
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 28 March 2024
Sec. Animal Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1286563
This article is part of the Research Topic Comparative Aspects of Physiology and Nutrition of Growing Animals View all 8 articles
A major global barrier to increased animal output is nutrition. The use of aquatic plants, which were previously considered to be waste and needed a lot of labor to eliminate, has recently come to light due to the lack of feed during the dry season in the majority of tropical regions of Africa. The objectives of this study were therefore to see how different dietary Eichhornia crassipise inclusion rates affect the hematological indicators of Ethiopian Doyogena sheep and Woyto-Guji goats. Blood samples were taken from the jugular veins of 12 Doyogena sheep and 12 Woyto-Guji goats in a 2 × 4 randomized crossover design with two animal species, four diets, and four random periods (15 - day adaption period followed by a 7- day experimental diet in each period). The dietary inclusion rates E. crassipise were 0, 25, 50, and 75% that was used as a replacement for commercial concentrate mix diet in the treatment groups. The data was analyzed using the SAS software programme PROC GLM, and Pearson's correlation coefficient was calculated between hematological markers. The hemoglobin (Hb), red blood cell count (RBC), packed cell volume (PCV), mean corpuscular volume (MCH), and mean corpuscular hemoglobin (MCHC) results revealed substantial, RDW-SD, and WBC differences between animal species (P < 0.001). Sheep had greater WBC, Hb, RBC, PCV, RDW-SD, and RDW-CV levels, while goats had higher MCH and MCHC values (P < 0.001). For the analyzed hematological measures, the Pearson's correlation coefficient ranged from low to strong in terms of positive and negative associations (P < 0.05). Since all hematological indicators were closer to those of clinically healthy native Ethiopian sheep and goat breeds, feeding water hyacinth to sheep and goats up to a 75% inclusion level in diets without producing sickness may provide a remedy for adverse feed shortages.
Small ruminants, such as sheep and goats, have significant importance in the livelihoods of many people in tropical regions, especially those who rely on native breed types. They contribute to income generation, food security, sustainable agriculture, and the empowerment of marginalized groups (1, 2). Doyogena Sheep and Woyto-Guji goat breeds are two potential indigenous livestock species in Ethiopia that play a vital role in the livelihoods and food security of Southern Ethiopian rural communities (3, 4). Despite its socioeconomic importance, small ruminant productivity is hampered by a number of complex and interconnected factors, such as poor genetics, poor reproductive performance, poor quality and varying seasonal availability of feed, high disease incidence and parasite challenges, and limited access to services and inputs (5). Among these, the seasonal shortage of feed was identified as one of the primary challenges in Ethiopia. This shortage refers to both the limited quantity and quality of available feed resources for livestock (6). Farm lands are used for crops during the rainy seasons, and the feed shortage persists even during those times (7).
Even though, agro-industrial by-products and commercial concentrate feeds are skyrocketing and thus restricting possibilities for resource-poor families. Under such circumstances, it becomes imperative for farmers to explore alternate, economically viable, and environmentally sound non-conventional feed resources like water hyacinth (Eichhornia crassipes), which could help alleviate feed shortages and improve the nutritional status of these animals. Water hyacinth is one of the noxious weeds that thrives and procreates in aquatic settings (8). It seriously jeopardizes the global environment, public health, the development of society, and access to clean water (9). Fresh plant matter may contain up to 95% water, which makes it challenging for harvesting and processing (10); transport, and post-harvest handling (11). Similarly, freshly harvested E. crassipes contains abrasive calcium oxalate crystals hurt the mouth and are which contribute to low palatability of various animal species (12). However, properly dried E. crassipes possesses potential as a feed resource for livestock due to its high nutritional value, such as proteins, carbohydrates, lipids, and so on Sotolu and Sule (13). Even in Ethiopia, study by Mekuriaw et al. (14) showed that wilted E. crassipes leave can effectively substitute concentrate mix up to 75% and promoting ideal growth of Washera sheep without notably toxicity. Considering its potential as an ingredient in livestock feed, there is a lack of comprehensive research on the influence of E. crassipes diets on the hematological indices of small ruminants such as sheep and goats. Hematological markers, particularly those linked to blood composition and immune response, provide significant information on the health and physiological status of farm animals (15). As a result, the purpose of this study was to look into the hematological indices of two sheep and goat breeds, Doyogena sheep and Woito-Guji goats that were fed varied amounts of water hyacinth in commercial concentrate mix and hay-based diets.
The experiment was conducted between October 2021 and February 2022 at the Arba Minch University livestock Research Farm at the Kulfo campus in Southern Ethiopia. The farm is located at a latitude of 6°2′21′′N and longitude 37°34′24′′E, and 435 km south of Addis Ababa, Ethiopia. It lies at an altitude of 1285 m above sea level, its average temperature is 29°c and average annual rainfall is 892 mm.
Twelve intact yearling sheep lambs and 12 intact yearling goat bucks, Ethiopian Doyogena sheep and Woito-Guju goat breeds with average weights of 20.78 kg and 19.23 kg, respectively, were employed in an 84-day feeding trial and a 7-day digesting trial. Rams and bucks were quarantined and acclimatized for 4 weeks before onset of the experiment, during which they received a broad-spectrum anthelmintic (albendazole) against internal parasites, were sprayed with an accaricide (diazzinole) against external parasites, and were vaccinated against anthrax and pasteurelosis. During the acclimatization period, the animals were given time to adjust to their new environment and introduced to the experimental diets in order to adapt their rumen microbes. During the period, animals were weighed after overnight fasting on 2 consecutive days, and the average was taken as the initial body weight, ear tagged, and placed in separate metabolic pens. Each experimental animal was randomly assigned to treatment groups, and feed was provided in equal portions twice daily, with clean water and salt licks provided ad libitum.
The study employed a randomized two-by-four crossover design with two species and four treatment groups comprising three sheep and three goats in each period. Rams and bucks were given the experimental diets for 21 days for each phase, with blood samples collected on the last day. Animal weights were measured separately after an overnight fast to ensure consistency. The diet provision for the next period was adjusted based on body weight. The daily experimental dietary treatment (basal + supplement) fed to sheep and goats was fed at 3% of body weight, well-adjusted to provide minimum 8.36 MJ/kg metabolizable energy and 70 g DM CP/kg on a DM basis (16). The commercial concentrate, feed formulation that contained 33% maize, 10% nuge cake, 15% wheat middling, 40% wheat bran, 1% mineral premixes, and 1% salt; was purchased from Muza livestock feed maker and distributor, a private enterprise in Arba Minch. Grass hay was prepared from plant harvests from the Kulfo campus that were made from various plant species mixes. It was made up of plant groups such as Asteraceae, Fabaceae, Poaceae, Commelinaceae, and others, with Fabacea and Asteraceae dominating ones. A list of treatment groupings can be seen in Table 1.
Hematological index analyses were carried out at the laboratory of the Nech Sar campus, Arba Minch University, Ethiopia. At the end of each experimental period, sheep and goats were restrained for blood collection. In order to perform hematological index analysis, 5 ml of blood samples were collected via the jugular vein into sterile test tubes containing the anti-coagulant Ethylenediaminetetraacetic acid (EDTA). Laboratory analyzed parameters included packed cell volume (PCV), white blood cell (WBC), hemoglobin (Hb), red blood cell (RBC), RBC indices such as mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width standard deviation (RDW-SD), and red blood cell distribution width coefficient of variation (RDW-CV). This was done by using an automated hematology analyzer complete blood count machine (17).
The chemical composition of the feed samples used in feeding was analyzed at the chemistry laboratory of Arba Minch University. To determine the dry matter (DM), ash, Ether extract (EE) and Kjeldahl N following the procedures outlined in AOAC (18). The Crude Protein (CP) was determined by multiplying %N by 6.25, and the organic matter (OM) was estimated by subtracting the ash content from 100. The neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined according to the procedure of Van Soest et al. (19).
Variance analysis was conducted following a 2 × 4 factorial layout in a randomized cross-over design using mixed model procedures (PROC MIXED) of SAS 2013 version 9.4. All hematological indices were firstly tested for normality and homoscedasticity with the Shapiro–Wilk's and Levene's test, respectively. Significant differences were declared at P < 0.05 and highly significant for P < 0.001. Means for hematological parameters were separated via the Tukey's HSD method and the level of significance was determined at P < 0.05. The Pearson correlation coefficient was used to evaluate the intensity of the association between hematological indices. The appropriate statistical model.
Where,
Yijk = the response due to the animal i, in period j, treatment k, and interaction effects, μ = the overall mean effect, αi = the fixed effect of the ith species groups (I = sheep or goat) (subject; i =1, 2, 3…12), βj = the random effect of the jth collection period (j = 1, 2, 3, 4), γk = the fixed effect of the kth treatment (k =1, 2, 3, 4), αβik = the interaction effect between species i and treatment k, and Σijkl = the random error.
Table 2 displays the chemical composition analyses of the experimental feedstuffs used in the dietary interventions. Hay, a commercial concentrate combination, and water hyacinth all had dry matter values of 92.23%, 90.17%, and 92.34%, respectively. Ether extract ranged from 3.78% (hay) to 7.9% (concentration mix) while organic matter ranged from 89.12 (water hyacinth) to 93.15% (concentrate mix). When compared to grass-based hay, concentrate mix (CP, 17.98%) and water hyacinth leaves (CP, 12.05%) had higher crude protein levels. As anticipated, water hyacinth leaf content (5.72%) was twice that of hay. The most ash was found in water hyacinth (10.88%), while the least was in concentrate mix (6.85%). The concentrations of neutral detergent fibers, acid detergent fibers, and acid detergent lignin were highest in hay (66.29%, 42.18%, and 11.57%, respectively), and lowest in commercial concentrate mixture (38.47%, 27.92%, and 6.89).
The hematological profiles of sheep and goat breeds fed varying levels of water hyacinth (E. Crassipes) are presented in Table 3. The results revealed a significant variation in hematological parameters among the two species. The differences were found to be statistically significant (P < 0.001) for hemoglobin (g/dl), red blood cells (106 /μL), packed cell volume (%), mean corpuscular hemoglobin (pg), and mean corpuscular hemoglobin concentration (g/L). Likewise, the white blood cell count (109 /L) showed significant variation (P < 0.005) between the two species. Furthermore, the red blood cell distribution width standard deviation values varied significantly (P < 0.001) among the animals. Sheep displayed higher values for WBC count, Hb count, RBC count, PCV, RDW-SD, and RDW-CV, while goats had higher values for MCH and MCHC. There were no significant differences in mean corpuscular volume (fL) or red blood cell distribution width coefficient of variation. It also, appears that there were no significant differences observed among the treatment groups and no significant interactions between species and treatment being studied for any of the hematological parameters.
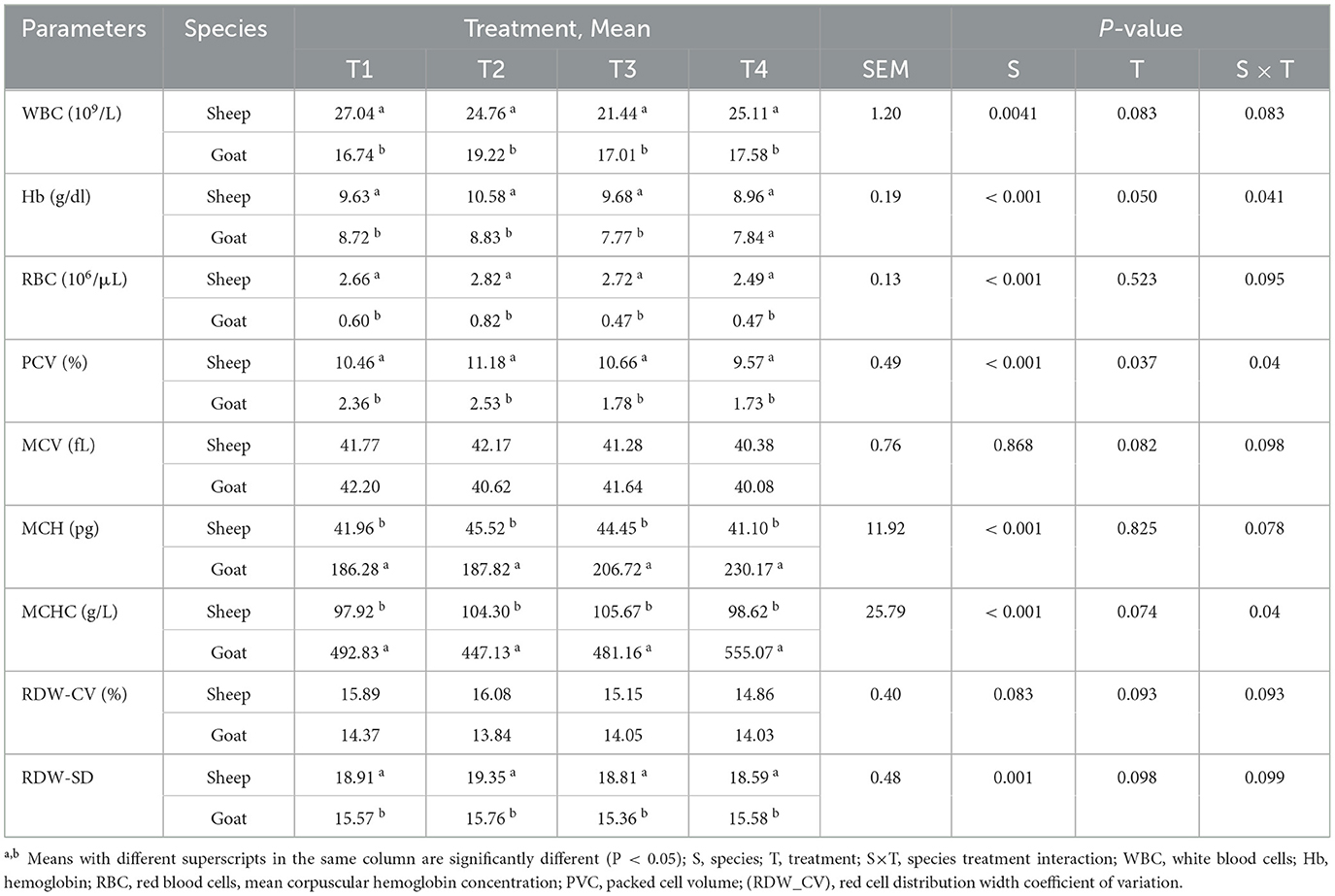
Table 3. Least square means for hematological parameters compared between sheep and goat fed graded levels of water hyacinth (E. crassipes).
Tables 4, 5 summarize the results of the correlation study between hematological markers in sheep and goats, respectively. White blood cell count (WBC) and red blood cell count (RBC) (r = 0.46), as well as WBC and packed cell volume (PCV) (r = 0.39), were discovered to have positive associations in sheep. WBC and mean corpuscular volume (MCV) (r = −0.5), mean corpuscular hemoglobin (MCH) (r = −0.43), mean corpuscular hemoglobin concentration (MCHC) (r = −0.3), red cell distribution width coefficient of variation (RDW_CV) (r = −0.36), and red cell distribution width standard deviation (RDW_SD) (r = −0.38) were found to have negative correlations. Hb also had favorable relationships with MCV (r = 0.25), MCH (r = 0.55), and MCHC (r = 0.63). Furthermore, RBC were positively correlated with PCV (r = 0.95) but negatively correlated with MCV (r = −0.75), MCH (r = −0.85), MCHC (r = −0.78), RDW_CV (r = −0.33), and RDW_SD (r = −0.31). PCV also exhibited negative correlations with MCV (r = −0.51), MCH (r = −0.74), MCHC (r = −0.76), RDW_CV (r = −0.35), and RDW_SD (r = −0.35). On the contrary, MCV showed positive correlations with MCH (r = 0.80), MCHC (r = 0.57), and RDW_CV (r = 0.25), while MCH was positively correlated with MCHC (r = 0.94). Additionally, RDW_SD was positively correlated with MCHC (r = 0.14) and RDW_CV (r = 0.96).
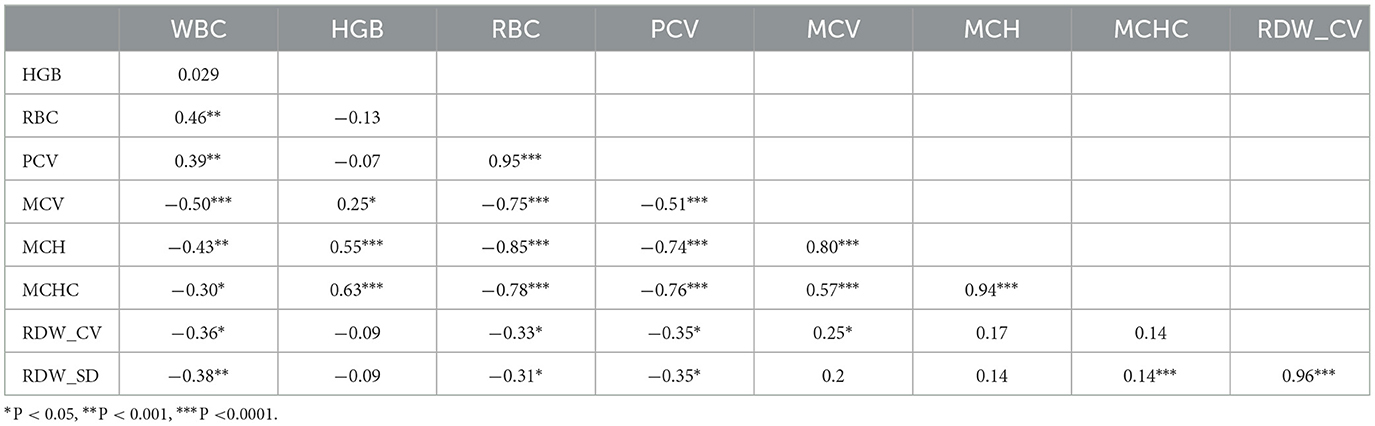
Table 4. Pearson's correlation relationship between hematological parameters of Doyogena sheep fed varying incorporation rates of water hyacinth.
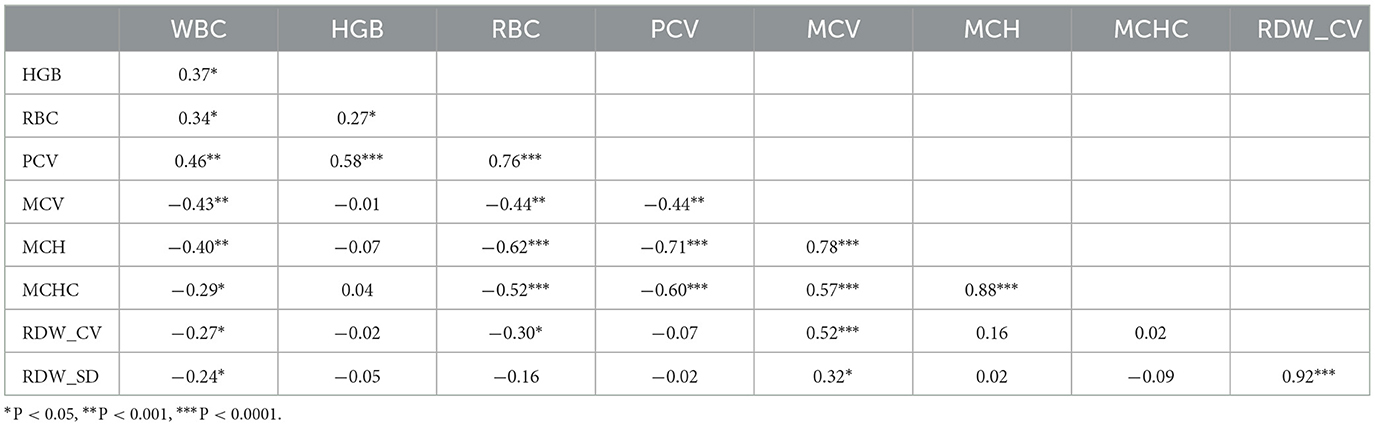
Table 5. Pearson's correlation coefficient among hematological parameters of Woyto-Guji goat breed fed different inclusion rate of water hyacinth.
In goats, positive correlations were observed between WBC and Hb (r = 0.37), RBC (r = 0.34), and PCV (r = 0.46). Negative correlations were found between WBC and MCV (r = −0.43), MCH (r = −0.40), MCHC (r = −0.29), RDW_CV (r = −0.27), and RDW_SD (r = −0.24). Hb showed positive correlations with RBC (r = 0.27) and MCH (r = 0.58), while RBC was positively correlated with PCV (r = 0.76). However, RBC exhibited negative correlations with MCV (r = −0.44), MCH (r = −0.62), MCHC (r = −0.52), and RDW_CV (r = −0.30). PCV also showed negative correlations with MCV (r = −0.44), MCH (r = −0.71), and MCHC (r = −0.60). On the other hand, MCV exhibited positive correlations with MCH (r = 0.78), MCHC (r = 0.57), RDW_CV (r = 0.52), and RDW_SD (r = 0.32), while MCH was positively correlated with MCHC (r = 0.88). Furthermore, RDW_SD was positively correlated with MCHC (r = 0.14) and RDW_CV (r = 0.92).
There is a wide range of values reported in different research for the crude protein (CP) content of grass-based hay in this study. Simone et al. (20) found a CP content of 4.60%, which is lower than ours. Kibret et al. (21) had the highest CP content at 9.53%, followed by Ayele et al. (22) at 7.9% and Yigzaw et al. (23) at 7.13%. Geleti et al. (24) and Teklehaymanot (25), for instance, reported CP levels of 5.8% and 5.56%, respectively, which are comparable to our findings. Variations in the CP content of grass hay among studies may be related to factors such as species composition, cultivation circumstances, and maturity stage at which the hay was harvested (26). The CP content of grass hay achieved in this investigation was < 7%CP required for microbial protein synthesis in the rumen to meet the basic maintenance demands of ruminants (19). The NDF, ADF, and ADL values in this study were greater than those published by Gulilat et al. (27), Woyessa et al. (28) and Mengistu et al. (29), which were 55.88%, 39.67%, and 6.4%, respectively. However, it had lower NDF, ADF, and ADL values when compared to Fikre et al. (30), which were 75.42%, 57.45%, and 14.42%, respectively. This inference validates the findings of Kibret et al. (21) and Teklehaymanot (25), who reported equivalent NDF, ADF, and ADL values of 65%, 42.6%, and 12.17%, respectively.
The CP concentration of the concentrate combination was comparable to the 19.2% reported by Ayele et al. (22) but less than the 23.40% and 24.54% reported by Simone et al. (20) and Abreha et al. (31), respectively. Amare and Girmay (32) reported a CP content of 16.3%, which is lower than our results. The variation in CP content of commercial concentrate mix among trials could be related to differences in the level and kind of components utilized in creating the concentrate combination, according to Simone et al. (20). When compared to previous research by Olafadehan and Okunade (33) and Abreha et al. (31), the concentrate mix in this study exhibited greater levels of NDF, ADF, and ADL. The corresponding figures were 30.6%, 14.2%, and 4.13%. Ayele et al. (22), Simone et al. (20), and Maurya et al. (34), on the other hand, reported identical NDF, ADF, and ADL values of 39.9%, 27.66%, and 6.23%, respectively.
The CP content of water hyacinth used in this study was comparable to the 11.9% reported by Patel et al. (35), but higher than the 10.3 to 10.4 and 10.5% reported by Hossain et al. (36) and Mako et al. (37), respectively, and lower than 14.4% reported by Mekuriaw et al. (14) and 18.03% CP reported by Aboud et al. (38). The water hyacinth NDF, ADF, and ADL values in this study were greater than those published by Banakar et al. (39), Mekuriaw et al. (14), and Tiwari et al. (40), which were 46.59%, 28.2%, and 5.35%, respectively. They were, however, lower than the NDF, ADF, and ADL values reported by Mako et al. (37) and Mako and Ikusika (41), which were respectively 65.9–77.9%, 36.5–39.7%, and 12.0%. Selim et al. (42) and Carneiro et al. (43), on the other hand, reported equivalent NDF, ADF, and ADL values of 54.8%, 29.8%, and 8.36%, respectively. The chemical makeup of water hyacinth may differ according on species, harvesting season, habitat and light, and water temperature, according to Shafy et al. (44), De Vasconcelos et al. (45), and Makkar et al. (46).
Lonsdale (47) classified feeds into low, medium, and high protein sources based on their protein composition. Feeds with < 12% CP were labeled as low protein sources, while those with 12 to 20% CP were labeled as medium protein sources. High protein sources were defined as feeds having a protein concentration >20% CP. Roughage feed can also be classed based on fiber fraction (NDF and ADF) value, which is crucial since it gives vital information on fiber content and animal digestibility. High-quality roughage feeds have NDF values below 45% and are easily digested, giving nutrients that are readily available. NDF-values in medium-quality roughage feeds range from 45% to 65%, striking a compromise between nutrient availability and fiber content. Low-quality roughage feeds have higher NDF-values and are more difficult to digest due to a higher proportion of indigestible components (48). Based on their ADF values, Kellems and Church (49) classified roughages similarly. Roughages with < 40% ADF were considered high quality, while those with more than 40% ADF were considered low quality. ADF denotes the less digestible components of the diet, such as cellulose and lignin. In this study, the CP content of grass hay was found to be low, indicating that the grass hay used in the study was of poor quality. The water hyacinth and concentrate mixture had a medium protein level.
Generally, the hematological profile of animals plays a crucial role in understanding their overall health and wellbeing, such as their physiological status, diagnosis of diseases, monitoring treatment efficacy, and even assessing the impact of environmental factors on their health (50, 51). Moreover, it can also be a valuable tool in assessing feed quality, such as nutrient availability, mineral deficiencies, feed composition, digestibility and absorption in animals (52).
Perhaps, the most striking finding to emerge from this analysis was a substantial disparity in hematological parameters among sheep and goats. As expected, these differences were statistically significant, in terms of various blood parameters, including Hb, RBC, PCV, MCH, MCHC, WBC, and RDW_SD. These findings suggest distinct physiological variations between the species. These results are in accord with the observation by Etim et al. (53) that indicates significant variations in hematological parameters, including Hb, RBC, PCV, MCH, and MCHC, these variations are not limited to a specific population but are generalizable across different settings. Hematological indices of various livestock species are typically influenced by genetic and non-genetic factors (54). This distinct genetic variation between species may cause variations in their hematological profiles, influencing the production and functionality of red blood cells, hemoglobin, and other blood components. Hematological indices such as WBC count, Hb count, RBC count, PCV, RDW-SD, and RDW-CV values were higher in sheep. These results are consistent with those of Kiran et al. (55) findings, which showed, sheep consistently displayed higher levels of hematological values compared to goats.
Surprisingly, the study did not detect any significant effects of treatments or treatment-by-species interactions on hematological indices among sheep and goats. These findings align with several recent studies that used different dietary inclusion rates. For instance, WBC, RBC, Hb, PCV, MCV, MCH, and MCHC (56) values were not affected when sheep were fed varying dietary inclusions of alternative feedstuffs. Similarly, certain parameters such as PCV, Hb, WBC, and RBC (57) were not affected when sheep were fed varying dietary inclusions of Artemisia sieberi leaves. Moreover, WBC, RBC, MCH, MCHC, Hb, and PCV (58) values were not influenced by goats supplemented with Lablab purpureus and Vigna unguiculata. Furthermore, PCV, Hb, RBC, MCH, MCHC, and WBC (59) values were not affected by goat fed Mombasa or blue panic as a salt-tolerant alternative to replacing Alfalfa. PCV, Hb, RBC, MCV, MCH, and MCHC (60) values were not affected by West African Dwarf goats fed varying levels of treated sweet orange peels. The lack of significant effects observed in our study suggests that treatments and treatment-by-species interactions may not play a significant role in influencing hematological indices in the studied species. However, the findings of the current study do not support previous research by Kurtoglu et al. (61) and Osita et al. (62) on hematological indices influenced by diet.
The values for WBC in this study ranged between 21.44 and 27.04 × 109/L for sheep and 16.74 and 19.22 × 109/L for goats. The values obtained in this study are far higher than laboratory reference value by Jackson and Cockcroft (63) 4–12 × 109 /L sheep and 4–13 × 109/L goats. Comparable to the value range of 20.44 ± 1.02 × 109/L and 27.0 ± 0.56 × 109/L reported by Njidda et al. (64) for Yankasa and Ouda sheep, respectively, in the semi-arid region of Nigeria. In this study, an elevated WBC suggests an immunological reaction against contamination or toxic chemicals in the body, whereas a smaller count implies the presence of infectious infections or antigens in the body (65). The elevated levels of WBC observed in both species indicate that they have an immune system that is functioning properly.
Hemoglobin, an iron-rich protein, is found in red blood cells. It allows the blood to carry oxygen to all of the body's tissues (66). The Hb values were between 8.96 and 10.58 (g/dl) sheep and 7.77–8.83 (g/dl) goats. The values obtained in this study are almost in accord with laboratory reference value by Jackson and Cockcroft (63) 9–15 g/dl sheep and 8–12 g/dl goats. Moreover, this result is in line with the value range between 8.8 and 10.73 g/dl) reported by Dikko et al. (67) for Yankasa Sheep fed Brewers' Dried Grain, and 8.6 and 9.7 g/dl were reported by Pudjihastuti et al. (68) for domestic goats supplemented with urea palm sugar block. Generally, elevated concentrations of HB are attributed to a more pronounced capacity for overcoming infection with a disease, while lower levels are a sign of illness, infection, and inadequate nutrition (69, 70). The study indicates that, neither sheep nor goats suffer from microcytic hypochromic anemia, which is related to iron scarcity and scant utilization during the formation of Hb Olafadehan (71).
The RBC value were between 2.49 and 2.82 (106/μL) sheep and 0.47–0.82 (106 /μL) goats and were not statistically different (P > 0.05) among the treatments. The values observed in this investigation are far below those observed by Jackson and Cockcroft (63) 9–15 × 106 /μl sheep and 8–18 × 106/μl goats. This abnormally low figure obtained in this study might be a clinical sign of eminent vulnerability to anemia-related disease conditions (72). Furthermore, the lowest levels of red blood cells may be a sign of anemia, internal bleeding, inadequate iron consumption, vitamin deficiency, or other health problems (64). However, comparison of the findings with those of other recent studies reported closer values of 3.40 ± 0.12, 3.41 ± 0.12, 3.11 ± 0.12, and 3.72 ± 0.12 for different sheep breeds reared by Agbaye et al. (73). Moreover, 2.39–3.51 × 106/μl WAD sheep fed Ensiled maize stover and concentrate supplement had no clinical signs of disease (74).
PCV is a metric representation of the quantity of volume the red blood cells occupy in relation to the overall volume of blood in a sample of capillary, venous, or arterial blood (75). PCV values ranged from 9.57 to 11.18 % in sheep and 1.73–2.53 % in goats. The values observed in this study were far below the reference range by Jackson and Cockcroft (63) for sheep (27.0–45.0%) and goats (22.0–38.0%). The study indicates that the animal may be at risk for developing macrocytic anemia due to a vitamin B12 or folate deficiency (76).
RBC indices are metrics that are crucial to diagnosing the underlying cause of anemia (77). MCV, MCH and MCHC are considered RBC indices give details about average cell size, hemoglobin content, and hemoglobin concentration, respectively (78). The MCV values of sheep 40.38 - 42.17 (fL) obtained in this study were closer to the range value 45.5–46.78 (fL) obtained by Orzuna et al. (79) for sheep supplemented with a Polyherbal Mixture; little bit higher than the reference range by Jackson and Cockcroft (63) for sheep 28–40 (fL) and goats 16–25 (fL), but lower than 62.2 (fL) reported by Egbe-Nwiyi et al. (80) of apparently healthy goats. The MCV value in obtained this study implies that the animal is exposed to macrocytic anemia due to vitamin B12 or folate deficiency (76). Contrary to expectations, results pertaining to MCH in this study were by far higher than the laboratory reference for hematological parameters of sheep 8–12 (pg) and goats 5.2–8 (pg) Jackson and Cockcroft (63). An elevated MCH can signify the existence of reticulocytes or hemolysis, whereas a lower MCH is thought to correspond to a severe iron deficiency (78). Similarly, MCHC has been suggested to be the most accurate of the RBC indices, and it might rise when there is an instance of hemolysis or fall in the case of reticulocytosis and iron deficient anemia (78). MCHC values in this study far lower for sheep 310–340 (g/L) and far higher than goats 300–360 (g/L) than the laboratory reference for hematological parameters (63).
RDW, an estimate derived from the red blood cell distribution curves obtained from automated hematology analyzers, was carried out to gauge the erythrocytes' size variability in circulation (81). RDW-CV levels in this study range between 14.86 and 16.08% in sheep and 13.84–14.37% in goats. These values are somewhat closer to Manchega Spanish sheep (16.44 ± 0.34%) Bórnez et al. (82) and Pramenka sheep (15.46%) in Livno fields, Ohran (83). However, it was lower than 24.16 ± 1.61 % that of Wonosobo sheep (84). On the other hand, RDW levels of goats obtained in this study were lower than those of Mohammed et al. (76) 28.75 ± 3.04% for the Damascus goat, and Reiten et al. (85), 23–35% for the Norwegian Dairy Goat Breed. Elevated concentration of RDW outside the typical reference range, prognostic for iron deficiency anemia, folic acid and/or vitamin B12 deficiencies, autoimmune or hemolytic anemia, myelodysplastic syndrome, or sickle cell disease (77).
The Pearson correlation coefficient for hematological indices in this study exhibited low to high, positive and negative levels of associations. So far, the study has broadly aligned and deviated from recent studies working with a variety of experimental designs and sheep and goat breeds. Antunović et al. (86) reported a significant negative association between WBC and MCV (r = 0.22) for the Merinolandschaf sheep breed. Karki et al. (87) reported a significant positive association between RBC and HCT (r = 0.81) for the Katahdin sheep breed. A negative association between RBC and MCV (r = 0.29), RBC and MCH (r = 0.59), and RBC and MCHC (r = 0.42) was found by Karki et al. (87) for the Katahdin sheep breed and HCT and MCHC (r = 0.24) by Antunović et al. (86) for Merinolandschaf sheep. However, the findings of the current study deviate from the previous research. Fadare et al. (88) report a significant negative association between WBC and RBC (r = 0.72) and WBC and HTC (r = 0.41) for white-colored West African Dwarf sheep. Antunović et al. (86) reported significant negative correlations between Hb and MCV (r = 0.21); Hb and MCHC (r = 0.22); and MCH and MCHC (r = 0.63) for Merinolandschaf sheep.
The results pertaining to certain hematological indices of goats in this study support recent studies using different goat breeds with different experimental designs. A significant positive correlation obtained between Hb and RBC (r = 0.805) and PCV (r = 0.424) in goats (89); between Hb and HCT (r = 0.57) for beef (90); and between MCV and MCH (r = 0.95) Perumal et al. (91) and Karki et al. (87) findings reported that RBC was negatively correlated with MCV (r = −0.58) and MCH (r = −0.71) in the kiko goat breed. In contrast, the results of this investigation differ from those of the other studies. A significant negative association between WBC and RBC (r = 0.16) (92); and between WBC and RBC (r = 0.35) (93) for WAD goats whereas a significant positive correlation was observed between RBC and MCHC (r = 0.36) for kiko goat breeds (87).
In conclusion, the study successfully determined that different levels of water hyacinth inclusion in the diets of sheep and goats did not have any detrimental effects on their hematological parameters. There were a substantial disparity observed among the hematological parameters of sheep and goats, but these differences were not influenced by the treatments or treatment-species interactions. While certain parameters such as PCV, RDW, RBC, and its indices deviated from the normal range, none of the animals showed any clinical signs of conditions like haemolysis, microcytic hypochromic anemia, or inflammatory response to the treatment diets. Pearson correlation coefficient for hematological indices showed both positive and negative relationships, ranging from low to high. Overall, these findings suggest that dried water hyacinth meal can be used as a substitute for up to 75% of commercial concentrate diets without affecting the hematological indices of sheep and goats. Further validation of these findings necessitates additional studies. These future studies should delve deeper into evaluate the effects of prolonged water hyacinth inclusion in the diets of sheep and goats on their hematological profiles. Additionally, it would be valuable to investigate the potential accumulation of any toxins or contaminants from water hyacinth in the animals' systems and how it may impact their hematological health.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://doi.org/10.5281/zenodo.8304949.
The Animal Research Ethics Committee of Arba Minch University approved the research methodologies and procedures, verifying that they follow ethical standards. The committee issued a certificate (ref. no AMU/AREC/4/2016, dated 20, September, 2021) stating that the study was approved and carried out in an ethical and responsible manner. The study was conducted in accordance with the local legislation and institutional requirements.
YF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YK: Supervision, Writing – review & editing. NY: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship and/or publication of this article. The authors would like to express their appreciation to the Arba Minch University VLIR-IUC project for funding their research work.
The authors would like to acknowledge the Arba Minch University Animal Research Farm, Medical and Chemistry Laboratory for their technical support in analyzing the Hematological indices and chemical composition of the feed samples, respectively. Furthermore, the authors would like to extend their gratitude to all those who have contributed to the realization of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mohammadabadi M. Tissue-specific mRNA expression profile of ESR2 gene in goat. Agric Biotechnol J. (2021) 12:167–81. doi: 10.22103/jab.2021.17011.1284
2. Masoudzadeh SH, Mohammadabadi M, Khezri A, Stavetska RV, Oleshko VP, Babenko OI, et al. Effects of diets with different levels of fennel (Foeniculum vulgare) seed powder on DLK1 gene expression in brain, adipose tissue, femur muscle and rumen of Kermani lambs. Small Rum Res. (2020) 193:106276. doi: 10.1016/j.smallrumres.2020.106276
3. Taye M, Yilma M, Rischkowsky B, Dessie T, Okeyo M, Mekuriaw G, et al. Morphological characteristics and linear body measurements of Doyogena sheep in Doyogena district of SNNPR, Ethiopia. Afr J Agric Res. (2016) 11:4873–85. doi: 10.5897/AJAR2016.11826
4. Lorato Y. Community Based Characterization of Woyto Guji goat in Loma district, SNNP. Accept Regional State of Ethiopia Accept for Award of Msc (Animal Breeding and Genetics). Jimma: Jimma University (2014), 142.
5. Mekuriaw Z, Harris-Coble L. Ethiopia's livestock Systems: Overview and Areas of Inquiry. Gainesville, FL: Feed the Future Innovation Lab for Livestock Systems (2021).
6. CSA. “Agricultural sample survey. Report on livestock and livestock characteristics (private peasant holdings),” Statistical Bulletin. Addis Ababa: Central Statistical Agency Federal Democratic Republic of Ethiopia (2016), 583.
7. Ayele AT. On Farm Evaluation of the Effects of Nutritional Flushing on Body Weight, Body Condition Score and Reproductive Performances of Doyogena ewes in Doyogena District, Southern Ethiopia' (Doctoral Dissertation). Beirut: International Center for Agricultural Research in the Dry Areas (2022).
8. Nandiyanto AB, Ragadhita R, Hofifah SN, Al Husaeni DF, Al Husaeni DN, Fiandini M, et al. Progress in the utilization of water hyacinth as effective biomass material. Environ Dev Sust. (2023) 28:1–48. doi: 10.1007/s10668-023-03655-6
9. Dersseh MG, Melesse AM, Tilahun SA, Abate M, Dagnew DC. Water hyacinth: review of its impacts on hydrology and ecosystem services—lessons for management of Lake Tana. Extreme Hydrol Clim Variability. (2019) 1:237–51. doi: 10.1016/B978-0-12-815998-9.00019-1
10. Nega DT, Ramayya AV, Manenti F, Amaral AF. Turning curse into cure: potential of water hyacinth for bio-refining-A contextual investigation of Lake Tana. Environ Challenges. (2021) 5:100387. doi: 10.1016/j.envc.2021.100387
11. Su W, Sun Q, Xia M, Wen Z, Yao Z. The resource utilization of water hyacinth (Eichhornia crassipes [Mart] Solms) and its challenges. Resources. (2018) 7:46. doi: 10.3390/resources7030046
12. Krajda G, Kasprzak K, Urbańczyk A, Kański J, Przybylo M. Maize green forage as a partial replacement for lettuce in the diet of West Indian manatee Trichechus manatus. J Zoo Aquar Res. (2023) 11:384–92. doi: 10.19227/jzar.v11i4.752
13. Sotolu AO, Sule SO. Digestibility and performance of water hyacinth meal in the diets of African catfish (Clarias gariepinus; Burchell, 1822). Tropical Subtropical Agro Ecosyst. (2011) 14:245–50.
14. Mekuriaw S, Tegegne F, Tsunekawa A, Ichinohe T. Effects of substituting concentrate mix with water hyacinth (Eichhornia crassipes) leaves on feed intake, digestibility and growth performance of Washera sheep fed rice straw-based diet. Trop Anim Health Prod. (2018) 50:965–72. doi: 10.1007/s11250-018-1519-5
15. Etim NN, Enyenihi G, Williams ME, Udo MD, Edem E. Haematological Parameters: Indicators of the Physiological Status of Farm Animals (2013).
16. NRC. Committee on Nutrient Requirements of Small Ruminants. Committee on Nutrient Requirements of Small Ruminants. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Rockville, MD: NRC (2007).
17. Wahed A, Dasgupta A. Hematology and coagulation. Benign White Blood Cell Platelet Dis. (2015) 5:81–92. doi: 10.1016/B978-0-12-800241-4.00005-X
19. Van Soest PV, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
20. Simone SK, Urge M. Yeheyis L. Effect of Alfalfa (Medicago sativa L) hay supplementation and urea molasses block on feed intake digestibility, and body weight change of yearling local sheep fed grass hay as basal diet. Turkish J Agric Food Sci Technol. (2023) 11:1067–73. doi: 10.24925/turjaf.v11i6.1067-1073.5989
21. Kibret W, Asmare B, Mekuriaw Y. Effects of Faba Bean Hull, Noug Seed (Guizotia Abyssinica) cake, wheat bran and their mixtures supplementation on biological performance and economic benefits of farta sheep fed natural pasture hay in Ethiopia. Sci Papers Anim Sci Biotechnol. (2021) 54:1–14.
22. Ayele S, Urge M, Animut G, Yusuf M. Feed intake, digestibility, growth performance and blood profiles of three Ethiopian fat tail hair sheep fed hay supplemented with two levels of concentrate supplement. Open J Anim Sci. (2017) 7:149–67. doi: 10.4236/ojas.2017.72013
23. Yigzaw D, Berhanu A, Asnakew A. Effect of different levels of lentil (Lensculinaries) hull and noug seed (Guizotiaabyssinica) cake mixture supplementation on feed intake, digestibility and body weight change of farta sheep fed hay as basal diet. Academic Res J Agric Sci Res. (2019) 7:75–86. doi: 10.14662/ARJASR2019.006
24. Geleti D, Hailemariam M, Mengistu A, Tolera A. Nutritive value of selected browse and herbaceous forage legumes adapted to medium altitude subhumid areas of western Oromia, Ethiopia. Glob Vet. (2013) 11:809–16. doi: 10.5829/idosi.gv.2013.11.6.8216
25. Teklehaymanot A. Feed intake, digestibility and growth performance of Begait sheep fed hay basal diet and supplemented with Tsara (Pterocarpus lucens), Pigeon pea (Cajanus cajan) leaves and concentrate mixture. Int J Livestock Prod. (2019) 10:204–12. doi: 10.5897/IJLP2018.0563
26. Worku D, Urge M. Effect of Substitution of Concentrate Mix With Dried Mulberry Leaves on Feed Intake, Digestibility, Body Weight Gain and Carcass Characteristics of Arsi-Bale Goats (Doctoral dissertation). Dire Dawa: Haramaya University. (2015).
27. Gulilat L, Walelign E, Amane A. Evaluation of the effects of concentrate supplementation on carcass merits of farta sheep. Acad Res J Agri Sci Res. (2018) 6:35–41. doi: 10.18685/EJARD(8)1_EJARD-17-013
28. Woyessa F, Tolera A, Diba D. Feed intake, digestibility and growth performance of Horro lambs fed natural pasture hay supplemented graded level of Vernonia amygdalina leaves and sorghum grain mixture. Science, Technol Arts Res J. (2013) 2:30–7. doi: 10.4314/star.v2i2.98870
29. Mengistu G, Assefa G, Tilahun S. Noug seed (Guizotia abyssinica) cake substituted with dried mulberry (Morus indica) and vernonia amygdalina mixed leaves' meal on growth performances of bonga sheep at Teppi, Ethiopia. J Nutr Metab. (2020) 24:8761. doi: 10.1155/2020/9308761
30. Fikre T, Alemu B, Ayele S. Effect of effective microorganism treated grass hay supplementation on feed intake, digestibility and growth performance of washera sheep fed natural grass hay as a basal diet. Int J Sci Res Pub. (2019) 9:845–53. doi: 10.29322/IJSRP.9.12.2019.p96109
31. Abreha HH, Animut G, Hailemichael A, Tedla DG, Baragabr FH. Effect of commercial and non-conventional feeds, leaves of indigenous and improved multipurpose tree supplementation on feed intake, digestibility and growth performance of sheep. The Open Agric J. (2010) 13:1–14. doi: 10.2174/1874331501913010207
32. Amare B, Girmay A. Effect of dietary supplemented cowpea (Vigna unguiculata) hay as replacement of concentrate on performance and economic efficiency of abergelle goats. Online J Anim Feed Res. (2020) 10:313–20. doi: 10.51227/ojafr.2020.42
33. Olafadehan OA, Okunade SA. Fodder value of three browse forage species for growing goats. J Saudi Soc Agric Sci. (2016) 17:43–50. doi: 10.1016/j.jssas.2016.01.001
34. Maurya VP, Sejian V, Kumar D, Naqvi SMK. Impact of heat stress, nutritional restriction and combined stresses (heat and nutritional) on growth and reproductive performance of Malpura rams under semi-arid tropical environment. J Anim Physiol Anim Nutr. (2016) 100:938–46. doi: 10.1111/jpn.12443
35. Patel V, Desai M, Madamwar D. Thermochemical pretreatment of water hyacinth for improved biomethanation. Appl Biochem Biotechnol. (1993) 42:67–74. doi: 10.1007/BF02788902
36. Hossain ME, Sikder H, Kabir MH, Sarma SM. Nutritive value of water hyacinth (Eichhornia crassipes). Online J Anim Feed Res. (2015) 5:40–4.
37. Mako AA, Babayemi OJ, Akinsoyinu AO. An evaluation of nutritive value of water hyacinth (Eichhornia crassipes Mart. Solms-Laubach) harvested from different water sources as animal feed. Livestock Res Rural Dev. (2011) 23:10.
38. Aboud AAO, Kidunda RS, Osarya J. Potential of water hyacinth (Eicchornia crassipes) in ruminant nutrition in Tanzania. Livestock Res Rural Dev. (2005) 17:2005.
39. Banakar PS, Kulangara A, Prasad CK, Soren NM, Dominic G, Terhuja M, et al. In vitro Evaluation of Newer Unconventional Feedstuffs for Livestock. Indian J Vet Sci Biotechnol. (2021) 17:78–83. doi: 10.21887/ijvsbt.17.3.17
40. Tiwari MR, Karki M, Pandey LN, Poudel N. Replacement of concentrate mixture with different levels of water hyacinth (Eichhornia crassipes) in basal diet on feed intake and production performance of piglets. J Agric Nat Res. (2020) 3:205–17. doi: 10.3126/janr.v3i2.32507
41. Mako AA, Ikusika OO. Utilization of Pleurotus sajor-caju biodegraded water hyacinth (Eichhornia crassipes) in a solid state fermentation by West African Dwarf goats in the humid tropics. Tropical Agric. (2020) 97:3.
42. Selim ASM, Hasan MN, Rahman MA, Rahman MM, Islam MR, Bostami AR, et al. Nutrient content and in vitro degradation study of some unconventional feed resources of Bangladesh. Heliyon. (2022) 8:5. doi: 10.1016/j.heliyon.2022.e09496
43. Carneiro MT, Barros AZ, Morais AI, Carvalho Melo AL, Bezerra RD, Osajima JA, et al. Application of water hyacinth biomass (Eichhornia crassipes) as an adsorbent for methylene blue dye from aqueous medium: kinetic and isothermal study. Polymers. (2022) 14:2732. doi: 10.3390/polym14132732
44. Shafy HI, Farid MR, El-Din AM. Water-hyacinth from Nile River: chemical contents, nutrient elements and heavy metals. Egypt J Chem. (2016) 59:131. doi: 10.21608/ejchem.2016.934
45. De Vasconcelos D, Véras GA, de Lima Silva RML, Cardoso J, de Castro Soares DB, de Morais P, et al. Effect of water hyacinth (Eichhornia crassipes) hay inclusion in the diets of sheep. Trop Anim Health Prod. (2016) 48:539–44. doi: 10.1007/s11250-015-0988-z
46. Makkar HP, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, et al. Seaweeds for livestock diets: a review. Anim Feed Sci Technol. (2016) 212:1–17. doi: 10.1016/j.anifeedsci.2015.09.018
47. Lonsdale C. Raw Materials for Animal Feed Compounders and Farmers. Marlow: Chalcombe Publications P (1989), 88.
48. Lemus RW. Hay Storage: Dry Matter Losses and Quality Changes. Oxford, MS: Mississippi State University Extension Service (2009).
49. Kellems RO, Church DC. Livestock Feeds and Feeding, 4th Edn. Hoboke„ NJ: Prentice-Hall, Inc (1998), 573.
50. Ewuola EO, Sokunbi OA, Sanni KM, Oyedemi OM, Lawal TT. Haematological and serum biochemical responses of rabbit does to crude Moringa Oleifera leaf extract at gestation and lactation. Trop Anim Health Prod. (2015) 47:637–42. doi: 10.1007/s11250-015-0759-x
51. Al-Eissa M, Alkahtani S, Al-Farraj S, Alarifi SA, Al-Dahmash B, Al-Yahya H. Seasonal variation effects on the composition of blood in Nubian ibex (Capra nubiana) in Saudi Arabia. Afr J Biotechnol. (2012) 11:1283–6. doi: 10.5897/AJB11.2004
52. Yusuf AO, Owolabi AJ, Adebayo KO, Aina ABJ, Sowande OS, Ajayi TO, et al. Growth performance of goats fed diets containing varying levels of water hyacinth. Agric Tropica et Subtropica. (2021) 54:228–37. doi: 10.2478/ats-2021-0024
53. Etim NN, Williams ME, Akpabio U, Offiong EE. Haematological parameters and factors affecting their values. Agric Sci. (2014) 2:37–47. doi: 10.12735/as.v2i1p37
54. Gbolabo O, Onasanya GO, Oke FO, Sanni TM, Muhammad AI. Parameters influencing haematological, serum and bio-chemical references in livestock animals under different management systems. Open J Vet Med. (2015) 5:181–9. doi: 10.4236/ojvm.2015.58025
55. Kiran S, Bhutta A, Khan B, Durrani S, Ali M, Iqbal F. Effect of age and gender on some blood biochemical parameters of apparently healthy small ruminants from Southern Punjab in Pakistan. Asian Pacific J Trop Biomed. (2012) 24:304–6. doi: 10.1016/S2221-1691(12)60028-8
56. Awawdeh MS, Dager HK, Obeidat BS. Dietary inclusion of alternative feedstuffs had no negative effects on hematological and biochemical parameters of growing Awassi lambs. Trop Anim Health Prod. (2020) 52:2157–62. doi: 10.1007/s11250-020-02236-3
57. Faryabi R, Mousaie A, Bahrampour J, Barazandeh A. The effect of dietary inclusion of Artemisia sieberi leaves on growth performance, feeding behaviors, ruminal fermentation, feed digestibility, and blood hemato-biochemical profile of growing male lambs. Trop Anim Health Prod. (2023) 55:1–11. doi: 10.1007/s11250-023-03455-0
58. Soul W, Mupangwa J, Muchenje V, Mpendulo TC. Biochemical indices and heamtological parameters of goats fed lablab purpureus and vigna unguiculata as supplements to a chloris gayana basal diet. Vet Anim Sci. (2019) 8:100073. doi: 10.1016/j.vas.2019.100073
59. Zaher HA, Mesalam A, Al Bloushi AI, Tolba A, Swelum AA, Abu-Alrub I. Hematological and biochemical indices, growth performance, and puberty of goats fed with Mombasa and blue panic as salt-tolerant alternatives to alfalfa under arid conditions. Front Vet Sci. (2022) 9:961583. doi: 10.3389/fvets.2022.961583
60. Oloche J, Atooshi MZ, Tyokase MU. Growth performance and blood profile of West African Dwarf (WAD) goats fed varying levels of treated sweet orange peels. Trop Anim Health Prod. (2019) 51:131–6. doi: 10.1007/s11250-018-1667-7
61. Kurtoglu F, Kurtoglu V, Celik I, Keçeci T, Nizamlioglu M. Effects of dietary boron supplementation on some biochemical parameters, peripheral blood lymphocytes, splenic plasma cells and bone characteristics of broiler chicks given diets with adequate or inadequate cholecalciferol (vitamin D3) content. Br Poult Sci. (2005) 46:87–96. doi: 10.1080/00071660400024001
62. Osita CO, Ani AO, Ezema C, Oyeagu CE, Uzochukwu IE, Ezemagu IE. Hematological and biochemical indices of West African Dwarf sheep fed diets containing yeast (Saccharomyces cerevisiae), grass, grass/legume (50: 50) and legume. Pakistan J Nutr. (2019) 18:34–41. doi: 10.3923/pjn.2019.34.41
63. Jackson PG, Cockcroft PD. Appendix 2: Laboratory Reference Values: Haematology. Clin Exam Farm Anim. (2002) 302:14–21. doi: 10.1002/9780470752425.app2
64. Njidda AA, Shuai'Bu AA, Isidahomen CE. Haematological and serum biochemical indices of sheep in semi-arid environment of northern Nigeria. Global J Sci Front Res. (2014) 14: 49–56.
65. Bradbury MG, Egan SV, Bradbury JH. Determination of all forms of cyanogens in cassava roots and cassava products using picrate paper kits. JS Clin Cases Small Rum Zaria Nigeria Bullet Anim Health Prod Africa. (1999) 30:111–6.
66. Lee G, Choi S, Kim K, Yun JM, Son JS, Jeong SM. Association of Hemoglobin Concentration and Its Change With Cardiovascular and All-Cause Mortality. J Am Heart Assoc. (2018) 7:e007723. doi: 10.1161/JAHA.117.007723
67. Dikko AH, Tsado DN, Adama TZ, Ibrahim MJ. Effect of Brewers' Dried Grain on Growth Performance, Hematology and Serum Biochemistry indices of Yankasa Sheep (2019). Available online at: http://repository.futminna.edu.ng:8080/jspui/handle/123456789/9076
68. Pudjihastuti E, Bujung JR, Kaunang CL. Haematological profile of goats fed with urea palms sugar block supplementation. AIP Conf Proc. (2023) 2694:1–15. doi: 10.1063/5.0119386
69. Cheesbrough M. District Laboratory Practice in tropical Countries. Cambridge: University Press Cambridge (2004), 266–342.
70. Tambuwal FM, Agale BM, Bangana A. Haematological and Biochemical values of Apparently Healthy Red Sokoto Goats. In: Proceeding of 27th Annual Conference Nigerian Society of Animal Production (NSAP), FUTA Akure, Nigeria (2002)
71. Olafadehan OA. Changes in haematological and biochemical diagnostic parameters of Red Sokoto goats fed tannin-rich Pterocarpus erinaceus forage diets. Vet Archives. (2011) 81:471–83. doi: 10.1007/s11250-016-1045-2
72. Fadiyimu AA, Alokan JA, Fajemisin AN. Digestibility, nitrogen balance and haematological profile of West African dwarf sheep fed dietary levels of Moringa oleifera as supplement to Panicum maximum. J Am Sci. (2010) 6:634–43.
73. Agbaye FP, Sokunbi AO, Onigemo MA, Alaba O, Anjola OAJ, Amao EA, et al. Blood profile of prevalent sheep breeds in Nigeria: a case study of Ikorodu local government area of Lagos State, Nigeria. Nigerian J Anim Prod. (2021) 48:293–9. doi: 10.51791/njap.v48i5.3173
74. Amuda AJ, Okunlola DO. Haematological parameters and serum biochemistry of West African dwarf sheep fed ensiled maize stover and concentrate supplements. J Agric Vet Sci. (2018) 11:57–63.
75. NCCLS. Procedure for Determining Packed Cell Volume by the Microhematocrit Method; Approved Standard—Third Edition. Wayne, PA: NCCLS (2000).
76. Mohammed SA, Razzaque MA, Omar AE, Albert S, Al-Gallaf WM. Biochemical and hematological profile of different breeds of goat maintained under intensive production system. Afr J Biotechnol. (2016) 15:1253–7. doi: 10.5897/AJB2016.15362
77. Sarma PR. Red Cell Indices. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd Edn. London: Butterworth Publishers (1990).
78. Polizopoulou ZS. Haematological tests in sheep health management. Small Rum Res. (2010) 92:88–91. doi: 10.1016/j.smallrumres.2010.04.015
79. Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, Hernández-García PA, et al. Growth performance, carcass characteristics, and blood metabolites of lambs supplemented with a Polyherbal Mixture. Animals. (2021) 11:955. doi: 10.3390/ani11040955
80. Egbe-Nwiyi TN, Nwaosu SC, Salami HA. Haematological values of appararently healthy sheep and goats as influenced by age and sex in arid zone of Nigeria. Afr J Biomed Res. (2000) 3:109–15.
81. Torres-Chable OM, García-Herrera RA, González-Garduño R, Ojeda-Robertos NF, Peralta-Torres JA, Chay-Canul AJ, et al. Relationships among body condition score, FAMACHA© score and haematological parameters in Pelibuey ewes. Trop Anim Health Prod. (2020) 52:3403–8. doi: 10.1007/s11250-020-02373-9
82. Bórnez R, Linares MB, Vergara H. Haematological, hormonal and biochemical blood parameters in lamb: Effect of age and blood sampling time. Livest Sci. (2009) 121:200–6. doi: 10.1016/j.livsci.2008.06.009
83. Ohran H. The influence of geographic area on blood parameters of Pramenka Sheep in the area of Bosnia and Herzegovina. Turkish J Vet Res. (2019) 3:1–8.
84. Sarmin SS, Astuti P, Airin CM. The hematological and biochemical profiles of wonosobo sheep blood in various physiological conditions. Buletin Peternakan. (2022) 46:169–78. doi: 10.21059/buletinpeternak.v46i3.73423
85. Reiten MR, Bakkebø MK, Brun-Hansen H, Lewandowska-Sabat AM, Olsaker I, Tranulis MA, et al. Hematological shift in goat kids naturally devoid of prion protein. Front Cell Dev Biol. (2015) 3:44. doi: 10.3389/fcell.2015.00044
86. Antunović Z, Novoselec J, Klir Ž. Hematological parameters in ewes during lactation in organic farming. Poljoprivreda. (2017) 23:46–52. doi: 10.18047/poljo.23.2.7
87. Karki U, Tiwari A, Norwood K, Johnson JN, Karki LB. 365 the extent and pattern of correlation among blood parameters differed between pasture-raised goats and sheep. J Anim Sci. (2021) 99(Supplement_3):202–3. doi: 10.1093/jas/skab235.367
88. Fadare AO, Peters SO, Yakubu A, Sonibare AO, Adeleke MA, Ozoje MO, et al. Physiological and haematological indices suggest superior heat tolerance of white-coloured West African Dwarf sheep in the hot humid tropics. Trop Anim Health Prod. (2012) 45:157–65. doi: 10.1007/s11250-012-0187-0
89. Javed MT, Ahmad L, Irfan M, Ali I, Khan A, Wasiq M, et al. Haematological and serum protein values in tuberculin reactor and non-reactor water buffaloes, cattle, sheep and goats. Pak Vet J. (2010) 30:100–4.
90. Ahmad S, Lashari MH, Farooq U, A. preliminary study on devising a hematological formula for estimation of hemoglobin from packed cell volume in beetal goats. Arquivo Brasileiro de Med Vet e Zootecnia. (2022) 74:77–82. doi: 10.1590/1678-4162-12568
91. Perumal P, De AK, Bhattacharya D, Sunder J, Bhowmick S, Kundu A, et al. Walking and dry season stresses modulates the physiological, heamatological and biochemical profiles of indigenous local goats in Andaman and Nicobar Islands. Int J Bio-Res Stress Manage. (2019) 10:597–605. doi: 10.23910/IJBSM/2019.10.6.2041a
92. El-Tarabany MS, El-Tarabany AA, Roushdy EM. Impact of lactation stage on milk composition and blood biochemical and hematological parameters of dairy Baladi goats. Saudi J Biol Sci. (2018) 25:1632–8. doi: 10.1016/j.sjbs.2016.08.003
Keywords: hematological indices, goat, nutrition, sheep, water hyacinth
Citation: Fanta Y, Kechero Y and Yemane N (2024) Hematological parameters of sheep and goats fed diets containing various amounts of water hyacinth (Eichhornia crassipes). Front. Vet. Sci. 11:1286563. doi: 10.3389/fvets.2024.1286563
Received: 31 August 2023; Accepted: 05 March 2024;
Published: 28 March 2024.
Edited by:
Gerhard Breves, University of Veterinary Medicine Hannover, GermanyReviewed by:
Damiano Cavallini, University of Bologna, ItalyCopyright © 2024 Fanta, Kechero and Yemane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yared Fanta, eWZhbnRhMjAxN0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.