- 1Research Center for High Altitude Medicine of Qinghai University, Xining, Qinghai, China
- 2Key Laboratory of High Altitude Medicine in Qinghai Provincial, Qinghai University, Xining, Qinghai, China
- 3Department of Medical Imaging Center, Qinghai University Affiliated Hospital, Xining, Qinghai, China
Introduction: Alveolar echinococcosis (AE) is a parasitic disease caused by E. multilocularis metacestodes and it is highly prevalent in the northern hemisphere. We have previously found that vaccination with E. multilocularis-Leucine aminopeptidase (EM-LAP) could inhibit the growth and invasion of E. multilocularis in host liver, and Ubenimex, a broad-spectrum inhibitor of LAP, could also inhibit E. multilocularis invasion but had a limited effect on the growth and development of E. multilocularis.
Methods: In this study, the therapeutic effect of Ubenimex combined with Albendazole on AE was evaluated. Mice were intraperitoneally injected with protoscoleces and imaging examination was performed at week 8 and week 16 to detect cyst change. During this period, mice were intraperitoneally injected with Ubenimex and intragastrically administered with Albendazole suspension. At last, the therapeutic effect was evaluated by morphological and pathological examination and liver function.
Results: The results revealed that the combined treatment could inhibit the growth and infiltration of cysts in BALB/c mice infected with E. multilocularis protoscoleces. The weight, number, invasion and fibrosis of cysts were reduced in mice treated with Ubenimex in combination with Albendazole. The same effect was achieved by the single Ubenimex treatment because of its inhibitory effect on LAP activity, but it was less effective in inhibiting the growth of cysts. The levels of ALT, AST, TBIL, DBIL, ALP, and γ-GT were reduced after the combined treatment, indicating that treatment with both Ubenimex and Albendazole could alleviate liver damage.
Discussion: This study suggests that the combined treatment with Ubenimex and Albendazole could be a potential therapeutic strategy for E. multilocularis infections.
Introduction
Alveolar echinococcosis (AE) is a zoonotic parasitic disease caused by Echinococcus multilocularis metacestodes and it is highly prevalent in the northern hemisphere, particularly in developing countries (1). AE is difficult to treat and can only be removed surgically in clinic. However, not all infection foci could be removed surgically in end-stage AE patients with multiple organ metastasis, and in this case liver transplantation becomes the only therapeutic strategy (2). Drug therapy still plays a major role in the treatment of AE (3). Imidazole derivatives, such as Albendazole and Phenimidazole, are included in the World Health Organization's (WHO) Standard List of Essential medicines, but they have a limited effect on AE as they could only inhibit the uptake of glucose and deplete the glycogen of the germinal layer cells but could not restrain the growth and invasion of parasites (4, 5). E. multilocularis protoscoleces grow like a malignant tumor in the host and will infect the whole liver within only 5–10 years. AE may develop into a “parasitic cancer” and metastasize to other organs, causing portal hypertension, cerebral echinococcosis, pulmonary echinococcosis and even death (4, 6). Therefore, AE has become a public health problem of worldwide concern, especially in some developing countries such as China, Algeria, Kazakhstan and Mongolia. This highlights the need to find effective ways to control the growth and invasion of parasites (4). The difficulty in treating AE arises from the insidious onset and infiltrative growth and the tiny lesions are likely to relapse. E. multilocularis metacestodes and cysts can invade the vasculature, resulting in metastases to other liver lobes and even other tissues and organs. E. multilocularis can also lead to liver fibrosis through E. multilocularis Leucine aminopeptidase (EM-LAP) (7, 8). Existing drugs like Albendazole have lower water solubility and drug concentration in hydatid lesions, making it difficult to completely clear all parasites and restrain infiltrative growth (9). Even if increase the concentration or prolonged the treatment periods of Albendazole, may not improve treatment effect, but some toxic effect such as liver toxicity, allergic reactions, and rarely severe myelosuppression may occur (10). Up to now, there still has not ideal therapeutic methods and medicines to heal it, surgical operation and organ transplantation was only option in end-stage of AE.
Some other research has reported that drug combination was an effective way to treat AE, the combination of Carvacrol and Albendazole can enhanced the efficacy of monotherapy in AE, this therapeutic method may allow to improve the efficacy of the treatment without increasing the doses of Albendazole or prolong the therapy period, reducing the occurrence of toxic effects and adverse effects (11). Metformin can improve the therapeutic effect of low-dose Albendazole against AE, combination therapy with Metformin and Albendazole can reduce the toxicity of the high-dose Albendazole monotherapy currently employed, and increase the efficacy of the treatment (12). Albendazole combined the Isethionate/Hypromellose Acetate Succinate can improvement of the bioavailability and anti-hepatic AE effect (13). Therefore, a potential therapeutic strategy is to combine different drugs to reduce or inhibit the infiltrative and invasive growth of E. multilocularis.
We have previously found that vaccination with EM-LAP induced specific immune response and inhibited the invasion of E. multilocularis (7). We have also evaluated the therapeutic effect of recombinant EM-LAP (rEM-LAP) on AE and found that Ubenimex, a broad-spectrum inhibitor of LAP, also showed similar inhibitory effect on the invasion of E. multilocularis (8). These studies suggest that EM-LAP could be a potential therapeutic target of E. multilocularis infection. LAP is a metalloprotease of the M17 family (14) and it plays a crucial role in many physiological processes including growth, nutrition and metabolism. It is especially important for the invasion of pathogenic parasites in the host (15). There is also evidence that vaccination with Fasciola hepatica leucine aminopeptidase induced specific IgG antibody responses against infection, invasion and migration of Fasciola hepatica in the host (16). LAP also acts as a therapeutic target in Malaria infection (17). A number of studies have reported that LAP inhibitors such as Ubenimex (18) and CHR-2863 (19) could inhibit the enzymatic activity of Malaria M17 leucine aminopeptidase (PfM17LAP) and thus effectively decreased the digests host cell hemoglobin and invasion growth (19–21). Our previous studies have also found that Ubenimex had inhibitory effect on the invision of E. multilocularis in host liver but little effect on the nutrition, metabolism and growth of the parasites. Thus, the purpose of this study is to find an effective treatment for E. multilocularis infection.
Ubenimex is a broad-spectrum inhibitor of LAP and it is often used as adjuvant therapy for malignant tumors (22, 23). Ubenimex can inhibit tumor aggressiveness and development through recede the aminopeptidase CD13 (24, 25), and it also plays an important role in the treatment of many parasitic diseases such as Malaria (26) and E. multilocularis infection (8, 27) because of its ability to reduce the invasiveness and digestion of LAP in the host. Albendazole is a well known anthelmintic that can inhibit many parasitic infections (28), and it also acts as a suppressor of glycogen metabolism in parasites that leads to nutritional failure and death, but it is less effective for E. multilocularis infection (29). However, there is evidence that Albendazole in combination with other drugs may be more effective in inhibiting parasite invasion and growth (30). Albendazole is effective in inhibiting the growth and development of E. multilocularis by inhibiting parasite's absorption of glucose that may lead to glycogen depletion or by inhibiting the fumarate reductase system that may lead to reduced production of ATP. However, it has a low drug concentration in hydatid lesions and therefore little inhibitory effect on the infiltrative growth of E multilocularis. Ubenimex can inhibit the invasion of E. multilocularis metacestodes in host liver by inhibiting LAP production and activity, but it has a limited effect on the growth and development of E. multilocularis. Thus, a better therapeutic outcome is expected by combining Ubenimex and Albendazole.
In this study, Ubenimex and Albendazole are used in combination in the treatment of the infection of E. multilocularis protoscoleces, which may provide new insights into the treatment of AE.
Materials and methods
Mouse model and treatment
All animal experiments were performed in compliance with the regulations of the Ministry of Science and Technology of China and approved by the Experimental Committee of Qinghai University (QHDX-2019-09). Male BALB/c specific pathogen free (SPF) mice of 6–8 weeks old were purchased from Beijing Spaefer Biotechnology Company (SCXK2019-0010) and fed with sterilized feed and water over a 24 h day/night cycle in an animal biosafety level II (ABSL-2) laboratory (7, 8, 31). All mice were intraperitoneally injected with 2,000 protoscoleces, which were isolated from Gerbillinae infected with E. multilocularis for over 20 weeks in the Research Center for High Altitude Medicine (7, 32). At week 8, mice were randomized into four groups of nine mice each, including Combination group (Ubenimex + Albendazole), Model group, Ubenimex group and Albendazole group (Table 1), and the first imaging examination (Ultrasonography and MRI) was performed. Mice received different treatments until 16 weeks and the second imaging examination was performed. After that, mice were sacrificed to evaluate the infection, growth and invasion of E. multilocularis.
A 9.13 mg of Ubenimex (Macklin Bio.; CAS: 58970-76-6) was dissolved in a solution consisting of 1,100 μl of DMSO, and then 4,400 μl of PEG300, 50 μl of Tween80, and 4,950 μl of normal saline were added (8, 33). Three gram of Albendazole (TCI Shanghai Chemical Industry Development Co. LTD.; CAS: 54965-21-8) was suspended in 1% sodium carboxymethylcellulose (11).
Treatment was initiated 4–6 weeks after protoscoleces inoculation. Mice in the Combination group were intraperitoneally injected with 7.5 mg/kg/day Ubenimex and intragastrically administered with 0.2 ml/20 g/day Albendazole suspension; mice in the Ubenimex group were intraperitoneally injected with only 7.5 mg/kg/day Ubenimex (8, 33, 34); mice in the Albendazole group were intragastrically administered with only 0.2 ml/20 g/day Albendazole suspension (11, 35, 36); while mice in the Model group only received pharmaceutical solvent (Table 1). All mice were treated for 8–9 consecutive weeks. At week 8 and week 16 after protoscoleces inoculation, imaging examination (Ultrasonography and MRI) was performed to observe the therapeutic efficiency and samples used for the comparison of therapeutic efficiency were taken from the same mice.
Ultrasonography and MRI
Mice were anesthetized with 1.5% isoflurane in O2 on a specially designed heated bed for measurement of the cyst area in the epigastrium. Ultrasonography was performed using a FUJIFILM Vevo® Ultrasonic imager for small animals; and MRI was performed using a BioSpec® MRI scanner (Bruker PharmaScan 70/16 USR) and 7.0T MR Scanning system for small animals, which was equipped with a set of RF RES 300 1H 075/040 QSN TR application-specific radiofrequency mouse body coil for in vivo imaging. The body surface projection of the liver was positioned as close as possible to the center of the coil, and the height of the mouse in the coil was adjusted to ensure the liver at the center of the magnetic field for measurement of cysts in the liver and adjacent tissues. Physiological signals, such as breathing rate, were monitored throughout the experiment. The whole acquisition monitor process is triggered by breath gating. The scanning sequence was T1WI: TE 3.0 ms, TR 300.0 ms, turning Angle 90°, layer thickness 1.0 mm, FOV 35 mm × 35 mm, Averages = 6; T2WI: TE 25.0 ms, TR 2,000.0 ms, layer thickness 1.0 mm, FOV 35 mm × 35 mm, Averages = 16; DWI: TE 30.0 ms, TR 1,000.0 ms, b value 600 mm/s, layer thickness 1.0 mm, FOV 35 mm × 35 mm, Averages = 16.
Morphological and pathological examination
At week 17 after the second imaging examination, all mice were euthanized and serum was separated from blood samples. The cysts of metacestodes in the liver and peritoneum were photographed to assess morphological alterations, and the weigh and number of cysts in the abdomen were also measured. Liver samples were optimal cutting temperature (OCT) embedded, sectioned into 4.5 μm-thick sections by a freezing microtome, and then stained with hematoxylin–eosin (Beijing Jiuzhoubailin Biotechnology Co. LTD.), Masson trichrome (Masson Trichrome Stain Kit Solarbio G1340) and Gomori-aldehyde-fuchsin (Modified Gomori-Aldehyde-Fuchsin Stain Kit Solarbio G1593) for histopathological examination of the invasion of metacestodes to adjacent tissues and the fibrosis level. Images were captured using a microscope (OLYMPUS MX63L) and the positive areas were identified using ImageJ software. The levels of alanine aminotransferase (ALT), aspartate amino transferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), albumin (ALB), alkaline phosphatase (ALP), and gamma-glutamyl transferase (γ-GT) in the serum were detected using a fully automatic biochemical analyzer (Chemray 800, Rayto Life and Analytical Sciences Co., Ltd.) to determine liver damages of mice in each group.
Statistical analyses
All statistical analyses were performed using SPSS 22.0 and Microsoft Excel 2019. All measurement data were expressed as mean ± standard deviation ( ± SD). Comparison between two groups was performed using the independent sample T-test; while that among multiple groups was performed using the one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
Ultrasound and MRI results
All mice underwent MRI (including T1, T2 and DWI, which could indicate the anatomical position, E. multilocularis lesions, and the invasion of cysts and metacestodes in the liver, respectively). The T2 sequence results (Figure 1A) showed that at week 8, cysts were distributed throughout the liver in all mice with almost no difference in size and location. At week 16, the cysts grew rapidly and almost occupied the whole liver in the model group. In comparison, cyst growth was significantly inhibited in mice treated with both Ubenimex and Albendazole, but there was no obvious change in cyst size in mice treated with only Ubenimex or Albendazole. A dark dividing line was observed surrounding liver tissues in the combination group and the Ubenimex group, indicating that infiltration of cysts was inhibited.
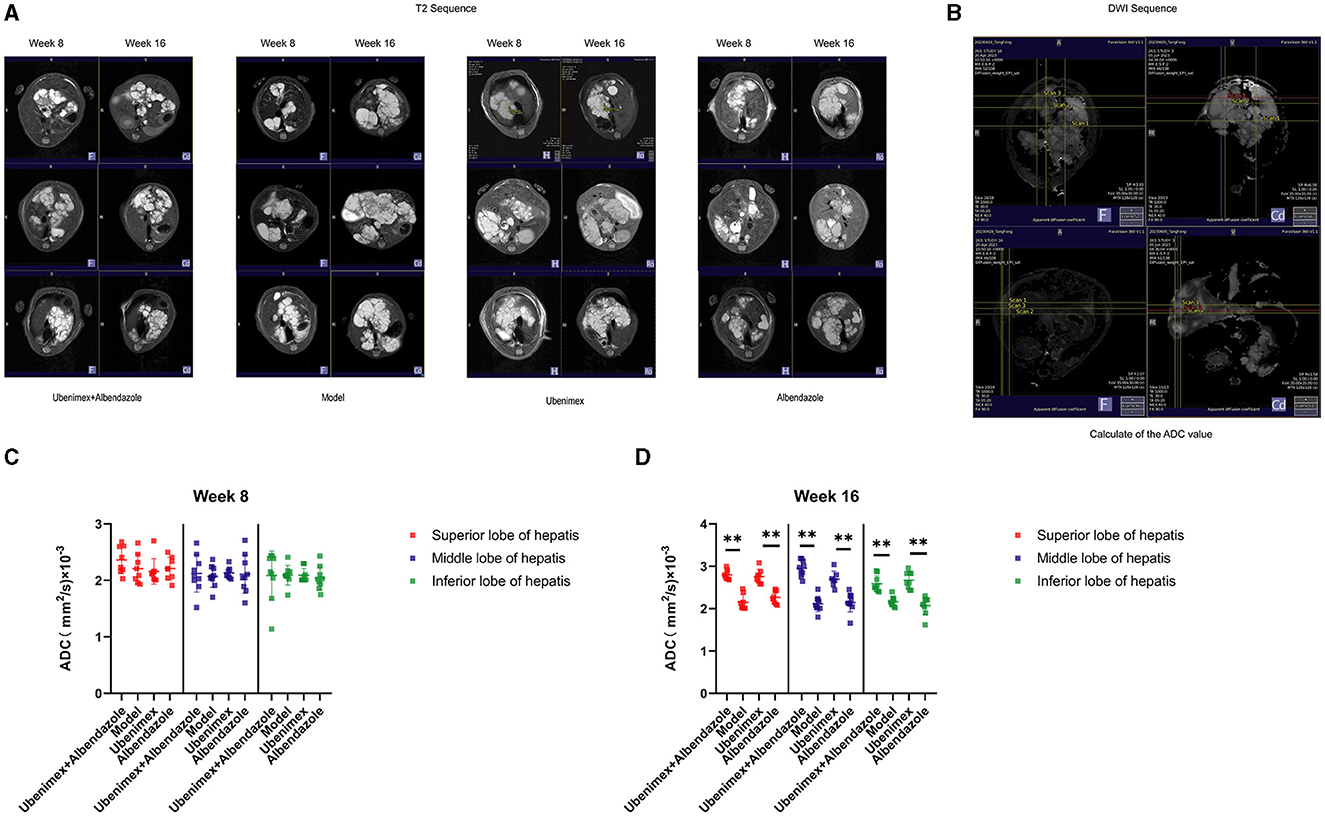
Figure 1. The MRI result in each group. (A) T2 scanning at week 8 and week 16. (B) DWI scanning. (C, D) The junctional area were measured to calculate the ADC values, and choice the superior, middle and inferior lobes of the liver DWI result to calculated at week 8 and week 16. **P < 0.05.
The DWI sequence was obtained and the ADC value was calculated from the superior, middle and inferior lobes of the liver (Figure 1B). The results indicated that at week 8 (Figure 1C), there was no significant difference in the ADC values among the four groups (P > 0.05); while at week 16 (Figure 1D), the ADC values of the combination group and Ubenimex group were significantly higher than that of the model group and Albendazole group (P < 0.05), but there was no significant difference between the model group and the Albendazole group (P > 0.05).
The ultrasonic results showed that (Figure 2A) at week 8, many cysts of different sizes were closely packed in the liver and some cysts invaded into liver tissues in all mice; while at week 16, the cyst sizes were significantly increased in the model group (P < 0.05); significantly decreased in the combination group (P < 0.05), but remained almost unchanged in the Ubenimex group and Albendazole group (P > 0.05) (Figure 2B).
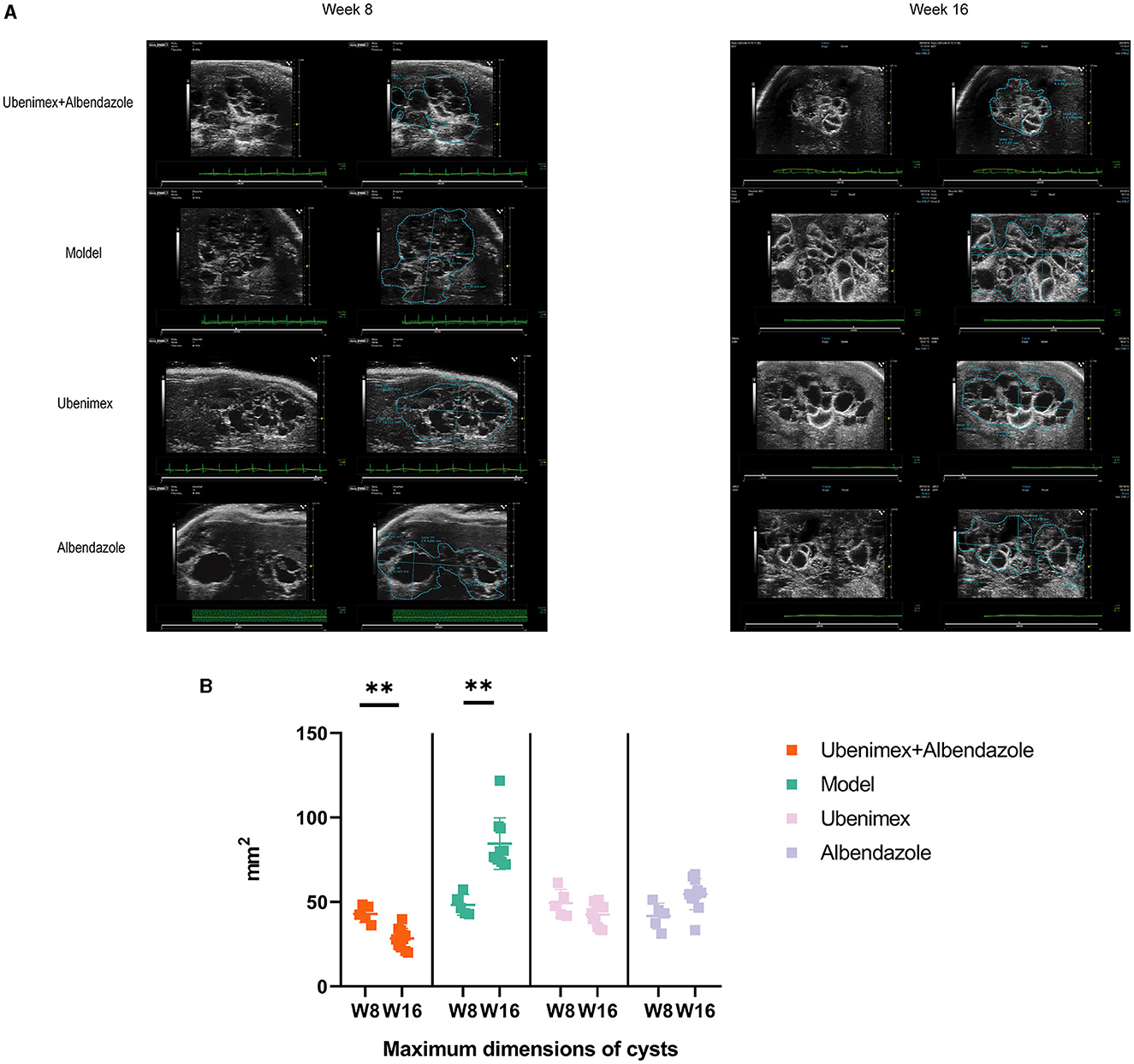
Figure 2. Ultrasonography. (A) Cysts in different groups and time points, at week 8 and week 16 to examine. (B) The maximum dimensions of cysts. **P < 0.05.
Morphological and pathological results
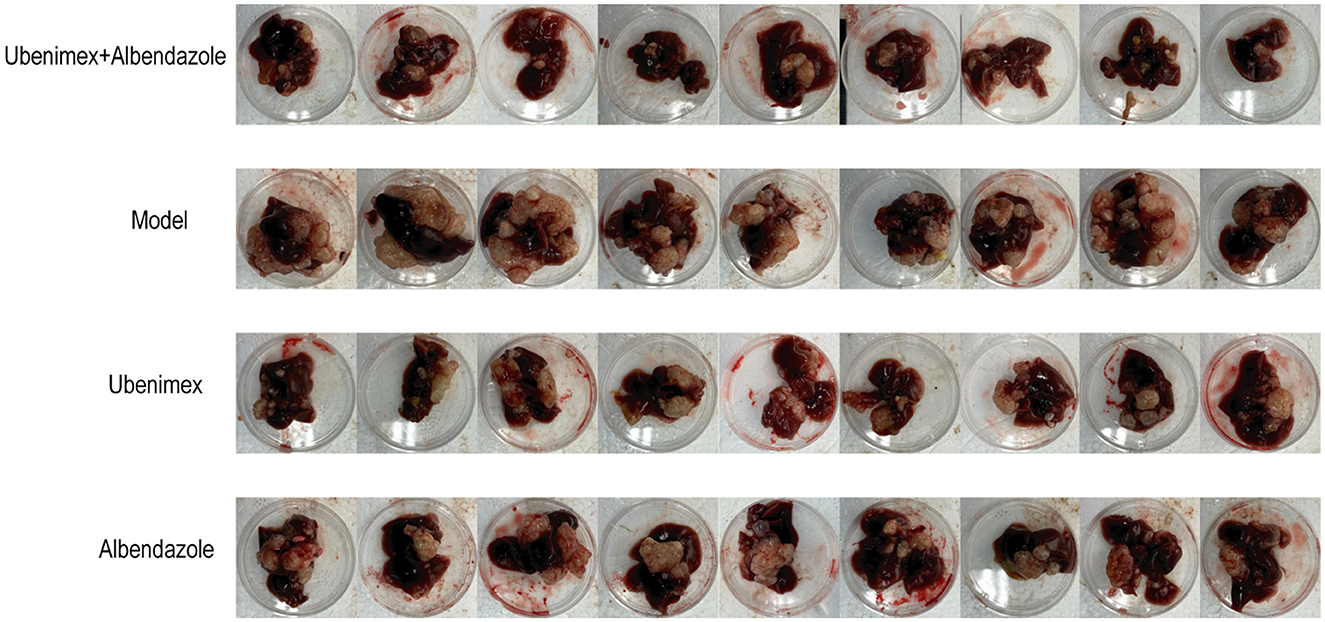
All mice were sacrificed at week 17 and the cysts in the liver and peritoneum were excised and photographed. A large number of cysts of different sizes were observed in the model group and they were closely connected to liver tissues with no apparent demarcation, indicating an infiltrative growth pattern (Figure 3). However, the numbers and sizes of cysts were decreased in most mice treated with Ubenimex and Albendazole. The cysts were not tightly connected to liver tissues and some cysts could be easily peeled off, indicating that the combined treatment of Ubenimex and Albendazole inhibited the invasion of cysts. The same phenomenon was also observed in the Ubenimex group, but the sizes and numbers of cysts were not obviously changed. However, mice in the Albendazole group showed no obvious changes in the size, number and infiltration of cysts. Thus, the combined treatment of Ubenimex and Albendazole led to a substantial decrease in the size, number and infiltration of cysts in the liver.
There was a significant difference in the number and weight of cysts collected from abdomen and liver of mice treated with different drugs (Figure 4A). Compared to the model group, the number (Figure 4B) of cysts was significantly decreased in the combination group, Albendazole group and Ubenimex group (P < 0.05); the weight (Figure 4C) was mostly significantly decreased in the combination group (P < 0.05); However, there was a significant difference in the weight of cysts between Albendazole group and Ubenimex group (P < 0.05). These results suggest that the combination of Ubenimex and Albendazole may be a viable treatment option for the infection of E. multilocularis protoscoleces.
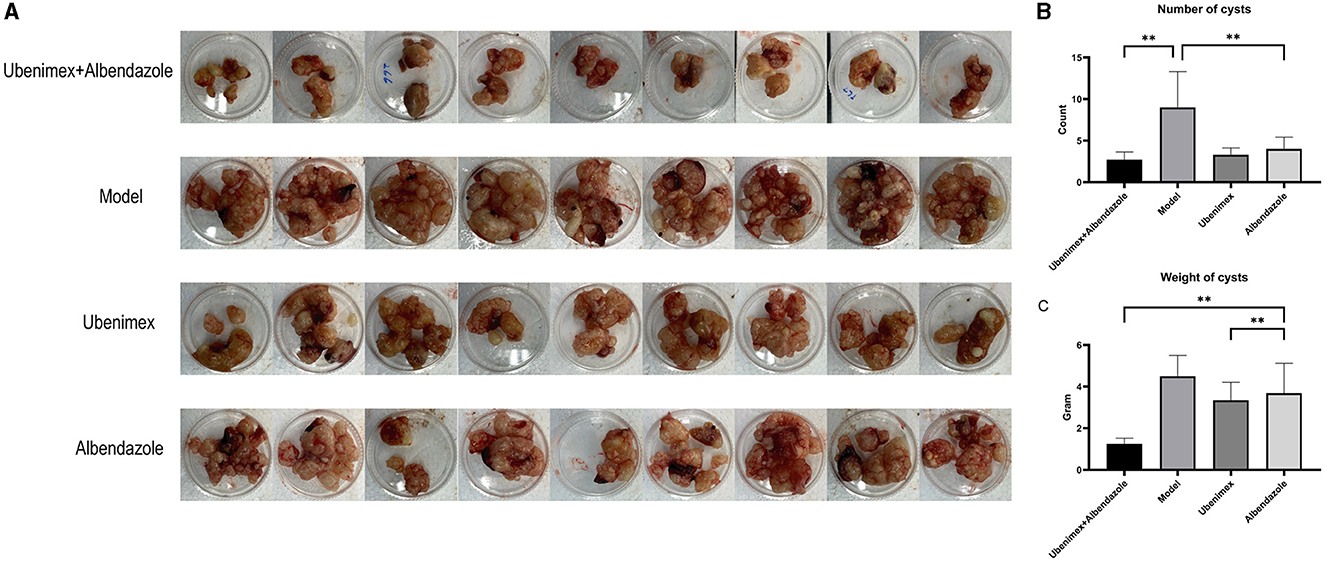
Figure 4. The cysts in abdomen. (A) The size and number of cysts at week 17. (B) The number of cysts in different groups. (C) The weight of cysts in different groups (**P < 0.05 v.s. Model group).
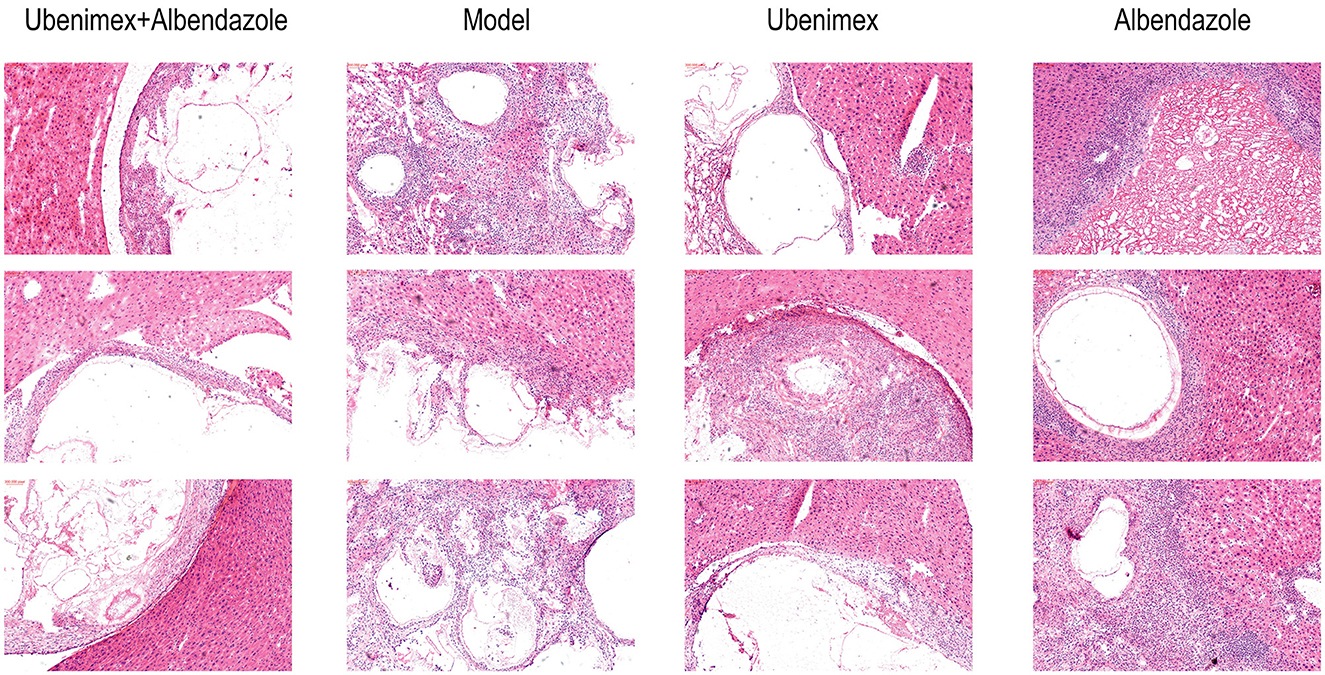
The HE staining results revealed that there was a clear boundary between cysts and liver tissues in mice treated with Ubenimex and Albendazole, which indicated a non-infiltrative growth pattern. The germinal layer of the cysts was swollen and the number of metacestodes was reduced. Broken cysts were infiltrated by host inflammatory cells such as lymphocytes, neutrophils, and eosinophils, leading to collapse of the laminate and amorphous necrosis of cysts. However, mice in the Ubenimex group showed less host inflammatory cells in the cysts, less amorphous necrosis, and less swelling of the germinal layer. In the model group, a large number of cysts were observed in the liver with apparent vascular invasion and no clear boundary with surrounding liver tissues, indicating severe infiltrative growth. Clear laminated and germinal layers were observed; the cell density of the germinal layer was high and the number of metacestodes was increased. In the Albendazole group, the cysts also showed an infiltrative growth pattern, but the germinal layer in the cysts was mildly swollen and metacestodes disappeared. Proliferation of fibrous tissue was seen in some cysts as a red hyaline deformation, and there were a large number of host inflammatory cells surrounding the cysts (Figure 5).
The Masson and Gomori-aldehyde-fuchsin staining results revealed that in the combination group and Ubenimex group, liver fibrosis and cyst invasion were significantly inhibited and the levels of collagenous and elastic fibers which were stained blue were decreased in the liver but increased in the cysts; while in the model group and Albendazole group, high levels of collagenous and elastic fibers were found in the liver and the cysts showed an infiltrative growth pattern (Figure 6A).
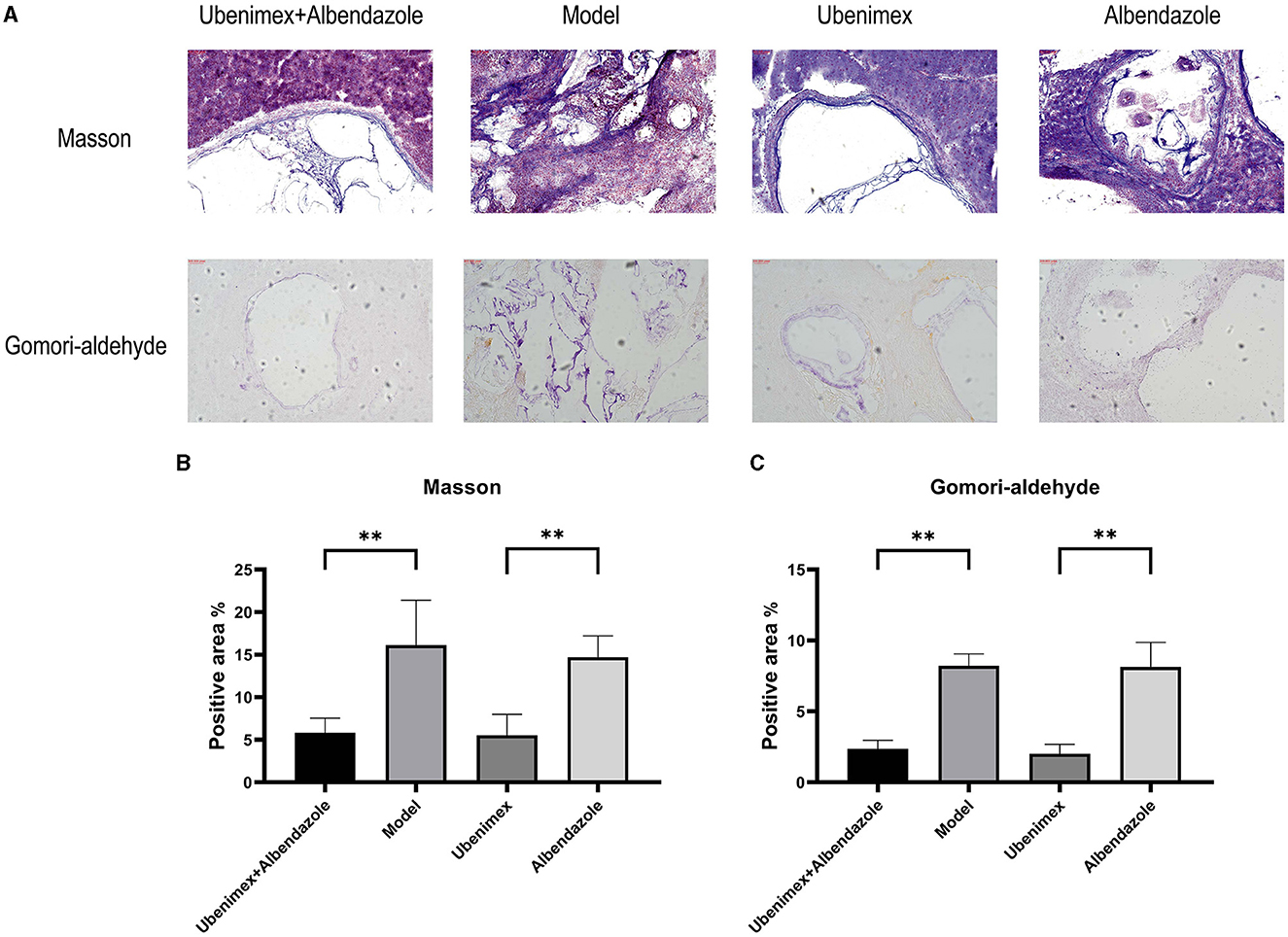
Figure 6. Histological sections. (A) Representative Masson- and Gomori-aldehyde-fuchsin-stained sections (100×); (B, C) Positive staining area (%) is presented in each image. The results are obtained from several images of 3 mice for each staining (**P < 0.05).
The positive levels of Masson–stained and Gomori-aldehyde-fuchsin–stained cysts were detected by ImageJ. The results (Figure 6B) showed that the levels of Masson-positive cysts of the combination group and Ubenimex group were significantly reduced compared to the model group and Albendazole group (P < 0.05), but there was no significant difference between model and Albendazole group or between combination and Ubenimex group. The same result was also observed for Gomori-Aldehyde-Fuchsin–positive cysts (Figure 6C).
Liver functions
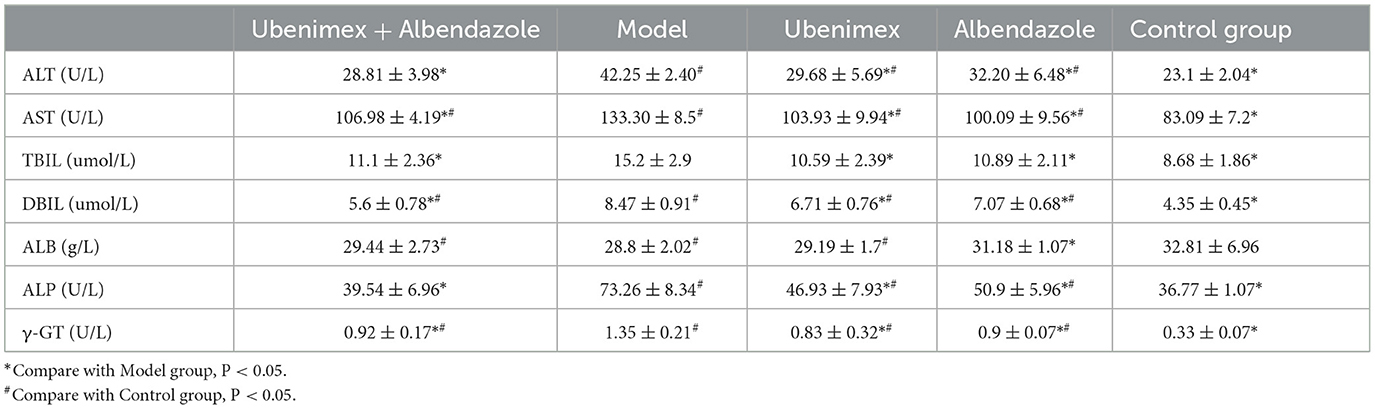
In this study, the levels of ALT, AST, TBIL, DBIL, ALB, ALP, and γ-GT in the serum were detected to determine liver damages of mice in each group. The results (Table 2) indicated that compared to the model group, the levels of ALT, AST, TBIL, DBIL, ALP, and γ-GT were significantly decreased in the combination group, Ubenimex group and Albendazole group. Compare to the blank group (healthy mice without any infection and treatment), the levels of AST, DBIL and ALB were significantly increased in the combination group; and the levels of ALT, AST, DBIL, ALP, and γ-GT were significantly increased in the Ubenimex group and Albendazole group.
Discussion
AE is a refractory parasitic disease and also a neglected parasitic zoonosis, as the parasite has high adaptability and immune escape capability (37) that make it difficult to clean, and it can infiltrate into other tissues and organizations in the host like a malignant tumor. In clinic, the single-drug therapy is of limited benefit and surgery is the only way to remove the parasites, but recurrence and metastasis are still common (38, 39). Liver transplantation is the only therapeutic strategy in end-stage patients (40). Albendazole is the only drug for the treatment of AE, but its effect is unsatisfactory. Therefore, there is a need for new therapeutic approaches to eliminate hydatid lesions and inhibit infiltration and fibrosis in the liver.
At present in clinical practice, only Albendazole and surgical operation be applied to treat AE, but has less curative effect, more than 20% of cases fail therapy (41), and beyond that, high dose and long-term medication with Albendazole may increase the toxic and side effect in host. So, many studies have focus on find a new therapy method, which can eliminate the hydatid lesions efficiency and less side effect in host. Liu et al. (31) found that, Thiacloprid, as one of insecticide which has favorable efficacy to treat AE in vitro and in vivo, and low mammalian toxicity to hosts, Thiacloprid can inhibited the acetylcholinesterase activity in hydatid protoscoleces and metacestodes that can damaged the germinal layer, but it still has some latent unknown side effect such as hypersensitivity need further research. Fabbri et al. (42) reported that the dichlorophen (DCP) and silica nanoparticles modified with DCP (NP-DCP) both demonstrated a time and dose-dependent in vitro effect against protoscoleces. Some new horizons of herbal drugs to cure CE are opening with every passing day (43), AE was no exception, some research reported that the Chinese traditional medicine-Crocin exert parasiticidal activity against E. multilocularis in vitro and in vivo (44); Jiang (45) indicated that a novel compound extracted from traditional Chinese medicine have efficacy of AE. Even though there's numerous research on innovate treatment AE, but still have insufficient, safety and efficient treatment method was essential, need long-term refinement and validation then can translate into clinical application.
In recent years more and more researches are also explore the conventional antiparasitic drugs' therapeutic potential, designed to increase efficacy and reduce toxic side effects, for instance Albendazole combination other drugs which was an effective way to treat AE (11–13). Therefore, in this study, a potential therapeutic strategy is to combine Ubenimex with Albendazole to reduce or inhibit the infiltrative and invasive growth of E. multilocularis.
We have previously found that vaccination with EM-LAP induced specific immune responses and inhibited the growth of E. multilocularis, and it is a potential therapeutic target for the treatment for AE (7, 8). LAP is an aminopeptidase from the M17 family, which is widely expressed in parasites and is an important metabolic enzyme that can digestive host tissues and then cause serious diseases (46). In our previous studies, we found that EM-LAP also allowed infiltrative growth of hydatid lesions in host liver. As a broad-spectrum inhibitor of EM-LAP, Ubenimex can reduce EM-LAP and inhibit the infiltrative growth of E. multilocularis (8), and thus it can act as a candidate drug for the treatment of AE. However, Ubenimex alone has little effect on the ontogeny and development of E. multilocularis but a better effect in inhibiting the invasion and infiltrative growth of hydatid lesions in liver. Thus, Ubenimex can be used in combination with other anthelmintic drugs to eliminate E multilocularis and hydatid lesions.
Ubenimex is one of LAP inhibitors that can reduce the activity of LAP, and therefore it can reduce the digestion of liver tissues, fibrogenesis and infiltrative growth (8, 47, 48). It has also reported that Ubenimex can reduce the invasiveness and damage of other parasites like Leishmania, Human liver fluke, plasmodium by inhibiting the LAP proteins levels (27, 49, 50). In some malignant tumors, Ubenimex can also inhibit the migration and invasion by alleviating the activity of the LAP (CD13)/NAB1/MAPK pathway (51–54).
Albendazole is a broad-spectrum anthelmintic which can inhibit multiple parasitic infections (55), but it is less effective for the infection of E. multilocularis. Many other studies have also explored how to enhance the efficacy of Albendazole in deworming (56, 57). Albendazole sulphoxide binds to parasite ß-microtubules, preventing them from aggregating into microtubules to disrupt the cascade of mid-stage cell division and ultimately leading to the death of individual cells and then the death of the parasite (58), Albendazole sulphoxide can also inhibit the glucose absorption of the parasite, leading to glycogen depletion, or inhibit the fumarate reductase system, blocking the production of ATP and therefore making it impossible for the parasite to survive and reproduce (59). However, Albendazole has low solubility in water and drug concentration in hydatid lesions (60), and different from Ubenimex, it has little inhibitory effect on hydatid infiltrative growth and invasion.
Single Ubenimex and Albendazole treatment has a limited therapeutic effect and could not inhibit the growth or invasion of cysts. In this study, the combination of Ubenimex and Albendazole can be an effective therapeutic approach for the treatment of AE in mice infected with E. multilocularis, which could reduce the growth, infiltration, invasion and fibrosis of hydatid cysts.
In this study, the combined use of two drugs demonstrated superior therapeutic effects compared to single drug administration, The potential reasons for this could be that, on one hand, both drugs participate in the parasite metabolism, but intervening from two different pathways, which can block the uptake of protein amino acids and glucose, making it difficult for the parasite to obtain nutrients from the host. On the other hand, the metabolic pathways blocked by the two drugs happen to be the pathways for the invasive growth of the parasite, significantly reducing the parasite's harm to the host. Therefore, the combined use of the drugs can exert a better synergistic effect. In addition, due to the different administration methods of the two drugs, the occurrence of unpredictable direct interactions between the two drugs is minimized, allowing each to achieve the desired effect to the greatest extent.
The two drugs act synergistically to provide an effective strategy for E. multilocularis infection and growth. However, neither single drug nor combined treatment led to significant changes in the liver function, indicating that these two drugs are relatively safe.
Nowadays, there have been numerous studies on the combined use of drugs to treat AE. However, these studies have primarily focused on enhancing the efficacy of Albendazole or increasing the blood concentration of Albendazole (61–63), without intervening in the infiltrative, fibrotic, and metastatic aspects of E. multilocularis infection. In conclusion, our study provides a new effective combined strategy for the treatment AE, which can not only reduce the growth of E. multilocularis metacestodes but can also inhibit the infiltration, invasion and fibrosis in host liver.
AE as one of regional public health issues that serious threat to the health of the ethnic minority areas population in China, the custom and living habits of ethnic minorities such as using no soap for cleaning dirty hands and eating raw food, are the main causes for the difficult to prophylaxis (64). For this reason, health education and propagating drug knowledge is an effective means of prevent the AE. Therefore, in this study we aim to come up with more effective drug combinations to treatment AE, not only reduce the growth of E. multilocularis metacestodes, but also inhibition of infiltration, invasion and fibrosis in host liver. On this account present the effective prevention and treatment measures of AE.
In this experiment, we focused on the therapeutic effect of drug combination. However, there still has some limitations. We did not investigate the mechanism of the combined drug therapy. In future studies, we will continue to explore the mechanism and pharmacological principles of combined drug therapy to optimize treatment strategies and further enhance therapeutic effects.
For the last few years, the parasitic disease was more and more paid attention, not only in E. multilocularis infection, but also other epidemic parasites. Meanwhile the conventional antiparasitic drugs occurrence anthelmintic resistance (65), such as Albendazole, due to the anthelmintic resistance, low solubility in water and less drug concentration in lesions, it was not exerted completely eliminate effect in some parasites infection. So, numerous researches were searching for a new therapeutic method, Velázquez-Antunez et al. (66) found that the secondary compounds of Guazuma ulmifolia leaves can inhibit the hatching of eggs of Haemonchus contortus; Al-Saeed et al. (67) reported that Haloxylon salicornicum leaves extract can exhibited a strong anthelmintic potential against haemonchosis in vitro. Thus, those methods can be developed as a novel drug for the treatment of some parasites. But antiparasitic drug development was a long process, need long-term refinement and validation then can translate into clinical application, so in future research, we will explore new Treatment methods and new drugs, so that increase therapeutic effect and decrease toxic-side effect and anthelmintic resistance.
Conclusions
The combined treatment of Ubenimex and Albendazole can well inhibit the growth, development, invasion and fibrosis of cysts and protect the liver from damage by E. multilocularis. Thus, it is a potential candidate for the treatment of AE.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
All animal experiments were performed in compliance with the regulations of the Ministry of Science and Technology of China and approved by the Experimental Committee of Qinghai University (QHDX-2019-09). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MH: Data curation, Formal analysis, Methodology, Writing – original draft. YM: Data curation, Writing – original draft. FT: Conceptualization, Writing – review & editing. R-LG: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundations of China (No. 82072107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang S, Wu J, Ding C, Cui Y, Zhou Y, Li Y, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. (2017) 17:716–25. doi: 10.1016/S1473-3099(17)30227-X
2. Aydinli B, Ozturk G, Arslan S, Kantarci M, Tan O, Ahiskalioglu A, et al. Liver transplantation for alveolar echinococcosis in an endemic region. Liver Transpl. (2015) 21:1096–102. doi: 10.1002/lt.24195
3. Xu X, Qian X, Gao C, Pang Y, Zhou H, Zhu L, et al. Advances in the pharmacological treatment of hepatic alveolar echinococcosis: from laboratory to clinic. Front Microbiol. (2022) 13:953846. doi: 10.3389/fmicb.2022.953846
4. Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, et al. Echinococcosis: advances in the 21st century. Clin Microbiolo Rev. (2019) 32:e00075-18. doi: 10.1128/CMR.00075-18
5. Horton J. Albendazole for the treatment of echinococcosis. Fundam Clin Pharmacol. (2003) 17:205–12. doi: 10.1046/j.1472-8206.2003.00171.x
6. Xu K, Ahan A, A. new dawn in the late stage of alveolar echinococcosis “parasite cancer”. Med Hypotheses. (2020) 142:109735. doi: 10.1016/j.mehy.2020.109735
7. Wang L, Wei W, Zhou P, Liu H, Yang B, Feng L, et al. Enzymatic characteristics and preventive effect of leucine aminopeptidase against Echinococcus multilocularis. Acta Trop. (2021) 222:106066. doi: 10.1016/j.actatropica.2021.106066
8. Zhou Z, Zhou P, Mu Y, Wang L, Cao Z, Dong S, et al. Therapeutic effect on Alveolar echinococcosis by targeting EM-leucine aminopeptidase. Front Immunol. (2022) 13:1027500. doi: 10.3389/fimmu.2022.1027500
9. Pensel P, Paredes A, Albani CM, Allemandi D, Sanchez Bruni S, Palma SD, et al. Albendazole nanocrystals in experimental alveolar echinococcosis: enhanced chemoprophylactic and clinical efficacy in infected mice. Vet Parasitol. (2018) 251:78–84. doi: 10.1016/j.vetpar.2017.12.022
10. Chai JY, Jung BK, Hong SJ. Albendazole and Mebendazole as anti-parasitic and anti-cancer agents: an update. Korean J Parasitol. (2021) 59:189–225. doi: 10.3347/kjp.2021.59.3.189
11. Lopez LM, Pensel PE, Fabbri J, Albani CM, Elissondo N, Gambino G, et al. The combination of carvacrol and albendazole enhanced the efficacy of monotherapy in experimental alveolar echinococcosis. Acta Trop. (2022) 225:106198. doi: 10.1016/j.actatropica.2021.106198
12. Loos JA, Coccimiglio M, Nicolao MC, Rodrigues CR, Cumino AC. Metformin improves the therapeutic efficacy of low-dose albendazole against experimental alveolar echinococcosis. Parasitology. (2022) 149:138–44. doi: 10.1017/S0031182021001633
13. Hu C, Zhang F, Fan H. Improvement of the bioavailability and anti-hepatic alveolar echinococcosis effect of albendazole-isethionate/hypromellose acetate succinate (HPMC-AS) complex. Antimicrob Agents Chemother. (2021) 65:e0223320. doi: 10.1128/AAC.02233-20
14. Wu M, Yan M, Xu J, Liang Y, Gu X, Xie Y, et al. Expression, tissue localization and serodiagnostic potential of Echinococcus granulosus leucine aminopeptidase. Int J Mol Sci. (2018) 19:1063. doi: 10.3390/ijms19041063
15. Lee YR, Na BK, Moon EK, Song SM, Joo SY, Kong HH, et al. Essential role for an M17 leucine aminopeptidase in encystation of Acanthamoeba castellanii. PLoS ONE. (2015) 10:e0129884. doi: 10.1371/journal.pone.0129884
16. Checa J, Salazar C, Goyeche A, Rivera M, Silveira F, Maggioli G. A promising new target to control fasciolosis: Fasciola hepatica leucine aminopeptidase 2. Vet Parasitol. (2023) 320:109959. doi: 10.1016/j.vetpar.2023.109959
17. Edgar RCS, Siddiqui G, Hjerrild K, Malcolm TR, Vinh NB, Webb CT, et al. Genetic and chemical validation of Plasmodium falciparum aminopeptidase PfA-M17 as a drug target in the hemoglobin digestion pathway. eLife. (2022) 11:e80813. doi: 10.7554/eLife.80813
18. Gardiner DL, Trenholme KR, Skinner-Adams TS, Stack CM, Dalton JP. Overexpression of leucyl aminopeptidase in Plasmodium falciparum parasites. Target for the antimalarial activity of bestatin. J Biol Chem. (2006) 281:1741–5. doi: 10.1074/jbc.M508955200
19. Skinner-Adams TS, Peatey CL, Anderson K, Trenholme KR, Krige D, Brown CL, et al. The aminopeptidase inhibitor CHR-2863 is an orally bioavailable inhibitor of murine malaria. Antimicrob Agents Chemother. (2012) 56:3244–9. doi: 10.1128/AAC.06245-11
20. Cunningham E, Drag M, Kafarski P, Bell A. Chemical target validation studies of aminopeptidase in malaria parasites using alpha-aminoalkylphosphonate and phosphonopeptide inhibitors. Antimicrob Agents Chemother. (2008) 52:3221–8. doi: 10.1128/AAC.01327-07
21. Mathew R, Wunderlich J, Thivierge K, Cwiklinski K, Dumont C, Tilley L, et al. Biochemical and cellular characterisation of the Plasmodium falciparum M1 alanyl aminopeptidase (PfM1AAP) and M17 leucyl aminopeptidase (PfM17LAP). Sci Rep. (2021) 11:2854. doi: 10.1038/s41598-021-82499-4
22. Yue K, Hou X, Jia G, Zhang L, Zhang J, Tan L, et al. Design, synthesis and biological evaluation of hybrid of ubenimex-fluorouracil for hepatocellular carcinoma therapy. Bioorg Chem. (2021) 116:105343. doi: 10.1016/j.bioorg.2021.105343
23. Yoneda J, Saiki I, Fujii H, Abe F, Kojima Y, Azuma I. Inhibition of tumor invasion and extracellular matrix degradation by ubenimex (bestatin). Clin Exp Metastasis. (1992) 10:49–59. doi: 10.1007/BF00163576
24. Yamashita M, Wada H, Eguchi H, Ogawa H, Yamada D, Noda T, et al. A CD13 inhibitor, ubenimex, synergistically enhances the effects of anticancer drugs in hepatocellular carcinoma. Int J Oncol. (2016) 49:89–98. doi: 10.3892/ijo.2016.3496
25. Guo Q, Jing FJ, Xu W, Li X, Li X, Sun JL, et al. Ubenimex induces autophagy inhibition and EMT suppression to overcome cisplatin resistance in GC cells by perturbing the CD13/EMP3/PI3K/AKT/NF-κB axis. Aging. (2019) 12:80–105. doi: 10.18632/aging.102598
26. Dalal S, Klemba M. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J Biol Chem. (2007) 282:35978–87. doi: 10.1074/jbc.M703643200
27. Ariefta NR, Pagmadulam B, Hatano M, Ikeda N, Isshiki K, Matoba K, et al. Antiplasmodial activity evaluation of a bestatin-related aminopeptidase inhibitor, phebestin. Antimicrob Agents Chemother. (2023) 67:e0160622. doi: 10.1128/aac.01606-22
28. Horton J. Albendazole: a broad spectrum anthelminthic for treatment of individuals and populations. Curr Opin Infect Dis. (2002) 15:599–608. doi: 10.1097/00001432-200212000-00008
29. Fabbri J, Pensel PE, Albani CM, Lopez LM, Simonazzi A, Bermudez JM, et al. Albendazole solid dispersions against alveolar echinococcosis: a pharmacotechnical strategy to improve the efficacy of the drug. Parasitology. (2020) 147:1026–31. doi: 10.1017/S0031182020000670
30. Abulaihaiti M, Wu XW, Qiao L, Lv HL, Zhang HW, Aduwayi N, et al. Efficacy of albendazole-chitosan microsphere-based treatment for alveolar Echinococcosis in mice. PLoS Negl Trop Dis. (2015) 9:e0003950. doi: 10.1371/journal.pntd.0003950
31. Liu C, Fan H, Ma J, Ma L, Ge RL. In vitro and in vivo efficacy of thiacloprid against Echinococcus multilocularis. Parasit Vectors. (2021) 14:450. doi: 10.1186/s13071-021-04952-7
32. Li R, Yang Q, Guo L, Feng L, Wang W, Liu K, et al. Immunological features and efficacy of the recombinant subunit vaccine LTB-EMY162 against Echinococcus multilocularis metacestode. Appl Microbiol Biotechnol. (2018) 102:2143–54. doi: 10.1007/s00253-018-8771-5
33. Lis M, Szczypka M, Suszko A, Obmińska-Mrukowicz B. The effects of bestatin on humoral response to sheep erythrocytes in non-treated and cyclophosphamide-immunocompromised mice. Immunopharmacol Immunotoxicol. (2013) 35:133–8. doi: 10.3109/08923973.2012.719524
34. Hossain A, Heron D, Davenport I, Huckaba T, Graves R, Mandal T, et al. Protective effects of bestatin in the retina of streptozotocin-induced diabetic mice. Exp Eye Res. (2016) 149:100–6. doi: 10.1016/j.exer.2016.06.016
35. Zhu L, Kuang X, Zhang G, Liang L, Liu D, Hu B, et al. Albendazole induces immunotherapy response by facilitating ubiquitin-mediated PD-L1 degradation. J Immunother Cancer. (2022) 10:e003819. doi: 10.1136/jitc-2021-003819
36. Jhan KY, Cheng CJ, Chou CJ, Jung SM, Lai GJ, Chen KY, et al. Improvements of cognitive functions in mice heavily infected by Angiostrongylus cantonensis after treatment with albendazole, dexamethasone, or co-therapy. J Microbiol Immunol Infect. (2022) 55:935–45. doi: 10.1016/j.jmii.2022.04.004
37. Zhang C, Wang H, Li J, Hou X, Li L, Wang W, et al. Involvement of TIGIT in natural killer cell exhaustion and immune escape in patients and mouse model with liver Echinococcus multilocularis Infection. Hepatology. (2021) 74:3376–93. doi: 10.1002/hep.32035
38. Atanasov G, Benckert C, Thelen A, Tappe D, Frosch M, Teichmann D, et al. Alveolar echinococcosis-spreading disease challenging clinicians: a case report and literature review. World J Gastroenterol. (2013) 19:4257–61. doi: 10.3748/wjg.v19.i26.4257
39. Kaethner M, Epping K, Bernthaler P, Rudolf K, Thomann I, Leitschuh N, et al. Transforming growth factor-β signalling regulates protoscolex formation in the Echinococcus multilocularis metacestode. Front Cell Infect Microbiol. (2023) 13:1153117. doi: 10.3389/fcimb.2023.1153117
40. Kamiyama T. Recent advances in surgical strategies for alveolar echinococcosis of the liver. Surg Today. (2020) 50:1360–7. doi: 10.1007/s00595-019-01922-6
41. Du C, Liu Z, Yang X, Yan L, Li B, Wen T, et al. Hepatectomy for patients with alveolar echinococcosis: long-term follow-up observations of 144 cases. Int J Surg. (2016) 35:147–52. doi: 10.1016/j.ijsu.2016.09.094
42. Fabbri J, Pensel PE, Albani CM, Arce VB, Mártire DO, Elissondo MC. Drug repurposing for the treatment of alveolar echinococcosis: in vitro and in vivo effects of silica nanoparticles modified with dichlorophen. Parasitology. (2019) 146:1620–30. doi: 10.1017/S0031182019001057
43. Alvi MA, Khan S, Ali RMA, Qamar W, Saqib M, Faridi NY, et al. Herbal medicines against hydatid disease: a systematic review (2000-2021). Life. (2022) 12:676. doi: 10.3390/life12050676
44. Liu C, Fan H, Guan L, Ge RL, Ma L. In vivo and in vitro efficacy of crocin against Echinococcus multilocularis. Parasit Vectors. (2021) 14:364. doi: 10.1186/s13071-021-04866-4
45. Jiang C. Experimental study on a novel compound extracted from Traditional Chinese Medicine for treatment of alveolar echinococcosis. Chin Med J. (2002) 115:1576–8.
46. McCarthy E, Stack C, Donnelly SM, Doyle S, Mann VH, Brindley PJ, et al. Leucine aminopeptidase of the human blood flukes, Schistosoma mansoni and Schistosoma japonicum. Int J Parasitol. (2004) 34:703–14. doi: 10.1016/j.ijpara.2004.01.008
47. Harbut MB, Velmourougane G, Dalal S, Reiss G, Whisstock JC, Onder O, et al. Bestatin-based chemical biology strategy reveals distinct roles for malaria M1- and M17-family aminopeptidases. Proc Natl Acad Sci U S A. (2011) 108:E526–34. doi: 10.1073/pnas.1105601108
48. Singh AK, Singh R, Tomar D, Pandya CD, Singh R. The leucine aminopeptidase of Staphylococcus aureus is secreted and contributes to biofilm formation. Int J Infect Dis. (2012) 16:e375–81. doi: 10.1016/j.ijid.2012.01.009
49. Hassan AHE, Mahmoud K, Phan TN, Shaldam MA, Lee CH, Kim YJ, et al. Bestatin analogs-4-quinolinone hybrids as antileishmanial hits: design, repurposing rational, synthesis, in vitro and in silico studies. Eur J Med Chem. (2023) 250:115211. doi: 10.1016/j.ejmech.2023.115211
50. Khampoosa P, Jones MK, Lovas EM, Piratae S, Kulsuntiwong J, Prasopdee S, et al. Egg-hatching mechanism of human liver fluke, opisthorchis viverrini: a role for leucine aminopeptidases from the snail host, bithynia siamensis goniomphalos. J Parasitol. (2018) 104:388–97. doi: 10.1645/16-125
51. Liu X, Guo Q, Jing F, Zhou C, Xiu T, Shi Y, et al. Ubenimex suppresses the ability of migration and invasion in gastric cancer cells by alleviating the activity of the CD13/NAB1/MAPK pathway. Cancer Manag Res. (2021) 13:4483–95. doi: 10.2147/CMAR.S300515
52. Wang X, Niu Z, Jia Y, Cui M, Han L, Zhang Y, et al. Ubenimex inhibits cell proliferation, migration and invasion by inhibiting the expression of APN and inducing autophagic cell death in prostate cancer cells. Oncol Rep. (2016) 35:2121–30. doi: 10.3892/or.2016.4611
53. Wan J, Ling XA, Wang J, Ding GG, Wang X. Inhibitory effect of Ubenimex combined with fluorouracil on multiple drug resistance and P-glycoprotein expression level in non-small lung cancer. J Cell Mol Med. (2020) 24:12840–7. doi: 10.1111/jcmm.15875
54. Tsukamoto H, Shibata K, Kajiyama H, Terauchi M, Nawa A, Kikkawa F. Aminopeptidase N (APN)/CD13 inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer. BMC Cancer. (2008) 8:74. doi: 10.1186/1471-2407-8-74
55. Babják M, Königová A, Burcáková L, Komáromyová M, Dolinská MU, Várady M. Assessing the efficacy of Albendazole against Fasciola hepatica in naturally infected cattle by in vivo and in vitro methods. Vet Sci. (2021) 8:249. doi: 10.3390/vetsci8110249
56. Movahedi F, Li L, Gu W, Xu ZP. Nanoformulations of albendazole as effective anticancer and antiparasite agents. Nanomedicine. (2017) 12:2555–74. doi: 10.2217/nnm-2017-0102
57. da Silva Santana RC, Prudente TP, de Sousa Guerra CH, de Lima NF, de Souza Lino Junior R, Vinaud MC. Albendazole - ivermectin combination decreases inflammation in experimental neurocysticercosis. Exp Parasitol. (2023) 251:108568. doi: 10.1016/j.exppara.2023.108568
58. Jacob J, Siraj MA, Steel A, Tan G, Jarvi S. Evaluation of the mechanism of action of albendazole on adult rat lungworm (Angiostrongylus cantonensis). Exp Parasitol. (2022) 242:108355. doi: 10.1016/j.exppara.2022.108355
59. Albanese G, Venturi C. Albendazole: a new drug for human parasitoses. Dermatol Clin. (2003) 21:283–90. doi: 10.1016/S0733-8635(02)00085-2
60. Hu C, Qin M, Zhang F, Gao R, Gan X, Du T. Improvement of antialveolar echinococcosis efficacy of novel albendazole-bile acids derivatives with enhanced oral bioavailability. PLoS Negl Trop Dis. (2023) 17:e0011031. doi: 10.1371/journal.pntd.0011031
61. Stettler M, Rossignol JF, Fink R, Walker M, Gottstein B, Merli M, et al. Secondary and primary murine alveolar echinococcosis: combined albendazole/nitazoxanide chemotherapy exhibits profound anti-parasitic activity. Int J Parasitol. (2004) 34:615–24. doi: 10.1016/j.ijpara.2004.01.006
62. Enkai S, Kouguchi H, Inaoka DK, Irie T, Yagi K, Kita K. In vivo efficacy of combination therapy with albendazole and atovaquone against primary hydatid cysts in mice. Eur J Clin Microbiol Infect Dis. (2021) 40:1815–20. doi: 10.1007/s10096-021-04230-5
63. Xin Q, Lv W, Xu Y, Luo Y, Zhao C, Wang B, et al. 2-Deoxy-D-glucose and combined 2-Deoxy-D-glucose/albendazole exhibit therapeutic efficacy against Echinococcus granulosus protoscoleces and experimental alveolar echinococcosis. PLoS Negl Trop Dis. (2022) 16:e0010618. doi: 10.1371/journal.pntd.0010618
64. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. (2003) 362:1295–304. doi: 10.1016/S0140-6736(03)14573-4
65. Qamar W. and Alkheraije KA. Anthelmintic resistance in Haemonchus contortus of sheep and goats from Asia–a review of in vitro and in vivo studies. Pak Vet J. (2023) 43:376–87.
66. Velázquez-Antunez JO-PJ, Olmedo-Juárez A, Rojas-Hernandez S, Villa-Mancera A, Romero-Rosales T, Zamilpa A, et al. Biological activity of the secondary compounds of Guazuma ulmifolia leaves to inhibit the hatching of eggs of Haemonchus contortus. Pak Vet J. (2023) 43:55–60.
Keywords: LAP, Ubenimex, Albendazole, E. multilocularis, liver, mice
Citation: Zhou Z, Huayu M, Mu Y, Tang F and Ge R-L (2024) Ubenimex combined with Albendazole for the treatment of Echinococcus multilocularis-induced alveolar echinococcosis in mice. Front. Vet. Sci. 11:1320308. doi: 10.3389/fvets.2024.1320308
Received: 27 October 2023; Accepted: 05 March 2024;
Published: 15 March 2024.
Edited by:
Yadong Zheng, Zhejiang Agriculture and Forestry University, ChinaReviewed by:
Mughees Aizaz Alvi, University of Agriculture, PakistanFadi Jebbawi, University of Bern, Switzerland
Copyright © 2024 Zhou, Huayu, Mu, Tang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ri-Li Ge, Z2VyaWxpZ2FvQGhvdG1haWwuY29t; Feng Tang, bGVpbGVpdGFuZzE5ODRAMTYzLmNvbQ==
 Zhen Zhou
Zhen Zhou Meiduo Huayu1,2
Meiduo Huayu1,2 Yalin Mu
Yalin Mu Feng Tang
Feng Tang Ri-Li Ge
Ri-Li Ge