- 1Programa de Pós-Graduação em Ciências Genômicas e Biotecnologia, Universidade Católica de Brasília, Brasília, DF, Brazil
- 2Bio Biotecnologia da Reprodução Animal, Brasília, DF, Brazil
- 3Programa de Pós-Graduação em Biologia Animal, Universidade de Brasília, Brasília, DF, Brazil
- 4Departamento de Medicina Veterinária, Centro Universitário de Brasília, Brasília, DF, Brazil
- 5Embrapa Recursos Genéticos e Biotecnologia, Brasília, DF, Brazil
Introduction: The aim of this study was to evaluate potential effects of diflubenzuron on the production and quality of gametes, and on in vitro embryo production (IVEP) outcomes, in cattle.
Methods: Two experiments were performed, the first to evaluate effects on semen, and the second on cumulus-oocyte complexes (COC) and on IVEP. Nelore (Bos taurus indicus) bulls (n = 14) or heifers (n = 16) were allocated into control (CG) or treatment (DIF) groups. All groups received a mineral mix supplement added (DIF) or not (CG) with diflubenzuron (30 mg/head/day), during 8 weeks. Animals were weighed and blood samples were collected throughout the experimental period. Every other week, bulls were subjected to semen collection and heifers to transvaginal ultrasound-guided follicle aspiration sessions. Semen underwent physical and morphological evaluation, and samples were stored for further computer-assisted sperm analysis. The COC recovered were evaluated according to morphology and those classified as viable were sent to an IVEP laboratory.
Results: Diflubenzuron had no effect (P > 0.05) on average body weight or in any blood hematological or biochemical endpoints, regardless of gender. In experiment 1, there was no difference (P > 0.05) between DIF and CG groups for sperm concentration, morphology, or kinetics. In experiment 2, there was also no effect of diflubenzuron on the number of total, viable, or grade I oocytes, as well as on cleavage or blastocyst rates (P > 0.05).
Discussion: In summary, the oral administration of diflubenzuron, within the recommended dose, has no short-term negative effects on sperm production and quality or on oocyte yield and developmental potential in vitro, in cattle.
1. Introduction
The diflubenzuron [1-(4-clorophenyl)-3-(2,6-difluorobenzoyl) urea] is a chemical from the benzoylphenyl urea (BPU) class widely used since the 1970′s for pest control, both in urban areas and in agriculture and livestock (1). It inhibits synthesis of chitin exoskeleton and acts as an arthropod larvae growth regulator, being used as an insecticide and acaricide. In cattle, diflubenzuron has been used as a diet additive to control horn fly (Haematobia irritans), housefly (Musca domestica), stablefly (Stomoxys calcitrans), as well as ticks (Rhipicephalus microplus). Diflubenzuron is poorly absorbed by the gastrointestinal track of cattle, extensively metabolized, and almost totally excreted in feces and urine, resulting in very low residue concentrations in milk and meat (2, 3). Within the recommended doses, such residues are usually undetectable or below the acceptable daily intake (ADI) values (4), and thus are unlikely to present a public health concern (1, 5). Therefore, there is no withdraw time, and diflubenzuron can be used in lactating dairy cattle (6).
Although diflubenzuron has been considered as safe for use in livestock, results from toxicological studies on non-target species are controversial. No carcinogenic effect of diflubenzuron was observed in vitro (7, 8), whereas dose-dependent genotoxic and mutagenic effects were observed in vivo in some animal models (9), but not others (10). Such inconsistencies may be associated with the indirect effect of metabolites, such as 4-chloroaniline, which are formed in vivo from the small proportion of the diflubenzuron that is actually absorbed (11). Recently, diflubenzuron was shown to induce cell apoptosis and ROS generation in mammary epithelial cells (12). Potentially detrimental effects for cell and especially for DNA integrity are particularly important for gametes and embryos, and thus for reproductive toxicity. It is also noteworthy that diflubenzuron, besides acting on chitin synthesis on larvae, also impairs oogenesis and reduces ovarian follicle population in adult insects (13). In this regard, potential actions on gametogenesis in other species should be a target for risk assessment. Nevertheless, very few studies have addressed the potential reproductive toxicity of diflubenzuron on livestock.
In vitro embryo production (IVEP) has become the technique of choice for embryo production in many countries, and in 2020 the number of embryos produced in vitro was three times greater than the number of embryos collected in vivo worldwide (14). Besides its key role as a tool for animal breeding programs, IVEP also provides an important platform for the study of environmental effects, including endocrine disruptors, on reproduction. In vitro maturation (IVM) of bovine oocytes, for example, has been proposed as a potential method to evaluate chemical hazards on fertility, requiring far fewer animals to demonstrate potential detrimental effects on reproduction than in vivo-models (15). The bovine species is also a suitable model for human reproductive toxicology studies, due to the similarities between bovine and human ovarian physiology (16). Similarly, the advances in bovine semen evaluation, such as the use of computer-assisted sperm analysis (CASA), has provided more accurate tools to identify potential interferences in male reproductive physiology, e.g., minor changes in sperm quality not detectable in routine andrological exams.
The aim of the present study was to evaluate possible short-term potential detrimental effects of diflubenzuron treatment on gamete quantity, quality, and developmental potential in cattle. Our hypothesis was that, within the current recommended doses, diflubenzuron treatment would not affect gamete or embryo quality and developmental potential in cattle. The rationale for this study was that the use of new analytical methods, such as CASA, and in vivo-in vitro experimental models such as the association of in vivo oocyte recovery with IVEP, are important to validate previous toxicological evaluations on the safety of diflubenzuron on livestock.
2. Material and methods
2.1. Animals and location
Experiment 1 was conducted on a private beef farm located in Formosa, GO, Brazil. Pubertal Nelore (Bos taurus indicus) bulls (n = 14), with no detectable abnormalities during andrological exams, were enrolled. The bulls were kept under Brachiaria sp. pasture with ad libitum access to water, and received 2 Kg/head/day of concentrate supplement with a mineral mix. Experiment 2 was conducted at the Embrapa's Sucupira Experimental Station, in Brasilia, DF, Brazil. Nulliparous, pubertal, cycling Nelore (Bos taurus indicus) heifers (n = 16), with no detected pathologies during gynecological examination, were enrolled. The heifers were confined and received a maintenance diet consisting of corn silage and mineral mix (100 g/day/head), starting at an adaptation period 2 weeks before the beginning of the experiment. Both experiments were conducted from June to August (winter, dry season).
2.2. Experimental design
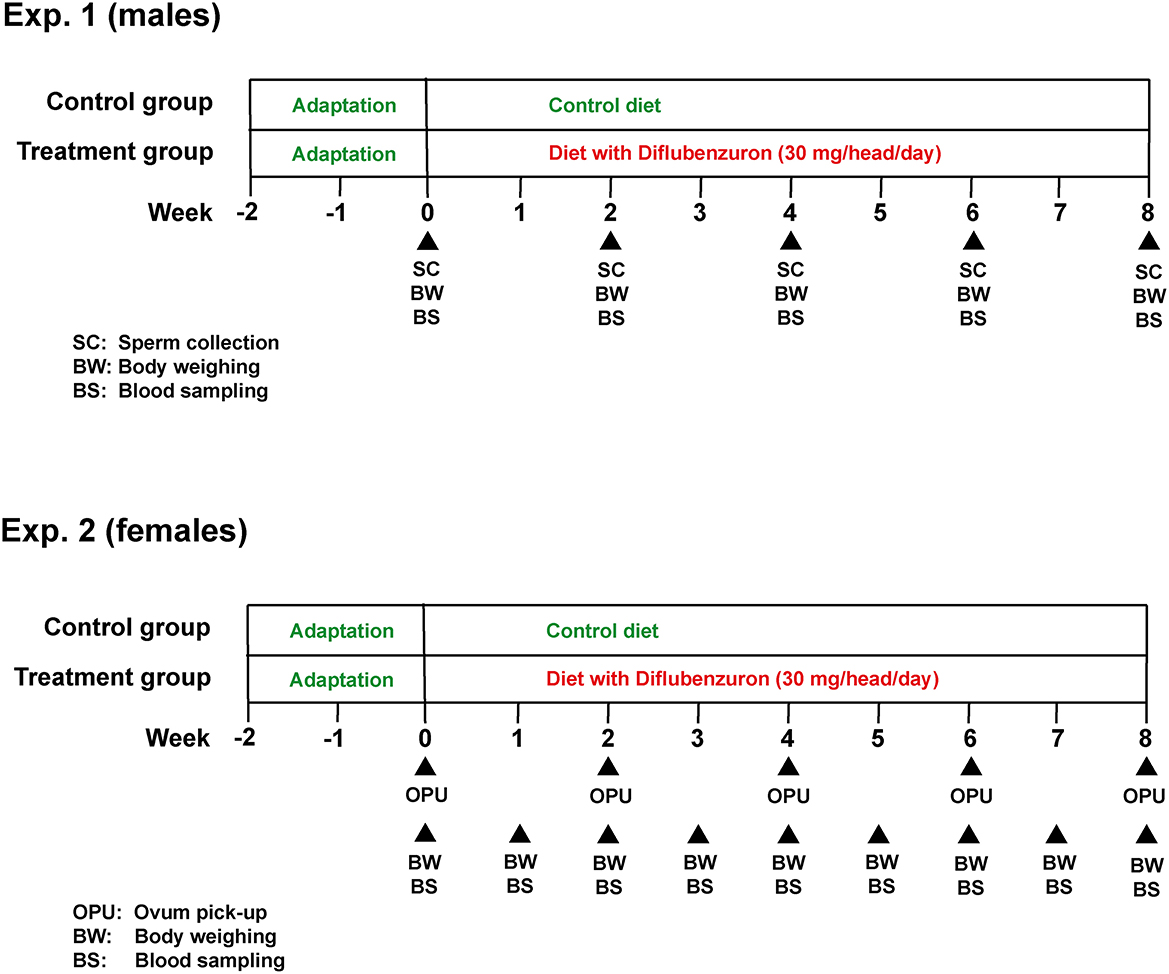
This study was subdivided into two experiments (for bulls and heifers, respectively), both following a similar experimental design (Figure 1). In both experiments, animals were distributed into a control (CG) or treatment (DIF) group. Bulls were allocated to the experimental groups at random, whereas heifers were first ranked according to antral follicle count (AFC), as defined elsewhere (17) and, from the higher to the lower position, alternately allocated to the CG or DIF groups. The balancing of distribution was confirmed by the lack of difference (P > 0.05) in AFC between the two groups at the beginning of the experiment. The experimental groups received a mineral mix added (DIF) or not (CG) with diflubenzuron, calculated to meet the daily mineral requirements and, in the DIF groups, to ensure the daily consumption of 1 g of a commercial formulation (Difly S3, Champion, Anápolis, Brazil) with 3% diflubenzuron (30 mg per head/day, according to product label recommendation, https://www.champion.ind.br/produto/difly-s3-6-kg-2/), during 8 weeks (56 days). The consumption of the mineral mix of each group was monitored daily, the confirm the ingestion of the expected diflubenzuron dose. Body weight was evaluated and blood samples were collected weekly in the heifers, and every 15 days in the bulls. Starting immediately before treatments, every other week bulls were subjected to semen collection, and heifers to transvaginal ultrasound-guided follicle aspiration (OPU), in a total of five sessions in each experiment. Semen underwent physical and morphological evaluation, and samples were stored for further computer-assisted sperm analysis (CASA). The cumulus-oocyte complexes (COC) recovered were evaluated according to morphology and sent to a commercial laboratory for IVEP.
2.3. Blood sampling and blood analyses
Blood samples were collected by puncture of the coccygeal vein or artery, using 21G double-ended needles. Two samples were collected from each animal into 5 mL vacuum tubes, one with and another without K3 EDTA (Vacutainer Systems; Becton Dickinson, São Paulo, SP, Brazil). Samples were kept under refrigeration (5°C) and transported to a commercial veterinary clinical analysis laboratory. Each sample was evaluated for hemogram and biochemical endpoints.
2.4. Semen collection and analysis
Semen samples were collected by electroejaculation, as described elsewhere (18). Briefly, bulls were contained in a beef cattle squeeze chute, and ejaculation was induced using an electroejaculator, at presetting #4 (Autojac 3, Neovet, Uberaba, Brazil). Samples (20 μL) of fresh semen were evaluated under x200 magnification for mass movement, vigor, and motility, and under x1000 magnification for morphological analysis of the sperm cells, as previously described (19). The semen was them diluted 1:1 in a semen extender (Optixcell, IMV Technologies Brasil, Campinas, Brazil) and kept under refrigeration (5°C) until transportation to the laboratory. Fresh semen from each bull was rediluted 1:10 in Optixcell and a 3 μL sample was analyzed in a counting chamber (Leja, IMV Technologies) using CASA (SpermVision Minitube GMBH, Tiefenbach, Germany), with the standard presetting of the equipment. The remaining semen was then diluted, according to the sperm concentration determined by CASA, to a final concentration of 50 x 106 sperm/mL, filled into 0.5 mL straws, stabilized at 4°C for 4 h, and frozen using a freezing machine (Cryogen, Neovet) with the standard freezing curve for cattle. Samples of frozen semen were also analyzed by CASA, immediately after thawing or after being submitted to a thermo-resistance test (TRT) during 4 h.
2.5. Oocyte recovery and in vitro embryo production
All ultrasonographic exams and OPU procedures were performed using a portable ultrasound device (MyLab 30 Gold Vet, Esaote, Genova, Italy) equipped with a 7.5 MHz linear rectal probe or a micro-convex vaginal 7.5 MHz probe mounted in a custom-made polyethylene needle guide (WTA Tecnologia Aplicada, Cravinhos, Brazil). Before the first OPU, the number of sonographic-detectable follicles (AFC) in the ovaries was recorded and used to balance the distribution of heifers between treatments.
The OPU-IVEP procedures used were the same as described elsewhere (20). Briefly, immature COC were collected by puncture and aspiration of visible follicles (>2 mm), using 20 G needles and a vacuum pressure of approximately 80 mm/Hg. The aspirated fluid with the follicle content was recovered into 50 mL tubes containing DPBS added with 1% FCS and 125 IU/mL sodium heparin. COC quality was evaluated under a stereomicroscope at x40 magnification and those morphologically classified as viable, as previously described (21), were transferred to 1.2 mL cryotubes (Corning, New York, USA) containing maturation medium and transported in a portable incubator (Minitub do Brasil, Porto Alegre, Brazil) at 38°C to the IVEP laboratory.
The COC from each donor underwent in vitro maturation (IVM), in vitro fertilization (IVF), and subsequent in vitro embryo culture (IVC) separately. IVM was performed for 20 h in TCM199 (Gibco, New York, USA) supplemented with 0.05 IU/mL FSH (Pluset, Hertape-Calier, Barcelona, Spain), 1 mg/mL estradiol, and 10% FCS in a humidified incubator at 5% CO2 in air and 38.5°C. Expanded COC were then partially denuded and transferred to drops with Tyrode's albumin lactate pyruvate (TALP) medium supplemented with 10 μg/mL heparin, 20 μM D-penicillamine, 10 μM hypotaurine, and 1 μM epinephrine. IVF was performed with 1 x 106 spermatozoa/mL for 18 h. Semen from a single Nelore sire with known fertility on IVEP was used for all fertilization batches. Presumptive zygotes were then IVC in 50 μL droplets of synthetic oviduct fluid (SOFaa) supplemented with essential and non-essential amino acids, 0.34 mM sodium tricitrate, 2.77 mM myo-inositol, and 10% FCS, under mineral oil. Cleavage and blastocyst rates were determined at 72 and 168 h post-insemination. Blastocysts were also classified according to developmental stage (initial blastocysts, blastocysts, expanded blastocysts, or hatched blastocysts). A subset of the expanded blastocysts (circa 30/batch/group) were transferred to glass slides, stained with Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA), and evaluated under the microscope (x1000) for total cell number (ICM plus trophoblast).
2.6. Data analysis
All endpoints related to weight, blood hematological and biochemical analyses, sperm production and quality in the bulls, and oocyte yield and in vitro embryo production in heifers, were compared between CG and DIF groups using the GLIMMIX procedure of SAS (SAS Studio 3.8, University Edition; SAS Institute Inc., Cary, NC, USA) with a repeated statement to account for measurements over time. The model included the effects of treatment (control or diflubenzuron), time (semen collection or OPU-IVEP session), and their interactions, and was adjusted for the type of distribution of each variable. Due to low individual frequencies, sperm pathologies were pooled as major or minor defects, as described elsewhere (22). Similarly, expanded blastocysts were grouped according to cell count in those with >100 or < 100 cells. Differences among means were determined using the Student T-test. Results are shown as mean ± SEM. Exact P-values are shown for each comparison, to denote how close mean differences were from significance (P < 0.05).
3. Results
The average body weight and blood parameters in Nelore bulls and heifers from the CG and DIF groups are shown in Table 1. In general, there was no effect (P > 0.05) of treatment on any of the endpoints analyzed for both males and females. A time-effect (P > 0.05) was observed on weight in males and on most blood parameters in both groups and gender, but a treatment x time interaction was only observed for creatinine and alkaline phosphatase (ALP) in males.
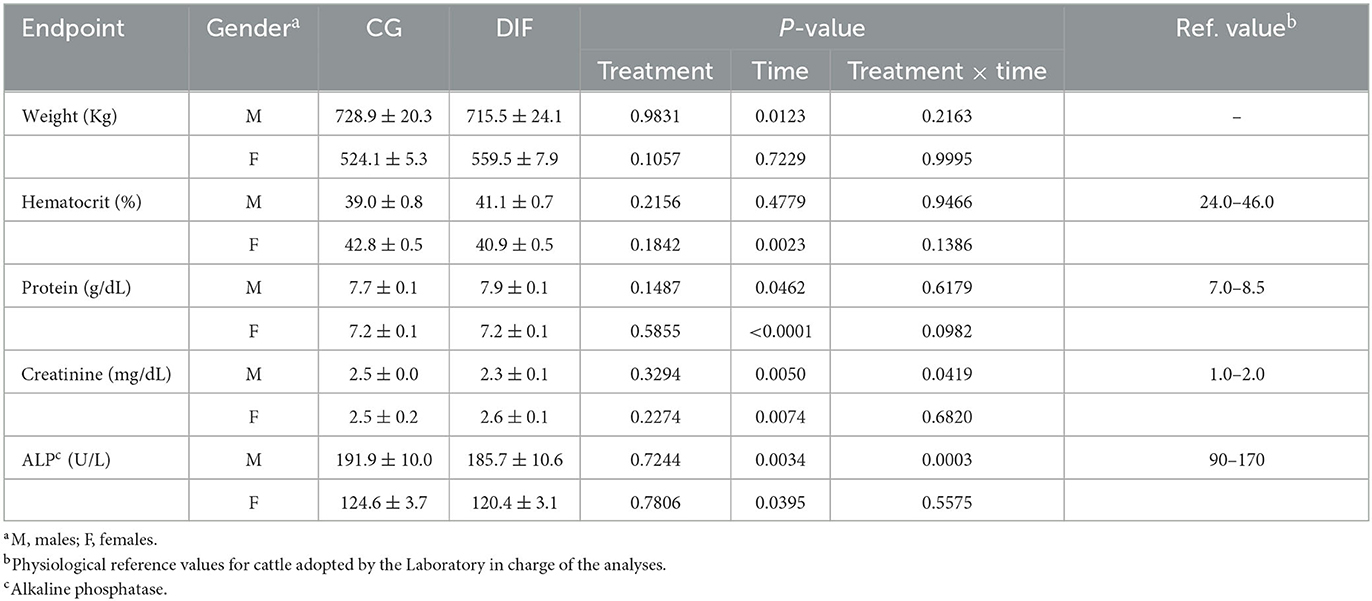
Table 1. Body weight and blood hematological and biochemical endpoints (mean ± SEM) of Nelore (Bos taurus indicus) bulls and heifers treated (DIF) or not (CG) with 30 mg diflubenzuron/day, during 8 weeks.
3.1. Experiment 1
Two bulls did not adapt to the semen collection routine and were removed from the experiment, therefore the CG and DIF groups consisted of five and seven bulls, respectively. The treatment with diflubenzuron had no effect (P > 0.05) on any physical or CASA sperm parameter, regardless of whether the analyses were carried out with fresh, frozen-thawed, or frozen-thawed plus TRT semen (Table 2). Conversely, there was a time-effect (P < 0.05) on most endpoints. Treatment x time effects were seldom observed, in all cases associated with fluctuations in values, rather than with increasing or decreasing trends throughout the experimental period.
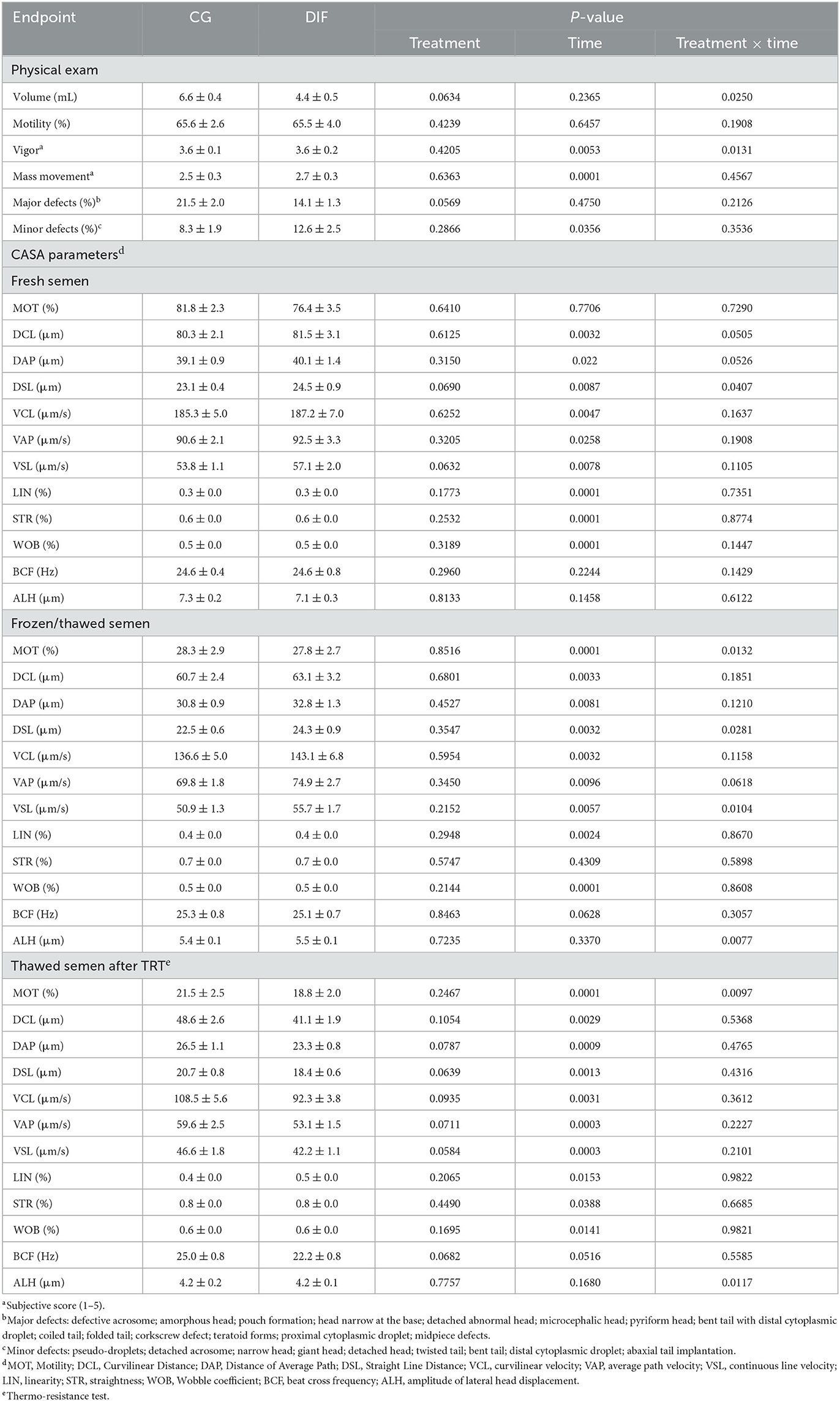
Table 2. Sperm endpoints (mean ± SEM) in Nelore (Bos taurus indicus) bulls treated (DIF, n = 7) or not (CG, n = 5) with 30 mg diflubenzuron/day, during 8 weeks.
3.2. Experiment 2
Data related to oocyte recovery and in vitro embryo production are shown in Table 3. During the five OPU sessions, the CG and DIF groups yielded 801 and 986 total COC, and 631 and 797 viable COC, respectively. The third IVEP session was affected by microbiological contamination in both groups during IVF, thus data from this IVEP session was discarded. Similarly to what was observed for semen, a time-effect was observed for most OPU-IVEP endpoints, whereas there was no effect (P > 0.05) of the diflubenzuron treatment on any parameter. A treatment x time interaction was observed in the number of follicles aspirated, which decreased (P < 0.05) consistently in CG but increased (P < 0.05) from sessions three to four in DIF group, and in the proportion of expanded blastocysts and of embryos with > 100 cells, which fluctuated in both groups.
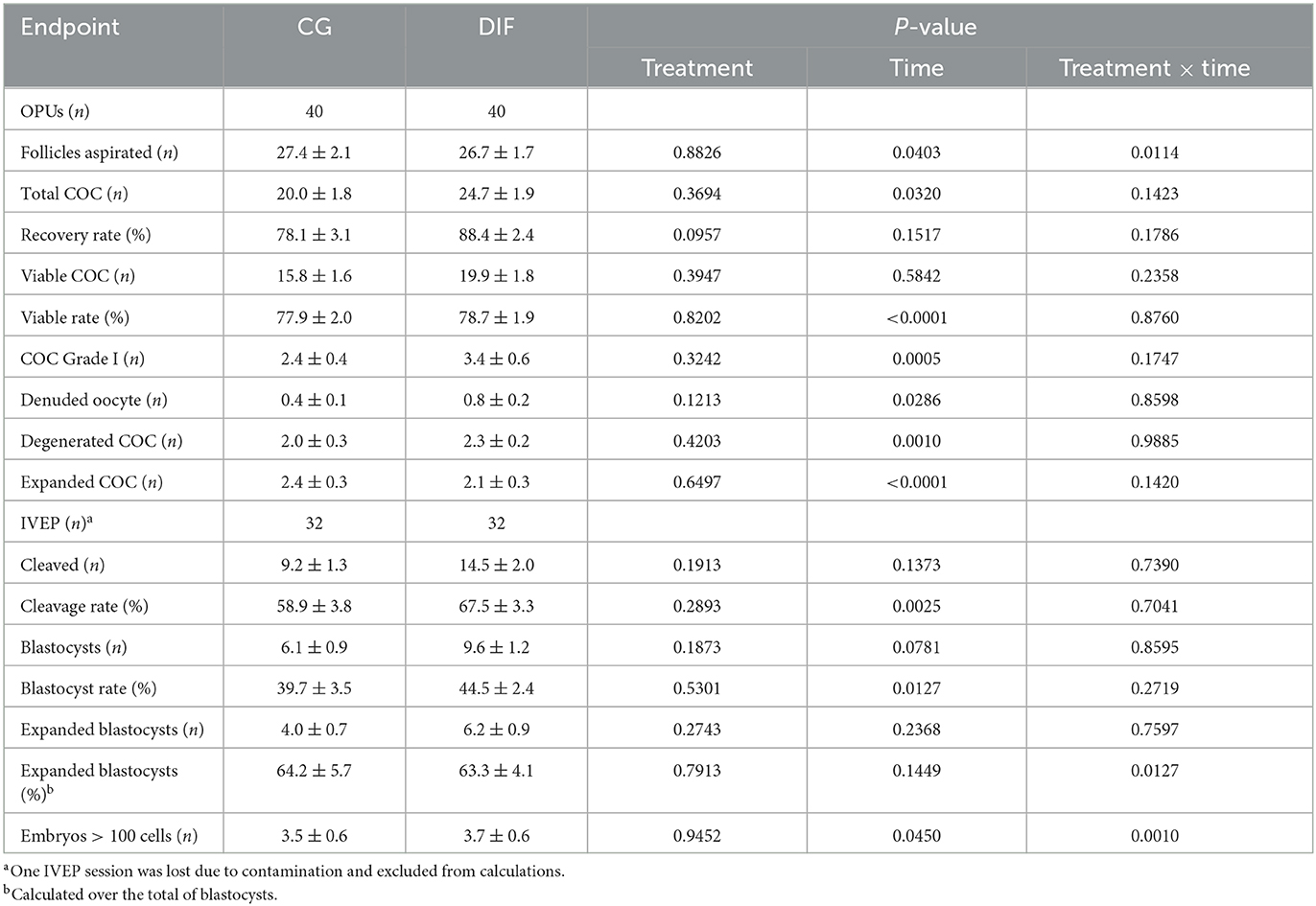
Table 3. OPU-IVEP outcomes (mean ± SEM) in Nelore (Bos taurus indicus) heifers treated (DIF) or not (CG) with 30 mg diflubenzuron/day, during 8 weeks.
4. Discussion
Benzoylurea chitin inhibitors, such as diflubenzuron, are selective pesticides with known detrimental effects on arthropods, as wells as in other organisms presenting chitin in their shells or exoskeleton, but not in mammals. Diflubenzuron was approved and is currently used for the control of a range of plagues in agriculture and parasites in livestock (5). However, toxicity studies have demonstrated potential side-effects in non-target animal models. Thus, the safety of these chemicals needs to be reassessed over time, particularly when new analytical methods or experimental models become available. Our hypothesis was that, within the current recommended doses, diflubenzuron treatment would not affect gamete or embryo quality and developmental potential in cattle, as determined by CASA or OPU-IVEP. The present study provided additional evidence of the lack of reproductive toxicity of diflubenzuron on livestock.
Although the focus of the current study was the potential effects on gametes and embryos, we also monitored weight and hematological parameters that could indicate systemic toxicity. We evaluated indicators of the hematopoietic system (hematocrit, protein), hepatic (alkaline phosphatase), and renal (creatinine) function. The liver is the main organ responsible for the metabolism of endogenous and exogenous compounds and, together with the kidney, is responsible for the excretion of most metabolites. Therefore, these organs are targets for the toxic actions of chemicals, resulting in altered plasma concentrations of biomarkers such as ALP and creatinine (23, 24). Moreover, ALT was found to have increased in male rats treated with Diflubenzuron (11). The hematopoietic system, on the other hand, is highly susceptible to toxic substances, being an important endpoint in toxicological studies (25). In the current study, no physiological endpoint was affected by the treatment with diflubenzuron. As expected, we observed fluctuations over time, all but for creatinine within the physiological range for cattle (26, 27). In both groups, creatinine plasma concentrations were consistently higher than the reference values used by the laboratory that performed the analyses. However, all animals remained sound and showed no other signs of potential intoxication throughout the experiment. One can speculate whether the higher creatinine concentrations observed were due to any of the environmental effects that may affect thresholds and the width of reference intervals, as reported elsewhere (27). There was also no effect of DIF treatment on body weight, which remained constant in heifers receiving a maintenance diet, whereas increased over time in bulls raised on pasture.
Fertility is a key component of toxicity tests. A number of chemicals have been described as endocrine disruptors for cattle (28), and thus with the potential to affect the reproductive process and to compromise fertility (29). However, the reproductive toxicity of diflubenzuron was evaluated mostly in non-target species, especially in rodents. de Barros et al. (11) observed that subacute exposure of male rats to diflubenzuron was associated with a decrease on testicle weight and daily sperm production, although no alterations were found in sperm morphology. The extrapolation of these results to other species, however, requires caution, particularly due to the differences in the physiology of both species and in the doses used. Interestingly, there are very few reports evaluating diflubenzuron toxicity on cattle reproduction. A study conducted in the late 1970′s with young Holstein male calves found no effect of diflubenzuron on sperm (30). However, in that study a single semen evaluation was performed. In the current study, the time-window (56 days) used almost encompassed the seminiferous epithelium cycle and the duration of spermatogenesis in cattle (31), so we were able to evaluate potential effects over time. Moreover, by using CASA, a much more sensitive analytic technique, we evaluated a range of sperm kinetic endpoints. Nevertheless, no evidence of detrimental effects of diflubenzuron was detected.
One potential bias of studies evaluating COC recovery and thus embryo yield is the uneven distribution of cows showing high or low AFC. Ovarian follicle population has a Weibull-like, rather than a Gaussian distribution, with a few animals presenting over 100 antral follicles at a given time (32). Donors yielding over 500 COC have been reported (33), thus a random distribution of donors within treatments may result in significant bias. In the current study, we used a balanced distribution strategy, ensuring that there were differences neither in AFC nor in the number of follicles aspirated or COC recovered at the first OPU-IVEP session between CG and DIF groups.
In heifers, as observed for bulls, there was no effect of diflubenzuron on any of the endpoints evaluated. Differences over time were expected, particularly due to the trend of reduction in COC recovery in Bos taurus indicus donors undergoing repeated OPU (34). To our best knowledge, this is the first study to address the potential hazard effects of diflubenzuron on oocyte quality and developmental potential in cattle. In insects, diflubenzuron acts not only on chitin formation, but also directly on reproduction in adult females, reducing follicle populations, yolk formation, and fecundity (13, 35). The mechanisms underlying the latter effects, however, are not fully understood. Potential detrimental effects on natural reproduction in large, mono-ovulating species such as cattle may be difficult to demonstrate, due to the high number of animals required and to the difficulty in controlling other environmental effects. In this regard, the OPU-IVEP model used was particularly important to ensure the evaluation of a high number of gametes (>800 per group), as well as the evaluation of their developmental potential during the critical period encompassing maturation, fertilization, and early embryo development. In both groups (CG and DIF), oocyte recovery and quality, as well as cleavage and embryo rates, were similar, and within those reported for the Nelore breed (20, 32, 36). Thus, the current results suggest that there is no interference of diflubenzuron treatment on the latter stages of folliculogenesis and oocyte maturation, differently to what is observed in non-chordate animals.
5. Conclusion
In summary, there is no evidence of a detrimental effect of short-term treatments with diflubenzuron, within the recommended dose, on gamete quality or embryo development potential in cattle.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Embrapa Ethics in the Use of Animals Committee (Protocol CEUA-002/2022). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MP and JV: conceptualization, supervision, and methodology. MP, RF, and JV: funding. MX, LM, RM, DM, JB, and JV: investigation. JV: statistical analysis. MX, RF, RA, and JV: writing—original draft and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
The financial support was provided by Embrapa Project 30.19.90.006.00.00 and FAPDF Project 00193-00000613/2023-95.
Acknowledgments
The authors thank Champion Farmacoquímica LTDA for supplying the commercial formulation of diflubenzuron used.
Conflict of interest
MX and MP were employed by Bio Biotecnologia da Reprodução Animal. RF and JV were employed by Embrapa Recursos Genéticos e Biotecnologia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun R, Liu C, Zhang H, Wang Q. Benzoylurea chitin synthesis inhibitors. J Agric Food Chem. (2015) 63:6847–65. doi: 10.1021/acs.jafc.5b02460
2. Ivie GW. Fate of diflubenzuron in cattle and sheep. J Agric Food Chem. (1978) 26:81–9. doi: 10.1021/jf60215a013
3. Tfouni SAV, Furlani RPZ, Carreiro LB, Loredo ISD, Gomes AG, Alves LA, et al. Determination of diflubenzuron residues in milk and cattle tissues. Arq Br Med Vet Zoot. (2013) 65:301–7. doi: 10.1590/S0102-09352013000100043
4. FAO. (2011). Available online at: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report11/Diflubenzuron.pdf (accessed May 2, 2023).
5. Junquera P, Hosking B, Gameiro M, Macdonald A. Benzoylphenyl ureas as veterinary antiparasitics. An overview and outlook with emphasis on efficacy, usage and resistance. Parasite. (2019) 26:26. doi: 10.1051/parasite/2019026
6. Van der Leek ML, Donovan GA, Saltman RL, Moja RJ. Effect of an insecticide controlled-release bolus on a milk antibiotic residue test. J Dairy Sci. (1991) 74:433–5. doi: 10.3168/jds.S0022-0302(91)78188-5
7. Perocco P, Colacci A, Grilli S. In vitro cytotoxic and cell transforming activities exerted by the pesticides cyanazine, dithianon, diflubenzuron, procymidone, and vinclozolin on BALB/c 3T3 cells. Environ Mol Mutagen. (1993) 21:81–6. doi: 10.1002/em.2850210111
8. Bayoumi AE, Pérez-Pertejo Y, Zidan HZ, Balaña-Fouce R, Ordóñez C, Ordóñez D. Cytotoxic effects of two antimolting insecticides in mammalian CHO-K1 cells. Ecotoxicol Environ Saf. (2003) 55:19–23. doi: 10.1016/S0147-6513(02)00068-4
9. de Barros AL, de Souza VV, Navarro SD, Oesterreich SA, Oliveira RJ, Kassuya CA, et al. Genotoxic and mutagenic effects of diflubenzuron, an insect growth regulator, on mice. J Toxicol Environ Health A. (2013) 76:1003–6. doi: 10.1080/15287394.2013.830585
10. Quarles JM, Norman JO, Kubena LF. Absence of transformation by diflubenzuron in a host-mediated transplacental carcinogen assay. Bull Environ Contam Toxicol. (1980) 25:252–6. doi: 10.1007/BF01985520
11. de Barros AL, Cavalheiro GF, de Souza AV, Traesel GK, Anselmo-Franci JA, Kassuya CA, et al. Subacute toxicity assessment of diflubenzuron, an insect growth regulator, in adult male rats. Environ Toxicol. (2016) 31:407–14. doi: 10.1002/tox.22054
12. Lee W, An G, Park H, Lim W, Song G. Diflubenzuron leads to apoptotic cell death through ROS generation and mitochondrial dysfunction in bovine mammary epithelial cells. Pestic Biochem Physiol. (2021) 177:104893. doi: 10.1016/j.pestbp.2021.104893
13. Smagghe G, Zotti M, Retnakaran A. Targeting female reproduction in insects with biorational insecticides for pest management: a critical review with suggestions for future research. Curr Opin Insect Sci. (2019) 31:65–9. doi: 10.1016/j.cois.2018.10.009
14. Viana J. 2020 Statistics of embryo collection and transfer in domestic farm animals: world embryo industry grows despite the pandemic. Embr Tech Newsl. (2021) 39:24–37.
15. Luciano AM, Franciosi F, Lodde V, Corbani D, Lazzari G, Crotti G, et al. Transferability and inter-laboratory variability assessment of the in vitro bovine oocyte maturation (IVM) test within ReProTect. Reprod Toxicol. (2010) 30:81–8. doi: 10.1016/j.reprotox.2010.01.015
16. Santos RR, Schoevers EJ, Roelen BA. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol. (2014) 12:117. doi: 10.1186/1477-7827-12-117
17. Evans AC, Mossa F, Fair T, Lonergan P, Butler ST, Zielak-Steciwko AE, et al. Causes and consequences of the variation in the number of ovarian follicles in cattle. Soc Reprod Fertil Suppl. (2010) 67:421–9. doi: 10.7313/UPO9781907284991.032
18. Doroteu EM, Viana JHM, Ferreira Junior JA, Macedo JTA, Oliveira RA, Pedroso PMO. Effect of a single or two doses of an anti-GnRH vaccine on testicle morpho-functional characteristics in Nelore bulls. Trop Anim Health Prod. (2021) 53:153. doi: 10.1007/s11250-021-02600-x
19. Menon AG, Barkema HW, Wilde R, Kastelic JP, Thundathil JC. Associations between sperm abnormalities, breed, age, and scrotal circumference in beef bulls. Can J Vet Res. (2011) 75:241–7.
20. Malard PF, Peixer MAS, Grazia JG, Brunel HDSS, Feres LF, Villarroel CL, et al. Intraovarian injection of mesenchymal stem cells improves oocyte yield and in vitro embryo production in a bovine model of fertility loss. Sci Rep. (2020) 10:8018. doi: 10.1038/s41598-020-64810-x
21. Viana JH, De Almeida Camargo LS, De Moraes Ferreira A, De Sa WF, De Carvalho Fernandes CA, De Pinho Marques Junior A. Short intervals between ultrasonographically guided follicle aspiration improve oocyte quality but do not prevent establishment of dominant follicles in the Gir breed (Bos indicus) of cattle. Anim Reprod Sci. (2004) 84:1–12. doi: 10.1016/j.anireprosci.2003.12.002
22. Johnson WH. The significance to bull fertility of morphologically abnormal sperm. Vet Clin North Am Food Anim Pract. (1997) 13:255–70. doi: 10.1016/S0749-0720(15)30339-X
23. Stark JL. BUN/creatinine: your keys to kidney function. Nursing. (1980) 10:33–8. doi: 10.1097/00152193-198005000-00007
24. Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. (2008) 245:194–205. doi: 10.1016/j.tox.2007.11.021
25. Gribaldo L. Haematotoxicology: scientific basis and regulatory aspects. Altern Lab Anim. (2002) 30 Suppl 2:111–3. doi: 10.1177/026119290203002S17
26. Rowlands GJ, A. review of variations in the concentrations of metabolites in the blood of beef and dairy cattle associated with physiology, nutrition and disease, with particular reference to the interpretation of metabolic profiles. World Rev Nutr Diet. (1980) 35:172–235. doi: 10.1159/000386410
27. Roland L, Drillich M, Iwersen M. Hematology as a diagnostic tool in bovine medicine. J Vet Diagn Invest. (2014) 26:592–8. doi: 10.1177/1040638714546490
28. Petro EM, Leroy JL, Van Cruchten SJ, Covaci A, Jorssen EP, Bols PE. Endocrine disruptors and female fertility: focus on (bovine) ovarian follicular physiology. Theriogenology. (2012) 78:1887–900. doi: 10.1016/j.theriogenology.2012.06.011
29. Mourikes VE, Flaws JA. Reproductive toxicology: effects of chemical mixtures on the ovary. Reproduction. (2021) 162:F91–F100. doi: 10.1530/REP-20-0587
30. Miller RW, Cecil HC, Carey AM, Corley C, Kiddy CA. Effects of feeding diflubenzuron to young male holstein cattle. Bull Environ Contam Toxicol. (1979) 23:482–6. doi: 10.1007/BF01769991
31. Staub C, Johnson L. Review: spermatogenesis in the bull. Animal. (2018) 12:s27–35. doi: 10.1017/S1751731118000435
32. Pontes JH, Melo Sterza FA, Basso AC, Ferreira CR, Sanches BV, Rubin KC, et al. Ovum pick up, in vitro embryo production, and pregnancy rates from a large-scale commercial program using Nelore cattle (Bos indicus) donors. Theriogenology. (2011) 75:1640–6. doi: 10.1016/j.theriogenology.2010.12.026
33. Resende AO, Bohrer RC, Viana JHM. A record of 485 viable cumulus-oocyte complexes recovered and 165 viable embryos produced in a single ovum pickup session from a Senepol breed donor. Reprod Fertil Develop. (2021) 33:111. doi: 10.1071/RDv33n2Ab7
34. Gimenes LU, Ferraz ML, Fantinato-Neto P, Chiaratti MR, Mesquita LG, Sá Filho MF, et al. The interval between the emergence of pharmacologically synchronized ovarian follicular waves and ovum pickup does not significantly affect in vitro embryo production in Bos indicus, Bos taurus, and Bubalus bubalis. Theriogenology. (2015) 83:385–93. doi: 10.1016/j.theriogenology.2014.09.030
35. Soltani-Mazouni N. Effects of ingested diflubenzuron on ovarian development during the sexual maturation of mealworms. Tissue Cell. (1994) 26:439–45. doi: 10.1016/0040-8166(94)90027-2
Keywords: Difly, reproductive disruptors, in vitro embryo production, semen, Nelore breed
Citation: Xavier MC, Martins LP, Moura RM, Morais DF, Barbosa JVL, Figueiredo RA, Peixer MAS, Andrade RVd and Viana JHM (2023) Evaluation of the effects of the recommended oral dose of diflubenzuron on bovine sperm and oocyte quality using CASA and OPU-IVEP. Front. Vet. Sci. 10:1215722. doi: 10.3389/fvets.2023.1215722
Received: 02 May 2023; Accepted: 26 June 2023;
Published: 11 July 2023.
Edited by:
Benito Soto-Blanco, Federal University of Minas Gerais, BrazilReviewed by:
Timothy Evans, University of Missouri, United StatesAndré Mariano Batista, Universidade Federal Rural de Pernambuco, Brazil
Copyright © 2023 Xavier, Martins, Moura, Morais, Barbosa, Figueiredo, Peixer, Andrade and Viana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joao Henrique Moreira Viana, aGVucmlxdWUudmlhbmFAZW1icmFwYS5icg==
 Marcelo Cunha Xavier1,2
Marcelo Cunha Xavier1,2 Rosangela Vieira de Andrade
Rosangela Vieira de Andrade Joao Henrique Moreira Viana
Joao Henrique Moreira Viana