
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci., 25 September 2023
Sec. Comparative and Clinical Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1257624
Multiple endocrine disorders are uncommon in veterinary medicine, and the disease combination is usually related to hypercortisolism or autoimmunity. Central-pituitary hypothyroidism, also refer to secondary hypothyroidism, can be caused by hypercortisolemic conditions and is well-recognized in human medicine. However, central hypothyroidism, including pituitary hypothyroidism, concurrent with hyperadrenocorticism, is rarely reported in veterinary medicine. A 7-year-old, intact female Miniature Schnauzer presented with generalized alopecia, scale, and pruritus and was diagnosed with superficial pyoderma and Malassezia dermatitis. Hormonal tests were performed, and the results indicated multiple endocrinopathies with a combination of non-adrenal dependent hyperadrenocorticism and central-pituitary hypothyroidism. Magnetic resonance imaging (7 T) and high-resolution research tomography positron emission tomography were performed to differentiate neuroendocrine tumors; however, no lesion was found in the hypothalamic to pituitary region. Hyperadrenocorticism was managed first to control endocrinopathy. After controlling hypercortisolism, a weak elevation of free thyroxine (T4) was revealed, whereas total T4 and thyroid-stimulating hormone (TSH) were still undetectable, and hypothyroidism management was added. About 9 months after the management, both endocrine diseases were well controlled, and clinical signs improved; however, serum TSH was unmeasured consistently. This case study describes a case of multiple endocrinopathies in a Miniature Schnauzer dog diagnosed with central-pituitary hypothyroidism concurrent with non-adrenal dependent hyperadrenocorticism without pituitary macroadenoma.
Multiple endocrine disorders, including more than one endocrine disorder, have been reported uncommonly and are not well-established in veterinary medicine. The prevalence of multiple endocrine disorders in dogs was low, representing 0.3% of canine patients and 2.3% of the total canine patients with endocrinopathy (1). A previous study reported that combination of disorders included diabetes mellitus and hyperadrenocorticism 57.1%, hypoadrenocorticism and hypothyroidism 22.9%, diabetes mellitus and hypothyroidism 28.6% in the dogs with multiple endocrinopathies (1). Another study reported that the dogs with diabetes mellitus and hyperadrenocorticism were 6.65%, diabetes mellitus and hypothyroidism were 0.6%, and hyperadrenocorticism and primary hypothyroidism were 0.53% among all dogs with endocrine disease (2). Canine hypothyroidism is a common canine endocrinopathy; however, central hypothyroidism has been rarely documented and is less than 5% of all cases of canine hypothyroidism (3, 4). Central-pituitary hypothyroidism, also refer to secondary hypothyroidism, results from a loss of pituitary thyroid-stimulating hormone (TSH) secretion and is usually associated with an expanding pituitary tumor, pituitary malformation, and pituitary destruction due to radiation therapy or surgery (3, 5). Additionally, a possible breed predisposing to central hypothyroidism in Miniature Schnauzer has been reported previously (6). In human medicine, central-pituitary hypothyroidism is a potential, uncommon, and reversible condition due to hypercortisolemic conditions; however, it is controversial in veterinary medicine (7–9). In veterinary medicine, central hypothyroidism concurrent with hyperadrenocorticism was rarely reported in two cases accompanied by pituitary macroadenoma (10, 11). This case report describes a case of multiple endocrinopathies concurrent with non-adrenal dependent hyperadrenocorticism and central-pituitary hypothyroidism without pituitary macroadenoma in Miniature Schnauzer.
A 7-year-old, intact female Miniature Schnauzer weighing 7.4 kg was admitted because of generalized alopecia, scale, and pruritus. Physical examination revealed bilateral symmetric truncal alopecia with hyperpigmentation in the dorsum, abdomen, axilla, and inguinal region (Figure 1A). Infectious dermatitis, which is associated with a mixed infection of bacteria and Malassezia, was identified on cytology and culture. In the hematologic examination, normocytic, normochromic, nonregenerative anemia (packed cell volume, 35.6%; reference interval [RI], 37.3–61.7%) was identified in complete blood count, and hypercholesterolemia (total cholesterol, 967 mg/dL; RI, 135–270 mg/dL), hypertriglyceridemia (triglyceride, 470 mg/dL; RI, 21–116 mg/dL), and increased activity of liver enzymes (alkaline phosphate, 316 IU/L; RI, 29–97 IU/L) were revealed from serum chemistry analysis. There were no abnormal findings on thoracic radiography, but hepatomegaly and urinary bladder calculi were identified on abdominal radiography. Abdominal ultrasonography revealed a hyperechoic liver and gall bladder mucocele. The size of both adrenal glands was normal (left cranial pole, 5.5 mm; right cranial pole, 4.9 mm), and the morphologic abnormalities were not evident. Secondary infection due to concurrent endocrine disease was suspected, and hormonal tests were performed. An exogenous adrenocorticotrophic hormone (ACTH; Synacthen, Novartis Pharm., Basel, Switzerland) stimulation test was performed and revealed high post-ACTH cortisol (1,156 nmol/L, 41.9 μg/dL) that exceeded 600 nmol/L and was approximately six times greater than pre-ACTH cortisol (183 nmol/L, 6.65 μg/dL). A low-dose dexamethasone suppression (LDDS) test was performed to distinguish between pituitary and adrenal disease. After administration of dexamethasone (Je-il Pharm., Daegu, Korea), the concentration of 4-h post-dexamethasone serum cortisol (<28 nmol/L) was suppressed by less than 50% of baseline concentration (129 nmol/L) and 6-h post-dexamethasone serum cortisol measured as 32 nmol/L. Therefore, adrenal-dependent hyperadrenocorticism was excluded, and pituitary-dependent hyperadrenocorticism (PDH) was suspected. Additional hormonal tests were performed because PDH can be associated with other pituitary-dependent endocrinopathies. Hormonal assessments of the hypothalamic–pituitary-thyroid (HPT) axis were performed and revealed low serum total thyroxine (T4) (<0.3 μg/dL; RI, 1.0–4.0 μg/dL), low free T4 (<0.3 ng/dL; RI, 0.6–3.7 ng/dL), and low serum levels of TSH (<0.03 ng/mL; RI, 0.05–0.42 ng/mL). Plasma TSH concentration after stimulation with thyrotropin-releasing hormone (TRH; Cerebrain, Shinpoong Pharm., Seoul, Korea) also revealed low levels at 10, 20, 30 min, and 4 h (<0.03 ng/mL, respectively). Total T4 and free T4 concentrations were also undetectable before and 4 h after stimulation with TRH (total T4, <0.3 μg/dL; free T4, <0.3 ng/dL, respectively). Based on these results, a pituitary endocrine tumor was suspected, and intracranial imaging was performed using 7 T magnetic resonance imaging (MRI) and high-resolution research tomography positron emission tomography (HRRT-PET) under general anesthesia. The dog was fasted for 12 h, and 18F-fluorodeoxyglucose (FDG) was administered at 0.14 mg/kg intravenously. Sixty min after the FDG injection, the FDG-PET scan was conducted for 30 min on the HRRT device (ECAT HRRT; Siemens, Knoxville, TN, United States). Immediately after the FDG-PET scan, pre- and post-contrast T1- weighted images of the brain were obtained using a 7 T-MRI scanner (Magnetom 7 T; Siemens, Berlin, Germany; Figures 2A–D). In the transverse pre-contrast T1-weighted image, a hyperintense region, indicating neurohypophysis, was present in the center of the pituitary gland. The size of the pituitary gland was measured (height, 3.7 mm, RI: 3.0–7.5 mm; width, 4.8 mm, RI: 4.0–9.0 mm) on the transverse post-contrast T1 weighted image and the pituitary height/brain area (P/B) ratio was 0.2 (RI, ≤0.31). The standardized uptake value of the pituitary gland (mean, 0.32; max, 0.47) was lower than those of the gray matter (mean, 0.96; max, 1.02) and caudal colliculus (mean, 1.69; max, 2.44; Figures 2E–H). No evidence of pituitary macroadenoma or abnormal findings was observed in the brain. As pituitary microadenoma cannot be ruled out, insulin-like growth factor (IGF-1) was measured to differentiate growth hormone-secreting abnormalities. It was measured by a reference laboratory (Diagnostic Center for Population & Animal Health, Michigan State University, Lansing, MI), and the result (7 nmol/L; RI, 4–95 nmol/L) was indicated as normal. Therefore, we concluded that the patient had multiple endocrinopathies, including non-adrenal dependent hyperadrenocorticism and central-pituitary hypothyroidism, without a pituitary macroadenoma.
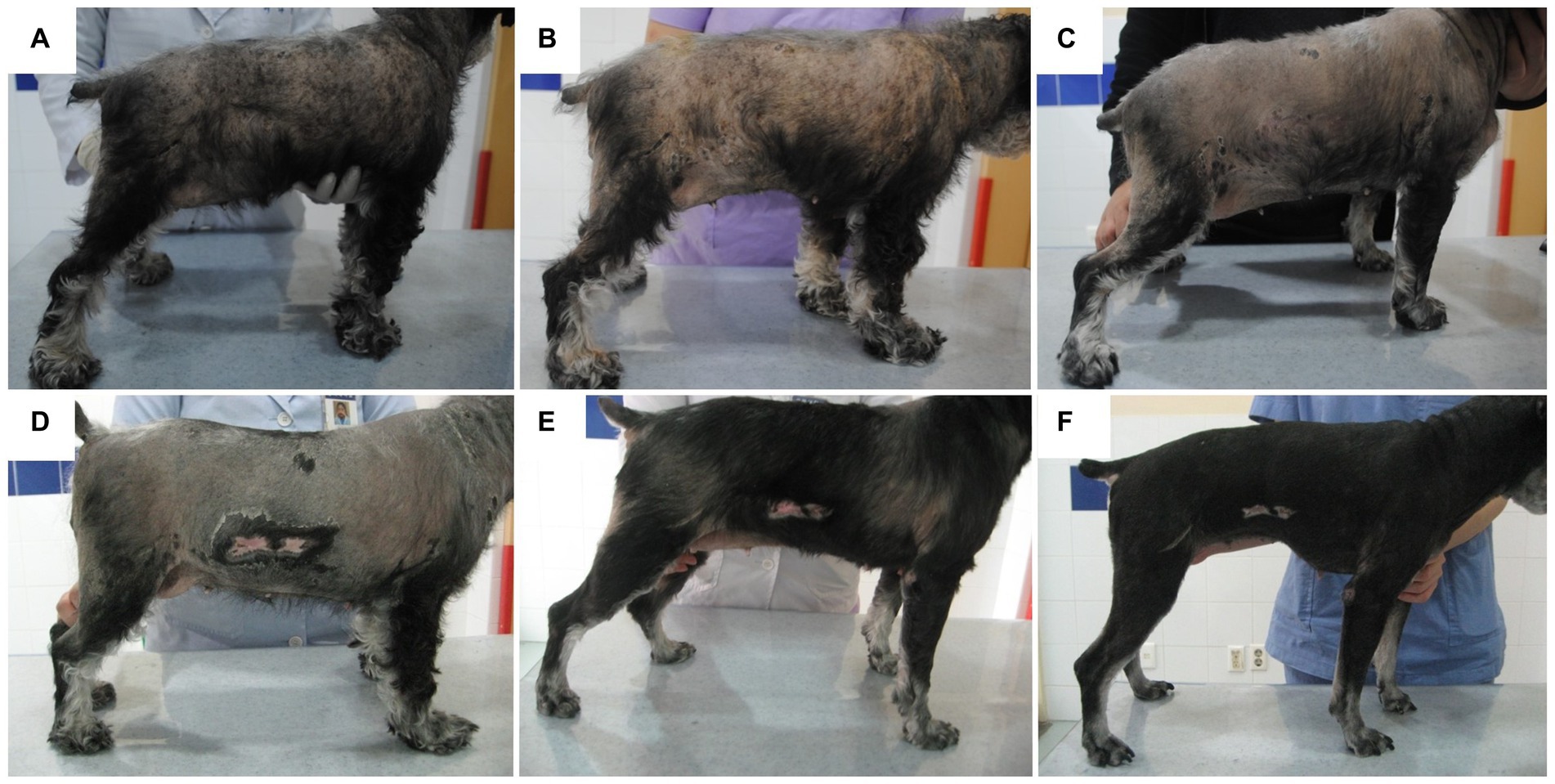
Figure 1. Schematic diagram photography of cutaneous lesion improvement. Generalized truncal alopecia and scale with hyperpigmentation were presented before the treatment (A). No significant improvement was observed 2 weeks after the management of superficial pyoderma and Malassezia dermatitis (B). Mild improvement of papule and scale was identified 1 month after hyperadrenocorticism management (C). Generalized skin lesions, including alopecia, were improved after additional hypothyroidism management at 1 month (D), 3 months (E), and 5 months (F).
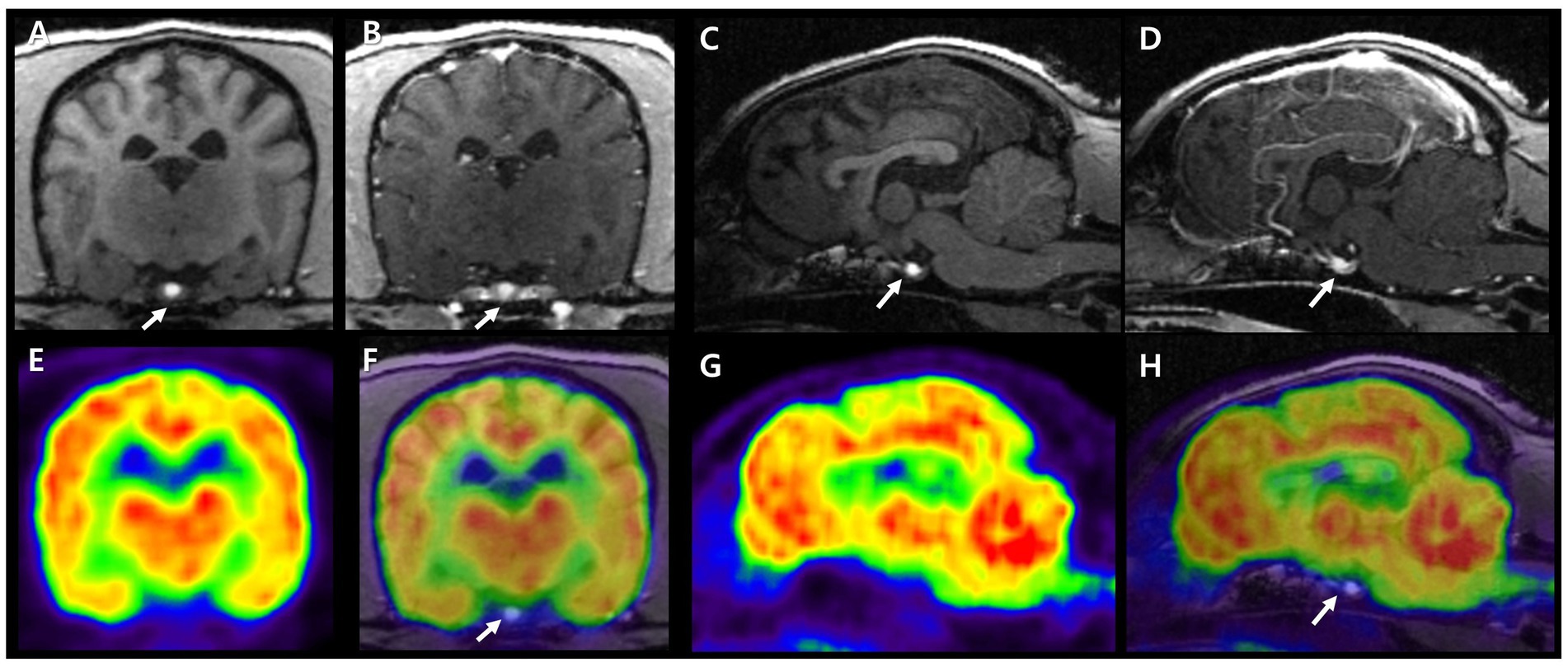
Figure 2. 7 T MRI and FDG-PET characteristics of a canine pituitary region. Pre- and post-contrast T1 images in transverse (A,B) and sagittal plane (C,D). Neurohypophysis is presented as a hyperintense signal in the center of the pituitary gland (arrows). The adenohypophysis is surrounding neurohypophysis with an isointense signal in T1 image and is enhanced after gadolinium injection. FDG-PET images in transverse and sagittal planes (E,G). Fusion of T1 MRI and FDG-PET images in transverse and sagittal planes (F,H). On PET images, reddish to yellowish color represents high FDG uptake and bluish to greenish color represents low FDG uptake. The pituitary gland showed lower glucose metabolism than cerebral cortex and white matter. MRI, magnetic resonance imaging; FDG-PET, fluorodeoxyglucose-positron emission tomography.
To treat infectious dermatitis, amoxicillin/clavulanic acid (25 mg/kg PO q12h), itraconazole (5 mg/kg PO q24h), and benzoyl peroxide shampoo were prescribed for 2 weeks; however, no significant improvement was observed (Figure 1B). To control the endocrinopathy and infectious dermatitis, which can be caused by hyperadrenocorticism or hypothyroidism, the medication for hyperadrenocorticism, trilostane (1 mg/kg PO q12h), was prescribed first because hypercortisolism can suppress the HPT axis. After the control of the hypercortisolism (post-ACTH stimulation test results 5.12 ug/dL), total T4 and TSH concentrations were still undetectable, whereas free T4 (0.435 ng/dL) revealed weak elevation (Table 1). L-thyroxine (0.02 mg/kg PO q12h) was administered after the stabilization of hyperadrenocorticism to control hypothyroidism and to pursue rapid clinical improvement. Some responses, such as improvement of papule and scale, were identified after the control of hyperadrenocorticism and infectious dermatitis (Figure 1C), whereas generalized alopecia was improved after levothyroxine administration (Figures 1D–F). After 6 months of levothyroxine administration (7 months of hyperadrenocorticism management), increased total T4 (2.80 ug/dL) and free T4 (5.78 ng/dL) were identified with a general improvement in body condition (Figure 1F), whereas serum TSH was still undetectable. About 9 months after the control of the hyperadrenocorticism, the serum cortisol concentration of pre- and post-ACTH stimulation test was revealed, 2.55 ug/dL and 2.89 ug/dL, respectively. At the same time point, total T4 was 2.24 ug/dL, and free T4 was 5.24 ng/dL, whereas TSH was unmeasured consistently (Table 1).
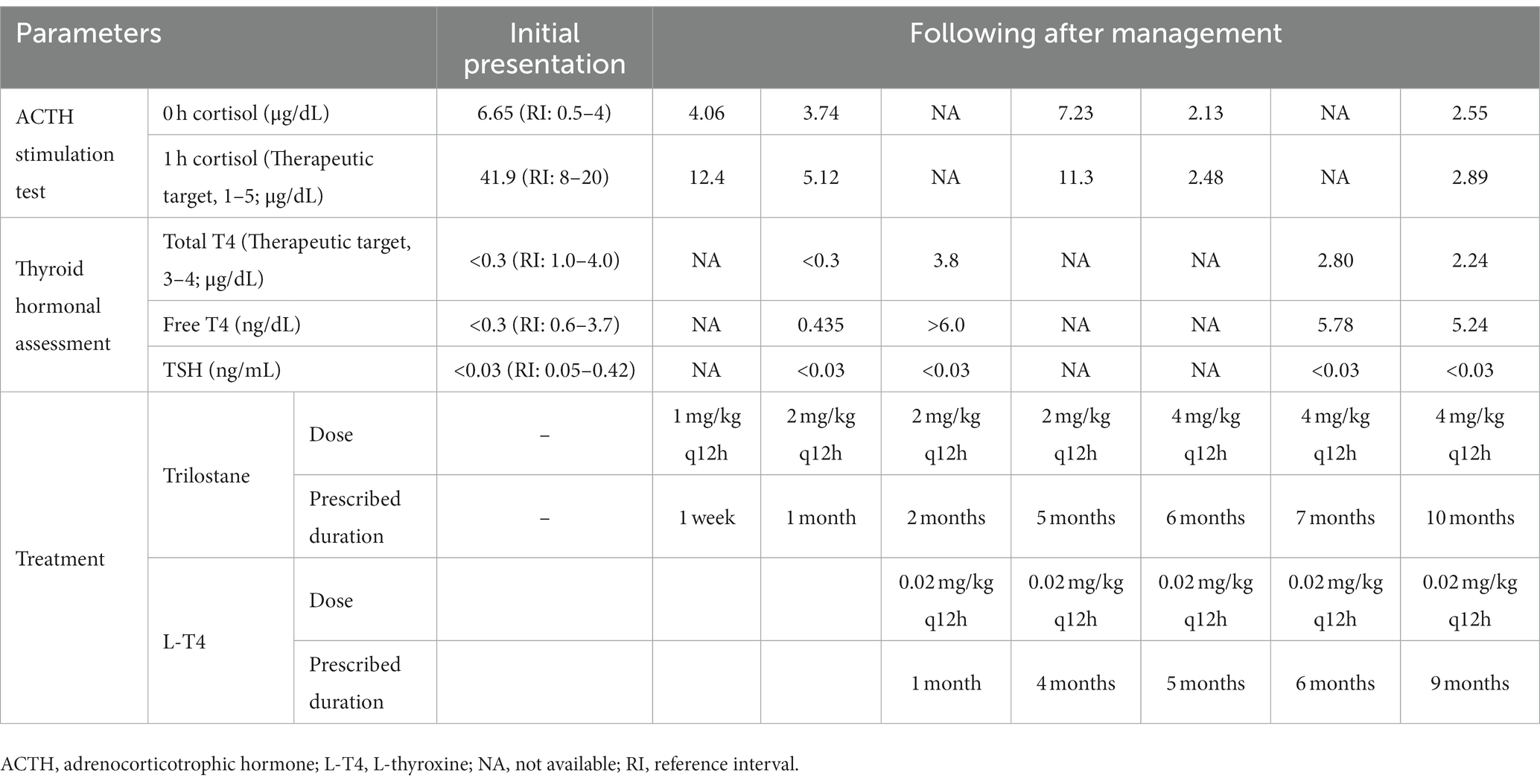
Table 1. Monitoring management for a dog with non-adrenal dependent hyperadrenocorticism and central-pituitary hypothyroidism.
Multiple endocrinopathies are uncommon in veterinary medicine, and the most common combination of endocrine disorders is hyperadrenocorticism and diabetes mellitus (1, 2). The etiology of these two diseases is usually unrelated; however, insulin resistance secondary to hyperadrenocorticism may influence diabetes mellitus occurrence (1, 2). The next most common multiple endocrinopathy is hypothyroidism with hypoadrenocorticism or diabetes mellitus, which can have a potential association with autoimmune poly-endocrinopathy (1, 2). In this case, the Miniature Schnauzer dog, which has breed predisposing hypothyroidism, was diagnosed with non-adrenal dependent hyperadrenocorticism and central-pituitary hypothyroidism without pituitary macroadenoma.
Because hyperadrenocorticism dogs have clinical signs similar to those of hypothyroid dogs and may have low total T4 concentrations, the differential diagnosis between hypothyroidism and euthyroid sick syndrome can be challenging. Moreover, dogs with primary hypothyroidism have low total T4 levels and high TSH concentrations (3). In dogs with non-thyroidal-illness syndrome, including hyperadrenocorticism, the occurrence of high TSH concentration with low T4 is uncommon (9, 12–14). The suppression of the HPT axis caused by hypercortisolism is well established in humans (7). In veterinary medicine, the effects of exogenous and endogenous glucocorticoids on the HPT axis have also been investigated in dogs (9, 14–19). However, central-pituitary hypothyroidism due to hyperadrenocorticism is diagnosed uncommonly, although it can be theoretically common. A previous case study reported a dog with central-pituitary hypothyroidism and PDH caused by pituitary macroadenoma (11). Differently, in our case, the dog with non-adrenal dependent hyperadrenocorticism and central-pituitary hypothyroidism was diagnosed by hormonal assessment and showed no evidence of abnormal lesions in the brain on 7 T MRI and HRRT-PET. However, even if no lesions are identified through brain MRI, microadenoma cannot be ruled out. In human medicine, microadenomas are more common than macroadenomas, and Cushing’s disease is often caused by microadenomas (7, 20, 21). Furthermore, a higher prevalence of central hypothyroidism is reported in ACTH-secreting microadenomas than in other microadenoma types, which indicates the role of hypercortisolism in the genesis of central hypothyroidism (8). At the hypothalamic level, chronic hypercortisolism can reduce TRH gene expression in the hypothalamic paraventricular nucleus and increase somatostatin release, which in turn inhibits TSH release (7, 22–25). At the pituitary level, hypercortisolism directly inhibits TSH secretion (7, 26). However, even if TSH isoforms are qualitatively defective and have impaired biological activity, they can be quantitatively normal or even elevated and maintain their immunoreactivity in the diagnostic test (27, 28). Therefore, differentiating central-pituitary hypothyroidism requires the TRH stimulation test; however, it is often considered underdiagnosed in dogs due to the low sensitivity of TSH measurement, which cannot detect all isoforms (28). In this case, the dog was diagnosed with central-pituitary hypothyroidism rather than non-thyroidal illness syndrome due to hypercortisolism because TSH and T4 were undetectable before and after TRH stimulation. However, it could not be concluded as the pituitary hypothyroidism secondary to PDH, because hypersecreting endogenous ACTH was not confirmed in this dog. Moreover, the reduction of TSH levels during uncontrolled hypercortisolism can return to normal thyroid function after the cure of PDH, which has been reported in both humans and dogs (7, 14, 29). Although, in this case, post-ACTH stimulation cortisol levels were maintained appropriately after hyperadrenocorticism management, TSH concentration was undetectable until 9 months after treatment. Serum TSH may not have had enough time to recover or may be influenced by levothyroxine medication which can suppress serum TSH concentration. However, suppression of serum TSH concentration which caused by levothyroxine application was identified in euthyroid or primary hypothyroid dogs (5, 30–33), but was not investigated in dogs with central-pituitary hypothyroidism. Therefore, further investigation of sustained TSH suppression and other causes of pituitary hypothyroidism should also be considered in our case.
Another possibility in the current case is a breed-specific predisposition. A previous study reported central hypothyroidism in Miniature Schnauzer (6). These dogs were diagnosed by hormonal assays, including a 3-day-TSH-stimulation test or TRH stimulation test and thyroid scintigraphy, and had no evidence of pituitary and hypothalamic abnormalities in CT scans (6). The genetic background of this breed was suspicious, whereas no disease-causing mutations were found in the TSH-beta gene and the exons of the TRH receptor gene (6). However, TSH release can be decreased in primary hypothyroidism because of TRH receptor desensitization, which is caused by persistent stimulation of thyrotropes via the negative feedback loop (30). In laboratory-induced primary hypothyroidism, TSH increases after thyroidectomy and decreases gradually after 3 years; however, it can be detectable and measured within the reference range (30). In this case, loss of TSH-secreting function was identified through the TRH stimulation test. However, differentiating thyroid atrophy caused by decreased TSH secretion from pituitary thyrotrope desensitization due to primary hypothyroidism is challenging because primary hypothyroidism was not discriminated through the TSH stimulation test. Although weak elevation of free T4 was identified after PDH management, total T4 and TSH were still undetectable. Furthermore, the pituitary gland can usually be enlarged secondary to primary hypothyroidism (30). However, in this case, no evidence of an enlarged pituitary gland in the 7 T-MRI scan and no increased avidity of FDG on the HRRT-PET scan were detected, which can help in a more accurate diagnosis of the pituitary lesion (34–37). Based on these findings, central-pituitary hypothyroidism associated with breed-specificity is highly suggested rather than primary hypothyroidism.
Another possible cause of hypercortisolism is adrenal TRH stimulation. TRH is transported to the pituitary via the hypothalamic–hypophyseal portal system, which consists of the primary capillary plexus penetrating the median eminence, and directly stimulating TRH receptors on melanotropes in the pars intermedia of the pituitary gland. It releases stored proopiomelanocortin, which is then converted into ACTH and other peptides by prohormone convertases (38). A previous study reported the presence of TRH receptors in the pituitary and adrenal glands of dogs (39). However, plasma cortisol concentration did increase significantly after TRH stimulation, whereas plasma ACTH concentration did not rise significantly (39). This suggests the possibility of hypercortisolism, which can be induced by direct TRH stimulation of the adrenal gland. In this case, hyperadrenocorticism was confirmed by the ACTH stimulation test, and adrenal-dependent hyperadrenocorticism was excluded by the LDDS test. This dog had no evidence of ectopic ACTH secretion such as intrathoracic tumors or neuroendocrine malignancies on radiography and abdominal ultrasonography. No brain lesions, including those in the pituitary to hypothalamic area, were identified on HRRT-PET and 7 T MRI. While it is plausible to speculate that the hypercortisolism identified in this case could have resulted from TRH elevation, which can be increased due to central-pituitary hypothyroidism, in practice, we were unable to measure the endogenous TRH concentration as the TRH assay is not validated in dogs and unmeasured endogenous ACTH because of laboratory limitation.
In human medicine, hypothyroidism causes elevated cortisol levels, presumably due to decreased clearance and blunted negative cortisol feedback in the hypothalamus-pituitary–adrenal axis (40). Furthermore, reduced serum cortisol levels after 5–7 months of levothyroxine therapy in patients with primary hypothyroidism have been reported (41). In veterinary medicine, hypercortisolemic status tend to be controlled prior to conclusive diagnosis or management of hypothyroidism, because of its HPT axis suppressing effect. Also, in this case, hyperadrenocorticism was managed first, but clinical improvement was not clearly identified. After managing concurrent hypothyroidism during treatment for hyperadrenocorticism, the post-ACTH cortisol level and clinical signs showed stabilization at 5 months. This suggests that the management of hypothyroidism influences the control of hyperadrenocorticism. Therefore, in this case, the additional consideration, that secondarily caused elevation of cortisol concentration which is influenced by breed related central hypothyroidism rather than a PDH-HAC from the beginning, should be suspected such as below. The cortisol level that was elevated initially (baseline and post-ACTH) could have been influenced by the generalized infectious dermatitis. Basal and post-ACTH cortisol concentration were reported already within the RI 1 week after trilostane, which usually takes slightly more time to act. At that time, the dog was already on antibiotics for 2 weeks. Additionally, clinical signs and laboratory results for this dog were generally inconsistent with HAC. This dog also seemed to improve significantly only when levothyroxine was added and not with trilostane only. Therefore, to control both concomitant endocrinopathies well, a definitive differential diagnosis between primary and central hypothyroidism is needed because central hypothyroidism can be misdiagnosed due to the effect of hypercortisolism in suppressing HPT axis and hypothyroidism can be speculated to increase the cortisol level.
Theoretically, hypercortisolism concurrent with primary or secondary hypothyroidism that have elevated endogenous TRH can be common in veterinary medicine. However, in authors knowledge, the concept of hyperadrenocorticism secondary to hypothyroidism, which is the state of oversecretion of TRH is not mentioned before in dogs. We think that it might be underdiagnosed these connected diseases because of the limitation of diagnosing methods, such as low sensitivity and specificity of TSH measuring, limited use of TSH and TRH stimulation tests, non-measurement of endogenous ACTH, in veterinary field. Clinicians can face the state of concurrent these two endocrinopathies more commonly than in the literature, but definitive diagnosis still can be challenged because diagnostic methods have limitations as we mentioned above. Therefore, further exploration on the new insight of comorbidity for hyperadrenocorticism and hypothyroidism should be needed.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because this case study was descripted retrospectively from the data obtained for clinical purposes. All the procedures being performed were part of the routine care. Informed consent for all datas in our manuscript were obtained from the owners. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
YC: Writing – original draft. TY: Writing – review & editing. YK: Writing – review & editing. DL: Writing – review & editing. M-PY: Writing – review & editing. HK: Writing – review & editing. B-TK: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT), number 2021R1A2C1012058, and by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through the Companion Animal Life Cycle Industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (322095-04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blois, SL, Dickie, E, Kruth, SA, and Allen, DG. Multiple endocrine diseases in dogs: 35 cases (1996–2009). J Am Vet Med Assoc. (2011) 238:1616–21. doi: 10.2460/javma.238.12.1616
2. Pöppl, ÁG, Coelho, IC, da Silveira, CA, Moresco, MB, and Carvalho, GLC. Frequency of endocrinopathies and characteristics of affected dogs and cats in southern Brazil (2004-2014). Acta Sci Vet. (2016) 44:9. doi: 10.22456/1679-9216.81099
3. Mooney, C. Canine hypothyroidism: a review of aetiology and diagnosis. N Z Vet J. (2011) 59:105–14. doi: 10.1080/00480169.2011.563729
4. McDonald, LE. Veterinary endocrinology and reproduction In: Vet Endocrinol Reprod. 3rd. eds. P. Mauricio and P. D. Michael (Philadelphia, USA: Lea & Febiger). (1980).
5. Feldman, EC, and Nelson, RW. Preface In: EC Feldman, RW Nelson, CE Reusch, and JCR Scott-Moncrieff, editors. Canine and Feline Endocrinology. 4th ed. St. Louis: W.B. Saunders (2015). vii.
6. Voorbij, AMWY, Leegwater, PAJ, Buijtels, JJCWM, Daminet, S, and Kooistra, HS. Central hypothyroidism in Miniature Schnauzers. J Vet Intern Med. (2016) 30:85–91. doi: 10.1111/jvim.13818
7. Paragliola, RM, Corsello, A, Papi, G, Pontecorvi, A, and Corsello, SM. Cushing’s syndrome effects on the thyroid. Int J Mol Sci. (2021) 22:3131. doi: 10.3390/ijms22063131
8. Mathioudakis, N, Thapa, S, Wand, GS, and Salvatori, R. ACTH-secreting pituitary microadenomas are associated with a higher prevalence of central hypothyroidism compared to other microadenoma types. Clin Endocrinol. (2012) 77:871–6. doi: 10.1111/j.1365-2265.2012.04442.x
9. Meij, B, Mol, J, Bevers, M, and Rijnberk, A. Alterations in anterior pituitary function of dogs with pituitary-dependent hyperadrenocorticism. J Endocrinol. (1997) 154:505–12. doi: 10.1677/joe.0.1540505
10. Shiel, RE, Acke, E, Puggioni, A, Cassidy, JP, and Mooney, CT. Tertiary hypothyroidism in a dog. Ir Vet J. (2007) 60:88–93. doi: 10.1186/2046-0481-60-2-88
11. Barr, SC. Pituitary tumour causing multiple endocrinopathies in a dog. Aust Vet J. (1985) 62:127–9. doi: 10.1111/j.1751-0813.1985.tb07260.x
12. Torres, SMF, Feeney, DA, Lekcharoensuk, C, Fletcher, TF, Clarkson, CE, Nash, NL, et al. Comparison of colloid, thyroid follicular epithelium, and thyroid hormone concentrations in healthy and severely sick dogs. J Am Vet Med Assoc. (2003) 222:1079–85. doi: 10.2460/javma.2003.222.1079
13. Kantrowitz, LB, Peterson, ME, Melián, C, and Nichols, R. Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease. J Am Vet Med Assoc. (2001) 219:765–9. doi: 10.2460/javma.2001.219.765
14. Kenefick, SJ, and Neiger, R. The effect of trilostane treatment on circulating thyroid hormone concentrations in dogs with pituitary-dependent hyperadrenocorticism. J Small Anim Pract. (2008) 49:139–43. doi: 10.1111/j.1748-5827.2007.00509.x
15. Ferguson, DC, and Peterson, ME. Serum free and total iodothyronine concentrations in dogs with hyperadrenocorticism. Am J Vet Res. (1992) 53:1636–40.
16. Kemppainen, RJ, Thompson, FN, Lorenz, MD, Munnell, JF, and Chakraborty, PK. Effects of prednisone on thyroid and gonadal endocrine function in dogs. J Endocrinol. (1983) 96:293–NP. doi: 10.1677/joe.0.0960293
17. Moore, GE, Ferguson, DC, and Hoenig, M. Effects of oral administration of anti-inflammatory doses of prednisone on thyroid hormone response to thyrotropin-releasing hormone and thyrotropin in clinically normal dogs. Am J Vet Res. (1993) 54:130–5.
18. Nelson, RW, Ihle, SL, Feldman, EC, and Bottoms, GD. Serum free thyroxine concentration in healthy dogs, dogs with hypothyroidism, and euthyroid dogs with concurrent illness. J Am Vet Med Assoc. (1991) 198:1401–7.
19. Peterson, ME, Ferguson, DC, Kintzer, PP, and Drucker, WD. Effects of spontaneous hyperadrenocorticism on serum thyroid hormone concentrations in the dog. Am J Vet Res. (1984) 45:2034–8.
20. Daly, AF, Rixhon, M, Adam, C, Dempegioti, A, Tichomirowa, MA, and Beckers, A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liège. Belgium J Clin Endocrinol Metab. (2006) 91:4769–75. doi: 10.1210/jc.2006-1668
21. Aljabri, KS, Bokhari, SA, Assiri, FY, Alshareef, MA, and Khan, PM. The epidemiology of pituitary adenomas in a community-based hospital: a retrospective single center study in Saudi Arabia. Ann Saudi Med. (2016) 36:341–5. doi: 10.5144/0256-4947.2016.341
22. Hollenberg, AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid. (2008) 18:131–9. doi: 10.1089/thy.2007.0251
23. Cintra, A, Fuxe, K, Wikström, AC, Visser, T, and Gustafsson, JA. Evidence for thyrotropin-releasing hormone and glucocorticoid receptor-immunoreactive neurons in various preoptic and hypothalamic nuclei of the male rat. Brain Res. (1990) 506:139–44. doi: 10.1016/0006-8993(90)91210-8
24. Kakucska, I, Qi, Y, and Lechan, RM. Changes in adrenal status affect hypothalamic thyrotropin-releasing hormone gene expression in parallel with corticotropin-releasing hormone. Endocrinology. (1995) 136:2795–802. doi: 10.1210/endo.136.7.7789304
25. Alkemade, A, Unmehopa, UA, Wiersinga, WM, Swaab, DF, and Fliers, E. Glucocorticoids decrease thyrotropin-releasing hormone messenger ribonucleic acid expression in the paraventricular nucleus of the human hypothalamus. J Clin Endocrinol Metab. (2005) 90:323–7. doi: 10.1210/jc.2004-1430
26. Ahlquist, JA, Franklyn, JA, Ramsden, DB, and Sheppard, MC. The influence of dexamethasone on serum thyrotrophin and thyrotrophin synthesis in the rat. Mol Cell Endocrinol. (1989) 64:55–61. doi: 10.1016/0303-7207(89)90064-6
27. Horimoto, M, Nishikawa, M, Ishihara, T, Yoshikawa, N, Yoshimura, M, and Inada, M. Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism: comparison between in vivo 3,5,3′-triiodothyronine response to TSH and in vitro bioactivity of TSH. J Clin Endocrinol Metab. (1995) 80:1124–8.
28. Boretti, FS, and Reusch, CE. Endogenous TSH in the diagnosis of hypothyroidism in dogs. Schweiz Arch Tierheilkd. (2004) 146:183–8. doi: 10.1024/0036-7281.146.4.183
29. Xiang, B, Tao, R, Liu, X, Zhu, X, He, M, Ma, Z, et al. A study of thyroid functions in patients with Cushing’s syndrome: a single-center experience. Endocr Connect. (2019) 8:1176–85. doi: 10.1530/EC-19-0309
30. Diaz-Espiñeira, MM, Mol, JA, van den Ingh, TSG, van der Vlugt-Meijer, RH, Rijnberk, A, and Kooistra, HS. Functional and morphological changes in the adenohypophysis of dogs with induced primary hypothyroidism: loss of TSH hypersecretion, hypersomatotropism, hypoprolactinemia, and pituitary enlargement with transdifferentiation. Domest Anim Endocrinol. (2008) 35:98–111. doi: 10.1016/j.domaniend.2008.03.001
31. Dixon, RM, Reid, SW, and Mooney, CT. Treatment and therapeutic monitoring of canine hypothyroidism. J Small Anim Pract. (2002) 43:334–40. doi: 10.1111/j.1748-5827.2002.tb00082.x
32. Reusch, CE, Fracassi, F, Sieber-Ruckstuhl, NS, Burkhardt, WA, Hofer-Inteeworn, N, Schuppisser, C, et al. Altered serum thyrotropin concentrations in dogs with primary hypoadrenocorticism before and during treatment. J Vet Intern Med. (2017) 31:1643–8. doi: 10.1111/jvim.14840.PMID:
33. Ziglioli, V, Panciera, DL, Troy, GC, Monroe, WE, Boes, KM, and Refsal, KR. Effects of levothyroxine administration and withdrawal on the hypothalamic-pituitary-thyroid Axis in euthyroid dogs. J Vet Intern Med. (2017) 31:705–10. doi: 10.1111/jvim.14711.
34. Taoda, T, Hara, Y, Masuda, H, Teshima, T, Nezu, Y, Teramoto, A, et al. Magnetic resonance imaging assessment of pituitary posterior lobe displacement in dogs with pituitary-dependent hyperadrenocorticism. J Vet Med Sci. (2011) 73:725–31. doi: 10.1292/jvms.10-0192.
35. Travetti, O, White, C, Labruyère, J, and Dunning, M. Variation in the MRI appearance of the canine pituitary gland. Vet Radiol Ultrasound. (2021) 62:199–209. doi: 10.1111/vru.12938
36. Chittiboina, P, Montgomery, BK, Millo, C, Herscovitch, P, and Lonser, RR. High-resolution(18)F-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging for pituitary adenoma detection in Cushing disease. J Neurosurg. (2015) 122:791–7. doi: 10.3171/2014.10.JNS14911
37. Seok, H, Lee, EY, Choe, EY, Yang, WI, Kim, JY, Shin, DY, et al. Analysis of 18F-fluorodeoxyglucose positron emission tomography findings in patients with pituitary lesions. Korean J Intern Med. (2013) 28:81–8. doi: 10.3904/kjim.2013.28.1.81
38. Sarapura, VD, and Samuel, MH. Chapter 6 - thyroid-stimulating hormone In: S Melmed, editor. The Pituitary. 4th ed. Amsterdam, Netherlands: Elsevier (2017). 163–201.
39. Pijnacker, T, Knies, M, Galac, S, Sanders, K, Mol, JA, and Kooistra, HS. TRH-induced secretion of adrenocorticotropin and cortisol in dogs with pituitary-dependent hypercortisolism. Vet Q. (2018) 38:72–8. doi: 10.1080/01652176.2018.1521537
40. Miller, H, Durant, JA, Cowan, JM, Knott, JMS, and Garnett, ES. THYROID FUNCTION AND STEROID HORMONE EXCRETION. J Endocrinol. (1970) 48:55–9. doi: 10.1677/joe.0.0480055
Keywords: central-pituitary hypothyroidism, hyperadrenocorticism, Miniature Schnauzer dog, multiple endocrinopathies, secondary hypothyroidism
Citation: Chae Y, Yun T, Koo Y, Lee D, Yang M-P, Kim H and Kang B-T (2023) Case report: Central-pituitary hypothyroidism concurrent with hyperadrenocorticism without pituitary macroadenoma in a Miniature Schnauzer dog. Front. Vet. Sci. 10:1257624. doi: 10.3389/fvets.2023.1257624
Received: 12 July 2023; Accepted: 30 August 2023;
Published: 25 September 2023.
Edited by:
Muhammad Saqib, University of Agriculture, Faisalabad, PakistanReviewed by:
Luca Giori, University of Tennessee, Knoxville, United StatesCopyright © 2023 Chae, Yun, Koo, Lee, Yang, Kim and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byeong-Teck Kang, a2FuZ2J0QGNodW5nYnVrLmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.