
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Vet. Sci. , 29 September 2023
Sec. Veterinary Infectious Diseases
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1249410
The aim of the present systematic review and meta-analysis was to identify the main infectious agents related to bovine abortion worldwide in the period between 2000 and 2022. First, we investigated the global prevalence of infectious agents related to bovine abortion. For this analysis, only 27 articles detected of a wide panel of agents were included. The random effects model revealed that the estimated prevalence of the abortifacient agents in bovine abortion was 45.7%. The heterogeneity among studies was high, but Egger’s test showed that there was no publication bias, even though the total number of samples analyzed in these articles was variable. There was no significant effect of the year of the study publication on the estimated prevalence, although an increasing trend was observed over time, possibly due to the implementation of new diagnostic techniques. Then, we analyzed the prevalence of the main transmissible agents in bovine abortion. For this analysis, 76 studies that analyzed 19,070 cases were included. Some infectious agent was detected in 7,319 specimens, and a final diagnosis was reached in 3,977 of these, when both the infectious agent and compatible histopathological changes were detected. We found that Neospora caninum was the most detected agent (22.2%), followed by opportunistic bacteria (21.4%), Chlamydiaceae family (10.9%) and Coxiella burnetii (9.5%). Regarding viral agents, bovine herpes virus type 1 and bovine viral diarrhea displayed similar prevalence rates (approximately 5%). After considering the description of specific histopathological changes, our analyzes showed that N. caninum was a confirmed cause of abortion in 16.7% of the analyzed cases, followed by opportunistic bacteria (12.6%) and Chlamydia spp. (6.8%); however, C. burnetii was only confirmed as a cause of abortion in 1.1% of the cases. For all agents, the heterogeneity among studies was high, and the subgroup analyzes discarded the diagnostic method as the cause of such heterogeneity. This study provides knowledge about the global prevalence of the different infectious agents related to bovine abortion, the most coming of which is N. caninum. In addition, this review reveals the existing deficiencies in the diagnosis of bovine abortion that must be addressed in the future.
The cattle industry is the main producer of milk worldwide and the third largest producer of meat, following poultry and pigs (1). Intensive production systems are common in dairy cattle, while extensive grazing is common in beef cattle (2). The main challenge to reaching profitability for both production systems is achieving the largest quantity of calves per bred cow in a year. However, the efficiency in most livestock systems often falls below the level of expectation due to bovine reproductive failure (3). Economic losses induced by bovine abortion and perinatal mortality are associated with costs of lost milk and beef production due to longer calving intervals, decreased average weight in calves, loss of offspring from aborted cows, costs of replacement cows, costs due to losing genetic improvement, and veterinarian costs associated with sanitary and reproductive treatments (4, 5). In addition, these losses lead to animal welfare and societal concerns (6).
Reproductive failure is common in the livestock industry; the prevalences of abortion and perinatal mortality range from 5 to 12% and from 2 to 5%, respectively (5, 7). Bovine abortion and perinatal mortality have multifactorial origins, and many factors can influence viability, such as hormonal fluctuations, genetic abnormalities, and exposure to pharmacologic, environmental, toxic, or infectious agents. Among them, infectious agents play an important role. In addition, many of the agents responsible for bovine reproductive failure have zoonotic potential, such as Brucella abortus, Campylobacter spp., Listeria monocytogenes and Coxiella burnetii (8). Consequently, these infectious agents are of importance in both public health and the livestock industry, and strategies to reduce reproductive infections should be based on close collaboration between both medics and veterinarians in accordance with the One Health approach (9). However, the aetiological diagnosis efficacy of these reproductive losses is still below 50% and does not appear to have improved over time despite the development of new diagnostic techniques (4, 6, 7, 10). Recently, a meta-analysis quantified reproductive losses during early pregnancy in beef cattle and the main associated factors (3). However, no updated information is available on the global situation of the main causes of the bovine infectious abortion. Therefore, the aim of the present study was to carry out a systematic review of the scientific literature and a meta-analysis of the prevalence of the main infectious agents involved in bovine abortion worldwide between 2000 and 2022.
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyzes (PRISMA) guidelines (PRISMA Transparent Reporting of Systematic Reviews and Meta-Analysis) (11). We searched the PubMed1 and Scopus2 databases from January 1, 2000, to December 31, 2022 to retrieve relevant studies. Search terms included a combination of words “bovine” or “cattle” and “abortion.”
A series of inclusion and exclusion criteria were established, and the reference lists of all included articles were manually searched for potentially eligible literature. The inclusion criteria were as follows: 1) full-text articles available online in English or Spanish languages, 2) studies examining infectious causes of abortion, 3) studies examining at least one infectious agent as a possible foetal death cause, 4) beginning and end date of study implementation should be mentioned, and 5) number of abortion cases in the study is specified. On the other hand, all the studies that did not include the above-mentioned criteria, review articles, systematic analyzes, and experimental infection studies were excluded. In addition, seroprevalence studies in which only serological tests were performed or studies of an outbreak case were also excluded. Furthermore, duplicates and articles that did not include in the title the words “bovine,” “cattle” or “ruminant,” and “abortion/aborted” or “reproductive failure” were eliminated. Then, the abstracts of the remaining studies were screened. Subsequently, the full texts of the potentially eligible studies were screened against the inclusion criteria.
Full text articles were examined and assessed for eligibility by two independent researchers (YPH and SGO). Discrepancies between the authors at any stage of the selection process were resolved by consulting a third and fourth reviewer (PHI, LMOM). Relevant data were extracted from text and placed into purpose-built tables using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, United States). The required information was extracted for each study, including name of the first author, country of the study, publication year, abortion moment (1st, 2nd, or 3rd trimester); type of production system (dairy or beef); number of analyzed farms; cattle management system (intensive or extensive); history of reproductive failures; type of samples analyzed and sample size (the number of examined animals), infectious pathogens analyzed, diagnostic methods and results, final diagnosis; and any additional data that could be of interest for the study.
In the present study, the quality of articles was assessed using a modified version of the Newcastle–Ottawa Scale (NOS) (12). This quality scale ranges from 0 to 9 points, and higher scores indicate better quality studies. Only articles with acceptable quality (≥4) were included in this study.
All analyzes were carried out using the “SPSS v. 28” and “Open Meta-Analyst 10.12” programs. First, an analysis of the global prevalence of infectious agents related to bovine abortion was performed. All studies that diagnosed a wide panel of agents (viruses, bacteria, protozoa and/or fungi) were included in this analysis. Using the website http://mapinseconds.com, a map that represents the number of cases analyzed in the different countries was created. Second, a meta-analysis was carried out to determine the prevalence of the main infectious agent in bovine abortion. In all cases a random effects model, since high heterogeneity between studies was expected, and confidence levels of 95% were applied.
The degree of heterogeneity between the analyzed studies was determined using the I2 statistic. Heterogeneity was considered low if the I2 value was less than 25%; average heterogeneity was indicated by an I2 value of approximately 50%, and high heterogeneity was indicated by an I2 value above 75% (13). Forest plots were used to show the results of each study and the heterogeneity among studies. For the analysis of the prevalence of infectious agents related to bovine abortion, due to the high rate of heterogeneity obtained, sensitivity analysis was performed using the LOOCV (leave-one-out cross-validation) method. This was performed to exclude small studies with extreme effect sizes that could affect the overall results. To determine if publication bias could affect the estimated prevalence, a funnel plot and Egger’s test were used.
To determine the influence of the diagnostic method on the high heterogeneity, subgroup analyzes were performed, and significant differences between groups were analyzed by using a chi-square test. p values <0.05 indicated statistical significance and the existence of a relationship between the diagnostic method used and the prevalence obtained (13). Sensitivity analysis was also performed using the LOOCV (leave-one-out cross-validation) method. Finally, we performed a meta-regression to evaluate the effect of the publication year of studies on the prevalence estimates by adding the publication year as a variable to the simple model regression.
The search of two databases (PubMed and Scopus) yielded 6,539 articles; 3,739 articles remained after removing duplicates. Following an initial screening based on titles and abstracts, 3,206 studies were excluded because they did not meet the inclusion criteria. Next, the remaining 533 full-text articles were assessed for eligibility. Ultimately, 76 of these articles met the inclusion criteria and were included in the meta-analysis (Figure 1). Information and characteristics of the included articles are presented in Table 1 (4, 7, 14–87). The quality assessment of studies with the NOS checklist indicated that the articles included in this meta-analysis were of acceptable quality (≥4 for each study) (Supplementary Table S1).
The included articles were published from 2000 to 2022, and all articles and all studies that examined one or more infectious agents in bovine abortion cases during this period were included. Overall, the selected works came from 4 continents, although not in the same proportion (Supplementary Figure S1). There were 32 studies from Europe (Belgium:4; Cyprus:1; Denmark:1; Finland:1; Hungary:4; Italy:2; Latvia:1; Netherland:1; Portugal:1; Scotland:2; Serbia:1; Spain:2; Switzerland:4; Turkey:5; United Kingdom:2), 23 studies from North and South America (Argentina:4; Brazil:10; Canada:2; Chile:1; Mexico:2; Peru:1; Uruguay:1; United States:2), 16 studies from Asia (China:3; Iran:11; Israel:1; Republic of Korea:1), and 5 studies from Africa (Algeria:2; Rwanda:1; South Africa:1; Tanzania:1). In 30 of the 76 articles, information about the time of gestation in which reproductive failure occurred was provided. Out of 11,376 aborted foetuses, 4.9% examined cases from 1st–2nd trimester of pregnancy (n = 553), 72.4% from the 2nd–3rd trimester (n = 8,232) and 22.8% from the 1st–2nd–3rd trimester (n = 2,591), without specifying the exact number in each trimester. Moreover, 32 (n = 8,576) of the 76 articles included the information about the type of herd production system (dairy or beef herds) from which the analyzed samples proceeded. Of these studies, 24.1% of cases (n = 2,064) were collected exclusively from dairy herds and 75.9% (n = 6,512) collected from dairy and beef herds without specifying the number of aborted specimens from each farm.
In relation to the analyzed samples, most of them were composed only of foetal tissues (41 studies with 10,700 cases). Only 26 studies (6,905 cases) included foetuses and some of their placentas for the diagnosis of abortion, although the exact number of placentas was not specified. In 5 studies (426 cases), the placenta was the only sample analyzed, in 4 studies (790 cases) only abomasal fluid was studied, and in one study, both placenta and abomasal fluid samples (249 cases) were investigated. A high or moderate grade of autolysis was mentioned in 17 studies in which some foetal tissues or placenta were not available for histopathological examination. In the same way, secondary contaminations that made the diagnosis difficult were mentioned in three studies.
Although 76 articles were included in this meta-analysis, only 27 were selected to determine the prevalence of infectious agents related to bovine abortion. These articles included in their analysis the detection of a wide panel of viral, bacterial, protozoan and/or fungal agents to reach the diagnosis of abortion. The other studies (n = 49) were excluded from this analysis because their aims were to study the implication of some specific agents in bovine abortion. A total of 10,667 aborted bovine foetuses from 17 countries from America, Europe, Africa, and Asia were examined to detect infections that caused abortion.
The global estimated prevalence of infectious agents that caused bovine abortion based on the random effects model was 45.7% (95% CI, 38.6–52.8%) (Figure 2). The heterogeneity among studies was high (I2 = 99.2%, p < 0.001); however, Egger’s test showed that there was no publication bias in the overall prevalence estimated (p = 0.239). This fact could be graphically observed in the funnel plot (Figure 3). In addition, the results of the LOOCV method showed that the overall estimated prevalence was not influenced by any specific study (Figure 4). Finally, we assessed the effect of the year of publication on the estimated prevalence of infectious agents related to bovine abortion, and although there was not a significant effect (coefficient = 0.006; p = 0.199), an increasing trend was observed over time (Figure 5).

Figure 2. Forest plot of the worldwide prevalence of infectious agents related to bovine abortion. The blue square is the point estimate, and the horizontal line is the 95% confidence interval (CI) for prevalence plotted for each dataset. The left columns show the bibliographic reference for each dataset, the prevalence, the standard error, 95% CI from each dataset, the p value and the weight of the study related to the global estimate. The green diamond at the bottom of the forest plot is the worldwide prevalence of infectious agents related to bovine abortion.
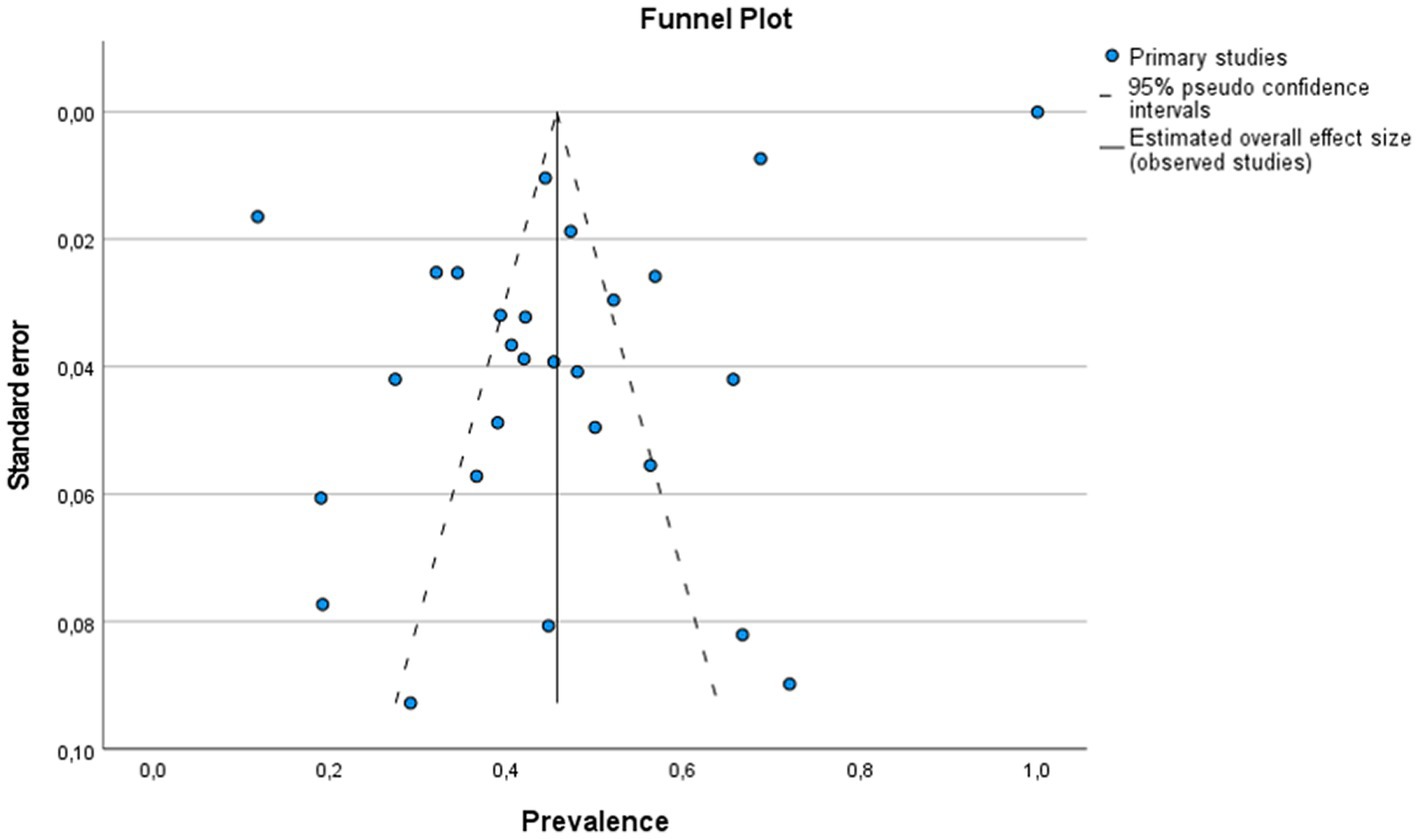
Figure 3. Funnel plot to detect publication bias in studies showing the prevalence of infectious agents on bovine infectious abortion. The circles represent the prevalence in each analyzed study. The continuous lines represent the overall prevalence of all analyzed studies. The prevalence of bovine infectious abortion is plotted on the horizontal axis. The standard error of each study is plotted on the vertical axis.
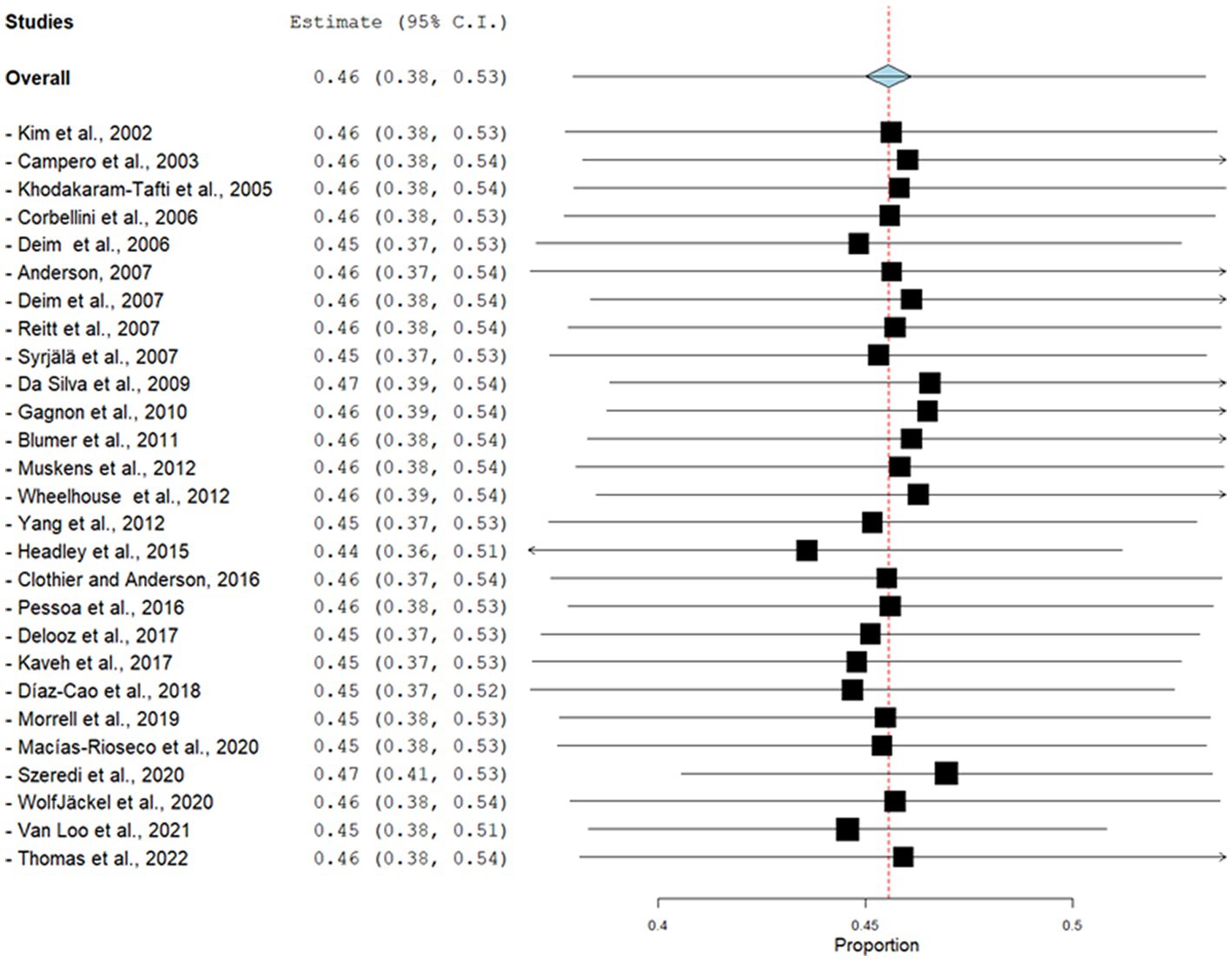
Figure 4. Forest plot of the results of the analysis using the leave-one-out cross-validation (LOOCV) method to determine the sensitivity of the meta-analysis of the estimated prevalence of infectious agents related to bovine abortion. The black square is the point estimate, and the horizontal line is the 95% confidence interval (CI) for prevalence plotted for each dataset. The left columns show the bibliographic reference, the prevalence and the 95% CI from each dataset. The blue diamond at the bottom of the forest plot is a worldwide-pooled prevalence of infectious agents related to bovine abortion.
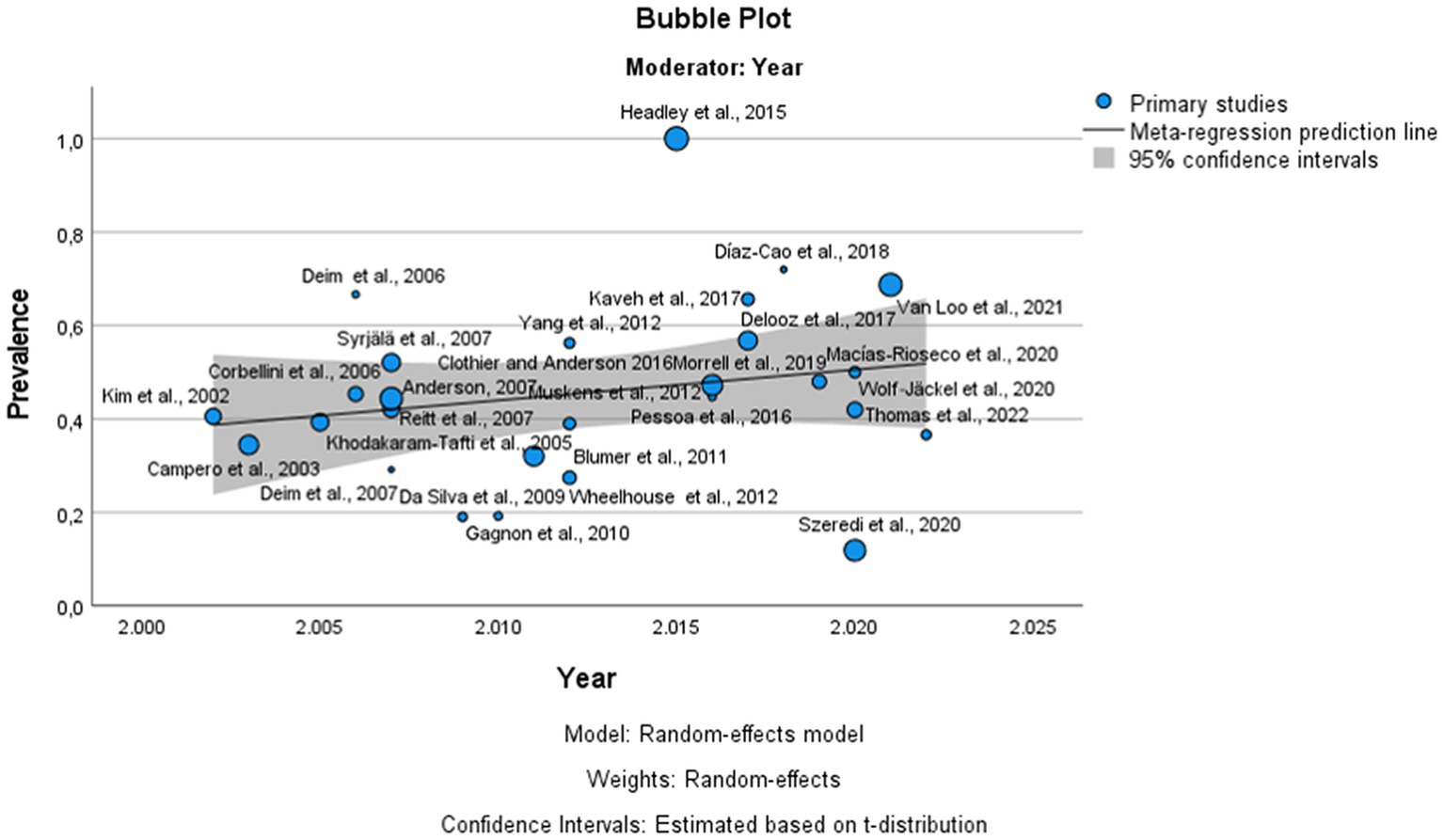
Figure 5. Bubble plot of the results of the meta-regression of published articles against the prevalence of bovine infectious abortion. The circles represent the individual studies. The continuous lines represent the regression lines. The year of publication is plotted on the horizontal axis. The prevalence of bovine infectious abortion is plotted on the vertical axis.
To determine the prevalence of the different transmissible agents in bovine abortion, studies in which some laboratory tests were performed (culture or polymerase chain reaction -PCR- or foetal serology or immunohistochemistry -IHC-) to detect a specific agent (on aborted foetus or placenta) were selected. However, in this analysis were excluded studies in which diagnosis of the abortion cause was made using only maternal serology. On the other hand, the detection of an agent in abortion samples does not confirm its participation in the production of abortion. Therefore, in this study only in the cases in which specific histopathological changes were reported, it was the cause of the abortion considered confirmed. From the 19,070 cases of bovine abortion included in the 76 selected studies, in 7,319 specimens some infectious agents were detected, but only 54.4% (3,977 cases) were confirmed as the cause of foetal death.
According to this meta-analysis, Neospora caninum was the most common infectious agent found in cases of bovine abortion, with an estimated prevalence of 22.2% (95% CI: 17.6–26.8) from 9,164 aborted bovine foetuses examined (Supplementary Figure S2a). However, the contribution of this protozoan parasite to foetal death was only confirmed in 16.7% (95% CI: 12.3–21.1) of the analyzed specimens when histopathological changes were found (Table 2).
The presence of sporadically isolated or opportunistic bacteria (Salmonella spp. Escherichia coli, Trueperella pyogenes, Bacillus licheniformis, Pajaroellobacter abortibovis, Acinetobacter spp., Histophilus somni, Actinomyces spp., Aeromona spp., Bordetella spp., Cardiobacterium spp., Klebsiella spp., Enterobacter spp., Pasteurella spp., Staphylococcus spp., Streptococcus spp., Pseudomona spp., and Yersinia paratuberculosis) was observed in 21.4% (95% CI: 10.4–32.5) of the 9,824 analyzed cases from 18 articles (Supplementary Figure S2b). In twelve of these studies (4,997 cases), the authors also searched for histopathological compatible changes, confirming some of these bacteria as causes of foetal death in 12.6% (95% CI: 7.9–17.3) (Table 2).
In third place, the prevalence of the Chlamydiaceae family was 10.9% (95% CI: 4.2–17.7) of 2,651 aborted foetuses (Supplementary Figure S2c). The detection of its infection was mostly carried out by PCR, and only compatible lesions were reported in 6.8% (95% CI: 0.0–13.5) of the cases (Table 2). The specific Chlamydia species were only reported in 10 studies (1,817 cases) mentioning the presence of C. abortus (90 cases), C. psittaci (10 cases), C. suis (1 case) and C. pecorum (1 case). In addition, five studies mentioned the detection of Parachlamydia spp., although its role as an abortigenic agent in these studies could not be confirmed (absence of compatible lesions). On the other hand, the estimated prevalence of Coxiella burnetii in bovine abortion was 9.5% (95% CI: 3.7–15.3) in the analyzed cases (n = 7,987) (Supplementary Figure S2d), and this agent was detected mostly by PCR (Table 2). Interestingly, this bacterium was confirmed as the cause of abortion (compatible histopathological changes) in only 1.1% (95% CI: −0.1–2.3) of the analyzed cases (n = 2,008).
Infection with Leptospira spp. was detected in 5.2% (95% CI: 1.7–8.6) of 5,522 aborted foetuses from 17 studies (Supplementary Figure S2e), but in any selected study was mentioned the Leptospira species found in the specimen analyzed. This spirochete was detected mostly by PCR and histopathology (HP) and was confirmed as a cause of death in 5.2% (95% CI: 0.5–9.9) of the cases (Table 2). In addition, the search of Brucella spp. was made in 4,238 specimens from 15 studies, yielding a prevalence rate of 5.1% (95% CI: 1.5–8.6) (Supplementary Figure S2f), although only it was confirmed as a cause of abortion in 3.2% (95% CI: 0.2–6.2). Only five studies (556 cases) detected Brucella spp., including the presence of B. abortus (39/556) and B. melitensis (22/556). Remarkably, the presence of Campylobacter spp. was detected in 1.3% (95% CI: 0.2–2.3) of 5,312 abortion cases (Supplementary Figure S2g) and only in 7 studies (1,805 cases) was the species of Campylobacter specified. The species found were C. fetus (21/1,805), C. fetus subsp. fetus (17/1,805) and C. fetus subsp. venerealis (10/1,805) and C. jejuni (4/1,805). The final diagnosis of campylobacteriosis was confirmed in 2.3% (95% CI: 0.3–4.3) of foetal tissues referred for pathological diagnosis (4,218 cases). Listeria monocytogenes was detected in 2.6% (95% CI: 0.5–5.2) of the 9,103 analyzed cases (Supplementary Figure S2h). On the other hand, Tritrichomonas foetus was detected in 2.3% (95% CI: −0.1–4.7) of 608 cases, although only five included studies reported the presence of this protozoa (Supplementary Figure S2i). Finally, the diagnosis of fungi as causal agents of bovine reproductive failure was investigated in 10,216 cases from 19 studies. The participation of these agents in reproductive bovine failure was 3.4% (95% CI: 1.5–5.3) (Supplementary Figure S2j), with different species of Aspergillus being the most commonly diagnosed. In fourteen of these studies (5,341 cases), the authors also searched for histopathological compatible changes, confirming some fungi as causes of foetal death in 1.4% (95% CI: 0.7–2.0) (Table 2).
In the case of viral pathogens, the most commonly involved in bovine reproductive failure were bovine herpes virus type 1 (BoHV-1) and bovine viral diarrhea virus (BVDV) (Table 2). The estimated prevalence of BoHV-1 was 5.9% (95% CI: 1.5–10.2) from 6,006 total cases (Supplementary Figure S2k) and histopathological changes were found in 0.9% (95% CI: 0.2–1.5) of the cases submitted for histopathological examination (n = 5,496). In the case of BVDV, the prevalence rate was 4.7% (95% CI: 2.7–6.6) among the 11,348 analyzed cases (Supplementary Figure S2l) and the diagnosis was confirmed in 1.9% (95% CI: 1.0–2.9) of foetal samples analyzed by histopathology (n = 5,232). For both viral pathogens, the viral antigen was detected mostly using PCR or direct ELISA or direct fluorescent antibody tests (DFAT). In addition, in four studies, the presence of BoHV-4 DNA was reported in aborted foetuses, although a clear link between this viral infection and abortion was not provided.
For all pathogens, the heterogeneity among studies was high (Table 2). However, the subgroup analysis based on the diagnostic method showed that regardless of the technique used for its analysis, the heterogeneity remained high, discarding this as the cause of such heterogeneity (Supplementary Figure S3). In addition, the results of the LOOCV method showed that the overall estimated prevalence for each infectious agent was not influenced by any specific study (Supplementary Figure S4). Finally, there was no effect of study year on the prevalence of all pathogens (p > 0.05) (Supplementary Figure S5).
Reproductive failure causes a major deleterious impact on cattle operations, potentially resulting in large scale economic losses. In this context, infectious abortion plays a key role because the infectious diagnostic rate in bovine abortion can vary between 32 and 58% (88). The aim of the present study was to estimate the general prevalence of bovine infectious abortion and the relative importance of the main infectious agents involved worldwide. After carrying out a systematic review of the available literature, we found a lack of studies that met our inclusion criteria in countries such as Australia that, together with United States, Argentina, China, and Brazil, represent over 50% of the productive cattle stock worldwide (1). Thus, the data compiled in our study are mainly focused on certain areas of the world for which there are high-quality data, although some countries that are important livestock producers are not represented herein. It is well known that the prevalence of an infectious agent related to abortions is influenced by the region, climatic differences, management measures in each farm and health program implemented (5, 25). Therefore, it is essential to increase the number of studies on bovine abortion to have better understand its prevalence and causes, thereby enabling the development of specific control strategies to reduce its impact on the efficiency of bovine production systems. In addition, it is important to consider that the studies included in this meta-analysis are based on samples submitted by convenience to the diagnostic laboratory. Therefore, the lack of random sampling in the selected studies could bias the results of the present meta-analysis. However, due to the nature of this type of studies it is the most common way to obtain samples for analysis (when abortions occur under field conditions, only some foetuses are recovered and sent to the diagnostic laboratory, and only some of them are in good conditions to be analyzed).
In the analysis of the prevalence of bovine infectious abortion, it was only possible to include data from 17 countries belonging to America, Europe, Africa and Asia based on the inclusion criteria of this meta-analysis; thus, as commented before, this work has been limited to the study of the prevalence in the areas analyzed. The results revealed that the global prevalence of infectious agents related to bovine abortion was 45.7%, which is consistent with previously reported results by other authors that mentioned a prevalence between 25 and 50% (7, 88). However, this estimated prevalence was higher than those reported by Kirkbride (89) and Campero et al. (17). Reasons for the difference in prevalence could be due to the geographical origin of samples, differences in the management of production systems in the different studies, differences in the type of analyzed samples, and the use of different diagnostic methods. Possibly, the aforementioned could also be the causes of the heterogeneity observed in this analysis (I2 = 99.2, p < 0.001) because the publication bias analysis showed that the prevalence of bovine infectious abortion was not affected by the number of samples analyzed in each study (p = 0.239). In addition, it has been mentioned that the implementation of new techniques for the diagnosis of infectious agents related to bovine abortion could be responsible for the higher prevalence in the most recent reports (4, 5, 7). In the present study, the meta-regression did not show a significant effect of the study year on the prevalence estimate of infectious agents related to bovine abortion, although an increasing trend was observed over time (Figure 5).
Among the analyzed agents, N. caninum was the agent with the highest prevalence (22.2%). Previous studies have mentioned N. caninum as one of the main transmissible agents responsible for bovine abortion, probably due to the high efficiency of vertical transmission of this protozoan parasite (90). However, although some infected cows could abort after transplacental transmission, many foetal infections produce only congenitally infected offspring. In the present meta-analysis, N. caninum was confirmed as the cause of bovine abortion in 16.7% of 6,769 specimens that were submitted to histopathological studies in which compatible lesions were found in foetal brain or in other target tissues like heart, lung, tongue, or placenta. The authors detected that in some studies, the interpretation of the laboratory results was not correct, establishing, for example, a definitive diagnosis based on the detection of maternal antibodies against N. caninum (80). These studies were excluded of our analysis. Only studies in which the parasite was detected in the foetus, or its placenta were included. This fact shows that there are still deficiencies in the interpretation of laboratory results that could contribute to further reducing diagnostic efficiency.
The prevalence of opportunistic bacteria was estimated in 21% of 9,824 analyzed samples, including bacteria in this group that are sporadically involved as causal agents of abortions, such as Salmonella spp. E. coli, T. pyogenes, B. licheniformis and P. abortibovis, the cause of Epizootic Bovine Abortion. The latter is an important bovine reproductive pathogen in cattle grazing foothill rangelands from United States but has not been reported in other geographical areas (4, 25). Nevertheless, the isolation of these agents does not confirm them as the cause of the abortion because many of them are common inhabitants of the cow reproductive tract or can be an accidental contamination of the sample (25). In the present study, autolysis or secondary contamination of some samples or incorrect sample submission (especially nonsubmission of the placenta) was mentioned in 20 of the 76 analyzed studies. Only 36.21% of 19,070 analyzed cases were sending the foetuses with some of their placenta. The placenta is not usually collected for diagnosis because 1) might be retained in some abortions, 2) placentophagy, 3) presence of predatory animals that feed on the placenta, or 4) inability to recover the placenta in abortion cases occurring in extensive field conditions. However, the placenta is a fundamental organ in the detection of certain agents, such as Chlamydia spp., C. burnetii, B. lichenifomis or fungi (4, 5, 25, 91). Therefore, the non-inclusion of this tissue in diagnosis could contribute to underestimating the importance of some agents in abortion production.
In this work, infection with bacteria of the Chlamydiaceae family was detected in 10.9% of the analyzed samples, while some studies have described a prevalence higher than 40% (26, 39). On the other hand, the prevalence of C. burnetii detected in the present study was 9.5% of 7,987 samples analyzed, although in most cases, only infection with this agent was detected by PCR. In fact, its diagnosis was confirmed only in 1.1% of cases submitted to the pathology laboratory. Agerholm (92) mentioned that confirmation of an association between lesions and the presence of the organism is mandatory to confirm C. burnetii as the cause of foetal disease. Additionally, the PCR technique is commonly used for the detection of other agents, such as BoHV-1, Leptospira spp., Chlamydia spp., or N. caninum (7, 25, 93, 94). This diagnostic technique is chosen in certain cases where traditional diagnostic methods are laborious and costly. Nonetheless, a positive PCR result indicates the presence of the analyzed infectious agent, but it does not confirm that this infection was the cause of the abortion.
In addition, six studies mentioned the presence of the related Chlamydia-like organisms Parachlamydia spp., Rhabdochlamydia spp. or Waddlia spp. However, they were identified in combination with other more extensively characterized infectious agents (26, 36, 37, 56, 63). The presence of these microorganisms could be associated with contamination of the placental tissues during parturition with environmental Parachlamydia spp. (49). A similar situation occurred with the detection of BoHV-4 in four studies that reported the presence of DNA of this virus in the analyzed specimens (22, 27, 57, 61). Previous studies hypothesized that BoHV-4 induces immunosuppression that could enhance the proliferation of opportunistic bacteria, thus inducing reproductive failure (61, 95). However, the role of BoHV-4 as an aetiological agent of bovine abortion needs further study.
On the other hand, the presence of BoHV-1 and BVDV was analyzed in the included studies, and similar prevalence rates (between 5 and 6%) and final diagnosis rates (between 1 and 2%) were observed for both viral pathogens, consistent with previous reports (4, 7, 17, 72). Although abortion induced by BoHV-1 is only a sequel to respiratory infection and viremia, some authors mentioned that BoHV-1 was responsible for abortion in dairy herds in 36.3% of the cases analyzed (50). However, our results agree with other studies that mentioned a low prevalence of this virus in bovine abortions (45, 96), probably because in some countries eradication efforts have been successful.
Otherwise, foetal infection with BVDV is a common finding, however its importance as cause of abortion must be carefully considered. The outcomes of its infection during gestation depend on the moment of the infection and the biotype involved (5, 25). An important epidemiological aspect of foetal BVDV infection is that infections in foetuses prior to 4 months of gestation with a non-cytopathic BVDV can result in persistently infected (PI) live offspring that although it does not present antibodies, it is continuously eliminating the virus being a major source of infection for other cattle. In addition, foetal infections after 4 months of gestation often result in the development of a foetal immune response, with development of specific foetal antibodies, and these infections cannot result in abortions although the virus antigen is detected. Therefore, the importance of this virus as cause of abortion may be overestimated as the virus may infect the foetus without causing its death and there are not specific foetal lesions attributed to infection making accurate diagnosis difficult. Therefore, to confirm BVDV as the cause of abortion, the diagnosis of viral infection needs to be combined with the herd history. The prevalence of BVDV-positive specimens has been decreasing in the most recent studies as eradication and vaccination programs progress in different countries (88). In the present meta-analysis, the heterogeneity among the studies diagnosing BVDV was high, and one of the reasons for this was the different national BVDV control context because the subgroup analysis showed that said heterogeneity could not be associated with the techniques used to make the diagnosis.
In the present study, most of the analyzed foetuses recovered during the 2nd and 3rd (72.36%) trimesters of gestation. This situation has been previously mentioned by others (5) because during the first trimester of pregnancy, most foetal deaths go unnoticed, and abortion in extensive grazing conditions is more difficult to return to the laboratory. This fact could influence the prevalence obtained for certain agents that could be underestimated. This is the case for T. foetus, Campylobacter spp. or BVDV (6). In the present meta-analysis study, infection with T. foetus was estimated in 2.3% of the 608 studied cases, while for C. fetus, it was estimated in 1.3% of the 5,312 aborted cases, in agreement with the prevalence obtained in previous studies (7, 50). It is important to consider that the prevalence of these agents can also be influenced by the biotype animal and management conditions of each farm, which are possible causes of heterogeneity found in the analysis of these pathogens.
In conclusion, the results of this meta-analysis showed that the prevalence of infectious agents related to bovine abortion was approximately 50% of the analyzed cases. According to this study, N. caninum was the most commonly detected agent in bovine abortion, followed by opportunistic bacteria, the Chlamydiaceae family and C. burnetii, although the last two bacteria in most cases only infection was determined by PCR. Although the application of new techniques has improved the identification of infectious agents in abortions, the diagnosis of transmissible bovine abortion remains often incomplete and in some cases is only based on the detection of the agent and/or serological analyzes. An enhanced diagnostic is the key to establishing specific control strategies to reduce the impact of abortifacient agents on the efficiency of bovine productive systems.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
PH and LOM conceived the study. YH, PH, and LOM coordinated and organized the systematic review and reviewed and edited the manuscript. YH and SGO surveyed the literature and extracted and reviewed the data. YH, SGO, PH, and LOM collected and selected the articles. YH drafted the manuscript and designed tables and figures. SC and YH performed the statistical analysis. All authors contributed to the article and approved the submitted version.
This research was supported by the projects funded by the Spanish Ministry of Science and Innovation (PID2019-104713RB-C21) and the Community of Madrid (PLATESA2-CM-P2018/BAA-4370). YH is funded by the 2023–2025 postdoctoral fellowship “Sello de Excelencia Instituto de Salud Carlos III (ISCIII-Health)” (IHMC22/00014) that is supported by funding from Next Generation EU program (Plan de Recuperación, Transformación y Resiliencia).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1249410/full#supplementary-material
2. Thornton, PK . Livestock production: recent trends, future prospects. Philos Trans R Soc Lond Ser B Biol Sci. (2010) 365:2853–67. doi: 10.1098/rstb.2010.0134
3. Reese, ST, Franco, GA, Poole, RK, Hood, R, Fernadez Montero, L, Oliveira Filho, RV, et al. Pregnancy loss in beef cattle: a meta-analysis. Anim Reprod Sci. (2020) 212:106251. doi: 10.1016/j.anireprosci.2019.106251
4. Clothier, K, and Anderson, M. Evaluation of bovine abortion cases and tissue suitability for identification of infectious agents in California diagnostic laboratory cases from 2007 to 2012. Theriogenology. (2016) 85:933–8. doi: 10.1016/j.theriogenology.2015.11.001
5. Campero, C, Cantón, G, and Moore, P. Abortos y otras pérdidas reproductivas en bovinos diagnóstico y control. First ed. Buenos Aires, Argentina: Editorial Hemisferio Sur (2017).
6. Mee, JF . Investigation of bovine abortion and stillbirth/perinatal mortality - similar diagnostic challenges, different approaches. Ir Vet J. (2020) 73:20. doi: 10.1186/s13620-020-00172-0
7. Morrell, EL, Campero, CM, Cantón, GJ, Odeón, AC, Moore, DP, Odriozola, E, et al. Current trends in bovine abortion in Argentina. Pesq Vet Bras. (2019) 39:12–9. doi: 10.1590/1678-5150-PVB-5668
8. Baumgartner, W. Fetal disease and abortion, bovine reproduction. John Wiley & Sons, Ltd Hoboken, NJ (2021). p. 665–716.
9. Aguirre, AA, Beasley, VR, Augspurger, T, Benson, WH, Whaley, J, and Basu, N. One health—transdisciplinary opportunities for SETAC leadership in integrating and improving the health of people, animals, and the environment. Environ Toxicol Chem. (2016) 35:2383–91. doi: 10.1002/etc.3557
10. Pohler, KG, Franco, GA, Reese, ST, and Smith, MF. Chapter 3, Physiology and pregnancy of beef cattle In: FW Bazer, GC Lamb, and G Wu, editors. Animal Agriculture. Amsterdam: Elsevier (2020). 37–55.
11. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Modesti, PA, Reboldi, G, Cappuccio, FP, Agyemang, C, Remuzzi, G, Rapi, S, et al. Panethnic differences in blood pressure in Europe: a systematic review and Meta-analysis. PLoS One. (2016) 11:e0147601. doi: 10.1371/journal.pone.0147601
13. Frías-navarro, D, and Capítulo VI, M-I-BH. Revisiones sistemáticas: introducción al meta-análisis In: E Palmero , editor. Reforma estadística en Psicología. North Charleston, SC: Ediciones Palmero (2018). 151–68.
14. Schock, A, Buxton, D, Spence, JA, Low, JC, and Baird, A. Histopathological survey of aborted bovine fetuses in Scotland with special reference to Neospora caninum. Vet Rec. (2000) 147:687–8.
15. Corbellini, LG, Driemeier, D, Cruz, CFE, Gondim, LFP, and Wald, V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet Parasitol. (2002) 103:195–202. doi: 10.1016/s0304-4017(01)00600-8
16. Kim, J-H, Lee, J-K, Lee, B-C, Park, B-K, Yoo, H-S, Hwang, W-S, et al. Diagnostic survey of bovine abortion in Korea: with special emphasis on Neospora caninum. J Vet Med Sci. (2002) 64:1123–7. doi: 10.1292/jvms.64.1123
17. Campero, CM, Moore, DP, Odeón, AC, Cipolla, AL, and Odriozola, E. Aetiology of bovine abortion in Argentina. Vet Res Commun. (2003) 27:359–69. doi: 10.1023/A:1024754003432
18. Pereira-Bueno, J, Quintanilla-Gozalo, A, Pérez-Pérez, V, Espi-Felgueroso, A, Álvarez-García, G, Collantes-Fernández, E, et al. Evaluation by different diagnostic techniques of bovine abortion associated with Neospora caninum in Spain. Vet Parasitol. (2003) 111:143–52. doi: 10.1016/S0304-4017(02)00361-8
19. Khodakaram-Tafti, A, and Ikede, BO. A retrospective study of sporadic bovine abortions, stillbirths, and neonatal abnormalities in Atlantic Canada, from 1990 to 2001. Can Vet J. (2005) 46:635–7.
20. Takiuchi, E, Médici, KC, Alfieri, AF, and Alfieri, AA. Bovine herpesvirus type 1 abortions detected by a semi-nested PCR in Brazilian cattle herds. Res Vet Sci. (2005) 79:85–8. doi: 10.1016/j.rvsc.2004.11.005
21. Corbellini, LG, Pescador, CA, Frantz, F, Wunder, E, Steffen, D, Smith, DR, et al. Diagnostic survey of bovine abortion with special reference to Neospora caninum infection: importance, repeated abortion and concurrent infection in aborted fetuses in southern Brazil. Vet J. (2006) 172:114–20. doi: 10.1016/j.tvjl.2005.03.006
22. Deim, Z, Szeredi, L, Tompó, V, and Egyed, L. Detection of bovine herpesvirus 4 in aborted bovine placentas. Microb Pathog. (2006) 41:144–8. doi: 10.1016/j.micpath.2006.03.006
23. Medina, L, Cruz-Vázquez, C, Quezada, T, Morales, E, and García-Vázquez, Z. Survey of Neospora caninum infection by nested PCR in aborted fetuses from dairy farms in Aguascalientes, Mexico. Vet Parasitol. (2006) 136:187–91. doi: 10.1016/j.vetpar.2005.11.003
24. Parisi, A, Fraccalvieri, R, Cafiero, M, Miccolupo, A, Padalino, I, Montagna, C, et al. Diagnosis of Coxiella burnetii-related abortion in Italian domestic ruminants using single-tube nested PCR. Vet Microbiol. (2006) 118:101–6. doi: 10.1016/j.vetmic.2006.06.023
25. Anderson, ML . Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology. (2007) 68:474–86. doi: 10.1016/j.theriogenology.2007.04.001
26. Borel, N, Ruhl, S, Casson, N, Kaiser, C, Pospischil, A, and Greub, G. Parachlamydia spp. and related Chlamydia-like organisms and bovine abortion. Emerg Infect Dis. (2007) 13:1904–7. doi: 10.3201/eid1312.070655
27. Deim, Z, Szeredi, L, and Egyed, L. Detection of bovine herpesvirus 4 DNA in aborted bovine fetuses. Can J Vet Res. (2007) 71:226–9.
28. Pescador, CA, Corbellini, LG, Oliveira, EC, Raymundo, DL, and Driemeier, D. Histopathological and immunohistochemical aspects of Neospora caninum diagnosis in bovine aborted fetuses. Vet Parasitol. (2007) 150:159–63. doi: 10.1016/j.vetpar.2007.08.028
29. Razmi, GR, Maleki, M, Farzaneh, N, Talebkhan Garoussi, M, and Fallah, AH. First report of Neospora caninum-associated bovine abortion in Mashhad area, Iran. Parasitol Res. (2007) 100:755–7. doi: 10.1007/s00436-006-0325-6
30. Reitt, K, Hilbe, M, Voegtlin, A, Corboz, L, Haessig, M, and Pospischil, A. Aetiology of bovine abortion in Switzerland from 1986 to 1995 – a retrospective study with emphasis on detection of Neospora caninum and toxoplasma gondii by PCR. J Vet Med A Physiol Pathol Clin Med. (2007) 54:15–22. doi: 10.1111/j.1439-0442.2007.00913.x
31. Sadrebazzaz, A, Habibi, G, Haddadzadeh, H, and Ashrafi, J. Evaluation of bovine abortion associated with Neospora caninum by different diagnostic techniques in Mashhad, Iran. Parasitol Res. (2007) 100:1257–60. doi: 10.1007/s00436-006-0417-3
32. Syrjälä, P, Anttila, M, Dillard, K, Fossi, M, Collin, K, Nylund, M, et al. Causes of bovine abortion, stillbirth and neonatal death in Finland 1999–2006. Acta Vet Scand. (2007) 49:S3. doi: 10.1186/1751-0147-49-S1-S3
33. TMA Da, S, Oliveira, RG d, Mol, JP d, Silva, TMA, Oliveira, RG, Mol, JPS, et al. Etiologic diagnosis of bovine infectious abortion by PCR. Cienc Rural. (2009) 39:2563–70. doi: 10.1590/S0103-84782009000900028
34. Yao, L, Yang, N, Liu, Q, Wang, M, Zhang, W, Qian, WF, et al. Detection of Neospora caninum in aborted bovine fetuses and dam blood samples by nested PCR and ELISA and seroprevalence in Beijing and Tianjin, China. Parasitology. (2009) 136:1251–6. doi: 10.1017/S0031182009990813
35. Gagnon, CA, Allam, O, Drolet, R, and Tremblay, D. Quebec: detection of bovine lymphotropic herpesvirus DNA in tissues of a bovine aborted fetus. Can Vet J. (2010) 51:1021–2.
36. Wheelhouse, N, Katzer, F, Wright, F, and Longbottom, D. Novel Chlamydia-like organisms as cause of bovine abortions. Emerg Infect Dis. (2010) 16:1323–4. doi: 10.3201/eid1608.091878
37. Blumer, S, Greub, G, Waldvogel, A, Hässig, M, Thoma, R, Tschuor, A, et al. Waddlia, Parachlamydia and Chlamydiaceae in bovine abortion. Vet Microbiol. (2011) 152:385–93. doi: 10.1016/j.vetmic.2011.05.024
38. Cantas, H, Muwonge, A, Sareyyupoglu, B, Yardimci, H, and Skjerve, E. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Vet Res. (2011) 7:13. doi: 10.1186/1746-6148-7-13
39. Clemente, MLT, Barahona, MJB, Andrade, MFC, Botelho, AR, and Vicari, N. Diagnosis by PCR-REA of Chlamydophila species infections in late-term abortions of domestic ruminants. Vet Rec. (2011) 168:619–9. doi: 10.1136/vr.d889
40. Dos, SDS, Andrade, MP, Varaschin, MS, Guimarães, AM, and Hirsch, C. Neospora caninum in bovine fetuses of Minas Gerais, Brazil: genetic characteristics of rDNA. Rev Bras Parasitol Vet. (2011) 20:281–8. doi: 10.1590/S1984-29612011000400005
41. Ghalmi, F, China, B, Kaidi, R, and Losson, B. Neospora caninum is associated with abortion in Algerian cattle. J Parasitol. (2011) 97:1121–4. doi: 10.1645/GE-2861.1
42. Mazuz, M, Fish, L, Molad, T, Savitsky, I, Wolkomirsky, R, Leibovitz, B, et al. Neospora caninum as causative-pathogen of abortion in cattle. Isr J Vet Med. (2011) 66:14–8.
43. Safarpoor, DF . Prevalence study of bovine viral diarrhea virus by evaluation of antigen capture ELISA and RT-PCR assay in bovine, ovine, caprine, buffalo and camel aborted fetuses in Iran. AMB Express. (2011) 1:32. doi: 10.1186/2191-0855-1-32
44. Albayrak, H, Gumusova, SO, Ozan, E, and Yazici, Z. Molecular detection of pestiviruses in aborted foetuses from provinces in northern Turkey. Trop Anim Health Prod. (2012) 44:677–80. doi: 10.1007/s11250-011-9955-5
45. Crook, T, Benavides, J, Russell, G, Gilray, J, Maley, M, and Willoughby, K. Bovine herpesvirus 1 abortion: current prevalence in the United Kingdom and evidence of hematogenous spread within the fetus in natural cases. J Vet Diagn Investig. (2012) 24:662–70. doi: 10.1177/1040638712448187
46. Momtaz, H, and Moshkelani, S. Detection and characterization of Leptospira spp. isolated from aborted bovine clinical samples. Acta Vet Brno. (2012) 81:21–5. doi: 10.2754/avb201281010021
47. Muskens, J, Wouda, W, von Bannisseht-Wijsmuller, T, and van Maanen, C. Prevalence of Coxiella burnetii infections in aborted fetuses and stillborn calves. Vet Rec. (2012) 170:260–17. doi: 10.1136/vr.100378
48. Safarpoor Dehkordi, F, Momtaz, H, and Doosti, A. Application of real-time PCR for detection of aspergillus species in aborted ruminant foetuses. Bulg J Vet Med. (2012) 15:30–6.
49. Wheelhouse, N, Howie, F, Gidlow, J, Greub, G, Dagleish, M, and Longbottom, D. Involvement of Parachlamydia in bovine abortions in Scotland. Vet J. (2012) 193:586–8. doi: 10.1016/j.tvjl.2012.01.008
50. Yang, N, Cui, X, Qian, W, Yu, S, and Liu, Q. Survey of nine abortifacient infectious agents in aborted bovine fetuses from dairy farms in Beijing, China, by PCR. Acta Vet Hung. (2012) 60:83–92. doi: 10.1556/avet.2012.007
51. Guven, E, Bastem, Z, Avcioglu, H, and Erdem, H. Molecular determination of Tritrichomonas spp. in aborted bovine foetuses in eastern Anatolian region of Turkey. Vet Parasitol. (2013) 196:278–82. doi: 10.1016/j.vetpar.2013.03.031
52. Safarpoor Dehkordi, F, Haghighi, N, Momtaz, H, Salari Rafsanjani, M, and Momeni, M. Conventional vs real-time PCR for detection of bovine herpes virus type 1 in aborted bovine, buffalo and camel Foetuses. Bulg J Vet Med. (2013) 16:102–11.
53. Šteingolde, Ž, Avsejenko, J, and Berziņš, A. Overview of Listeria monocytogenes caused abortions in cattle in Latvia in 2013. S. Treija and S. Skujeniece. Research for Rural Development: Proceedings of the annual 20th International Scientific Conference; 2014 21-23 May; Jelgava, Latvia.
54. Kamali, A, Seifi, HA, Movassaghi, AR, Razmi, GR, and Naseri, Z. Histopathological and molecular study of Neospora caninum infection in bovine aborted fetuses. Asian Pac J Trop Biomed. (2014) 4:990–4. doi: 10.12980/APJTB.4.201414B378
55. Headley, SA, Voltarelli, D, de Oliveira, VHS, Bronkhorst, DE, Alfieri, AF, Filho, LCN, et al. Association of Histophilus somni with spontaneous abortions in dairy cattle herds from Brazil. Trop Anim Health Prod. (2015) 47:403–13. doi: 10.1007/s11250-014-0740-0
56. Kreizinger, Z, Szeredi, L, Bacsadi, Á, Nemes, C, Sugár, L, Varga, T, et al. Occurrence of Coxiella burnetii and Chlamydiales species in abortions of domestic ruminants and in wild ruminants in Hungary, Central Europe. J Vet Diagn Investig. (2015) 27:206–10. doi: 10.1177/1040638714563566
57. Cvetojević, Đ, Savić, B, Milićević, V, Kureljušić, B, Jezdimirović, N, Jakić-Dimić, D, et al. Prevalence of bovine herpesvirus type 4 in aborting dairy cows. Pol J Vet Sci. (2016) 19:731–6. doi: 10.1515/pjvs-2016-0092
58. Medina-Esparza, L, Regidor-Cerrillo, J, García-Ramos, D, Álvarez-García, G, Benavides, J, Ortega-Mora, LM, et al. Genetic characterization of Neospora caninum from aborted bovine foetuses in Aguascalientes, Mexico. Vet Parasitol. (2016) 228:183–7. doi: 10.1016/j.vetpar.2016.09.009
59. Pessoa, GA, Martini, AP, Trentin, JM, Dalcin, VC, Leonardi, CEP, Vogel, FSF, et al. Impact of spontaneous Neospora caninum infection on pregnancy loss and subsequent pregnancy in grazing lactating dairy cows. Theriogenology. (2016) 85:519–27. doi: 10.1016/j.theriogenology.2015.09.034
60. Barati, S, Moori-Bakhtiari, N, Najafabadi, MG, Momtaz, H, and Shokuhizadeh, L. The role of zoonotic chlamydial agents in ruminant abortion. Iran J Microbiol. (2017) 9:288–94.
61. Delooz, L, Czaplicki, G, Houtain, JY, Dal Pozzo, F, and Saegerman, C. Laboratory findings suggesting an association between BoHV-4 and bovine abortions in southern Belgium. Transbound Emerg Dis. (2017) 64:1100–9. doi: 10.1111/tbed.12469
62. Kaveh, AA, Merat, E, Samani, S, Danandeh, R, and Soltannezhad, S. Infectious causes of bovine abortion in Qazvin Province, Iran. Arch Razi Inst. (2017) 72:225–30. doi: 10.22092/ari.2017.113299
63. Vidal, S, Kegler, K, Greub, G, Aeby, S, Borel, N, Dagleish, MP, et al. Neglected zoonotic agents in cattle abortion: tackling the difficult to grow bacteria. BMC Vet Res. (2017) 13:373. doi: 10.1186/s12917-017-1294-y
64. Díaz Cao, JM, Prieto Lago, A, López Lorenzo, G, Díaz Fernández, P, López Sández, CM, Morrondo Pelayo, MP, et al. Broadening the diagnosis panel of reproductive pathogens associated with abortion in ruminants. Span J Agric Res. (2018) 16:e05SC01. doi: 10.5424/sjar/2018162-12180
65. Moroni, M, Navarro, M, Paredes, E, Romero, A, Alberdi, A, Lischinsky, T, et al. Identification of Neospora caninum in aborted bovine fetuses of southern Chile. Braz J Vet Pathol. (2018) 11:37–41. doi: 10.24070/bjvp.1983-0246.v11i2p37-41
66. Rahal, M, Tahir, D, Eldin, C, Bitam, I, Raoult, D, and Parola, P. Genotyping of Coxiella burnetii detected in placental tissues from aborted dairy cattle in the north of Algeria. Comp Immunol Microbiol Infect Dis. (2018) 57:50–4. doi: 10.1016/j.cimid.2018.06.001
67. Rojas, MDC, Fort, M, Bettermann, S, Entrocassi, C, Costamagna, SR, Sachse, K, et al. Detection of Chlamydia abortus in bovine reproductive losses in the province of La Pampa, Argentina. Rev Argent Microbiol. (2018) 50:269–74. doi: 10.1016/j.ram.2017.10.002
68. Açici, M, Bölükbaş, C, Pekmezci, G, Gürler, H, Genç, O, Gürler, A, et al. A diagnostic survey of Neospora caninum infection in aborted fetuses in the middle Black Sea region and Sivas Province, Turkey. Turk J Vet Anim Sci. (2019) 43:761–6. doi: 10.3906/vet-1908-16
69. Serrano-Martínez, ME, Cisterna, CAB, Romero, RCE, Huacho, MAQ, Bermabé, AM, and Albornoz, LAL. Evaluation of abortions spontaneously induced by Neospora caninum and risk factors in dairy cattle from Lima, Peru. Rev Bras Parasitol Vet. (2019) 28:215–20. doi: 10.1590/S1984-29612019026
70. Dorsch, MA, Regidor-Cerrillo, J, Ortega-Mora, LM, Scioli, MV, Morrell, EL, Cantón, GJ, et al. Microsatellite genotyping reveals extensive genetic diversity in bovine Neospora caninum from the humid Pampa region in Argentina. Parasitol Res. (2020) 119:4049–59. doi: 10.1007/s00436-020-06922-x
71. Grégoire, F, Bakinahe, R, Petitjean, T, Boarbi, S, Delooz, L, Fretin, D, et al. Laboratory diagnosis of bovine abortions caused by non-maintenance pathogenic Leptospira spp.: necropsy, serology and molecular study out of a Belgian experience. Pathogens. (2020) 9:413. doi: 10.3390/pathogens9060413
72. Macías-Rioseco, M, Silveira, C, Fraga, M, Casaux, L, Cabrera, A, Francia, ME, et al. Causes of abortion in dairy cows in Uruguay. Pesq Vet Bras. (2020) 40:325–32. doi: 10.1590/1678-5150-PVB-6550
73. Szeredi, L, Dán, Á, Malik, P, Jánosi, S, and Hornyák, Á. Low incidence of Schmallenberg virus infection in natural cases of abortion in domestic ruminants in Hungary. Acta Vet Hung. (2020) 68:105–11. doi: 10.1556/004.2020.00002
74. Wolf-Jäckel, GA, Hansen, MS, Larsen, G, Holm, E, Agerholm, JS, and Jensen, TK. Diagnostic studies of abortion in Danish cattle 2015-2017. Acta Vet Scand. (2020) 62:1. doi: 10.1186/s13028-019-0499-4
75. Zhang, H, Deng, X, Cui, B, Shao, Z, Zhao, X, Yang, Q, et al. Abortion and various associated risk factors in dairy cow and sheep in Ili, China. PLoS One. (2020) 15:e0232568. doi: 10.1371/journal.pone.0232568
76. Jonker, A, and Michel, A. Retrospective study of bacterial and fungal causes of abortion in domestic ruminants in northern regions of South Africa (2006–2016). Aust Vet J. (2021) 99:66–71. doi: 10.1111/avj.13035
77. Mohabati Mobarez, A, Khalili, M, Mostafavi, E, and Esmaeili, S. Molecular detection of Coxiella burnetii infection in aborted samples of domestic ruminants in Iran. PLoS One. (2021) 16:e0250116. doi: 10.1371/journal.pone.0250116
78. Salehi, B, Amouei, A, Dodangeh, S, Daryani, A, Sarvi, S, Safari-Kharyeki, MR, et al. Molecular identification of Neospora caninum infection in aborted fetuses of sheep, cattle, and goats in Mazandaran province, northern Iran. Iran J Parasitol. (2021) 16:483–9. doi: 10.18502/ijpa.v16i3.7102
79. Şevik, M . Genomic characterization of pestiviruses isolated from bovine, ovine and caprine foetuses in Turkey: a potentially new genotype of Pestivirus I species. Transbound Emerg Dis. (2021) 68:417–26. doi: 10.1111/tbed.13691
80. Van Loo, H, Pascottini, OB, Ribbens, S, Hooyberghs, J, Pardon, B, and Opsomer, G. Retrospective study of factors associated with bovine infectious abortion and perinatal mortality. Prev Vet Med. (2021) 191:105366. doi: 10.1016/j.prevetmed.2021.105366
81. Villa, L, Maksimov, P, Luttermann, C, Tuschy, M, Gazzonis, AL, Zanzani, SA, et al. Spatial distance between sites of sampling associated with genetic variation among Neospora caninum in aborted bovine foetuses from northern Italy. Parasit Vectors. (2021) 14:47. doi: 10.1186/s13071-020-04557-6
82. Mioni, MDSR, Henker, LC, WSR, T, Lorenzett, MP, Labruna, MB, Pavarini, SP, et al. Molecular detection of Coxiella burnetii in aborted bovine fetuses in Brazil. Acta Trop. (2022) 227:106258. doi: 10.1016/j.actatropica.2021.106258
83. Irehan, B, Sonmez, A, Atalay, MM, Ekinci, AI, Celik, F, Durmus, N, et al. Investigation of toxoplasma gondii, Neospora caninum and Tritrichomonas foetus in abortions of cattle, sheep and goats in Turkey: analysis by real-time PCR, conventional PCR and histopathological methods. Comp Immunol Microbiol Infect Dis. (2022) 89:101867. doi: 10.1016/j.cimid.2022.101867
84. Ntivuguruzwa, JB, Kolo, FB, Mwikarago, EI, and van Heerden, H. Characterization of Brucella spp. and other abortigenic pathogens from aborted tissues of cattle and goats in Rwanda. Vet Med Sci. (2022) 8:1655–63. doi: 10.1002/vms3.805
85. Saegerman, C, Grégoire, F, and Delooz, L. Diagnosis of Coxiella burnetii cattle abortion: a one-year observational study. Pathogens. (2022) 11:429. doi: 10.3390/pathogens11040429
86. Thomas, KM, Kibona, T, Claxton, JR, de Glanville, WA, Lankester, F, Amani, N, et al. Prospective cohort study reveals unexpected aetiologies of livestock abortion in northern Tanzania. Sci Rep. (2022) 12:11669. doi: 10.1038/s41598-022-15517-8
87. da Costa, LS, Withoeft, JA, Bilicki, JV, Melo, IC, Snak, A, das Neves, GB, et al. Neospora caninum-associated abortions in cattle from southern Brazil: Anatomopathological and molecular characterization. Vet Parasitol Reg Stud Reports. (2022) 36:100802. doi: 10.1016/j.vprsr.2022.100802
88. Mee, JF . Review-ESDAR 2023 invited review: bovine abortion - incidence, risk factors and causes. Reprod Domest Anim. (2023). doi: 10.1111/rda.14366
89. Kirkbride, CA . Etiologic agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Investig. (1992) 4:175–80. doi: 10.1177/104063879200400210
90. Lindsay, DS, and Dubey, JP. Neosporosis, toxoplasmosis, and Sarcocystosis in ruminants: an update. Vet Clin North Am Food Anim Pract. (2020) 36:205–22. doi: 10.1016/j.cvfa.2019.11.004
91. Agerholm, JS, Krogh, HV, and Jensen, HE. A retrospective study of bovine abortions associated with Bacillus licheniformis. J Veterinary Med Ser B. (1995) 42:225–34. doi: 10.1111/j.1439-0450.1995.tb00706.x
92. Agerholm, JS . Coxiella burnetii associated reproductive disorders in domestic animals--a critical review. Acta Vet Scand. (2013) 55:13. doi: 10.1186/1751-0147-55-13
93. Yoo, HS . Infectious causes of reproductive disorders in cattle. J Reprod Dev. (2010) 56:S53–60. doi: 10.1262/jrd.1056s53
94. Borel, N, Frey, CF, Gottstein, B, Hilbe, M, Pospischil, A, Franzoso, FD, et al. Laboratory diagnosis of ruminant abortion in Europe. Vet J. (2014) 200:218–29. doi: 10.1016/j.tvjl.2014.03.015
95. Donofrio, G, Cavirani, S, van Santen, V, and Flammini, CF. Potential secondary pathogenic role for bovine herpesvirus 4. J Clin Microbiol. (2005) 43:3421–6. doi: 10.1128/JCM.43.7.3421-3426.2005
Keywords: bovine abortion, infectious agents, prevalence, diagnosis, meta-analysis
Citation: Hecker YP, González-Ortega S, Cano S, Ortega-Mora LM and Horcajo P (2023) Bovine infectious abortion: a systematic review and meta-analysis. Front. Vet. Sci. 10:1249410. doi: 10.3389/fvets.2023.1249410
Received: 28 June 2023; Accepted: 18 September 2023;
Published: 29 September 2023.
Edited by:
Dirk Werling, Royal Veterinary College (RVC), United KingdomReviewed by:
Jørgen S. Agerholm, University of Copenhagen, DenmarkCopyright © 2023 Hecker, González-Ortega, Cano, Ortega-Mora and Horcajo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Miguel Ortega-Mora, bHVpcy5vcnRlZ2FAdWNtLmVz; Pilar Horcajo, cGhvcmNham9AdWNtLmVz
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.