- 1Paul G. Allen School for Global Health, Washington State University, Pullman, WA, United States
- 2Washington State University Global Health–Kenya, Nairobi, Kenya
- 3University of Nairobi Institute of Tropical and Infectious Diseases, Nairobi, Kenya
Introduction: Raw diets have become popular in companion animal nutrition, but these diets may be contaminated with harmful bacteria because heat processing is not utilized to mitigate pathogens during the production process. We analyzed 24 commercially available frozen raw canine and feline diets for extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-E).
Methods: Samples were incubated in tryptic soy broth augmented with 50 μg/mL ampicillin to enrich for ESBL-E. ESBL-E were isolated using CHROMagar ESBL plates and isolate identification and antibiotic susceptibility testing were confirmed using the VITEK®2 instrument.
Results: ESBL-E were isolated from 42% (10/24) of raw diets, with E. coli, Enterobacter cloacae complex and Klebsiella pneumoniae predominating. Most ESBL-E isolates (71%, 32/45) were multidrug-resistant. Direct plating of samples onto tryptic soy agar yielded bacterial counts >6 log10 for 2 samples from two different manufacturers.
Conclusion: This preliminary study justifies further investigation into the potential contribution of raw diets to the dissemination of antibiotic resistant bacteria in companion animals and domestic living spaces.
Introduction
A growing number of pet owners are choosing raw meat-based diets (RMBDs), also known as “raw diets,” for their pets (1–3). RMBDs are formulated using uncooked ingredients including muscle, organ meat and bone sourced from domestic and wild animals (1). Pet owners often elect to feed RMBDs due to mistrust of conventional diets and the belief that RMBDs are healthier and more natural diets for pets (4–6). There are perceived health benefits to feeding RMBDs, including improved dental health, coat quality and muscle mass. However, these claims are unsubstantiated by current literature (1, 5, 6). Approximately 25% of North American agility dog owners feed their dogs RMBDs and a 2020 survey found that 9% of pet owners across Canada, New Zealand, Australia, and the US feed RMBDs exclusively (2, 3). Notably, the Centers for Disease Control and Prevention, the American Veterinary Medical Association, and the U.S. Food and Drug Administration advise against RMBDs due to the risk of bacterial contamination and transmission to pets and owners (7–9). Raw meat can become contaminated with pathogens during slaughter, processing and transportation and there is existing evidence of RMBD cross-contamination during manufacturing (1, 6, 10). Previous studies have detected DNA from undeclared protein sources in RMBDs and genetically-identical bacteria has been isolated from RMBDs with different protein sources that were produced by the same manufacturer (10, 11). Salmonella species are often a prominent concern, as these bacteria cause 26,500 hospitalizations in the United States annually (12). Furthermore, the risk of bacterial contamination is made more serious by the threat of antimicrobial resistance. In 2019, antimicrobial-resistant bacteria contributed to an estimated 4.95 million human deaths globally (13). Extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) are especially concerning and have a public health importance because they can inactivate critical β-lactam antibiotics such as cephalosporins and carbapenems. In 2017, ESBL-E were responsible for 9,100 estimated deaths in the United States (14).
The extent to which RMBDs disseminate ESBL-E remains largely unknown (15). Studies in Europe have isolated ESBL-E from 61% (31/51) of sampled RMBDs and ESBL-producing Escherichia coli from 80% (28/35) of sampled RMBDs (16, 17). Additionally, a 2017 study in the Netherlands detected ESBL-E in 78% (14/18) of RMBD products but none in non-raw pet foods (18). Data on ESBL-E contamination of RMBDs in the United States are scant; a recent study isolated ESBL-E from 10% (20/200) of RMBD products (11). These findings indicate the potential public health threat posed by RMBDs in the prevailing antibiotic resistance crisis. We analyzed commercially available frozen RMBDs in the US to assess ESBL-E contamination, examine the antibiotic resistance profiles of ESBL-E isolates and characterize aerobic bacterial contamination of RMBDs.
Methods
Product selection
For this preliminary study, a convenience sample of RMBDs that met the following criteria were selected: (i) frozen products intended for consumption by dogs, or dogs and cats, (ii) products containing a single animal protein and whose meat was sourced from the US, and (iii) products that were not freeze-dried, pasteurized, fermented, or high pressure processed. RMBDs were either purchased online and shipped via ground transport from the distributor, or from a retail store and transported to the laboratory within 3 h of purchase. Transportation was done under chilled conditions. Upon arrival, each diet was photographed, and the integrity of packaging was assessed. Diets were immediately placed in a −20°C freezer. Lot numbers and expiration dates were recorded where available.
Sample processing
Each product was stored in a −20°C freezer for a median of 18 days (range: 1–38 days) in its original packaging. Prior to processing, the products were thawed at 4°C for 26–28 h and transferred into sterile 24 oz Whirl Pak sample bags (Fort Atkinson, WA) in a biosafety cabinet. The products were homogenized by hand for 60 s. Six 2-g samples were collected from each raw diet for processing in duplicates; three samples were added to 10-ml tryptic soy broth (TSB) augmented with ampicillin (50 μg/ml) and three samples to 10-ml of sterile water to enumerate total bacterial counts (colony forming units; CFUs). Each sample was assigned a unique laboratory identification code based upon the food brand, protein source and order that the samples were collected from the homogenized diet.
The TSB tubes were mixed for 30 s using a vortex to form slurries, then incubated in a 37°C shaker overnight (18–24 h). Ten microliters (10 μl) of the resulting TSB-ampicillin cultures were streaked onto CHROMagar ESBL plates (CHROMagar, Paris, France). The plates were incubated at 37°C overnight. Blue colonies on ESBL plates—presumptively identified as Klebsiella, Citrobacter, or Enterobacter sp.—or pink—presumptively E. coli—were collected from each positive plate and purified on trypticase soy agar (TSA) plates. A maximum of four colonies of different colors and morphologies were selected from a single ESBL plate for purification on a TSA plate. After overnight incubation at 37°C, one colony from each TSA plate was transferred to TSB broth with 50% v/v sterile glycerol and archived in a −80°C freezer. Archived isolates were revived by streaking onto TSA plates and incubating at 37°C overnight. One colony from each TSA plate was re-streaked for isolation on a second plate prior to identification and antibiotic susceptibility testing using VITEK®2 GN ID and AST GN84 cards, respectively. The GN84 card was selected for its ESBL confirmatory test and inclusion of antibiotics from three most frequently used antibiotic classes in food animals (19). Breakpoints for each antibiotic and bacterial species followed CLSI M100 (20). ATCC-BAA-2469 was a positive control for ESBL plates while ATCC 2912 and ATCC 25922 were negative controls. All controls were also used with the VITEK®2. Isolates were tested for susceptibility to 16 antibiotics belonging to nine antibiotic classes, including aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, monobactams, nitrofurans, penicillins, sulfonamides and tetracyclines.
The sterile water slurries were used for CFU counts. Ten-fold serial dilutions (up to 10−5) of slurries were prepared using 0.9% sterile saline in a 96-well plate. Five microliters (5 μl) of each dilution were transferred onto TSA plates using a multi-channel pipette. The plates were inverted and incubated at 37°C for 16–18 h. CFU counts were calculated from dilutions containing 25–250 discrete colonies.
Data analysis
Isolates of the same bacterial species with identical antibiotic resistance phenotypes that were recovered from the same initial 2-g raw food sample were assumed to be duplicates. Multidrug resistance was defined as resistance to at least one antibiotic from ≥3 antibiotic classes (21). Isolates with intermediate resistance were classified as susceptible. Data entry and summaries of the antimicrobial resistance profiles, recovered bacterial species, protein sources, lot numbers and expiration dates were performed in Microsoft Excel. Data analysis was limited to descriptive data.
Results
Twenty-four RMBDs from seven manufacturers were purchased between May and July 2021. Between 2–5 RMBDs were evaluated from each manufacturer, with a mode of three products per manufacturer. Fifty-eight percent (14/24) products were purchased online while 42% (10/24) were purchased in a retail store. Fifty-four percent (13/24) had lot numbers and 67% (16/24) had expiration or packaging dates. The RMBDs included five protein sources (beef, chicken, duck, lamb, and turkey). Forty-six percent (11/24) diets included vegetables in their ingredients, 42% (10/24) had meat only, while 4% (3/24) had no ingredients listed. One manufacturer (n = 4 products) used bacteriophages and blanched their vegetables as a food safety measure; ESBL-E were isolated from one product by this manufacturer.
ESBL-E were isolated from 42% (10/24) of the RMBDs representing 71% (5/7) manufacturers, with 56% (25/45) ESBL-E isolates sourced from a single manufacturer and 88% (39/45) ESBL-E isolates from three manufacturers. In terms of ingredients, ESBL-E were isolated from 27% (3/11) diets with meat and vegetables, 33% (3/10) diets with meat only, and 67% (2/3) diets with unlisted ingredients (Table 1). Of the five protein sources, ESBL-E were detected in 50% (3/6) of beef, 33% (2/6) of chicken, 25% (1/4) of lamb, 33% (1/3) of duck, and 60% (3/5) of turkey diets. Fifty-six presumptive ESBL-E isolates were analyzed using the VITEK®2. Of these, 93% (52/56) were confirmed ESBL-positive. After excluding six putative clonal isolates and an outlier Citrobacter sp. isolate, 45 ESBL-E isolates were analyzed. E. coli (42%, 19/45), Enterobacter cloacae complex (36%, 16/45), and Klebsiella pneumoniae (22%, 10/45) were the predominant bacterial species. E. coli was the only ESBL-E isolated from chicken diets; 42% (8/19) of all E. coli isolates were from chicken (Table 1).
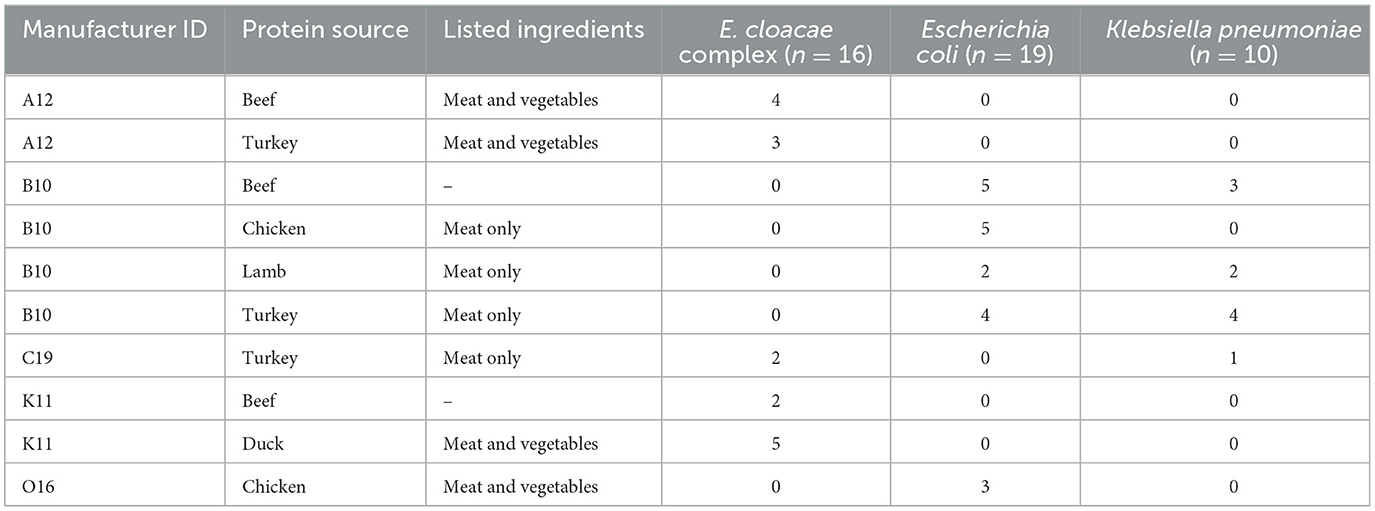
Table 1. Distribution of recovered ESBL-E bacteria by manufacturer, protein source, other ingredients, and bacterial species.
CFU counts were possible for 42% (10/24) total diets. ESBL-E were detected in 33% (3/10) of these diets, including one turkey diet, one lamb diet with <50 CFUs, and one beef diet with 4.0 × 106 CFUs. The remaining diets had CFU counts ranging from <50 CFUs/g to too numerous to count, consistent with significant bacterial contamination (Table 2).
All isolates were resistant to cefazolin and susceptible to ertapenem, imipenem and meropenem. Forty per cent (18/45) were resistant to amoxicillin-clavulanic acid, including 100% (16/16) Enterobacter sp., 10% (1/10) Klebsiella sp. and 5% (1/19) E. coli isolates. Most isolates (71%, 32/45) were resistant to ceftriaxone and 47% (21/45) to aztreonam, with Klebsiella and E. coli predominating. Several isolates (36%, 16/45) were resistant to tetracycline with E. coli and Enterobacter predominating (Figure 1). Most ESBL-E isolates (71%, 32/45) were multidrug-resistant with 31% (14/45) being resistant to three antibiotic classes, and 29% (13/45), 7% (3/45), and 4% (4/45) being resistant to four, five, and six antibiotic classes, respectively.
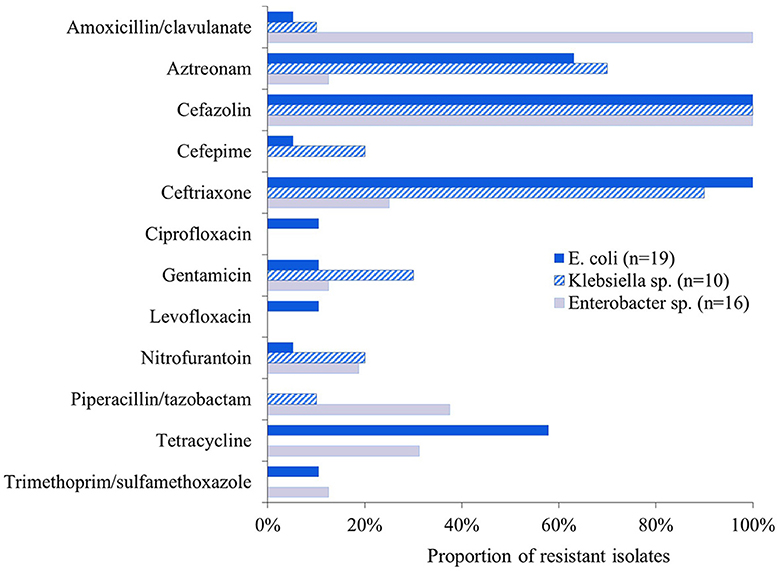
Figure 1. Proportion of ESBL-E isolates (n = 45) that were resistant to the 16 antibiotics tested. All isolates were susceptible to ertapenem, imipenem, and meropenem.
Discussion
In European studies, ESBL-E prevalence values of >60% have been reported while an American study found a prevalence of 10% in the sampled products (11, 16–18). Consequently, the isolation of ESBL-E in the present study was expected. Nevertheless, our study reported a higher prevalence (42%) than that previously reported in America, potentially due to disproportionate ESBL-E contamination among RMBD manufacturers. In our study, over half (56%) of ESBL-E isolates were detected in diets sourced from a single manufacturer. This is consistent with a study in which 75% of contaminated products came from 4/61 manufacturers; multiple products of different protein sources from the same manufacturers also contained genetically identical bacteria (11). Furthermore, a 2020 North American study recovered the DNA of at least one undeclared animal source in >60% of RMBDs analyzed (10). These results indicate that the manufacturing process and cross-contamination may significantly influence ESBL-E contamination of RMBDs.
ESBL-E have more frequently been isolated from frozen raw diets than freeze-dried or other raw diet preparations (11, 22). A previous American study recovered ESBL-E from frozen but not from freeze-dried raw diets (11). This difference may exist because the reduced water content in freeze-dried foods creates suboptimal conditions for bacterial survival (11, 23). It is also possible that different thawing methods may impact the quantity of bacteria recovered from frozen RMBDs. One study noted a significant increase in aerobic bacterial CFU counts 24 h after RMBD defrosting began, at 2 and 7°C (24). These results demonstrate the need for further research to elucidate the safest RMBD processing and feeding practices.
Several studies have isolated ESBL-producing E. coli from RMBDs (11, 16, 17), so it is not surprising to isolate E. coli. The proportions of K. pneumoniae (22%) and E. cloacae (35%) isolated in the present study were higher than previously reported (6%−10% and 0%−2%, respectively) (11, 16). Enterobacter sp. were isolated from three manufacturers and three distinct protein sources, indicating that Enterobacter sp. contamination was not unique to a single manufacturer or protein source. Conversely, Klebsiella was only isolated from two manufacturers, with 90% of the isolates recovered from a single manufacturer. It is possible that this disproportionate contamination is responsible for the higher overall prevalence of Klebsiella in our study.
The proportion of multi-drug resistant ESBL-E (71%) and pan-susceptibility to carbapenems observed in the present study were consistent with previous findings (11, 16). Previous literature from the Netherlands reported no ESBL-E. coli resistance to tetracycline (25), whereas our study found that 57% of E. coli isolates were resistant to tetracycline. This discrepancy is intriguing given that tetracyclines had the highest food animal antibiotic sales volume in both the Netherlands and United States when these studies were conducted (26, 27). With regards to sulfonamides, a Swiss study found that 50% of ESBL-E isolates were resistant to trimethoprim-sulfamethoxazole whereas only 9% were resistant in our study. This disparity may exist because sulfonamides had the second and third highest food animal antibiotic sales volume in the countries where RMBDs were sourced for the study in Switzerland (28, 29). In contrast, sulfonamides comprise 5% of medically-important food animal antibiotic sales in the U.S. (26). These results indicate the need to investigate how national antibiotic usage impacts antibiotic resistance phenotypes in RMBDs.
Data on bacterial contamination of RMBDs are unavailable in the US. Studies in Europe have reported aerobic mesophilic and Enterobacterales counts of 8.2 × 104-7.4 × 108 CFU/g, and aerobic bacterial counts of 7.9 × 102-5.0 × 106 CFU/g and 4.22 × 104 CFU/g−3.77 × 106 CFU/g (16, 17, 30). The counts of aerobic bacteria in the present study varied from <50 CFU/g to numerous. The quantifiable upper range (4.0 × 106 CFU/g) is comparable to the upper range of aerobic bacteria in previous studies (5.0 × 106 CFU/g and 3.77 × 106 CFU/g) (17, 30). The wide range of contamination recorded in the present study suggests that further work is needed to identify sources of bacterial contamination of RMBDs.
RMBDs pose a potential risk to public health, as people can be exposed to harmful bacteria by handling contaminated diets or the feces of RMBD-fed pets (31). Pets fed RMBDs are more likely to shed harmful bacteria in their feces (18, 32–34); a 2022 study demonstrated clonal relationships between Salmonella sp. isolated from RMBDs and canine fecal samples from the same household (31). Moreover, a study in Brazil reported that dogs fed RMBDs were 30 times more likely to shed Salmonella in their feces compared to dogs fed commercial dry food (33). Similarly, a cohort study that compared RMBD-fed cats and those not fed RMBDs isolated ESBL-E in 90 and 6% of cat stool samples, respectively (18). Contaminated feces may pose health risks to children; a Canadian survey found that 52% of household reported that their children (<16 years) play in the same areas where their dogs defecate (35). While the risk of human infection with antibiotic-resistant bacteria shed by pets consuming RMBDs has not been reported, household transmission of antibiotic-resistant Enterobacterales between dogs and humans has been documented (36). A 2020 study in New Zealand found that in 22% of households, clonal strains of ESBL-producing E. coli were cultured from both a person and pet within the household (36). Likewise, a 2019 study in the Netherlands noted that “eating raw meat” was a predictor of ESBL-E carriage in canines and that human-canine ESBL-E co-carriage was higher than predicted based on chance (0.9%) (37). Although these studies show that the proportion of household human-canine co-carriage of ESBL bacteria is relatively small, 69 million households in the US own a dog and—based on a survey of 1,250 dog owners in the US-−63% included raw food as a part of their dog's diet (2, 38). Based on this data, even if the proportion of affected household is as low as 1%, potentially >400,000 American households could be affected. These results emphasize the need for long-term studies to establish the directionality of ESBL-E transfer between humans and pets, identify sources of ESBL-E, and further describe the persistence of ESBL-shedding in pets.
In the present study, 46% of RMBD samples did not include lot numbers and 33% did not have expiration or packaging dates. The FDA recommends including lot numbers on pet food for ease of recall and to facilitate the reporting of product concerns (39). It requires that pet food includes an ingredients list, nutritional adequacy statement and guaranteed analysis on packaging (22, 40, 41); 13% of samples representing two different brands did not meet any of these requirements. The nutritional adequacy statement is important as it indicates whether the diet will meet the pet's daily nutrient needs (41). Comparably, a study in Minnesota found that 27% of RMBD brands evaluated did not include a nutritional adequacy statement or guaranteed analysis (22). These results demonstrate that some RMBD manufacturers omit important information that could impact the health and safety of pets.
The present study was intended as a small-scale preliminary study and as such, there were several constraints. As we did not conduct whole genome sequencing, we were unable to confirm isolate uniqueness for data analysis or genetic variation of the ESBL-E recovered from RMBDs. These are potential research areas for future studies. Additionally, the relative abundance of ESBL-E organisms compared to normal flora in the RMBDs is unclear, as the CFU counts in the present study only allowed for a snapshot of overall microbiological contamination of the RMBDs. The present study was also limited to seven brands and 24 diets that could be purchased and delivered to Washington state in the US. It is possible that the samples analyzed in the present study were not representative of commercially available frozen RMBDs. The present study did not allow for an overall prevalence estimate of ESBL-E contamination of RMBDs in the US. Additional research is required to establish these prevalence estimates. Freeze-dried, pasteurized, high pressure processed and fermented RMBD preparations were not investigated in the present study. Further research is needed to elucidate the prevalence of ESBL-E in these RMBD preparations. The number of diets in the present study did not allow for a comparative analysis of contamination in RMBDs with different protein sources and ingredients. This is an area that future research could explore.
Conclusion
This study advances our knowledge of the ESBL-E bacteria that humans and animals may encounter through RMBDs. This can guide the development of intervention strategies, educate veterinarians, and advise pet owners about the risks involved with feeding RMBDs. While more data are needed to establish the true prevalence of ESBL-E in frozen RMBDs in the U.S., this study and the previous literature emphasize the need for good hygiene practices when feeding RMBDs and handling pets that are fed RMBDs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. DC: Methodology, Project administration, Resources, Supervision, Writing – review & editing. SO: Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was funded by The Paul G. Allen School for Global Health and a Washington State University College of Veterinary Medicine Summer Research Fellowship.
Acknowledgments
We would like to thank the Call lab members for laboratory assistance: Jennifer Horton, Jesus Mendoza, Colin McDowell, and Johannesty Avillan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Freeman LM, Chandler ML, Hamper BA, Weeth LP. Current knowledge about the risks and benefits of raw meat–based diets for dogs and cats. J Am Vet Med Assoc. (2013) 243:1549–58. doi: 10.2460/javma.243.11.1549
2. Dodd S, Cave N, Abood S, Shoveller AK, Adolphe J, Verbrugghe A. An observational study of pet feeding practices and how these have changed between 2008 and 2018. Vet Rec. (2020) 186:643–643. doi: 10.1136/vr.105828
3. Laflamme D, Abood S, Fascetti A, Fleeman L, Freeman L, Michel K, et al. Pet feeding practices of dog and cat owners in the United States and Australia. J Am Vet Med Assoc. (2008) 232:687–94. doi: 10.2460/javma.232.5.687
4. Morgan SK, Willis S, Shepherd ML. Survey of owner motivations and veterinary input of owners feeding diets containing raw animal products. PeerJ. (2017) 5:e3031. doi: 10.7717/peerj.3031
5. Morgan G, Williams N, Schmidt V, Cookson D, Symington C, Pinchbeck G, et al. Dog's Dinner: factors affecting food choice and feeding practices for UK dog owners feeding raw meat-based or conventional cooked diets. Prev Vet Med. (2022) 208:105741. doi: 10.1016/j.prevetmed.2022.105741
6. Morelli G, Bastianello S, Catellani P, Ricci R. Raw meat-based diets for dogs: survey of owners' motivations, attitudes and practices. BMC Vet Res. (2019) 15:74. doi: 10.1186/s12917-019-1824-x
7. Pet Food Safety | Healthy Pets Healthy People | CDC. (2018). Available online at: https://www.cdc.gov/healthypets/publications/pet-food-safety.html (accessed January 10, 2021).
8. American Veterinary Medical Association. Raw or undercooked animal-source protein in cat and dog diets. Available online at: https://www.avma.org/resources-tools/avma-policies/raw-or-undercooked-animal-source-protein-cat-and-dog-diets (accessed January 10, 2021).
9. Center for Veterinary Medicine. Get the Facts! Raw Pet Food Diets can be Dangerous to You and Your Pet. Rockville, MD: FDA (2020).
10. Cox A, Defalque VE, Udenberg TJ, Barnum S, Wademan C. Detection of DNA from undeclared animal species in commercial canine and feline raw meat diets using qPCR. Can Vet J. (2020) 61:977–84.
11. Cole SD, Healy I, Dietrich JM, Redding LE. Evaluation of canine raw food products for the presence of extended-spectrum beta-lactamase- and carbapenemase-producing bacteria of the order Enterobacterales. Am J Vet Res. (2022) 83:ajvr.21.12.0205. doi: 10.2460/ajvr.21.12.0205
12. Salmonella By the Numbers | Food Safety and Inspection Service. Available online at: http://www.fsis.usda.gov/inspection/inspection-programs/inspection-poultry-products/reducing-salmonella-poultry/salmonella (accessed July 2, 2023).
13. Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
14. Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.) (2019). Available online at: https://stacks.cdc.gov/view/cdc/82532 (accessed January 10, 2021).
15. Nemser SM, Doran T, Grabenstein M, McConnell T, McGrath T, Pamboukian R, et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog Dis. (2014) 11:706–9. doi: 10.1089/fpd.2014.1748
16. Nüesch-Inderbinen M, Treier A, Zurfluh K, Stephan R. Raw meat-based diets for companion animals: a potential source of transmission of pathogenic and antimicrobial-resistant Enterobacteriaceae. R Soc Open Sci. (2019) 6:191170. doi: 10.1098/rsos.191170
17. van Bree FPJ, Bokken GCAM, Mineur R, Franssen F, Opsteegh M, van der Giessen JWB, et al. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet Rec. (2018) 182:50. doi: 10.1136/vr.104535
18. Baede VO, Broens EM, Spaninks MP, Timmerman AJ, Graveland H, Wagenaar JA, et al. Raw pet food as a risk factor for shedding of extended-spectrum beta-lactamase-producing Enterobacteriaceae in household cats. PLoS ONE. (2017) 12:e0187239. doi: 10.1371/journal.pone.0187239
19. 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Rockville, MD: U.S. Food and Drug Administration Center for Veterinary Medicine (2020).
20. VITEK2 cards selection guide. Available online at: https://www.biomerieux-usa.com/sites/subsidiary_us/files/vitek2_cards_selection_guide.pdf (accessed February 24, 2024).
21. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
22. Mehlenbacher S, Churchill J, Olsen KE, Bender JB. Availability, brands, labelling and Salmonella contamination of raw pet food in the Minneapolis/St. Paul Area: availability, brands, labelling and Salmonella. Zoonoses Public Health. (2012) 59:513–20. doi: 10.1111/j.1863-2378.2012.01491.x
23. Kananub S, Pinniam N, Phothitheerabut S, Krajanglikit P. Contamination factors associated with surviving bacteria in Thai commercial raw pet foods. Vet World. (2020) 13:1988. doi: 10.14202/vetworld.2020.1988-1991
24. Morelli G, Catellani P, Scapin RM, Bastianello S, Conficoni D, Contiero B, et al. Evaluation of microbial contamination and effects of storage in raw meat-based dog foods purchased online. J Anim Physiol Anim Nutr. (2020) 104:690–7. doi: 10.1111/jpn.13263
25. Nilsson O. Hygiene quality and presence of ESBL-producing Escherichia coli in raw food diets for dogs. Infect Ecol Epidemiol. (2015) 5:28758. doi: 10.3402/iee.v5.28758
26. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Rockville, MD: U.S. Food and Drug Administration Center for Veterinary Medicine (2022).
27. Havelaar AH, Graveland H, van de Kassteele J, Zomer TP, Veldman K, Bouwknegt M. A summary index for antimicrobial resistance in food animals in the Netherlands. BMC Vet Res. (2017) 13:305. doi: 10.1186/s12917-017-1216-z
28. Köper LM, Bode C, Bender A, Reimer I, Heberer T, Wallmann J. Eight years of sales surveillance of antimicrobials for veterinary use in Germany—what are the perceptions? PLoS ONE. (2020) 15:e0237459. doi: 10.1371/journal.pone.0237459
29. StAR. Swiss Antibiotic Resistance Report 2020. Available online at: https://www.star.admin.ch/star/en/home/star/Newsletter-Beitraege/swiss-antibiotic-resistance-report-2020.html (accessed August 6, 2023).
30. Bottari B, Bancalari E, Barera A, Ghidini S, Gatti M. Evaluating the presence of human pathogens in commercially frozen, biologically appropriate raw pet food sold in Italy. Vet Rec. (2020) 187:e50. doi: 10.1136/vr.105893
31. Solís D, Toro M, Navarrete P, Faúndez P, Reyes-Jara A. Microbiological quality and presence of foodborne pathogens in raw and extruded canine diets and canine fecal samples. Front Vet Sci. (2022) 9:799710. doi: 10.3389/fvets.2022.799710
32. Groat EF, Williams NJ, Pinchbeck G, Warner B, Simpson A, Schmidt VM, et al. dogs eating raw meat diets have higher risk of Salmonella and antimicrobial-resistant Escherichia coli faecal carriage. J Small Anim Pract. (2022) 63:435–41. doi: 10.1111/jsap.13488
33. Viegas FM, Ramos CP, Xavier RGC, Lopes EO, Júnior CAO, Bagno RM, et al. Fecal shedding of Salmonella spp, Clostridium perfringens, and Clostridioides difficile in dogs fed raw meat-based diets in Brazil and their owners' motivation. PLoS ONE. (2020) 15:e0231275. doi: 10.1371/journal.pone.0231275
34. Runesvärd E, Wikström C, Fernström LL, Hansson I. Presence of pathogenic bacteria in faeces from dogs fed raw meat-based diets or dry kibble. Vet Rec. (2020) 187:e71–e71. doi: 10.1136/vr.105644
35. Stull JW, Peregrine AS, Sargeant JM, Weese JS. Pet husbandry and infection control practices related to zoonotic disease risks in Ontario, Canada. BMC Public Health. (2013) 13:520. doi: 10.1186/1471-2458-13-520
36. Toombs-Ruane LJ, Benschop J, French NP, Biggs PJ, Midwinter AC, Marshall JC, et al. Carriage of extended-spectrum-beta-lactamase- and AmpC beta-lactamase-producing Escherichia coli strains from humans and pets in the same households. Appl Environ Microbiol. (2020) 86:e01613-20. doi: 10.1128/AEM.01613-20
37. van den Bunt G, Fluit AC, Spaninks MP, Timmerman AJ, Geurts Y, Kant A, et al. Faecal carriage, risk factors, acquisition and persistence of ESBL-producing Enterobacteriaceae in dogs and cats and co-carriage with humans belonging to the same household. J Antimicrob Chemother. (2020) 75:342–50. doi: 10.1093/jac/dkz462
38. Facts + Statistics: Pet Ownership and Insurance | III. Available online at: https://www.iii.org/fact-statistic/facts-statistics-pet-ownership-and-insurance (accessed February 18, 2023).
Keywords: extended-spectrum beta-lactam-resistance, ESBL, Enterobacterales, pet food, raw diet, RMBD
Citation: Fisher CD, Call DR and Omulo S (2024) Detection of antibiotic resistant Enterobacterales in commercial raw pet food: a preliminary study. Front. Vet. Sci. 11:1294575. doi: 10.3389/fvets.2024.1294575
Received: 14 September 2023; Accepted: 27 May 2024;
Published: 12 June 2024.
Edited by:
Valentina Virginia Ebani, University of Pisa, ItalyReviewed by:
Genever Morgan, University of Liverpool, United KingdomMohammad Nasim Sohail, University of Illinois at Urbana–Champaign, United States
Copyright © 2024 Fisher, Call and Omulo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvia Omulo, c3lsdmlhLm9tdWxvQHdzdS5lZHU=
 Carolyn D. Fisher
Carolyn D. Fisher Douglas R. Call
Douglas R. Call Sylvia Omulo
Sylvia Omulo