
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 15 August 2023
Sec. Veterinary Humanities and Social Sciences
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1203481
This article is part of the Research TopicHow Animals Affect Us: Examining the Influence of Human-Animal Interactions on Human’s HealthView all 13 articles
 Alexandra N. Stergiou1,2*
Alexandra N. Stergiou1,2* Sanna Mattila-Rautiainen3*
Sanna Mattila-Rautiainen3* Dimitrios N. Varvarousis4
Dimitrios N. Varvarousis4 Meropi Tzoufi5
Meropi Tzoufi5 Panagiota Plyta1,2
Panagiota Plyta1,2 Alexandros Beris6
Alexandros Beris6 Avraam Ploumis1
Avraam Ploumis1Purpose: To evaluate the efficacy of Equine Assisted Therapy in children with Cerebral Palsy, in terms of gross motor function, performance, and spasticity as well as whether this improvement can be maintained for 2 months after the end of the intervention.
Methods: Children with Cerebral Palsy participated in this prospective cohort study. The study lasted for 28 weeks, of which the equine assisted therapy lasted 12 weeks taking place once a week for 30 min. Repeated measures within the subject design were used for the evaluation of each child’s physical performance and mental capacity consisting of six measurements: Gross Motor Function Measure-88 (GMFM-88), Gross Motor Performance Measure (GMPM), Gross Motor Function Classification System (GMFCS), Modified Ashworth Scale (MAS) and Wechsler Intelligence Scale for Children (WISC III).
Results: Statistically significant improvements were achieved for 31 children in Gross Motor Function Measure and all its subcategories (p < 0.005), also in total Gross Motor Performance Measure and all subcategories (p < 0.005). These Gross Motor Function Measure results remained consistent for 2 months after the last session of the intervention. Regarding spasticity, although an improving trend was seen, this was not found to be statistically significant.
Conclusion and implications: Equine Assisted Therapy improves motor ability (qualitatively and quantitatively) in children with Cerebral Palsy, with clinical significance in gross motor function.
Cerebral Palsy (CP) is a permanent, non-progressive encephalopathy that occurs in the brain during its development, before, during the birth, and up to 2 years after the birth (1–3). Children with CP have atypical posture and gait patterns due to abnormal muscle tone, reduced control of their muscles, static and dynamic imbalance, incoordination and asymmetry between agonist and antagonist muscles and poor equilibrium reflexes (4, 5). The main target of any therapeutic intervention is to enable patients to carry out daily activities and participation as independently as possible (International Classification of Functioning d230) (6–8).
In equine assisted therapy (EAT) the movement of the horse is utilized to improve functional and sensory limitations of individuals with movement disorders (9, 10). During EAT the muscles strengthen and the range of motion of joints is improved. Also, their stability, the coordination of the movement, the synergy of muscles, the displacement of weight shift and the control of the balance (11, 12) are improved while the oscillation of the patient is reduced due to its effort to maintain posture on the horseback (13–15). EAT also enhances the stability of the hip and trunk with hip and pelvic flexibility (16, 17).
Studies have shown that EAT is beneficial for children with CP for motor function abilities (13, 18–22), standing (13, 20, 23, 24) and sitting balance (25–27), gait parameters (13, 19, 28), the reduction of spasticity (23, 29–31), the symmetry of muscle activity (32), the joint range of motion (29) as well as in psychosocial domains and quality of life (18, 19, 21, 33, 34). The common outcome measures in the above studies were the Gross Motor Function Measure (GMFM) and Pediatric Balance Scale (PBS).
Furthermore, the duration of the positive effect of the EAT after its termination is questionable, having not been extensively investigated. In the literature, only two studies (35, 36) using GMFM found that participants had positive results in their gross motor function that was maintained from seven to 10 weeks, following the completion of the intervention.
This study aimed to investigate the effect of EAT on gross motor function, performance, and spasticity in children with CP in terms of GMFM, Gross Motor Performance Measure (GMPM) and Modified Ashworth Scale (MAS). This study also aims to investigate whether these improvements continue to exist after a two-month follow-up from the completion of the intervention.
This prospective cohort study was registered in the clinical trials database (NCT01621984 Unique Protocol ID: 274/21-9-2011) and was approved by the Scientific Committee [12/24-8-2011 (θ.17)] and Administration Board (38/3–102,011 θ.33) of the University Hospital of Ioannina. All procedures performed in studies involving human or animal participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Participants were sought within the registry of the Department of Physical Medicine and Rehabilitation and the Pediatrics Department of the General University Hospital of Ioannina, as well as through the non-profit organization “MERIMNA.” Informed consent was signed by the parents or caregivers after informing the purposes of the study and were assured the confidentiality of the personal data. Assessment and selection of children who met the inclusion criteria followed as outlined below. This study conforms to all CONSORT and STROBE guidelines and reports the required information accordingly.
The Inclusion criteria included: (1) children aged from 3 to 18 years old with CP; (2) written parental consent; (3) children with adequate range of motion to sit astride on the horse (participants should also have at least partial head control).
Exclusion criteria included: (1) unregulated epileptic seizures; (2) any musculo-skeletal disorder which could be aggravated by the motion of the horse; (3) allergy to the dust of the riding arena; (4) previous experience in EAT (5) botulinum toxin injections in any muscle during the last six months and (6) any surgery within a year prior to the study.
The Gross Motor Function Measure is the most common quantitative outcome measure used for children with CP (37, 38) to evaluate a change occurring over time in gross motor ability after various clinical interventions (37, 38). It quantitatively measures gross motor function, an activity a child can do (GMFM-88) or a level of motor ability achieved (GMFM-66) (38). GMFM-88, which was used for the present study, includes 88-point assessment criteria, being distributed across 5 categories: (Α) lying and rolling, (Β) sitting, (C) crawling and kneeling, (D) standing and (E) walking, running & jumping. Each category is comprised of several elements graded from 0 to 3 units (0 = the child is not able to start an activity and 3 = the child is able to fully complete the activity). It has been studied for its reliability and validity (37–39).
The Gross Motor Performance Measure assessed the quality of movement, which means how well an activity is completed. It has been designed to be used in combination with GMFM (40, 41). It is a criterion-based observational measure evaluating five different aspects of quality of movement: alignment, stability, coordination, weight shift and dissociation over 20 GMFM items (40, 41). It additionally has been studied for its reliability and validity (41, 42).
The Modified Ashworth Scale is a measure of resistance to passive stretch which has been studied for its reliability and validity. A six-point numerical scale (0, 1, 1+, 2, 3, 4) grade spasticity from zero to four, with zero being no resistance and four being a joint rigid in flexion or extension (43, 44). While resistance and passive movement from the hip joint were both measured from five repetitions.
Gross motor function and performance, as well as spasticity of all participants who were included in the study, were assessed. All participants were categorized cognitively, with Wisc III, and motorically, with the GMFCS. The total number of children was subdivided into two subgroups, the first included children with severe deficits and the second included children with mild and moderate deficits, according to their cognitive or/and functional capacity. This was completed to identify changes in cognitive and gross motor capacity (progress of the functionality) within these two groups of children.
For each patient, six measurements took place using the GMFM scale and the MAS. The GMPM scale was measured at two different time points, before and after the intervention (Table 1). Successive assessments of the children were carried out by two independent researchers (AP, DV) who were experienced in the use of the assessments and were blinded to the results of previous assessments. The evaluators of Wisc III were child psychiatrists who were blinded to the study and this assessment took place in State Pediatrics Educational Center in Ioannina.
The equine assisted therapy lasted for 3 months (12 weeks) each session consisting of 30 min of exercise on the horseback taking place every week at the Ioannina Therapeutic Riding Center, Greece. Participants continued to receive their conventional rehabilitation program throughout the pre-and post-intervention as well as during the intervention.
Three horses, trained for therapy purposes, of varying sizes were used to match the sizes of the participants. Two qualified professionals in EAT carried out the intervention which was individualized to the needs of every child. Trained side-walkers ensured the safety of the mounted child and horse leaders followed the instructions from the EAT practitioner for the individualized walking rhythm of the horse. A soft saddle pad with a vaulting girdle was used for the children to be able to perceive the horse’s temperature and transmitted movement more easily (13, 45). Adjustable stirrups in the vaulting girdle were used for performing exercises, such as sitting and standing up (45).
All children wore protective riding helmets. Children according to their ability mounted the horse from a mounting ramp with assistance, independently or were passively placed on the horseback by the professional leading the session. The EAT sessions were carried out depending on the children’s classification of performance ability according to GMFCS and mental capacity according to Wisc III.
The horse was being led in straight lines, in circles, or a “figure of eight” between cones and serpentines and the child was sitting on the horseback with the eyes open or closed. The horse’s gait also varied (moderate to fast walking and trotting) (45, 46). One goal for the participant was to be able to sit independently, with good alignment and symmetry (47).
The ones that were able to follow directions, either because their mental capacity (Wisc III) allowed them to do so or because of their functional capacity (GMFCS I, II, III) or as an outcome of a combination of the above, played a more active role in their therapy and performed more complicated activities.
Each child, depending on motor ability, actively or passively changed position while on the horse (i.e., sitting astride or laying back or in front on the neck of the horse or sitting sideways on the horse) (48, 49). Different body positions on the moving horse ensured that the child would receive multiple vestibular stimuli (46, 48, 49) Stirrups were used (50) so that the child would be able to lift himself and sit back again (48, 51) to strengthen the lower limbs and to improve in shifting the centre of gravity and balance (48).
In order to attain the objectives, set for the EAT intervention, a series of exercises were performed. Exercises that were masked to a form of play were easier to perform. The exercises performed consisted of catching and tossing a ball, throwing rings on the cones, throwing bean bags on the basket, and searching for hidden objects on the horse by catching and tossing a ball to a basket from a moving horse the eye-hand coordination, planning, timing and needed force to perform the task were trained. Hiding objects underneath a saddle pad was aimed to train body orientation and problem-solving.
Children of classification IV and V in GMFCS in combination with respective Wisc III classification, children presenting serious and severe mental disabilities, received passive mobilization of their body on the horseback, directional changes, gradually building up the stimuli depending on their needs and limitations.
To enable active participation with performance wherever possible, a passive or active-assisted approach was applied for exercises of the trunk and extremities (reaching, weight shifting), while on the horse, to increase the range of motion. An effort was made for their active participation wherever this was possible.
Statistical analyses were conducted using Stata 14.1 (StataCorp, College Station, TX, United States). A longitudinal analysis was performed for the GMFM. Univariate and multivariate mixed-effects linear regression models were used (time series analysis was used based on single and multiple linear mixed-effects models with individuals as random effects). In the univariate models, the time of measurement was the primary variable. The comparison for the subgroup was done with paired t-test. The results were considered significant at the level 0.05.
The comparison of the GMPM scale values was done in a univariate manner with paired t-tests and in a multivariate way using longitudinal analysis methods.
To compare the values of the MAS at different time points, Fisher’s exact test was used. In the multivariate models, the results were adjusted for possible confounding variables, such as gender, age, and assessment based on Wisc III and the GMFCS level for the aforementioned three types of assessment tools. Data on MAS, GMFM and its subcategories were available for six-time points. The first three took place before the intervention. For increased accuracy with respect to the initial measurement of MAS and GMFM, the mean of the three GMFM measurements prior to intervention was used.
Thirty-five children fulfilled the initial inclusion criteria and were included in the study. One child withdrew immediately after the first assessment; another one after the second; and two others after the third assessment, all prior to the start of the intervention because the caregiver did not see a benefit to the intervention. Finally, 31 children participated in the EAT intervention (Table 2).
From the 31 children, two subgroups were divided according to their mental or/and functional capacity. Eleven children with severe deficits, and 20 children with mild and moderate deficits. The term severe impairment referred to children with a low score in WISC III (profound and severe, that usually are not able to follow rules and communicate) or/and are classified as Level III, IV, V in GMFCS. Children with severe deficits needed more assistance from the therapist in contrast with children with mild and moderate impairments that they could be more active in the intervention due to their higher cognitive and motor function level (Table 2).
Of 31 children, 29 participated in the last assessment (assessment no 4) that took place 2 months after the end of the intervention. The total score of GMFM increased significantly (p < 0.0001) after 6 weeks (assessment no 2) (mean difference = 4.92) as well as after 12 weeks (assessment no 3) of the intervention (mean difference = 8.05) (Table 3; Figure 1). Nevertheless, a statistically significant decrease (p = 0.0217) in the total GMFM score was observed between the 3rd third (at the 12th week, the end of the intervention) and the fourth 4th assessment (2 months after the end of the intervention). However, the total GMFM in the 4th assessment was still significantly better (p < 0.005) than the 1st assessment and about the same as the 2nd assessment (mean difference = 2.18). Statistically significant improvements were also observed in all subcategories of GMFM (A-D) but not in subcategory E (walking running and jumping) of non-ambulatory children (level V of GMFCS) (p > 0.005). A greater improvement of the total score was found for children classified as GMFCS III (13.65), then IV (10.61), then ΙΙ (9.98), V (4.26) and lastly Ι (3.03) (differences between assessments 1st and 3rd).
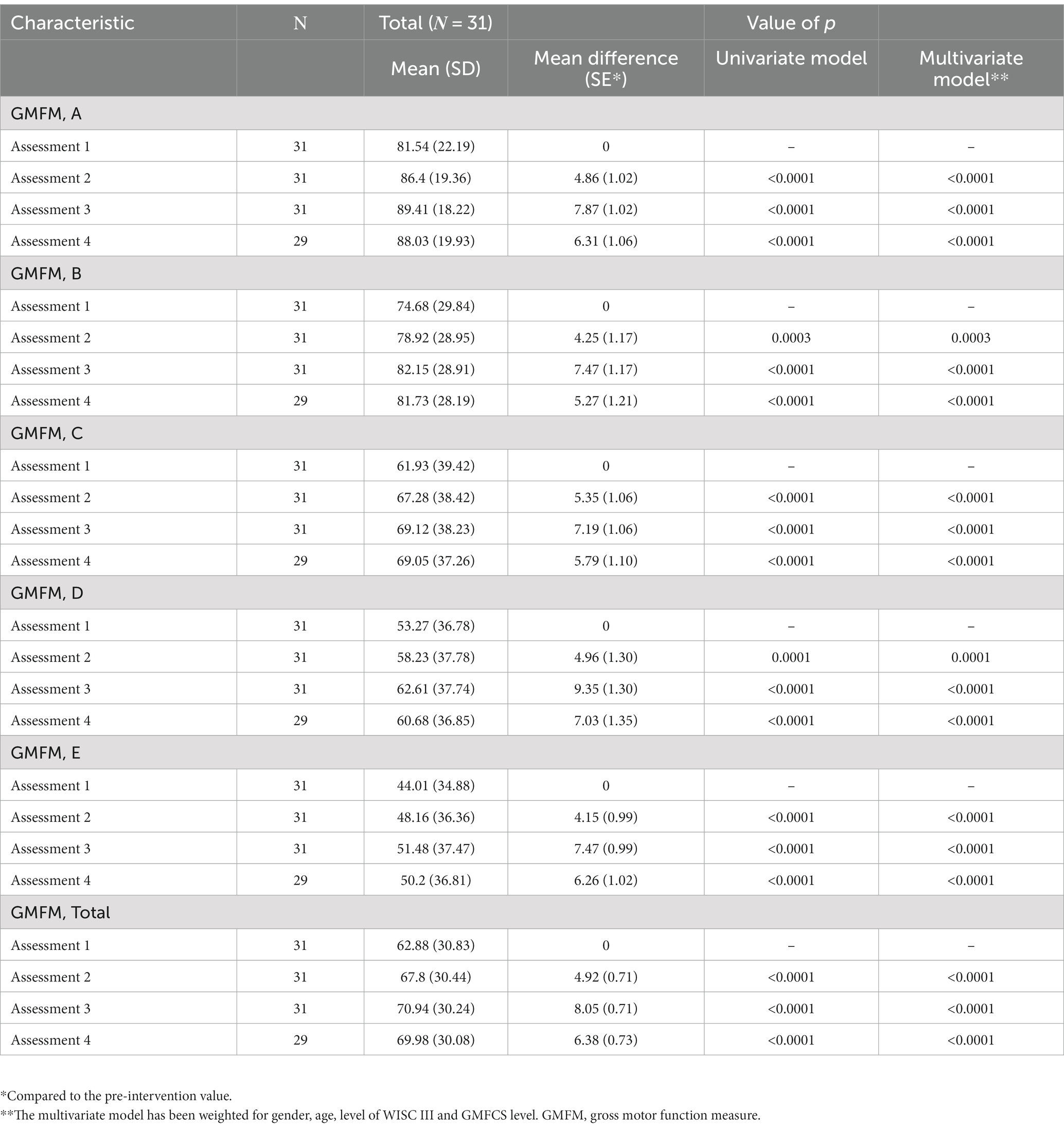
Table 3. Comparison of the GMFM and dimensions (A, B, C, D, E) between the different time points (assessments 2, 3, 4) and the initial measurement (assessment 1).
Gross motor performance measure was measured of 29 participants. Distribution across the GMPM scale and its subcategories, for two different time points is presented in Table 4. A statistically significant increase in GMPM and all its subcategories was achieved (Table 4). Children classified as level I (8.17) on the GMFCS showed greater improvement in the total score, followed by levels V (6.66), IV (6.25), III (5.58) and lastly, II (4.93) (differences between assessments 1st and 3rd).
Nineteen of the participants had spasticity and three of them did not participate at the last assessment 2 months after the end of the intervention. Ashworth scale values of the different time points are presented in Table 5. A decrease in spasticity is seen over the time points, but it was not found to be statistically significant per Fisher’s exact test criterion (p = 0.350).
All participants, regardless of mild–severe deficits, demonstrated statistically significant improvement in the GMFM (mean difference = 7.16 and 9.67 respectively) and GMPM (mean difference 6.53 and 6.19 respectively) (p < 0.05) (Table 6). No statistical significance was observed between the two groups.
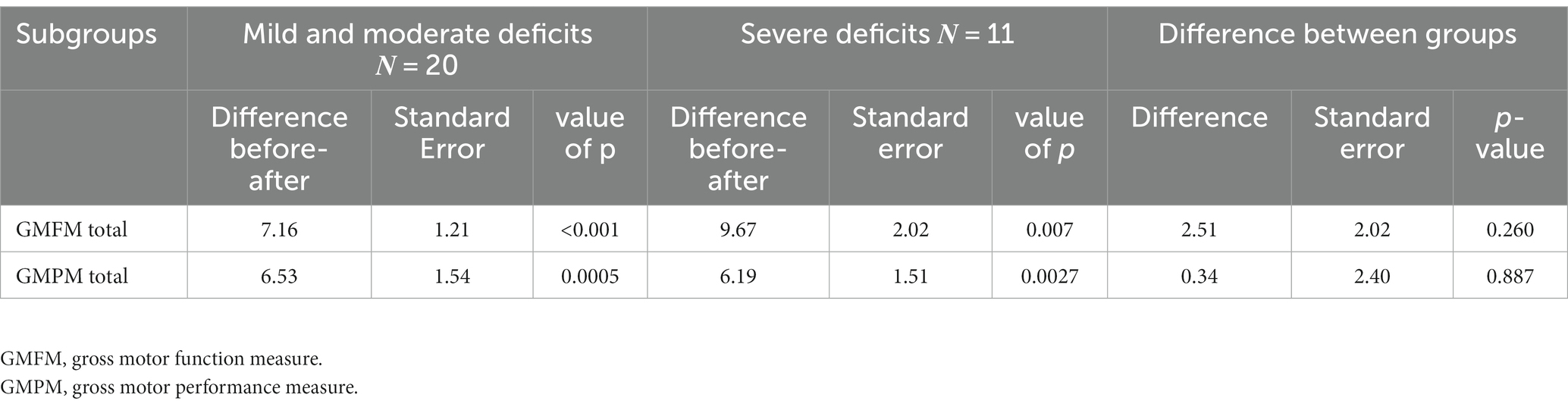
Table 6. Mean (SD) total GMFM and GMPM in subcategories of children with 1. mild and moderate deficits and 2. severe deficits.
According to the literature (52), clinically important improvement was observed (average low-value differential >1.29 and high value >3.99) in all GMFM analyses between the initial assessment (assessment no 1) and the 12th-week assessment (assessment no 3), regarding the total number of the children (8.06), but also in the subgroups of children with mild and moderate deficits (7.16) and children with severe deficits (9.67). The same was observed between the initial and the final assessment. This is a high-power study (100.00 and 95.86%, respectively, for average low differential and average high differential MCID) according to the post-hoc power estimation for Minimal Clinically Important Differences.
There were no adverse events related to the intervention. None of the participants suffered any injury or had any other complication during the study.
This prospective study aimed to assess the effectiveness of EAT intervention in children with CP. The aforementioned results state, that the participants demonstrated improved GMFM scores that met the criteria of MCID at the last follow-up. All groups show statistical significance (p < 0.005) between the assessments (Table 4). An important note is that the results of the intervention show, that there is no statistical difference in the outcomes between the CP subgroups (mild and moderate vs. severe). As it is not correct to compare unsimilar groups we can see significant improvement in both subgroups, stating that the objectives for the rehabilitation were achieved regardless of the level of CP. The results for spasticity showed improvement but were not statistically significant.
In the literature, many studies have shown statistically significant improvement in some subcategories of GMFM and total score of GMFM-66 and GMFM-88 depending on the classification (level Ι to ΙV in GMFCS) (13, 20, 35, 47, 49, 50, 53). In our study, statistically significant improvement was observed in all subcategories and the total score of GMFM-88, but, as was expected, there was no statistically significant improvement in subcategory E (walking, running and jumping) of non-ambulatory children (level V of GMFCS).
In two other studies (35, 36), GMFM measurements took place at 7 and 10 weeks following the completion of the intervention, respectively, and found that positive results of GMFM were maintained (no statistically significant difference was noticed from the termination of intervention to the last follow-up). Similarly, in our study, the significant improvement of GMFM was maintained 8 weeks after the completion of the intervention, which was also clinically significant (according to MCID). This means that the increase of GMFM of patients being rehabilitated with EAT (following equine assisted exercises) was leading to better functional abilities.
Regarding GMPM, the current study showed that EAT intervention improved the quality of movement of children with motor dysfunctions. Similar studies in the international literature using GMPM, investigate the benefits of different types of therapeutic exercise with heterogeneous results (54–56).
In the study of MacKinnon et al. (21) children who were able to cooperate better (due to their mental capacity and functional skills) showed increased motor development. Based on our results using GMFM and GMPM and according to GMFCS level, it was observed that in quantitative measurement of motion (GMFM) children classified as II and III improved more compared to other subcategories, while children classified as I and V improved less. The opposite happens concerning the quality of movement (GMPM). A possible theory could be that EAT intervention benefits more children with mild and moderate functional deficits in gross motor function, while children with independent functionality or severe motor disorders are mainly benefited in terms of the quality of movement. Probably children with independent functionality (level I) as well as with severe motor disorders (V) cannot give statistically significant results in contrast to mild and moderate motor deficits (II, III) where the intervention seems to give statistically significant results.
While statistically significant differences were found in our measures, this does not necessarily translate to clinically important differences. Minimal Clinically Important Differences (MCID) provide the threshold for determining if clinically important differences take place before and after an intervention (57). Our study has shown the clinical significance of the change in gross motor function of children by the equine assisted therapy intervention. In the study of Davis et al. (33) both the statistical and clinical significance of gross motor function changes were not proven results, while in a review study by Little et al. (58), the clinically meaningful effect of hippotherapy on gross motor function in the short term was small.
Regarding spasticity and MAS, one randomized trial (59) and one meta-analysis (60) showed a statistically significant improvement in adductor spasticity (32, 59) and generally in the muscles of the pelvis and lower limbs (29, 61, 62). Nevertheless, in another study (63), results were similar to ours, since no statistically significant improvement had been observed concerning adductor spasticity. Antunes et al. (64) noticed an improvement in adductor spasticity when horse walking and trotting were included. It is worth mentioning that the benefits of this intervention were short-term (32, 59–62, 64). This is shown by the fact that measurements in these studies were made just before and after the treatment was completed (32, 61, 64), in contrast to our study, where spasticity was measured at a pre-determined appointment after the intervention. The above studies are possibly based on the fact that prolonged muscle stretching for a period of 10–30 min is effective in reducing spasticity and may last up to 35 min after exercise completion (65). It has also been mentioned that spasticity improvement has been maintained up to at least 4 days (62). The fact that in our study final assessments for each patient took place in a period of 1–5 days after the end of the intervention, may hide the possible beneficial immediate effect of EAT on spasticity.
The strength of the study is the relatively large number of participants in comparison to other studies (10, 11, 17, 60, 66–68), as well as the participation of children of various functional levels and with a diversity of motor dysfunctions. It also categorized the results in children with different functional levels. The intervention was led by different professionals than those conducting the assessments and were blinded. Another strength was, that the horses with staff stayed the same for the children throughout the intervention.
A disadvantage of our study was that children varied greatly in functional classification in all subcategories according to GMFCS and Wisc III. So, in children of lower classification in Wisc III, communication and cooperation were difficult. We may have had more significant improvement with an intervention, which would have lasted several months longer or the conditions were designed for the children to participate more frequently in the therapy. This research, as others (47, 49, 50), uses the same children as a control group before and after the intervention, instead of other participants who would not have gone through any EAT as in a typical control group. This may be initially seen as a study limitation, but in reality, it may also be a sensitive way of detecting even the slightest therapeutic changes (49), since the development of each child with CP may vary. The children continued to receive their conventional therapies throughout the intervention period and follow-up. Even though the strength of the statistical analysis for the modified Ashworth scale changes is low, the results are valid for our sample.
The findings of this study support that EAT may improve gross motor function and performance in children with CP, even 2 months after the end of the intervention. These findings should be reinforced with more research, as many clinically significant results were found.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Scientific Committee [12/24-8-2011 (θ.17)] and Administration Board (38/3–102,011 θ.33) of the University Hospital of Ioannina. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
AS: conceptualization, formal analysis, investigation, methodology, visualization, and roles and writing – original draft. SM-R: data curation, methodology, software, and writing – review and editing. DV: conceptualization, investigation, resources, software, visualization, roles and writing – original draft, and writing – review and editing. MT: conceptualization, investigation, project administration, and supervision. PP: data curation, investigation, and resources. AB: formal analysis, software, supervision, and writing – review and editing AP: conceptualization, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, and writing – review and editing. All authors contributed to the article and approved the submitted version.
This work was funded by the Ann Kern-Godal’s grant (No:11297) Unifor, Norway. One of the authors (SM-R) holds a shared doctoral researcher position at the University of Eastern Finland.
This manuscript and research, including the intervention, would not have been possible without the children and their families, who were brave to take part in and commit to the rehabilitation. Special thanks to the Kallirroi Zikidou, Anneta Rita, and Andreas Paterousis of the non-profit organization “MERIMNA”, for their collaboration to organize the participation of the children of “MERIMINA” in this study. We would also like to thank George Markozanes for his contribution to the statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CP, Cerebral Palsy; EAT, Equine Assisted Therapy; GMFCS, Gross Motor Function Classification System; GMFM, Gross Motor Function Measure; GMPM, Gross Motor Performance Measure; MAS, Modified Ashworth Scale; Wisc III, Wechsler Intelligence Scale for Children, 3rd edition.
1. Rosenbaum, P , Paneth, N , Leviton, A , Goldstein, M , Bax, M , Damiano, D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. (2007) 109:8–14.
3. Unnithan, VB , Clifford, C , and Bar-Or, O . Evaluation by exercise testing of the child with cerebral palsy. Sports Med. (1998) 26:239–51. doi: 10.2165/00007256-199826040-00003
4. Krivickas, LS . Exercise in neuromuscular disease. J Clin Neuromuscul Dis. (2003) 5:29–39. doi: 10.1097/00131402-200309000-00004
5. Gage, JR . Gait analysis in cerebral palsy. Clinics in developmental medicine. London: McKeith Press (1991). 121 p.
6. Levitt, S . Treatment of cerebral palsy and motor delay, vol. xiv. 3rd ed. Oxford, UK; Cambridge, MA, USA: Blackwell Science (1995). 341 p.
7. Imms, C . Children with cerebral palsy participate: a review of the literature. Disabil Rehabil. (2008) 30:1867–84. doi: 10.1080/09638280701673542
8. Bickenbach, JE . ICF core sets: manual for clinical practice. 2nd ed. Boston: Hogrefe Publishing (2020).
9. PATH International (2013). Available at: http://www.pathintl.org/ (Acessed January 19, 2013).
10. Sterba, JA . Does horseback riding therapy or therapist-directed hippotherapy rehabilitate children with cerebral palsy? Dev Med Child Neurol. (2007) 49:68–73. doi: 10.1017/S0012162207000175.x
11. Zadnikar, M , and Kastrin, A . Effects of hippotherapy and therapeutic horseback riding on postural control or balance in children with cerebral palsy: a meta-analysis. Dev Med Child Neurol. (2011) 53:684–91. doi: 10.1111/j.1469-8749.2011.03951.x
12. Silkwood-Sherer, DJ , Killian, CB , Long, TM , and Martin, KS . Hippotherapy—an intervention to habilitate balance deficits in children with movement disorders: a clinical trial. Phys Ther. (2012) 92:707–17. doi: 10.2522/ptj.20110081
13. Kwon, JY , Chang, HJ , Lee, JY , Ha, Y , Lee, PK , and Kim, YH . Effects of hippotherapy on gait parameters in children with bilateral spastic cerebral palsy. Arch Phys Med Rehabil. (2011) 92:774–9. doi: 10.1016/j.apmr.2010.11.031
14. Janura, M , Peham, C , Dvorakova, T , and Elfmark, M . An assessment of the pressure distribution exerted by a rider on the back of a horse during hippotherapy. Hum Mov Sci. (2009) 28:387–93. doi: 10.1016/j.humov.2009.04.001
15. Sakakura, M , Barros Santos, R , Cyrillo, F , Perdigao, A , and Torriani, C . Electromyography comparative analysis of lumbar erector muscle with a cerebral palsy patient performing different postures on horseback. Scientific & Educational Journal of Therapeutic Riding for the Disabled International (2006): 38–44.
16. Encheff, JL , Armstrong, C , Masterson, M , Fox, C , and Gribble, P . Hippotherapy effects on trunk, pelvic, and hip motion during ambulation in children with neurological impairments. Pediatr Phys Ther. (2012) 24:242–50. doi: 10.1097/PEP.0b013e31825c1dc3
17. Snider, L , Korner-Bitensky, N , Kammann, C , Warner, S , and Saleh, M . Horseback riding as therapy for children with cerebral palsy: is there evidence of its effectiveness? Phys Occup Ther Pediatr. (2007) 27:5–23. doi: 10.1300/J006v27n02_02
18. Deutz, U , Heussen, N , Weigt-Usinger, K , Leiz, S , Raabe, C , Polster, T, et al. Impact of Hippotherapy on gross motor function and quality of life in children with bilateral cerebral palsy: a randomized open-label crossover study. Neuropediatrics. (2018) 49:185–92. doi: 10.1055/s-0038-1635121
19. Mutoh, T , Mutoh, T , Tsubone, H , Takada, M , Doumura, M , Ihara, M, et al. Impact of Long-term Hippotherapy on the walking ability of children with cerebral palsy and quality of life of their caregivers. Front Neurol. (2019) 10:834. doi: 10.3389/fneur.2019.00834
20. Kwon, JY , Chang, HJ , Yi, SH , Lee, JY , Shin, HY , and Kim, YH . Effect of hippotherapy on gross motor function in children with cerebral palsy: a randomized controlled trial. J Altern Complement Med. (2015) 21:15–21. doi: 10.1089/acm.2014.0021
21. Mackinnon, JR , Noh, S , Lariviere, J , Macphail, A , Allan, DE , and Laliberte, D . A study of therapeutic effects of horseback riding for children with cerebral palsy. Phys Occup Ther Pediatr. (1995) 15:17–34. doi: 10.1080/J006v15n01_02
22. Santos de Assis, G , Schlichting, T , Rodrigues Mateus, B , Gomes Lemos, A , and Dos Santos, AN . Physical therapy with hippotherapy compared to physical therapy alone in children with cerebral palsy: systematic review and meta-analysis. Dev Med Child Neurol. (2022) 64:156–61. doi: 10.1111/dmcn.15042
23. Alemdaroglu, E , Yanikoglu, I , Oken, O , Ucan, H , Ersoz, M , Koseoglu, BF, et al. Horseback riding therapy in addition to conventional rehabilitation program decreases spasticity in children with cerebral palsy: a small sample study. Complement Ther Clin Pract. (2016) 23:26–9. doi: 10.1016/j.ctcp.2016.02.002
24. Silkwood-Sherer, DJ , and McGibbon, NH . Can hippotherapy make a difference in the quality of life of children with cerebral palsy? A Pragmatic Study Physiother Theory Pract. (2022) 38:390–400. doi: 10.1080/09593985.2020.1759167
25. Kang, H , Jung, J , and Yu, J . Effects of Hippotherapy on the sitting balance of children with cerebral palsy: a randomized control trial. J Phys Ther Sci. (2012) 24:833–6. doi: 10.1589/jpts.24.833
26. Matusiak-Wieczorek, E , Dziankowska-Zaborszczyk, E , Synder, M , and Borowski, A . The influence of Hippotherapy on the body posture in a sitting position among children with cerebral palsy. Int J Environ Res Public Health. (2020) 17:6846. doi: 10.3390/ijerph17186846
27. Matusiak-Wieczorek, E , Malachowska-Sobieska, M , and Synder, M . Influence of Hippotherapy on body balance in the sitting position among children with cerebral palsy. Ortop Traumatol Rehabil. (2016) 18:165–75. doi: 10.5604/15093492.1205024
28. Hyun, C , Kim, K , Lee, S , Ko, N , Lee, IS , and Koh, SE . The short-term effects of Hippotherapy and therapeutic horseback riding on spasticity in children with cerebral palsy: a meta-analysis. Pediatr Phys Ther. (2022) 34:172–8. doi: 10.1097/PEP.0000000000000880
29. Baik, K , Byeun, JK , and Baek, JK . The effects of horseback riding participation on the muscle tone and range of motion for children with spastic cerebral palsy. J Exerc Rehabil. (2014) 10:265–70. doi: 10.12965/jer.140124
30. Lucena-Anton, D , Rosety-Rodriguez, I , and Moral-Munoz, JA . Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. (2018) 31:188–92. doi: 10.1016/j.ctcp.2018.02.013
31. Cunha, AB , Novaes, GF , Rezende, LC , Correa, MMD , Garbellini, D , Maluf, E, et al. Hippotherapy results on muscular tonus of lower limbs and motor performance in children with spastic cerebral palsy. Hores in education and therapy International. (2007) 13:2–4.
32. Benda, W , McGibbon, NH , and Grant, KL . Improvements in muscle symmetry in children with cerebral palsy after equine-assisted therapy (hippotherapy). J Altern Complement Med. (2003) 9:817–25. doi: 10.1089/107555303771952163
33. Davis, E , Davies, B , Wolfe, R , Raadsveld, R , Heine, B , Thomason, P, et al. A randomized controlled trial of the impact of therapeutic horse riding on the quality of life, health, and function of children with cerebral palsy. Dev Med Child Neurol. (2009) 51:111–9. doi: 10.1111/j.1469-8749.2008.03245.x
34. Mattila-Rautiainen, S , Venojarvi, M , Rautiainen, H , and Keski-Valkama, A . The impact on physical performance, pain and psychological wellbeing of chronic low back pain patients during 12-weeks of equine-facilitated therapy intervention. Front Vet Sci. (2023) 10:1085768. doi: 10.3389/fvets.2023.1085768
35. Champagne, D , Corriveau, H , and Dugas, C . Effect of Hippotherapy on motor proficiency and function in children with cerebral palsy who walk. Phys Occup Ther Pediatr. (2017) 37:51–63. doi: 10.3109/01942638.2015.1129386
36. Winchester, P , Kendall, K , Peters, H , Sears, N , and Winkley, T . The effect of therapeutic horseback riding on gross motor function and gait speed in children who are developmentally delayed. Phys Occup Ther Pediatr. (2002) 22:37–50. doi: 10.1300/J006v22n03_04
37. Alotaibi, M , Long, T , Kennedy, E , and Bavishi, S . The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): a literature review. Disabil Rehabil. (2014) 36:617–27. doi: 10.3109/09638288.2013.805820
38. Russell, DJ , Rosenbaum, PL , Cadman, DT , Gowland, C , Hardy, S , and Jarvis, S . The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. (1989) 31:341–52. doi: 10.1111/j.1469-8749.1989.tb04003.x
39. Nordmark, E , Jarnlo, GB , and Hagglund, G . Comparison of the gross motor function measure and Paediatric evaluation of disability inventory in assessing motor function in children undergoing selective dorsal rhizotomy. Dev Med Child Neurol. (2000) 42:245–52. doi: 10.1017/S0012162200000426
40. Boyce, WF , Gowland, C , Hardy, S , Rosenbaum, PL , Lane, M , Plews, N, et al. Development of a quality-of-movement measure for children with cerebral palsy. Phys Ther. (1991) 71:813–9. doi: 10.1093/ptj/71.11.820
41. Boyce, WF , Gowland, C , Rosenbaum, PL , Lane, M , Plews, N , Goldsmith, CH, et al. The gross motor performance measure: validity and responsiveness of a measure of quality of movement. Phys Ther. (1995) 75:603–13. doi: 10.1093/ptj/75.7.603
42. Gowland, C , Boyce, WF , Wright, V , Russell, DJ , Goldsmith, CH , and Rosenbaum, PL . Reliability of the gross motor performance measure. Phys Ther. (1995) 75:597–602. doi: 10.1093/ptj/75.7.597
43. Pandyan, AD , Johnson, GR , Price, CI , Curless, RH , Barnes, MP , and Rodgers, H . A review of the properties and limitations of the Ashworth and modified Ashworth scales as measures of spasticity. Clin Rehabil. (1999) 13:373–83. doi: 10.1191/026921599677595404
44. Bohannon, RW , and Smith, MB . Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. (1987) 67:206–7. doi: 10.1093/ptj/67.2.206
45. Moraes, AG , Copetti, F , Angelo, VR , Chiavoloni, LL , and David, AC . The effects of hippotherapy on postural balance and functional ability in children with cerebral palsy. J Phys Ther Sci. (2016) 28:2220–6. doi: 10.1589/jpts.28.2220
46. Shurtleff, TL , Standeven, JW , and Engsberg, JR . Changes in dynamic trunk/head stability and functional reach after hippotherapy. Arch Phys Med Rehabil. (2009) 90:1185–95. doi: 10.1016/j.apmr.2009.01.026
47. Casady, RL , and Nichols-Larsen, DS . The effect of hippotherapy on ten children with cerebral palsy. Pediatr Phys Ther. (2004) 16:165–72. doi: 10.1097/01.PEP.0000136003.15233.0C
48. Bertoti, DB . Effect of therapeutic horseback riding on posture in children with cerebral palsy. Phys Ther. (1988) 68:1505–12.
49. Sterba, JA , Rogers, BT , France, AP , and Vokes, DA . Horseback riding in children with cerebral palsy: effect on gross motor function. Dev Med Child Neurol. (2002) 44:301–8. doi: 10.1111/j.1469-8749.2002.tb00815.x
50. McGibbon, NH , Andrade, CK , Widener, G , and Cintas, HL . Effect of an equine-movement therapy program on gait, energy expenditure, and motor function in children with spastic cerebral palsy: a pilot study. Dev Med Child Neurol. (1998) 40:754–62. doi: 10.1111/j.1469-8749.1998.tb12344.x
51. Frank, A , McCloskey, S , and Dole, RL . Effect of hippotherapy on perceived self-competence and participation in a child with cerebral palsy. Pediatr Phys Ther. (2011) 23:301–8. doi: 10.1097/PEP.0b013e318227caac
52. Wang, HY , and Yang, YH . Evaluating the responsiveness of 2 versions of the gross motor function measure for children with cerebral palsy. Arch Phys Med Rehabil. (2006) 87:51–6. doi: 10.1016/j.apmr.2005.08.117
53. Chang, HJ , Kwon, JY , Lee, JY , and Kim, YH . The effects of Hippotherapy on the motor function of children with spastic bilateral cerebral palsy. J Phys Ther Sci. (2012) 24:1277–80. doi: 10.1589/jpts.24.1277
54. Bower, E , Michell, D , Burnett, M , Campbell, MJ , and McLellan, DL . Randomized controlled trial of physiotherapy in 56 children with cerebral palsy followed for 18 months. Dev Med Child Neurol. (2001) 43:4–15. doi: 10.1017/S0012162201000020
55. Kwon, HY , and Ahn, SY . Effect of task-oriented training and high-variability practice on gross motor performance and activities of daily living in children with spastic diplegia. J Phys Ther Sci. (2016) 28:2843–8. doi: 10.1589/jpts.28.2843
56. Sorsdahl, AB , Moe-Nilssen, R , Kaale, HK , Rieber, J , and Strand, LI . Change in basic motor abilities, quality of movement and everyday activities following intensive, goal-directed, activity-focused physiotherapy in a group setting for children with cerebral palsy. BMC Pediatr. (2010) 10:26. doi: 10.1186/1471-2431-10-26
57. Oeffinger, D , Bagley, A , Rogers, S , Gorton, G , Kryscio, R , Abel, M, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Dev Med Child Neurol. (2008) 50:918–25. doi: 10.1111/j.1469-8749.2008.03150.x
58. Little, K , Nel, N , Ortell, V , Van Wyk, H , Badenhorst, M , and Louw, QA . The clinical effect on hippotherapy on gross motor function of children with cerebral palsy. SA. J Physiother. (2013) 69:26–34. doi: 10.4102/sajp.v69i2.321
59. McGibbon, NH , Benda, W , Duncan, BR , and Silkwood-Sherer, D . Immediate and long-term effects of hippotherapy on symmetry of adductor muscle activity and functional ability in children with spastic cerebral palsy. Arch Phys Med Rehabil. (2009) 90:966–74. doi: 10.1016/j.apmr.2009.01.011
60. Tseng, SH , Chen, HC , and Tam, KW . Systematic review and meta-analysis of the effect of equine assisted activities and therapies on gross motor outcome in children with cerebral palsy. Disabil Rehabil. (2013) 35:89–99. doi: 10.3109/09638288.2012.687033
61. Lechner, HE , Feldhaus, S , Gudmundsen, L , Hegemann, D , Michel, D , Zach, GA, et al. The short-term effect of hippotherapy on spasticity in patients with spinal cord injury. Spinal Cord. (2003) 41:502–5. doi: 10.1038/sj.sc.3101492
62. Lechner, HE , Kakebeeke, TH , Hegemann, D , and Baumberger, M . The effect of hippotherapy on spasticity and on mental well-being of persons with spinal cord injury. Arch Phys Med Rehabil. (2007) 88:1241–8. doi: 10.1016/j.apmr.2007.07.015
63. Cherng, RJ , Liao, HF , Leung, HW , and Hwang, AW . The effectiveness of therapeutic horseback riding in children with spastic cerebral palsy. Adapt Phys Act Q. (2004) 21:103–21. doi: 10.1123/apaq.21.2.103
64. Antunes, FN , Pinho, AS , Kleiner, AF , Salazar, AP , Eltz, GD , de Oliveira Junior, AA, et al. Different horse's paces during hippotherapy on spatio-temporal parameters of gait in children with bilateral spastic cerebral palsy: a feasibility study. Res Dev Disabil. (2016) 59:65–72. doi: 10.1016/j.ridd.2016.07.015
65. Tremblay, F , Malouin, F , Richards, CL , and Dumas, F . Effects of prolonged muscle stretch on reflex and voluntary muscle activations in children with spastic cerebral palsy. Scand J Rehabil Med. (1990) 22:171–80. doi: 10.2340/165019779022171180
66. Bronson, C , Brewerton, K , Ong, J , Palanca, C , and Sullivan, SJ . Does hippotherapy improve balance in persons with multiple sclerosis: a systematic review. Eur J Phys Rehabil Med. (2010) 46:347–53.
67. Stergiou, A , Tzoufi, M , Ntzani, E , Varvarousis, D , Beris, A , and Ploumis, A . Therapeutic effects of horseback riding interventions: a systematic review and Meta-analysis. Am J Phys Med Rehabil. (2017) 96:717–25. doi: 10.1097/PHM.0000000000000726
Keywords: Cerebral Palsy, equine assisted therapies, gross motor function, gross motor performance, spasticity
Citation: Stergiou AN, Mattila-Rautiainen S, Varvarousis DN, Tzoufi M, Plyta P, Beris A and Ploumis A (2023) The efficacy of Equine Assisted Therapy intervention in gross motor function, performance, and spasticity in children with Cerebral Palsy. Front. Vet. Sci. 10:1203481. doi: 10.3389/fvets.2023.1203481
Received: 10 April 2023; Accepted: 01 August 2023;
Published: 15 August 2023.
Edited by:
Selcuk Akpinar, Nevşehir Hacı Bektaş Veli University, TürkiyeReviewed by:
Kürşat Özcan, Nevşehir Hacı Bektaş Veli University, TürkiyeCopyright © 2023 Stergiou, Mattila-Rautiainen, Varvarousis, Tzoufi, Plyta, Beris and Ploumis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanna Mattila-Rautiainen, c2FubmEubWF0dGlsYS1yYXV0aWFpbmVuQHVlZi5maQ==; Alexandra N. Stergiou, YWxleHN0ZXJnaW91QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.