
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci. , 11 October 2023
Sec. Veterinary Experimental and Diagnostic Pathology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1235110
Abomasal ulcers, an economic concern for all calf-raising farms, are usually silent until perforation occurs, at which time management is complicated and often unrewarding. This case study describes perforating ulcer in a 3-day-old Brahman heifer, occurring secondary to a congenital narrowing of the pylorus and proximal duodenum and leading to marked abomasal distention, leakage, and eventual peritonitis and sepsis.
Abomasal ulcers are lesions in the abomasum that penetrate the entire thickness of the mucosa and may extend through the submucosa and muscularis externa and reach the serosa. These may be single or multiple lesions (1, 2), and their size may range from a few millimeters to several centimeters (3). Abomasal ulcers in calves are classified into four types. Type 1 ulcers are non-perforating ulcers, with minimal intraluminal hemorrhage and local wall thickening and serositis, type 2 ulcers are non-perforating ulcers with severe intraluminal hemorrhage, type 3 ulcers are perforating ulcers with local, confined peritonitis, and type 4 ulcers are perforating ulcers with a generalized peritonitis after ingesta spills into the abdominal cavity (1, 4, 5). Ulcer management is complicated in calves and the causes are multifactorial.
Ante-mortem diagnosis is often challenging. Clinical signs may be absent, subtle, or severe, ranging from general signs of gastrointestinal discomfort such as non-specific abdominal pain, dehydration, anorexia, and hypomotility of the rumen (4, 6) to heavy bleeding or perforation of the abomasum, with signs of anemia, peritonitis, and death (7, 8). In dairy calves, abomasal ulcers are often inapparent and commonly identified in animals dying from other problems or at slaughter (1). They can represent 22% of losses in veal calves (9). At the time of slaughter, the prevalence of abomasal lesions in cows has been reported in the range of 11%−49% (6, 10, 11).
The factors contributing to the development of the abomasal ulcers are numerous, with stress factors topping the list (9, 12). Diet is also thought to play an important role, with many ulcers occurring at the transition from preruminant to ruminant digestion, i.e., at weaning (13). Other factors noted in the literature include low feeding frequency, feeding of abrasive agents, ingestion of stones, mineral deficiencies, notably copper, and administration of NSAIDs (12–16). Infections associated with some fungi and bacteria have also been associated with abomasal ulcers but likely invade the ulceration (17, 18). The case presented here was very unusual and occurred secondary to congenital pyloric and duodenal stenosis.
A 3-day-old Brahman heifer was presented for necropsy after written informed consent was obtained from the owner. History included failure to thrive and suspected sepsis that progressed to signs of abdominal distension and discomfort. The calf was treated at the University of Georgia Veterinary Teaching Hospital with a variety of therapeutic modalities, most notably intravenous fluid therapy, including dextrose supplementation, antibiotics, and non-steroidal anti-inflammatory drugs. Abomasal decompression was performed as well. However, in the absence of significant clinical improvement, the calf was humanely euthanized.
External examination at necropsy revealed a state of dehydration, with sunken eyes and tacky subcutaneous tissues. The umbilicus was dry and unremarkable. A distinct red line was present at the gingiva adjacent to the teeth (“toxic line”), indicating probable sepsis, as suspected clinically.
Internally, the most remarkable gross finding was a massively expanded, milk-filled abomasum. The expected size of the abomasum was 3–4X, and distinct plaques of fibrin were present at one area of the serosa along the greater curvature. Small amounts of fibrin were seen elsewhere in the abdominal cavity. The pyloric opening was markedly small (8 mm in diameter), and the proximal duodenum was similarly constricted, with a markedly decreased diameter compared with normal, for the proximal 20 cm (see Figure 1). Although data on the normal diameter of the pylorus in cattle could not be found, the figure for normal diameter in domestic cats is 9 mm (19). Milk was also present in the rumen. Multiple large joints were opened. A small amount of fibrin was evident at the occipito-atlanto articulation and in the limbs; there was marked peri-articular redness and edema and occasionally excessive and slightly turbid joint fluid. Aerobic culture from a joint fluid swab yielded no significant growth.
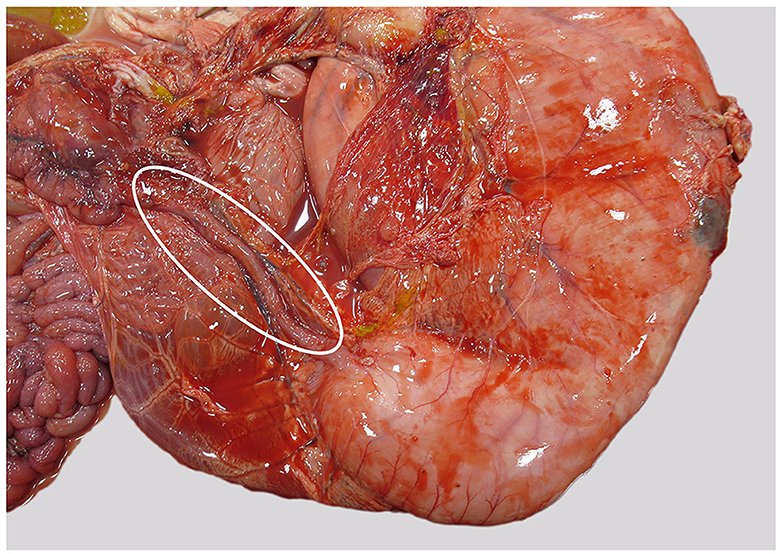
Figure 1. Abomasum and duodenum, as seen at necropsy. Duodenum is markedly narrowed (within oval), abomasum is distended, and fibrin is present along the greater curvature.
Histologically, the abomasum in a focal area showed a marked diminution of the tunica mucosa and scattered inflammatory cells, predominantly neutrophils, present throughout the submucosa and muscularis, both of which were markedly stretched and with abundant edema (see Figure 2). Subjacent to this, there were fibrin plaques with embedded neutrophils adherent to the serosa and also extending out beyond the serosa. Diffuse congestion and edema were present throughout the lungs. Scattered small aggregates of inflammatory cells, predominantly mononuclear, were present within some parts of the brain, especially in the midbrain. The tissue from the umbilicus was expanded by edema with scattered inflammatory cells, predominantly lymphocytes, and a few distinct clusters of neutrophils (presumed normal postpartum inflammation). Other examined tissues, including the intestines, kidney, bladder, thyroid/parathyroid, adrenal, liver, heart, and spleen, were all histologically unremarkable.
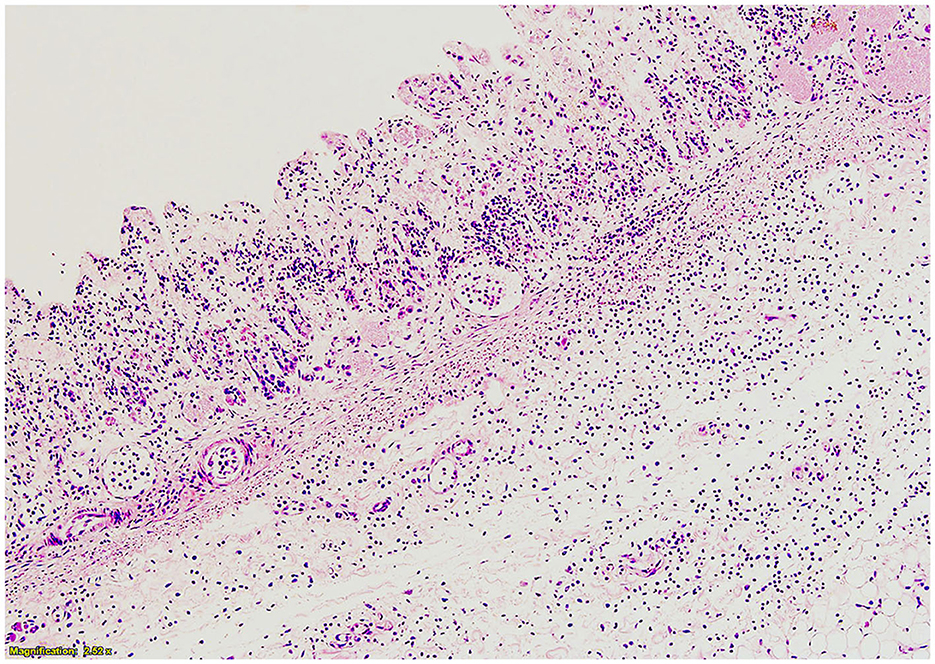
Figure 2. Histopathology of abomasum at greater curvature. Tunica mucosa is diminished, and neutrophils and edema are present throughout the submucosa.
The cause of this calf's clinical problem was presumed sepsis. The marked narrowing of pylorus and duodenum created difficulty in passage of milk, undoubtedly also contributing to the animal's decline. Minimal ingesta was able to pass through the small opening, which resulted in milk accumulation and marked distension of the abomasum, with sufficient compromise of the wall to allow bacteria or milk molecules to enter the peritoneal space. Fibrin plaques directly over the area of the greater curvature which was where the most mucosal compromise was noted are strongly suggestive that leakage was occurring. The lack of significant aerobic bacterial growth from the joint swab is likely because the calf received antibiotics prior to death, resulting in inhibited growth in vitro.
In general, abomasal ulceration is a common cause of death in suckling calves aged 4–8 weeks (15, 20, 21). This case is unusual in the very young age of the calf. This heifer was only 3 days old when it was euthanized, after 2 days of intensive hospital therapy. Diagnosis of perforating abomasal ulcer in the calf of <1 week old is striking. Other cases of abomasal ulceration in such young calves are single reports and attributed to severe in utero stress (22) or in utero fungal infection (18). To the best of our knowledge, this is the first report of abomasal ulceration secondary to congenital pyloric and duodenal stenosis.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Marshall S. Abomasal ulceration and tympany of calves. Vet Clin North Am Food Anim Pract. (2009) 25:209–20. doi: 10.1016/j.cvfa.2008.10.010
2. Mattiello S, Canali E, Ferrante V, Caniatti M, Gottardo F, Cozzi G, et al. The provision of solid feeds to veal calves: II. Behavior, physiology, and abomasal damage. J Anim Sci. (2002) 80:367–75. doi: 10.2527/2002.802367x
3. Webb LE, Bokkers EAM, Heutinck LFM, Engel B, Buist WG, Rodenburg TB, et al. Effects of roughage source, amount, and particle size on behavior and gastrointestinal health of veal calves. J Dairy Sci. (2013) 96:7765–76. doi: 10.3168/jds.2012-6135
4. Smith DF, Munson L, Erb HN. Abomasal ulcer disease in adult dairy cattle. Cornell Vet. (1983) 73:213–24.
5. Van Immerseel F, Pardon B, Maes S, Heyndrickx M, Timbermont L, Boyen F, et al. Isolation of a clonal population of Clostridium perfringens type A from a Belgian Blue calf with abomasal ulceration. J Comp Pathol. (2010) 143:289–93. doi: 10.1016/j.jcpa.2010.02.004
6. Braun U, Eicher R, Ehrensperger F. Type 1 abomasal ulcers in dairy cattle. Zentralbl Veterinarmed A. (1991) 38:357–66. doi: 10.1111/j.1439-0442.1991.tb01022.x
7. Palmer JE, Whitlock RH. Perforated abomasal ulcers in adult dairy cows. J Am Vet Med Assoc. (1984) 184:171–4.
8. Smith DF, Munson L, Erb HN. Predictive values for clinical signs of abomasal ulcer disease in adult dairy-cattle. Prev Vet Med. (1986) 3:573–80. doi: 10.1016/0167-5877(86)90035-8
9. Bähler C, Steiner A, Luginbühl A, Ewy A, Posthaus H, Strabel D, et al. Risk factors for death and unwanted early slaughter in Swiss veal calves kept at a specific animal welfare standard. Res Vet Sci. (2012) 92:162–8. doi: 10.1016/j.rvsc.2010.10.009
10. Mesarič M. Role of serum pepsinogen in detecting cows with abomasal ulcer. Vet Arh. (2005) 75:111–8.
11. Hund A, Beer T, Wittek T. Abomasal ulcers in slaughtered cattle in Austria. Tierarztl Prax Ausg G Grosstiere Nutztiere. (2016) 44:279–85. doi: 10.15653/TPG-150800
12. Bähler C, Regula G, Stofel MH, Steiner A, Von Rotz A. Efects of the two production programs ‘naturafarm' and ‘conventional' on the prevalence of non-perforating abomasal lesions in Swiss veal calves at slaughter. Res Vet Sci. (2010) 88:352–60. doi: 10.1016/j.rvsc.2009.08.009
13. Jelinski MD, Ribble CS, Campbell JR, Janzen ED. Descriptive epidemiology of fatal abomasal ulcers in Canadian beef calves. PrevVet Med. (1996) 26:9–15. doi: 10.1016/0167-5877(95)00500-5
14. Ahmed AF, Constable PD, Misk NA. Effect of feeding frequency and route of administration on abomasal luminal pH in dairy calves fed milk replacer. J Dairy Sci. (2002) 85:1502–8. doi: 10.3168/jds.S0022-0302(02)74219-7
15. Mills KW, Johnson JL, Jensen RL, Woodard LF, Doster AR. (1990). Laboratory findings associated with abomasal ulcers/tympany in range calves. J Vet Diagn Invest. (1990) 2:208–12. doi: 10.1177/104063879000200310
16. Walsh P, Carvallo Chaigneau FR, Anderson M, Behrens N, McEligot H, Gunnarson B, et al. Adverse effects of a 10-day course of ibuprofen in Holstein calves. J Vet Pharmacol Ther. (2016) 39:518–21. doi: 10.1111/jvp.12295
17. Assis RA, Lobato FCF, Facury Filho EJ, Uzal FA, Santana FJF, Dias LD, et al. Isolation of Clostridium perfringens type D from a suckling calve with ulcerative abomasitis. Arch Med Vet. (2002) 2:287–92. doi: 10.4067/S0301-732X2002000200015
18. Lawhon SD, Corapi WV, Hoffmann AR, Libal MC, Alvarez E, Guarro J, et al. In utero infection of a calf by Saksenaea erythrospora resulting in neonatal abomasitis and dermatitis. J Vet Diagn Invest. (2012) 24:990–3. doi: 10.1177/1040638712452106
19. Lamoureux A, Benchekroun G, German AJ, Freichi V. An endoscopic method for semi-quantitatively measuring internal pyloric diameter in healthy cats: a prospective study of 24 cases. Res Vet Sci. (2019) 122:165–9. doi: 10.1016/j.rvsc.2018.11.023
20. Tulleners EP, Hamilton GF. Surgical resection of perforated abomasal ulcers in calces. Can Vet J. (1980) 21:262–4.
21. Jelinski MD, Ribble CS, Chirino-Trejo M, Clark EG, Janzen ED. The relationship between the presence of Helicobacter pylori, Clostridium perfringens type A, Campylobacter spp, or fungi and fatal abomasal ulcers in unweaned beef calves. Can Vet J. (1995) 36:379–82.
Keywords: abomasal ulcer, congenital abnormality, duodenum, peritonitis, heifer
Citation: Laabouri FZ, Folmar C, Reyes VA, Beasley E, Ryan C and Brown C (2023) Case report: Abomasal ulcer secondary to congenital pyloric and duodenal stenosis in a 3-day-old heifer. Front. Vet. Sci. 10:1235110. doi: 10.3389/fvets.2023.1235110
Received: 05 June 2023; Accepted: 19 September 2023;
Published: 11 October 2023.
Edited by:
Tabaran Alexandru Flaviu, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Pouya Hassandarvish, University of Malaya, MalaysiaCopyright © 2023 Laabouri, Folmar, Reyes, Beasley, Ryan and Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Corrie Brown, Y29yYnJvd25AdWdhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.