
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci., 05 September 2023
Sec. Veterinary Neurology and Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1209935
This article is part of the Research TopicChemotherapy and Other Pharmacotherapies for Canine Neurological DisordersView all 7 articles
Intrathecal chemotherapy is used in human medicine for the treatment or prophylaxis of CNS hematopoietic neoplasia. However, the clinical benefits in veterinary medicine have been scarcely documented. A 4-year-old male entire cross-breed dog presented with a 24-h history of severe lethargy, pelvic limb weakness, and urinary retention. Examination revealed generalized peripheral lymphadenomegaly, and the neurological findings were suggestive of a myelopathy in the region of T3-L3. Following the diagnosis of multicentric lymphoblastic B-cell lymphoma (stage Vb), a modified L-LOP with cytosine arabinoside was started, and complete clinical remission was achieved. After 4 weeks, there was acute neurological deterioration (spinal pain and proprioceptive deficits) without peripheral lymphadenomegaly. MRI findings and CSF analysis were consistent with meningeal and spinal cord lymphoma infiltration at the level of L3. Intrathecal chemotherapy (cytosine arabinoside and methotrexate) were administered in the cisterna magna with systemic dexamethasone and analgesia. Clinical signs were resolved within 24 h, and the patient remained asymptomatic for 3.5 weeks. After this period, CNS relapse (proprioceptive deficits and severe thoracolumbar pain) was suspected, and repeat intrathecal chemotherapy was declined. The patient was humanely euthanized 9 weeks after the initial diagnosis. This is the first report on the clinical benefit of intrathecal chemotherapy with a combination of methotrexate and cytarabine for the management of CNS lymphoma in dogs. Based on our case, intrathecal chemotherapy with methotrexate and cytarabine can induce a short-lasting CNS clinical remission (3 weeks).
Primary or secondary central nervous system lymphoma (CNS) is clinically challenging to treat as cytotoxic medications that cross the blood–brain barrier (BBB) are limited (1–3). In humans, intrathecal chemotherapy has an important role in hematopoietic CNS neoplasia, particularly in treating children or older patients to minimize exposure to radiotherapy (4–8). Considering specifically lymphoblastic lymphoma, intrathecal chemotherapy composes most of the protocols prescribed in human medicine, mainly as a prophylactic measure due to the low incidence of CNS involvement during presentation (3–9%) (1, 3, 9, 10).
In dogs, Genoni et al. (11) documented a low complication rate (1/112) following intrathecal chemotherapy for the treatment of CNS neoplastic or inflammatory conditions. However, the literature on the clinical benefit of intrathecal chemotherapy for the management of CNS neoplasia in dogs is limited to six cases (12, 13). Of these, five had a multimodal approach (systemic chemotherapy, CNS radiotherapy, and intrathecal chemotherapy), and the sixth case was of a dog that had intrathecal chemotherapy and L-asparaginase. All these cases had intrathecal chemotherapy with a single agent, cytarabine arabinoside (11–13).
In this report, we have described the clinical benefit of cytarabine arabinoside and methotrexate administered intrathecally alongside systemic chemotherapy for the management of lymphoblastic lymphoma involving the CNS in a dog.
A 4-year-old male entire cross-breed dog presented with a 24-hour history of severe lethargy, pelvic limb weakness, and urinary retention. Previous clinical history was unknown as the patient had been recently rescued from aboard.
The dog was quiet, alert, and responsive on presentation. General clinical examination findings included generalized lymphadenopathy (right mandibular trilobed, 5 cm; left mandibular, 4.2 cm; right pre-scapular, 4.5 cm; left pre-scapular, 4.7 cm; and bilateral popliteal, 3 cm) and tachycardia (heart rate, 160 beats/min). On neurological examination, the patient was ambulatory paraparetic, had proprioceptive ataxia, absent paw replacement, and had markedly delayed hopping on the pelvic limbs with conserved segmental spinal reflexes. Pain was evident on cranial lumbar palpation. The cranial nerves and the remaining neurological examination were unremarkable. Neuroanatomical localization was compatible with T3-L3 myelopathy.
The patient deteriorated after admission to the clinic; he became depressed and developed spontaneous horizontal nystagmus with the fast left phase, right-sided vestibular ataxia, and reduced right menace response. Our main differential diagnosis was lymphoma with central nervous system involvement; however, the neurological signs could represent a different process, such as multifocal vascular, inflammatory, infectious, or less likely degenerative.
A complete blood count and blood film analysis (Table 1) showed pancytopenia characterized by mild regenerative anemia associated with moderate polychromasia and marked anisocytosis, moderate neutropenia with a low proportion of neutrophils with a toxic appearance, and moderate thrombocytopenia with a minimum manual estimate count of 110 x 109/L. There were also circulating large, mononuclear, atypical cells (measuring x 1.5–2 diameter of a neutrophil), characterized by a large round nucleus containing finely stippled chromatin and variably prominent nucleoli (1–3 nucleoli) on a scant deep basophilic cytoplasm. The extended biochemistry panel and electrolytes did not show any significant or specific changes except for elevated C-reactive protein (77 mg/L) (ref.: <10). Toxoplasma spp. and Neospora spp. serologies were negative, and the urine analysis was unremarkable.
Cytology analysis of peripheral lymph nodes identified a high number of monomorphic, large lymphoid cells (x2–3 small lymphocytes) with round nuclei with finely dispersed chromatin and prominent nucleoli. The cytoplasm was scant and basophilic. The mitotic count was high (8–10/5 x 60 HPF). The sample was also composed of small lymphocytes (<5%) and rare binucleated and mott cells. Flow cytometry of the peripheral lymph nodes was positive for CD79 (90%), CD45 (62%), and CD34 (96%) and negative for CD3, CD4, CD5, CD8, CD21, and MHCII.
The patient underwent thoracic radiographs (both DV and right lateral) to assess for intrathoracic lymphadenopathy. Additionally, a orthogonal radiographic lumbar spine study was performed to investigate the possible causes of urinary retention. Finally, an abdominal ultrasound was performed also under sedation. The diagnostic imaging findings included presence of mediastinal and intraabdominal lymphadenopathy, along with hepatomegaly characterized by heterogenous echogenicity. Splenomegaly was also observed, showing a diffuse mottled appearance. Furthermore, there was a small amount of pleural and peritoneal effusion.
Our findings were consistent with a multicentric lymphoblastic B-cell lymphoma (stage Vb) with suspected CNS involvement and bone marrow infiltration.
The patient was started on a modified L-LOP chemotherapy protocol (L-asparaginase, lomustine, vincristine, and prednisolone) with cytarabine arabinoside. The first treatments were administered, while the patient was hospitalized (Table 2, day 0). Although there was a moderate improvement of CNS signs with cytarabine (normal cranial nerves but persistent mild paraparesis with right-sided delayed postural reactions, considered a partial CNS response), he had persistent pancytopenia, which precluded further cytotoxic medication. Therefore, after being pre-medicated with chlorphenamine (0.4 mg/kg IV), L-asparaginase was administered (Table 2, day 0). During hospitalization, the patient also received analgesia for thoracolumbar pain (paracetamol 10 mg/kg PO q12h; gabapentin 10 mg/kg PO q12h; and buprenorphine 0.02 mg/kg IV q6h) and anti-nausea medication, as he showed signs of nausea (ondansetron 1 mg/kg PO q12h and maropitant 1 mg/kg IV q24h). An indwelling urinary catheter was placed to manage urinary retention, suspected to be secondary to thoracolumbar pain.
The patient was reassessed on a weekly basis for the continuation of the prescribed systemic chemotherapy protocol (Table 2). The clinical systemic response was assessed by measuring palpable peripheral lymph nodes and quantifying circulating atypical cells. The central nervous system response was measured by an improvement in neurological deficits and thoracolumbar pain. He had intermittent paraparesis during the first 2 weeks, but the remaining neurological examination was unremarkable. Analgesia for thoracolumbar pain was discontinued in Week 3. On day 28, there was progressive peripheral lymphadenopathy, and atypical cells were found circulating in the blood. As a rescue treatment, a CHOP-based protocol (cyclophosphamide, vincristine, doxorubicin, and prednisolone) was initiated. Despite variable responses to treatment (Table 2) during this period, the patient maintained a good quality of life without the side effects of chemotherapy.
The patient presented as an emergency 5 days after receiving cyclophosphamide. The dog showed severe thoracolumbar pain and bilateral hind limb proprioceptive deficits but without peripheral lymphadenopathy. To investigate the condition, various tests were conducted, including hematology and an extended biochemistry panel. Additionally, an MRI of the T3-S3 region was performed using sagittal T2W and STIR sequences, as well as transverse T2W, T1W, and T1W + C sequences). Furthermore, a CSF analysis was conducted, involving cytology and biochemistry, obtained from the cisterna magna. The MRI revealed compression of the spinal cord at the L3 level due to an interspersed, slightly right-sided, and ventral extradural material. The abnormal material observed in the spinal cord was slightly hyperintense on T2W and T1W MRI sequences to the spinal cord gray matter, and it exhibited clear contrast enhancement (Figure 1). This material caused compression at the L3 level, and, extending cranially, the spinal cord showed mild intramedullary patchy hyperintensity on both T2W and T1W images but without contrast enhancement. The CSF analysis revealed significant abnormalities, with a nucleated cell count of 1,900/uL (R.I: 0–5) and a protein of 0.34 g/L (R.I: 0.14–0.30). The cytology of the CSF also showed an abundance of atypical large lymphoid cells with irregularly round nuclei containing dispersed fine chromatin and several nucleoli, along with a small amount of basophilic cytoplasm. The presence of a high number of mitotic figures was also evident. These findings were consistent with meningeal and spinal cord lymphoma infiltration at the level of L3.
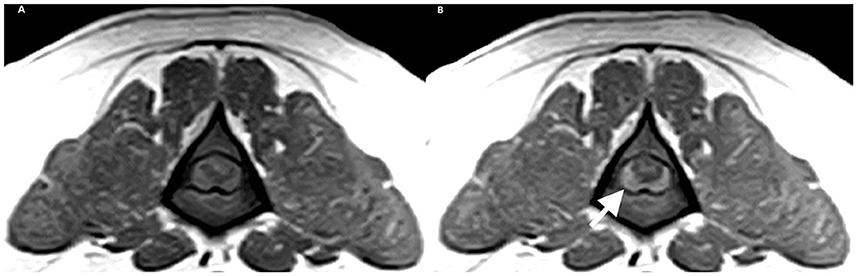
Figure 1. Transverse MRI of L3. (A) T1W and (B) T1W+C. White arrow showing a right 291 lateralized, contrast enhancing extramedullary compression.
In view of this diagnosis, intrathecal chemotherapy was recommended. Under general anesthesia, the patient was placed in right lateral recumbency, and following the aseptic preparation of the area, 1 mL of CSF was retrieved from the cisterna magna. Through the same needle used for CSF collection, we administered 100 mg of cytarabine arabinoside (100 mg/mL solution) and 2.5 mg of methotrexate (2.5 mg/mL, 25 mg/mL diluted in 0.9% sodium chloride) intrathecally. The injection was administered over the course of 1 min. The patient tolerated the procedure and recovered from general anesthesia uneventfully. He received a single dose of intravenous dexamethasone and continued the same dose orally for 7 days (0.2 mg/kg q24h IV/PO), amantadine hydrochloride (5 mg/kg PO q12h), and gabapentin (10 mg/kg PO q12h). As the patient had afebrile neutropenia during intrathecal chemotherapy (Table 1, day 42), prophylactic cefalexin (10 mg/kg PO q8h) was started according to the practice guidelines.
The dog remained hospitalized for 48 h for monitoring, and no complications were noted. Neurological clinical signs resolved within 24 h of treatment administration. Three days after intrathecal chemotherapy, systemic chemotherapy was continued as persistent atypical cells were circulating (Tables 1, 2, day 44). This corresponded to an overall delay of 2 days from the scheduled protocol. The dexamethasone dose was reduced to 0.1 mg/kg q24h PO, and cefalexin was discontinued after 9 days when resolution of neutropenia was documented (neutrophil count 3.32 × 109/L, R.I.: 3.0–11.5). During these assessments, the neurological examination remained unremarkable. 7 days after the administration of doxorubicin a reassessment confirmed a persistent complete clinical remission, and no neurological signs were reported or observed.
Twenty-five days after ITC, the patient presented with a relapse of neurological signs (severe thoracolumbar pain and hind limb deficits). There was no evidence of peripheral lymphadenopathy. A repeat ITC was declined. The dexamethasone dose was re-increased to 0.2 mg/kg PO q24h from 0.1 mg/kg PO q24h), and analgesia with amantadine hydrochloride (5 mg/kg PO q12h), paracetamol (10 mg/kg PO q12h), and gabapentin (10 mg/kg PO q8h) were introduced but did not have any clinical benefits. The patient was humanely euthanized after 48 h, 9 weeks after the initial presentation.
Methotrexate and cytarabine arabinoside are examples of chemotherapy agents with proven clinical benefits in canine lymphoma and penetration of the BBB when administered intravenously (14–17). The diffusion of medications through the BBB is influenced by their molecular weight, ionization at physiological pH, protein binding, liposolubility, and interaction with MDR1 receptors (18). In human medicine, some will advocate for the use of high doses of methotrexate (e.g., 6–8 g/m2) systemically instead of intrathecal administration (6, 19, 20). However, such doses in dogs are associated with high toxicity, and this is not a treatment option (16). Cytarabine is thus administered intravenously as part of multi-agent protocols for the management of CNS neoplasia in dogs (17). In the case described, the response to cytarabine and prednisolone was mild and of short duration. Lomustine (cytotoxic agent with CNS penetration) was considered to induce CNS remission; however, our patient developed progressive peripheral disease. Therefore, at the time of the diagnosis of CNS relapse in the current case, intrathecal administration of chemotherapy was the best treatment option available. A standard dose of 100 mg of cytarabine and 2.5 mg of methotrexate is recommended. Dose adjustments, hypothetically extrapolating from human medicine, may be required in younger patients as the CSF's volume remains stable from the age of 3 years and is not influenced by body weight. Genoni et al. (11) administered the standard dose to patients aged 7 months to 14 years without significant complications in younger patients or those with a lower surface area (ranging from 3.5 to 53 kg). However, in cats, the dose of cytarabine was 50 mg and methotrexate 2.5 mg (11). Based on the information available, no dose adjustments are recommended based on age or body weight in dogs.
As previously described, the combination of cytarabine arabinoside and methotrexate administered intrathecally is well tolerated, and the clinical decision was made to use both medications (11). LaRue et al. (12) described a case of relapsed CNS lymphoma treated with ITC with cytarabine arabinoside only, and no response was reported (euthanized after 3 days). The other five cases reported received additional local treatment, including CNS radiotherapy, which led to a rapid improvement of clinical signs and clearance of CSF lymphoid neoplastic cells between 2 and 10 days later (12, 13). One case received six ITC treatments (2 x weekly) and six CNS RTs (Monday, Wednesday, and Friday) alongside systemic chemotherapy and lived for 84 days (13). A second case lived for 286 days after receiving ITC, radiotherapy (total dose of 44 Gy), and systemic chemotherapy (CHOP and rescue CCNU). However, information about the treatment schedule and response is not described. The remaining three cases were euthanized within 10 days due to neurological deterioration or suspected adverse effects of systemic chemotherapy (13). This combination of local therapy seems to induce rapid clinical remission, but radiotherapy was not available at our hospital. In our case, CNS clinical signs resolved within 24 h after ITC, and the response lasted for 3.5 weeks. As described in the human literature, repeat administrations may have been required for a sustained response.
Of the six dogs with CNS lymphoma treated with ITC reported in the literature, one was believed to have died due to tentorial herniation secondary to ITC (13). As suggested by the authors and described in the human literature, pre-treatment (for example, glucocorticoids) is essential for decreasing CSF pressure and theoretically decreasing this risk. Our case did not have any complications but had been pre-treated for 5 weeks. Case selection for a low complication rate associated with ITC may be detrimental to a favorable outcome. Factors such as disease burden, pre-treatment, the volume of CSF removed, the volume of medication administered, and overall health for general anesthesia should be considered before ITC.
LaRue et al. (12) identified a longer survival time in dogs with CNS lymphoma if treated with a multimodal approach, particularly chemotherapy. As intrathecal chemotherapy is not associated with myelotoxicity, concurrent treatment with systemic chemotherapy can be administered to manage the extra-CNS disease. In our case, there was no evidence of myelotoxicity after 2 days, and the prescribed CHOP-based rescue protocol could be continued. At the time of euthanasia, the patient did not have peripheral lymphadenopathy. The difference between the improvement of CNS deficits and peripheral disease (lymphadenopathy and circulating atypical cells) in our case suggests that there may be a benefit in combining local treatment (CNS radiotherapy, ITC) with systemic treatment (3, 5, 8).
Intrathecal chemotherapy may be considered in the selected cases of CNS lymphoma refractory to systemic chemotherapy with the penetration of the BBB. The combination of methotrexate and cytarabine arabinoside administered intrathecally induced a rapid, short-lasting (3.5 weeks) improvement in clinical signs and was safe overall.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Acquisition of data, analysis and interpretation of data, revising the article for intellectual content, and final approval of the completed article: FL, CD, and GBC. Drafting the article: FL. All authors contributed to the article and approved the submitted version.
The authors declare that this study received funding from Linnaeus Veterinary Limited. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
All authors were employed by company Linnaeus Veterinary Limited.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Portell CA, Sweetenham JW. Adult lymphoblastic lymphoma. Cancer J. (2012) 18:432–8. doi: 10.1097/PPO.0b013e31826b1232
2. Olmos-Jiménez R, Espuny-Miró A, Cárceles RC, Díaz-Carrasco MS. Practical aspects of the use of intrathecal chemotherapy. Farm Hosp. (2017) 41:105–29. doi: 10.7399/fh.2017.41.1.10616
3. Paul S, Short NJ. Central nervous system involvement in adults with acute leukemia: diagnosis, prevention, and management. Curr Oncol Rep. (2020) 24:427–36. doi: 10.1007/s11912-022-01220-4
4. Berg SL, Chamberlain MC. Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy and symptom management. Cancer Treat Res. (2005) 125:121–46. doi: 10.1007/0-387-24199-X_8
5. Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude total therapy study 16. J Clin Oncol. (2019) 37:3377–91. doi: 10.1200/JCO.19.01692
6. Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma. (2018) 59:3–13. doi: 10.1080/10428194.2017.1326597
7. Reid JH, Perissinotti AJ, Benitez L, Bixby DL, Burke P, Pettit K, et al. Impact of prophylactic intrathecal chemotherapy on CNS relapse rates in AML patients presenting with hyperleukocytosis. Leuk Lymphoma. (2020) 61:862–8. doi: 10.1080/10428194.2019.1691199
8. Tang J, Yu J, Cai J, Zhang L, Hu S, Gao J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. (2021) 138:331–43. doi: 10.1182/blood.2020010438
9. Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol. (2009) 88:193–201. doi: 10.1007/s00277-008-0645-y
10. Cortelazzo S, Ferreri A, Hoelzer D, Ponzoni M. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. (2017) 113:304–17. doi: 10.1016/j.critrevonc.2017.03.020
11. Genoni S, Palus V, Eminaga S, Cherubini GB. Safety of intrathecal administration of cytosine arabinoside and methotrexate in dogs and cats. Vet Comp Oncol. (2016) 14: 331–6. doi: 10.1111/vco.12109
12. LaRue MK, Taylor AR, Back AR, Lindley SE, Boudreaux BL, Almond GT, et al. Central nervous system lymphoma in 18 dogs (2001 to 2015). J Small Anim Pract. (2018) 59:547–52. doi: 10.1111/jsap.12868
13. Couto CG, Cullen J, Pedroia V, Turrel JM. Central nervous system lymphosarcoma in the dog. J Am Vet Med Assoc. (1984) 184:809–13.
14. Scott-Moncrieff JC, Chan TC, Samuels ML, Cook JR, Coppoc GL, DeNicola DB, et al. Plasma and cerebrospinal fluid pharmacokinetics of cytosine arabinoside in dogs. Cancer Chemother Pharmacol. (1991) 29:13–8. doi: 10.1007/BF00686329
15. Pawlak A, Kutkowska J, Obmińska-Mrukowicz B, Rapak A. Methotrexate induces high level of apoptosis in canine lymphoma/leukemia cell lines. Res Vet Sci. (2017) 114:518–23. doi: 10.1016/j.rvsc.2017.09.026
16. Rostang A, Mosca M, Jeannin M, Luissiez C, Berny P, Fourel I, et al. Pharmacokinetics of low-dose methotrexate in healthy beagle dogs. J Vet Pharmacol Ther. (2018) 41:659–69. doi: 10.1111/jvp.12673
17. Guillen A, Finotello R, Wynne P, Harper A, Killick D, Amores-Fuster I, et al. Toxicity of cytarabine constant rate infusion in dogs with high-grade non-Hodgkin lymphoma with bone marrow or central nervous system involvement. Aust Vet J. (2020) 98:69–78. doi: 10.1111/avj.12895
18. Ruggiero A, Conter V, Milani M, Biagi E, Lazzareschi I, Sparano P, et al. Intrathecal chemotherapy with antineoplastic agents in children. Paediatr Drugs. (2001) 3:237–46. doi: 10.2165/00128072-200103040-00001
19. Sierra DRM, Ricard D, Houillier C, Navarro S, Gonzalez-Aguilar A, Idbaih A, et al. Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol. (2012) 106:143–6. doi: 10.1007/s11060-011-0649-7
20. Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from children's oncology group study AALL0232. J Clin Oncol. (2016) 34:2380–8. doi: 10.1200/JCO.2015.62.4544
21. Vail DM, Michels GM, Khanna C, Selting KA, London CA, Veterinary Cooperative Oncology Group. Response evaluation criteria for peripheral nodal lymphoma in dogs (v10)–a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. (2010) 8:28–37. doi: 10.1111/j.1476-5829.2009.00200.x
Keywords: intrathecal chemotherapy, CNS lymphoblastic lymphoma, neuro-oncology, methotrexate, cytarabine arabinoside
Citation: Lyseight FLS, Dupont COC and Cherubini GB (2023) Intrathecal chemotherapy for the management of lymphoblastic lymphoma in a 4-year-old dog: a case report. Front. Vet. Sci. 10:1209935. doi: 10.3389/fvets.2023.1209935
Received: 21 April 2023; Accepted: 25 July 2023;
Published: 05 September 2023.
Edited by:
R. Timothy Bentley, Purdue University, United StatesCopyright © 2023 Lyseight, Dupont and Cherubini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filipa Louise Simões Lyseight, ZmlsaXBhLmx5c2VpZ2h0QGR3ci5jby51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.