriate concentration added to the basic diet of jade screen polysaccharide can improve chicken growth performance.
Growth hormone and insulin-like growth factor are both peptides that can promote growth and play an active role in the body’s growth, development and metabolism. Growth hormone, also known as somatotropin, has widely distributed receptors that stimulate the growth and differentiation of muscle, bone, and cartilage. On the other hand, growth hormone also stimulates the production of insulin-like growth factors (IGFs), which can indirectly mediate many of the growth-promoting effects of growth hormone (46). There are two forms of IGF, IGF-I and IGF-II. Among them, the production of IGF-I is more dependent on GH, and its growth-promoting effect is stronger. Some studies have shown that dietary supplementation of Radix Rehmanniae Preparata polysaccharides (RRPP) can promote and significantly improve growth performance (p < 0.05); the mRNA expression levels of serum growth hormone (GH), insulin-like growth factor IGF-I and IGF-II were significantly increased by RRPP supplementation (47). Similarly, the research of Zhao et al. (48) also has the same conclusion: in early weaned piglets, diets supplemented with different concentrations of Mulberry leaf polysaccharides (MLPs) and antibiotics found that IGF-I and GH levels in Mulberry leaf polysaccharide treated (MT) group were significantly higher than those in control treated (CT) group and antibiotic treated (AT) group (p < 0.05). Ouyang et al. (49) also reported that supplementation with 1.0% and 1.5% Ouyang et al. also reported that supplementation with 1.0% and 1.5% water soluble Alfalfa olysaccharides (WSAP) significantly or extremely significantly increased the expression of GH and IGF-I genes in liver, adipose tissue and kidney of broilers (p < 0.05 or p < 0.01), and improved the growth performance and carcass traits of broilers. In this experiment, the same conclusion was also obtained, when the added amount of YPF-P was 8 g/kg, the expression levels of IGF-I were significantly increased (p < 0.05), and the concentration of GH was not significantly changed, but there was a trend of increase. This is also consistent with our results in the previous part, when the amount of Yupingfeng polysaccharide added to the diet was 8 g/kg, the weight gain of Qingyuan Partridge chicken was the most obvious, and the feed reward was the lowest. Therefore, it was concluded that Yupingfeng polysaccharide could promote growth.
A large microbial community exists in the gastrointestinal tract and these microorganisms play an important role in the growth and health of chickens, promoting the absorption of nutrients and improving the immune system (50). The addition of Chinese herbal ingredients and their derivatives can improve the community composition of intestinal microorganisms of broilers, affect the digestion and absorption of nutrients, and thus affect the growth performance and immune performance of broilers (51–56). α diversity results showed that compared with the control group, there were no significant differences in Shannon, and Simpson indices in the YC group, indicating that adding Yupingfeng polysaccharide did not change the species diversity and richness of intestinal flora. The PCoA map of β diversity results showed that the intestinal flora of the control group and the experimental group were significantly separated, with little overlap, and there was a significant difference between the groups, suggesting that there was a substantial difference in the microbial community between the two groups, and the analysis results were reliable. At the phylum level, Firmicutes, Bacteroidetes, Desulfobacterota, Campylobacter, Actinobacteria, and Proteobacteria were the main microbial groups in the cecum, among which Firmicutes (43.18%) and Bacteroidetes (41.94%) were in the control group, and Firmicutes (55.45%) and Bacteroidetes (25.30%) were in the YC group. Studies by Li et al. showed that Bacteroides, Firmicutes, Proteus, and Ferribacter, accounted for more than 97% of the cecal microbiota of all chickens (57); the top two are Firmicutes and Bacteroidetes, which are consistent with our results. Hou et al. found that in Arbor Acres broilers fed the same diet in FL and LL lines, Firmicutes and Bacteroides accounted for 71.36% and 23.40% of the intestinal flora in fat chickens, 53.44% and 41.09% in lean chickens, respectively (58). Consistent with the findings of Furet and Chika Kasai, non-obese subjects had higher Bacteroides abundances at the phylum level in their gut microbes compared to obese subjects (59, 60). In this experiment, the addition of Yupingfeng polysaccharide tended to reduce the relative abundance of Bacteroidetes, consistent with the study’s results, and the ratio of Firmicutes to Bacteroidetes increased, and the weight of birds increased. At the genus level, the predominant bacteria groups of cecum contents are unclassified_Muribaculaceae, unclassified_Lachnospiraceae, Bacteroides, Lachnospiraceae_NK4A136_group, and unclassified_Oscillospiraceae. Among them, the first three belong to Bacteroides, while the last two belong to Firmicutes and Clostridium. The flora that differed between the CC and YC group were unclassified_Muribaculaceaeand unclassified_Oscillospiraceae. Hu et al. found that Grifola frondosa powder suspension (GFPS, powder of a medicinal fungus with anti-obesity properties) can reduce body weight and increase the abundance of unclassified_Muribaculaceae in the intestinal flora of mice (61), which is exactly consistent with our results. unclassified_Muribaculaceae is a bacterium that produces short-chain fatty acids, which are important in regulating glucose and lipid metabolism. These results indicate that Yupingfeng polysaccharide can regulate the abundance of different intestinal flora by increasing the proportion of beneficial bacteria and reducing the proportion of harmful bacteria to promote the growth performance of chickens.
In summary, this study shows that adding Yupingfeng polysaccharide to the basic diet can improve the growth performance of broilers and have a particular effect on the composition of their intestinal flora. When 8 g/kg Yupingfeng polysaccharide was added to the diet, the final body weight, the total weight gain and the average daily gain of Qingyuan Partridge chickens was increased (p < 0.05), and the feed reward was also decreased (p < 0.05). At the same time, it can promote the expression of GH and IGF-I in serum. Although adding Yupingfeng polysaccharide did not change the diversity and richness of intestinal flora, it could significantly affect the distribution proportion of dominant flora and increase the proportion of dominant flora. This study can provide the basic theoretical basis for utilizing Yupingfeng polysaccharide in broiler production.
The original contributions presented in the study are publicly available. This data can be found at: https://www.ncbi.nlm.nih.gov/bioproject/; PRJNA1067511.
The animal studies were approved by Institutional Experimental Animal Ethics Committee of Foshan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
YG: Data curation, Formal analysis, Methodology, Writing – original draft. WZ: Methodology, Visualization, Writing – review & editing. YB: Data curation, Writing – review & editing. BW: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key-Area Research and Development Program of Guangdong Province (2019B110209005), National Natural Science Foundation of China Youth Science Foundation (32102752), and the Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding (2019B030301010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
YPF-P, Yupingfeng polysaccharide; GW, weight gain; ADG, average daily gain; ADFI, average daily feed intake; GH, growth hormone; IGF-1, insulin-like growth factor 1; CC group, control group; YC group, Yupingfeng polysaccharide addition group
1. Van, TTH , Yidana, Z , Smooker, PM , and Coloe, PJ . Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J Global Antimicrobial Resistance. (2020) 20:170–7. doi: 10.1016/j.jgar.2019.07.031
2. Cheng, G , Hao, H , Xie, S , Wang, X , Dai, M , Huang, L, et al. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol. (2014) 5:217. doi: 10.3389/fmicb.2014.00217
3. Fernández Miyakawa, ME , Casanova, NA , and Kogut, MH . How did antibiotic growth promoters increase growth and feed efficiency in poultry? Poult Sci. (2024) 103:103278. doi: 10.1016/j.psj.2023.103278
4. Abd El-Ghany, WA . Paraprobiotics and Postbiotics: contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet J. (2020) 10:323–30. doi: 10.4314/ovj.v10i3.11
5. Manyi-Loh, C , Mamphweli, S , Meyer, E , and Okoh, A . Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules. (2018) 23:795. doi: 10.3390/molecules23040795
6. Aljumaah, MR , Alkhulaifi, MM , and Abudabos, AM . In vitro antibacterial efficacy of non-antibiotic growth promoters in poultry industry. J Poult Sci. (2020) 57:45–54. doi: 10.2141/jpsa.0190042
7. Liu, Y , Li, Y , Niu, J , Liu, H , Jiao, N , Huang, L, et al. Effects of dietary Macleaya Cordata extract containing Isoquinoline alkaloids supplementation as an alternative to antibiotics in the diets on growth performance and liver health of broiler chickens. Front Vet Sci. (2022) 9:950174. doi: 10.3389/fvets.2022.950174
8. Rounds, T , and Straus, SK . Lipidation of antimicrobial peptides as a design strategy for future alternatives to antibiotics. Int J Mol Sci. (2020) 21:9692. doi: 10.3390/ijms21249692
9. Evangelista, AG , Corrêa, JAF , Pinto, A , and Luciano, FB . The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance – a review. Crit Rev Food Sci Nutr. (2022) 62:5267–83. doi: 10.1080/10408398.2021.1883548
10. Gupta, M , Anzelc, M , Stetkevich, S , and Burkhart, C . Bacteriophages: an alternative to combat antibiotic resistance? J Drugs Dermatol. (2022) 21:1311–5. doi: 10.36849/jdd.6638
11. Abd El-Hack, ME , El-Saadony, MT , Salem, HM , El-Tahan, AM , Soliman, MM , Youssef, GBA, et al. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult Sci. (2022) 101:101696. doi: 10.1016/j.psj.2022.101696
12. Kuralkar, P , and Kuralkar, SV . Role of herbal products in animal production – an updated review. J Ethnopharmacol. (2021) 278:114246. doi: 10.1016/j.jep.2021.114246
13. Zhengzao, Y , Haichao, S , Minghua, Y , Yanguang, Z , and Sumei, Z . Research progress on effect of herbal feed additives from plants. Feed Industry. (2022) 43:29–34. doi: 10.13302/j.cnki.fi.2022.02.006
14. Olawuwo, OS , Abdalla, MA , Mühling, KH , and McGaw, LJ . Proximate analysis of nutrients and in vitro radical scavenging efficacy in selected medicinal plant powders with potential for use as poultry feed additives. S Afr J Bot. (2022) 146:103–10. doi: 10.1016/j.sajb.2021.09.038
15. Xing, Y , Zheng, Y , Yang, S , Zhang, L , Guo, S , Shi, L, et al. Artemisia Ordosica polysaccharide ameliorated Lps-induced growth inhibition and intestinal injury in broilers through enhancing immune-regulation and antioxidant capacity. J Nutr Biochem. (2023) 115:109284. doi: 10.1016/j.jnutbio.2023.109284
16. Jahja, EJ , Yuliana, R , Simanjuntak, WT , Fitriya, N , Rahmawati, A , and Yulinah, E . Potency of Origanum Vulgare and Andrographis Paniculata extracts on growth performance in poultry. Vet Animal Sci. (2023) 19:100274. doi: 10.1016/j.vas.2022.100274
17. Li, P , Qiu, Q , Qin, C , Mo, H , Liu, X , Shi, J, et al. Research Progress of Yupingfengsan and predictive analysis on quality markers. Chin Arch Tradit Chin Med. (2024):1–18.
18. Wang, H , Zhao, K , Gao, X , Li, R , and Xu, X . Effects of melatonin and Yupingfeng powder on Lmmune system in mice based Onnetwork pharmacology. World Chin Med. (2022) 17:1540–6.
19. Wang, D , Wang, D , Cao, Q , and Yan, K . Network pharmacology mechanism of Yupingfeng powder for the prevention of Covid 19 based on Ace2 receptor. Western J Tradit Chin Med. (2023) 36:18–24.
20. Zhao, Y , Liang, M , Wang, F , and Hu, K . A study on the action mechanism of Yupingfeng san in treating non-small cell Lungcancer based on network pharmacology. Clin J Chin Med. (2023) 15:1–8.
21. Ma, Z , Zhao, Q , and Lin, Y . Experimental study of Yupingfeng powder and its research progress in prevention and treatment of senility. Clin Res Pract. (2022) 7:188–90. doi: 10.19347/j.cnki.2096-1413.202209053
22. Sun, H , Ni, X , Zeng, D , Zou, F , Yang, M , Peng, Z, et al. Bidirectional immunomodulating activity of fermented polysaccharides from Yupingfeng. Res Vet Sci. (2017) 110:22–8. doi: 10.1016/j.rvsc.2016.10.015
23. Yang, Y , Yang, M , Yi, M , Huang, Y , Yu, H , and Lai, M . Effect of Yupingfeng polysaccharide compound microecological preparation on growth and intestinal microflora of grass carp. Feed Res. (2023) 46:66–70. doi: 10.13557/j.cnki.issn1002-2813.2023.11.014
24. Wang, D , Zhang, BB , Qu, XX , Gao, F , and Yuan, MY . Microwave-assisted extraction of polysaccharides from Yupingfeng powder and their antioxidant activity. Pharmacogn Mag. (2015) 11:546–54. doi: 10.4103/0973-1296.160468
25. Zheng, W , Guan, Y , and Wu, B . Effects of Yupingfeng polysaccharides as feed supplement on immune function and intestinal microbiome in chickens. Microorganisms. (2023) 11:2774. doi: 10.3390/microorganisms11112774
26. Su, C , Fan, D , Pan, L , Lu, Y , Wang, Y , and Zhang, M . Effects of Yu-ping-Feng polysaccharides (Yps) on the immune response, intestinal microbiota, disease resistance and growth performance of Litopenaeus Vannamei. Fish Shellfish Immunol. (2020) 105:104–16. doi: 10.1016/j.fsi.2020.07.003
27. Filardy, AA , Ferreira, JRM , Rezende, RM , Kelsall, BL , and Oliveira, RP . The intestinal microenvironment shapes macrophage and dendritic cell identity and function. Immunol Lett. (2023) 253:41–53. doi: 10.1016/j.imlet.2023.01.003
28. Guevara-Garcia, A , Soleilhac, M , Minc, N , and Delacour, D . Regulation and functions of cell division in the intestinal tissue. Semin Cell Dev Biol. (2023) 150–151:3–14. doi: 10.1016/j.semcdb.2023.01.004
29. McCarty, MF , and Lerner, A . Perspective: prospects for nutraceutical support of intestinal barrier function. Adv Nutr. (2021) 12:316–24. doi: 10.1093/advances/nmaa139
30. Ghosh, S , Whitley, CS , Haribabu, B , and Jala, VR . Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. (2021) 11:1463–82. doi: 10.1016/j.jcmgh.2021.02.007
31. Xi, Y , Shuling, N , Kunyuan, T , Qiuyang, Z , Hewen, D , Chen Cheng, G, et al. Characteristics of the intestinal Flora of specific pathogen free chickens with age. Microb Pathog. (2019) 132:325–34. doi: 10.1016/j.micpath.2019.05.014
32. Guo, FC , Williams, BA , Kwakkel, RP , Li, HS , Li, XP , Luo, JY, et al. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult Sci. (2004) 83:175–82. doi: 10.1093/ps/83.2.175
33. Wu, G , Gu, W , Chen, G , Cheng, H , Li, D , and Xie, Z . Interactions of tea polysaccharides with gut microbiota and their health-promoting effects to host: advances and perspectives. J Funct Foods. (2023) 102:105468. doi: 10.1016/j.jff.2023.105468
34. Yao, S , Yang, X , Wu, W , Jiang, Q , Deng, S , Zheng, B, et al. Effect of Paecilomyces cicadae polysaccharide Pc0-1 on cyclophosphamide-induced immunosuppression and regulation of intestinal Flora in mice. Food Biosci. (2023) 51:102340. doi: 10.1016/j.fbio.2022.102340
35. Quast, C , Pruesse, E , Yilmaz, P , Gerken, J , Schweer, T , Yarza, P, et al. The Silva ribosomal Rna gene database project: improved data processing and web-based tools. Nucl Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
36. Taoerdahong, H , Kadeer, G , Chang, J , Kang, J , Ma, X , and Yang, F . A review concerning the polysaccharides found in edible and medicinal plants in Xinjiang. Molecules. (2023) 28:2054. doi: 10.3390/molecules28052054
37. Bai, C , Su, F , Zhang, W , and Kuang, H . A systematic review on the research Progress on polysaccharides from fungal traditional Chinese medicine. Molecules. (2023) 28:196816. doi: 10.3390/molecules28196816
38. Yang, Y , Xiao, G , Cheng, P , Zeng, J , and Liu, Y . Protective application of Chinese herbal compounds and formulae in intestinal inflammation in humans and animals. Molecules. (2023) 28:196811. doi: 10.3390/molecules28196811
39. Zhou, M , Yue, Y , Wang, Y , and Yan, S . Polysaccharides from Chinese herbs as natural weapons against colorectal Cancer. Biosci Rep. (2023) 43:e41. doi: 10.1042/bsr20230041
40. Yang, X , Cao, D , Ji, H , Xu, H , Feng, Y , and Liu, A . Physicochemical characterization, rheological properties, and hypolipidemic and antioxidant activities of compound polysaccharides in Chinese herbal medicines by fractional precipitation. Int J Biol Macromol. (2023) 242:124838. doi: 10.1016/j.ijbiomac.2023.124838
41. Ba, X , Yang, Y , and Zhang, A . Advances in lmmunoregulatory effects of polysaccharides from Chinese herbal medicines on dendritic cells. Prog Vet Med. (2018) 39:102–6. doi: 10.16437/j.cnki.1007-5038.2018.05.020
42. Xue, H , Mei, CF , Wang, FY , and Tang, XD . Relationship among Chinese herb polysaccharide (Chp), gut microbiota, and chronic diarrhea and impact of Chp on chronic diarrhea. Food Sci Nutr. (2023) 11:5837–55. doi: 10.1002/fsn3.3596
43. Chen, L . Effect of Lonicera Fulvotomentosa extract on growth performance, immune function and intestinal Flora of weaned piglets, Master’s thesis Guizhou Normal University (2017).
44. Basit, MA , Arifah, AK , Loh, TC , Saleha, AA , Salleh, A , Kaka, U, et al. Effects of graded dose dietary supplementation of Piper Betle leaf meal and Persicaria Odorata leaf meal on growth performance, apparent Ileal digestibility, and gut morphology in broilers. Saudi J Biol Sci. (2020) 27:1503–13. doi: 10.1016/j.sjbs.2020.04.017
45. Reda, FM , El-Saadony, MT , El-Rayes, TK , Farahat, M , Attia, G , and Alagawany, M . Dietary effect of licorice (Glycyrrhiza Glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult Sci. (2021) 100:101266. doi: 10.1016/j.psj.2021.101266
46. Welniak, LA , and Murphy, WJ . Chapter 5—growth hormone. AW Thomson and MT Lotze, (Eds.), The cytokine handbook (4th Edn). London: Academic Press (2003). 103–113
47. Wu, C , Shan, J , Feng, J , Wang, J , Qin, C , Nie, G, et al. Effects of dietary radix rehmanniae preparata polysaccharides on the growth performance, immune response and disease resistance of Luciobarbus Capito. Fish Shellfish Immunol. (2019) 89:641–6. doi: 10.1016/j.fsi.2019.04.027
48. Zhao, X , Li, L , Luo, Q , Ye, M , Luo, G , and Kuang, Z . Effects of mulberry (Morus Alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livest Sci. (2015) 177:88–94. doi: 10.1016/j.livsci.2015.03.001
49. Ouyang, K , Xiong, X , Wang, W , Hu, Y , Lai, Y , and Wu, D . Effect of water soluble alfalfa polysaccharide on growth performance, carcass quality, growth hormone and insulin-like growth factor-1 gene expression of broilers. Chin J Animal Nutr. (2014) 26:1272–8.
50. Fathima, S , Shanmugasundaram, R , Adams, D , and Selvaraj, RK . Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens. Foods. (2022) 11:101401. doi: 10.3390/foods11101401
51. Murugesan, GR , Syed, B , Haldar, S , and Pender, C . Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front Vet Sci. (2015) 2:21. doi: 10.3389/fvets.2015.00021
52. Ballou, AL , Ali, RA , Mendoza, MA , Ellis, JC , Hassan, HM , Croom, WJ, et al. Development of the Chick microbiome: how early exposure influences future microbial diversity. Front Vet Sci. (2016) 3:2. doi: 10.3389/fvets.2016.00002
53. Chen, HL , Li, DF , Chang, BY , Gong, LM , Dai, JG , and Yi, GF . Effects of Chinese herbal polysaccharides on the immunity and growth performance of young broilers. Poult Sci. (2003) 82:364–70. doi: 10.1093/ps/82.3.364
54. Hashemi, SR , and Davoodi, H . Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet Res Commun. (2011) 35:169–80. doi: 10.1007/s11259-010-9458-2
55. Liu, Y , Miao, Y , Xu, N , Ding, T , Cui, K , Chen, Q, et al. Corrigendum to “effects of dietary Astragalus polysaccharides (aps) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys Crocea) larvae”. Aquaculture. (2020) 520:734997. doi: 10.1016/j.aquaculture.2020.734997
56. Long, LN , Zhang, HH , Wang, F , Yin, YX , Yang, LY , and Chen, JS . Research note: effects of polysaccharide-enriched Acanthopanax Senticosus extract on growth performance, immune function, Antioxidation, and Ileal microbial populations in broiler chickens. Poult Sci. (2021) 100:101028. doi: 10.1016/j.psj.2021.101028
57. Xiang, L , Si, C , Zhao, Z-T , Meng, Z , Yi, H , Ye, X-M, et al. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int J Biol Macromol. (2020) 155:61–70. doi: 10.1016/j.ijbiomac.2020.03.198
58. Hou, Q , Kwok, LY , Zheng, Y , Wang, L , Guo, Z , Zhang, J, et al. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci Rep. (2016) 6:37376. doi: 10.1038/srep37376
59. Furet, JP , Kong, LC , Tap, J , Poitou, C , Basdevant, A , Bouillot, JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. (2010) 59:3049–57. doi: 10.2337/db10-0253
60. Kasai, C , Sugimoto, K , Moritani, I , Tanaka, J , Oya, Y , Inoue, H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. (2015) 15:100. doi: 10.1186/s12876-015-0330-2
Keywords: Yupingfeng polysaccharide, chicken, antibiotics, production performance, gut microbiota
Citation: Guan Y, Zheng W, Bai Y and Wu B (2024) Yupingfeng polysaccharide promote the growth of chickens via regulating gut microbiota. Front. Vet. Sci. 11:1337698. doi: 10.3389/fvets.2024.1337698
Received: 13 November 2023; Accepted: 08 February 2024;
Published: 23 February 2024.
Edited by:
Liwei Guo, Yangtze University, ChinaReviewed by:
Mustafa Shukry, Kafrelsheikh University, EgyptCopyright © 2024 Guan, Zheng, Bai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wu, d2IwMjA5MDIwOUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.