- 1Department of Pathobiology, Faculty of Veterinary Medicine, Bu-Ali Sina University, Hamedan, Iran
- 2Department of Pathobiology, Faculty of Veterinary Medicine, Ferdowsi University, Mashhad, Iran
- 3Department of Clinical Sciences, Faculty of Veterinary Medicine, Bu-Ali Sina University, Hamedan, Iran
- 4Provincial Department of Environment, Hamedan, Iran
- 5Department of Parasitology, Veterinary Faculty, Selçuk University, Konya, Türkiye
Between September 2019 and December 2023, a total of 612 wild birds representing 16 orders, 33 families, 60 genera, and 78 species from nine provinces of Iran with different climates namely Hamedan (n = 54), Sistan-va-Baluchestan (n = 372), Kerman (n = 73), South Khorasan (n = 52), Mazandaran (n = 7), Chaharmahal-va-Bakhtiari (n = 2), Gilan (n = 2), Golestan (n = 18), North Khorasan (n = 9), and Razavi Khorasan (n = 23) were examined for chewing lice infestation. Naked eye examination revealed that 58 birds (9.5%) were infested with at least one chewing louse species. Collected lice specimens belonged to 28 species from the families Philopteridae, Menoponidae and Laemobothriidae including Strigiphilus strigis (n = 55, 15.6%), Falcolipeurus quadripustulatus (n = 41, 11.6%), Craspedorrhynchus platystomus (n = 40, 11.3%), Colpocephalum turbinatum (n = 36, 10.2%), Laemobothrion maximum (n = 25, 7.1%), Nosopon lucidum (n = 20, 5.6%), Degeeriella fulva (n = 18, 5.1%), Colpocephalum eucarenum (n = 16, 4.5%), Laemobothrion vulturis (n = 15, 4.2%), Anaticola crassicornis (n = 13, 3.7%), Craspedorrhynchus aquilinus (n = 9, 2.5%), Degeeriella fusca (n = 7, 2.0%), Aegypoecus trigonoceps (n = 7, 2.0%), Quadraceps obscurus (n = 6, 1.7%), Colpocephalum impressum (n = 6, 1.7%), Trinoton querquedulae (n = 6, 1.7%), Colpocephalum heterosoma (n = 5, 1.4%), Colpocephalum nanum (n = 5, 1.4%), Lunaceps holophaeus (n = 4, 1.1%), Quadraceps spp. (n = 4, 1.1%), Actornithophilus uniseriatus (n = 2, 0.6%), Nosopon chanabense (n = 2, 0.6%), Actornithophilus cornutus (n = 1, 0.3%), Cuclotogaster heterographus (n = 1, 0.3%), Falcolipeurus suturalis (n = 1, 0.3%), Laemobothrion atrum (n = 1, 0.3%), Colpocephalum gypsi (n = 1, 0.3%), and Rallicola cuspidatus (n = 1, 0.3%). All of these species except six, i.e., Trinoton spp., C. aquilinus, L. vulturis, L. maximum, C. impressum, C. turbinatum, and C. heterographus are recorded for the first time from Iran. This study is the largest epidemiological study to date performed in the country. Data reported herein contribute to our knowledge about diversity of avian chewing lice from wild birds in Iran. In this paper, an updated checklist of louse species reported from Iran according to their avian hosts is presented.
Introduction
Lice are small (0.35–11 mm long as adults), wingless, dorsoventrally flattened insects. They are obligatory, permanent ectoparasites of birds and mammals throughout the world which typically, parasitize individuals in small numbers and cause no apparent discomfort however, some of the lice can cause skin lesions and act as vectors or intermediate hosts of several bacteria, viruses and filarial parasites (1, 2). In addition, it has been shown that Piagetiella titan infesting white pelicans may invade the oral cavity causing erosions and petechial hemorrhages (3–5).
Lice (Insecta: Psocoptera: Phthiraptera) with about 5,000 known species, present on roughly 4,000 species of birds and 800 mammals, are categorized in four suborders (6). Species of the suborder Anoplura have adopted to suck blood from capillaries of mammals and ingest it, while Amblycera, Ischnocera, and Rhynchophthirina (formerly known as Mallophaga) have chewing mouth pieces, adapted to eat hairs and feathers, and sometimes also the skin and blood of birds and mammals (7). Avian chewing lice belong to one of two sub-orders: Amblycera, which occur on feathers and skin, or Ischnocera, which are more restricted to feathers (1). Most of the lice species are strongly associated with hosts, their phylogeny parallels that of hosts, sometimes with different speeds however, “host specificity” cannot be assumed (7, 8). Among different fields of wildlife parasitology, studying avian chewing lice is important as their epizootiology is largely associated with geographical distribution of their hosts.
Iran is a country in western Asia with a territory of 1,648,195 km2. It is the second largest country in the Middle East and the 17th largest in the world. In the country, 550 avian species are distributed which is almost equal to the richness of birds in Europe (9, 10). However, there is limited and scanty information about their parasites fauna specially the chewing lice (11–14) with several published in Persian language and presented in local congresses (15–19). Considering the scarcity of published records of lice in Iran, we aimed to gather new data and present an updated checklist of birds’ Phthiraptera occurring in the country.
Materials and methods
Between September 2019 and December 2023, totally 612 wild birds belonging 16 orders, 33 families, 60 genera, and 78 species from Hamedan (n = 54), nine different regions of Sistan-va-Baluchestan (n = 372), Kerman (n = 73), South Khorasan (n = 52), Mazandaran (n = 7), Chaharmahal-va-Bakhtiari (n = 2), Gilan (n = 2), Golestan (n = 18), North Khorasan (n = 9), and Razavi Khorasan (n = 23) were collected (Figure 1). The birds were euthanized by the Provincial Department of Environment because of general health failure or were found dead in the environment. The time lapse from death to examination of birds for lice infestation could not be estimated however, only fresh carcasses were examined. Individual birds were sent to Laboratory of Parasitology, Faculty of Veterinary Medicine, Bu-Ali Sina University in sealed plastic bags for examination or were examined in the field. The bird identifications were made using the reference book Atlas of Birds of Iran (9), and a standard examination for searching chewing lice was performed (20). The collected lice were placed in tubes containing 70% ethanol, cleared in 10% KOH for at least 1 day, mounted in Canada balsam on glass slides (21), and identified according to the original descriptions or keys (7, 22–35) using a Leica DM750 camera mounted trinocular microscope with Leica DFC295 application unit.
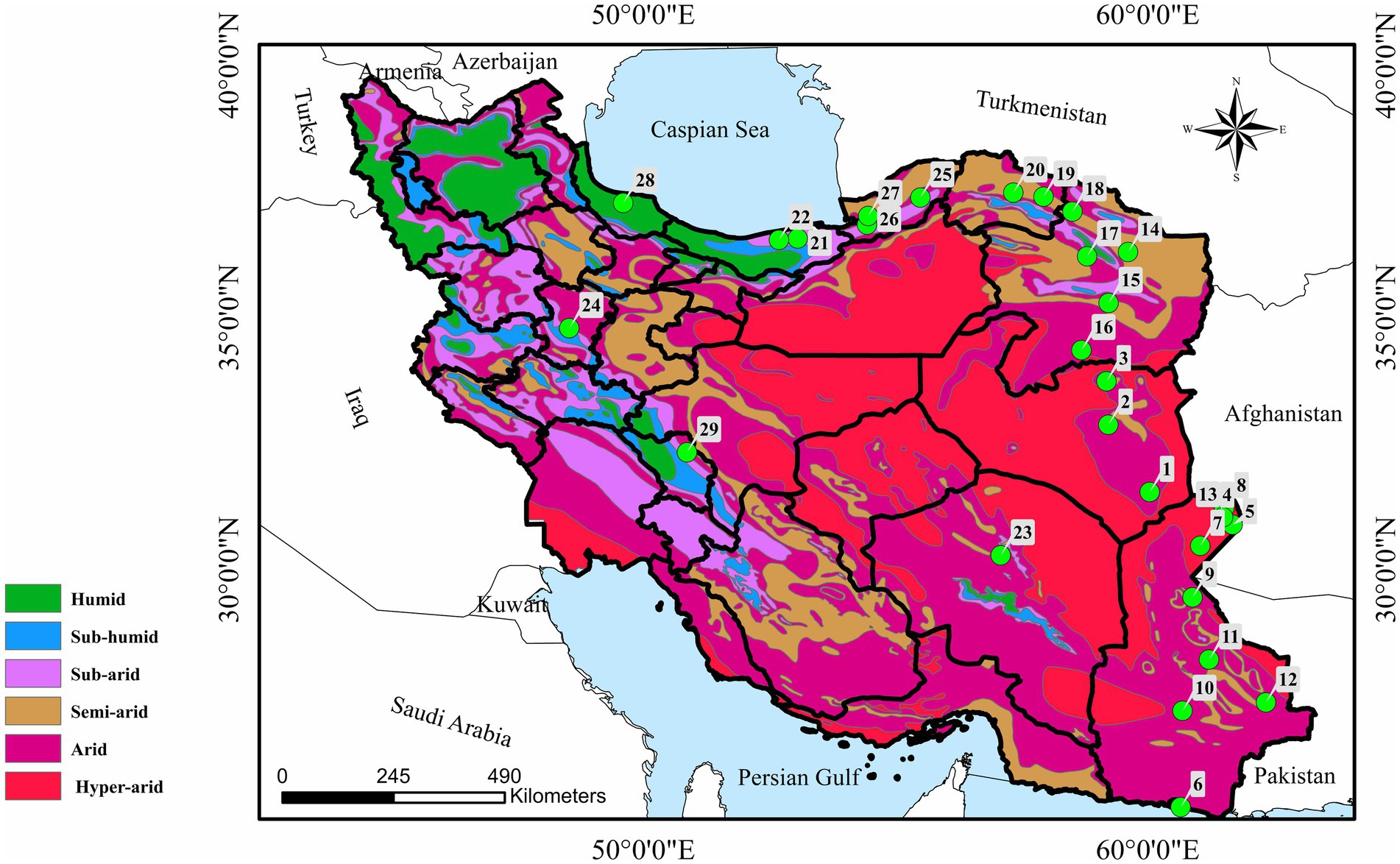
Figure 1. Map of Iran showing 29 sampling localities in nine provinces with number of birds examined in each city. (1) Nehbandan (n = 9), (2) Birjand (n = 10), (3) Qaen (n = 6), (4) Zabol (n = 93), (5) Zehak (n = 136), (6) Chabahar (n = 15), (7) Hamun (n = 59), (8) Hirmand (n = 29), (9) Zahedan (n = 26), (10) Iranshahr (n = 4), (11) Khash (n = 5), (12) Saravan (n = 5), (13) Nimruz (n = 27), (14) Mashhad (n = 12), (15) Torbat-e Heydariyeh (n = 4), (16) Gonabad (n = 2), (17) Neyshabur (n = 2), (18) Quchan (n = 3), (19) Shirvan (n = 5), (20) Bojnoord (n = 4), (21) Sari (n = 4), (22) Babol (n = 3), (23) Kerman (n = 73), (24) Hamedan (n = 54), (25) Kalaleh (n = 7), (26) Gorgan (n = 7), (27) Aqqala (n = 4), (28) Rasht (n = 2), and (29) Shahrekord (n = 2).
We also collected all the available information about chewing lice infesting birds in Iran. The databases and search engines employed for the literature review were Phthiraptera.info,1 PubMed,2 Google,3 Scientific Information Database of Iran,4 the collection of defended theses at all Iranian Universities,5 and the collection of proceedings of Iranian congresses.6 Valid names of the louse and bird species were obtained from Global Biodiversity Information Facility resources (36).
Results
In total, 352 lice specimens including Strigiphilus strigis n = 55; 15.6% (Pontoppidan, 1763), Falcolipeurus quadripustulatus n = 41; 11.6% (Burmeister, 1838), Craspedorrhynchus platystomus n = 40; 11.3% (Burmeister, 1838), Colpocephalum turbinatum n = 36; 10.2% (Denny, 1842), Laemobothrion maximum n = 25; 7.1% (Scopoli, 1763), Nosopon lucidum n = 20; 5.6% (Rudow, 1869), Degeeriella fulva n = 18; 5.1% (Giebel, 1874), Colpocephalum eucarenum n = 16; 4.5% (Burmeister, 1838), Laemobothrion vulturis n = 15; 4.2% (Fabricius, 1775), Anaticola crassicornis n = 13; 3.7% (Scopoli, 1763), Craspedorrhynchus aquilinus n = 9; 2.5% (Denny, 1842), Degeeriella fusca n = 7; 2.0% (Denny, 1842), Aegypoecus trigonoceps n = 7; 2.0% (Giebel, 1874), Quadraceps obscurus n = 6; 1.7% (Burmeister, 1838), Colpocephalum impressum n = 6; 1.7% (Rudow, 1866), Trinoton querquedulae n = 6; 1.7% (Linnaeus, 1758), Colpocephalum heterosoma n = 5; 1.4% (Clay, 1951), Colpocephalum nanum n = 5; 1.4% (Piaget, 1890), Lunaceps holophaeus n = 4; 1.1% Burmeister, 1838, Quadraceps spp. (new species) n = 4; 1.1% (Clay and Meinertzhagen, 1939), Actornithophilus uniseriatus n = 2; 0.6% (Piaget, 1880), Nosopon chanabense n = 2; 0.6% (Ansari, 1951), Actornithophilus cornutus n = 1; 0.3% (Giebel, 1866), Cuclotogaster heterographus n = 1; 0.28% (Nitzsch, 1866), Falcolipeurus suturalis n = 1; 0.3% (Rudow, 1869), Laemobothrion atrum n = 1; 0.3% (Nitzsch, 1818), Colpocephalum gypsi n = 1; 0.3% (Eichler & Zlotorzycka, 1971), and Rallicola cuspidatus n = 1; 0.3% (Scopoli, 1763) were collected from 58/612 birds (9.5%). Collected lice specimens belonged to 31 species from the families Philopteridae, Menoponidae, and Laemobothriidae. All of the identified lice species except C. aquilinus, L. vulturis, L. maximum, C. impressum, C. turbinatum, and C. heterographus are recorded for the first time from Iran (Table 1).
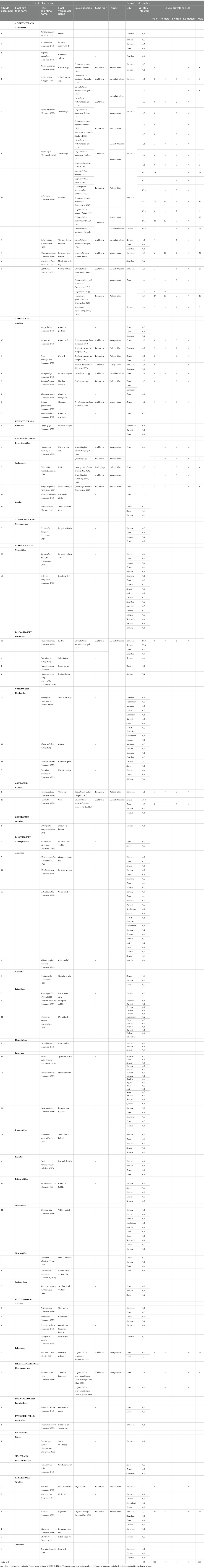
Table 1. Distribution of louse species of wild birds in some regions of Iran (September 2019 and December 2023) according to their host bird species.
Number of lice specimens collected from examined birds ranged from 1 to 55, the latter was a Bubo bubo Linnaeus, 1758. Mixed lice infestation was found in 11 birds, i.e., in one Philomachus pugnax (Linnaeus, 1758), two Himantopus himantopus Linnaeus, 1758, two Anas crecca Linnaeus, 1758, one Aquila nipalensis Hodgson, 1833, one Aquila rapax Temminck, 1828, two Gyps fulvus Hablitz, 1783, and two Buteo buteo Linnaeus, 1758. Photomicrographs of examined lice specimens are presented in Figures 2–14.
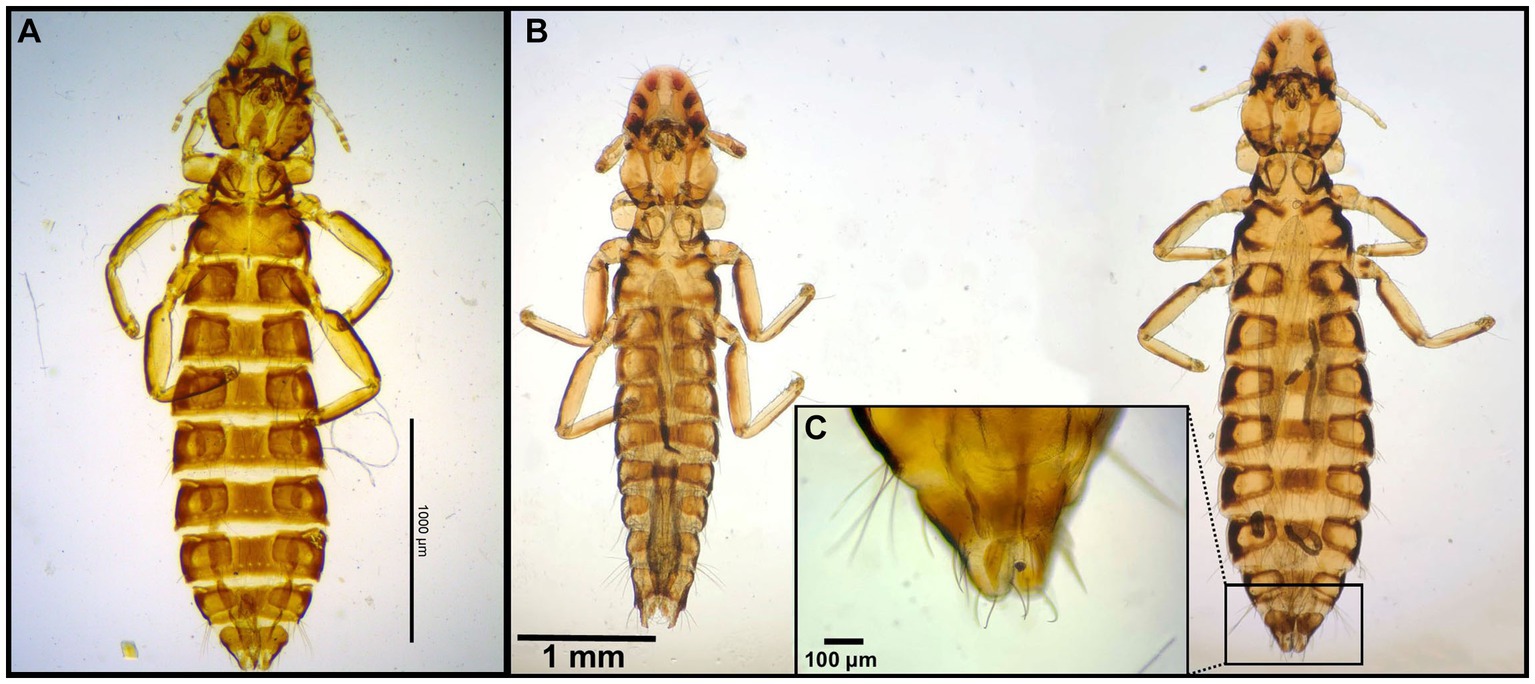
Figure 2. Chewing lice identified in this study part I: (A) Falcolipeurus suturalis ♀; (B) Falcolipeurus quadripustulatus left ♂, right ♀; and (C) ♀ posterior end. The map was drawn by using ArcGIS software version 10.3 (https://enterprise.arcgis.com/en/portal/).
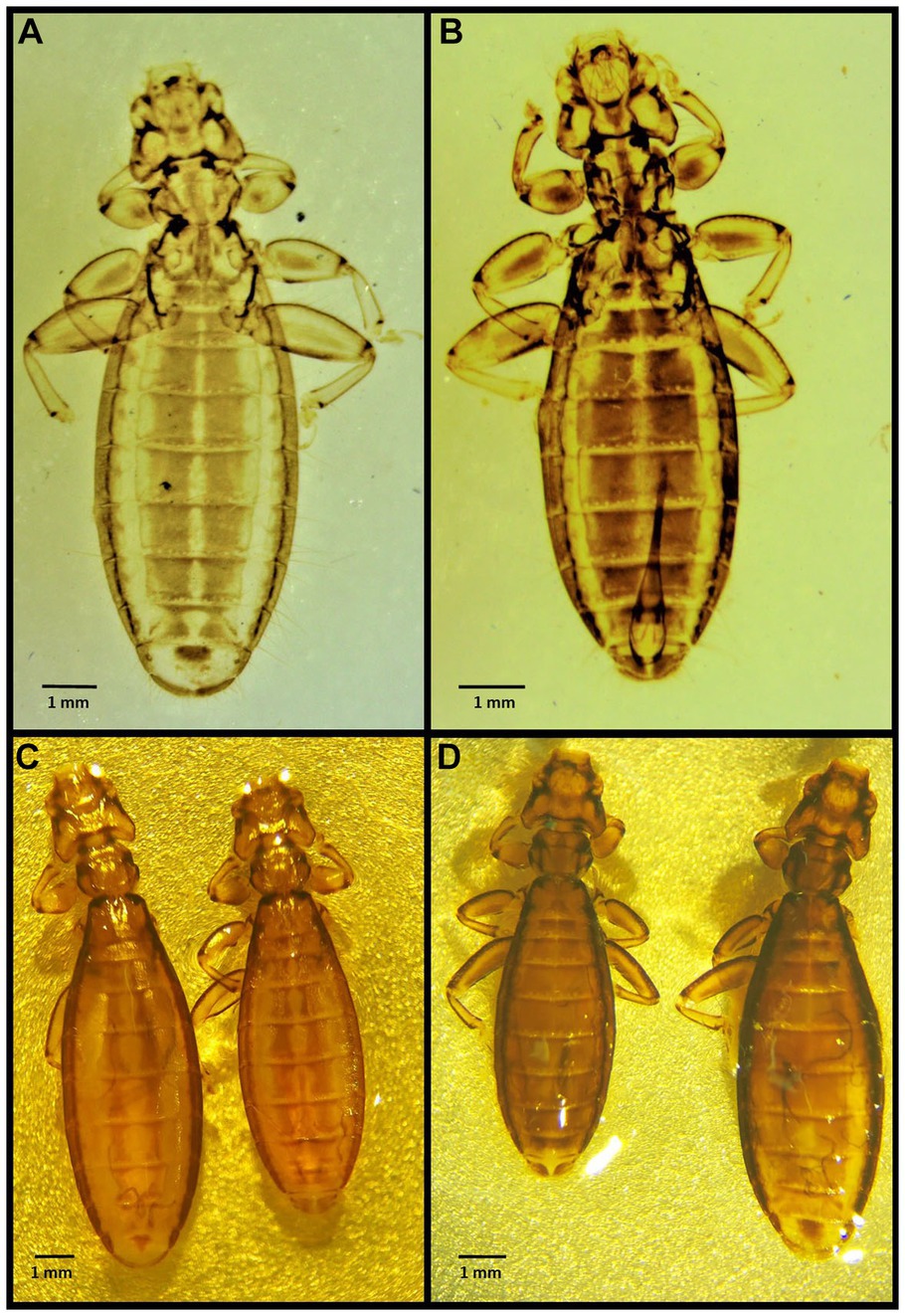
Figure 3. Chewing lice identified in this study part II: (A) Laemobothrion maximum ♀; (B) Laemobothrion maximum ♂; (C) Stereomicroscope picture of Laemobothrion vulturis left ♀, right ♂; and (D) Stereomicroscope picture of Laemobothrion maximum left ♂, right ♀.
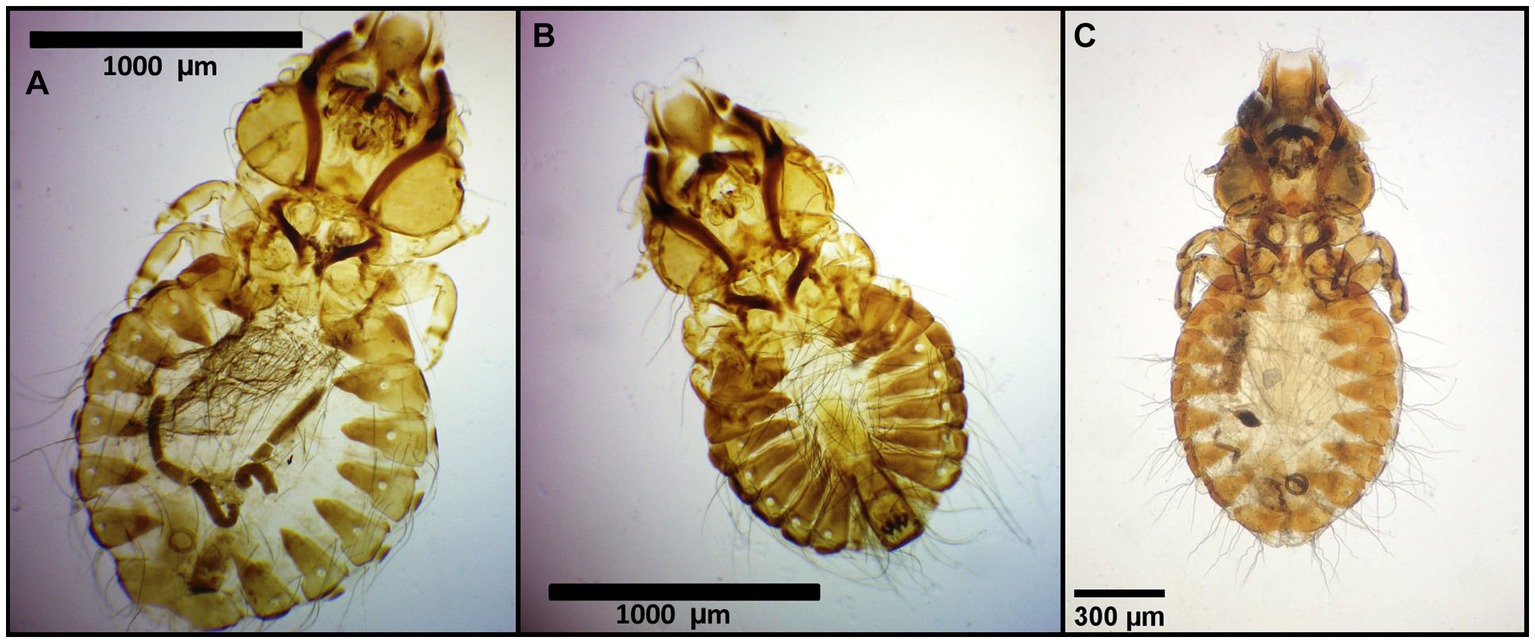
Figure 4. Chewing lice identified in this study part III: (A) Craspedorrhynchus aquilinus ♀, (B) Craspedorrhynchus aquilinus ♂; and (C) Craspedorrhynchus platystomus ♀.
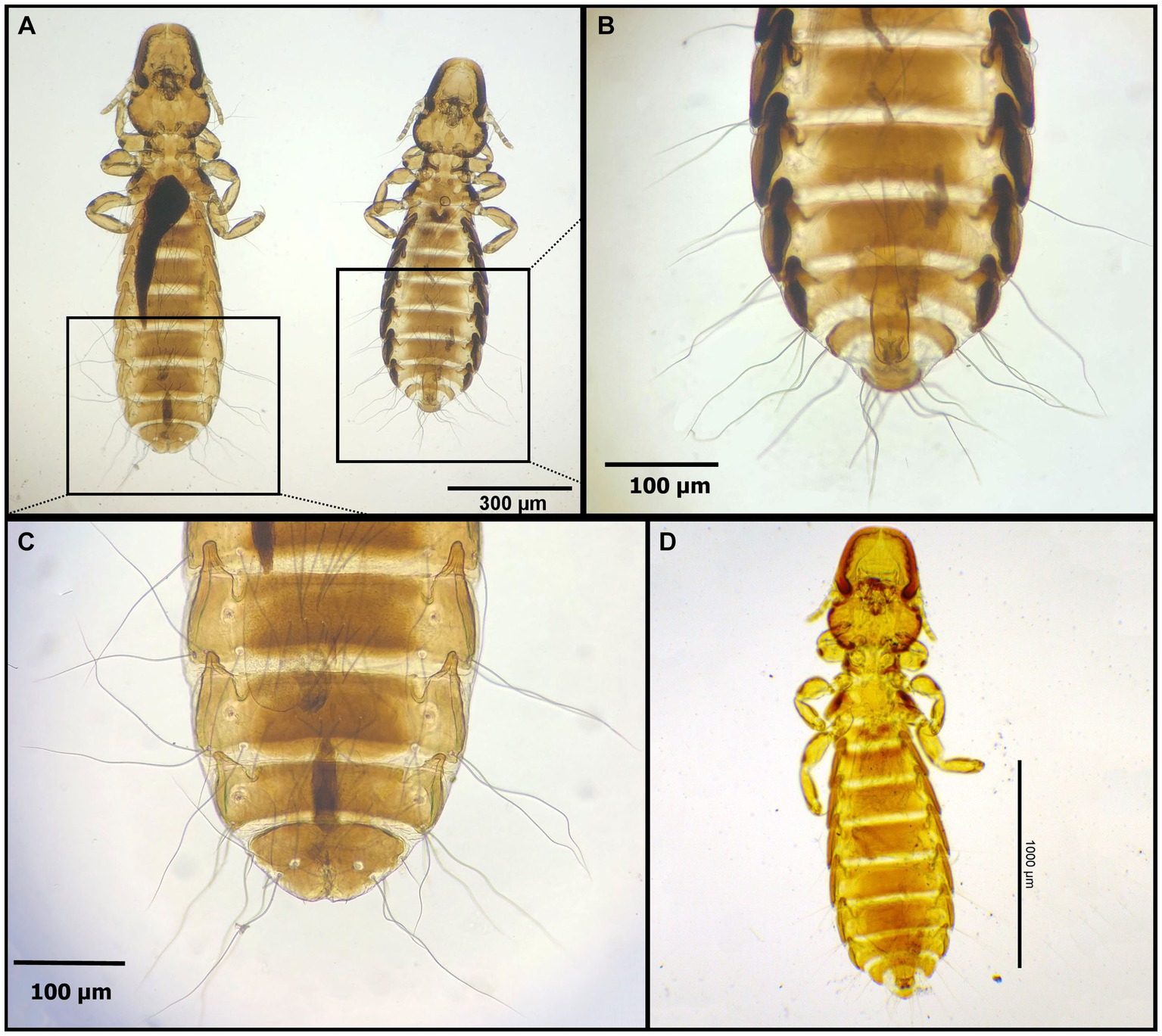
Figure 5. Chewing lice identified in this study part IV: (A–C) Degeeriella fusca, (A) left ♀, right ♂; (B) ♂, posterior part of the abdomen; (C) ♀, posterior part of the abdomen; and (D) Degeeriella fulva ♂.

Figure 6. Chewing lice identified in this study part V: (A–C) Nosopon chanabense ♀; (A) Stereomicroscope picture; (B) Light microscope picture; (C) Head; and (D) Nosopon lucidum ♂.
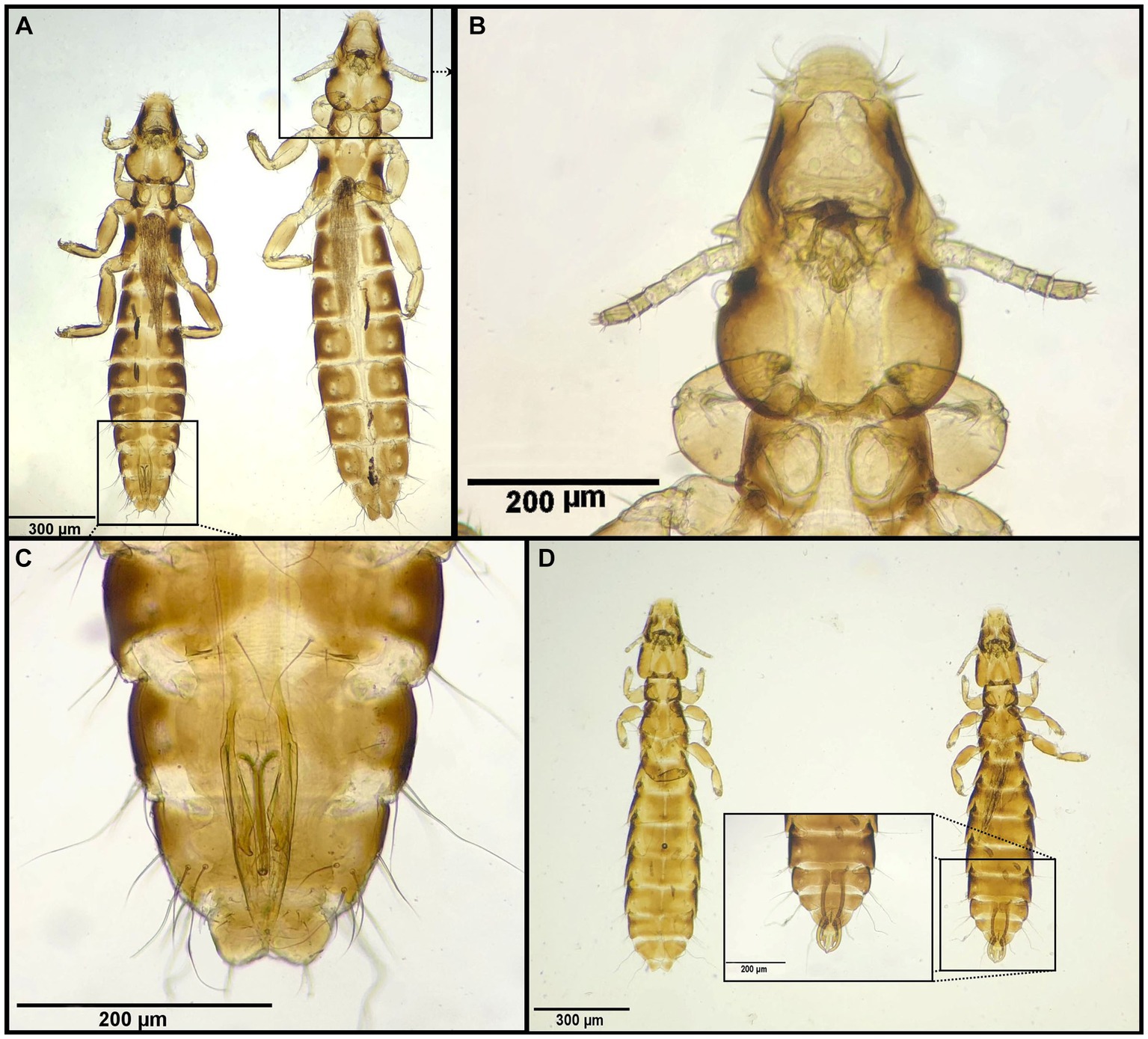
Figure 7. Chewing lice identified in this study part VI: (A–C) Anaticola crassicornis left ♂, right ♀; (B) ♀, Head; (C) ♂, posterior end; and (D) Quadraceps obscurus left ♀, right ♂.

Figure 8. Chewing lice identified in this study part VII: (A) Colpocephalum nanum ♀; (B) Colpocephalum gypsi ♂; (C) Colpocephalum eucarenum left ♂, right ♀; (D–G) Colpocephalum impressum; (D) ♀; (E) ♂; (F) ♂, posterior end; and (G) ♂, head.

Figure 9. Chewing lice identified in this study part VIII: (A–E) Colpocephalum heterosoma; (B) Head of large specimen ♂; (C) Head of small specimen, ♂; (D) Posterior end of small specimen ♂, and (E) Posterior end of small specimen ♀.
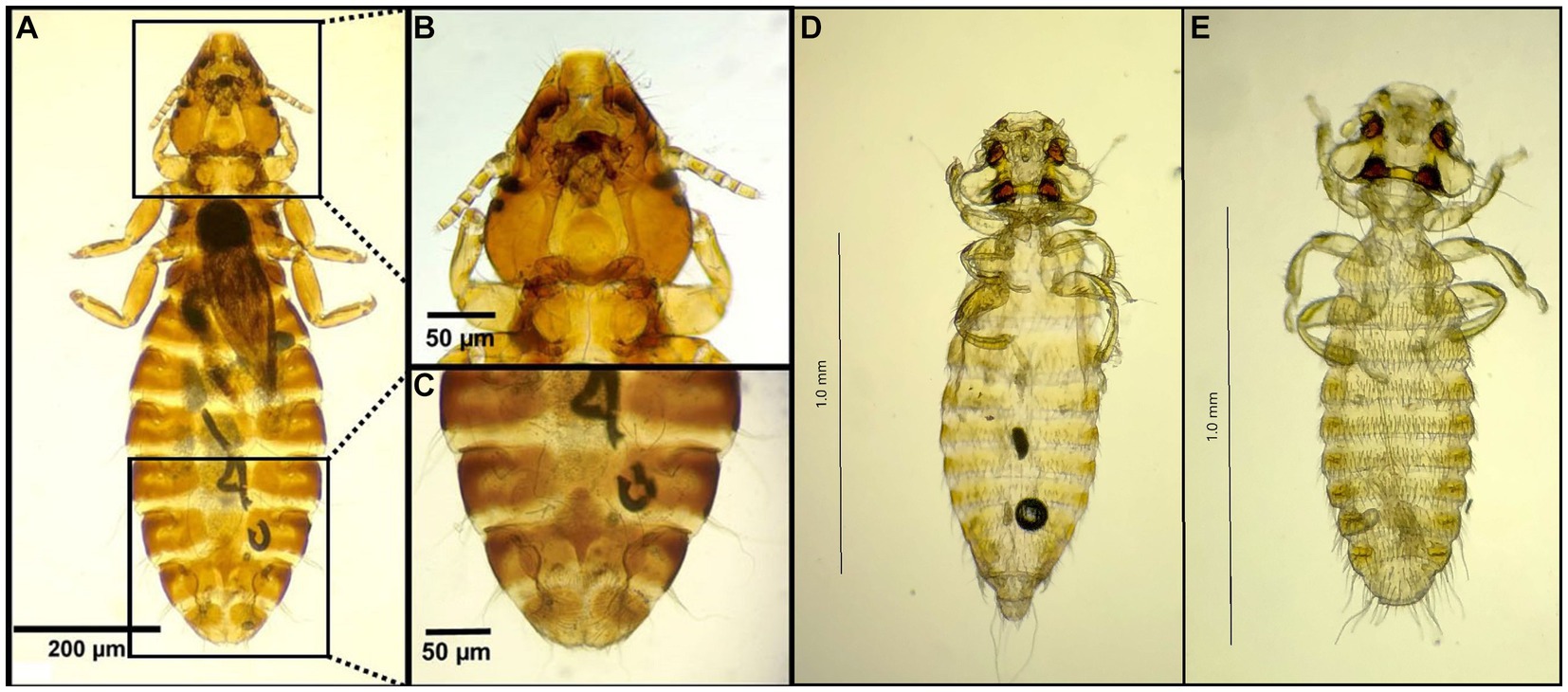
Figure 10. Chewing lice identified in this study part IX: (A–C) Pectinopygus spp. ♀; (B) Head; (C) Posterior end; (D) Colpocephalum turbinatum ♀; and (E) Colpocephalum turbinatum ♂.
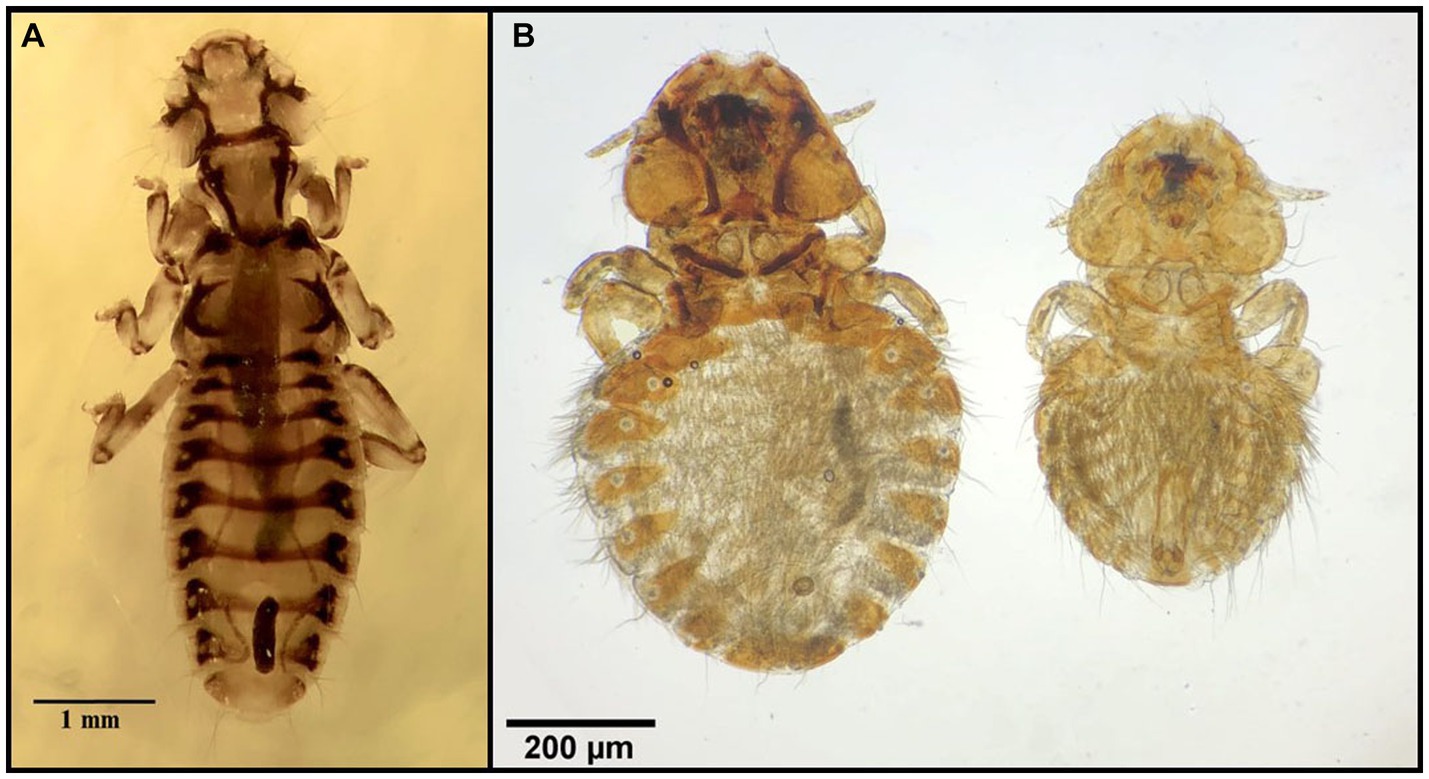
Figure 11. Chewing lice identified in this study part X: (A) Trinoton querquedulae ♀; (B) Aegypoecus trigonoceps left ♀, right ♂.
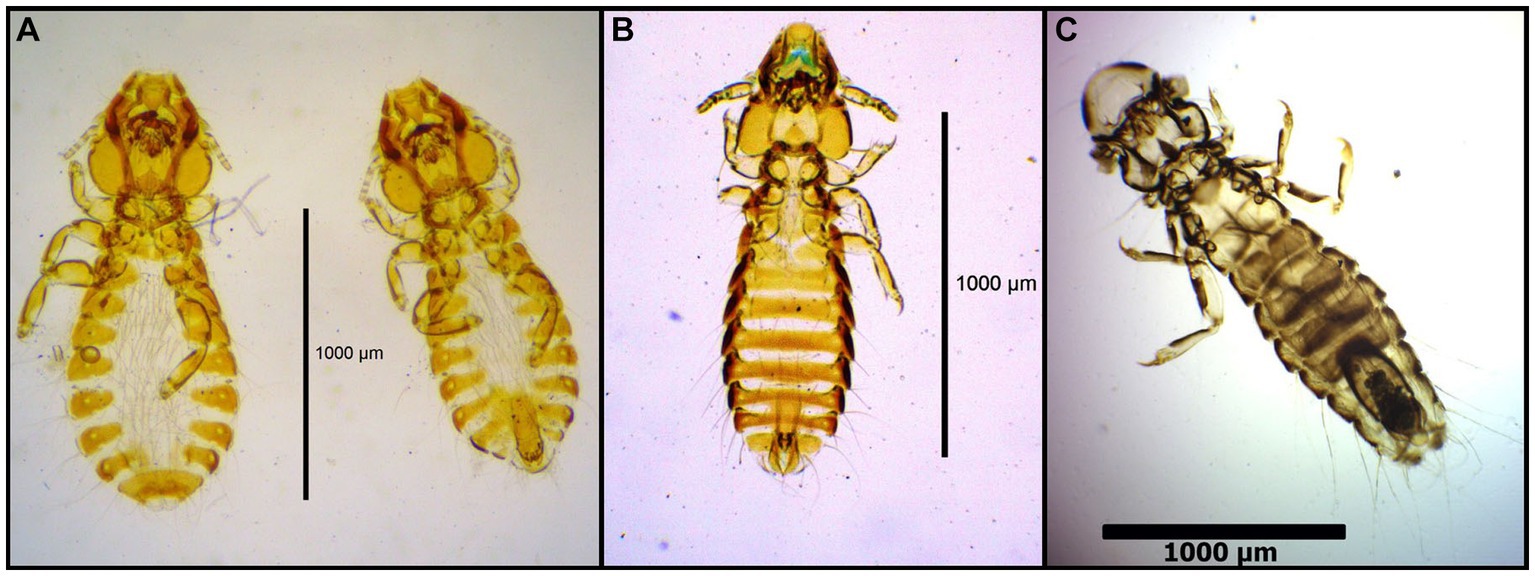
Figure 12. Chewing lice identified in this study part XI: (A) Strigiphilus strigis left ♀, right ♂; (B) Rallicola cuspidatus ♂; and (C) Cuclotogaster heterographus ♂.

Figure 13. Chewing lice identified in this study part XII: (A) Lunaceps holophaeus left ♂, right ♀; (B) Laemobothrion spp. nymph.

Figure 14. Chewing lice identified in this study part XIII: (A) Actornithophilus uniseriatus ♂; (B) Actornithophilus cornutus ♂; (C) Lamobothrion atrum ♀; and (D) Quadraceps spp. ♂.
Identification of few specimens could not be performed to species level including one damaged female Strigiphilus sp. collected from Asio otus Linnaeus, 1758, one female Pectinopygus spp. collected from Anas clypeata Linnaeus, 1758 which is an unusual host and possibly was a contamination, one Laemobothrion spp. nymph, and four Quadraceps spp. (Figures 10A–C, 13B, 14D). In addition, nits collected from one Falco tinnunculus Linnaeus, 1758 could not be identified.
In Supplementary Table 1, the information in Table 1 in addition to world conservation status according to International Union for Conservation of Nature (IUCN) and names of birds in Persian language are presented.
Discussion
This study is the largest epidemiological study to date performed in Iran. However, low number of collected lice from birds could be due to the fact that most of the ectoparasites including lice leave dead hosts rather quickly. Data reported herein contribute to our knowledge about diversity of avian chewing lice from wild birds in Iran and in a broader context in western Asia. Lice species in this study belonged to both Ischnocera (15 species), Amblycera (14 species). We compiled our data and previous information about avian lice species in Iran in Table 2. So far, lice infestation of birds belonging to 16 orders, 33 families, 60 genera, and 78 species and subspecies has been recorded from Iran. In Supplementary Table 2, the information in Table 2 in addition to world conservation status and names of birds in Persian language are presented.
Review of all relevant publications indicated that in some reports from Iran, researchers identified the lice specimens only to genus level, i.e., Brueelia (nine documents), Philopterus, Menacanthus (six documents), Ricinus, Lipeurus (three documents), Sturnidoecus, Trinoton, Menopon, and Goniodes (two documents), Ardeicola, Colpocephalum, Craspedorrhynchus, Laemobothrion, Strigiphilus, and Myrsidea (one document) (14, 16, 17, 19, 38, 40, 47, 50, 51, 62). The reason could be damage of the specimens, observation of a louse with morphological differences from identification keys or difficulty in identification of the species. It is necessary that researchers will try their best to identify the lice to species level correctly and provide the drawings, measurements, or photos.
Observation of one male poultry head louse specimen, Cuclotogaster heterographus (Nitzsch, 1866) which was collected from the buzzard Buteo buteo (Linnaeus, 1758) in this study was probably because the buzzard preyed with a galliform bird and the louse was mechanically transferred to the predator. In some reports from Iran, lice species that normally infest other bird orders were documented on abnormal bird species. For instance, Trinoton sp. that infest Anseriform birds were collected from raptors Buteo rufinus and Gyps fulvus (16, 17). It can be assumed that lice infestation occurred during feeding the raptors from their preys. In addition, in some reports, Menacanthus stramineus, Menopon gallinae, and Cuclotogaster heterographus that live on Galliformes were collected from mallards and geese (42, 43) as well as pigeons (47). These findings could be due to keeping mixed species together by nomads which is a normal practice in Iran although misidentification cannot be ruled out. Special caution should be taken for interpretation of such findings.
It is known that both amblyceran and ischnoceran lice can act as vectors or intermediate hosts of helminths, bacteria, and viruses, so it was suggested to delouse the wild birds with insecticides (1) however, we disagree with manipulating host–parasite interactions in the wildlife. Additionally, from the conservation point of view some authors expressed their concerns about co-extinction of the lice with their hosts, e.g., Rallicola extinctus (64) and their extinction during the conservation efforts to save the host, e.g., Rallicola pilgrimi (Clay, 1972) and Colpocephalum californici (31, 64, 65). According to International Union for Conservation of Nature (IUCN), there are concerns regarding decreasing population of several predators such as Aquila nipalensis Hodgson, 1833 steppe eagle (endangered) and Aquila heliaca Savigny, and 1809 Asian imperial eagle (vulnerable) (66). Hence, it is suggested that conservationists consider preserving host-specific lice as part of their efforts to save vertebrate hosts (65).
This study provides the first information about lice infestation of wild birds in different regions of Iran and reports Craspedorrhynchus platystomus, Colpocephalum nanum, Colpocephalum gypsi, Colpocephalum eucarenum, Colpocephalum heterosoma, Degeeriella fulva, Degeeriella fusca, Nosopon chanabense, Nosopon lucidum, Falcolipeurus quadripustulatus Falcolipeurus suturalis, Aegypoecus trigonoceps, Trinoton querquedulae, Anaticola crassicornis, Quadraceps obscurus, Rallicola cuspidatus, and Strigiphilus strigis for the first time from the country. Review of the published data on avian lice fauna of Iran shows that the information is available for almost 14% of the bird species. In contrast, researchers from the neighboring country Turkey have identified over 150 lice species from more than half of the bird species inhabiting the country (21). As Iran and Turkey share many bird species, it seems that many louse species remain to be discovered. Molecular phylogenetic analysis of avian lice from Iran will bring clearer understanding of the role of migratory birds in biogeographic distributions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because examined birds Hamedan province were euthanized by a certified veterinarian of the Provincial Department of Environment because of general health failure prior to transfer to the Faculty of Veterinary Medicine, Bu-Ali Sina University. Birds in other provinces were dead animals.
Author contributions
ZB: Methodology, Writing – original draft. AS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. JK: Methodology, Writing – original draft. MB: Methodology, Writing – original draft. EM: Methodology, Writing – original draft. BD: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Bu-Ali Sina University, Hamedan, Iran (Grant Number 1/1/28864).
Acknowledgments
The authors wish to thank Dr. Mehdi Safikhani, Majid Shabanloo, Reza Daneshpajooh, Dr. Alireza Mohammadi, Javad Noori Azhar (Provincial Department of Agriculture, Hamedan, Iran) and Shirdarreh for their kind cooperation in sample collection; Atabak Roohi-Aminjan (Bu-Ali Sina University, Hamedan, Iran) and Abolghasem Khaleghizadeh (Iranian Research Institute of Plant Protection, Agricultural Research, Education and Extension Organization, Tehran, Iran) for the confirmation of bird species; Fatemeh Nikbin and the staff of Laboratory of Parasitology, Faculty of Veterinary Science, Bu-Ali Sina University for their assistance. The authors also thank Mohamamd-Parsa Meeaadfar for providing us with the Trinoton querquedulae specimen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1324619/full#supplementary-material
Footnotes
References
1. Clayton, DH, Adams, RJ, and Bush, SE. Phthiraptera, the chewing lice In: CT Atkinson, NJ Thomas, and DB Hunter, editors. Parasitic Diseases of Wild Birds. Ames, USA: Wiley-Blackwell (2009)
2. Marcondes, CB, and Linardi, PM. Sucking and chewing lice In: CB Marcondes, editor. Arthropod Borne Diseases. Cham, Switzerland: Springer (2016). 503–15.
3. Dik, B. Erosive Stomatitis in a White Pelican (Pelecanus onocrotalus) Caused by Piagetiella titan (Mallophaga: Menoponidae). J Vet Med. (2006) 53:153–4. doi: 10.1111/j.1439-0450.2006.00927.x
4. Tavassoli, M, Salmanzadeh, R, and Jabbary, H. Infestations of Piagetiella titan (Menoponidae: Mallophaga) on juvenile white pelicans (Pelecanus nocrotalus, L.) in Urmia Lake National Park, northwest Iran. Iran J Vet Med. (2011) 5:105–8. doi: 10.22059/IJVM.2011.23106
5. Wobeser, G, Johnson, GR, and Acompanado, G. Stomatitis in a juvenile white pelican due to Piagetiella peralis (Mallophaga: Menoponidae). J Wildl Dis. (1974) 10:135–8. doi: 10.7589/0090-3558-10.2.135
6. Smith, V. S., Broom, Y., and Dalgleish, R. (2023). Louse-host associations. International Society of Phthirapterists. Available at: http://phthiraptera.myspecies.info (Accessed December 10, 2023).
7. Price, R., Hellenthal, R., Palma, R., Johnson, K., and Clayton, D. (2003). The chewing lice: world checklist and biological overview. Illinois Natural History Survey, Illinois, USA.
8. Barker, SC. Phylogeny and classification, origins, and evolution of host associations of lice. Int J Parasitol. (1994) 24:1285–91. doi: 10.1016/0020-7519(94)90195-3
9. Kaboli, M., Aliabadian, M., Tohidifar, M., Hashemi, A., Musavi, S. B., and Roselaar, C. C. (2016). Atlas of birds of Iran. Department of Environment of Iran, Tehran, Iran.
10. Khaleghizadeh, A., Roselaar, C., Scott, D. A., Tohidifar, M., Mlíkovský, J., Blair, M., et al. (2017). Birds of Iran: annotated checklist of the species and subspecies. Iranian Research Institute of Plant Protection, Tehran, Iran.
12. Ardalan, A. Mallophaga of Iran. II. 5 new records of Mallophaga from Iran. Bull Soc Pathol Exotique. (1975) 68:93–4.
13. Dik, B, and Halajian, A. Chewing lice (Phthiraptera) of several species of wild birds in Iran, with new records. J Arthropod Borne Dis. (2013) 7:83–9.
14. Moodi, B, Aliabadian, M, Moshaverinia, A, and Kakhki, OM. New data on the chewing lice (Phthiraptera) of passerine birds in East of Iran. Sci Parasitol. (2013) 14:63–8.
15. Ardalan, A. (1972). “Notes on the Mallophaga of Iran” in 4th National Congress of Plant Medicine. Tehran, Iran.
16. Ghaemi, P., and Roshanian, S. (2009). “First report of Trinoton sp infestation in Gyps fulvus in Golestan National Park” in 1st Natioanal Congress of Veterinary Laboratory Sciences, Tehran, Iran. pp. VETLAB01_32.
17. Ghaemi, P., Roshanian, S., and Ghaemi, P. (2010). “First report of Trinoton sp lice infestation in Buteo rufinus in Golestan province” in 16th Iranian Veterinary Congress, Tehran, Iran. pp. THVC16_0485
18. Oormazdi, H. (1958). Mallophaga of poultry in Tehran and outskirt. DVM Thesis. University of Tehran.
19. Youssefi, MR, Asadi-Irayi, M, Rezazadeh-Kalashami, A, Mashayekhnia, MJ, Roudaki-Sarvandani, MR, and Eslami-Amoli, A. Study of endo- and ectoparasites of Scolopax rusticola in northern Iran. J Vet Lab Res. (2018) 10:166.
20. Tomás, A, Palma, RL, Rebelo, MT, and da Fonseca, IP. Chewing lice (Phthiraptera) from wild birds in southern Portugal. Parasitol Int. (2016) 65:295–301. doi: 10.1016/j.parint.2016.02.007
21. Dik, B, Erciyas-Yavuz, K, and Per, E. Chewing lice (Phthiraptera: Amblycera, Ischnocera) on birds in the Kızılırmak delta, Turkey. Rev Med Vet. (2017) 167:53–62.
22. Clay, T. Revisions of the genera of Mallophaga.—I. The Rallicola-complex. Proc Zool Soc London. (1953) 123:563–88. doi: 10.1111/j.1096-3642.1953.tb00188.x
23. Clay, T. Revisions of Mallophaga genera. Degeeriella from the Falconiformes. Bull Br Mus Nat Hist. (1958) 7:123–207.
24. Clay, T. A new species of Strigiphilus (Philopteridae: Mallophaga). Pacific Insects. (1966) 8:835–47.
25. Dik, B, and Uslu, U. Strigiphilus strigis (Mallophaga: Philopteridae) in a Eurasian Eagle owl (Bubo bubo interpositus) in Turkey. Türk Parazitol Derg. (2007) 31:69–71.
26. Emerson, K. A review of the genus Rallicola (Philopteridae, Mallophaga) found on Aramidae, Psophiidae and Rallidae. Ann Entomol Soc Am. (1955) 48:284–99. doi: 10.1093/aesa/48.4.284
27. Gallego, M, Martin Mateo, M, and Aguirre, Y. Malofagos de rapaces Espanolas. II. Las especies del género Craspedorrynchus Keler, 1938 parasitas de falconiformes, con descripsion de tres especies nuevas. EOS-Rev Esp Entomol. (1987) 63:31–66.
28. Mey, E. A new Craspedorrhynchus species (Phthiraptera, Ischnocera) from Australia, with an annotated checklist of this chewing louse genus. Deutsch Entomol Zeitsch. (2001) 48:117–32. doi: 10.1002/dez.200100012
29. Nelson, RC, and Price, RD. The Laemobothrion (Mallophaga: Laemobothriidae) of the Falconiformes. J Med Entomol. (1965) 2:249–57. doi: 10.1093/jmedent/2.3.249
30. Price, R. A new species of Colpocephalum (Phthiraptera) on Threskiornis (Aves) from Aldabra. Syst Entomol. (1976) 1:61–3. doi: 10.1111/j.1365-3113.1976.tb00031.x
31. Price, RD, and Beer, JR. Species of Colpocephalum (Mallophaga: Menoponidae) parasitic upon the Falconiformes. Can Entomol. (1963) 95:731–63. doi: 10.4039/Ent95731-7
33. Tandan, B. Mallophaga from birds of the Indian subregion. Part VI Falcolipeurus Bedford. Proc Roy Entomolog Soc London Ser B Taxon. (1964) 33:173–80. doi: 10.1111/j.1365-3113.1964.tb01599.x
34. Tendeiro, J. Études sur les mallophages. Sur quelques espèces et sous-espèces du genre Nosopon Hopkins (Amblycera, Menoponidae), parasites de Falconiformes. Bolet Cult Guiné Portug. (1959) 14:193–211.
35. Zlotorzycka, J. Revision der europaischen Strigiphilini (Mallophaga, Strigiphilinae). Polskie pismo entomologiczne. Bull Entomol Pologne. (1974) 44:319–58.
36. GBIF.org (2023). Global Biodiversity Information Facility. Available at: https://www.gbif.org (Accessed December 10, 2023).
37. Azizi, H, Adel, M, Sayahi, E, Moghadam, AZ, Dehkordi, AE, and Hematzadeh, M. Laemobothrion maximum (chewing lice) in Iranian golden eagles. J Anim Poultry Sci. (2013) 2:85–90.
38. Rak, H, Anwar, M, and Niak, A. The species of mallophaga in wild birds in Iran. Bull Soc Pathol Exot Fil. (1975) 68:588–91.
39. Alborzi, A. R., and Naddaf, H. (2008). “First report of Laemobothrion (Laemobothrion) maximum (Scopoli, 1763) in Bonelli’s eagle (Hieraaetus fasciatus) from Khuzestan province—Iran” in 4th National Congress of Poultry Health and Diseases, Shahrekord, Iran.
40. Ahoo, MB, Hosseini, SH, Mobedi, I, Fathi, S, Soltani, M, and Tolouei, T. Parasites of wildlife birds in samples referred to the Iranian National Parasitology Museum (INPM). Iran J Vet Med. (2018) 12:20.
41. Rafyi, A, Alavi, A, and Rak, H. Bird lice in Iran. J Faculty Vet Med Univ Tehran. (1968) 25:107–22.
42. Shemshadi, B, Ranjbar-Bahadori, S, and Delfan-Abazari, M. Prevalence and intensity of parasitic infection in domestic ducks (Anas platyrhynchas) in Gilan Province, Northern Iran. Comp Clin Pathol. (2017) 26:165–7. doi: 10.1007/s00580-016-2361-7
43. Hosseini, SH, Saifuri, P, Eslami, A, and Nabieian, S. Parasitic infections of graylag goose (Anser anser) in Gilan Province, Iran. J Faculty Vet Med Univ Tehran. (2001) 56:57–60.
44. Borji, H, Moghaddas, E, Razmi, GR, and Azad, M. A survey of ecto-and endo-parasites of domestic pigeons (Columba livia) in Mashhad, Iran. Iran J Vet Sci Technol. (2013) 4:37–42. doi: 10.22067/VETERINARY.V4I2.3215
45. Radfar, MH, Asl, EN, Seghinsara, HR, Dehaghi, MM, and Fathi, S. Biodiversity and prevalence of parasites of domestic pigeons (Columba livia domestica) in a selected semiarid zone of South Khorasan, Iran. Trop Anim Health Prod. (2012) 44:225–9. doi: 10.1007/s11250-011-0002-3
46. Rezaei, F, Hashemnia, M, Chalechale, A, Seidi, S, and Gholizadeh, M. Prevalence of ectoparasites in free-range backyard chickens, domestic pigeons (Columba livia domestica) and turkeys of Kermanshah province, west of Iran. J Parasit Dis. (2016) 40:448–53. doi: 10.1007/s12639-014-0524-5
47. Chaechi-Nosrati, MR, Eslami, A, Rahbari, S, Houshmand, E, and Yousefi, A. The survey of parasitic infections of wild pigeons (Columba livia) in Lahijan city, Guilan, Iran. Comp Clin Pathol. (2018) 27:1405–8. doi: 10.1007/s00580-018-2779-1
48. Mahmoudian, J. (2015). Investigation on external and internal parasites of wild pigeon (Columba livia) and dove (Streptopelia senegalensis). M.Sc. Thesis, Urmia University, Iran.
49. Nazarbeigy, M., Halajian, A., and Yakhchali, M. (2019). “Lice and Mites Infestation in Bee-Eaters (Aves: Meropidae) from Western Iran” in 4th International and 11th National Congress of Parasitology and Parasitic Diseases of Iran, Urmia, Iranpp. 94.
51. Vazirianzadeh, B, Rahdar, M, and Molaee, S. Mallophaga of domestic birds of Ahvaz. Iran J Exp Zoo India. (2007) 10:75–7.
52. Eslami, A, Ghaemi, P, and Rahbari, S. Parasitic infections of free–range chickens from Golestan Province, Iran. Iran J Parasitol. (2009) 3:10–4.
53. Hashemzadeh-Farhang, H, Namdarian, M, Shirazi, S, and Shahbazi, P. Ectoparasites of local chickens from Tabriz county. Iran Vet J. (2009) 4:97–100.
54. Mamashly, M, Ranjbar-Bahadori, S, Safdari, A, and Aghaebrahimi-Samani, R. Study on parasitic infections of native poultry in Golestan province. J Comp Pathobiol. (2010) 7:189–92.
55. Nazarbeigy, M, Eslami, A, and Rahbari, S. Study of parasitic infections of local chickens from Ilam county. J Compar Pathobiol. (2013) 10:907–12.
56. Ebrahimi, M, Samiei, K, Anousheh, D, and Razi Jalali, M. Identification of ectoparasites in indigenous poultry in southern areas of West Azerbaijan, Iran: a study on the prevalence and importance of these parasites. Arch Razi Instit. (2016) 71:253–8. doi: 10.22034/ARI.2016.107510
57. Zakian, N, Nayebzadeh, H, Dezfoulian, O, and Aghaebrahimi-Samani, R. Parasitic infection of local chickens from Lorestan province. Iran Vet Res Biol Products. (2016) 28:18–20. doi: 10.22092/VJ.2015.103025
58. Hossienzadeh Marzenaki, J. Survey of ectoparasites in native poultry of Langroud city in 1395. Q J Vet Histobiol. (2017) 5:43–6.
59. Shamsi, L, Samaeinasab, S, and Haghighatkhah, A. Prevalence of ectoparasites in free-ranging backyard chickens of Sabzevar city, Iran. J Med Microbiol Infect Dis. (2020) 8:124–19. doi: 10.29252/JoMMID.8.3.124
60. Rassouli, M, Darvishi, MM, and Rosstami Lima, SR. Ectoparasite (louse, mite and tick) infestations on female turkeys (Galliformes, Phasianidae. Meleagris gallopavo) in Iran. J Parasit Dis. (2016) 40:1226–9. doi: 10.1007/s12639-015-0657-1
61. Ganjali, M, Keighobadi, M, and Hajipour, N. First report of new species of Goniodes pavonis (the chewing lice) from Indian Peacock in Iran. J New Biol Rep. (2015) 4:76–8.
62. Sadaghian, M., and Nouri, M. (2014). “Grey partridge pediculosis case report” in 4th International Veterinary Poultry Congress, Tehran, Iran, 231.
63. Imanibaran, A. Report of chewing louse infestation Philopterus ocellatus (Mallophaga: Ischnocera) from Black Crows (Corvus corone) in Miandoab region, West Azerbaijan province in 2010. J Vet Clin Pathol. (2014) 8:604–11.
64. Mey, E. Eine neue ausgestorbene vogel-Ischnozere von neuseeland, Huiacola extinctus (Insecta, Phthiraptera). Zool Anz. (1990) 224:49–73.
65. Rózsa, L, and Vas, Z. Co-extinct and critically co-endangered species of parasitic lice, and conservation-induced extinction: should lice be reintroduced to their hosts? Oryx. (2015) 49:107–10. doi: 10.1017/S0030605313000628
Keywords: birds of prey, chewing louse species, fauna, host–parasite associations, Iran, Middle-east, new record, Phthiraptera
Citation: Bahiraei Z, Sazmand A, Khedri J, Babaei M, Moeinifard E and Dik B (2024) Chewing lice of wild birds in Iran: new data and a checklist of avian louse species reported in Iran. Front. Vet. Sci. 10:1324619. doi: 10.3389/fvets.2023.1324619
Edited by:
Calin Mircea Gherman, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Bersissa Kumsa, Addis Ababa University, EthiopiaLuis Garcia Prieto, National Autonomous University of Mexico, Mexico
Copyright © 2024 Bahiraei, Sazmand, Khedri, Babaei, Moeinifard and Dik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alireza Sazmand, QWxpcmV6YS5TYXptYW5kQGJhc3UuYWMuaXI=
†These authors have contributed equally to this work
‡ORCID: Zahra Bahiraei orcid.org/0000-0002-7023-1029
Alireza Sazmand orcid.org/0000-0002-8450-2993
Mohammad Babaei orcid.org/0000-0002-1517-7162
Bilal Dik orcid.org/0000-0002-7553-5611
 Zahra Bahiraei1†‡
Zahra Bahiraei1†‡ Alireza Sazmand
Alireza Sazmand Bilal Dik
Bilal Dik